An Overview of Altered Pathways Associated with Sensitivity to Platinum-Based Chemotherapy in Neuroendocrine Tumors: Strengths and Prospects
Abstract
1. Introduction
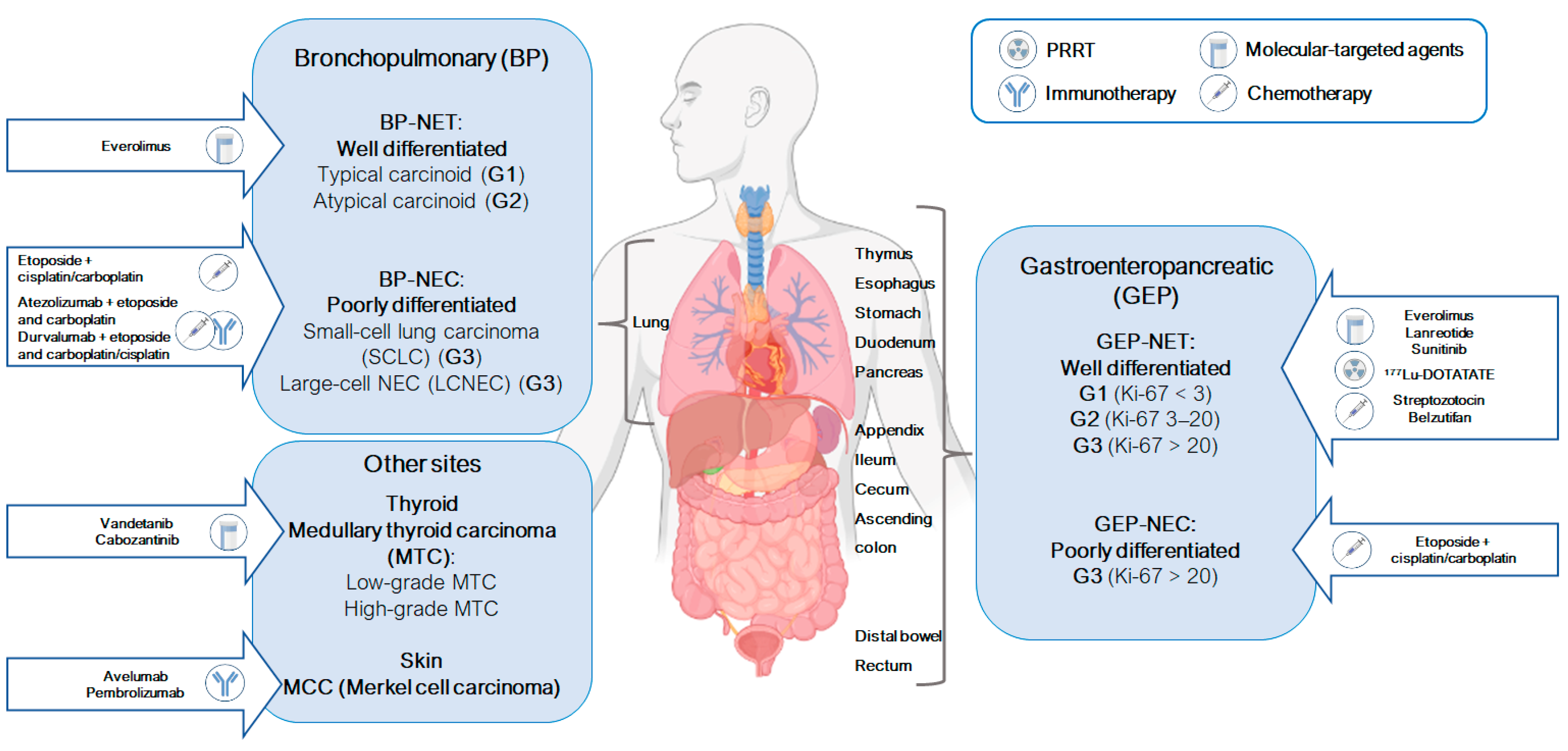
1.1. Most Common Neuroendocrine Neoplasms
1.1.1. Gastroenteropancreatic Neuroendocrine Neoplasms (GEP-NENs)
1.1.2. Bronchopulmonary Neuroendocrine Neoplasms (BP-NENs)
2. Approved Therapeutic Options for Neuroendocrine Neoplasms
3. Platinum-Based Chemotherapy
General Mechanism of Action of Cisplatin and Carboplatin
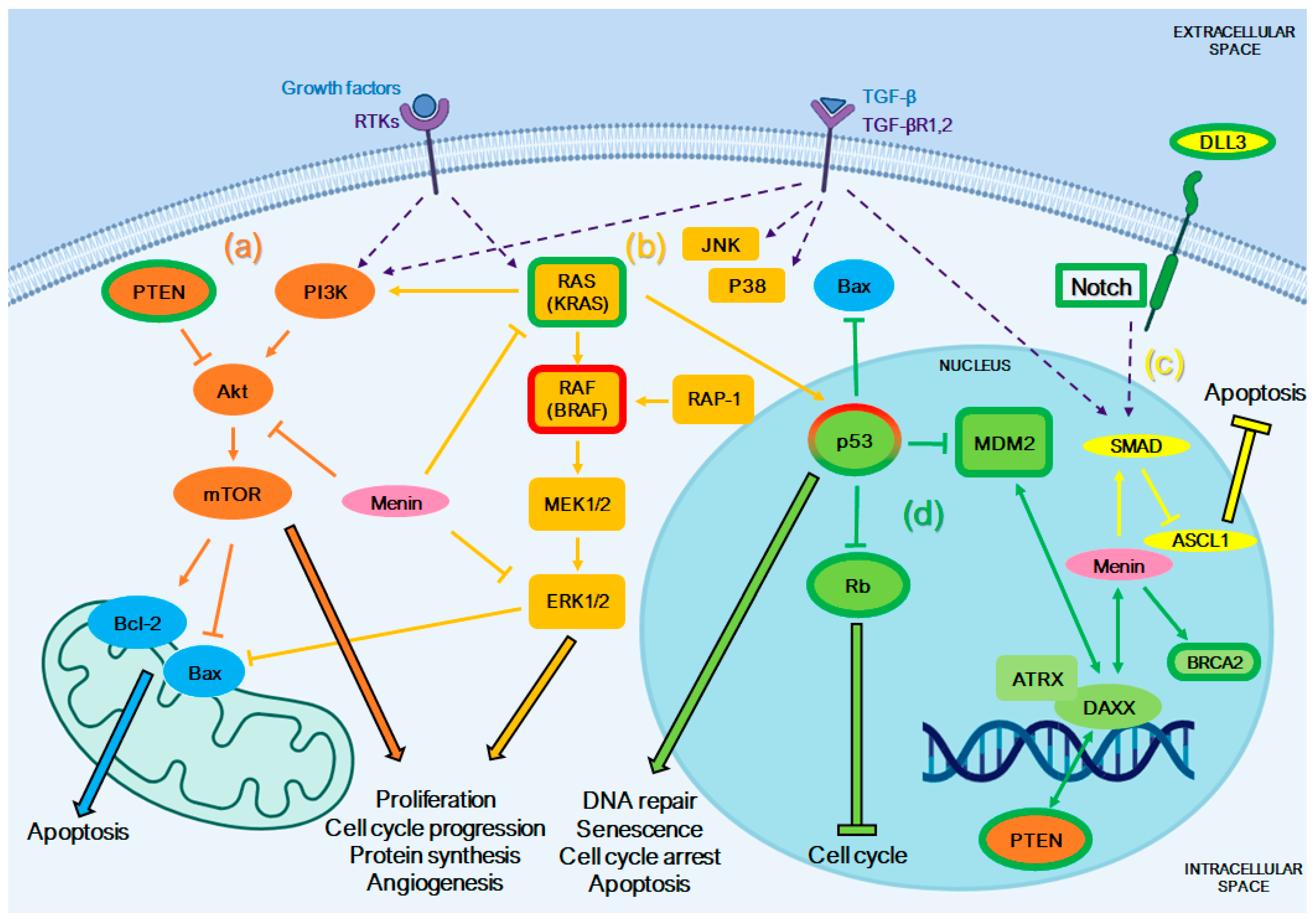
4. Altered Pathways in NENs and Platinum-Based Chemotherapy Sensitivity
4.1. PTEN/PI3K/Akt/mTOR Pathway
4.2. Mitogen-Activated Protein Kinase (MAPK) Pathway
4.3. Notch/ASCL1 Pathway
4.4. Pathways Involved in DNA Repair
4.5. Other Genomic Alterations
5. Influence of Specific Cellular Pathways in the Response to Platinum-Based Therapy in NENs
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef]
- Lamberti, G.; Brighi, N.; Maggio, I.; Manuzzi, L.; Peterle, C.; Ambrosini, V.; Ricci, C.; Casadei, R.; Campana, D. The Role of mTOR in Neuroendocrine Tumors: Future Cornerstone of a Winning Strategy? Int. J. Mol. Sci. 2018, 19, 747. [Google Scholar] [CrossRef]
- Kaltsas, G.A.; Besser, G.M.; Grossman, A.B. The Diagnosis and Medical Management of Advanced Neuroendocrine Tumors. Endocr. Rev. 2004, 25, 458–511. [Google Scholar] [CrossRef] [PubMed]
- Schimmack, S.; Svejda, B.; Lawrence, B.; Kidd, M.; Modlin, I.M. The Diversity and Commonalities of Gastroenteropancreatic Neuroendocrine Tumors. Langenbeck’s Arch. Surg. 2011, 396, 273–298. [Google Scholar] [CrossRef]
- Rossi, R.E.; Massironi, S. The Increasing Incidence of Neuroendocrine Neoplasms Worldwide: Current Knowledge and Open Issues. J. Clin. Med. 2022, 11, 3794. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Olarte, P.; La Salvia, A.; Riesco-Martinez, M.C.; Anton-Pascual, B.; Garcia-Carbonero, R. Chemotherapy in NEN: Still Has a Role? Rev. Endocr. Metab. Disord. 2021, 22, 595–614. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- La Rosa, S.; Uccella, S. Classification of Neuroendocrine Neoplasms: Lights and Shadows. Rev. Endocr. Metab. Disord. 2021, 22, 527–538. [Google Scholar] [CrossRef]
- Rorstad, O. Prognostic Indicators for Carcinoid Neuroendocrine Tumors of the Gastrointestinal Tract. J. Surg. Oncol. 2005, 89, 151–160. [Google Scholar] [CrossRef]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A Common Classification Framework for Neuroendocrine Neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) Expert Consensus Proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef]
- Kunz, P.L.; Reidy-Lagunes, D.; Anthony, L.B.; Bertino, E.M.; Brendtro, K.; Chan, J.A.; Chen, H.; Jensen, R.T.; Kim, M.K.; Klimstra, D.S.; et al. Consensus Guidelines for the Management and Treatment of Neuroendocrine Tumors. Pancreas 2013, 42, 557–577. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Nagura, H. A Comparative Study of Neuroendocrine Carcinoma and Carcinoid Tumor with Special Reference to Expression of HLA-DR Antigen and PCNA. Zentralbl. Pathol. 1993, 139, 171–175. [Google Scholar] [PubMed]
- La Rosa, S.; Sessa, F.; Uccella, S. Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (MiNENs): Unifying the Concept of a Heterogeneous Group of Neoplasms. Endocr. Pathol. 2016, 27, 284–311. [Google Scholar] [CrossRef] [PubMed]
- Bocchini, M.; Nicolini, F.; Severi, S.; Bongiovanni, A.; Ibrahim, T.; Simonetti, G.; Grassi, I.; Mazza, M. Biomarkers for Pancreatic Neuroendocrine Neoplasms (PanNENs) Management—An Updated Review. Front. Oncol. 2020, 10, 831. [Google Scholar] [CrossRef]
- La Salvia, A.; Siciliani, A.; Rinzivillo, M.; Verrico, M.; Baldelli, R.; Puliani, G.; Modica, R.; Zanata, I.; Persano, I.; Fanciulli, G.; et al. Thyroid Transcription Factor-1 Expression in Lung Neuroendocrine Tumours: A Gender-Related Biomarker? Endocrine 2024, 83, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Mete, O. Cytokeratin Profiles in Pituitary Neuroendocrine Tumors. Hum. Pathol. 2021, 107, 87–95. [Google Scholar] [CrossRef]
- Gut, P.; Komarowska, H.; Czarnywojtek, A.; Waligórska-Stachura, J.; Bączyk, M.; Ziemnicka, K.; Fischbach, J.; Wrotkowska, E.; Ruchała, M. Familial Syndromes Associated with Neuroendocrine Tumours. Contemp. Oncol./Współczesna Onkol. 2015, 3, 176–183. [Google Scholar] [CrossRef]
- Murakumo, Y.; Jijiwa, M.; Asai, N.; Ichihara, M.; Takahashi, M. RET and Neuroendocrine Tumors. Pituitary 2006, 9, 179–192. [Google Scholar] [CrossRef]
- Estrella, J.S.; Broaddus, R.R.; Mathews, A.; Milton, D.R.; Yao, J.C.; Wang, H.; Rashid, A. Progesterone Receptor and PTEN Expression Predict Survival in Patients with Low- and Intermediate-Grade Pancreatic Neuroendocrine Tumors. Arch. Pathol. Lab. Med. 2014, 138, 1027–1036. [Google Scholar] [CrossRef]
- Pulvirenti, A.; Raj, N.; Cingarlini, S.; Pea, A.; Tang, L.H.; Luchini, C.; Chou, J.F.; Grego, E.; Marinova, I.; Capanu, M.; et al. Platinum-Based Treatment for Well- and Poorly Differentiated Pancreatic Neuroendocrine Neoplasms. Pancreas 2021, 50, 138–146. [Google Scholar] [CrossRef]
- Rinke, A.; Gress, T.M. Neuroendocrine Cancer, Therapeutic Strategies in G3 Cancers. Digestion 2017, 95, 109–114. [Google Scholar] [CrossRef]
- Brown, A.; Kumar, S.; Tchounwou, P.B. Cisplatin-Based Chemotherapy of Human Cancers. J. Cancer Sci. Ther. 2019, 11, 97. [Google Scholar]
- Sorbye, H.; Welin, S.; Langer, S.W.; Vestermark, L.W.; Holt, N.; Osterlund, P.; Dueland, S.; Hofsli, E.; Guren, M.G.; Ohrling, K.; et al. Predictive and Prognostic Factors for Treatment and Survival in 305 Patients with Advanced Gastrointestinal Neuroendocrine Carcinoma (WHO G3): The NORDIC NEC Study. Ann. Oncol. 2013, 24, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Strosberg, J.; Baudin, E.; Klimstra, D.S.; Yao, J.C. Gastroenteropancreatic High-Grade Neuroendocrine Carcinoma. Cancer 2014, 120, 2814–2823. [Google Scholar] [CrossRef]
- Fottner, C.; Ferrata, M.; Weber, M.M. Hormone Secreting Gastro-Entero-Pancreatic Neuroendocrine Neoplasias (GEP-NEN): When to Consider, How to Diagnose? Rev. Endocr. Metab. Disord. 2017, 18, 393–410. [Google Scholar] [CrossRef]
- Kulke, M.H.; Shah, M.H.; Benson, A.B.; Bergsland, E.; Berlin, J.D.; Blaszkowsky, L.S.; Emerson, L.; Engstrom, P.F.; Fanta, P.; Giordano, T.; et al. Neuroendocrine Tumors, Version 1.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 78–108. [Google Scholar] [CrossRef]
- Daskalakis, K. Functioning and Nonfunctioning pNENs. Curr. Opin. Endocr. Metab. Res. 2021, 18, 284–290. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Kunz, P.L. Making Sense of a Complex Disease: A Practical Approach to Managing Neuroendocrine Tumors. JCO Oncol. Pract. 2022, 18, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Ooki, A.; Osumi, H.; Fukuda, K.; Yamaguchi, K. Potent Molecular-Targeted Therapies for Gastro-Entero-Pancreatic Neuroendocrine Carcinoma. Cancer Metastasis Rev. 2023, 42, 1021–1054. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. The Epidemiology of Metastases in Neuroendocrine Tumors: Epidemiology of Metastases. Int. J. Cancer 2016, 139, 2679–2686. [Google Scholar] [CrossRef]
- Rekhtman, N. Lung Neuroendocrine Neoplasms: Recent Progress and Persistent Challenges. Mod. Pathol. 2022, 35, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Fisseler-Eckhoff, A.; Demes, M. Neuroendocrine Tumors of the Lung. Cancers 2012, 4, 777–798. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.L.; Van Suylen, R.J.; Thunnissen, E.; den Bakker, M.A.; Groen, H.J.; Smit, E.F.; Damhuis, R.A.; Van Den Broek, E.C.; Speel, E.-J.M.; Dingemans, A.-M.C.; et al. Chemotherapy for Pulmonary Large Cell Neuroendocrine Carcinomas: Does the Regimen Matter? Eur. Respir. J. 2017, 49, 1601838. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Santos-Zabala, M.L.; Iyriboz, T.; Woo, K.M.; Sima, C.S.; Fiore, J.J.; Kris, M.G.; Riely, G.J.; Lito, P.; Iqbal, A.; et al. Large Cell Neuroendocrine Carcinoma of the Lung: Clinico-Pathologic Features, Treatment, and Outcomes. Clin. Lung Cancer 2016, 17, e121–e129. [Google Scholar] [CrossRef] [PubMed]
- Zappi, A.; Persano, I.; Galvani, L.; Parlagreco, E.; Andrini, E.; Campana, D.; Brizzi, M.P.; Lamberti, G.; La Salvia, A. Chemotherapy in Well Differentiated Neuroendocrine Tumors (NET) G1, G2, and G3: A Narrative Review. J. Clin. Med. 2023, 12, 717. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.H.; Basturk, O.; Sue, J.J.; Klimstra, D.S. A Practical Approach to the Classification of WHO Grade 3 (G3) Well-Differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. Am. J. Surg. Pathol. 2016, 40, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Vijayvergia, N.; Boland, P.M.; Handorf, E.; Gustafson, K.S.; Gong, Y.; Cooper, H.S.; Sheriff, F.; Astsaturov, I.; Cohen, S.J.; Engstrom, P.F. Molecular Profiling of Neuroendocrine Malignancies to Identify Prognostic and Therapeutic Markers: A Fox Chase Cancer Center Pilot Study. Br. J. Cancer 2016, 115, 564–570. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive Genomic Profiles of Small Cell Lung Cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Torniai, M.; Rinaldi, S.; Morgese, F.; Ricci, G.; Onofri, A.; Ghroé, C.; Berardi, R. Medical Therapy for Advanced Gastro-Entero-Pancreatic and Bronchopulmonary Neuroendocrine Tumors. J. Cancer Metastasis Treat. 2016, 2, 329. [Google Scholar] [CrossRef][Green Version]
- Spada, F.; Valente, M. Review of Recents Advances in Medical Treatment for Neuroendocrine Neoplasms: Somatostatin Analogs and Chemotherapy. J. Cancer Metastasis Treat. 2016, 2, 313. [Google Scholar] [CrossRef][Green Version]
- Zandee, W.T.; de Herder, W.W. The Evolution of Neuroendocrine Tumor Treatment Reflected by ENETS Guidelines. Neuroendocrinology 2018, 106, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Porras, M.; Cárdenas-Salas, J.; Álvarez-Escolá, C. Somatostatin Analogs in Clinical Practice: A Review. Int. J. Mol. Sci. 2020, 21, 1682. [Google Scholar] [CrossRef]
- Rogoza, O.; Megnis, K.; Kudrjavceva, M.; Gerina-Berzina, A.; Rovite, V. Role of Somatostatin Signalling in Neuroendocrine Tumours. Int. J. Mol. Sci. 2022, 23, 1447. [Google Scholar] [CrossRef]
- Nuñez, J.E.; Donadio, M.; Filho, D.R.; Rego, J.F.; Barros, M.; Formiga, M.N.; Lopez, R.; Riechelmann, R. The Efficacy of Everolimus and Sunitinib in Patients with Sporadic or Germline Mutated Metastatic Pancreatic Neuroendocrine Tumors. J. Gastrointest. Oncol. 2019, 10, 645–651. [Google Scholar] [CrossRef]
- Yoo, C.; Cho, H.; Song, M.J.; Hong, S.-M.; Kim, K.; Chang, H.-M.; Chae, H.; Kim, T.W.; Hong, Y.S.; Ryu, M.-H.; et al. Efficacy and Safety of Everolimus and Sunitinib in Patients with Gastroenteropancreatic Neuroendocrine Tumor. Cancer Chemother. Pharmacol. 2017, 79, 139–146. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.-L.; Bang, Y.-J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the Treatment of Advanced, Non-Functional Neuroendocrine Tumours of the Lung or Gastrointestinal Tract (RADIANT-4): A Randomised, Placebo-Controlled, Phase 3 Study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- Okafor, C.; Hogan, J.; Raygada, M.; Thomas, B.J.; Akshintala, S.; Glod, J.W.; Del Rivero, J. Update on Targeted Therapy in Medullary Thyroid Cancer. Front. Endocrinol. 2021, 12, 708949. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A.; Gosnell, J.E.; Gagel, R.F.; Moley, J.; Pfister, D.; Sosa, J.A.; Skinner, M.; Krebs, A.; Vasselli, J.; Schlumberger, M. Vandetanib for the Treatment of Patients with Locally Advanced or Metastatic Hereditary Medullary Thyroid Cancer. J. Clin. Oncol. 2010, 28, 767–772. [Google Scholar] [CrossRef]
- Carra, S.; Gaudenzi, G.; Dicitore, A.; Saronni, D.; Cantone, M.C.; Plebani, A.; Ghilardi, A.; Borghi, M.O.; Hofland, L.J.; Persani, L.; et al. Vandetanib versus Cabozantinib in Medullary Thyroid Carcinoma: A Focus on Anti-Angiogenic Effects in Zebrafish Model. Int. J. Mol. Sci. 2021, 22, 3031. [Google Scholar] [CrossRef]
- Pavel, M.; Valle, J.W.; Eriksson, B.; Rinke, A.; Caplin, M.; Chen, J.; Costa, F.; Falkerby, J.; Fazio, N.; Gorbounova, V.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms: Systemic Therapy—Biotherapy and Novel Targeted Agents. Neuroendocrinology 2017, 105, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Al-Toubah, T.; El-Haddad, G.; Strosberg, J. 177Lu-DOTATATE for the Treatment of Gastroenteropancreatic Neuroendocrine Tumors. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Albertelli, M.; Dotto, A.; Nista, F.; Veresani, A.; Patti, L.; Gay, S.; Sciallero, S.; Boschetti, M.; Ferone, D. Present and Future of Immunotherapy in Neuroendocrine Tumors. Rev. Endocr. Metab. Disord. 2021, 22, 615–636. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Lebbé, C.; Mortier, L.; Brohl, A.S.; Fazio, N.; Grob, J.-J.; Prinzi, N.; Hanna, G.J.; Hassel, J.C.; Kiecker, F.; et al. First-Line Avelumab in a Cohort of 116 Patients with Metastatic Merkel Cell Carcinoma (JAVELIN Merkel 200): Primary and Biomarker Analyses of a Phase II Study. J. Immunother. Cancer 2021, 9, e002646. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Arvind, D.; Dowlati, A.; Mohamed, A. Role of Immunotherapy in Gastro-enteropancreatic Neuroendocrine Neoplasms (Gep-nens): Current Advances and Future Directions. J. Neuroendocrinol. 2021, 33, e12943. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Al-Toubah, T.; Strosberg, J. Chemotherapy in Neuroendocrine Tumors. Cancers 2021, 13, 4872. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.G.; Hanley, J.A.; Johnson, L.A. Streptozocin Alone Compared with Streptozocin plus Fluorouracil in the Treatment of Advanced Islet-Cell Carcinoma. N. Engl. J. Med. 1980, 303, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Murraylyon, I. Treatment of Multiple-Hormone-Producing Malignant Islet-Cell Tumour with Streptozotocin. Lancet 1968, 292, 895–898. [Google Scholar] [CrossRef]
- Capdevila, J.; Ducreux, M.; García Carbonero, R.; Grande, E.; Halfdanarson, T.; Pavel, M.; Tafuto, S.; Welin, S.; Valentí, V.; Salazar, R. Streptozotocin, 1982–2022: Forty Years from the FDA’s Approval to Treat Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2022, 112, 1155–1167. [Google Scholar] [CrossRef]
- Moertel, C.G.; Lefkopoulo, M.; Lipsitz, S.; Hahn, R.G.; Klaassen, D. Streptozocin–Doxorubicin, Streptozocin–Fluorouracil, or Chlorozotocin in the Treatment of Advanced Islet-Cell Carcinoma. N. Engl. J. Med. 1992, 326, 519–523. [Google Scholar] [CrossRef]
- Pelle, E.; Al-Toubah, T.; Morse, B.; Strosberg, J. Belzutifan in a Patient with VHL-Associated Metastatic Pancreatic Neuroendocrine Tumor. J. Natl. Compr. Cancer Netw. 2022, 20, 1285–1287. [Google Scholar] [CrossRef]
- Cives, M.; Ghayouri, M.; Morse, B.; Brelsford, M.; Black, M.; Rizzo, A.; Meeker, A.; Strosberg, J. Analysis of Potential Response Predictors to Capecitabine/Temozolomide in Metastatic Pancreatic Neuroendocrine Tumors. Endocr.-Relat. Cancer 2016, 23, 759–767. [Google Scholar] [CrossRef]
- Bajetta, E.; Catena, L.; Procopio, G.; De Dosso, S.; Bichisao, E.; Ferrari, L.; Martinetti, A.; Platania, M.; Verzoni, E.; Formisano, B.; et al. Are Capecitabine and Oxaliplatin (XELOX) Suitable Treatments for Progressing Low-Grade and High-Grade Neuroendocrine Tumours? Cancer Chemother. Pharmacol. 2007, 59, 637–642. [Google Scholar] [CrossRef]
- Lassen, U.; Kristjansen, P.E.G.; Østerlind, K.; Bergman, B.; Sigsgaard, T.C.; Hirsch, F.R.; Hansen, M.; Dombernowsky, P.; Hansen, H.H. Superiority of Cisplatin or Carboplatin in Combination with Teniposide and Vincristine in the Induction Chemotherapy of Small-Cell Lung Cancer. A Randomized Trial with 5 Years Follow Up. Ann. Oncol. 1996, 7, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.J.; Lippard, S.J. Synthetic Methods for the Preparation of Platinum Anticancer Complexes. Chem. Rev. 2014, 114, 4470–4495. [Google Scholar] [CrossRef]
- Baik, M.-H.; Friesner, R.A.; Lippard, S.J. Theoretical Study of Cisplatin Binding to Purine Bases: Why Does Cisplatin Prefer Guanine over Adenine? J. Am. Chem. Soc. 2003, 125, 14082–14092. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Tomida, A.; Tsuruo, T. Drug Resistance Mediated by Cellular Stress Response to the Microenvironment of Solid Tumors. Anti-Cancer Drug Des. 1999, 14, 169–177. [Google Scholar]
- De Castro, F.; Stefàno, E.; De Luca, E.; Benedetti, M.; Fanizzi, F.P. Platinum-Nucleos(t)Ide Compounds as Possible Antimetabolites for Antitumor/Antiviral Therapy: Properties and Perspectives. Pharmaceutics 2023, 15, 941. [Google Scholar] [CrossRef]
- Stefàno, E.; De Castro, F.; De Luca, E.; Muscella, A.; Marsigliante, S.; Benedetti, M.; Fanizzi, F.P. Synthesis and Comparative Evaluation of the Cytotoxic Activity of Cationic Organometallic Complexes of the Type [Pt(η1-CH2-CH2-OR)(DMSO)(Phen)]+ (R = Me, Et, Pr, Bu). Inorganica Chim. Acta 2023, 546, 121321. [Google Scholar] [CrossRef]
- De Castro, F.; De Luca, E.; Benedetti, M.; Fanizzi, F.P. Platinum Compounds as Potential Antiviral Agents. Coord. Chem. Rev. 2022, 451, 214276. [Google Scholar] [CrossRef]
- De Castro, F.; Stefàno, E.; Migoni, D.; Iaconisi, G.N.; Muscella, A.; Marsigliante, S.; Benedetti, M.; Fanizzi, F.P. Synthesis and Evaluation of the Cytotoxic Activity of Water-Soluble Cationic Organometallic Complexes of the Type [Pt(η1-C2H4OMe)(L)(Phen)]+ (L = NH3, DMSO; Phen = 1,10-Phenanthroline). Pharmaceutics 2021, 13, 642. [Google Scholar] [CrossRef]
- Benedetti, M.; De Castro, F.; Romano, A.; Migoni, D.; Piccinni, B.; Verri, T.; Lelli, M.; Roveri, N.; Fanizzi, F.P. Adsorption of the Cis-[Pt(NH3)2(P2O7)]2− (Phosphaplatin) on Hydroxyapatite Nanocrystals as a Smart Way to Selectively Release Activated Cis-[Pt(NH3)2Cl2] (Cisplatin) in Tumor Tissues. J. Inorg. Biochem. 2016, 157, 73–79. [Google Scholar] [CrossRef]
- Benedetti, M.; Antonucci, D.; De Castro, F.; Girelli, C.R.; Lelli, M.; Roveri, N.; Fanizzi, F.P. Metalated Nucleotide Chemisorption on Hydroxyapatite. J. Inorg. Biochem. 2015, 153, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Girelli, C.R.; Antonucci, D.; De Pascali, S.A.; Fanizzi, F.P. New Method for the Synthesis of [PtCl{η1-CH2C(O)R}(N-N)] Ketonyl Derivatives Starting from the Zeise’s Salt. Inorganica Chim. Acta 2014, 413, 109–114. [Google Scholar] [CrossRef]
- Carrisi, C.; Antonucci, D.; Lunetti, P.; Migoni, D.; Girelli, C.R.; Dolce, V.; Fanizzi, F.P.; Benedetti, M.; Capobianco, L. Transport of Platinum Bonded Nucleotides into Proteoliposomes, Mediated by Drosophila Melanogaster Thiamine Pyrophosphate Carrier Protein (DmTpc1). J. Inorg. Biochem. 2014, 130, 28–31. [Google Scholar] [CrossRef]
- Benedetti, M.; Lamacchia, V.; Antonucci, D.; Papadia, P.; Pacifico, C.; Natile, G.; Fanizzi, F.P. Insertion of Alkynes into Pt–X Bonds of Square Planar [PtX2(N^N)] (X = Cl, Br, I) Complexes. Dalton Trans. 2014, 43, 8826–8834. [Google Scholar] [CrossRef]
- Paprocka, R.; Wiese-Szadkowska, M.; Janciauskiene, S.; Kosmalski, T.; Kulik, M.; Helmin-Basa, A. Latest Developments in Metal Complexes as Anticancer Agents. Coord. Chem. Rev. 2022, 452, 214307. [Google Scholar] [CrossRef]
- De Castro, F.; Ciardullo, G.; Fanizzi, F.P.; Prejanò, M.; Benedetti, M.; Marino, T. Incorporation of N7-Platinated Guanines into Thermus Aquaticus (Taq) DNA Polymerase: Atomistic Insights from Molecular Dynamics Simulations. Int. J. Mol. Sci. 2023, 24, 9849. [Google Scholar] [CrossRef]
- De Castro, F.; Benedetti, M.; Antonaci, G.; Del Coco, L.; De Pascali, S.; Muscella, A.; Marsigliante, S.; Fanizzi, F. Response of Cisplatin Resistant Skov-3 Cells to [Pt(O,O′-Acac)(γ-Acac)(DMS)] Treatment Revealed by a Metabolomic 1H-NMR Study. Molecules 2018, 23, 2301. [Google Scholar] [CrossRef]
- De Castro, F.; Vergaro, V.; Benedetti, M.; Baldassarre, F.; Del Coco, L.; Dell’Anna, M.M.; Mastrorilli, P.; Fanizzi, F.P.; Ciccarella, G. Visible Light-Activated Water-Soluble Platicur Nanocolloids: Photocytotoxicity and Metabolomics Studies in Cancer Cells. ACS Appl. Bio Mater. 2020, 3, 6836–6851. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-Based Drugs for Cancer Therapy and Anti-Tumor Strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Lucaciu, R.L.; Hangan, A.C.; Sevastre, B.; Oprean, L.S. Metallo-Drugs in Cancer Therapy: Past, Present and Future. Molecules 2022, 27, 6485. [Google Scholar] [CrossRef]
- González-Ballesteros, M.M.; Mejía, C.; Ruiz-Azuara, L. Metallodrugs: An Approach against Invasion and Metastasis in Cancer Treatment. FEBS Open Bio 2022, 12, 880–899. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Sadler, P.J. Exploration of the Medical Periodic Table: Towards New Targets. Chem. Commun. 2013, 49, 5106. [Google Scholar] [CrossRef]
- Saad, J.S.; Benedetti, M.; Natile, G.; Marzilli, L.G. NMR Studies of Models Having the Pt(d(GpG)) 17-Membered Macrocyclic Ring Formed in DNA by Platinum Anticancer Drugs: Pt Complexes with Bulky Chiral Diamine Ligands. Inorg. Chem. 2011, 50, 4559–4571. [Google Scholar] [CrossRef]
- Skowron, M.A.; Oing, C.; Bremmer, F.; Ströbel, P.; Murray, M.J.; Coleman, N.; Amatruda, J.F.; Honecker, F.; Bokemeyer, C.; Albers, P.; et al. The Developmental Origin of Cancers Defines Basic Principles of Cisplatin Resistance. Cancer Lett. 2021, 519, 199–210. [Google Scholar] [CrossRef]
- Schabel, F.M.; Trader, M.W.; Laster, W.R.; Corbett, T.H.; Griswold, D.P. Cis-Dichlorodiammineplatinum(II): Combination Chemotherapy and Cross-Resistance Studies with Tumors of Mice. Cancer Treat. Rep. 1979, 63, 1459–1473. [Google Scholar]
- Ciarimboli, G. Membrane Transporters as Mediators of Cisplatin Effects and Side Effects. Scientifica 2012, 2012, 473829. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Hirota, T.; Sakiyama, R.; Baba, M.; Ieiri, I. Identification of Drug Transporters Contributing to Oxaliplatin-induced Peripheral Neuropathy. J. Neurochem. 2019, 148, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Planells-Cases, R.; Lutter, D.; Guyader, C.; Gerhards, N.M.; Ullrich, F.; Elger, D.A.; Kucukosmanoglu, A.; Xu, G.; Voss, F.K.; Reincke, S.M.; et al. Subunit Composition of VRAC Channels Determines Substrate Specificity and Cellular Resistance to P T-based Anti-cancer Drugs. EMBO J. 2015, 34, 2993–3008. [Google Scholar] [CrossRef]
- Frezza, M.; Hindo, S.; Chen, D.; Davenport, A.; Schmitt, S.; Tomco, D.; Ping Dou, Q. Novel Metals and Metal Complexes as Platforms for Cancer Therapy. Curr. Pharm. Des. 2010, 16, 1813–1825. [Google Scholar] [CrossRef]
- Sousa, G.F.D.; Wlodarczyk, S.R.; Monteiro, G. Carboplatin: Molecular Mechanisms of Action Associated with Chemoresistance. Braz. J. Pharm. Sci. 2014, 50, 693–701. [Google Scholar] [CrossRef]
- Yu, F.; Megyesi, J.; Price, P.M. Cytoplasmic Initiation of Cisplatin Cytotoxicity. Am. J. Physiol. Ren. Physiol. 2008, 295, F44–F52. [Google Scholar] [CrossRef]
- Raudenska, M.; Balvan, J.; Fojtu, M.; Gumulec, J.; Masarik, M. Unexpected Therapeutic Effects of Cisplatin. Metallomics 2019, 11, 1182–1199. [Google Scholar] [CrossRef]
- Becker, J.P.; Weiss, J.; Theile, D. Cisplatin, Oxaliplatin, and Carboplatin Unequally Inhibit in Vitro mRNA Translation. Toxicol. Lett. 2014, 225, 43–47. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Michels, J.; Brenner, C.; Szabadkai, G.; Harel-Bellan, A.; Castedo, M.; Kroemer, G. Systems Biology of Cisplatin Resistance: Past, Present and Future. Cell Death Dis. 2014, 5, e1257. [Google Scholar] [CrossRef]
- Lazarević, T.; Rilak, A.; Bugarčić, Ž.D. Platinum, Palladium, Gold and Ruthenium Complexes as Anticancer Agents: Current Clinical Uses, Cytotoxicity Studies and Future Perspectives. Eur. J. Med. Chem. 2017, 142, 8–31. [Google Scholar] [CrossRef]
- Hah, S.S.; Stivers, K.M.; De Vere White, R.W.; Henderson, P.T. Kinetics of Carboplatin−DNA Binding in Genomic DNA and Bladder Cancer Cells As Determined by Accelerator Mass Spectrometry. Chem. Res. Toxicol. 2006, 19, 622–626. [Google Scholar] [CrossRef]
- Aldossary, S.A. Review on Pharmacology of Cisplatin: Clinical Use, Toxicity and Mechanism of Resistance of Cisplatin. Biomed. Pharmacol. J. 2019, 12, 07–15. [Google Scholar] [CrossRef]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of Cytotoxic Action and Molecular Basis of Resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef]
- Singh, S.; Upadhyay, A.K.; Ajay, A.K.; Bhat, M.K. P53 Regulates ERK Activation in Carboplatin Induced Apoptosis in Cervical Carcinoma: A Novel Target of P53 in Apoptosis. FEBS Lett. 2007, 581, 289–295. [Google Scholar] [CrossRef]
- Guégan, J.-P.; Ezan, F.; Théret, N.; Langouët, S.; Baffet, G. MAPK Signaling in Cisplatin-Induced Death: Predominant Role of ERK1 over ERK2 in Human Hepatocellular Carcinoma Cells. Carcinogenesis 2013, 34, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-B.; Byun, H.-J.; Park, S.H.; Park, C.-Y.; Lee, S.-H.; Rho, S.B. CYR61 Controls P53 and NF-κB Expression through PI3K/Akt/mTOR Pathways in Carboplatin-Induced Ovarian Cancer Cells. Cancer Lett. 2012, 315, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Kleih, M.; Böpple, K.; Dong, M.; Gaißler, A.; Heine, S.; Olayioye, M.A.; Aulitzky, W.E.; Essmann, F. Direct Impact of Cisplatin on Mitochondria Induces ROS Production That Dictates Cell Fate of Ovarian Cancer Cells. Cell Death Dis. 2019, 10, 851. [Google Scholar] [CrossRef]
- Muslimović, A.; Nyström, S.; Gao, Y.; Hammarsten, O. Numerical Analysis of Etoposide Induced DNA Breaks. PLoS ONE 2009, 4, e5859. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Li, S.-Z.; Zhang, Z.-Y.; Hu, X.-M.; Hou, P.-N.; Gao, L.; Du, R.-L.; Zhang, X.-D. Nemo-like Kinase Is Critical for P53 Stabilization and Function in Response to DNA Damage. Cell Death Differ. 2014, 21, 1656–1663. [Google Scholar] [CrossRef]
- Jamil, S.; Lam, I.; Majd, M.; Tsai, S.-H.; Duronio, V. Etoposide Induces Cell Death via Mitochondrial-Dependent Actions of P53. Cancer Cell Int. 2015, 15, 79. [Google Scholar] [CrossRef]
- Karpinich, N.O.; Tafani, M.; Rothman, R.J.; Russo, M.A.; Farber, J.L. The Course of Etoposide-Induced Apoptosis from Damage to DNA and P53 Activation to Mitochondrial Release of Cytochromec. J. Biol. Chem. 2002, 277, 16547–16552. [Google Scholar] [CrossRef]
- Day, T.W.; Wu, C.-H.; Safa, A.R. Etoposide Induces Protein Kinase Cδ- and Caspase-3-Dependent Apoptosis in Neuroblastoma Cancer Cells. Mol. Pharmacol. 2009, 76, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.D.; Enoksson, M.; Suomela, M.; Zhivotovsky, B.; Orrenius, S. Caspase-2 Acts Upstream of Mitochondria to Promote Cytochromec Release during Etoposide-Induced Apoptosis. J. Biol. Chem. 2002, 277, 29803–29809. [Google Scholar] [CrossRef]
- Xie, B.-S.; Zhao, H.-C.; Yao, S.-K.; Zhuo, D.-X.; Jin, B.; Lv, D.-C.; Wu, C.-L.; Ma, D.-L.; Gao, C.; Shu, X.-M.; et al. Autophagy Inhibition Enhances Etoposide-Induced Cell Death in Human Hepatoma G2 Cells. Int. J. Mol. Med. 2011, 27, 599–606. [Google Scholar] [CrossRef]
- Montecucco, A.; Zanetta, F.; Biamonti, G. Molecular Mechanisms of Etoposide. EXCLI J. 2015, 14, 95. [Google Scholar] [CrossRef]
- Katayama, M.; Kawaguchi, T.; Berger, M.S.; Pieper, R.O. DNA Damaging Agent-Induced Autophagy Produces a Cytoprotective Adenosine Triphosphate Surge in Malignant Glioma Cells. Cell Death Differ. 2007, 14, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O.; Koski, S.L. Cisplatin-Based versus Carboplatin-Based Chemotherapy for Extrapulmonary Neuroendocrine Carcinomas: A Real-World Study. Neuroendocrinology 2022, 112, 777–783. [Google Scholar] [CrossRef]
- Robbins, H.L.; Hague, A. The PI3K/Akt Pathway in Tumors of Endocrine Tissues. Front. Endocrinol. 2016, 6, 188. [Google Scholar] [CrossRef]
- Wolin, E.M. PI3K/Akt/mTOR Pathway Inhibitors in the Therapy of Pancreatic Neuroendocrine Tumors. Cancer Lett. 2013, 335, 1–8. [Google Scholar] [CrossRef]
- Antonuzzo, L.; Del Re, M.; Barucca, V.; Spada, F.; Meoni, G.; Restante, G.; Danesi, R.; Di Costanzo, F.; Fazio, N. Critical Focus on Mechanisms of Resistance and Toxicity of M-TOR Inhibitors in Pancreatic Neuroendocrine Tumors. Cancer Treat. Rev. 2017, 57, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Gajate, P.; Alonso-Gordoa, T.; Martínez-Sáez, O.; Molina-Cerrillo, J.; Grande, E. Prognostic and Predictive Role of the PI3K–AKT–mTOR Pathway in Neuroendocrine Neoplasms. Clin. Transl. Oncol. 2018, 20, 561–569. [Google Scholar] [CrossRef]
- Zarebczan, B.; Chen, H. Signaling Mechanisms in Neuroendocrine Tumors as Targets for Therapy. Endocrinol. Metab. Clin. N. Am. 2010, 39, 801–810. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef]
- Wiedmer, T.; Blank, A.; Pantasis, S.; Normand, L.; Bill, R.; Krebs, P.; Tschan, M.P.; Marinoni, I.; Perren, A. Autophagy Inhibition Improves Sunitinib Efficacy in Pancreatic Neuroendocrine Tumors via a Lysosome-Dependent Mechanism. Mol. Cancer Ther. 2017, 16, 2502–2515. [Google Scholar] [CrossRef] [PubMed]
- Matrood, S.; De Prisco, N.; Wissniowski, T.T.; Wiese, D.; Jabari, S.; Griesmann, H.; Wanzel, M.; Stiewe, T.; Neureiter, D.; Klieser, E.; et al. Modulation of Pancreatic Neuroendocrine Neoplastic Cell Fate by Autophagy-Mediated Death. Neuroendocrinology 2021, 111, 965–985. [Google Scholar] [CrossRef]
- Gagliano, T.; Bellio, M.; Gentilin, E.; Molè, D.; Tagliati, F.; Schiavon, M.; Cavallesco, N.G.; Andriolo, L.G.; Ambrosio, M.R.; Rea, F.; et al. mTOR, p70S6K, AKT, and ERK1/2 Levels Predict Sensitivity to mTOR and PI3K/mTOR Inhibitors in Human Bronchial Carcinoids. Endocr.-Relat. Cancer 2013, 20, 463–475. [Google Scholar] [CrossRef][Green Version]
- Perren, A.; Komminoth, P.; Saremaslani, P.; Matter, C.; Feurer, S.; Lees, J.A.; Heitz, P.U.; Eng, C. Mutation and Expression Analyses Reveal Differential Subcellular Compartmentalization of PTEN in Endocrine Pancreatic Tumors Compared to Normal Islet Cells. Am. J. Pathol. 2000, 157, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Missiaglia, E.; Dalai, I.; Barbi, S.; Beghelli, S.; Falconi, M.; Della Peruta, M.; Piemonti, L.; Capurso, G.; Di Florio, A.; Delle Fave, G.; et al. Pancreatic Endocrine Tumors: Expression Profiling Evidences a Role for AKT-mTOR Pathway. J. Clin. Oncol. 2010, 28, 245–255. [Google Scholar] [CrossRef]
- Jayakumar, R.; Lanjewar, S.; Axiotis, C.A. Loss of PTEN and Increased pAKT Expression Distinguishes Aggressive Low-Grade Neuroendocrine Tumors. Ann. Clin. Lab. Sci. 2018, 48, 565–572. [Google Scholar]
- Tendler, S.; Kanter, L.; Lewensohn, R.; Ortiz-Villalón, C.; Viktorsson, K.; De Petris, L. The Prognostic Implications of Notch1, Hes1, Ascl1, and DLL3 Protein Expression in SCLC Patients Receiving Platinum-Based Chemotherapy. PLoS ONE 2020, 15, e0240973. [Google Scholar] [CrossRef]
- Gajra, A.; Tatum, A.H.; Newman, N.; Gamble, G.P.; Lichtenstein, S.; Rooney, M.T.; Graziano, S.L. The Predictive Value of Neuroendocrine Markers and P53 for Response to Chemotherapy and Survival in Patients with Advanced Non-Small Cell Lung Cancer. Lung Cancer 2002, 36, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Nowak, I.; Huang, J.; Keng, P.C.; Sun, H.; Xu, H.; Wei, G.; Lee, S.O. Erk/MAP Kinase Signaling Pathway and Neuroendocrine Differentiation of Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2014, 9, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Nakakura, E.K.; Sriuranpong, V.R.; Kunnimalaiyaan, M.; Hsiao, E.C.; Schuebel, K.E.; Borges, M.W.; Jin, N.; Collins, B.J.; Nelkin, B.D.; Chen, H.; et al. Regulation of Neuroendocrine Differentiation in Gastrointestinal Carcinoid Tumor Cells by Notch Signaling. J. Clin. Endocrinol. Metab. 2005, 90, 4350–4356. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Shi, C.; Edil, B.H.; De Wilde, R.F.; Klimstra, D.S.; Maitra, A.; Schulick, R.D.; Tang, L.H.; Wolfgang, C.L.; Choti, M.A.; et al. DAXX/ATRX, MEN1, and mTOR Pathway Genes Are Frequently Altered in Pancreatic Neuroendocrine Tumors. Science 2011, 331, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Yang, K.C.; Shen, Y.; Zhao, E.Y.; Loree, J.M.; Kennecke, H.F.; Kalloger, S.E.; Karasinska, J.M.; Lim, H.J.; Mungall, A.J.; et al. Molecular Characterization of Metastatic Pancreatic Neuroendocrine Tumors (PNETs) Using Whole-Genome and Transcriptome Sequencing. Cold Spring Harb. Mol. Case Stud. 2018, 4, a002329. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, S.E.; Kakar, S.; Basturk, O.; Kim, G.E.; Chatterjee, D.; Wen, K.W.; Hale, G.; Shafizadeh, N.; Cho, S.-J.; Whitman, J.; et al. Integrated Genomic and Clinicopathologic Approach Distinguishes Pancreatic Grade 3 Neuroendocrine Tumor from Neuroendocrine Carcinoma and Identifies a Subset with Molecular Overlap. Mod. Pathol. 2023, 36, 100065. [Google Scholar] [CrossRef] [PubMed]
- Couce, M.E.; Bautista, D.; Costa, J.; Carter, D. Analysis of K-Ras, N-Ras, H-Ras, and P53 in Lung Neuroendocrine Neoplasms. Diagn. Mol. Pathol. 1999, 8, 71–79. [Google Scholar] [CrossRef]
- Hu, W.; Feng, Z.; Modica, I.; Klimstra, D.S.; Song, L.; Allen, P.J.; Brennan, M.F.; Levine, A.J.; Tang, L.H. Gene Amplifications in Well-Differentiated Pancreatic Neuroendocrine Tumors Inactivate the P53 Pathway. Genes Cancer 2010, 1, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Yachida, S.; Vakiani, E.; White, C.M.; Zhong, Y.; Saunders, T.; Morgan, R.; de Wilde, R.F.; Maitra, A.; Hicks, J.; Demarzo, A.M.; et al. Small Cell and Large Cell Neuroendocrine Carcinomas of the Pancreas Are Genetically Similar and Distinct from Well-Differentiated Pancreatic Neuroendocrine Tumors. Am. J. Surg. Pathol. 2012, 36, 173–184. [Google Scholar] [CrossRef]
- Pal’tsev, M.A.; Demura, S.A.; Kogan, E.A.; Jaques, G.; Zende, B. Role ofBcl-2, Bax, andBak in Spontaneous Apoptosis and Proliferation in Neuroendocrine Lung Tumors: Immunohistochemical Study. Bull. Exp. Biol. Med. 2000, 130, 697–700. [Google Scholar] [CrossRef]
- Brambilla, E.; Negoescu, A.; Gazzeri, S.; Lantuejoul, S.; Moro, D.; Brambilla, C.; Coll, J.L. Apoptosis-Related Factors P53, Bcl2, and Bax in Neuroendocrine Lung Tumors. Am. J. Pathol. 1996, 149, 1941–1952. [Google Scholar] [PubMed]
- Roland, C.L.; Starker, L.F.; Kang, Y.; Chatterjee, D.; Estrella, J.; Rashid, A.; Katz, M.H.; Aloia, T.A.; Lee, J.E.; Dasari, A.; et al. Loss of DPC4/SMAD4 Expression in Primary Gastrointestinal Neuroendocrine Tumors Is Associated with Cancer-Related Death after Resection. Surgery 2017, 161, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Hofving, T.; Elias, E.; Rehammar, A.; Inge, L.; Altiparmak, G.; Persson, M.; Kristiansson, E.; Johansson, M.E.; Nilsson, O.; Arvidsson, Y. SMAD4 Haploinsufficiency in Small Intestinal Neuroendocrine Tumors. BMC Cancer 2021, 21, 101. [Google Scholar] [CrossRef]
- Banck, M.S.; Kanwar, R.; Kulkarni, A.A.; Boora, G.K.; Metge, F.; Kipp, B.R.; Zhang, L.; Thorland, E.C.; Minn, K.T.; Tentu, R.; et al. The Genomic Landscape of Small Intestine Neuroendocrine Tumors. J. Clin. Investig. 2013, 123, 2502–2508. [Google Scholar] [CrossRef] [PubMed]
- Cejas, P.; Drier, Y.; Dreijerink, K.M.A.; Brosens, L.A.A.; Deshpande, V.; Epstein, C.B.; Conemans, E.B.; Morsink, F.H.M.; Graham, M.K.; Valk, G.D.; et al. Enhancer Signatures Stratify and Predict Outcomes of Non-Functional Pancreatic Neuroendocrine Tumors. Nat. Med. 2019, 25, 1260–1265. [Google Scholar] [CrossRef]
- Marinoni, I.; Kurrer, A.S.; Vassella, E.; Dettmer, M.; Rudolph, T.; Banz, V.; Hunger, F.; Pasquinelli, S.; Speel, E.; Perren, A. Loss of DAXX and ATRX Are Associated with Chromosome Instability and Reduced Survival of Patients with Pancreatic Neuroendocrine Tumors. Gastroenterology 2014, 146, 453–460.e5. [Google Scholar] [CrossRef]
- Cives, M.; Partelli, S.; Palmirotta, R.; Lovero, D.; Mandriani, B.; Quaresmini, D.; Pelle’, E.; Andreasi, V.; Castelli, P.; Strosberg, J.; et al. DAXX Mutations as Potential Genomic Markers of Malignant Evolution in Small Nonfunctioning Pancreatic Neuroendocrine Tumors. Sci. Rep. 2019, 9, 18614. [Google Scholar] [CrossRef]
- Krausch, M.; Kroepil, F.; Lehwald, N.; Lachenmayer, A.; Schott, M.; Anlauf, M.; Cupisti, K.; Knoefel, W.T.; Raffel, A. Notch 1 Tumor Expression Is Lacking in Highly Proliferative Pancreatic Neuroendocrine Tumors. Endocrine 2013, 44, 182–186. [Google Scholar] [CrossRef]
- Panelos, J.; Batistatou, A.; Paglierani, M.; Zioga, A.; Maio, V.; Santi, R.; Pimpinelli, N.; De Giorgi, V.; Santucci, M.; Massi, D. Expression of Notch-1 and Alteration of the E-Cadherin/β-Catenin Cell Adhesion Complex Are Observed in Primary Cutaneous Neuroendocrine Carcinoma (Merkel Cell Carcinoma). Mod. Pathol. 2009, 22, 959–968. [Google Scholar] [CrossRef]
- Kikuchi, H.; Sakakibara-Konishi, J.; Furuta, M.; Yokouchi, H.; Nishihara, H.; Yamazaki, S.; Uramoto, H.; Tanaka, F.; Harada, M.; Akie, K.; et al. Expression of Notch1 and Numb in Small Cell Lung Cancer. Oncotarget 2017, 8, 10348–10358. [Google Scholar] [CrossRef]
- George, J.; Walter, V.; Peifer, M.; Alexandrov, L.B.; Seidel, D.; Leenders, F.; Maas, L.; Müller, C.; Dahmen, I.; Delhomme, T.M.; et al. Integrative Genomic Profiling of Large-Cell Neuroendocrine Carcinomas Reveals Distinct Subtypes of High-Grade Neuroendocrine Lung Tumors. Nat. Commun. 2018, 9, 1048. [Google Scholar] [CrossRef]
- Lohmann, D.R.; Funk, A.; Niedermeyer, H.P.; Häupel, S.; Höfler, H. Identification of P53 Gene Mutations in Gastrointestinal and Pancreatic Carcinoids by Nonradioisotopic SSCA. Virchows Arch. B Cell Pathol. 1993, 64, 293–296. [Google Scholar] [CrossRef]
- Weckström, P.; Hedrum, A.; Makridis, C.; Åkerström, G.; Rastad, J.; Scheibenpflug, L.; Uhlén, M.; Juhlin, C.; Wilander, E. Midgut Carcinoids and Solid Carcinomas of the Intestine: Differences in Endocrine Markers and P53 Mutations. Endocr. Pathol. 1996, 7, 273–279. [Google Scholar] [CrossRef]
- Przygodzki, R.M.; Finkelstein, S.D.; Langer, J.C.; Swalsky, P.A.; Fishback, N.; Bakker, A.; Guinee, D.G.; Koss, M.; Travis, W.D. Analysis of P53, K-Ras-2, and C-Raf-1 in Pulmonary Neuroendocrine Tumors. Correlation with Histological Subtype and Clinical Outcome. Am. J. Pathol. 1996, 148, 1531–1541. [Google Scholar]
- Voortman, J.; Lee, J.-H.; Killian, J.K.; Suuriniemi, M.; Wang, Y.; Lucchi, M.; Smith, W.I.; Meltzer, P.; Wang, Y.; Giaccone, G. Array Comparative Genomic Hybridization-Based Characterization of Genetic Alterations in Pulmonary Neuroendocrine Tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 13040–13045. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-L.; Sood, A.; Rahimi, H.A.; Wang, W.; Gupta, N.; Hicks, J.; Mosier, S.; Gocke, C.D.; Epstein, J.I.; Netto, G.J.; et al. Rb Loss Is Characteristic of Prostatic Small Cell Neuroendocrine Carcinoma. Clin. Cancer Res. 2014, 20, 890–903. [Google Scholar] [CrossRef]
- Takizawa, N.; Ohishi, Y.; Hirahashi, M.; Takahashi, S.; Nakamura, K.; Tanaka, M.; Oki, E.; Takayanagi, R.; Oda, Y. Molecular Characteristics of Colorectal Neuroendocrine Carcinoma; Similarities with Adenocarcinoma Rather than Neuroendocrine Tumor. Hum. Pathol. 2015, 46, 1890–1900. [Google Scholar] [CrossRef]
- Rekhtman, N.; Pietanza, M.C.; Hellmann, M.D.; Naidoo, J.; Arora, A.; Won, H.; Halpenny, D.F.; Wang, H.; Tian, S.K.; Litvak, A.M.; et al. Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma–like and Non–Small Cell Carcinoma–like Subsets. Clin. Cancer Res. 2016, 22, 3618–3629. [Google Scholar] [CrossRef]
- Miyoshi, T.; Umemura, S.; Matsumura, Y.; Mimaki, S.; Tada, S.; Makinoshima, H.; Ishii, G.; Udagawa, H.; Matsumoto, S.; Yoh, K.; et al. Genomic Profiling of Large-Cell Neuroendocrine Carcinoma of the Lung. Clin. Cancer Res. 2017, 23, 757–765. [Google Scholar] [CrossRef]
- Shamir, E.R.; Devine, W.P.; Pekmezci, M.; Umetsu, S.E.; Krings, G.; Federman, S.; Cho, S.-J.; Saunders, T.A.; Jen, K.-Y.; Bergsland, E.; et al. Identification of High-Risk Human Papillomavirus and Rb/E2F Pathway Genomic Alterations in Mutually Exclusive Subsets of Colorectal Neuroendocrine Carcinoma. Mod. Pathol. 2019, 32, 290–305. [Google Scholar] [CrossRef] [PubMed]
- Hillman, R.T.; Cardnell, R.; Fujimoto, J.; Lee, W.-C.; Zhang, J.; Byers, L.A.; Ramalingam, P.; Leitao, M.; Swisher, E.; Futreal, P.A.; et al. Comparative Genomics of High Grade Neuroendocrine Carcinoma of the Cervix. PLoS ONE 2020, 15, e0234505. [Google Scholar] [CrossRef]
- Xing, J.; Chen, J.; You, T.; Lu, T.; Wu, H.; Bai, C.; Sun, Z.; Cheng, Y. P53 and Rb Immunohistochemistry Staining Reveal Subtypes of Gastric Neuroendocrine Carcinoma with Distinct Prognosis. J. Clin. Oncol. 2022, 40, e16210. [Google Scholar] [CrossRef]
- Tannapfel, A.; Vomschloss, S.; Karhoff, D.; Markwarth, A.; Hengge, U.R.; Wittekind, C.; Arnold, R.; Hörsch, D. BRAF Gene Mutations Are Rare Events in Gastroenteropancreatic Neuroendocrine Tumors. Am. J. Clin. Pathol. 2005, 123, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Kleist, B.; Kempa, M.; Novy, M.; Oberkanins, C.; Xu, L.; Li, G.; Loland, C.; Poetsch, M. Comparison of Neuroendocrine Differentiation and KRAS/NRAS/BRAF/PIK3CA/TP53 Mutation Status in Primary and Metastatic Colorectal Cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 5927–5939. [Google Scholar]
- Ganesh, K.; Raj, N.P.; Yaeger, R.D.; Berger, M.F.; Shia, J.; Reidy, D.L. BRAF Mutations in Patients with Large Cell Neuroendocrine Carcinoma of the Colon (LCNECC). J. Clin. Oncol. 2016, 34, 567. [Google Scholar] [CrossRef]
- Kim, M.K.; Ye, F.; Wang, D.; Cui, M.; Ward, S.C.; Warner, R.R.P.; Roayaie, S.; Shafir, M.; Schwartz, M.; Zhang, D.; et al. Differential Protein Expression in Small Intestinal Neuroendocrine Tumors and Liver Metastases. Pancreas 2016, 45, 528–532. [Google Scholar] [CrossRef][Green Version]
- Shida, T.; Kishimoto, T.; Furuya, M.; Nikaido, T.; Koda, K.; Takano, S.; Kimura, F.; Shimizu, H.; Yoshidome, H.; Ohtsuka, M.; et al. Expression of an Activated Mammalian Target of Rapamycin (mTOR) in Gastroenteropancreatic Neuroendocrine Tumors. Cancer Chemother. Pharmacol. 2010, 65, 889–893. [Google Scholar] [CrossRef]
- Catena, L.; Bajetta, E.; Milione, M.; Ducceschi, M.; Valente, M.; Dominoni, F.; Colonna, V. Mammalian Target of Rapamycin Expression in Poorly Differentiated Endocrine Carcinoma: Clinical and Therapeutic Future Challenges. Target. Oncol. 2011, 6, 65–68. [Google Scholar] [CrossRef]
- Kasajima, A.; Pavel, M.; Darb-Esfahani, S.; Noske, A.; Stenzinger, A.; Sasano, H.; Dietel, M.; Denkert, C.; Rocken, C.; Wiedenmann, B.; et al. mTOR Expression and Activity Patterns in Gastroenteropancreatic Neuroendocrine Tumours. Endocr.-Relat. Cancer 2011, 18, 181–192. [Google Scholar] [CrossRef]
- Zhou, C.-F.; Ji, J.; Yuan, F.; Shi, M.; Zhang, J.; Liu, B.-Y.; Zhu, Z.-G. mTOR Activation in Well Differentiated Pancreatic Neuroendocrine Tumors: A Retrospective Study on 34 Cases. Hepato-Gastroenterology 2011, 58, 2140–2143. [Google Scholar] [CrossRef]
- Peng, D.-J.; Wang, J.; Zhou, J.-Y.; Wu, G.S. Role of the Akt/mTOR Survival Pathway in Cisplatin Resistance in Ovarian Cancer Cells. Biochem. Biophys. Res. Commun. 2010, 394, 600–605. [Google Scholar] [CrossRef]
- Yu, C.; Yu, Q.; Lu, J.; Zhou, J. KRT17 Enhances Carboplatin Resistance in Ovarian Cancer through the AKT/mTOR Pathway. Eur. J. Gynaecol. Oncol. 2022, 43, 49–57. [Google Scholar] [CrossRef]
- Nasrpour Navaei, Z.; Khalili-Tanha, G.; Sadra Zangouei, A.; Reza Abbaszadegan, M.; Moghbeli, M. PI3K/AKT Signaling Pathway as a Critical Regulator of Cisplatin Response in Tumor Cells. Oncol. Res. 2021, 29, 235–250. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Xie, Y.; Xiang, J.; Zhu, Y.; Yang, J. Enhanced Tumor Suppression by Adenoviral PTEN Gene Therapy Combined with Cisplatin Chemotherapy in Small-Cell Lung Cancer. Cancer Gene Ther. 2013, 20, 251–259. [Google Scholar] [CrossRef]
- Minami, D.; Takigawa, N.; Takeda, H.; Takata, M.; Ochi, N.; Ichihara, E.; Hisamoto, A.; Hotta, K.; Tanimoto, M.; Kiura, K. Synergistic Effect of Olaparib with Combination of Cisplatin on PTEN -Deficient Lung Cancer Cells. Mol. Cancer Res. 2013, 11, 140–148. [Google Scholar] [CrossRef]
- Omura, M.; Kosaka, T.; Aimono, E.; Nakamura, K.; Hongo, H.; Mikami, S.; Nishihara, H.; Oya, M. First Successful Case of Platinum-based Chemotherapy for Neuroendocrine Prostate Cancer with BRCA2 and PTEN Alterations. IJU Case Rep. 2022, 5, 41–44. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.; Liu, C.; Zhang, B.; Zheng, J.; Singh, P.K.; Bshara, W.; Wang, J.; Gomez, E.C.; Zhang, X.; et al. PTEN Loss Expands the Histopathologic Diversity and Lineage Plasticity of Lung Cancers Initiated by Rb1/Trp53 Deletion. J. Thorac. Oncol. 2023, 18, 324–338. [Google Scholar] [CrossRef]
- Chang, T.-M.; Chu, P.-Y.; Lin, H.-Y.; Huang, K.-W.; Hung, W.-C.; Shan, Y.-S.; Chen, L.-T.; Tsai, H.-J. PTEN Regulates Invasiveness in Pancreatic Neuroendocrine Tumors through DUSP19-Mediated VEGFR3 Dephosphorylation. J. Biomed. Sci. 2022, 29, 92. [Google Scholar] [CrossRef]
- Song, M.S.; Salmena, L.; Pandolfi, P.P. The Functions and Regulation of the PTEN Tumour Suppressor. Nat. Rev. Mol. Cell Biol. 2012, 13, 283–296. [Google Scholar] [CrossRef]
- Puc, J.; Keniry, M.; Li, H.S.; Pandita, T.K.; Choudhury, A.D.; Memeo, L.; Mansukhani, M.; Murty, V.V.V.S.; Gaciong, Z.; Meek, S.E.M.; et al. Lack of PTEN Sequesters CHK1 and Initiates Genetic Instability. Cancer Cell 2005, 7, 193–204. [Google Scholar] [CrossRef]
- Hijioka, S.; Hosoda, W.; Matsuo, K.; Ueno, M.; Furukawa, M.; Yoshitomi, H.; Kobayashi, N.; Ikeda, M.; Ito, T.; Nakamori, S.; et al. Rb Loss and KRAS Mutation Are Predictors of the Response to Platinum-Based Chemotherapy in Pancreatic Neuroendocrine Neoplasm with Grade 3: A Japanese Multicenter Pancreatic NEN-G3 Study. Clin. Cancer Res. 2017, 23, 4625–4632. [Google Scholar] [CrossRef]
- Tanaka, H.; Hijioka, S.; Hosoda, W.; Ueno, M.; Kobayashi, N.; Ikeda, M.; Ito, T.; Kodama, Y.; Morizane, C.; Notohara, K.; et al. Pancreatic Neuroendocrine Carcinoma G3 May Be Heterogeneous and Could Be Classified into Two Distinct Groups. Pancreatology 2020, 20, 1421–1427. [Google Scholar] [CrossRef]
- Elvebakken, H.; Perren, A.; Scoazec, J.-Y.; Tang, L.H.; Federspiel, B.; Klimstra, D.S.; Vestermark, L.W.; Ali, A.S.; Zlobec, I.; Myklebust, T.Å.; et al. A Consensus-Developed Morphological Re-Evaluation of 196 High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms and Its Clinical Correlations. Neuroendocrinology 2021, 111, 883–894. [Google Scholar] [CrossRef]
- Ogawa, H.; Sakai, Y.; Nishio, W.; Fujibayashi, Y.; Nishikubo, M.; Nishioka, Y.; Tane, S.; Kitamura, Y.; Sudo, T.; Sakuma, T.; et al. DLL3 Expression Is a Predictive Marker of Sensitivity to Adjuvant Chemotherapy for Pulmonary LCNEC. Thorac. Cancer 2020, 11, 2561–2569. [Google Scholar] [CrossRef]
- Daimon, T.; Kosaka, T.; Hongo, H.; Aimono, E.; Nakamura, K.; Mikami, S.; Nishihara, H.; Oya, M. Prominent Response to Platinum-based Chemotherapy in a Patient with BRCA2 Mutant-neuroendocrine Prostate Cancer and MDM2 Amplification. IJU Case Rep. 2021, 4, 216–219. [Google Scholar] [CrossRef]
- Chen, D.; Xu, J.; Qiao, R.; Zhao, Y.; Chu, T.; Han, B.; Zhong, R. Detection of Genetic Mutations by Next-Generation Sequencing for Predicting Prognosis of Extensive-Stage Small-Cell Lung Cancer. J. Oncol. 2020, 2020, 8811487. [Google Scholar] [CrossRef]
- Hongo, H.; Kosaka, T.; Nakatsuka, S.; Oya, M. A Long-Term Survivor of Metastatic Neuroendocrine Prostate Cancer Treated with Multimodal Therapy: Genetic Consideration from next-Generation Sequencing. Int. Cancer Conf. J. 2021, 10, 174–180. [Google Scholar] [CrossRef]
- Elvebakken, H.; Venizelos, A.; Perren, A.; Couvelard, A.; Lothe, I.M.B.; Hjortland, G.O.; Myklebust, T.Å.; Svensson, J.; Garresori, H.; Kersten, C.; et al. Treatment Outcome According to Genetic Tumour Alterations and Clinical Characteristics in Digestive High-Grade Neuroendocrine Neoplasms. Br. J. Cancer 2024. [Google Scholar] [CrossRef]
- Dowlati, A.; Lipka, M.B.; McColl, K.; Dabir, S.; Behtaj, M.; Kresak, A.; Miron, A.; Yang, M.; Sharma, N.; Fu, P.; et al. Clinical Correlation of Extensive-Stage Small-Cell Lung Cancer Genomics. Ann. Oncol. 2016, 27, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, C.; De Rycke, O.; Couvelard, A.; Turpin, A.; Cazes, A.; Hentic, O.; Gounant, V.; Zalcman, G.; Ruszniewski, P.; Cros, J.; et al. Biomarkers of Response to Etoposide-Platinum Chemotherapy in Patients with Grade 3 Neuroendocrine Neoplasms. Cancers 2021, 13, 643. [Google Scholar] [CrossRef]
- Kosaka, T.; Hongo, H.; Aimono, E.; Matsumoto, K.; Hayashida, T.; Mikami, S.; Nishihara, H.; Oya, M. A First Japanese Case of Neuroendocrine Prostate Cancer Accompanied by Lung and Brain Metastasis with Somatic and Germline BRCA2 Mutation. Pathol. Int. 2019, 69, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Turina, C.B.; Coleman, D.J.; Thomas, G.V.; Fung, A.W.; Alumkal, J.J. Molecular Testing Identifies Determinants of Exceptional Response and Guides Precision Therapy in a Patient with Lethal, Treatment-Emergent Neuroendocrine Prostate Cancer. Cureus 2019, 11, e5197. [Google Scholar] [CrossRef] [PubMed]
- Pandya, D.; Shah, M.; Kaplan, F.; Martino, C.; Levy, G.; Kazanjian, M.; Batter, S.; Martignetti, J.; Frank, R.C. Treatment-Emergent Neuroendocrine Prostate Cancer with a Germline BRCA2 Mutation: Identification of a Candidate Reversion Mutation Associated with Platinum/PARP-Inhibitor Resistance. Cold Spring Harb. Mol. Case Stud. 2021, 7, a005801. [Google Scholar] [CrossRef]
- Wood, R.; Arnason, T.; DeCoste, R.; Gaston, D.; Carter, M.D.; Rayson, D. Complete Response of a Colonic High-Grade Neuroendocrine Carcinoma to Platinum-Based Therapy: Insights from Comprehensive Genomic Profiling. Am. J. Clin. Pathol. 2021, 156, S143. [Google Scholar] [CrossRef]
- Furukawa, T.; Ozaka, M.; Takamatsu, M.; Okamoto, T.; Ishitsuka, T.; Yamada, M.; Nakagawa, H.; Mie, T.; Takeda, T.; Kasuga, A.; et al. MO69-3 Pathological Predictors of the Response to Platinum-Based Chemotherapy in Pancreatobiliary Neuroendocrine Carcinoma. Ann. Oncol. 2023, 34, S1438. [Google Scholar] [CrossRef]
- Wang, X.; Martindale, J.L.; Holbrook, N.J. Requirement for ERK Activation in Cisplatin-Induced Apoptosis. J. Biol. Chem. 2000, 275, 39435–39443. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Prieto, R.; Rojas, J.M.; Taya, Y.; Gutkind, J.S. A Role for the P38 Mitogen-Acitvated Protein Kinase Pathway in the Transcriptional Activation of P53 on Genotoxic Stress by Chemotherapeutic Agents. Cancer Res. 2000, 60, 2464–2472. [Google Scholar]
- Jones, E.V.; Dickman, M.J.; Whitmarsh, A.J. Regulation of P73-Mediated Apoptosis by c-Jun N-Terminal Kinase. Biochem. J. 2007, 405, 617–623. [Google Scholar] [CrossRef]
- Kim, K.W.; Krajewski, K.M.; Nishino, M.; Jagannathan, J.P.; Shinagare, A.B.; Tirumani, S.H.; Ramaiya, N.H. Update on the Management of Gastroenteropancreatic Neuroendocrine Tumors with Emphasis on the Role of Imaging. Am. J. Roentgenol. 2013, 201, 811–824. [Google Scholar] [CrossRef]
- Fazio, N.; Abdel-Rahman, O.; Spada, F.; Galdy, S.; De Dosso, S.; Capdevila, J.; Scarpa, A. RAF Signaling in Neuroendocrine Neoplasms: From Bench to Bedside. Cancer Treat. Rev. 2014, 40, 974–979. [Google Scholar] [CrossRef]
- Ning, L.; Chen, H.; Kunnimalaiyaan, M. Focal Adhesion Kinase, a Downstream Mediator of Raf-1 Signaling, Suppresses Cellular Adhesion, Migration, and Neuroendocrine Markers in BON Carcinoid Cells. Mol. Cancer Res. 2010, 8, 775–782. [Google Scholar] [CrossRef][Green Version]
- Stefàno, E.; Muscella, A.; Benedetti, M.; De Castro, F.; Fanizzi, F.P.; Marsigliante, S. Antitumor and Antimigration Effects of a New Pt Compound on Neuroblastoma Cells. Biochem. Pharmacol. 2022, 202, 115124. [Google Scholar] [CrossRef]
- Inoue, Y.; Lockwood, W. MA22.02 Activation of MAPK Suppresses Neuroendocrine Transcription Factors and Causes Transdifferentiation of Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, S433–S434. [Google Scholar] [CrossRef]
- Huang, L.; Feng, Y.; Xie, T.; Zhu, H.; Tang, L.; Shi, Y. Incidence, Survival Comparison, and Novel Prognostic Evaluation Approaches for Stage iii-iv Pulmonary Large Cell Neuroendocrine Carcinoma and Small Cell Lung Cancer. BMC Cancer 2023, 23, 312. [Google Scholar] [CrossRef]
- Caiola, E.; Salles, D.; Frapolli, R.; Lupi, M.; Rotella, G.; Ronchi, A.; Garassino, M.C.; Mattschas, N.; Colavecchio, S.; Broggini, M.; et al. Base Excision Repair-Mediated Resistance to Cisplatin in KRAS(G12C) Mutant NSCLC Cells. Oncotarget 2015, 6, 30072–30087. [Google Scholar] [CrossRef]
- Leonetti, A.; Facchinetti, F.; Minari, R.; Cortellini, A.; Rolfo, C.D.; Giovannetti, E.; Tiseo, M. Notch Pathway in Small-Cell Lung Cancer: From Preclinical Evidence to Therapeutic Challenges. Cell. Oncol. 2019, 42, 261–273. [Google Scholar] [CrossRef]
- Carter, Y.; Jaskula-Sztul, R.; Chen, H.; Mazeh, H. Signaling Pathways as Specific Pharmacologic Targets for Neuroendocrine Tumor Therapy: RET, PI3K, MEK, Growth Factors, and Notch. Neuroendocrinology 2013, 97, 57–66. [Google Scholar] [CrossRef]
- Kunnimalaiyaan, M.; Chen, H. Tumor Suppressor Role of Notch-1 Signaling in Neuroendocrine Tumors. Oncologist 2007, 12, 535–542. [Google Scholar] [CrossRef]
- Meder, L.; König, K.; Ozretić, L.; Schultheis, A.M.; Ueckeroth, F.; Ade, C.P.; Albus, K.; Boehm, D.; Rommerscheidt-Fuss, U.; Florin, A.; et al. NOTCH, ASCL1, P53 and RB Alterations Define an Alternative Pathway Driving Neuroendocrine and Small Cell Lung Carcinomas. Int. J. Cancer 2016, 138, 927–938. [Google Scholar] [CrossRef]
- Borges, M.; Linnoila, R.I.; Van De Velde, H.J.K.; Chen, H.; Nelkin, B.D.; Mabry, M.; Baylin, S.B.; Ball, D.W. An Achaete-Scute Homologue Essential for Neuroendocrine Differentiation in the Lung. Nature 1997, 386, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Linnoila, R.I.; Zhao, B.; DeMayo, J.L.; Nelkin, B.D.; Baylin, S.B.; DeMayo, F.J.; Ball, D.W. Constitutive Achaete-Scute Homologue-1 Promotes Airway Dysplasia and Lung Neuroendocrine Tumors in Transgenic Mice. Cancer Res. 2000, 60, 4005–4009. [Google Scholar] [PubMed]
- Demelash, A.; Rudrabhatla, P.; Pant, H.C.; Wang, X.; Amin, N.D.; McWhite, C.D.; Naizhen, X.; Linnoila, R.I. Achaete-Scute Homologue-1 (ASH1) Stimulates Migration of Lung Cancer Cells through Cdk5/P35 Pathway. Mol. Biol. Cell 2012, 23, 2856–2866. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.S.; Singleton, C.S.; Miele, L. Notch Signaling in Neuroendocrine Tumors. Front. Oncol. 2016, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Ito, T. Intratumoral Heterogeneity of Notch1 Expression in Small Cell Lung Cancer. J. Thorac. Dis. 2018, 10, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Ibaseta, A.; Fischer, M.M.; Cancilla, B.; O’Young, G.; Cristea, S.; Luca, V.C.; Yang, D.; Jahchan, N.S.; Hamard, C.; et al. Intratumoural Heterogeneity Generated by Notch Signalling Promotes Small-Cell Lung Cancer. Nature 2017, 545, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Lawson, M.H.; Cummings, N.M.; Rassl, D.M.; Russell, R.; Brenton, J.D.; Rintoul, R.C.; Murphy, G. Two Novel Determinants of Etoposide Resistance in Small Cell Lung Cancer. Cancer Res. 2011, 71, 4877–4887. [Google Scholar] [CrossRef]
- Lakiza, O.; Lutze, J.; Vogle, A.; Williams, J.; Abukdheir, A.; Miller, P.; Liao, C.-Y.; Pitroda, S.P.; Martinez, C.; Olivas, A.; et al. Loss of MEN1 Function Impairs DNA Repair Capability of Pancreatic Neuroendocrine Tumors. Endocr.-Relat. Cancer 2022, 29, 225–239. [Google Scholar] [CrossRef]
- Briest, F.; Grass, I.; Sedding, D.; Möbs, M.; Christen, F.; Benecke, J.; Fuchs, K.; Mende, S.; Kaemmerer, D.; Sänger, J.; et al. Mechanisms of Targeting the MDM2-P53-FOXM1 Axis in Well-Differentiated Intestinal Neuroendocrine Tumors. Neuroendocrinology 2018, 107, 1–23. [Google Scholar] [CrossRef]
- Phatak, V.; Von Grabowiecki, Y.; Janus, J.; Officer, L.; Behan, C.; Aschauer, L.; Pinon, L.; Mackay, H.; Zanivan, S.; Norman, J.C.; et al. Mutant P53 Promotes RCP-Dependent Chemoresistance Coinciding with Increased Delivery of P-Glycoprotein to the Plasma Membrane. Cell Death Dis. 2021, 12, 207. [Google Scholar] [CrossRef]
- Briest, F.; Grabowski, P. The P53 Network as Therapeutic Target in Gastroenteropancreatic Neuroendocrine Neoplasms. Cancer Treat. Rev. 2015, 41, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Pellot Ortiz, K.I.; Rechberger, J.S.; Nonnenbroich, L.F.; Daniels, D.J.; Sarkaria, J.N. MDM2 Inhibition in the Treatment of Glioblastoma: From Concept to Clinical Investigation. Biomedicines 2023, 11, 1879. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, Y.; Kishishita, S.; Obata, T.; Isazawa, Y.; Suzuki, T.; Tanaka, K.; Masuyama, N.; Gotoh, Y. Akt Enhances Mdm2-Mediated Ubiquitination and Degradation of P53. J. Biol. Chem. 2002, 277, 21843–21850. [Google Scholar] [CrossRef] [PubMed]
- Brazina, J.; Svadlenka, J.; Macurek, L.; Andera, L.; Hodny, Z.; Bartek, J.; Hanzlikova, H. DNA Damage-Induced Regulatory Interplay between DAXX, P53, ATM Kinase and Wip1 Phosphatase. Cell Cycle 2015, 14, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Švajdler, M.; Mezencev, R.; Ondič, O.; Šašková, B.; Mukenšnábl, P.; Michal, M. P16 Is a Useful Supplemental Diagnostic Marker of Pulmonary Small Cell Carcinoma in Small Biopsies and Cytology Specimens. Ann. Diagn. Pathol. 2018, 33, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Chedgy, E.C.P.; Annala, M.; Beja, K.; Warner, E.W.; Gleave, M.E.; Chi, K.N.; Wyatt, A.W. Moving Toward Personalized Care: Liquid Biopsy Predicts Response to Cisplatin in an Unusual Case of BRCA2-Null Neuroendocrine Prostate Cancer. Clin. Genitourin. Cancer 2016, 14, e233–e236. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Kosaka, T.; Aimono, E.; Hongo, H.; Mikami, S.; Nishihara, H.; Oya, M. Japanese Case of Enzalutamide-Resistant Prostate Cancer Harboring a SPOP Mutation with Scattered Allelic Imbalance: Response to Platinum-Based Therapy. Clin. Genitourin. Cancer 2019, 17, e897–e902. [Google Scholar] [CrossRef]
- Mani, R.-S. The Emerging Role of Speckle-Type POZ Protein (SPOP) in Cancer Development. Drug Discov. Today 2014, 19, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Salmena, L.; Carracedo, A.; Egia, A.; Lo-Coco, F.; Teruya-Feldstein, J.; Pandolfi, P.P. The Deubiquitinylation and Localization of PTEN Are Regulated by a HAUSP–PML Network. Nature 2008, 455, 813–817. [Google Scholar] [CrossRef]
- Kim, J.T.; Li, J.; Jang, E.R.; Gulhati, P.; Rychahou, P.G.; Napier, D.L.; Wang, C.; Weiss, H.L.; Lee, E.Y.; Anthony, L.; et al. Deregulation of Wnt/β-Catenin Signaling through Genetic or Epigenetic Alterations in Human Neuroendocrine Tumors. Carcinogenesis 2013, 34, 953–961. [Google Scholar] [CrossRef]
- Taghavi, S.F.; Ghorbani, M.; Panahi, M.; Nazem, S.; Karimi, M.; Salimi, V.; Tavakoli-Yaraki, M. Differential Expression Levels of β-Catenin Are Associated with Invasive Behavior of Both Functional and Non-Functional Pituitary Neuroendocrine Tumor (PitNET). Mol. Biol. Rep. 2023, 50, 6425–6434. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, P.; Bozdoğan, Ö.; Öztürk, S.; Ensari, A. BCL2 Expression and Its Correlation with Neuroendocrine Differentiation in Colon Carcinomas. Tumori 2004, 90, 233–238. [Google Scholar] [CrossRef]
- Gumulec, J.; Balvan, J.; Sztalmachova, M.; Raudenska, M.; Dvorakova, V.; Knopfova, L.; Polanska, H.; Hudcova, K.; Ruttkay-Nedecky, B.; Babula, P.; et al. Cisplatin-Resistant Prostate Cancer Model: Differences in Antioxidant System, Apoptosis and Cell Cycle. Int. J. Oncol. 2014, 44, 923–933. [Google Scholar] [CrossRef] [PubMed]
- De M Rêgo, J.F.; Salles Scortegagna De Medeiros, R.; Braghiroli, M.I.; Galvão, B.; Evangelista Bezerra Neto, J.; Ramella Munhoz, R.; Guerra, J.; Nonogaki, S.; Kimura, L.; Pfiffer, T.E.; et al. Expression of ERCC1, Bcl-2, Lin28a, and Ki-67 as Biomarkers of Response to First-Line Platinum-Based Chemotherapy in Patients with High-Grade Extrapulmonary Neuroendocrine Carcinomas or Small Cell Lung Cancer. Ecancermedicalscience 2017, 11, 767. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sartorius, U.A.; Krammer, P.H. Upregulation of Bcl-2 Is Involved in the Mediation of Chemotherapy Resistance in Human Small Cell Lung Cancer Cell Lines. Int. J. Cancer 2002, 97, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Ohmoto, A.; Rokutan, H.; Yachida, S. Pancreatic Neuroendocrine Neoplasms: Basic Biology, Current Treatment Strategies and Prospects for the Future. Int. J. Mol. Sci. 2017, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Benitez, J.A.; Ma, J.; D’Antonio, M.; Boyer, A.; Camargo, M.F.; Zanca, C.; Kelly, S.; Khodadadi-Jamayran, A.; Jameson, N.M.; Andersen, M.; et al. PTEN Regulates Glioblastoma Oncogenesis through Chromatin-Associated Complexes of DAXX and Histone H3.3. Nat. Commun. 2017, 8, 15223. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, C.; Ear, P.; Tran, C.; Howe, J.; Chandrasekharan, C.; Quelle, D. Pancreatic Neuroendocrine Tumors: Molecular Mechanisms and Therapeutic Targets. Cancers 2021, 13, 5117. [Google Scholar] [CrossRef] [PubMed]
- Pipinikas, C.P.; Berner, A.M.; Sposito, T.; Thirlwell, C. The Evolving (Epi)Genetic Landscape of Pancreatic Neuroendocrine Tumours. Endocr.-Relat. Cancer 2019, 26, R519–R544. [Google Scholar] [CrossRef]
- Cherif, C.; Nguyen, D.T.; Paris, C.; Le, T.K.; Sefiane, T.; Carbuccia, N.; Finetti, P.; Chaffanet, M.; Kaoutari, A.E.; Vernerey, J.; et al. Menin Inhibition Suppresses Castration-Resistant Prostate Cancer and Enhances Chemosensitivity. Oncogene 2022, 41, 125–137. [Google Scholar] [CrossRef]
- Baas, R.; Sijm, A.; Van Teeffelen, H.A.A.M.; Van Es, R.; Vos, H.R.; Marc Timmers, H.T. Quantitative Proteomics of the SMAD (Suppressor of Mothers against Decapentaplegic) Transcription Factor Family Identifies Importin 5 as a Bone Morphogenic Protein Receptor SMAD-Specific Importin. J. Biol. Chem. 2016, 291, 24121–24132. [Google Scholar] [CrossRef] [PubMed]
- Blank, U.; Karlsson, S. The Role of Smad Signaling in Hematopoiesis and Translational Hematology. Leukemia 2011, 25, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Murai, F.; Koinuma, D.; Shinozaki-Ushiku, A.; Fukayama, M.; Miyaozono, K.; Ehata, S. EZH2 Promotes Progression of Small Cell Lung Cancer by Suppressing the TGF-β-Smad-ASCL1 Pathway. Cell Discov. 2015, 1, 15026. [Google Scholar] [CrossRef] [PubMed]


| Altered Gene/ Biomarker | NE Tumor Type | Type of Analyses | Observations | References |
|---|---|---|---|---|
| MAPK Pathway | ||||
| KRAS | G3 P-NET (n = 21) P-NEC (n = 31 SCNEC; n = 18 LCNEC) | PCR IHC | KRAS mutations are not detected in NET-G3 (0%), while NEC-G3 harbors KRAS mutations in 48.7% of cases. There are no significant differences between SCNEC and LCNEC in the prevalence of KRAS mutations. KRAS mutations are associated with a higher response to platinum-based chemotherapy compared to those without mutations (mutated KRAS, 77% vs. wild-type, 23%). | [182] |
| G3 P-NET (n = 21) P-NEC (n = 18 LCNEC; n = 31 SCNEC) | IHC Real-Time PCR | KRAS is mutated in 48.7% of G3 P-NEC. Patients with a KRAS mutation exhibit a better response to first-line platinum-based therapy compared to those with wild-type KRAS but tend to have shorter overall survival rates. | [183] | |
| G3 NET (n = 6) NEC (n = 77) | Real-Time PCR NGS | KRAS mutations do not affect treatment effectiveness or survival rates following initial chemotherapy. | [184] | |
| BRAF | G3 NET (n = 6) NEC (n = 77) | Real-Time PCR NGS | A higher frequency of BRAF mutations is found in colon NEC and predicts failure to first-line treatment with cisplatin/carboplatin and etoposide. | [184] |
| PTEN/PI3K/Akt/mTOR Pathway | ||||
| PTEN | prostate NEC (n = 1) | NGS IHC | Somatic mutations in PTEN (and BRCA2) were identified in the tumor tissue. The tumor cells exhibited decreased staining for PTEN, indicating a loss of protein expression, which is also associated with a significant response to platinum therapy. | [177] |
| Notch/ASCL1 pathway | ||||
| Notch1 | SCLC (n = 46) | IHC | Hes1, ASCL1, and DLL3 protein expression levels are not associated with sensitivity to platinum chemotherapy or prognosis. However, SCLC with low Notch-1 expression has a better survival rate. | [131] |
| DLL3 | LCNEC (n = 70) | IHC | DLL3 is a predictive marker for sensitivity to platinum-based adjuvant chemotherapy in LCNEC. Patients with DLL3-negative tumors who receive chemotherapy show significantly higher overall survival and recurrence-free survival rates. | [185] |
| Members of pathways involved in DNA repair | ||||
| MDM2 | prostate NEC (n = 1) | NGS | Platinum-based chemotherapy was found to be effective in a patient with pancreatic neuroendocrine carcinoma (NEC) exhibiting an aggressive course and MDM2 amplification. | [186] |
| p53 | NSCLC-NE (n = 157) | IHC | There is no statistically significant correlation between the p53 marker and response to chemotherapy. However, patients with an increased expression of p53 are more likely to experience progressive disease after undergoing chemotherapy. | [132] |
| G3 NET (n = 10) LCNEC (n = 31) SCNEC (n = 48) | IHC | There is no statistically significant correlation between the p53 marker and response to chemotherapy. However, patients with an increased expression of p53 are more likely to experience progressive disease after undergoing chemotherapy. | [132] | |
| ES-SCLC (n = 75) | NGS | Patients with mutant TP53 had a better PFS than those with wild-type TP53. | [187] | |
| P-NET (n = 50) P-NEC (n = 29) | IHC | Abnormal p53 expression is not associated with response to platinum-based therapy. | [20] | |
| Prostate NEC (n = 1) | NGS IHC | The TP53 p.P72R variant is correlated with higher platinum sensitivity and longer survival of patient with aggressive prostate cancer. | [188] | |
| G3 GEP-NET (n = 41) GEP-NEC (n = 188) | NGS | TP53 mutation predicts an inferior response rate to cisplatin/carboplatin for NEC but does not correlate with overall survival (except for small-cell NEC). | [189] | |
| Rb | SCLC (n = 50) | Whole/Targeted Genome Sequencing IHC Western Blotting | The RB1 mutation status had the most significant impact of any gene. SCLC patients with wild-type RB1 demonstrated a significantly lower response to chemotherapy compared to patients with mutant RB1. | [190] |
| G3 P-NET (n = 21) P-NEC (n = 31 SCNEC; n = 18 LCNEC) | PCR IHC | The loss of Rb expression was not observed in NET-G3 (0%), while NEC-G3 showed a loss of expression in 54.5% of cases. There were no significant differences in the prevalence of abnormal Rb expression between SCNEC and LCNEC. The loss of Rb in NECs was associated with a significantly higher response rate to platinum-based chemotherapy compared to those without (80% vs. 24% with normal Rb expression). | [182] | |
| G3 P-NET (n = 21) P-NEC (n = 18 LCNEC; n = 31 SCNEC) | IHC Real-Time PCR | The rate of Rb loss in G3 P-NEC is 54.5% and is associated with a higher response rate to first-line platinum-based regimens compared to those without Rb loss. However, patients with Rb loss tended to have shorter overall survival rates than those without Rb loss. | [183] | |
| prostate NEC (n = 1) | NGS | A patient with heterozygosity loss in the RB1 gene displayed an aggressive course and responded favorably to chemotherapy containing platinum. | [186] | |
| G3 NET (n = 10) LCNEC (n = 31) SCNEC (n = 48) | IHC | Patients with G3 neuroendocrine neoplasms (NENs) exhibit varying responses to treatment with etoposide and platinum. However, the objective response rate was notably higher in NENs lacking the retinoblastoma (Rb) gene (63% vs. 42%). | [191] | |
| BRCA | prostate NEC with metastatic lung nodule and brain metastases (n = 1) | NGS | Combined platinum and etoposide chemotherapy yields partial and complete remissions of brain and lung metastases, respectively, in a patient with a somatic and germline BRCA2 mutation. | [192] |
| prostate NEC (n = 1) | NGS | A patient with a complete copy number loss of BRCA2 and ATM in prostate NEC (but not in his original adenocarcinoma) exhibited a complete response to carboplatin plus etoposide chemotherapy. | [193] | |
| prostate NEC (n = 1) | NGS | A patient with a BRCA2 mutation (along with a PTEN mutation) displays an aggressive disease progression and showed a positive response to chemotherapy containing platinum. | [186] | |
| prostate SCNEC (n = 1) | PCR | The patient with a germline BRCA2 mutation achieved a complete response to platinum-based chemotherapy but experienced a limited duration of remission when treated with olaparib (a PARP inhibitor) as maintenance therapy. | [194] | |
| colon LCNEC (n = 1) | CGP | Treatment with platinum-based therapy leads to a full radiographic remission of the metastases, with no indication of recurrence after 6.5 years. The response to the therapy is probably attributed to the loss of BRCA1 and/or BAP1 function. | [195] | |
| prostate NEC (n = 1) | NGC IHC | BRCA2 is mutated in tumors but not in normal tissue. BRCA2 somatic mutations are associated with a strong response to platinum therapy. | [177] | |
| Other Markers | ||||
| β-catenin | pancreatobiliary NEC (n = 30) | IHC | Higher levels of β-catenin are a predictive factor for response to platinum-based chemotherapy. | [196] |
| p16 | G3 NET (n = 10) LCNEC (n = 31) SCNEC (n = 48) | IHC | The objective response rate is significantly higher in NEN with high p16 levels (66% vs. 35%). | [191] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefàno, E.; De Castro, F.; Ciccarese, A.; Muscella, A.; Marsigliante, S.; Benedetti, M.; Fanizzi, F.P. An Overview of Altered Pathways Associated with Sensitivity to Platinum-Based Chemotherapy in Neuroendocrine Tumors: Strengths and Prospects. Int. J. Mol. Sci. 2024, 25, 8568. https://doi.org/10.3390/ijms25168568
Stefàno E, De Castro F, Ciccarese A, Muscella A, Marsigliante S, Benedetti M, Fanizzi FP. An Overview of Altered Pathways Associated with Sensitivity to Platinum-Based Chemotherapy in Neuroendocrine Tumors: Strengths and Prospects. International Journal of Molecular Sciences. 2024; 25(16):8568. https://doi.org/10.3390/ijms25168568
Chicago/Turabian StyleStefàno, Erika, Federica De Castro, Antonella Ciccarese, Antonella Muscella, Santo Marsigliante, Michele Benedetti, and Francesco Paolo Fanizzi. 2024. "An Overview of Altered Pathways Associated with Sensitivity to Platinum-Based Chemotherapy in Neuroendocrine Tumors: Strengths and Prospects" International Journal of Molecular Sciences 25, no. 16: 8568. https://doi.org/10.3390/ijms25168568
APA StyleStefàno, E., De Castro, F., Ciccarese, A., Muscella, A., Marsigliante, S., Benedetti, M., & Fanizzi, F. P. (2024). An Overview of Altered Pathways Associated with Sensitivity to Platinum-Based Chemotherapy in Neuroendocrine Tumors: Strengths and Prospects. International Journal of Molecular Sciences, 25(16), 8568. https://doi.org/10.3390/ijms25168568








