EWS-FLI1 and Activator Protein-1 (AP-1) Reciprocally Regulate Extracellular-Matrix Proteins in Ewing sarcoma Cells
Abstract
1. Introduction
2. Results
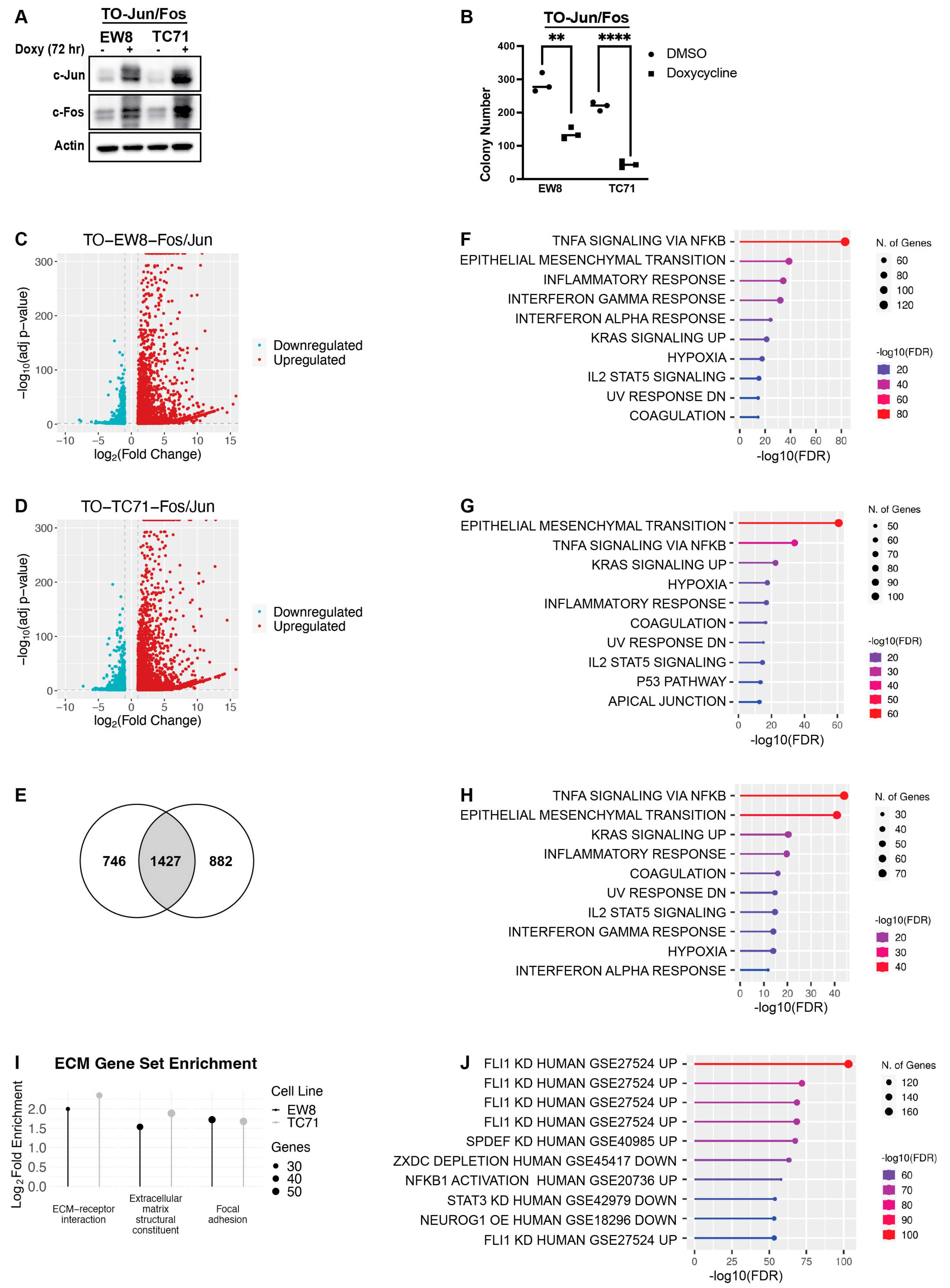
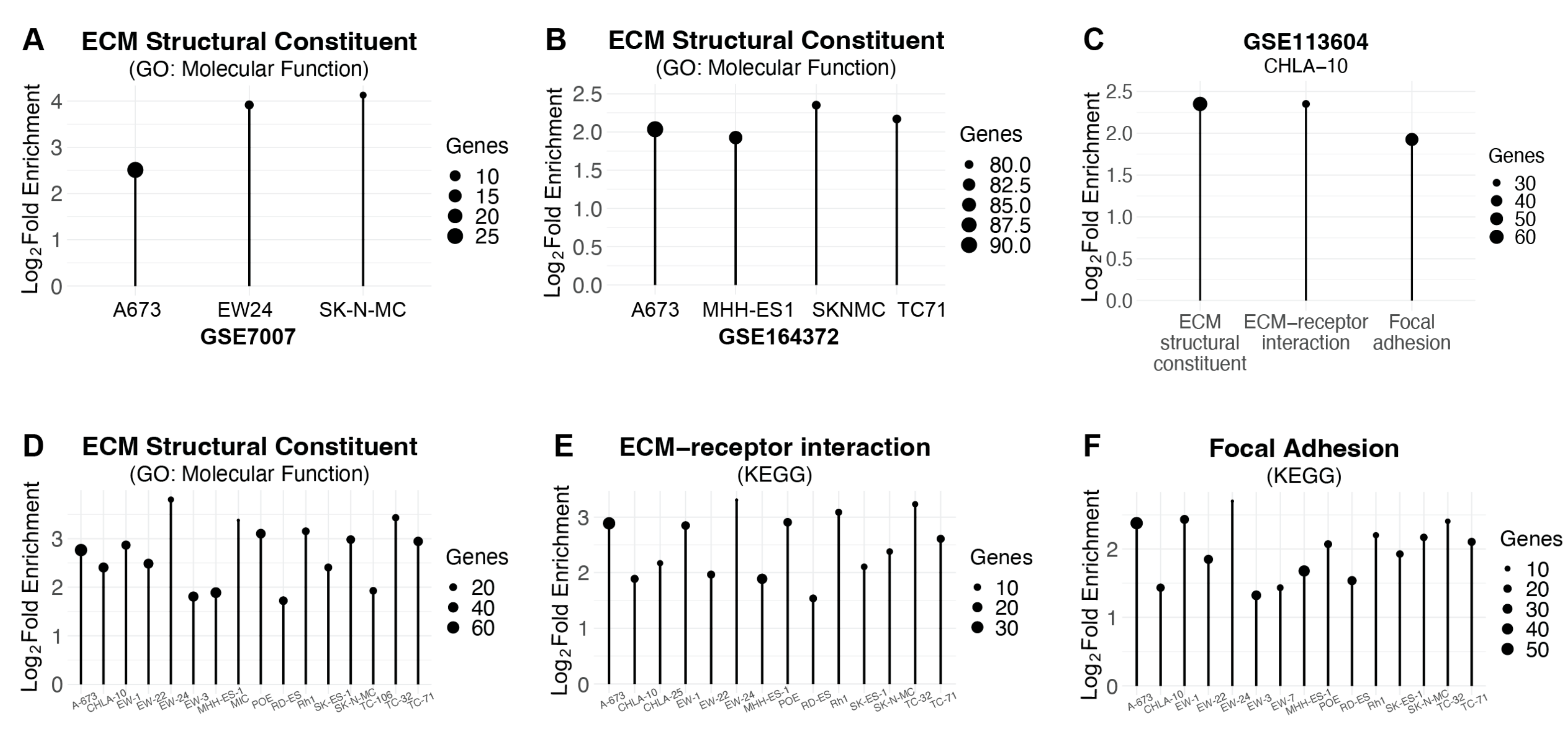
2.1. Genes Upregulated by AP-1 Are Enriched for EMT-Related Genes and Overlap with Genes Repressed by EWS-FLI1
2.2. The Set of Genes Upregulated by RRM1 Loss, AP-1 Overexpression, and EWS-FLI1 Knockdown Is Enriched for EMT-Associated Genes
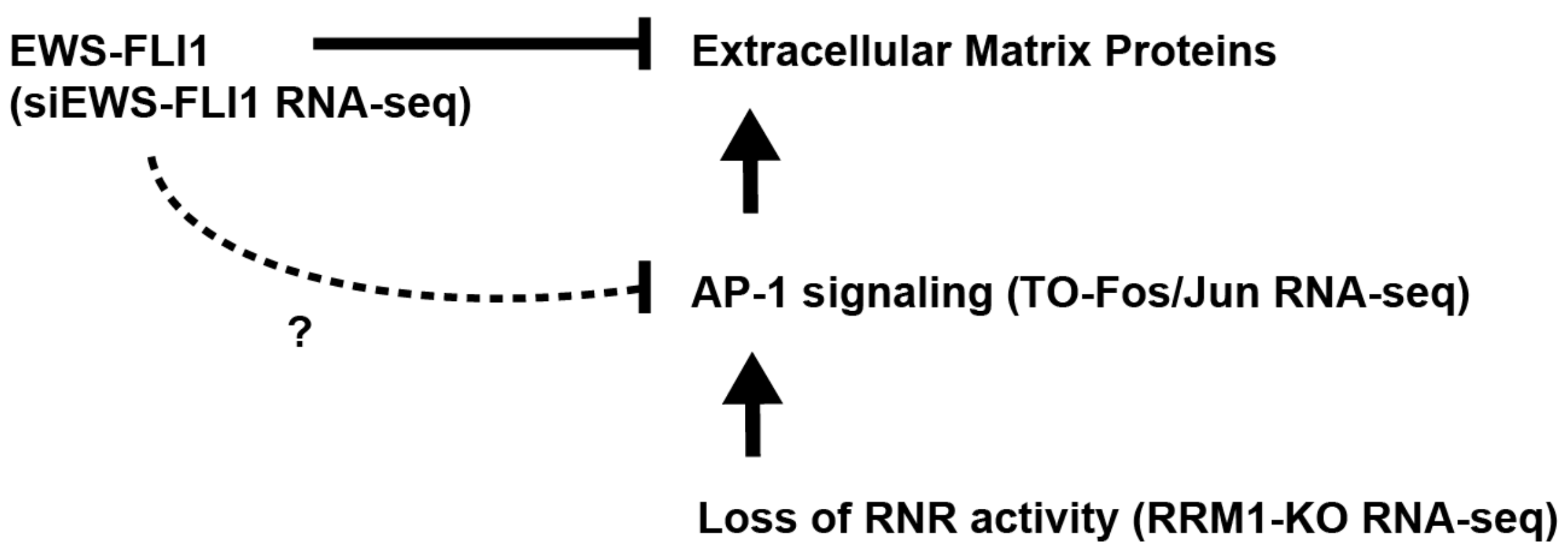
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture
4.2. Protein Isolation and Immunoblotting
4.3. RNA Sequencing and Analysis
4.4. Analysis of Previously Published RNA Sequencing Data
4.5. Analysis of Publicly Available Microarray Data
4.6. Clonogenic Assay
4.7. siRNA Transfection
4.8. TO-RRM1-KO Cell Lines
4.9. Doxycycline-Inducible Expression of c-Jun and c-Fos
4.10. RT-qPCR
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balamuth, N.J.; Womer, R.B. Ewing’s Sarcoma. Lancet Oncol. 2010, 11, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Delattre, O.; Zucman, J.; Plougastel, B.; Desmaze, C.; Melot, T.; Peter, M.; Kovar, H.; Joubert, I.; de Jong, P.; Rouleau, G.; et al. Gene Fusion with an ETS DNA-Binding Domain Caused by Chromosome Translocation in Human Tumours. Nature 1992, 359, 162–165. [Google Scholar] [CrossRef]
- Mitsiades, N.; Poulaki, V.; Mitsiades, C.; Tsokos, M. Ewing’s Sarcoma Family Tumors Are Sensitive to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand and Express Death Receptor 4 and Death Receptor 5. Cancer Res. 2001, 61, 2704–2712. [Google Scholar] [PubMed]
- Grünewald, T.G.P.; Cidre-Aranaz, F.; Surdez, D.; Tomazou, E.M.; de Álava, E.; Kovar, H.; Sorensen, P.H.; Delattre, O.; Dirksen, U. Ewing sarcoma. Nat. Rev. Dis. Primers 2018, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Stegmaier, K.; Wong, J.S.; Ross, K.N.; Chow, K.T.; Peck, D.; Wright, R.D.; Lessnick, S.L.; Kung, A.L.; Golub, T.R. Signature-Based Small Molecule Screening Identifies Cytosine Arabinoside as an EWS/FLI Modulator in Ewing sarcoma. PLoS Med. 2007, 4, e122. [Google Scholar] [CrossRef] [PubMed]
- Grohar, P.J.; Woldemichael, G.M.; Griffin, L.B.; Mendoza, A.; Chen, Q.-R.; Yeung, C.; Currier, D.G.; Davis, S.; Khanna, C.; Khan, J.; et al. Identification of an Inhibitor of the EWS-FLI1 Oncogenic Transcription Factor by High-Throughput Screening. J. Natl. Cancer Inst. 2011, 103, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Kovar, H. Downstream EWS/FLI1-Upstream Ewing’s Sarcoma. Genome Med. 2010, 2, 8. [Google Scholar] [CrossRef]
- Kennedy, A.L.; Vallurupalli, M.; Chen, L.; Crompton, B.; Cowley, G.; Vazquez, F.; Weir, B.A.; Tsherniak, A.; Parasuraman, S.; Kim, S.; et al. Functional, Chemical Genomic, and Super-Enhancer Screening Identify Sensitivity to Cyclin D1/CDK4 Pathway Inhibition in Ewing sarcoma. Oncotarget 2015, 6, 30178–30193. [Google Scholar] [CrossRef] [PubMed]
- Wilky, B.A.; Kim, C.; McCarty, G.; Montgomery, E.A.; Kammers, K.; DeVine, L.R.; Cole, R.N.; Raman, V.; Loeb, D.M. RNA Helicase DDX3: A Novel Therapeutic Target in Ewing sarcoma. Oncogene 2016, 35, 2574–2583. [Google Scholar] [CrossRef]
- Chansky, H.A.; Barahmand-Pour, F.; Mei, Q.; Kahn-Farooqi, W.; Zielinska-Kwiatkowska, A.; Blackburn, M.; Chansky, K.; Conrad, E.U.; Bruckner, J.D.; Greenlee, T.K.; et al. Targeting of EWS/FLI-1 by RNA Interference Attenuates the Tumor Phenotype of Ewing’s Sarcoma Cells in Vitro. J. Orthop. Res. 2004, 22, 910–917. [Google Scholar] [CrossRef]
- Tancredi, R.; Zambelli, A.; DaPrada, G.A.; Fregoni, V.; Pavesi, L.; Riccardi, A.; Burdach, S.; Grohar, P.J.; D’Incalci, M. Targeting the EWS-FLI1 Transcription Factor in Ewing sarcoma. Cancer Chemother. Pharmacol. 2015, 75, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Grohar, P.J.; Segars, L.E.; Yeung, C.; Pommier, Y.; D’Incalci, M.; Mendoza, A.; Helman, L.J. Dual Targeting of EWS-FLI1 Activity and the Associated DNA Damage Response with Trabectedin and SN38 Synergistically Inhibits Ewing sarcoma Cell Growth. Clin. Cancer Res. 2014, 20, 1190–1203. [Google Scholar] [CrossRef] [PubMed]
- Povedano, J.M.; Li, V.; Lake, K.E.; Bai, X.; Rallabandi, R.; Kim, J.; Xie, Y.; De Brabander, J.K.; McFadden, D.G. TK216 Targets Microtubules in Ewing sarcoma Cells. Cell Chem. Biol. 2022, 29, 1325–1332.e4. [Google Scholar] [CrossRef]
- Koppenhafer, S.L.; Goss, K.L.; Terry, W.W.; Gordon, D.J. Inhibition of the ATR-CHK1 Pathway in Ewing sarcoma Cells Causes DNA Damage and Apoptosis via the CDK2-Mediated Degradation of RRM2. Mol. Cancer Res. 2020, 18, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Croushore, E.E.; Koppenhafer, S.L.; Goss, K.L.; Geary, E.L.; Gordon, D.J. Activator Protein-1 (AP-1) Signaling Inhibits the Growth of Ewing sarcoma Cells in Response to DNA Replication Stress. Cancer Res. Commun. 2023, 3, 1580–1593. [Google Scholar] [CrossRef]
- Tomazou, E.M.; Sheffield, N.C.; Schmidl, C.; Schuster, M.; Schönegger, A.; Datlinger, P.; Kubicek, S.; Bock, C.; Kovar, H. Epigenome Mapping Reveals Distinct Modes of Gene Regulation and Widespread Enhancer Reprogramming by the Oncogenic Fusion Protein EWS-FLI1. Cell Rep. 2015, 10, 1082–1095. [Google Scholar] [CrossRef]
- Ohmura, S.; Marchetto, A.; Orth, M.F.; Li, J.; Jabar, S.; Ranft, A.; Vinca, E.; Ceranski, K.; Carreño-Gonzalez, M.J.; Romero-Pérez, L.; et al. Translational Evidence for RRM2 as a Prognostic Biomarker and Therapeutic Target in Ewing sarcoma. Mol. Cancer 2021, 20, 97. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.J.; Motwani, M.; Pellman, D. Modeling the Initiation of Ewing sarcoma Tumorigenesis in Differentiating Human Embryonic Stem Cells. Oncogene 2016, 35, 3092–3102. [Google Scholar] [CrossRef]
- Goss, K.L.; Gordon, D.J. Gene Expression Signature Based Screening Identifies Ribonucleotide Reductase as a Candidate Therapeutic Target in Ewing sarcoma. Oncotarget 2016, 7, 63003–63019. [Google Scholar] [CrossRef] [PubMed]
- Goss, K.L.; Koppenhafer, S.L.; Waters, T.; Terry, W.W.; Wen, K.-K.; Wu, M.; Ostergaard, J.; Gordon, P.M.; Gordon, D.J. The Translational Repressor 4E-BP1 Regulates RRM2 Levels and Functions as a Tumor Suppressor in Ewing sarcoma Tumors. Oncogene 2021, 40, 564–577. [Google Scholar] [CrossRef]
- Goss, K.L.; Koppenhafer, S.L.; Harmoney, K.M.; Terry, W.W.; Gordon, D.J. Inhibition of CHK1 Sensitizes Ewing sarcoma Cells to the Ribonucleotide Reductase Inhibitor Gemcitabine. Oncotarget 2017, 8, 87016–87032. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, E.; Karin, M. AP-1 in Cell Proliferation and Survival. Oncogene 2001, 20, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, E. AP-1--The Jun Proteins: Oncogenes or Tumor Suppressors in Disguise? Cell. Signal 2010, 22, 894–899. [Google Scholar] [CrossRef]
- Eferl, R.; Wagner, E.F. AP-1: A Double-Edged Sword in Tumorigenesis. Nat. Rev. Cancer 2003, 3, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Saratov, V.; Ngo, Q.A.; Pedot, G.; Sidorov, S.; Wachtel, M.; Niggli, F.K.; Schäfer, B.W. CRISPR Activation Screen Identifies TGFβ-Associated PEG10 as a Crucial Tumor Suppressor in Ewing sarcoma. Sci. Rep. 2022, 12, 10671. [Google Scholar] [CrossRef]
- Tirode, F.; Laud-Duval, K.; Prieur, A.; Delorme, B.; Charbord, P.; Delattre, O. Mesenchymal Stem Cell Features of Ewing Tumors. Cancer Cell 2007, 11, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Buchou, C.; Laud-Duval, K.; van der Ent, W.; Grossetête, S.; Zaidi, S.; Gentric, G.; Corbé, M.; Müller, K.; Del Nery, E.; Surdez, D.; et al. Upregulation of the Mevalonate Pathway through EWSR1-FLI1/EGR2 Regulatory Axis Confers Ewing Cells Exquisite Sensitivity to Statins. Cancers 2022, 14, 2327. [Google Scholar] [CrossRef] [PubMed]
- Gollavilli, P.N.; Pawar, A.; Wilder-Romans, K.; Natesan, R.; Engelke, C.G.; Dommeti, V.L.; Krishnamurthy, P.M.; Nallasivam, A.; Apel, I.J.; Xu, T.; et al. EWS/ETS-Driven Ewing sarcoma Requires BET Bromodomain Proteins. Cancer Res. 2018, 78, 4760–4773. [Google Scholar] [CrossRef] [PubMed]
- Orth, M.F.; Surdez, D.; Faehling, T.; Ehlers, A.C.; Marchetto, A.; Grossetête, S.; Volckmann, R.; Zwijnenburg, D.A.; Gerke, J.S.; Zaidi, S.; et al. Systematic Multi-Omics Cell Line Profiling Uncovers Principles of Ewing sarcoma Fusion Oncogene-Mediated Gene Regulation. Cell Rep. 2022, 41, 111761. [Google Scholar] [CrossRef]
- Moorthy, N.S.H.N.; Cerqueira, N.M.F.S.A.; Ramos, M.J.; Fernandes, P.A. Development of Ribonucleotide Reductase Inhibitors: A Review on Structure Activity Relationships. Mini-Rev. Med. Chem. 2013, 13, 1862–1872. [Google Scholar] [CrossRef]
- Aye, Y.; Li, M.; Long, M.J.C.; Weiss, R.S. Ribonucleotide Reductase and Cancer: Biological Mechanisms and Targeted Therapies. Oncogene 2015, 34, 2011–2021. [Google Scholar] [CrossRef]
- Fu, Y.; Long, M.J.C.; Wisitpitthaya, S.; Inayat, H.; Pierpont, T.M.; Elsaid, I.M.; Bloom, J.C.; Ortega, J.; Weiss, R.S.; Aye, Y. Nuclear RNR-α Antagonizes Cell Proliferation by Directly Inhibiting ZRANB3. Nat. Chem. Biol. 2018, 14, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lin, D.-C.; Cao, Q.; Pang, B.; Gae, D.D.; Lee, V.K.M.; Lim, H.J.; Doan, N.; Said, J.W.; Gery, S.; et al. Identification of a Novel SYK/c-MYC/MALAT1 Signaling Pathway and Its Potential Therapeutic Value in Ewing sarcoma. Clin. Cancer Res. 2017, 23, 4376–4387. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Tanaka, K.; Itonaga, I.; Iwasaki, T.; Tsumura, H. C-Myc Represses Tumor-Suppressive microRNAs, Let-7a, miR-16 and miR-29b, and Induces Cyclin D2-Mediated Cell Proliferation in Ewing’s Sarcoma Cell Line. PLoS ONE 2015, 10, e0138560. [Google Scholar] [CrossRef] [PubMed]
- Dauphinot, L.; De Oliveira, C.; Melot, T.; Sevenet, N.; Thomas, V.; Weissman, B.E.; Delattre, O. Analysis of the Expression of Cell Cycle Regulators in Ewing Cell Lines: EWS-FLI-1 Modulates p57KIP2and c-Myc Expression. Oncogene 2001, 20, 3258–3265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bailly, R.A.; Bosselut, R.; Zucman, J.; Cormier, F.; Delattre, O.; Roussel, M.; Thomas, G.; Ghysdael, J. DNA-Binding and Transcriptional Activation Properties of the EWS-FLI-1 Fusion Protein Resulting from the t(11;22) Translocation in Ewing sarcoma. Mol. Cell. Biol. 1994, 14, 3230–3241. [Google Scholar] [CrossRef]
- Bejjani, F.; Evanno, E.; Zibara, K.; Piechaczyk, M.; Jariel-Encontre, I. The AP-1 Transcriptional Complex: Local Switch or Remote Command? Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 11–23. [Google Scholar] [CrossRef]
- Madrigal, P.; Alasoo, K. AP-1 Takes Centre Stage in Enhancer Chromatin Dynamics. Trends Cell Biol. 2018, 28, 509–511. [Google Scholar] [CrossRef]
- Bahassi, E.M.; Karyala, S.; Tomlinson, C.R.; Sartor, M.A.; Medvedovic, M.; Hennigan, R.F. Critical Regulation of Genes for Tumor Cell Migration by AP-1. Clin. Exp. Metastasis 2004, 21, 293–304. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, W.; Yan, Y.; Chen, C.; Yu, Y.; Wang, B.; Zhao, H. Knockdown of EWSR1/FLI1 Expression Alters the Transcriptome of Ewing sarcoma Cells in Vitro. J. Bone Oncol. 2016, 5, 153–158. [Google Scholar] [CrossRef]
- Markov, G.J.; Mai, T.; Nair, S.; Shcherbina, A.; Wang, Y.X.; Burns, D.M.; Kundaje, A.; Blau, H.M. AP-1 Is a Temporally Regulated Dual Gatekeeper of Reprogramming to Pluripotency. Proc. Natl. Acad. Sci. USA 2021, 118, e2104841118. [Google Scholar] [CrossRef] [PubMed]
- Vierbuchen, T.; Ling, E.; Cowley, C.J.; Couch, C.H.; Wang, X.; Harmin, D.A.; Roberts, C.W.M.; Greenberg, M.E. AP-1 Transcription Factors and the BAF Complex Mediate Signal-Dependent Enhancer Selection. Mol. Cell 2017, 68, 1067–1082.e12. [Google Scholar] [CrossRef] [PubMed]
- MacLaren, A.; Black, E.J.; Clark, W.; Gillespie, D.A.F. C-Jun-Deficient Cells Undergo Premature Senescence as a Result of Spontaneous DNA Damage Accumulation. Mol. Cell. Biol. 2004, 24, 9006–9018. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chondronasiou, D.; Martínez de Villarreal, J.; Melendez, E.; Lynch, C.J.; Del Pozo, N.; Kovatcheva, M.; Aguilera, M.; Prats, N.; Real, F.X.; Serrano, M. Deciphering the Roadmap of in Vivo Reprogramming toward Pluripotency. Stem Cell Rep. 2022, 17, 2501–2517. [Google Scholar] [CrossRef] [PubMed]
- Antonova, D.V.; Gnatenko, D.A.; Kotova, E.S.; Pleshkan, V.V.; Kuzmich, A.I.; Didych, D.A.; Sverdlov, E.D.; Alekseenko, I.V. Cell-Specific Expression of the FAP Gene Is Regulated by Enhancer Elements. Front. Mol. Biosci. 2023, 10, 1111511. [Google Scholar] [CrossRef] [PubMed]
- Jochum, W.; Passegué, E.; Wagner, E.F. AP-1 in Mouse Development and Tumorigenesis. Oncogene 2001, 20, 2401–2412. [Google Scholar] [CrossRef] [PubMed]
- Hilberg, F.; Aguzzi, A.; Howells, N.; Wagner, E.F. C-Jun Is Essential for Normal Mouse Development and Hepatogenesis. Nature 1993, 365, 179–181. [Google Scholar] [CrossRef]
- Behrens, A.; Sibilia, M.; David, J.; Möhle-Steinlein, U.; Tronche, F.; Schütz, G.; Wagner, E.F. Impaired Postnatal Hepatocyte Proliferation and Liver Regeneration in Mice Lacking C-jun in the Liver. EMBO J. 2002, 21, 1782–1790. [Google Scholar] [CrossRef]
- Matsuo, K.; Owens, J.M.; Tonko, M.; Elliott, C.; Chambers, T.J.; Wagner, E.F. Fosl1 Is a Transcriptional Target of C-Fos during Osteoclast Differentiation. Nat. Genet. 2000, 24, 184–187. [Google Scholar] [CrossRef]
- Mohamed, S.G.-K.; Sugiyama, E.; Shinoda, K.; Taki, H.; Hounoki, H.; Abdel-Aziz, H.O.; Maruyama, M.; Kobayashi, M.; Ogawa, H.; Miyahara, T. Interleukin-10 Inhibits RANKL-Mediated Expression of NFATc1 in Part via Suppression of c-Fos and c-Jun in RAW264.7 Cells and Mouse Bone Marrow Cells. Bone 2007, 41, 592–602. [Google Scholar] [CrossRef]
- Sabatakos, G.; Sims, N.A.; Chen, J.; Aoki, K.; Kelz, M.B.; Amling, M.; Bouali, Y.; Mukhopadhyay, K.; Ford, K.; Nestler, E.J.; et al. Overexpression of ΔFosB Transcription Factor(s) Increases Bone Formation and Inhibits Adipogenesis. Nat. Med. 2000, 6, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Carrozza, M.L.; Jacobs, H.; Acton, D.; Verma, I.; Berns, A. Overexpression of the FosB2 Gene in Thymocytes Causes Aberrant Development of T Cells and Thymic Epithelial Cells. Oncogene 1997, 14, 1083–1091. [Google Scholar] [CrossRef][Green Version]
- Tournier, C. The 2 Faces of JNK Signaling in Cancer. Genes Cancer 2013, 4, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Bakiri, L.; Takada, Y.; Radolf, M.; Eferl, R.; Yaniv, M.; Wagner, E.F.; Matsuo, K. Role of Heterodimerization of C-Fos and Fra1 Proteins in Osteoclast Differentiation. Bone 2007, 40, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Cowles, E.A.; Brailey, L.L.; Gronowicz, G.A. Integrin-Mediated Signaling Regulates AP-1 Transcription Factors and Proliferation in Osteoblasts. J. Biomed. Mater. Res. 2000, 52, 725–737. [Google Scholar] [CrossRef]
- Kushibiki, T.; Tu, Y.; Abu-Yousif, A.O.; Hasan, T. Photodynamic Activation as a Molecular Switch to Promote Osteoblast Cell Differentiation via AP-1 Activation. Sci. Rep. 2015, 5, 13114. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, T.D.; Fahnestock, M.; Kumar, R. An AP-1 Site in the Nerve Growth Factor Promoter Is Essential for 1, 25-Dihydroxyvitamin D3-Mediated Nerve Growth Factor Expression in Osteoblasts. Biochemistry 1998, 37, 5988–5994. [Google Scholar] [CrossRef]
- FitzGerald, U.F.; Barnett, S.C. AP-1 Activity during the Growth, Differentiation, and Death of O-2A Lineage Cells. Mol. Cell. Neurosci. 2000, 16, 453–469. [Google Scholar] [CrossRef]
- Sark, M.W.; Fischer, D.F.; de Meijer, E.; van de Putte, P.; Backendorf, C. AP-1 and Ets Transcription Factors Regulate the Expression of the Human SPRR1A Keratinocyte Terminal Differentiation Marker. J. Biol. Chem. 1998, 273, 24683–24692. [Google Scholar] [CrossRef]
- Karin, M. The Regulation of AP-1 Activity by Mitogen-Activated Protein Kinases*. J. Biol. Chem. 1995, 270, 16483–16486. [Google Scholar] [CrossRef]
- Szabo, E.; Preis, L.H.; Brown, P.H.; Birrer, M.J. The Role of Jun and Fos Gene Family Members in 12-O-Tetradecanoylphorbol-13-Acetate Induced Hemopoietic Differentiation. Cell Growth Differ. 1991, 2, 475–482. [Google Scholar] [PubMed]
- Franzetti, G.-A.; Laud-Duval, K.; van der Ent, W.; Brisac, A.; Irondelle, M.; Aubert, S.; Dirksen, U.; Bouvier, C.; de Pinieux, G.; Snaar-Jagalska, E.; et al. Cell-to-Cell Heterogeneity of EWSR1-FLI1 Activity Determines Proliferation/Migration Choices in Ewing sarcoma Cells. Oncogene 2017, 36, 3505–3514. [Google Scholar] [CrossRef] [PubMed]
- Aynaud, M.-M.; Mirabeau, O.; Gruel, N.; Grossetête, S.; Boeva, V.; Durand, S.; Surdez, D.; Saulnier, O.; Zaïdi, S.; Gribkova, S.; et al. Transcriptional Programs Define Intratumoral Heterogeneity of Ewing sarcoma at Single-Cell Resolution. Cell Rep. 2020, 30, 1767–1779.e6. [Google Scholar] [CrossRef] [PubMed]
- Wrenn, E.D.; Apfelbaum, A.A.; Rudzinski, E.R.; Deng, X.; Jiang, W.; Sud, S.; Van Noord, R.A.; Newman, E.A.; Garcia, N.M.; Miyaki, A.; et al. Cancer-Associated Fibroblast-Like Tumor Cells Remodel the Ewing sarcoma Tumor Microenvironment. Clin. Cancer Res. 2023, 29, 5140–5154. [Google Scholar] [CrossRef] [PubMed]
- Apfelbaum, A.A.; Wrenn, E.D.; Lawlor, E.R. The Importance of Fusion Protein Activity in Ewing sarcoma and the Cell Intrinsic and Extrinsic Factors That Regulate It: A Review. Front. Oncol. 2022, 12, 1044707. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP: An Integrated Web Application for Differential Expression and Pathway Analysis of RNA-Seq Data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating Viruses and Cellular Organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef]



| Log2Fold Change | ||
|---|---|---|
| Gene | EW8 | TC71 |
| ATF1 | −0.7 | −0.6 |
| ATF2 | 0.2 | 0.2 |
| ATF3 | −2.4 | −2.6 |
| ATF4 | −1.4 | −1.2 |
| ATF5 | −1.2 | −2.9 |
| ATF6 | −0.5 | −0.9 |
| BATF2 | 2.1 | −1.4 |
| FOS | −2.2 | 0.4 |
| FOSL1 | 0.9 | 1.9 |
| FOSL2 | 0.9 | 0.8 |
| JDP2 | 1.2 | −1.6 |
| JUN | −2.2 | −0.8 |
| MAFA | 1.6 | 2.1 |
| MAFB | −0.9 | −0.5 |
| MAFG | −1.0 | −1.3 |
| MAFK | −1.4 | −0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croushore, E.E.; Stipp, C.S.; Gordon, D.J. EWS-FLI1 and Activator Protein-1 (AP-1) Reciprocally Regulate Extracellular-Matrix Proteins in Ewing sarcoma Cells. Int. J. Mol. Sci. 2024, 25, 8595. https://doi.org/10.3390/ijms25168595
Croushore EE, Stipp CS, Gordon DJ. EWS-FLI1 and Activator Protein-1 (AP-1) Reciprocally Regulate Extracellular-Matrix Proteins in Ewing sarcoma Cells. International Journal of Molecular Sciences. 2024; 25(16):8595. https://doi.org/10.3390/ijms25168595
Chicago/Turabian StyleCroushore, Emma E., Christopher S. Stipp, and David J. Gordon. 2024. "EWS-FLI1 and Activator Protein-1 (AP-1) Reciprocally Regulate Extracellular-Matrix Proteins in Ewing sarcoma Cells" International Journal of Molecular Sciences 25, no. 16: 8595. https://doi.org/10.3390/ijms25168595
APA StyleCroushore, E. E., Stipp, C. S., & Gordon, D. J. (2024). EWS-FLI1 and Activator Protein-1 (AP-1) Reciprocally Regulate Extracellular-Matrix Proteins in Ewing sarcoma Cells. International Journal of Molecular Sciences, 25(16), 8595. https://doi.org/10.3390/ijms25168595







