Glutamate, Gangliosides, and the Synapse: Electrostatics at Work in the Brain
Abstract
1. Introduction
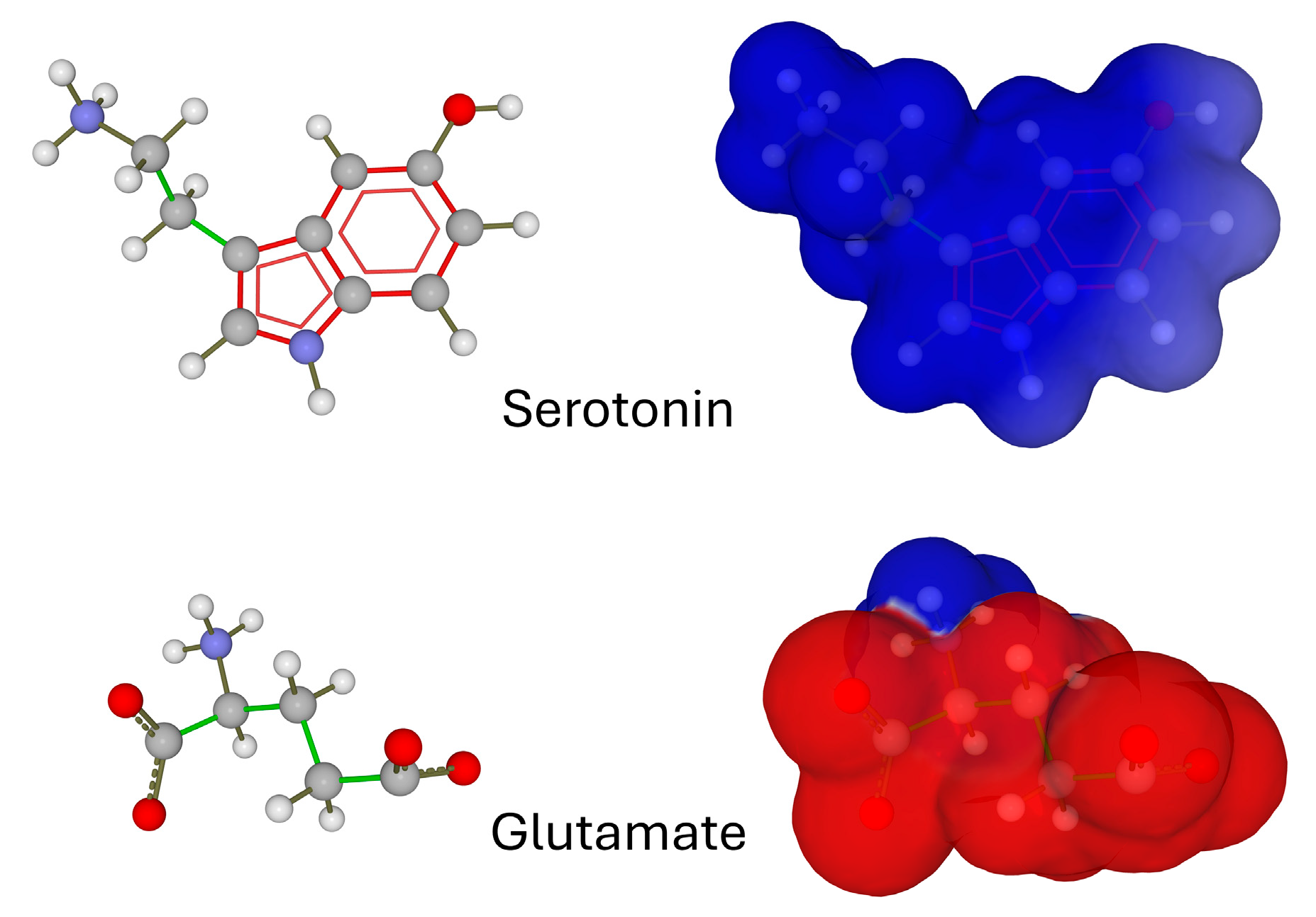
2. Electrostatic Surface Potential of Neurotransmitters
3. Electrostatic Surface Potential of Lipid Rafts
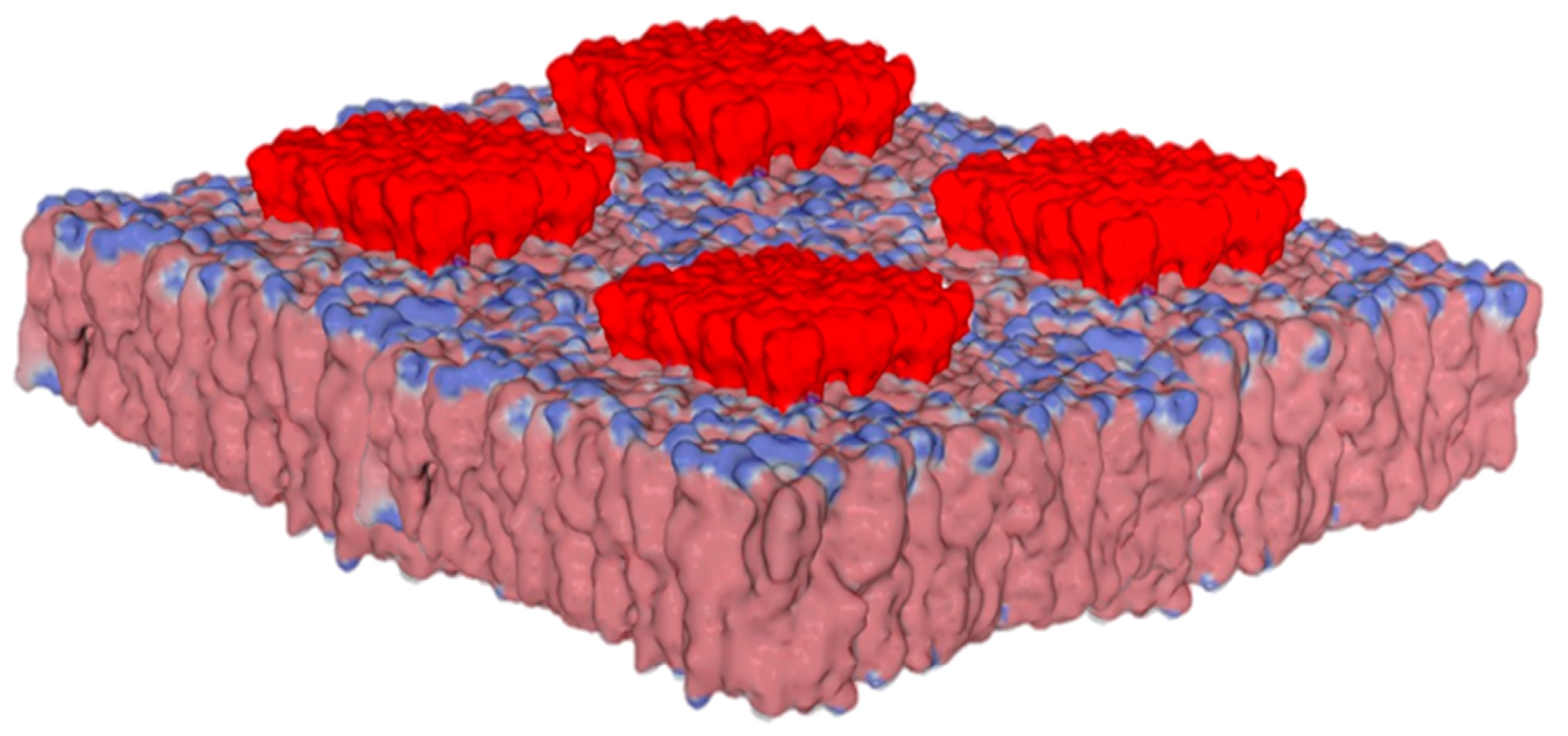
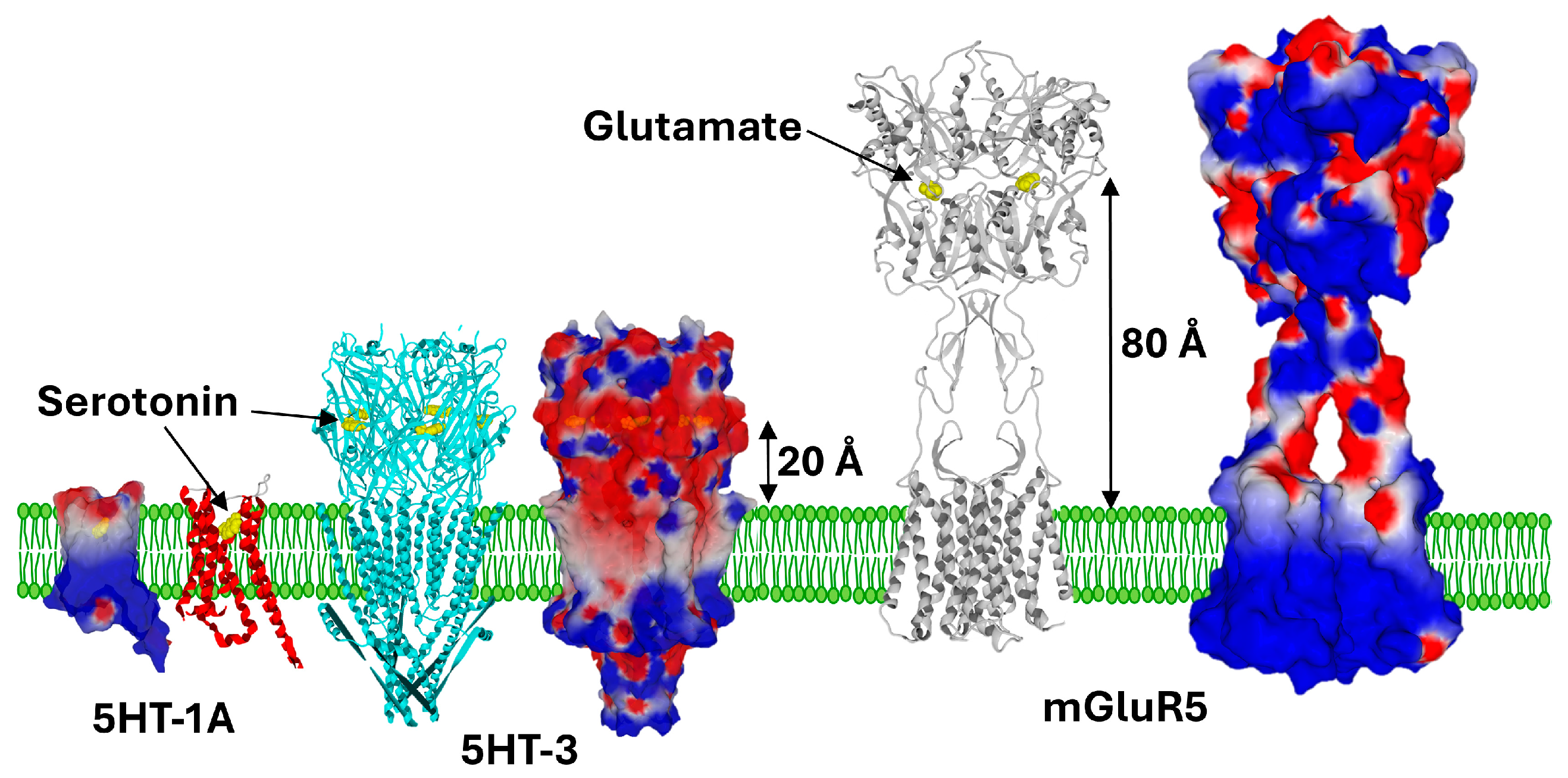
4. Electrostatic Constraints Applied to Post-Synaptic Glutamate Receptors
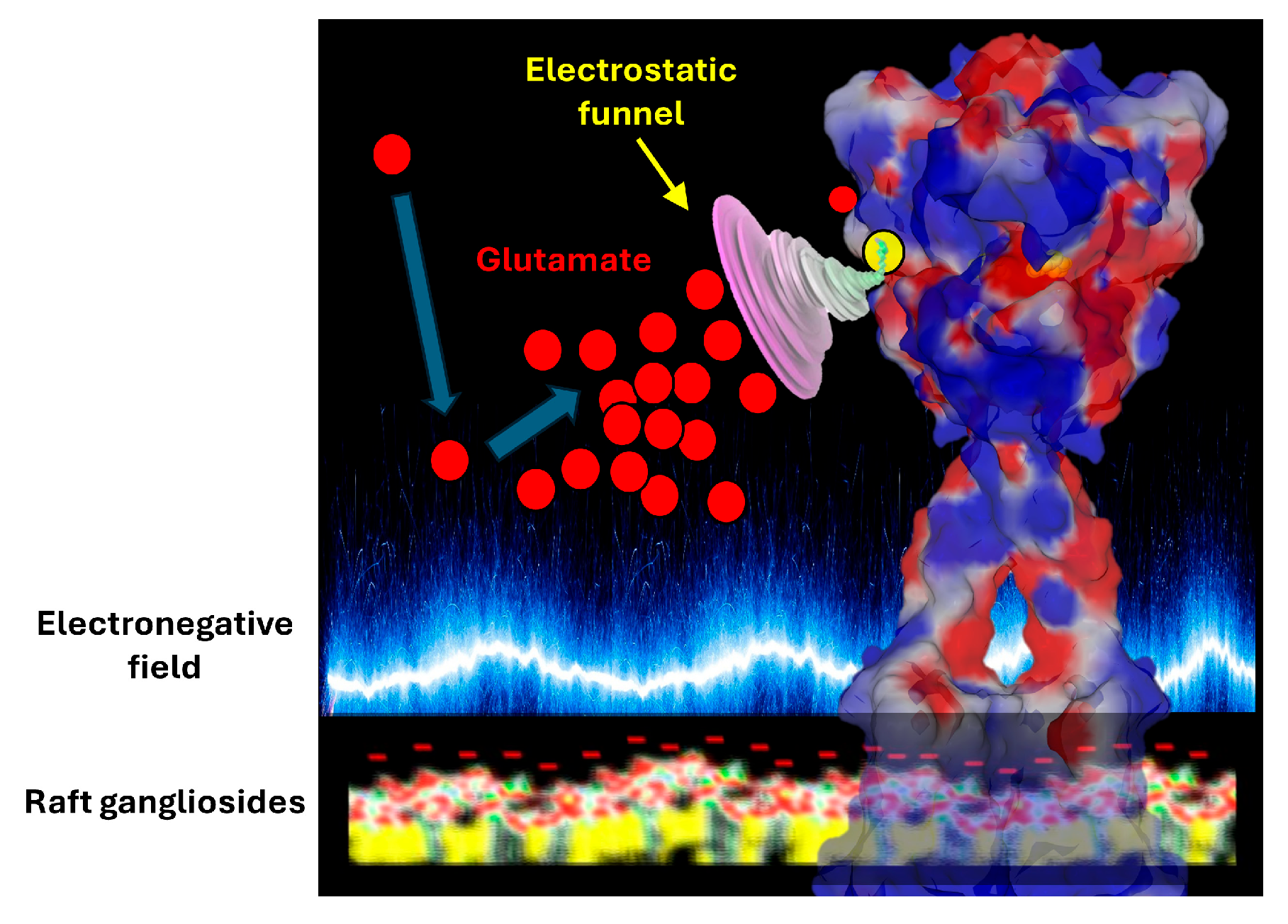
5. Electrostatic Control of Glutamate Flux in the Synaptic Cleft
6. How Astrocytes Buffer Glutamate and Manage Its Recycling
7. Pathological Implications
8. Consideration of the Electrostatic Parameter of Lipid Rafts: Overview of the Literature
9. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sotelo, C. The history of the synapse. Anat. Rec. 2020, 303, 1252–1279. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.J.; Grant, S.G. The origin and evolution of synapses. Nat. Rev. Neurosci. 2009, 10, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Nedergaard, M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience 2004, 129, 877–896. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Matveeva, M.; Lefebvre, M.; Chahinian, H. What Is life? Rethinking Biology in Light of Fundamental Parameters. Life 2024, 14, 280. [Google Scholar] [CrossRef] [PubMed]
- Takamura, K.; Kusu, F. Surface behavior of neurotransmitters, their agonists and antagonists on a gold electrode surface. J. Electrochem. Soc. 1990, 137, 2132. [Google Scholar] [CrossRef]
- Hermsmeier, M.A.; Gund, T.M. A graphical representation of the electrostatic potential and electric field on a molecular surface. J. Mol. Graph. 1989, 7, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Rukmini, R.; Mukherjee, S. Photophysics of a neurotransmitter: Ionization and spectroscopic properties of serotonin. Biophys. J. 1996, 71, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. Glutamate: A truly functional amino acid. Amino Acids 2013, 45, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z. Ganglioside GM1 and the central nervous system. Int. J. Mol. Sci. 2023, 24, 9558. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic acids in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.-J.; Tsao, S.-H.; Chen, A.; Hu, S.-K.; Chao, L.; Chao, P.-H.G. Lipid rafts sense and direct electric field-induced migration. Proc. Natl. Acad. Sci. USA 2017, 114, 8568–8573. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Azzaz, F.; Chahinian, H.; Yahi, N. Electrostatic Surface Potential as a Key Parameter in Virus Transmission and Evolution: How to Manage Future Virus Pandemics in the Post-COVID-19 Era. Viruses 2023, 15, 284. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Chahinian, H.; Yahi, N. Convergent Evolution Dynamics of SARS-CoV-2 and HIV Surface Envelope Glycoproteins Driven by Host Cell Surface Receptors and Lipid Rafts: Lessons for the Future. Int. J. Mol. Sci. 2023, 24, 1923. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, M.; Lefebvre, M.; Chahinian, H.; Yahi, N.; Fantini, J. Host membranes as drivers of virus evolution. Viruses 2023, 15, 1854. [Google Scholar] [CrossRef] [PubMed]
- Righetto, I.; Filippini, F. Normal modes analysis and surface electrostatics of haemagglutinin proteins as fingerprints for high pathogenic type A influenza viruses. BMC Bioinform. 2020, 21, 354. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.M.; Edwardson, J.M.; Geisse, N.A.; Saslowsky, D.E. Lipid rafts: Feeling is believing. Physiology 2004, 19, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Juhola, H.; Postila, P.A.; Rissanen, S.; Lolicato, F.; Vattulainen, I.; Róg, T. Negatively charged gangliosides promote membrane association of amphipathic neurotransmitters. Neuroscience 2018, 384, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Yahi, N. Brain Lipids in Synaptic Function and Neurological Disease: Clues to Innovative Therapeutic Strategies for Brain Disorders; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Rusakov, D.A.; Savtchenko, L.P.; Zheng, K.; Henley, J.M. Shaping the synaptic signal: Molecular mobility inside and outside the cleft. Trends Neurosci. 2011, 34, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Sylantyev, S.; Savtchenko, L.P.; Niu, Y.-P.; Ivanov, A.I.; Jensen, T.P.; Kullmann, D.M.; Xiao, M.-Y.; Rusakov, D.A. Electric fields due to synaptic currents sharpen excitatory transmission. Science 2008, 319, 1845–1849. [Google Scholar] [CrossRef][Green Version]
- McDaniel, R.; McLaughlin, S. The interaction of calcium with gangliosides in bilayer membranes. Biochim. Biophys. Acta 1985, 819, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Pristerá, A.; Okuse, K. Building excitable membranes: Lipid rafts and multiple controls on trafficking of electrogenic molecules. Neurosci. 2012, 18, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef] [PubMed]
- Simon, I.A.; Bjørn-Yoshimoto, W.E.; Harpsøe, K.; Iliadis, S.; Svensson, B.; Jensen, A.A.; Gloriam, D.E. Ligand selectivity hotspots in serotonin GPCRs. Trends Pharmacol. Sci. 2023, 44, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Huang, S.; Zhang, H.; Mao, C.; Zhou, X.E.; Cheng, X.; Simon, I.A.; Shen, D.D.; Yen, H.Y.; Robinson, C.V.; et al. Structural insights into the lipid and ligand regulation of serotonin receptors. Nature 2021, 592, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Gicheru, Y.; Rao, S.; Sansom, M.S.P.; Chakrapani, S. Cryo-EM reveals two distinct serotonin-bound conformations of full-length 5-HT(3A) receptor. Nature 2018, 563, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Koehl, A.; Hu, H.; Feng, D.; Sun, B.; Zhang, Y.; Robertson, M.J.; Chu, M.; Kobilka, T.S.; Laeremans, T.; Steyaert, J.; et al. Structural insights into the activation of metabotropic glutamate receptors. Nature 2019, 566, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Felder, C.B.; Graul, R.C.; Lee, A.Y.; Merkle, H.P.; Sadee, W. The Venus flytrap of periplasmic binding proteins: An ancient protein module present in multiple drug receptors. AAPS Pharmsci 1999, 1, E2. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, P.J.; Sheppard, P.O.; Thøgersen, H.; Venezia, D.; Haldeman, B.A.; McGrane, V.; Houamed, K.M.; Thomsen, C.; Gilbert, T.L.; Mulvihill, E.R. The ligand-binding domain in metabotropic glutamate receptors is related to bacterial periplasmic binding proteins. Neuron 1993, 11, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Huang, S.; Qian, J.; Huang, J.; Jin, L.; Su, Z.; Yang, J.; Liu, J. Evolution of the class C GPCR Venus flytrap modules involved positive selected functional divergence. BMC Evol. Biol. 2009, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Pin, J.P.; Acher, F. The metabotropic glutamate receptors: Structure, activation mechanism and pharmacology. Curr. Drug Targets. CNS Neurol. Disord. 2002, 1, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.S.; Lightstone, F.C. An Electrostatic Funnel in the GABA-Binding Pathway. PLoS Comput. Biol. 2016, 12, e1004831. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.D.; Lester, R.A.; Tong, G.; Jahr, C.E.; Westbrook, G.L. The time course of glutamate in the synaptic cleft. Science 1992, 258, 1498–1501. [Google Scholar] [CrossRef] [PubMed]
- Savtchenko, L.P.; Rusakov, D.A. The optimal height of the synaptic cleft. Proc. Natl. Acad. Sci. USA 2007, 104, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Beitinger, H.; Vogel, V.; Möbius, D.; Rahmann, H. Surface potentials and electric dipole moments of ganglioside and phospholipid monolayers: Contribution of the polar headgroup at the water/lipid interface. Biochim. Biophys. Acta (BBA)-Biomembr. 1989, 984, 293–300. [Google Scholar] [CrossRef]
- Lalo, U.; Koh, W.; Lee, C.J.; Pankratov, Y. The tripartite glutamatergic synapse. Neuropharmacology 2021, 199, 108758. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Fellin, T.; Haydon, P.G. The tripartite synapse: Roles for gliotransmission in health and disease. Trends Mol. Med. 2007, 13, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Ledeen, R.W.; Diebler, M.F.; Wu, G.; Lu, Z.H.; Varoqui, H. Ganglioside composition of subcellular fractions, including pre- and postsynaptic membranes, from Torpedo electric organ. Neurochem. Res. 1993, 18, 1151–1155. [Google Scholar] [CrossRef]
- Asou, H.; Hirano, S.; Uyemura, K. Ganglioside composition of astrocytes. Cell Struct. Funct. 1989, 14, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Maggio, B. The surface behavior of glycosphingolipids in biomembranes: A new frontier of molecular ecology. Prog. Biophys. Mol. Biol. 1994, 62, 55–117. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Campuzano, A.G.; Ortega, A. Glutamate transporters: Critical components of glutamatergic transmission. Neuropharmacology 2021, 192, 108602. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Boudker, O. Large domain movements through the lipid bilayer mediate substrate release and inhibition of glutamate transporters. Elife 2020, 9, e58417. [Google Scholar] [CrossRef] [PubMed]
- Garaeva, A.A.; Slotboom, D.J. Elevator-type mechanisms of membrane transport. Biochem. Soc. Trans. 2020, 48, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Sonnewald, U.; Westergaard, N.; Schousboe, A. Glutamate transport and metabolism in astrocytes. Glia 1997, 21, 56–63. [Google Scholar] [CrossRef]
- Hertz, L.; Dringen, R.; Schousboe, A.; Robinson, S.R. Astrocytes: Glutamate producers for neurons. J. Neurosci. Res. 1999, 57, 417–428. [Google Scholar] [CrossRef]
- Azzaz, F.; Yahi, N.; Di Scala, C.; Chahinian, H.; Fantini, J. Ganglioside binding domains in proteins: Physiological and pathological mechanisms. Adv. Protein Chem. Struct. Biol. 2022, 128, 289–324. [Google Scholar]
- Borroni, M.V.; Vallés, A.S.; Barrantes, F.J. The lipid habitats of neurotransmitter receptors in brain. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 2662–2670. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.A.; Halverson-Tamboli, R.A.; Rasenick, M.M. Lipid raft microdomains and neurotransmitter signalling. Nat. Rev. Neurosci. 2007, 8, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Butchbach, M.E.; Tian, G.; Guo, H.; Lin, C.-l.G. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: Importance for excitatory amino acid transporter localization and function. J. Biol. Chem. 2004, 279, 34388–34396. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J. Lipid rafts and human diseases: Why we need to target gangliosides. FEBS Open Bio 2023, 13, 1636–1650. [Google Scholar] [CrossRef] [PubMed]
- Björkholm, P.; Ernst, A.M.; Hacke, M.; Wieland, F.; Brügger, B.; von Heijne, G. Identification of novel sphingolipid-binding motifs in mammalian membrane proteins. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 2066–2070. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, X.; Jafurulla, M.; Sengupta, D.; Chattopadhyay, A. The ganglioside GM1 interacts with the serotonin1A receptor via the sphingolipid binding domain. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 2818–2826. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Chattopadhyay, A. Cholesterol interaction motifs in G protein-coupled receptors: Slippery hot spots? Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1481. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Pucadyil, T.J.; Paila, Y.D.; Ganguly, S.; Chattopadhyay, A. Chronic cholesterol depletion using statin impairs the function and dynamics of human serotonin(1A) receptors. Biochemistry 2010, 49, 5426–5435. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Barrantes, F.J. How cholesterol interacts with membrane proteins: An exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front. Physiol. 2013, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.S.; Park, S.; Wu, E.L.; Yeom, M.S.; Widmalm, G.; Klauda, J.B.; Im, W. Influence of ganglioside GM1 concentration on lipid clustering and membrane properties and curvature. Biophys. J. 2016, 111, 1987–1999. [Google Scholar] [CrossRef] [PubMed]
- Sandhoff, R.; Sandhoff, K. Emerging concepts of ganglioside metabolism. FEBS Lett. 2018, 592, 3835–3864. [Google Scholar] [CrossRef] [PubMed]
- Maggio, B.; Cumar, F.A.; Caputto, R. Surface behaviour of gangliosides and related glycosphingolipids. Biochem. J. 1978, 171, 559–565. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, R.V.; McLaughlin, A.; Winiski, A.P.; Eisenberg, M.; McLaughlin, S. Bilayer membranes containing the ganglioside GM1: Models for electrostatic potentials adjacent to biological membranes. Biochemistry 1984, 23, 4618–4624. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Lu, Z.-H.; Wang, J.; Wang, Y.; Xie, X.; Meyenhofer, M.F.; Ledeen, R.W. Enhanced susceptibility to kainate-induced seizures, neuronal apoptosis, and death in mice lacking gangliotetraose gangliosides: Protection with LIGA 20, a membrane-permeant analog of GM1. J. Neurosci. 2005, 25, 11014–11022. [Google Scholar] [CrossRef] [PubMed]
- Negrete-Díaz, J.V.; Falcón-Moya, R.; Rodríguez-Moreno, A. Kainate receptors: From synaptic activity to disease. FEBS J. 2022, 289, 5074–5088. [Google Scholar] [CrossRef]
- Copits, B.A.; Swanson, G.T. Kainate receptor post-translational modifications differentially regulate association with 4.1 N to control activity-dependent receptor endocytosis. J. Biol. Chem. 2013, 288, 8952–8965. [Google Scholar] [CrossRef]
- Favaron, M.; Manev, H.; Alho, H.; Bertolino, M.; Ferret, B.; Guidotti, A.; Costa, E. Gangliosides prevent glutamate and kainate neurotoxicity in primary neuronal cultures of neonatal rat cerebellum and cortex. Proc. Natl. Acad. Sci. USA 1988, 85, 7351–7355. [Google Scholar] [CrossRef] [PubMed]
- Manev, H.; Favaron, M.; Vicini, S.; Guidotti, A. Ganglioside-mediated protection from glutamate-induced neuronal death. Acta Neurobiol. Exp. 1990, 50, 475–488. [Google Scholar]
- Costa, E.; Armstrong, D.; Guidotti, A.; Kharlamov, A.; Kiedrowski, L.; Manev, H.; Polo, A.; Wroblewski, J. Gangliosides in the protection against glutamate excitotoxicity. Prog. Brain Res. 1994, 101, 357–373. [Google Scholar] [PubMed]
- Wu, G.; Lu, Z.H.; Xie, X.; Ledeen, R.W. Susceptibility of cerebellar granule neurons from GM2/GD2 synthase-null mice to apoptosis induced by glutamate excitotoxicity and elevated KCl: Rescue by GM1 and LIGA20. Glycoconj. J. 2004, 21, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Bachis, A.; Rabin, S.J.; Del Fiacco, M.; Mocchetti, I. Gangliosides prevent excitotoxicity through activation of TrkB receptor. Neurotox. Res. 2002, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, E.; Maggioni, M.; di Biase, E.; Lunghi, G.; Fazzari, M.; Loberto, N.; Elisa, M.; Scalvini, F.G.; Tedeschi, G.; Sonnino, S. The Neuroprotective Role of the GM1 Oligosaccharide, II(3)Neu5Ac-Gg(4), in Neuroblastoma Cells. Mol. Neurobiol. 2019, 56, 6673–6702. [Google Scholar] [CrossRef]
- Lunghi, G.; Di Biase, E.; Carsana, E.V.; Henriques, A.; Callizot, N.; Mauri, L.; Ciampa, M.G.; Mari, L.; Loberto, N.; Aureli, M.; et al. GM1 ganglioside exerts protective effects against glutamate-excitotoxicity via its oligosaccharide in wild-type and amyotrophic lateral sclerosis motor neurons. FEBS Open Bio 2023, 13, 2324–2341. [Google Scholar] [CrossRef] [PubMed]
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the brain: Physiology, pathophysiology and therapeutic applications. Front. Neurosci. 2020, 14, 572965. [Google Scholar] [CrossRef]
- Li, T.A.; Schnaar, R.L. Congenital disorders of ganglioside biosynthesis. Prog. Mol. Biol. Transl. Sci. 2018, 156, 63–82. [Google Scholar] [PubMed]
- Magistretti, P.J.; Geisler, F.H.; Schneider, J.S.; Li, P.A.; Fiumelli, H.; Sipione, S. Gangliosides: Treatment avenues in neurodegenerative disease. Front. Neurol. 2019, 10, 859. [Google Scholar] [CrossRef] [PubMed]
- Fazzari, M.; Lunghi, G.; Chiricozzi, E.; Mauri, L.; Sonnino, S. Gangliosides and the Treatment of Neurodegenerative Diseases: A Long Italian Tradition. Biomedicines 2022, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Chahinian, H.; Yahi, N. Progress toward Alzheimer’s disease treatment: Leveraging the Achilles’ heel of Aβ oligomers? Protein Sci. 2020, 29, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Yahi, N.; Di Scala, C.; Chahinian, H.; Fantini, J. Innovative treatment targeting gangliosides aimed at blocking the formation of neurotoxic α-synuclein oligomers in Parkinson’s disease. Glycoconj. J. 2021, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Azzaz, F.; Chahinian, H.; Yahi, N.; Fantini, J.; Di Scala, C. AmyP53 Prevents the Formation of Neurotoxic β-Amyloid Oligomers through an Unprecedent Mechanism of Interaction with Gangliosides: Insights for Alzheimer’s Disease Therapy. Int. J. Mol. Sci. 2023, 24, 1760. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Aras, R.; Williams, C.K.; Koprich, J.B.; Brotchie, J.M.; Singh, V. GM1 ganglioside modifies α-synuclein toxicity and is neuroprotective in a rat α-synuclein model of Parkinson’s disease. Sci. Rep. 2019, 9, 8362. [Google Scholar] [CrossRef]
- Fazzari, M.; Di Biase, E.; Zaccagnini, L.; Henriques, A.; Callizot, N.; Ciampa, M.G.; Mauri, L.; Carsana, E.V.; Loberto, N.; Aureli, M. GM1 oligosaccharide efficacy against α-synuclein aggregation and toxicity in vitro. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2023, 1868, 159350. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Lu, Z.-H.; Seo, J.H.; Alselehdar, S.K.; DeFrees, S.; Ledeen, R.W. Mice deficient in GM1 manifest both motor and non-motor symptoms of Parkinson’s disease; successful treatment with synthetic GM1 ganglioside. Exp. Neurol. 2020, 329, 113284. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Saywell, V.; Villard, L.; Cozzone, P.J.; Lutz, N.W. Metabolic fingerprints of altered brain growth, osmoregulation and neurotransmission in a Rett syndrome model. PLoS ONE 2007, 2, e157. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, R.; Riikonen, R.S. High levels of cerebrospinal fluid glutamate in Rett syndrome. Pediatr. Neurol. 1996, 15, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Lekman, A.Y.; Hagberg, B.A.; Svennerholm, L.T. Membrane cerebral lipids in Rett syndrome. Pediatr. Neurol. 1991, 7, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Naidu, S.; Kaufmann, W.E.; Abrams, M.T.; Pearlson, G.D.; Lanham, D.C.; Fredericksen, K.A.; Barker, P.B.; Horska, A.; Golay, X.; Mori, S.; et al. Neuroimaging studies in Rett syndrome. Brain Dev. 2001, 23 (Suppl. S1), S62–S71. [Google Scholar] [CrossRef] [PubMed]
- Rapport, M.M.; Donnenfeld, H.; Brunner, W.; Hungund, B.; Bartfeld, H. Ganglioside patterns in amyotrophic lateral sclerosis brain regions. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1985, 18, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.C.; Treleaven, C.M.; Pacheco, J.; Cooper, S.; Bao, C.; Abraham, M.; Cromwell, M.; Sardi, S.P.; Chuang, W.-L.; Sidman, R.L. Glycosphingolipids are modulators of disease pathogenesis in amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2015, 112, 8100–8105. [Google Scholar] [CrossRef] [PubMed]
- Boukhris, A.; Schule, R.; Loureiro, J.L.; Lourenço, C.M.; Mundwiller, E.; Gonzalez, M.A.; Charles, P.; Gauthier, J.; Rekik, I.; Lebrigio, R.F.A. Alteration of ganglioside biosynthesis responsible for complex hereditary spastic paraplegia. Am. J. Hum. Genet. 2013, 93, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Trinchera, M.; Parini, R.; Indellicato, R.; Domenighini, R.; Dall’Olio, F. Diseases of ganglioside biosynthesis: An expanding group of congenital disorders of glycosylation. Mol. Genet. Metab. 2018, 124, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E. Sphingolipid organization in biomembranes: What physical studies of model membranes reveal. J. Cell Sci. 1998, 111, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Garmy, N.; Mahfoud, R.; Yahi, N. Lipid rafts: Structure, function and role in HIV, Alzheimer’s and prion diseases. Expert Rev. Mol. Med. 2002, 4, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Munro, S. Lipid rafts: Elusive or illusive? Cell 2003, 115, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Chen, D.; Boda, A.R.; Foster, L.J.; Davis, M.J.; Hill, M.M. RaftProt: Mammalian lipid raft proteome database. Nucleic Acids Res. 2014, 43, D335–D338. [Google Scholar] [CrossRef] [PubMed]
- Funk, R.H. Biophysical mechanisms complementing “classical” cell biology. Front. Biosci. 2018, 23, 921–939. [Google Scholar] [CrossRef] [PubMed]
- Funk, R.H. Essay on information processes in the human brain. Arch. Anat. Physiol. 2024, 9, 001–006. [Google Scholar]
- Maggio, B. Modulation of phospholipase A2 by electrostatic fields and dipole potential of glycosphingolipids in monolayers. J. Lipid Res. 1999, 40, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Wilke, N.; Maggio, B. Electrostatic field effects on membrane domain segregation and on lateral diffusion. Biophys. Rev. 2011, 3, 185–192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McDaniel, R.; Sharp, K.; Brooks, D.; McLaughlin, A.; Winiski, A.; Cafiso, D.; McLaughlin, S. Electrokinetic and electrostatic properties of bilayers containing gangliosides GM1, GD1a, or GT1. Comparison with a nonlinear theory. Biophys. J. 1986, 49, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Thomas, P.D. Role of gangliosides in adhesion and conductance changes in large spherical model membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 1984, 776, 279–287. [Google Scholar] [CrossRef]
- Jordan, L.R.; Blauch, M.E.; Baxter, A.M.; Cawley, J.L.; Wittenberg, N.J. Influence of brain gangliosides on the formation and properties of supported lipid bilayers. Colloids Surf. B Biointerfaces 2019, 183, 110442. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chahinian, H.; Yahi, N.; Fantini, J. Glutamate, Gangliosides, and the Synapse: Electrostatics at Work in the Brain. Int. J. Mol. Sci. 2024, 25, 8583. https://doi.org/10.3390/ijms25168583
Chahinian H, Yahi N, Fantini J. Glutamate, Gangliosides, and the Synapse: Electrostatics at Work in the Brain. International Journal of Molecular Sciences. 2024; 25(16):8583. https://doi.org/10.3390/ijms25168583
Chicago/Turabian StyleChahinian, Henri, Nouara Yahi, and Jacques Fantini. 2024. "Glutamate, Gangliosides, and the Synapse: Electrostatics at Work in the Brain" International Journal of Molecular Sciences 25, no. 16: 8583. https://doi.org/10.3390/ijms25168583
APA StyleChahinian, H., Yahi, N., & Fantini, J. (2024). Glutamate, Gangliosides, and the Synapse: Electrostatics at Work in the Brain. International Journal of Molecular Sciences, 25(16), 8583. https://doi.org/10.3390/ijms25168583








