The Role of Proteomics in Identification of Key Proteins of Bacterial Cells with Focus on Probiotic Bacteria
Abstract
:1. Introduction
2. Proteomic Methods, Including MS-Based Approaches
3. Proteins of Probiotic Bacterial Cells and the Examples Studied by Proteomics
3.1. Whole Cell Lysates and Intracellular Proteins Identification
3.2. Surface Proteins Identification
3.3. Extracellular/Secreted Proteins Identification
3.4. The Proteins of EVs
4. Health-Related Functions of Probiotic Bacteria Examined by Proteomics
5. Concluding Remarks and Future Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The Internal Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Lebeer, S.; Bron, P.A.; Marco, M.L.; Van Pijkeren, J.P.; O’Connell Motherway, M.; Hill, C.; Pot, B.; Roos, S.; Klaenhammer, T. Identification of probiotic effector molecules: Present state and future perspectives. Curr. Opin. Biotechnol. 2018, 49, 217–223. [Google Scholar] [CrossRef]
- Gupta, R.S. Protein phylogenies and signature sequences: A reappraisal of evolutionary relationships among archaebacteria, eubacteria, and eukaryotes. Microbiol. Mol. Biol. Rev. 1998, 62, 1435–1491. [Google Scholar] [CrossRef]
- Scott, J.R.; Barnett, T.C. Surface proteins of gram-positive bacteria and how they get there. Annu. Rev. Microbiol. 2006, 60, 397–423. [Google Scholar] [CrossRef]
- Fischetti, V.A. Surface proteins on gram-positive bacteria. Microbiol. Spectr. 2019, 7, GPP3-0012-2018. [Google Scholar] [CrossRef] [PubMed]
- Flach, J.; van der Waal, M.B.; Kardinaal, A.F.M.; Schloesser, J.; Ruijschop, R.M.A.J.; Claasen, E. Probiotic research priorities for the healthy adult population: A review on the health benefits of Lactobacillus rhamnosus GG and Bifidobacterium animalis subspecies lactis BB-12. Cogent Food Agric. 2018, 66, 1452839. [Google Scholar] [CrossRef]
- Siciliano, R.A.; Mazzeo, M.F. Molecular mechanisms of probiotic action: A proteomic perspective. Curr. Opin. Microbiol. 2012, 15, 390–396. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Li, H.; Liu, X. The potential of proteins, hydrolysates and peptides as growth factors for Lactobacillus and Bifidobacterium: Current research and future perspectives. Food Funct. 2020, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Dominguez Rubio, A.P.D.; D’Antoni, C.L.; Piuri, M.; Perez, O.E. Probiotics, their extracellular vesicles and infectious diseases. Front. Microbiol. 2022, 13, 864720. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Sancez, M.; Carrasco-Navarro, U.; Juarez-Castelan, C.; Lozano-Aguirre Beltran, L.; Perez-Chabela, M.L.; Ponce-Alquicira, E. Probiotic properties and proteomic analysis of Pediococcus pentosaceus 1101. Foods 2022, 12, 46. [Google Scholar] [CrossRef]
- Szajewska, H.; Horvath, A. Lactobacillus rhamnosus GG in the primary prevention of eczema in children: A systematic review and meta-analysis. Nutrients 2018, 10, 1319. [Google Scholar] [CrossRef]
- Liu, S.; Hu, P.; Du, X.; Zhou, T.; Pei, X. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: A meta-analysis of randomized, placebo-controlled trials. Indian Pediatr. 2013, 50, 377–381. [Google Scholar] [CrossRef]
- van Baarlen, P.; Troost, F.J.; van Hemert, S.; Kleerebezem, M. Differential NF-kappaB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc. Natl. Acad. Sci. USA 2009, 106, 2371–2376. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.; Jiang, Z. Dietary additive probiotics modulation of the intestinal microbiota. Protein Pept. Lett. 2017, 24, 382–387. [Google Scholar] [CrossRef]
- Chan, H.H.Y.; Siu, P.L.K.; Choy, C.T.; Chan, U.K.; Zhou, J.; Wong, C.H.; Lee, Y.W.; Chan, H.W.; Tsui, J.C.C.; Loo, S.K.F.; et al. Novel multi-strain E3 probiotic formulation improved mental health symptoms and sleep quality in Hong Kong Chinese. Nutrients 2023, 15, 5037. [Google Scholar] [CrossRef]
- Ponda, P.P.; Mayer, L. Mucosal epithelium in health and disease. Curr. Mol. Med. 2005, 5, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, R.A.; Lippolis, R.; Mazzeo, M.F. Proteomics for the investigation of surface-exposed proteins in probiotics. Front. Nutr. 2019, 6, 52. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Probiotics and immune health. Curr. Opin. Gastroenterol. 2011, 27, 496–501. [Google Scholar] [CrossRef]
- Gandhi, A.; Shah, N.P. Integrating omics to unravel the stress-response mechanisms in probiotic bacteria: Approaches, challenges, and prospects. Crit. Rev. Food Sci. Nutr. 2017, 57, 3464–3471. [Google Scholar] [CrossRef] [PubMed]
- Remus, D.M.; Bongers, R.S.; Meijerink, M.; Fusetti, F.; Poolman, B.; de Vos, P.; Wells, J.M.; Kleerebezem, M.; Bron, P.A. Impact of Lactobacillus plantarum sortase on target protein sorting, gastrointestinal persistence, and host immune response modulation. J. Bacteriol. 2013, 195, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wei, M.; Yang, D.; Wu, X.; Wei, H.; Xu, F. Lactiplantibacillus plantarum strain FLPL05 promotes longevity in mice by improving intestinal barrier. Probiotics Antimicrob. Proteins 2023, 15, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.L. Antagonistic activities of lactobacilli and bifidobacterial against microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 405–440. [Google Scholar] [CrossRef] [PubMed]
- Corre, S.C.; Li, Y.; Riedel, C.U.; Gahan, C.G.M. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarus UCC118. Proc. Natl. Acad. Sci. USA 2007, 104, 7617–7621. [Google Scholar] [CrossRef] [PubMed]
- Makras, L.; Triantafyllou, V.; Fayol-Messaoudi, D.; Adriany, T.; Zoumpopoulou, G.; Tsakalidou, E.; Servin, A.; De Vuyst, L. Kinetic analysis of the antibacterial activity of probiotic lactobacilli towards Salmonella enterica serovar Typhimurium reveals a role for lactic acid and other inhibitory compounds. Res. Microbiol. 2006, 157, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak-Siedlecka, K.; Skonieczna-Zydecka, K.; Hupp, T.; Duchnowska, R.; Marek-Trzonkowska, N.; Polom, K. Next-generation probiotics—Do they open new therapeutic strategy for cancer patients? Gut Microbes 2022, 14, 2035659. [Google Scholar] [CrossRef] [PubMed]
- Dudik, B.; Kinova Sepova, H.; Greifova, G.; Bilka, F.; Bilkova, A. Next generation probiotics: An overview of the most promising candidates. Epidemiol. Mikrobiol. Imunol. 2022, 71, 48–56. [Google Scholar] [PubMed]
- Das, A.; Behera, R.N.; Kapoor, A.; Ambatipudi, K. The potential of meta-proteomics and artificial intelligence to establish the next generation of probiotics for personalized healthcare. J. Agric. Food Chem. 2023, 71, 17528–17542. [Google Scholar] [CrossRef]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A beneficial gut organism from the discoveries in genus and species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Pavan, S.; Kleerebezem, M. Towards understanding molecular modes of probiotic action. Curr. Opin. Biotechnol. 2006, 17, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Remaut, H.; Tang, C.; Henderson, N.S.; Pinkner, J.S.; Wang, T.; Hultgren, S.J.; Thanassi, D.G.; Waksman, G.; Li, H. Fiber formation across the bacterial outer membrane by the chaperone/usher pathway. Cell 2008, 133, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Sleytr, U.B.; Beveridge, T.J. Bacterial S-layers. Trends Microbiol. 1999, 7, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Cao, H.; Cover, T.L.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 2007, 132, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Grabik, K.A.; Waypa, T.S.; Musch, M.W.; Alverdy, J.C.; Schneewind, O.; Chang, E.B.; Petrof, E.O. Soluble factors from Lactobacillus GG activate MAPKS and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am. J. Physiol. Cell. Physiol. 2006, 290, C1018-30. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Polk, D.B. Characterization of a probiotic-derived soluble protein which reveals a mechanism of preventive and treatment effects of probiotics on intestinal inflammatory diseases. Gut Microbes 2012, 3, 25–28. [Google Scholar] [CrossRef]
- Aires, J.; Butel, M.J. Proteomics, human gut microbiota and probiotics. Expert Rev. Proteom. 2011, 8, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Hidalgo, C.; Blanco-Miguez, A.; Lourenco, A.; Sanchez, B.; Margolles, A. Tackling probiotic and gut microbiota functionality through proteomics. J. Proteom. 2016, 147, 29–39. [Google Scholar] [CrossRef]
- De Angelis, M.; Calasso, M.; Cavallo, N.; Di Cagno, R.; Gobbetti, M. Functional proteomics within the genus Lactobacillus. Proteomics 2016, 16, 946–962. [Google Scholar] [CrossRef]
- Martin, R.; Langella, P. Emerging health concepts in the probiotics field: Streamlining the definitions. Front. Microbiol. 2019, 10, 1047. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.; Kliem, M. Mass spectrometry tools for the classification and identification of bacteria. Nat. Rev. Microbiol. 2010, 8, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Welker, M. Proteomics for routine identification of microorganisms. Proteomics 2011, 11, 3143–3152. [Google Scholar] [CrossRef] [PubMed]
- Welker, M.; Van Belkum, A.; Girard, V.; Charrier, J.P.; Pincus, D. An update on the routine application of MALDI-TOF MS in clinical microbiology. Expert Rev. Proteom. 2019, 16, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, E.; Horvatovich, P.; Marchioni, E.; Aoude-Werner, D.; Sanz, Y.; Ennahar, S. 2-DE and MS analysis of key proteins in the adhesion of Lactobacillus plantarum, a first step toward early selection of probiotics based on bacterial biomarkers. Electrophoresis 2009, 30, 949–956. [Google Scholar] [CrossRef]
- Maffei, B.; Francetic, O.; Subtil, A. Tracking proteins secreted by bacteria: What’s in the toolbox? Front. Cell. Infect. Microbiol. 2017, 7, 221. [Google Scholar] [CrossRef] [PubMed]
- Abele, M.; Doll, E.; Bayer, F.P.; Meng, C.; Lomp, N.; Neuhaus, K.; Scherer, S.; Kuster, B.; Ludwig, C. Unified workflow for the rapid and in-depth characterization of bacterial proteomes. Mol. Cell. Proteom. 2023, 22, 100612. [Google Scholar] [CrossRef] [PubMed]
- Solis, N.; Cordwell, S.J. Current methodologies for proteomics of bacterial surface-exposed and cell envelope proteins. Proteomics 2011, 11, 3169–3189. [Google Scholar] [CrossRef] [PubMed]
- Bonn, F.; Maaß, S.; van Dijl, J.M. Enrichment of cell surface-associated proteins in Gram-positive bacteria by biotinylation or trypsin shaving for mass spectrometry analysis. Methods Mol. Biol. 2018, 1841, 35–43. [Google Scholar] [CrossRef]
- Solis, N.; Larsen, M.R.; Cordwell, S.J. Improved accuracy of cell surface shaving proteomics in Staphylococcus aureus using a false-positive control. Proteomics 2010, 10, 2037–2049. [Google Scholar] [CrossRef]
- Ong, S.; Blagoev, B.; Kratchmarova, I.; Kristensen, D.B.; Steen, H.; Pandey, A.; Mann, M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteom. 2002, 1, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, D.C.; Link, A.J.; Graumann, J.; Tirrell, D.A.; Schuman, E.M. Selective identification of newly synthesized proteins in mammalian cells using biorthogonal noncanonical amino acid tagging (BONCAT). Proc. Natl. Acad. Sci. USA 2006, 103, 9482–9487. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; McClatchy, D.B.; Barkallah, S.; Wood, W.W.; Yates, J.R., 3rd. Quantitative analysis of newly synthesized proteins. Nat. Protoc. 2018, 13, 1744–1762. [Google Scholar] [CrossRef] [PubMed]
- Lange, V.; Picotti, P.; Domon, B.; Aebersold, R. Selected reaction monitoring for quantitative proteomics: A tutorial. Mol. Syst. Biol. 2008, 4, 222. [Google Scholar] [CrossRef] [PubMed]
- Hamon, E.; Horvatovich, P.; Izquierdo, E.; Bringel, F.; Marchioni, E.; Aoude-Werner, D.; Ennahar, S. Comparative proteomic analysis of Lactobacillus plantarum for the identification of key proteins in bile tolerance. BMC Microbiol. 2011, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.M.; Sousa, C.S.; Oliveira, L.C.; Soares, S.C.; Souza, G.F.M.H.; Tavares, G.C.; Resende, C.P.; Folador, E.L.; Pereira, F.L.; Figueiredo, H.; et al. Comparative proteomic analysis of four biotechnological strains Lactococcus lactis through label-free quantitative proteomics. Microb. Biotechnol. 2019, 12, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yi, J.; Suo, K.; Kang, Q.; Lu, L.; Lu, J. Probiotic properties and proteomic analysis of ethanol-induced Lactococcus lactis subsp. lactis IL1403. World J. Microbiol. Biotechnol. 2023, 39, 197. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yi, J.; Kang, Q.; Song, M.; Raubenheimer, D.; Lu, J. Identification of a novel peptide with alcohol dehydrogenase activating ability from ethanol-induced Lactococcus lactis: A combined in silico prediction and in vivo validation. J. Agric. Food Chem. 2024, 72, 5746–5756. [Google Scholar] [CrossRef] [PubMed]
- Mbye, M.; Baig, M.A.; AbuQamar, S.F.; El-Tarabily, K.A.; Obaid, R.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Turner, M.S.; Shah, N.P.; Ayyash, M.M. Updates on understanding of probiotic lactic acid bacteria responses to environmental stresses and highlights on proteomic analyses. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1110–1124. [Google Scholar] [CrossRef]
- Beck, H.C.; Madsen, S.M.; Glenting, J.; Petersen, J.; Israelsen, H.; Norrelykke, M.R.; Antonsson, M.; Hansen, A.M. Proteomic analysis of cell surface-associated proteins from probiotic Lactobacillus plantarum. FEMS Microbiol. Lett. 2009, 297, 61–66. [Google Scholar] [CrossRef]
- Desvaux, M.; Dumas, E.; Chafsey, I.; Hebraud, M. Protein cell surface display in Gram-positive bacteria: From single protein to macromolecular protein structure. FEMS Microbiol. Lett. 2006, 256, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kwoji, I.D.; Aiyegoro, O.A.; Okpeku, M.; Adeleke, M.A. Elucidating the mechanisms of cell-to-cell crosstalk in probiotics co-culture: A proteomics study of Limosilactobacillus reuteri ZJ625 and Ligilactobacillus salivarius ZJ614. Probiotics Antimicrob. Proteins 2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xia, Y.; Cui, J.; Gu, Z.; Song, Y.; Chen, Y.Q.; Chen, H.; Zhang, H.; Chen, W. The roles of moonlighting proteins in bacteria. Curr. Issues Mol. Biol. 2014, 16, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, C.J. Intracellular/surface moonlighting proteins that aid in the attachment of gut microbiota to the host. AIMS Microbiol. 2019, 5, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Dramsi, S.; Bierne, H. Spatial organization of cell wall-anchored proteins at the surface of Gram-positive bacteria. In Protein and Sugar Export and Assembly in Gram-Positive Bacteria; Bagnoli, F., Rappuoli, R., Eds.; Current Topics in Microbiology and Immunology; Springer: Cham, Switzerland, 2016; Volume 404. [Google Scholar] [CrossRef]
- Fagan, R.P.; Fairweather, N.F. Biogenesis and functions of bacterial S-layers. Nat. Rev. Microbiol. 2014, 12, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, M.F.; Reale, A.; Di Renzo, T.; Siciliano, R.A. Surface layer protein pattern of Levilactobacillus brevis strains investigated by proteomics. Nutrients 2022, 14, 3679. [Google Scholar] [CrossRef] [PubMed]
- Klotz, C.; O’Flaherty, S.; Goh, Y.J.; Barrangou, R. Investigating the effect of growth phase on the surface-layer associated proteome of Lactobacillus acidophilus using quantitative proteomics. Front. Microbiol. 2017, 8, 2174. [Google Scholar] [CrossRef]
- Proft, T.; Baker, E.N. Pili in Gram-negative and Gram-positive bacteria—Structure, assembly and their role in disease. Cell. Mol. Life Sci. 2009, 66, 613–635. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Claes, I.; Tytgat, H.L.P.; Verhoeven, T.L.A.; Marien, E.; von Ossowski, I.; Reunanen, J.; Palva, A.; de Vos, W.M.; De Keersmaecker, S.C.J.; et al. Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 2012, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, Y.L.; Selle, K.; Yang, T.; Goh, Y.J.; Sahay, B.; Zadeh, M.; Owen, J.L.; Colliou, N.; Li, E.; Johannssen, T.; et al. SIGNR3-dependent immune regulation by Lactobacillus acidophilus surface layer protein A in colitis. EMBO J. 2015, 34, 881–895. [Google Scholar] [CrossRef]
- Calvo, E.; Pucciarelli, M.G.; Bierne, H.; Cossart, P.; Albar, J.P.; Garcia-Del Portillo, F. Analysis of the Listeria cell wall proteome by two-dimensional nanoliquid chromatography coupled to mass spectrometry. Proteomics 2005, 5, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Tjalsma, H.; van Dijl, J.M. Proteomic-based consensus prediction of protein retention in a bacterial membrane. Proteomics 2005, 5, 4472–4482. [Google Scholar] [CrossRef] [PubMed]
- Candela, M.; Bergmann, S.; Vici, M.; Vitali, B.; Turroni, S.; Eikmanns, B.J.; Hammerschmidt, S.; Brigidi, P. Binding of human plasminogen to Bifidobacterium. J. Bacteriol. 2007, 189, 5929–5936. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Escobedo, S.; Suarez, J.E.; Quiros, L.M. Widespread use of Lactobacillus OppA, a surface located protein, as an adhesin that recognizes epithelial cell surface glycosaminoglycans. Benef. Microbes 2019, 10, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Dubey, V.; Mishra, A.K.; Ghosh, A.R. Cell adherence efficacy of probiotic Pediococcus pentosaceus GS4 (MTCC 12683) and demonstrable role of its surface layer protein (Slp). J. Proteom. 2020, 226, 103894. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Xiong, Y.; Gu, Y.; Lei, Y.; An, H.; Yi, H.; Zhao, L.; Ren, F.; Hao, Y. Up-regulation of sortase-dependent pili in Bifidobacterium longum BBMN68 in response to bile stress enhances its adhesion to HT-29 cells. Int. J. Biol. Macromol. 2024, 257 Pt 2, 127527. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Dou, X.; Song, X.; Chang, J.; Zeng, X.; Zhu, L.; Xu, C. Selenite bioremediation by food-grade probiotic Lactobacillus casei ATCC 393: Insights from proteomics analysis. Microbiol. Spectr. 2023, 11, e0065923. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, X.; Wang, G.; Xia, Y.; Xiong, Z.; Ai, L. Understanding Ligilactobacillus salivarius from probiotic properties to omics technology: A review. Foods 2024, 13, 895. [Google Scholar] [CrossRef] [PubMed]
- Gilad, O.; Svensson, B.; Viborg, A.H.; Stuer-Lauridsen, B.; Jacobsen, S. The extracellular proteome of Bifidobacterium animalis subsp. lactis BB-12 reveals proteins with putative roles in probiotic effects. Proteomics 2011, 11, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- Bagon, B.B.; Oh, J.K.; Valeriano, V.D.V.; Pajarillo, E.A.B.; Kang, D.K. Exploring the bile stress response of Lactobacillus mucosae LM1 through exoproteome analysis. Molecules 2021, 26, 5695. [Google Scholar] [CrossRef]
- Sanchez, B.; Urdaci, M.C.; Margolles, A. Extracellular proteins secreted by probiotic bacteria as mediators of effects that promote mucosa-bacteria interactions. Microbiology 2010, 156, 3232–3242. [Google Scholar] [CrossRef] [PubMed]
- Krzyzek, P.; Marinacci, B.; Vitele, I.; Grande, R. Extracellular vesicles of probiotics: Shedding light on the biological activity and future applications. Pharmaceutics 2023, 15, 522. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating extracellular vesicles in human disease. N. Engl. J. Med. 2018, 379, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Nah, G.; Park, S.C.; Kim, K.; Kim, S.; Park, J.; Lee, S.; Won, S. Type-2 diabetics reduces spatial variation of microbiome based on extracellular vesicles from gut microbes across human body. Sci. Rep. 2019, 9, 20136. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Drummen, G.P.C.; Mathivanan, S. Focus on extracellular vesicles: Introducing the next small big thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Stastna, M. Advances in separation and identification of biologically important milk proteins and peptides. Electrophoresis 2023, 45, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and their potential applications in early life nutrition and beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed]
- Vindertola, G.; Sanders, M.E.; Salminen, S. The concept of postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Kulig, K.; Kowalik, K.; Surowiec, M.; Karnas, E.; Barczyk-Woznicka, O.; Zuba-Surma, E.; Pyza, E.; Kozik, A.; Rapala-Kozik, M.; Karkowska-Kuleta, J. Isolation and characteristics of extracellular vesicles produced by probiotics: Yeast Saccharomyces boulardii CNCM I-745 and Bacterium streptococcus salivarius K12. Probiotics Antimicrob. Proteins 2023, 16, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Chen, Y.Z.; Shen, T.L.; Pan, T.M.; Hsu, W.H. Proteomic characterization of extracellular vesicles derived from lactic acid bacteria. Food Chem. 2023, 427, 136685. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, A.; He, D.; Wu, X.; Yan, H.; Zhu, L. Isolation and proteomic analysis of extracellular vesicles from Lactobacillus salivarius SNK-6. J. Microbiol. Biotechnol. 2024, 34, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Rodovalho, V.R.; da Luz, B.S.R.; Nicolas, A.; Jardin, J.; Briard-Bion, V.; Folador, E.L.; Santos, A.R.; Jan, G.; Loir, Y.L.; Azevedo, V.A.C.; et al. Different culture media and purification methods unveil the core proteome of Propionibacterium freudenreichii-derived extracellular vesicles. Microlife 2023, 4, uqad029. [Google Scholar] [CrossRef]
- de Rezende Rodovalho, V.; da Luz, B.S.R.; Nicolas, A.; do Carmo, F.L.R.; Jardin, J.; Briard-Bion, V.; Jan, G.; Le Loir, Y.; de Carvalho Azevedo, V.A.; Guedon, E. Environmental conditions modulate the protein content and immunomodulatory activity of extracellular vesicles produced by the probiotic Propionibacterium freudenreichii. Appl. Environ. Microbiol. 2021, 87, e02263-20. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Sugrue, I.; Tobin, C.; Hill, C.; Stanton, C.; Ross, R.P. The Lactobacillus casei group: History and health related applications. Front. Microbiol. 2018, 9, 2107. [Google Scholar] [CrossRef]
- Huang, R.; Wang, K.; Hu, J. Effect of probiotics on depression: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef]
- Borgeraas, H.; Johnson, L.K.; Skattebu, J.; Hertel, J.K.; Hjemesaeth, J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2018, 19, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Cuello-Garcia, C.A.; Brozek, J.L.; Fiocchi, A.; Pawankar, R.; Yepes-Nunez, J.J.; Terracciano, L.; Gandhi, S.; Agarwal, A.; Zhang, Y.; Schunemann, H.J. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J. Allergy Clin. Immunol. 2015, 136, 952–961. [Google Scholar] [CrossRef] [PubMed]
- So, S.S.Y.; Wan, M.L.Y.; El-Nezami, H. Probiotics-mediated suppression of cancer. Curr. Opin. Oncol. 2017, 29, 62–72. [Google Scholar] [CrossRef]
- Raslan, M.A.; Raslan, S.A.; Shehata, E.M.; Mahmoud, A.S.; Viana, M.V.C.; Barh, D.; Sabri, N.A.; Azevedo, V. Applications of proteomics in probiotics having anticancer and chemopreventive properties. Adv. Exp. Med. Biol. 2024, 1443, 243–256. [Google Scholar] [CrossRef]
- Beltran-Velasco, A.I.; Reiriz, M.; Uceda, S.; Echeverry-Alzate, V. Lactiplantibacillus (Lactobacillus) plantarum as a complementary treatment to improve symptomatology in neurodegenerative disease: A systematic review of open access literature. Int. J. Mol. Sci. 2024, 25, 3010. [Google Scholar] [CrossRef]
- Cafaro, G.; Cruciani, G.; Bruno, L.; Dal Pozzolo, R.; Colangelo, A.; Tromby, F.; Nicchi, M.; Pianese, B.; Perricone, C.; Gerli, R.; et al. Microbiota and arthritis: Cause or consequence? Clin. Exp. Rheumatol. 2024, 42, 1097–1103. [Google Scholar] [CrossRef]
- Jarosz, L.S.; Socala, K.; Michalak, K.; Wiater, A.; Ciszewski, A.; Majewska, M.; Marek, A.; Gradzki, Z.; Wlaz, P. The effect of psychoactive bacteria, Bifidobacterium longum Rosell®-175 and Lactobacillus rgamnosus JB-1, on brain proteome profiles in mice. Psychopharmacology 2024, 241, 925–945. [Google Scholar] [CrossRef] [PubMed]
- Cufaro, M.C.; Prete, R.; Di Marco, F.; Sabatini, G.; Corsetti, A.; Gonzalez, N.G.; Del Boccio, P.; Battista, N. A proteomic insight reveals the role of food-associated Lactiplantibacillus plantarum C9O4 in reverting intestinal inflammation. iScience 2023, 26, 108481. [Google Scholar] [CrossRef] [PubMed]
- Averina, O.A.; Kovtun, A.S.; Mavletova, D.A.; Ziganshin, R.H.; Danilenko, V.N.; Mihaylova, D.; Blazheva, D.; Slavchev, A.; Brazkova, M.; Ibrahim, S.A.; et al. Oxidative stress response of probiotic strain Bifidobacterium longum subsp. longum GT15. Foods 2023, 12, 3356. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Siciliano, R.A.; Reale, A.; Mazzeo, M.F.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A new perspective for functional foods and nutraceuticals. Nutrients 2021, 13, 1225. [Google Scholar] [CrossRef]
- Haranahalli Nataraj, B.; Behare, P.V.; Yadav, H.; Srivastava, A.K. Emerging pre-clinical safety assessment for potential probiotic strains: A review. Crit. Rev. Food Sci. Nutr. 2023, 1–29. [Google Scholar] [CrossRef]
- Zucko, J.; Starcevic, A.; Diminic, J.; Oros, D.; Mortazavian, A.M.; Putnik, P. Probiotic—Friend or foe? Curr. Opin. Food Sci. 2020, 32, 45–49. [Google Scholar] [CrossRef]
- Wu, F.; Xie, X.; Du, T.; Jiang, X.; Miao, W.; Wang, T. Lactococcus lactis, a bacterium with probiotic functions and pathogenicity. World J. Microbiol. Biotechnol. 2023, 39, 325. [Google Scholar] [CrossRef]
- Pasala, S.; Singer, L.; Arshad, T.; Roach, K. Lactobacillus endocarditis in a healthy patient with probiotic use. IDCases 2020, 22, e00915. [Google Scholar] [CrossRef]
- Rahman, A.; Alqaisi, S.; Nath, J. A case of Lactobacillus casei endocarditis associated with probiotic intake in an immunocompromised patient. Cureus 2023, 15, e38049. [Google Scholar] [CrossRef] [PubMed]
- Stastna, M. Post-translational modifications of proteins in cardiovascular diseases examined by proteomic approaches. FEBS J. 2024. Early View. [Google Scholar] [CrossRef]
- Kwoji, I.D.; Aiyegoro, O.A.; Okpeku, M.; Adeleke, M.A. ‘Multi-omics’ data integration: Application in probiotics studies. npj Sci. Food 2023, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Ferrocino, I.; Rentsiou, K.; McClure, R.; Kostic, T.; de Souza, R.S.C.; Lange, L.; FitzGerald, J.; Kriaa, A.; Cotter, P.; Maguin, E.; et al. Microbiome Support Consortium. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1082–1103. [Google Scholar] [CrossRef] [PubMed]
- Rajczewski, A.T.; Jagtap, P.D.; Griffin, T.J. An overview of technologies for MS-based proteomics-centric multiomics. Expert Rev. Proteomics 2022, 19, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Laghi, L.; Correani, V.; Schifano, E.; Landi, C.; Uccelletti, D.; Mattei, B. A combined proteomics, metabolomics and in vivo analysis approach for the characterization of probiotics in large-scale production. Biomolecules 2020, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Preidis, G.A.; Weizman, A.V.; Kashyap, P.C.; Morgan, R.L. AGA technical review on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology 2020, 159, 708–738. [Google Scholar] [CrossRef] [PubMed]
- Al-Fakhrany, O.; Elekhnawy, E. Next-generation probiotics: The upcoming biotherapeutics. Mol. Biol. Rep. 2024, 51, 505. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Helmy, Y.A. Next-generation probiotics as novel therapeutics for improving human health: Current trends and future perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef]
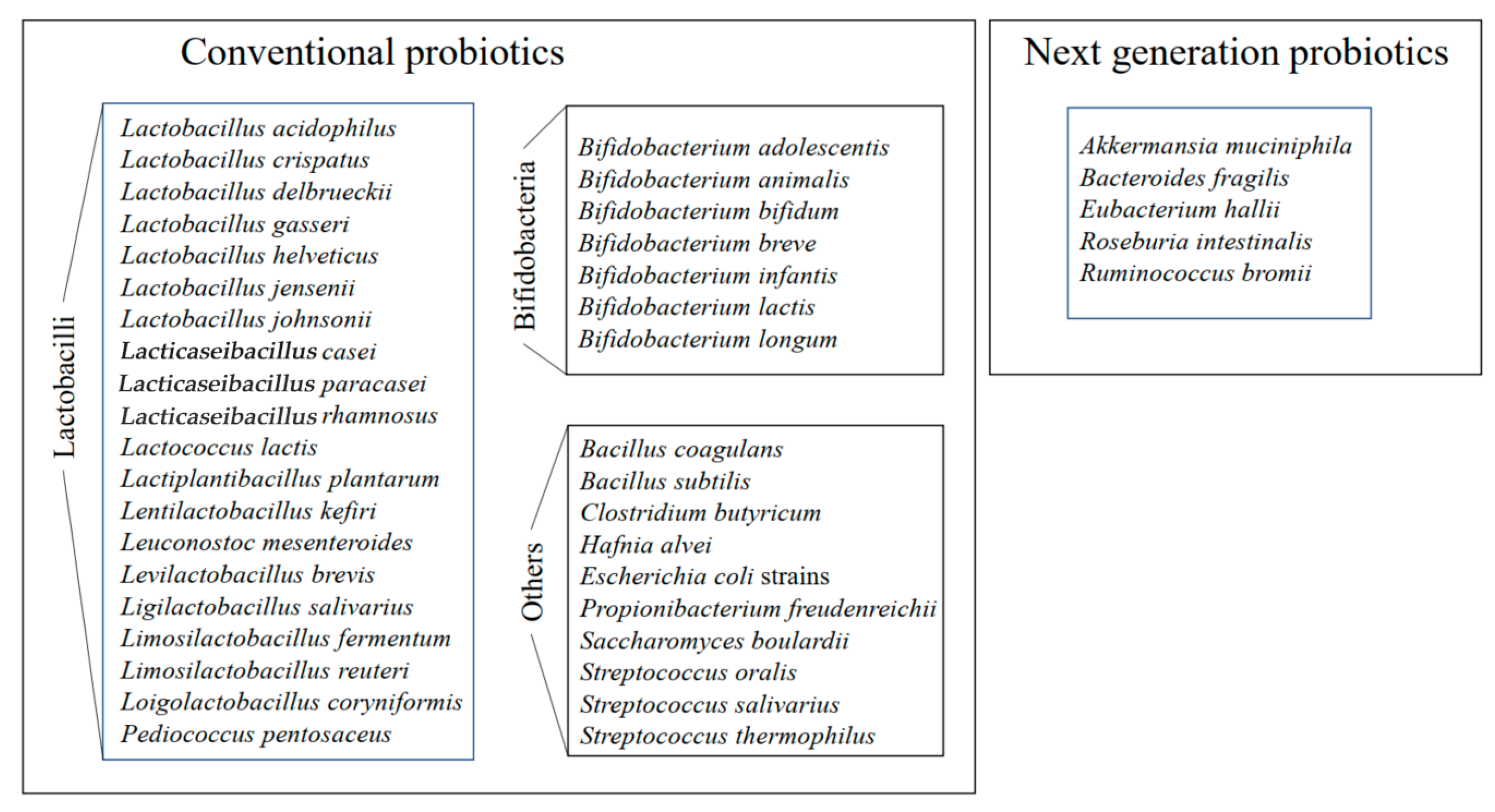
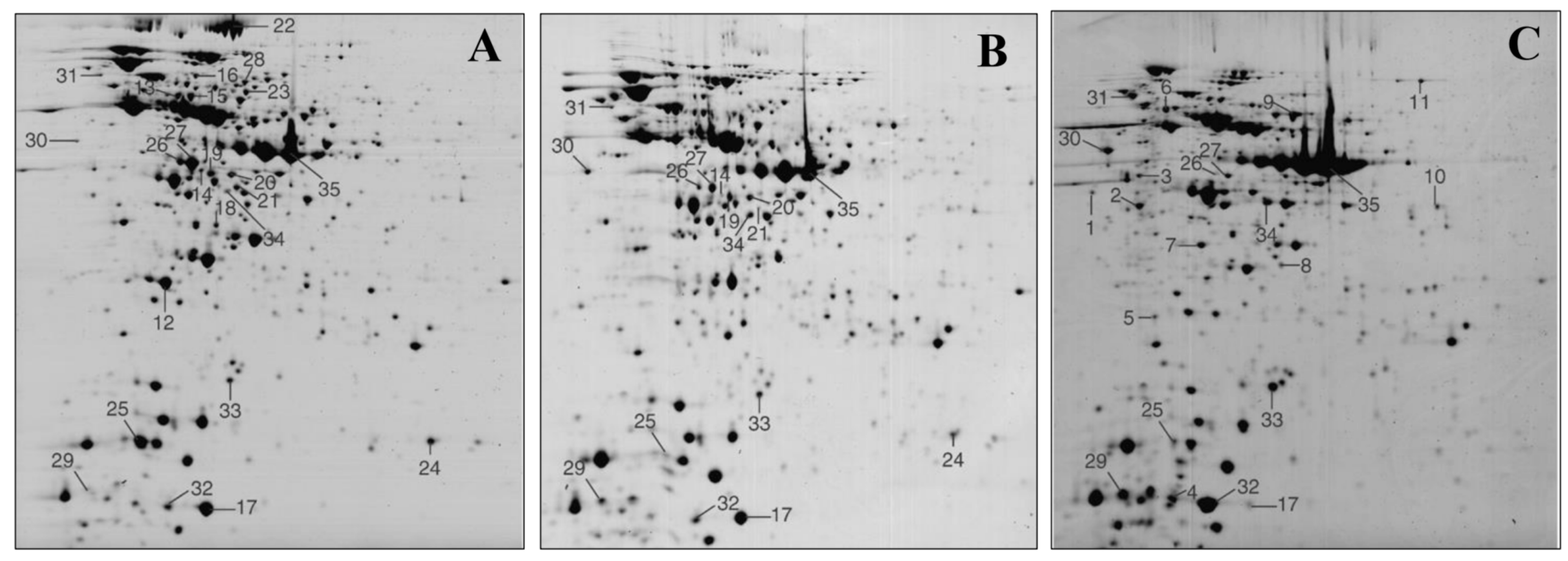

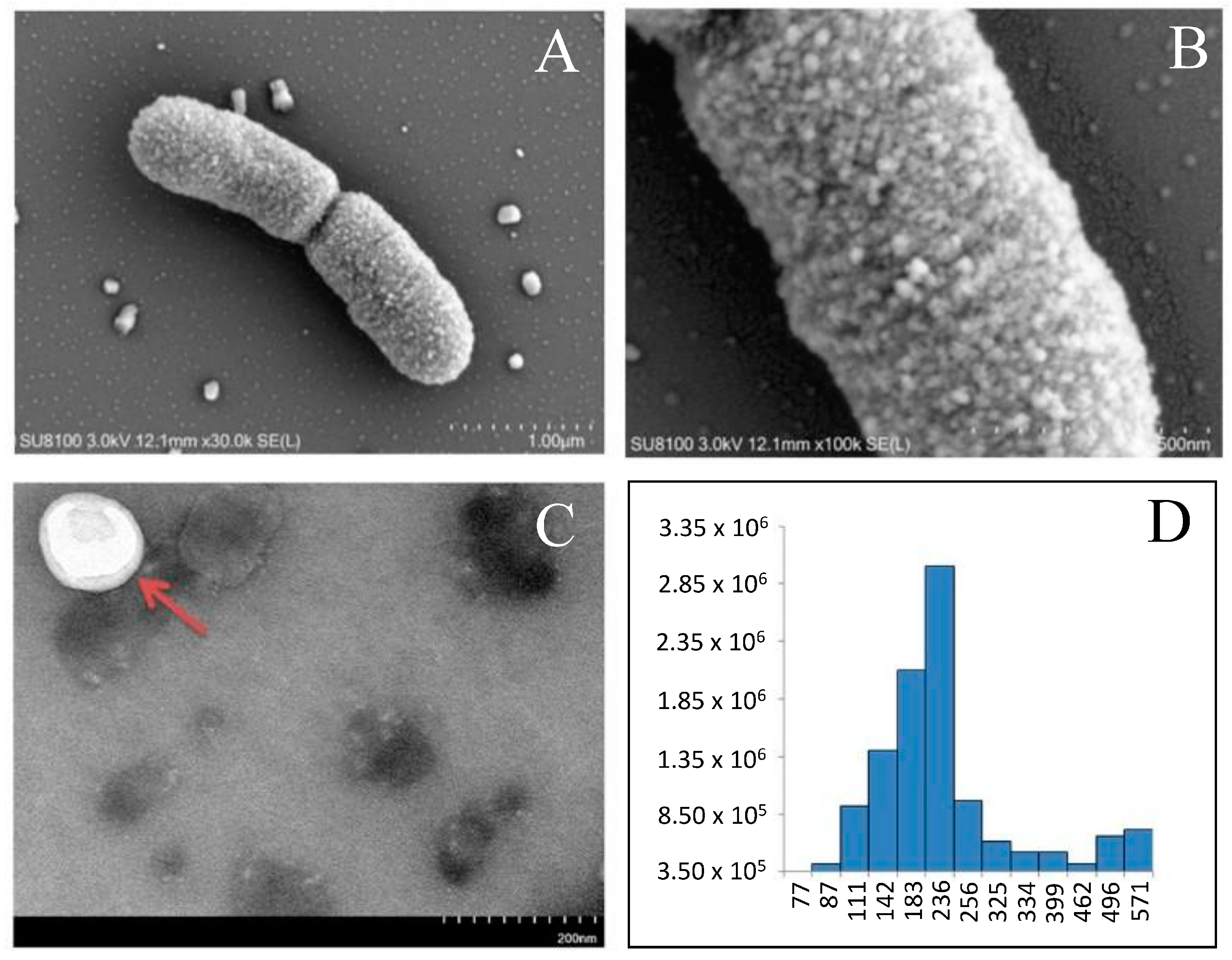
| Probiotics | Type of Proteins Examined | Findings | Ref./ Year |
|---|---|---|---|
| Pediococcus pentosaceus 1101 | intracellular proteins and whole-cell lysates | A total of 100 proteins were differentially expressed under exposure of the probiotic strain to 0.5% bile salts and acidity; two kinases and N-acetylmuramoyl-L-alanine amidase were identified as having crucial roles in adaptation of probiotic strain to conditions in the gastrointestinal tract. | [10]/ 2022 |
| Lactococcus lactis subsp. lactis NCDO2118; lactis IL1403; cremoris NZ9000; cremoris MG1363 | intracellular proteins and whole-cell lysates | The core proteome for L. lactis strains was determined (586 proteins) as responsible for strains stress resistance; strain-unique proteins were identified in three strains; 19 and 3 novel proteins to IL1403 and MG1363 strain, respectively, were found. | [56]/ 2019 |
| Lactococcus lactis subsp. lactis IL1403 | intracellular proteins and whole-cell lysates | Over 400 proteins were altered in the probiotic strain under ethanol-induced stress; stress-related proteins increased the activity of alcohol dehydrogenase. | [57]/ 2023 |
| Lactococcus lactis subsp. lactis IL1403 | intracellular proteins and whole-cell lysates | The molecular mechanism of ethanol-induced stress on the probiotic strain was determined using both in silico prediction and in vivo validation in a mice model. The FAPEG peptide exhibited the strongest ability to enhance alcohol dehydrogenase activity and prevent alcoholic liver injury. | [58]/ 2024 |
| Levilactobacillus brevis strains PA6; A4; A7; M4; DSMZ 20054 | surface proteins | Ten different S-layer proteins (Slps) were identified on these probiotic strains; simultaneous detection of eight Slps were found on the S-layer of A7 strain; 40 S-layer-associated proteins (SLAPs) were detected as common to all strains, however, with different quantities. | [67]/ 2022 |
| Lactobacillus crispatus Lv25; Lactobacillus reuteri RC14 | surface proteins | The presence of surface oligopeptide-binding proteins (OppA-like proteins) was confirmed in these strains; OppA proteins participated in the binding of diverse lactobacilli to the surface of human epithelial cells. | [75]/ 2019 |
| Pediococcus pentosaceus GS4 | surface proteins | The probiotic strain produced 98 kDa surface layer protein Slp that contributed to its adherence to epithelial cells in vitro. | [76]/ 2020 |
| Bifidobacterium longum BBMN68 | surface proteins | Out of 829 proteins identified with altered expression, 56 proteins were upregulated in the probiotic strain under a bile stress; the crucial role of pili (Pil1) was recognized in increased B. longum BBMN68 adhesion to HT-29 cells under bile stress conditions. | [77]/ 2024 |
| Lactobacillus casei ATCC 393 | surface proteins | Probiotic strain under sodium selenite treatment synthesized Se nanoparticles (SeNPs) by decreasing toxic sodium selenite into elemental selenium; SeNPs were coated by proteins in range 11–17 kDa with the most abundant 50S ribosomal protein L7/L12. | [78]/ 2023 |
| Limosilactobacillus reuteri ZJ625; Ligilactobacillus salivarius ZJ614 | extracellular/secreted proteins | Differentially expressed proteins were identified in co-culture of these two strains as compared to the cultures of each individual strain; the crosstalk between two strains in their co-culture was mediated by S-ribosylhomocysteine lyase. | [62]/ 2023 |
| Lactobacillus mucosae LM1 | extracellular/secreted proteins | The differences in exoproteome composition of probiotic strain under treatment by various bile concentrations indicated that this bacterium regulated its exoproteome by secretion of various intracellular proteins. | [81]/ 2021 |
| Lactobacillus plantarum; Lactobacillus fermentum; Lactobacillus gasseri | proteins of EVs | As the most abundant protein, GAPDH was identified in the EVs of L. plantarum, the enolase in EVs of L. gasseri, and the citrate lyase alpha chain in the EVs of L. fermentum; EVs from L. fermentum and L. gasseri were composed of plasma membrane proteins, the EVs from L. plantarum contained more cytosolic proteins. | [91]/ 2023 |
| Lactobacillus salivarius SNK-6 | proteins of EVs | In total, 320 proteins were identified (10–38 kDa) in the spherical EVs with diameters in the range of 100–256 nm; various anti-inflammatory proteins were detected. | [92]/ 2024 |
| Propionibacterium freudenreichii CIRM-BIA129 | proteins of EVs | EVs from probiotics were prepared by two purification methods and from two different culture media; the differences in protein content were related rather to EVs purification methods than to the type of culture media used. | [93]/ 2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stastna, M. The Role of Proteomics in Identification of Key Proteins of Bacterial Cells with Focus on Probiotic Bacteria. Int. J. Mol. Sci. 2024, 25, 8564. https://doi.org/10.3390/ijms25168564
Stastna M. The Role of Proteomics in Identification of Key Proteins of Bacterial Cells with Focus on Probiotic Bacteria. International Journal of Molecular Sciences. 2024; 25(16):8564. https://doi.org/10.3390/ijms25168564
Chicago/Turabian StyleStastna, Miroslava. 2024. "The Role of Proteomics in Identification of Key Proteins of Bacterial Cells with Focus on Probiotic Bacteria" International Journal of Molecular Sciences 25, no. 16: 8564. https://doi.org/10.3390/ijms25168564





