Liquid Biopsy in the Clinical Management of Cancers
Abstract
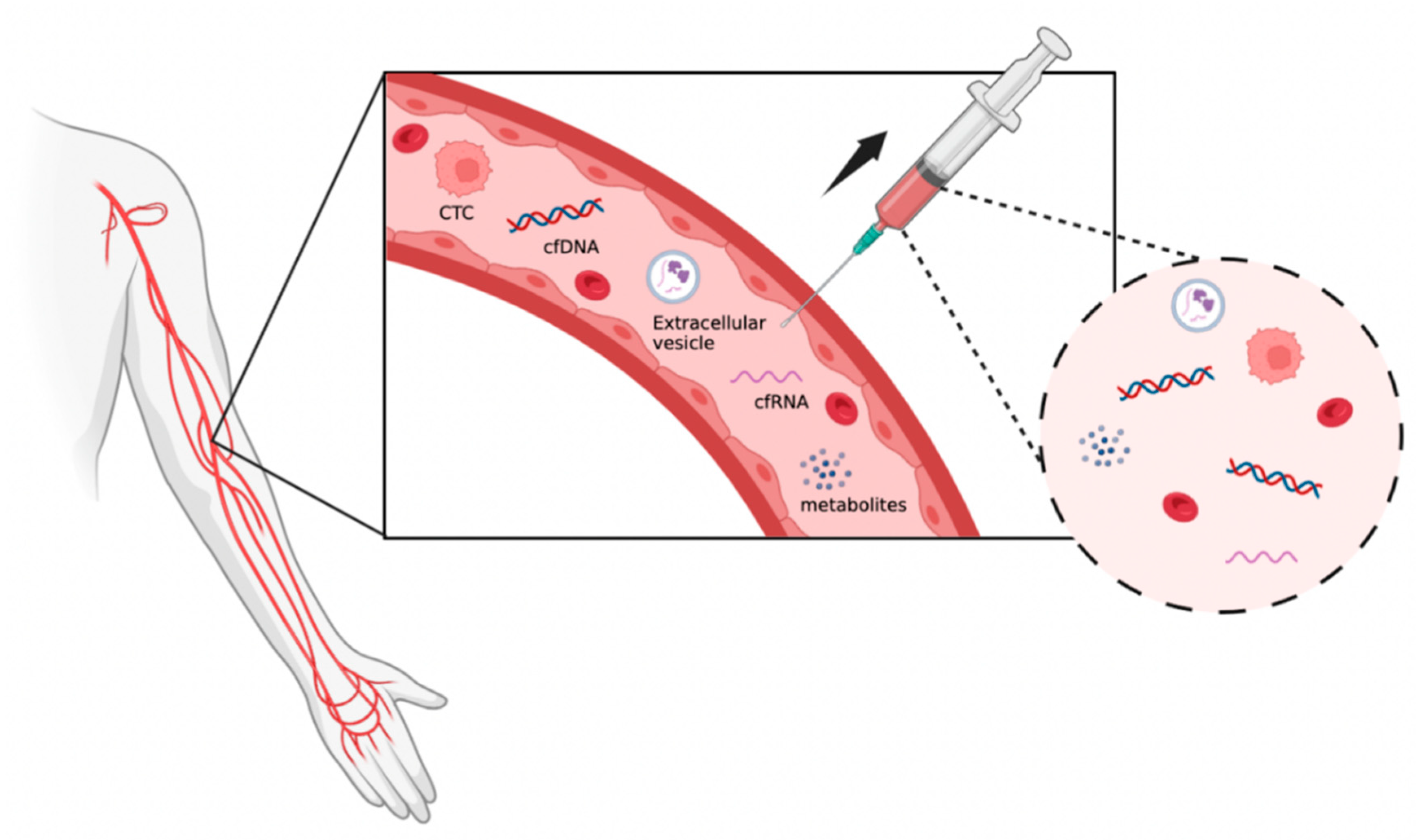
:1. Introduction
2. Background
3. Technology to Collect and Detect Liquid Biopsy
3.1. Circulating Tumor Cells (CTCs)
3.1.1. Capture and Isolation of CTCs
Advanced Microfluidic Technologies
Examples of Microfluidic Devices
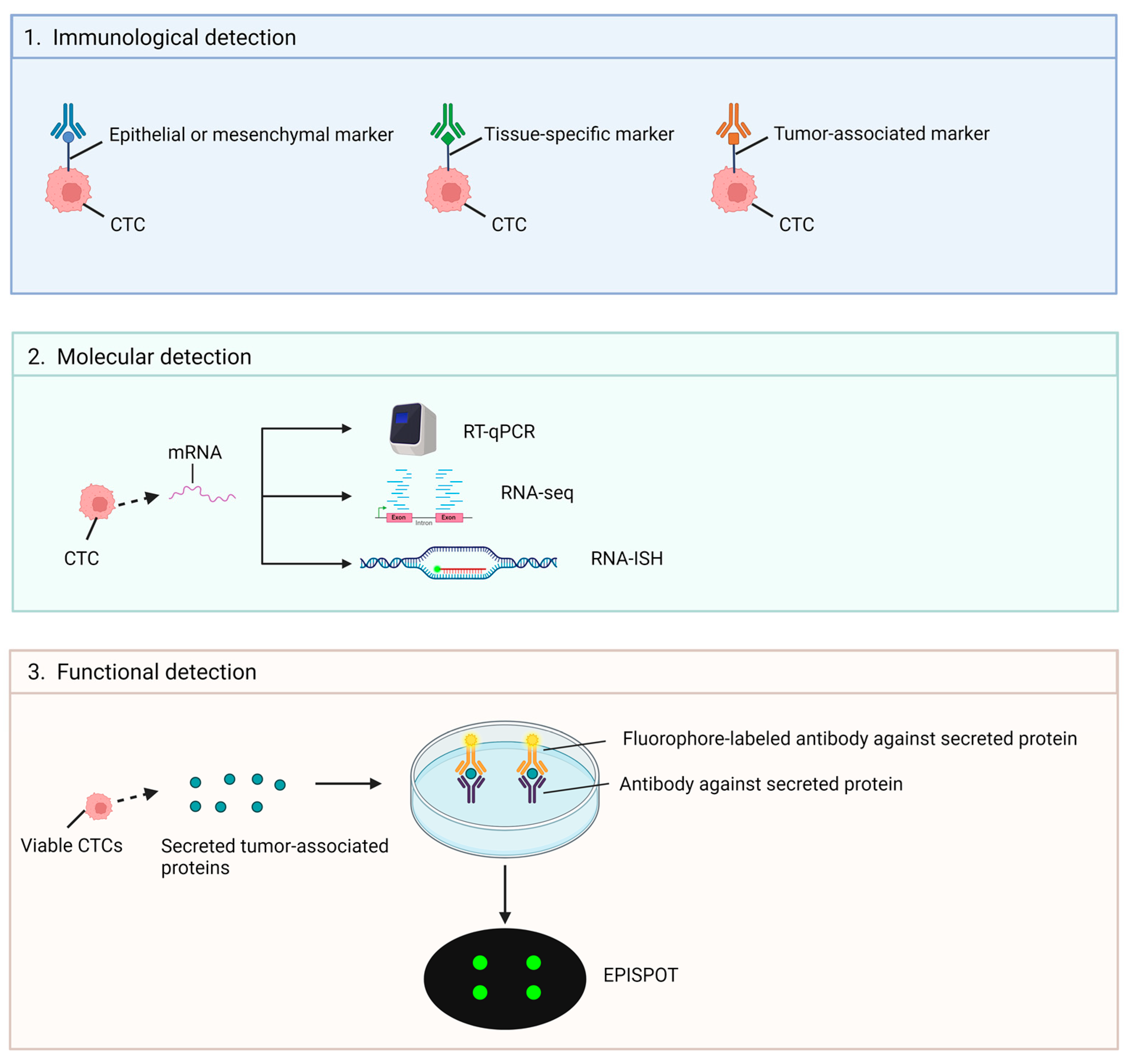
3.1.2. Strategies on CTCs Analysis
Immunological Technologies
3.1.3. Molecular Technologies (RNA-Based)
Single-Cell Analysis of CTCs Using Microfluidic Devices
Functional Assays
3.2. Circulating Nucleic Acids
3.3. Circulating Tumor DNA (ctDNA)
3.4. Circulating Tumor RNA (ctRNA)
3.4.1. Isolation of Circulating Cell-Free DNA
3.4.2. Circulating Nucleic Acids Detection and Analysis
Digital PCR and Next-Generation Sequencing (NGS)
Methylation Profiling
Fragmentomics Analysis
3.5. Extracellular Vesicles (EVs)
3.5.1. EV Isolation and Characterization Technologies
EV Proteomic and RNA Profiling
3.6. Metabolites
4. Applications in Cancer Management
4.1. Early Detection and Diagnosis
4.2. Prognostication and Predictive Biomarkers
4.3. Detection of Minimal Residual Disease (MRD)
4.4. Treatment Selection and Personalized Medicine
4.5. Lung Cancer
4.6. Breast Cancer
4.7. Colorectal Cancer (CRC)
4.8. Prostate Cancer
5. Example of Clinical Trials Evaluating Liquid Biopsy for Cancer
6. Liquid Biopsy Regulatory Considerations and Challenges
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Delmonico, L.; Alves, G.; Bines, J. Cell free DNA biology and its involvement in breast carcinogenesis. Adv. Clin. Chem. 2020, 97, 171–223. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xi, N.; Han, Z.; Luo, W.; Shen, J.; Wang, S.; Li, J.; Guo, Z.; Cheng, H. The Role of Liquid Biopsy Analytes in Diagnosis, Treatment and Prognosis of Colorectal Cancer. Front. Endocrinol. 2022, 13, 875442. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; Manfredini, M.; Tomasi, A. Non-blood sources of cell-free DNA for cancer molecular profiling in clinical pathology and oncology. Crit. Rev. Oncol. Hematol. 2019, 141, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Fang, Y.; Guo, K.; Zhang, N.; Xiang, N. Next-generation Liquid Biopsy Instruments: Challenges and Opportunities. Electrophoresis 2023, 44, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chen, Y.; Yang, J.; Zhuo, C.; Zhang, H.; Shi, Y. Clinical Perspectives on Liquid Biopsy in Metastatic Colorectal Cancer. Front. Genet. 2021, 12, 634642. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A. Changing Role of Histopathology in the Diagnosis and Management of Hepatocellular Carcinoma. World J. Gastroenterol. 2018, 24, 4000–4013. [Google Scholar] [CrossRef] [PubMed]
- Hirahata, T.; Ul Quraish, R.; Quraish, A.U.; Ul Quraish, S.; Naz, M.; Razzaq, M.A. Liquid Biopsy: A Distinctive Approach to the Diagnosis and Prognosis of Cancer. Cancer Inform. 2022, 21, 11769351221076062. [Google Scholar] [CrossRef] [PubMed]
- Adhit, K.K.; Wanjari, A.; Menon, S.; Siddhaarth, K. Liquid Biopsy: An Evolving Paradigm for Non-invasive Disease Diagnosis and Monitoring in Medicine. Cureus 2023, 15, e50176. [Google Scholar] [CrossRef] [PubMed]
- Russano, M.; Napolitano, A.; Ribelli, G.; Iuliani, M.; Simonetti, S.; Citarella, F.; Pantano, F.; Dell’Aquila, E.; Anesi, C.; Silvestris, N.; et al. Liquid biopsy and tumor heterogeneity in metastatic solid tumors: The potentiality of blood samples. J. Exp. Clin. Cancer Res. 2020, 39, 95. [Google Scholar] [CrossRef]
- Jin, N.; Kan, C.-M.; Pei, X.M.; Cheung, W.L.; Ng, S.S.M.; Wong, H.T.; Cheng, H.Y.-L.; Leung, W.W.; Wong, Y.N.; Tsang, H.F.; et al. Cell-free circulating tumor RNAs in plasma as the potential prognostic biomarkers in colorectal cancer. Front. Oncol. 2023, 13, 1134445. [Google Scholar] [CrossRef]
- Ilie, M.; Hofman, P. Pros: Can tissue biopsy be replaced by liquid biopsy? Transl. Lung Cancer Res. 2016, 5, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Vidlarova, M.; Rehulkova, A.; Stejskal, P.; Prokopova, A.; Slavik, H.; Hajduch, M.; Srovnal, J. Recent Advances in Methods for Circulating Tumor Cell Detection. Int. J. Mol. Sci. 2023, 24, 3902. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Sun, S.; Chen, Z.; Xiang, S.; Ding, Z.; Huang, Z.; Zhang, B. Understanding the versatile roles and applications of EpCAM in cancers: From bench to bedside. Exp. Hematol. Oncol. 2022, 11, 97. [Google Scholar] [CrossRef]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef]
- Wu, T.M.; Liu, J.B.; Liu, Y.; Shi, Y.; Li, W.; Wang, G.R.; Ma, Y.S.; Fu, D. Power and Promise of Next-Generation Sequencing in Liquid Biopsies and Cancer Control. Cancer Control 2020, 27, 1073274820934805. [Google Scholar] [CrossRef] [PubMed]
- Descamps, L.; Le Roy, D.; Deman, A.-L. Microfluidic-Based Technologies for CTC Isolation: A Review of 10 Years of Intense Efforts towards Liquid Biopsy. Int. J. Mol. Sci. 2022, 23, 1981. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, A.; Siddiqui, M.H. Detection of circulating tumour cells in colorectal cancer: Emerging techniques and clinical implications. World J. Clin. Oncol. 2021, 12, 1169–1181. [Google Scholar] [CrossRef]
- Wiegmans, A.P.; Ivanova, E.; Naei, V.Y.; Monkman, J.; Fletcher, J.; Mullally, W.; Warkiani, M.E.; O’Byrne, K.; Kulasinghe, A. Poor patient outcome correlates with active engulfment of cytokeratin positive CTCs within cancer-associated monocyte population in lung cancer. Clin. Exp. Metastasis 2024, 41, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Armakolas, A.; Kotsari, M.; Koskinas, J. Liquid Biopsies, Novel Approaches and Future Directions. Cancers 2023, 15, 1579. [Google Scholar] [CrossRef] [PubMed]
- Habli, Z.; AlChamaa, W.; Saab, R.; Kadara, H.; Khraiche, M.L. Circulating Tumor Cell Detection Technologies and Clinical Utility: Challenges and Opportunities. Cancers 2020, 12, 1930. [Google Scholar] [CrossRef]
- Lemma, S.; Perrone, A.M.; De Iaco, P.; Gasparre, G.; Kurelac, I. Current methodologies to detect circulating tumor cells: A focus on ovarian cancer. Am. J. Cancer Res. 2021, 11, 4111–4126. [Google Scholar] [PubMed]
- Chu, P.Y.; Hsieh, C.H.; Wu, M.H. The Combination of Immunomagnetic Bead-Based Cell Isolation and Optically Induced Dielectrophoresis (ODEP)-Based Microfluidic Device for the Negative Selection-Based Isolation of Circulating Tumor Cells (CTCs). Front. Bioeng. Biotechnol. 2020, 8, 921. [Google Scholar] [CrossRef] [PubMed]
- Eslami-S, Z.; Cortés-Hernández, L.E.; Alix-Panabières, C. Epithelial Cell Adhesion Molecule: An Anchor to Isolate Clinically Relevant Circulating Tumor Cells. Cells 2020, 9, 1836. [Google Scholar] [CrossRef] [PubMed]
- Fridrichova, I.; Kalinkova, L.; Ciernikova, S. Clinical Relevancy of Circulating Tumor Cells in Breast Cancer: Epithelial or Mesenchymal Characteristics, Single Cells or Clusters? Int. J. Mol. Sci. 2022, 23, 12141. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Xu, F.; Tian, J.; Chen, H.; Yang, C.; Huang, S.; Gao, K.; Wan, Z.; Li, M.; He, M.; et al. Pathology of circulating tumor cells and the available capture tools (Review). Oncol. Rep. 2020, 43, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Blick, T.; Williams, E.D.; Thompson, E.W. Applications of RNA characterisation in circulating tumour cells. Front. Biosci.-Landmark 2020, 25, 874–892. [Google Scholar] [CrossRef] [PubMed]
- Kalinich, M.; Kwan, T.T.; Toner, M.; Haber, D.A.; Maheswaran, S. Quantitative Analysis of Circulating Tumor Cells Using RNA-Based Digital Scoring. In Tumor Liquid Biopsies; Schaffner, F., Merlin, J.-L., von Bubnoff, N., Eds.; Springer: Cham, Switzerland, 2020; pp. 77–88. [Google Scholar]
- Payne, R.E.; Wang, F.; Su, N.; Krell, J.; Zebrowski, A.; Yagüe, E.; Ma, X.J.; Luo, Y.; Coombes, R.C. Viable circulating tumour cell detection using multiplex RNA in situ hybridisation predicts progression-free survival in metastatic breast cancer patients. Br. J. Cancer 2012, 106, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Wang, P.; Toh, A.; Thompson, E.W. New Insights Into the Role of Phenotypic Plasticity and EMT in Driving Cancer Progression. Front. Mol. Biosci. 2020, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Chen, Y.-C.; Lin, E.; Brien, R.; Jung, S.; Chen, Y.-T.; Lee, W.; Hao, Z.; Sahoo, S.; Min Kang, H.; et al. Hydro-Seq enables contamination-free high-throughput single-cell RNA-sequencing for circulating tumor cells. Nat. Commun. 2019, 10, 2163. [Google Scholar] [CrossRef]
- Mihalcioiu, C.; Li, J.; Badescu, D.; Camirand, A.; Kremer, N.; Bertos, N.; Omeroglu, A.; Sebag, M.; Di Battista, J.; Park, M.; et al. Improved platform for breast cancer circulating tumor cell enrichment and characterization with next-generation sequencing technology. Am. J. Cancer Res. 2023, 13, 25–44. [Google Scholar]
- Chauhan, A.; Kaur, R.; Ghoshal, S.; Pal, A. Exploration of Circulating Tumour Cell (CTC) Biology: A Paradigm Shift in Liquid Biopsy. Indian J. Clin. Biochem. 2021, 36, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating Tumor Cell Clusters Are Oligoclonal Precursors of Breast Cancer Metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Amintas, S.; Bedel, A.; Moreau-Gaudry, F.; Boutin, J.; Buscail, L.; Merlio, J.-P.; Vendrely, V.; Dabernat, S.; Buscail, É. Circulating Tumor Cell Clusters: United We Stand Divided We Fall. Int. J. Mol. Sci. 2020, 21, 2653. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, S.; Qin, L.; Zhou, J.; Shi, H.; Yang, Y.; Sun, J.; Tian, F.; Liu, C. Poly(ethylene Oxide) Concentration Gradient-Based Microfluidic Isolation of Circulating Tumor Cells. Anal. Chem. 2023, 95, 3468–3475. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Hou, J.; Luo, Z.; Yang, G.; Zhou, S. Conductive Nanofibers-Enhanced Microfluidic Device for the Efficient Capture and Electrical Stimulation-Triggered Rapid Release of Circulating Tumor Cells. Biosensors 2023, 13, 497. [Google Scholar] [CrossRef]
- Burr, R.; Edd, J.F.; Chirn, B.; Mishra, A.; Haber, D.A.; Toner, M.; Maheswaran, S. Negative-Selection Enrichment of Circulating Tumor Cells from Peripheral Blood Using the Microfluidic CTC-iChip. In Mammary Stem Cells: Methods and Protocols; Vivanco, M.D., Ed.; Springer: New York, NY, USA, 2022; pp. 309–321. [Google Scholar]
- Eslami-S, Z.; Cortés-Hernández, L.E.; Thomas, F.; Pantel, K.; Alix-Panabières, C. Functional analysis of circulating tumour cells: The KEY to understand the biology of the metastatic cascade. Br. J. Cancer 2022, 127, 800–810. [Google Scholar] [CrossRef]
- Mazard, T.; Cayrefourcq, L.; Perriard, F.; Senellart, H.; Linot, B.; de la Fouchardière, C.; Terrebonne, E.; François, E.; Obled, S.; Guimbaud, R.; et al. Clinical Relevance of Viable Circulating Tumor Cells in Patients with Metastatic Colorectal Cancer: The COLOSPOT Prospective Study. Cancers 2021, 13, 2966. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, P.; Zhang, K.; Zhang, W. Circulating tumour cell isolation, analysis and clinical application. Cell. Oncol. 2023, 46, 533–544. [Google Scholar] [CrossRef]
- Budna-Tukan, J.; Świerczewska, M.; Mazel, M.; Cieślikowski, W.A.; Ida, A.; Jankowiak, A.; Antczak, A.; Nowicki, M.; Pantel, K.; Azria, D.; et al. Analysis of Circulating Tumor Cells in Patients with Non-Metastatic High-Risk Prostate Cancer before and after Radiotherapy Using Three Different Enumeration Assays. Cancers 2019, 11, 802. [Google Scholar] [CrossRef]
- Cayrefourcq, L.; De Roeck, A.; Garcia, C.; Stoebner, P.-E.; Fichel, F.; Garima, F.; Perriard, F.; Daures, J.-P.; Meunier, L.; Alix-Panabières, C. S100-EPISPOT: A New Tool to Detect Viable Circulating Melanoma Cells. Cells 2019, 8, 755. [Google Scholar] [CrossRef]
- Garrel, R.; Mazel, M.; Perriard, F.; Vinches, M.; Cayrefourcq, L.; Guigay, J.; Digue, L.; Aubry, K.; Alfonsi, M.; Delord, J.-P.; et al. Circulating Tumor Cells as a Prognostic Factor in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: The CIRCUTEC Prospective Study. Clin. Chem. 2019, 65, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, F.S.; Barauna, V.G.; Dos Santos, L.; Costa, G.; Vassallo, P.F.; Campos, L.C.G. Properties and Application of Cell-Free DNA as a Clinical Biomarker. Int. J. Mol. Sci. 2021, 22, 9110. [Google Scholar] [CrossRef]
- Ramirez-Garrastacho, M.; Bajo-Santos, C.; Line, A.; Martens-Uzunova, E.S.; de la Fuente, J.M.; Moros, M.; Soekmadji, C.; Tasken, K.A.; Llorente, A. Extracellular vesicles as a source of prostate cancer biomarkers in liquid biopsies: A decade of research. Br. J. Cancer 2022, 126, 331–350. [Google Scholar] [CrossRef]
- Connal, S.; Cameron, J.M.; Sala, A.; Brennan, P.M.; Palmer, D.S.; Palmer, J.D.; Perlow, H.; Baker, M.J. Liquid biopsies: The future of cancer early detection. J. Transl. Med. 2023, 21, 118. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef]
- Kolenda, T.; Guglas, K.; Baranowski, D.; Sobocinska, J.; Kopczynska, M.; Teresiak, A.; Blizniak, R.; Lamperska, K. cfRNAs as biomarkers in oncology-still experimental or applied tool for personalized medicine already? Rep. Pract. Oncol. Radiother. 2020, 25, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sun, C.; Zhao, Y.; Wang, Q.; Guo, J.; Ye, B.; Yu, G. Overview of MicroRNAs as Diagnostic and Prognostic Biomarkers for High-Incidence Cancers in 2021. Int. J. Mol. Sci. 2022, 23, 11389. [Google Scholar] [CrossRef]
- Bautista-Sánchez, D.; Arriaga-Canon, C.; Pedroza-Torres, A.; De La Rosa-Velázquez, I.A.; González-Barrios, R.; Contreras-Espinosa, L.; Montiel-Manríquez, R.; Castro-Hernández, C.; Fragoso-Ontiveros, V.; Álvarez-Gómez, R.M.; et al. The Promising Role of miR-21 as a Cancer Biomarker and Its Importance in RNA-Based Therapeutics. Mol. Ther. Nucleic Acids 2020, 20, 409–420. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Liu, C.; Zhang, X.; Wu, Y.; Diao, M.; Tan, S.; Huang, S.; Cheng, Y.; You, T. MicroRNA-21 as a diagnostic and prognostic biomarker of lung cancer: A systematic review and meta-analysis. Biosci. Rep. 2022, 42, BSR20211653. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Kacimi, S.E.O.; Nguyen, T.L.; Suman, K.H.; Lemus-Martin, R.; Saleem, H.; Do, D.N. MiR-21 in the Cancers of the Digestive System and Its Potential Role as a Diagnostic, Predictive, and Therapeutic Biomarker. Biology 2021, 10, 417. [Google Scholar] [CrossRef]
- Ratti, M.; Lampis, A.; Ghidini, M.; Salati, M.; Mirchev, M.B.; Valeri, N.; Hahne, J.C. MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside. Target. Oncol. 2020, 15, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Beylerli, O.; Gareev, I.; Sufianov, A.; Ilyasova, T.; Guang, Y. Long noncoding RNAs as promising biomarkers in cancer. Noncoding RNA Res. 2022, 7, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, M.; Cheung, M.; Hillman, J.; Rassekh, S.R.; Deyell, R.J.; Batist, G.; Karsan, A.; Wyatt, A.W.; Johnson, N.; Scott, D.W.; et al. Evaluating the quantity, quality and size distribution of cell-free DNA by multiplex droplet digital PCR. Sci. Rep. 2020, 10, 12564. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, V.; Bronkhorst, A.J.; Holdenrieder, S. Preanalytical variables that affect the outcome of cell-free DNA measurements. Crit. Rev. Clin. Lab. Sci. 2020, 57, 484–507. [Google Scholar] [CrossRef]
- Meddeb, R.; Pisareva, E.; Thierry, A.R. Guidelines for the Preanalytical Conditions for Analyzing Circulating Cell-Free DNA. Clin. Chem. 2019, 65, 623–633. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, H.; Wang, Y.; Lin, Y.; Li, Q.; Li, L.; Zeng, G.; Ou, R.; Cheng, X.; Zhang, Y.; et al. Effects of blood-processing protocols on cell-free DNA fragmentomics in plasma: Comparisons of one- and two-step centrifugations. Clin. Chim. Acta 2024, 560, 119729. [Google Scholar] [CrossRef] [PubMed]
- Martignano, F. Cell-Free DNA: An Overview of Sample Types and Isolation Procedures. In Cell-Free DNA as Diagnostic Markers: Methods and Protocols; Casadio, V., Salvi, S., Eds.; Springer: New York, NY, USA, 2019; pp. 13–27. [Google Scholar]
- Diaz, I.M.; Nocon, A.; Held, S.A.E.; Kobilay, M.; Skowasch, D.; Bronkhorst, A.J.; Ungerer, V.; Fredebohm, J.; Diehl, F.; Holdenrieder, S.; et al. Pre-Analytical Evaluation of Streck Cell-Free DNA Blood Collection Tubes for Liquid Profiling in Oncology. Diagnostics 2023, 13, 1288. [Google Scholar] [CrossRef]
- Ullius, A.; Provencher, E.; Voss, T. Abstract 1965: Multimodal analysis of circulating cell-free RNA (ccfRNA), cell-free DNA (ccfDNA) and genomic DNA (gDNA) from blood samples collected in PAXgene Blood ccfDNA Tubes. Cancer Res. 2020, 80, 1965. [Google Scholar] [CrossRef]
- Parackal, S.; Zou, D.; Day, R.; Black, M.; Guilford, P. Comparison of Roche Cell-Free DNA collection Tubes® to Streck Cell-Free DNA BCT®s for sample stability using healthy volunteers. Pract. Lab. Med. 2019, 16, e00125. [Google Scholar] [CrossRef]
- Sorber, L.; Zwaenepoel, K.; Jacobs, J.; De Winne, K.; Van Casteren, K.; Augustus, E.; Lardon, F.; Prenen, H.; Peeters, M.; Van Meerbeeck, J. Specialized blood collection tubes for liquid biopsy: Improving the pre-analytical conditions. Mol. Diagn. Ther. 2020, 24, 113–124. [Google Scholar] [CrossRef]
- Lee, H.; Park, C.; Na, W.; Park, K.H.; Shin, S. Precision cell-free DNA extraction for liquid biopsy by integrated microfluidics. NPJ Precis. Oncol. 2020, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- van der Leest, P.; Boonstra, P.A.; ter Elst, A.; van Kempen, L.C.; Tibbesma, M.; Koopmans, J.; Miedema, A.; Tamminga, M.; Groen, H.J.M.; Reyners, A.K.L.; et al. Comparison of Circulating Cell-Free DNA Extraction Methods for Downstream Analysis in Cancer Patients. Cancers 2020, 12, 1222. [Google Scholar] [CrossRef] [PubMed]
- Terp, S.K.; Pedersen, I.S.; Stoico, M.P. Extraction of Cell-Free DNA: Evaluation of Efficiency, Quantity, and Quality. J. Mol. Diagn. 2024, 26, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.S.; Pham, H.-A.T.; Tran, V.T.T.; Tran, T.T.T.; Dang, A.-T.H.; Le, D.-T.V.; Nguyen, S.-L.; Nguyen, N.-V.; Nguyen, T.; Vo, B.T.; et al. Ultra-Deep Massively Parallel Sequencing with Unique Molecular Identifier Tagging Achieves Comparable Performance to Droplet Digital PCR for Detection and Quantification of Circulating Tumor DNA from Lung Cancer Patients. PLoS ONE 2019, 14, e0226193. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Araf, Y.; Promon, S.K. Circulating tumor DNA in cancer diagnosis, monitoring, and prognosis. J. Egypt. Natl. Cancer Inst. 2022, 34, 8. [Google Scholar] [CrossRef] [PubMed]
- Tamkovich, S.; Tupikin, A.; Kozyakov, A.; Laktionov, P. Size and Methylation Index of Cell-Free and Cell-Surface-Bound DNA in Blood of Breast Cancer Patients in the Contest of Liquid Biopsy. Int. J. Mol. Sci. 2022, 23, 8919. [Google Scholar] [CrossRef]
- Markou, A. DNA Methylation Analysis of Tumor Suppressor Genes in Liquid Biopsy Components of Early Stage NSCLC: A Promising Tool for Early Detection. Clin. Epigenet. 2022, 14, 61. [Google Scholar] [CrossRef]
- Liu, X.; Lang, J.; Li, S.; Wang, Y.; Peng, L.; Wang, W.; Han, Y.; Qi, C.; Song, L.; Yang, S.; et al. Fragment Enrichment of Circulating Tumor DNA with Low-Frequency Mutations. Front. Genet. 2020, 11, 147. [Google Scholar] [CrossRef]
- Kim, J.; Hong, S.-P.; Lee, S.; Lee, W.; Lee, D.; Kim, R.; Park, Y.J.; Moon, S.; Park, K.; Cha, B.; et al. Multidimensional Fragmentomic Profiling of Cell-Free DNA Released from Patient-Derived Organoids. Human Genom. 2023, 17, 96. [Google Scholar] [CrossRef]
- Qi, T.; Pan, M.; Shi, H.; Wang, L.; Bai, Y.M.; Ge, Q. Cell-Free DNA Fragmentomics: The Novel Promising Biomarker. Int. J. Mol. Sci. 2023, 24, 1503. [Google Scholar] [CrossRef]
- Shi, X.; Guo, S.; Duan, Q.; Zhang, W.; Gao, S.; Jing, W.; Jiang, G.; Kong, X.; Li, P.; Li, Y.; et al. Detection and Characterization of Pancreatic and Biliary Tract Cancers Using Cell-Free DNA Fragmentomics. J. Exp. Clin. Cancer Res. 2024, 43, 145. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, J.-K.; Lee, N.; Lee, S.-H.; Kim, H.-P.; Kim, S.Y.; Kim, T.-Y.; Kim, H.; Jung, H.A.; Sun, J.-M.; et al. Predicting Disease Recurrence in Limited Disease Small Cell Lung Cancer Using Cell-Free DNA-based Mutation and Fragmentome Analyses. Transl. Lung Cancer Res. 2024, 13, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.F.; Luo, P.; Znassi, N.; Arteaga, D.P.; Gray, D.; Danesh, A.; Han, M.; Zhao, E.Y.; Pedersen, S.; Prokopec, S.D.; et al. Integrated, Longitudinal Analysis of Cell-Free DNA in Uveal Melanoma. Cancer Res. Commun. 2023, 3, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Sundby, R.T.; Szymanski, J.J.; Pan, A.; Jones, P.A.; Mahmood, S.Z.; Reid, O.H.; Srihari, D.; Armstrong, A.E.; Chamberlain, S.; Burgic, S.; et al. Early Detection of Malignant and Pre-Malignant Peripheral Nerve Tumors Using Cell-Free DNA Fragmentomics. medRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Maansson, C.T.; Thomsen, L.S.; Meldgaard, P.; Nielsen, A.L.; Sorensen, B.S. Integration of Cell-Free DNA End Motifs and Fragment Lengths Can Identify Active Genes in Liquid Biopsies. Int. J. Mol. Sci. 2024, 25, 1243. [Google Scholar] [CrossRef]
- Zhou, Q.; Kang, G.; Jiang, P.; Qiao, R.; Lam, W.K.; Yu, S.C.Y.; Mary-Jane, L.; Lu, J.; Cheng, S.H.; Gai, W.; et al. Epigenetic Analysis of Cell-Free DNA by Fragmentomic Profiling. Proc. Natl. Acad. Sci. USA 2022, 119, e2209852119. [Google Scholar] [CrossRef]
- Stevic, I.; Buescher, G.; Ricklefs, F.L. Monitoring Therapy Efficiency in Cancer through Extracellular Vesicles. Cells 2020, 9, 130. [Google Scholar] [CrossRef]
- Liu, J.; Ren, L.; Li, S.; Li, W.; Zheng, X.; Yang, Y.; Fu, W.; Yi, J.; Wang, J.; Du, G. The biology, function, and applications of exosomes in cancer. Acta Pharm. Sin. B 2021, 11, 2783–2797. [Google Scholar] [CrossRef] [PubMed]
- Oliva, S.; D’Agostino, M.; Boccadoro, M.; Larocca, A. Clinical Applications and Future Directions of Minimal Residual Disease Testing in Multiple Myeloma. Front. Oncol. 2020, 10, 1. [Google Scholar] [CrossRef]
- Guo, W.; Li, Y.; Pang, W.; Shen, H. Exosomes: A Potential Therapeutic Tool Targeting Communications between Tumor Cells and Macrophages. Mol. Ther. 2020, 28, 1953–1964. [Google Scholar] [CrossRef]
- Li, J.; Guan, X.; Fan, Z.; Li, C.; Li, Y.; Wang, X.; Cao, W.; Liu, D.X. Non-Invasive Biomarkers for Early Detection of Breast Cancer. Cancers 2020, 12, 2767. [Google Scholar] [CrossRef] [PubMed]
- Deun, J.V.; Jo, A.; Li, Y.; Lin, H.K.; Weissleder, R.; Im, H.; Lee, H. Integrated Dual-Mode Chromatography to Enrich Extracellular Vesicles from Plasma. Adv. Biosyst. 2020, 4, 1900310. [Google Scholar] [CrossRef]
- Vestad, B.; Nyman, T.A.; Hove-Skovsgaard, M.; Stensland, M.; Hoel, H.; Trøseid, A.-M.S.; Aspelin, T.; Aass, H.C.D.; Puhka, M.; Hov, J.R.; et al. Potential Role of Plasma Extracellular Vesicles in Microbial Translocation and Cardiovascular Risk in People Living with HIV and Type 2 Diabetes. arXiv 2021. [Google Scholar] [CrossRef]
- Benecke, L.; Chiang, D.; Ebnoether, E.; Pfaffl, M.W.; Müller, L. Isolation and Analysis of Tumor-derived Extracellular Vesicles from Head and Neck Squamous Cell Carcinoma Plasma by Galectin-based Glycan Recognition Particles. Int. J. Oncol. 2022, 61, 133. [Google Scholar] [CrossRef]
- Havers, M.; Broman, A.; Lenshof, A.; Laurell, T. Advancement and Obstacles in Microfluidics-Based Isolation of Extracellular Vesicles. Anal. Bioanal. Chem. 2022, 415, 1265–1285. [Google Scholar] [CrossRef] [PubMed]
- Antounians, L.; Tzanetakis, A.; Pellerito, O.; Catania, V.D.; Sulistyo, A.; Montalva, L.; McVey, M.J.; Zani, A. The Regenerative Potential of Amniotic Fluid Stem Cell Extracellular Vesicles: Lessons Learned by Comparing Different Isolation Techniques. Sci. Rep. 2019, 9, 1837. [Google Scholar] [CrossRef]
- Charrier, H.; Beseme, O.; Michel, J.B.; Mulder, P.; Amouyel, P.; Pinet, F.; Turkieh, A. Lim Domain Binding 3 (Ldb3) Identified as a Potential Marker of Cardiac Extracellular Vesicles. Int. J. Mol. Sci. 2022, 23, 7374. [Google Scholar] [CrossRef]
- Serratì, S.; Palazzo, A.J.; Lapenna, A.; Mateos, H.; Mallardi, A.; Marsano, R.M.; Quarta, A.; Rosso, M.D.; Azzariti, A. Salting-Out Approach Is Worthy of Comparison with Ultracentrifugation for Extracellular Vesicle Isolation from Tumor and Healthy Models. Biomolecules 2021, 11, 1857. [Google Scholar] [CrossRef] [PubMed]
- Xue, V.W.; Yang, C.; Wong, S.C.C.; Cho, W.C. Proteomic Profiling in Extracellular Vesicles for Cancer Detection and Monitoring. Proteomics 2021, 21, 2000094. [Google Scholar] [CrossRef]
- Bryl-Górecka, P.; Olde, B.; Gidlöf, O.; Törngren, K.; Erlinge, D. Increased Expression of miR-224-5p in Circulating Extracellular Vesicles of Patients with Reduced Coronary Flow Reserve. BMC Cardiovasc. Disord. 2022, 22, 321. [Google Scholar] [CrossRef]
- Tesovnik, T.; Bizjan, B.J.; Šket, R.; Debeljak, M.; Battelino, T.; Kovač, J. Technological Approaches in the Analysis of Extracellular Vesicle Nucleotide Sequences. Front. Bioeng. Biotechnol. 2021, 9, 787551. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid Biopsy Enters the Clinic—Implementation Issues and Future Challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Mahmud, I.; Marchica, J.; Dereziński, P.; Qi, F.; Wang, F.; Joshi, P.; Valerio, F.; Rivera, I.; Patel, V.; et al. Integrated RNA and Metabolite Profiling of Urine Liquid Biopsies for Prostate Cancer Biomarker Discovery. Sci. Rep. 2020, 10, 3716. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, J.; Zhang, K.; Yang, Q.; Yang, J.; Cao, R.; Gu, F.; Liang, J.; Liu, Y.; Hu, Y.; et al. Application of Metabolomics by UHPLC-MS/MS in Diagnostics and Biomarker Discovery of Non-Small Cell Lung Cancer. Transl. Cancer Res. 2019, 8, 2371–2379. [Google Scholar] [CrossRef]
- Wei, M.; Zhao, X.; Wang, P.; Song, X.; Hu, J.; Zhong, K.; Lei, L.-L.; Xu, R.; Han, W.; Yang, M.; et al. Novel Metabolic Biomarker for Early Detection and Prognosis to the Patients with Gastric Cardia Adnocarcinoma. arXiv 2021. [Google Scholar] [CrossRef]
- Uchiyama, K.; Yagi, N.; Mizushima, K.; Higashimura, Y.; Hirai, Y.; Okayama, T.; Yoshida, N.; Katada, K.; Kamada, K.; Handa, O.; et al. Serum metabolomics analysis for early detection of colorectal cancer. J. Gastroenterol. 2017, 52, 677–694. [Google Scholar] [CrossRef]
- Geijsen, A.; van Roekel, E.H.; van Duijnhoven, F.J.B.; Achaintre, D.; Bachleitner-Hofmann, T.; Baierl, A.; Bergmann, M.M.; Boehm, J.; Bours, M.J.L.; Brenner, H.; et al. Plasma metabolites associated with colorectal cancer stage: Findings from an international consortium. Int. J. Cancer 2020, 146, 3256–3266. [Google Scholar] [CrossRef]
- Ren, F.; Fei, Q.; Qiu, K.; Zhang, Y.; Zhang, H.; Sun, L. Liquid Biopsy Techniques and Lung Cancer: Diagnosis, Monitoring and Evaluation. J. Exp. Clin. Cancer Res. 2024, 43, 96. [Google Scholar] [CrossRef]
- Lin, B.; Jiang, J.; Jia, J.M.; Zhou, X. Recent Advances in Exosomal miRNA Biosensing for Liquid Biopsy. Molecules 2022, 27, 7145. [Google Scholar] [CrossRef]
- Khachfe, H.H. Use of Liquid Biopsies in Gastrointestinal Cancers. World J. Gastrointest. Oncol. 2021, 13, 1210–1212. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhao, W. The Application of Liquid Biopsy Techniques in High-Risk Population for Hepatocellular Carcinoma. Cancer Manag. Res. 2022, 14, 2735–2748. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Huang, J.; Huang, S.; Ahir, B.K.; Li, L.-M.; Zhong, J.H. Advances in the Detection of Pancreatic Cancer Through Liquid Biopsy. Front. Oncol. 2021, 11, 801173. [Google Scholar] [CrossRef]
- Du, S.; Zhao, Y.; Lv, C.; Wei, M.; Gao, Z.; Meng, X. Applying Serum Proteins and MicroRNA as Novel Biomarkers for Early-Stage Cervical Cancer Detection. Sci. Rep. 2020, 10, 9033. [Google Scholar] [CrossRef] [PubMed]
- Gawel, S.H.; Jackson, L.; Jeanblanc, N.; Davis, G. Current and Future Opportunities for Liquid Biopsy of Circulating Biomarkers to Aid in Early Cancer Detection. J. Cancer Metast. Treat. 2022, 8, 26. [Google Scholar] [CrossRef]
- Jeffrey, G.P.; Gordon, L.G.; Hill, M.M.; Ramm, G.A. Liquid Biopsies for Hepatocellular Cancer and Their Potential in Clinical Practice. Hepatology 2020, 71, 2160–2162. [Google Scholar] [CrossRef] [PubMed]
- Wade, R.; Nevitt, S.; Liu, Y.; Harden, M.; Khouja, C.; Raine, G.; Churchill, R.; Dias, S. Multi-Cancer Early Detection Tests for General Population Screening: A Systematic Literature Review. medRxiv 2024. [Google Scholar] [CrossRef]
- Qvick, A.; Bratulic, S.; Carlsson, J.; Stenmark, B.; Karlsson, C.; Nielsen, J.; Gatto, F.; Helenius, G. Plasma Glycosaminoglycans and Cell-Free DNA to Discriminate Benign and Malignant Lung Diseases. medRxiv 2024. [Google Scholar] [CrossRef]
- Kan, C.M.; Pei, X.M.; Yeung, M.H.Y.; Jin, N.; Ng, S.S.M.; Tsang, H.F.; Cho, W.C.S.; Yim, A.K.; Yu, A.C.; Wong, S.C.C. Exploring the Role of Circulating Cell-Free RNA in the Development of Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 11026. [Google Scholar] [CrossRef]
- Shen, Y.; Shi, R.; Zhao, R.; Wang, H. Clinical Application of Liquid Biopsy in Endometrial Carcinoma. Med. Oncol. 2023, 40, 92. [Google Scholar] [CrossRef]
- Bagheri, A.; Khorshid, H.R.K.; Tavallaie, M.; Mowla, S.J.; Sherafatian, M.; Rashidi, M.; Zargari, M.; Boroujeni, M.E.; Hosseini, S.M. A panel of noncoding RNAs in non-small-cell lung cancer. J. Cell. Biochem. 2019, 120, 8280–8290. [Google Scholar] [CrossRef] [PubMed]
- Marcuello, M.; Vymetalkova, V.; Neves, R.P.L.; Duran-Sanchon, S.; Vedeld, H.M.; Tham, E.; van Dalum, G.; Flügen, G.; Garcia-Barberan, V.; Fijneman, R.J.A.; et al. Circulating biomarkers for early detection and clinical management of colorectal cancer. Mol. Asp. Med. 2019, 69, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Galvão-Lima, L.J.; Morais, A.H.F.; Valentim, R.A.M.; Barreto, E.J.S.S. miRNAs as biomarkers for early cancer detection and their application in the development of new diagnostic tools. BioMed. Eng. Online 2021, 20, 21. [Google Scholar] [CrossRef]
- Preethi, K.A.; Selvakumar, S.C.; Ross, K.; Jayaraman, S.; Tusubira, D.; Sekar, D. Liquid Biopsy: Exosomal microRNAs as Novel Diagnostic and Prognostic Biomarkers in Cancer. Mol. Cancer 2022, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Wu, Y. Recent Advances in Nanotechnology-Enabled Biosensors for Detection of Exosomes as New Cancer Liquid Biopsy. Exp. Biol. Med. 2022, 247, 2152–2172. [Google Scholar] [CrossRef]
- Li, S.; Yi, M.; Dong, B.; Tan, X.; Luo, S.; Wu, K. The Role of Exosomes in Liquid Biopsy for Cancer Diagnosis and Prognosis Prediction. Int. J. Cancer 2020, 148, 2640–2651. [Google Scholar] [CrossRef]
- Takeuchi, T.; Mori, K.; Sunayama, H.; Takano, E.; Kitayama, Y.; Shimizu, T.; Hirose, Y.; Inubushi, S.; Sasaki, R.; Tanino, H. Antibody-Conjugated Signaling Nanocavities Fabricated by Dynamic Molding for Detecting Cancers Using Small Extracellular Vesicle Markers from Tears. J. Am. Chem. Soc. 2020, 142, 6617–6624. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, D.; Arigami, T.; Okubo, K.; Sasaki, K.; Noda, M.; Kita, Y.; Mori, S.; Uenosono, Y.; Ohtsuka, T.; Natsugoe, S. The Diagnostic and Prognostic Value of a Liquid Biopsy for Esophageal Cancer: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 3070. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.T.; Delijani, K.; Mecum, A.; Goldkorn, A. Current Status of Liquid Biopsies for the Detection and Management of Prostate Cancer. Cancer Manag. Res. 2019, 11, 5271–5291. [Google Scholar] [CrossRef] [PubMed]
- Rink, M.; Schwarzenbach, H.; Vetterlein, M.W.; Soave, A. The Current Role of Circulating Biomarkers in Non-Muscle Invasive Bladder Cancer. Transl. Androl. Urol. 2019, 8, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Kim, C.-J.; Sunkara, V.; Kim, M.H.; Cho, Y.K. Liquid Biopsy in Lung Cancer: Clinical Applications of Circulating Biomarkers (CTCs and ctDNA). Micromachines 2018, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.; Ribeiro, I.P.; Jorge, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Melo, J.B.; Carreira, I.M. Liquid Biopsies: Applications for Cancer Diagnosis and Monitoring. Genes 2021, 12, 349. [Google Scholar] [CrossRef] [PubMed]
- Lampis, A.; Ghidini, M.; Ratti, M.; Mirchev, M.; Okuducu, A.F.; Valeri, N.; Hahne, J.C. Circulating Tumour DNAs and Non-Coding RNAs as Liquid Biopsies for the Management of Colorectal Cancer Patients. Gastrointest. Disord. 2020, 2, 212–235. [Google Scholar] [CrossRef]
- Agashe, R.; Kurzrock, R. Circulating Tumor Cells: From the Laboratory to the Cancer Clinic. Cancers 2020, 12, 2361. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, S.; Low, S.K.; Kiyotani, K. Precision Medicine for Colorectal Cancer with Liquid Biopsy and Immunotherapy. Cancers 2021, 13, 4803. [Google Scholar] [CrossRef] [PubMed]
- Sardarabadi, P.; Kojabad, A.A.; Jafari, D.; Liu, C.-H. Liquid Biopsy-Based Biosensors for MRD Detection and Treatment Monitoring in Non-Small Cell Lung Cancer (NSCLC). Biosensors 2021, 11, 394. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulou, D.; Markou, A.; Strati, A.; Zavridou, M.; Tzanikou, E.; Mastoraki, S.; Kallergi, G.; Georgoulias, V.; Lianidou, E. Comprehensive Liquid Biopsy Analysis as a Tool for the Early Detection of Minimal Residual Disease in Breast Cancer. Sci. Rep. 2023, 13, 1258. [Google Scholar] [CrossRef] [PubMed]
- Paschold, L.; Binder, M. Circulating Tumor DNA in Gastric and Gastroesophageal Junction Cancer. Curr. Oncol. 2022, 29, 1430–1441. [Google Scholar] [CrossRef]
- Pardini, B.; Sabo, A.A.; Birolo, G.; Calin, G.A. Noncoding RNAs in Extracellular Fluids as Cancer Biomarkers: The New Frontier of Liquid Biopsies. Cancers 2019, 11, 1170. [Google Scholar] [CrossRef]
- Eigėlienė, N.; Saarenheimo, J.; Jekunen, A. Potential of Liquid Biopsies for Breast Cancer Screening, Diagnosis, and Response to Treatment. Oncology 2019, 96, 115–124. [Google Scholar] [CrossRef]
- Yang, J.; Hu, J.; Li, Y.; Luo, W.; Liu, J.; Ye, D. Clinical Applications of Liquid Biopsy in Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 781820. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; You, Q.; Song, S.; Wang, H.; Wang, C.; Zhu, L.; Yang, Y. Nanostructures and Nanotechnologies for the Detection of Extracellular Vesicle. Adv. Biol. 2022, 7, 2200201. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Di Capua, D.; Bracken-Clarke, D.; Ronan, K.; Baird, A.-M.; Finn, S. The Liquid Biopsy for Lung Cancer: State of the Art, Limitations and Future Developments. Cancers 2021, 13, 3923. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.B.; Hou, L.K.; Yu, F.; Zhang, J.; Wu, W.; Tang, X.M.; Sun, F.; Lu, H.M.; Deng, J.; et al. Liquid biopsy in lung cancer: Significance in diagnostics, prediction, and treatment monitoring. Mol. Cancer 2022, 21, 25. [Google Scholar] [CrossRef]
- Esagian, S.M.; Grigoriadou, G.; Nikas, I.P.; Boikou, V.; Sadow, P.M.; Won, J.K.; Economopoulos, K.P. Comparison of liquid-based to tissue-based biopsy analysis by targeted next generation sequencing in advanced non-small cell lung cancer: A comprehensive systematic review. J. Cancer Res. Clin. Oncol. 2020, 146, 2051–2066. [Google Scholar] [CrossRef] [PubMed]
- Kolesar, J.; Peh, S.; Thomas, L.; Baburaj, G.; Mukherjee, N.; Kantamneni, R.; Lewis, S.; Pai, A.; Udupa, K.S.; Kumar An, N.; et al. Integration of liquid biopsy and pharmacogenomics for precision therapy of EGFR mutant and resistant lung cancers. Mol. Cancer 2022, 21, 61. [Google Scholar] [CrossRef]
- Jensen, S.G.; Epistolio, S.; Madsen, C.L.; Kyneb, M.H.; Riva, A.; Paganotti, A.; Barizzi, J.; Petersen, R.K.; Børgesen, M.; Molinari, F.; et al. A new sensitive and fast assay for the detection of EGFR mutations in liquid biopsies. PLoS ONE 2021, 16, e0253687. [Google Scholar] [CrossRef]
- Schmid, S.; Li, J.J.N.; Leighl, N.B. Mechanisms of osimertinib resistance and emerging treatment options. Lung Cancer 2020, 147, 123–129. [Google Scholar] [CrossRef]
- Del Re, M.; Crucitta, S.; Gianfilippo, G.; Passaro, A.; Petrini, I.; Restante, G.; Michelucci, A.; Fogli, S.; de Marinis, F.; Porta, C.; et al. Understanding the Mechanisms of Resistance in EGFR-Positive NSCLC: From Tissue to Liquid Biopsy to Guide Treatment Strategy. Int. J. Mol. Sci. 2019, 20, 3951. [Google Scholar] [CrossRef]
- Mazzitelli, C.; Santini, D.; Corradini, A.G.; Zamagni, C.; Trerè, D.; Montanaro, L.; Taffurelli, M. Liquid Biopsy in the Management of Breast Cancer Patients: Where Are We Now and Where Are We Going. Diagnostics 2023, 13, 1241. [Google Scholar] [CrossRef] [PubMed]
- Shegekar, T.; Vodithala, S.; Juganavar, A. The Emerging Role of Liquid Biopsies in Revolutionising Cancer Diagnosis and Therapy. Cureus 2023, 15, e43650. [Google Scholar] [CrossRef]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer [Internet]; Exon Publications: Brisbane, Australia, 2022. [Google Scholar]
- Sanches, S.M.; Braun, A.C.; Calsavara, V.F.; Barbosa, P.N.V.P.; Chinen, L.T.D. Comparison of hormonal receptor expression and HER2 status between circulating tumor cells and breast cancer metastases. Clinics 2021, 76, e2971. [Google Scholar] [CrossRef]
- Papadaki, M.A.; Koutsopoulos, A.V.; Tsoulfas, P.G.; Lagoudaki, E.; Aggouraki, D.; Monastirioti, A.; Koutoulaki, C.; Apostolopoulou, C.A.; Merodoulaki, A.C.; Papadaki, C. Clinical relevance of immune checkpoints on circulating tumor cells in breast cancer. Cancers 2020, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.J.A.d.; Causin, R.L.; Varuzza, M.B.; Calfa, S.; Hidalgo Filho, C.M.T.; Komoto, T.T.; Souza, C.d.P.; Marques, M.M.C. Liquid Biopsy as a Tool for the Diagnosis, Treatment, and Monitoring of Breast Cancer. Int. J. Mol. Sci. 2022, 23, 9952. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lim, A.R.; You, J.Y.; Lee, J.H.; Song, S.E.; Lee, N.K.; Jung, S.P.; Cho, K.R.; Kim, C.Y.; Park, K.H. PIK3CA Mutation is Associated with Poor Response to HER2-Targeted Therapy in Breast Cancer Patients. Cancer Res. Treat. 2023, 55, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Nakai, M.; Yamada, T.; Sekiya, K.; Sato, A.; Hankyo, M.; Kuriyama, S.; Takahashi, G.; Kurita, T.; Yanagihara, K.; Yoshida, H.; et al. PIK3CA mutation detected by liquid biopsy in patients with metastatic breast cancer. J. Nippon Med. Sch. 2021, 89, 66–71. [Google Scholar] [CrossRef]
- Venetis, K.; CursanSo, G.; Pescia, C.; D’Ercole, M.; Porta, F.M.; Blanco, M.C.; Frascarelli, C.; Ivanova, M.; Guerini Rocco, E.; Fusco, N. Liquid biopsy: Cell-free DNA based analysis in breast cancer. J. Liq. Biopsy 2023, 1, 100002. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2–negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef]
- Alsmadi, O.; Abdel-Razeq, H.; Talab, Y.; Abdulelah, H.; Shaheen, Z.; Tbakhi, A. PIK3CA Mutational Profiling in a Patient Cohort with HR+/HER2-Advanced Metastatic Breast Cancer at a Tertiary Cancer Center. Fortune J. Health Sci. 2024, 7, 158–163. [Google Scholar] [CrossRef]
- Liao, H.; Huang, W.; Pei, W.; Li, H. Detection of ESR1 mutations based on liquid biopsy in estrogen receptor-positive metastatic breast cancer: Clinical impacts and prospects. Front. Oncol. 2020, 10, 587671. [Google Scholar] [CrossRef]
- Urso, L.; Vernaci, G.; Carlet, J.; Lo Mele, M.; Fassan, M.; Zulato, E.; Faggioni, G.; Menichetti, A.; Di Liso, E.; Griguolo, G. ESR1 gene mutation in hormone receptor-positive HER2-negative metastatic breast cancer patients: Concordance between tumor tissue and circulating tumor DNA analysis. Front. Oncol. 2021, 11, 625636. [Google Scholar] [CrossRef]
- Mazouji, O.; Ouhajjou, A.; Incitti, R.; Mansour, H. Updates on clinical use of liquid biopsy in colorectal cancer screening, diagnosis, follow-up, and treatment guidance. Front. Cell Dev. Biol. 2021, 9, 660924. [Google Scholar] [CrossRef]
- Lou, S.; Shaukat, A. Noninvasive strategies for colorectal cancer screening: Opportunities and limitations. Curr. Opin. Gastroenterol. 2021, 37, 44–51. [Google Scholar] [CrossRef]
- Hanna, M.; Dey, N.; Grady, W.M. Emerging tests for noninvasive colorectal cancer screening. Clin. Gastroenterol. Hepatol. 2023, 21, 604–616. [Google Scholar] [CrossRef]
- Tsai, W.-S.; You, J.-F.; Hung, H.-Y.; Hsieh, P.-S.; Hsieh, B.; Lenz, H.-J.; Idos, G.; Friedland, S.; Yi-Jiun Pan, J.; Shao, H.-J.; et al. Novel Circulating Tumor Cell Assay for Detection of Colorectal Adenomas and Cancer. Clin. Transl. Gastroenterol. 2019, 10, e00088. [Google Scholar] [CrossRef]
- Tsai, W.-S.; Hung, W.-S.; Wang, T.-M.; Liu, H.; Yang, C.-Y.; Wu, S.-M.; Hsu, H.-L.; Hsiao, Y.-C.; Tsai, H.-J.; Tseng, C.-P. Circulating tumor cell enumeration for improved screening and disease detection of patients with colorectal cancer. Biomed. J. 2021, 44, S190–S200. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Wang, K.; Xu, C.; Hao, M.; Ding, L. Perspectives of the application of liquid biopsy in colorectal cancer. BioMed Res. Int. 2020, 2020, 6843180. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Epi proColon® for colorectal cancer screening: A profile of its use in the USA. Mol. Diagn. Ther. 2020, 24, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Kamel, F.; Eltarhoni, K.; Nisar, P.; Soloviev, M. Colorectal Cancer Diagnosis: The Obstacles We Face in Determining a Non-Invasive Test and Current Advances in Biomarker Detection. Cancers 2022, 14, 1889. [Google Scholar] [CrossRef]
- van den Puttelaar, R.; Nascimento de Lima, P.; Knudsen, A.B.; Rutter, C.M.; Kuntz, K.M.; de Jonge, L.; Escudero, F.A.; Lieberman, D.; Zauber, A.G.; Hahn, A.I.; et al. Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening with a Blood Test That Meets the Centers for Medicare & Medicaid Services Coverage Decision. Gastroenterology 2024, 167, 368–377. [Google Scholar] [CrossRef]
- Laugsand, E.A.; Brenne, S.S.; Skorpen, F. DNA methylation markers detected in blood, stool, urine, and tissue in colorectal cancer: A systematic review of paired samples. Int. J. Colorectal Dis. 2021, 36, 239–251. [Google Scholar] [CrossRef]
- Toth, J.F., III; Trivedi, M.; Gupta, S. Screening for Colorectal Cancer: The Role of Clinical Laboratories. Clin. Chem. 2024, 70, 150–164. [Google Scholar] [CrossRef]
- Fatemi, N.; Tierling, S.; Es, H.A.; Varkiani, M.; Mojarad, E.N.; Aghdaei, H.A.; Walter, J.; Totonchi, M. DNA methylation biomarkers in colorectal cancer: Clinical applications for precision medicine. Int. J. Cancer 2022, 151, 2068–2081. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.-Y.; Li, Q.-Q.; Zeng, Y. Clinical application of liquid biopsy in colorectal cancer: Detection, prediction, and treatment monitoring. Mol. Cancer 2024, 23, 145. [Google Scholar] [CrossRef]
- Boysen, A.K.; Pallisgaard, N.; Andersen, C.S.A.; Spindler, K.-L.G. Circulating tumor DNA as a marker of minimal residual disease following local treatment of metastases from colorectal cancer. Acta Oncol. 2020, 59, 1424–1429. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, C.; Lin, G.; Xiao, Y.; Jia, W.; Xiao, G.; Liu, Q.; Wu, B.; Wu, A.; Qiu, H.; et al. Serial Circulating Tumor DNA in Predicting and Monitoring the Effect of Neoadjuvant Chemoradiotherapy in Patients with Rectal Cancer: A Prospective Multicenter Study. Clin. Cancer Res. 2021, 27, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Movassaghi, M.; Chung, R.; Anderson, C.B.; Stein, M.; Saenger, Y.; Faiena, I. Overcoming Immune Resistance in Prostate Cancer: Challenges and Advances. Cancers 2021, 13, 4757. [Google Scholar] [CrossRef]
- Gasperoni, L.; Giunta, E.F.; Montanari, D.; Masini, C.; De Giorgi, U. New-generation androgen receptor signaling inhibitors (ARSIs) in metastatic hormone-sensitive prostate cancer (mHSPC): Pharmacokinetics, drug-drug interactions (DDIs), and clinical impact. Expert Opin. Drug Metab. Toxicol. 2024, 20, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Sperger, J.M.; Emamekhoo, H.; McKay, R.R.; Stahlfeld, C.N.; Singh, A.; Chen, X.E.; Kwak, L.; Gilsdorf, C.S.; Wolfe, S.K.; Wei, X.X.; et al. Prospective Evaluation of Clinical Outcomes Using a Multiplex Liquid Biopsy Targeting Diverse Resistance Mechanisms in Metastatic Prostate Cancer. J. Clin. Oncol. 2021, 39, 2926–2937. [Google Scholar] [CrossRef] [PubMed]
- Tulpule, V.; Morrison, G.J.; Falcone, M.; Quinn, D.I.; Goldkorn, A. Integration of Liquid Biopsies in Clinical Management of Metastatic Prostate Cancer. Curr. Oncol. Rep. 2022, 24, 1287–1298. [Google Scholar] [CrossRef]
- Ionescu, F.; Zhang, J.; Wang, L. Clinical Applications of Liquid Biopsy in Prostate Cancer: From Screening to Predictive Biomarker. Cancers 2022, 14, 1728. [Google Scholar] [CrossRef]
- Melnyk, J.E.; Steri, V.; Nguyen, H.G.; Hwang, Y.C.; Gordan, J.D.; Hann, B.; Feng, F.Y.; Shokat, K.M. Targeting a splicing-mediated drug resistance mechanism in prostate cancer by inhibiting transcriptional regulation by PKCβ1. Oncogene 2022, 41, 1536–1549. [Google Scholar] [CrossRef]
- Lu, D.; Krupa, R.; Harvey, M.; Graf, R.P.; Schreiber, N.; Barnett, E.; Carbone, E.; Jendrisak, A.; Gill, A.; Orr, S.; et al. Development of an immunofluorescent AR-V7 circulating tumor cell assay-A blood-based test for men with metastatic prostate cancer. J. Circ. Biomark. 2020, 9, 13–19. [Google Scholar] [CrossRef]
- Liu, H.E.; Vuppalapaty, M.; Hoerner, C.R.; Bergstrom, C.P.; Chiu, M.; Lemaire, C.; Che, J.; Kaur, A.; Dimmick, A.; Liu, S. Detecting androgen receptor (AR), AR variant 7 (AR-V7), prostate-specific membrane antigen (PSMA), and prostate-specific antigen (PSA) gene expression in CTCs and plasma exosome-derived cfRNA in patients with metastatic castration-resistant prostate cancer (mCRPC) by integrating the VTX-1 CTC isolation system with the QIAGEN AdnaTest. BMC Cancer 2024, 24, 482. [Google Scholar]
- Nimir, M.; Ma, Y.; Jeffreys, S.A.; Opperman, T.; Young, F.; Khan, T.; Ding, P.; Chua, W.; Balakrishnar, B.; Cooper, A.; et al. Detection of AR-V7 in Liquid Biopsies of Castrate Resistant Prostate Cancer Patients: A Comparison of AR-V7 Analysis in Circulating Tumor Cells, Circulating Tumor RNA and Exosomes. Cells 2019, 8, 688. [Google Scholar] [CrossRef]
- Abida, W.; Antonarakis, E.; Sartor, A.O. Management of Advanced Prostate Cancer with Germline or Somatic Homologous Recombination Repair Deficiency; UpToDate: Wellesley, MA, USA, 2023. [Google Scholar]
- von Werdt, A.; Brandt, L.; Schärer, O.D.; Rubin, M.A. PARP Inhibition in Prostate Cancer with Homologous Recombination Repair Alterations. JCO Precis. Oncol. 2021, 5, 1639–1649. [Google Scholar] [CrossRef]
- Stewart, M.D.; Merino Vega, D.; Arend, R.C.; Baden, J.F.; Barbash, O.; Beaubier, N.; Collins, G.; French, T.; Ghahramani, N.; Hinson, P.; et al. Homologous Recombination Deficiency: Concepts, Definitions, and Assays. Oncologist 2022, 27, 167–174. [Google Scholar] [CrossRef]
- Woodhouse, R.; Li, M.; Hughes, J.; Delfosse, D.; Skoletsky, J.; Ma, P.; Meng, W.; Dewal, N.; Milbury, C.; Clark, T.; et al. Clinical and analytical validation of FoundationOne Liquid CDx, a novel 324-Gene cfDNA-based comprehensive genomic profiling assay for cancers of solid tumor origin. PLoS ONE 2020, 15, e0237802. [Google Scholar] [CrossRef]
- Mekonnen, N.; Yang, H.; Shin, Y.K. Homologous recombination deficiency in ovarian, breast, colorectal, pancreatic, non-small cell lung and prostate cancers, and the mechanisms of resistance to PARP inhibitors. Front. Oncol. 2022, 12, 880643. [Google Scholar] [CrossRef]
- Armstrong, A.J. Predictive biomarkers in advanced prostate cancer. Clin. Adv. Hematol. Oncol. HO 2023, 6, 290–293. [Google Scholar]
- Vandekerkhove, G.; Giri, V.N.; Halabi, S.; McNair, C.; Hamade, K.; Bitting, R.L.; Wyatt, A.W. Toward Informed Selection and Interpretation of Clinical Genomic Tests in Prostate Cancer. JCO Precis. Oncol. 2024, 8, e2300654. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-J.; Dong, L.; Amend, S.R.; Cho, Y.-K.; Pienta, K.J. The role of liquid biopsies in prostate cancer management. Lab Chip 2021, 21, 3263–3288. [Google Scholar] [CrossRef] [PubMed]
- de Kruijff, I.E.; Sieuwerts, A.M.; Onstenk, W.; Kraan, J.; Smid, M.; Van, M.N.; van der Vlugt-Daane, M.; Oomen-de Hoop, E.; Mathijssen, R.H.J.; Lolkema, M.P.; et al. Circulating Tumor Cell Enumeration and Characterization in Metastatic Castration-Resistant Prostate Cancer Patients Treated with Cabazitaxel. Cancers 2019, 11, 1212. [Google Scholar] [CrossRef]
- Nagaya, N.; Nagata, M.; Lu, Y.; Kanayama, M.; Hou, Q.; Hotta, Z.-u.; China, T.; Kitamura, K.; Matsushita, K.; Isotani, S. Prostate-specific membrane antigen in circulating tumor cells is a new poor prognostic marker for castration-resistant prostate cancer. PLoS ONE 2020, 15, e0226219. [Google Scholar] [CrossRef]
- Yang, Y.J.; Kong, Y.Y.; Li, G.X.; Wang, Y.; Ye, D.W.; Dai, B. Phenotypes of circulating tumour cells predict time to castration resistance in metastatic castration-sensitive prostate cancer. BJU Int. 2019, 124, 258–267. [Google Scholar] [CrossRef]
- Shoukry, M.; Broccard, S.; Kaplan, J.; Gabriel, E. The Emerging Role of Circulating Tumor DNA in the Management of Breast Cancer. Cancers 2021, 13, 3813. [Google Scholar] [CrossRef] [PubMed]
- Vacante, M.; Ciuni, R.; Burzotta, F.; Biondi, A. The Liquid Biopsy in the Management of Colorectal Cancer: An Overview. Biomedicines 2020, 8, 308. [Google Scholar] [CrossRef]
- Palacín-Aliana, I.; García-Romero, N.; Asensi-Puig, A.; Carrión-Navarro, J.; González-Rumayor, V.; Ayuso-Sacido, Á. Clinical Utility of Liquid Biopsy-Based Actionable Mutations Detected via ddPCR. Biomedicines 2021, 9, 906. [Google Scholar] [CrossRef]
- Nordgård, O.; Forthun, R.B.; Lapin, M.; Grønberg, B.H.; Kalland, K.H.; Kopperud, R.K.; Thomsen, L.C.V.; Tjensvoll, K.; Gilje, B.; Gjertsen, B.T.; et al. Liquid Biopsies in Solid Cancers: Implementation in a Nordic Healthcare System. Cancers 2021, 13, 1861. [Google Scholar] [CrossRef]
- Goodsaid, F. The Labyrinth of Product Development and Regulatory Approvals in Liquid Biopsy Diagnostics. Clin. Transl. Sci. 2019, 12, 431–439. [Google Scholar] [CrossRef]
- Paracchini, L.; D’Incalci, M.; Marchini, S. Liquid Biopsy in the Clinical Management of High-Grade Serous Epithelial Ovarian Cancer—Current Use and Future Opportunities. Cancers 2021, 13, 2386. [Google Scholar] [CrossRef]
- Kan, C.M.; Tsang, H.F.; Pei, X.M.; Ng, S.S.M.; Yim, A.K.; Yu, A.C.; Wong, S.C.C. Enhancing Clinical Utility: Utilization of International Standards and Guidelines for Metagenomic Sequencing in Infectious Disease Diagnosis. Int. J. Mol. Sci. 2024, 25, 3333. [Google Scholar] [CrossRef]
- Temilola, D.O.; Wium, M.; Coulidiati, T.H.; Adeola, H.A.; Carbone, G.M.; Catapano, C.V.; Zerbini, L.F. The Prospect and Challenges to the Flow of Liquid Biopsy in Africa. Cells 2019, 8, 862. [Google Scholar] [CrossRef] [PubMed]
- Liebs, S.; Nonnenmacher, A.; Klauschen, F.; Keilholz, U.; Vecchione, L. Liquid Biopsy Assessment of Synchronous Malignancies: A Case Report and Review of the Literature. Esmo Open 2019, 4, e000528. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Zhang, H.; Cao, H.; Mao, J.; Chen, X.; Wang, L.; Zhang, N.; Luo, P.; Xue, J.; et al. Liquid Biopsy for Human Cancer: Cancer Screening, Monitoring, and Treatment. Medcomm 2024, 5, e564. [Google Scholar] [CrossRef]
- Mikilps-Mikgelbs, R.; Pupola, D.; Antone, E.; Kirshners, A.; Luguzis, A.; Salna, E.; Krams, A.; Ērglis, A. Liquid Biopsy—A Novel Diagnostic Tool for Management of Early-Stage Peripheral Lung Cancer. Proc. Latv. Acad. Sci. Sect. B Nat. Exact. Appl. Sci. 2022, 76, 325–332. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.; Bridgewater, J.; Normanno, N. Practical Considerations in Screening for Genetic Alterations in Cholangiocarcinoma. Ann. Oncol. 2021, 32, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, B.; Wu, A.; Wetterskog, D.; Attard, G. Blood-Based Liquid Biopsies for Prostate Cancer: Clinical Opportunities and Challenges. Br. J. Cancer 2022, 127, 1394–1402. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, D.; Guo, H.; Ma, W. Beyond Blood: Advancing the Frontiers of Liquid Biopsy in Oncology and Personalized Medicine. Cancer Sci. 2024, 115, 1060–1072. [Google Scholar] [CrossRef]
- Bao, H.; Min, L.; Bu, F.; Wang, S.; Meng, J. Recent Advances of Liquid Biopsy: Interdisciplinary Strategies Toward Clinical Decision-making. Interdiscip. Med. 2023, 1, e20230021. [Google Scholar] [CrossRef]



| Liquid Biopsy | Tissue Biopsy |
|---|---|
| Samples derived from body fluid | Samples derived from needle biopsy or surgical biopsy |
| Minimally invasive | Invasive |
| Preserved by preservation reagents | Preserved by paraffin embedding or cryopreservation |
| Can be performed after surgical resection and when there is no detectable metastatic mass | Impossible to be performed after surgical resection and when there is no detectable metastatic mass |
| Does not allow tumor histotype specification and staging | Allows histological diagnosis and staging |
| Easily performed at multiple or sequential time points during treatment | Not suitable for frequent monitoring |
| Allows real-time monitoring of disease | Single information point over time and space |
| Potential to reflect tumor heterogeneity | Not suitable to reflect tumor heterogeneity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, H.-Y.; Chung, K.-S.; Kan, C.-M.; Wong, S.-C. Liquid Biopsy in the Clinical Management of Cancers. Int. J. Mol. Sci. 2024, 25, 8594. https://doi.org/10.3390/ijms25168594
Ho H-Y, Chung K-S, Kan C-M, Wong S-C. Liquid Biopsy in the Clinical Management of Cancers. International Journal of Molecular Sciences. 2024; 25(16):8594. https://doi.org/10.3390/ijms25168594
Chicago/Turabian StyleHo, Ho-Yin, Kei-See (Kasey) Chung, Chau-Ming Kan, and Sze-Chuen (Cesar) Wong. 2024. "Liquid Biopsy in the Clinical Management of Cancers" International Journal of Molecular Sciences 25, no. 16: 8594. https://doi.org/10.3390/ijms25168594
APA StyleHo, H.-Y., Chung, K.-S., Kan, C.-M., & Wong, S.-C. (2024). Liquid Biopsy in the Clinical Management of Cancers. International Journal of Molecular Sciences, 25(16), 8594. https://doi.org/10.3390/ijms25168594




