Research Progress on Heat Stress Response Mechanism and Control Measures in Medicinal Plants
Abstract
1. Introduction
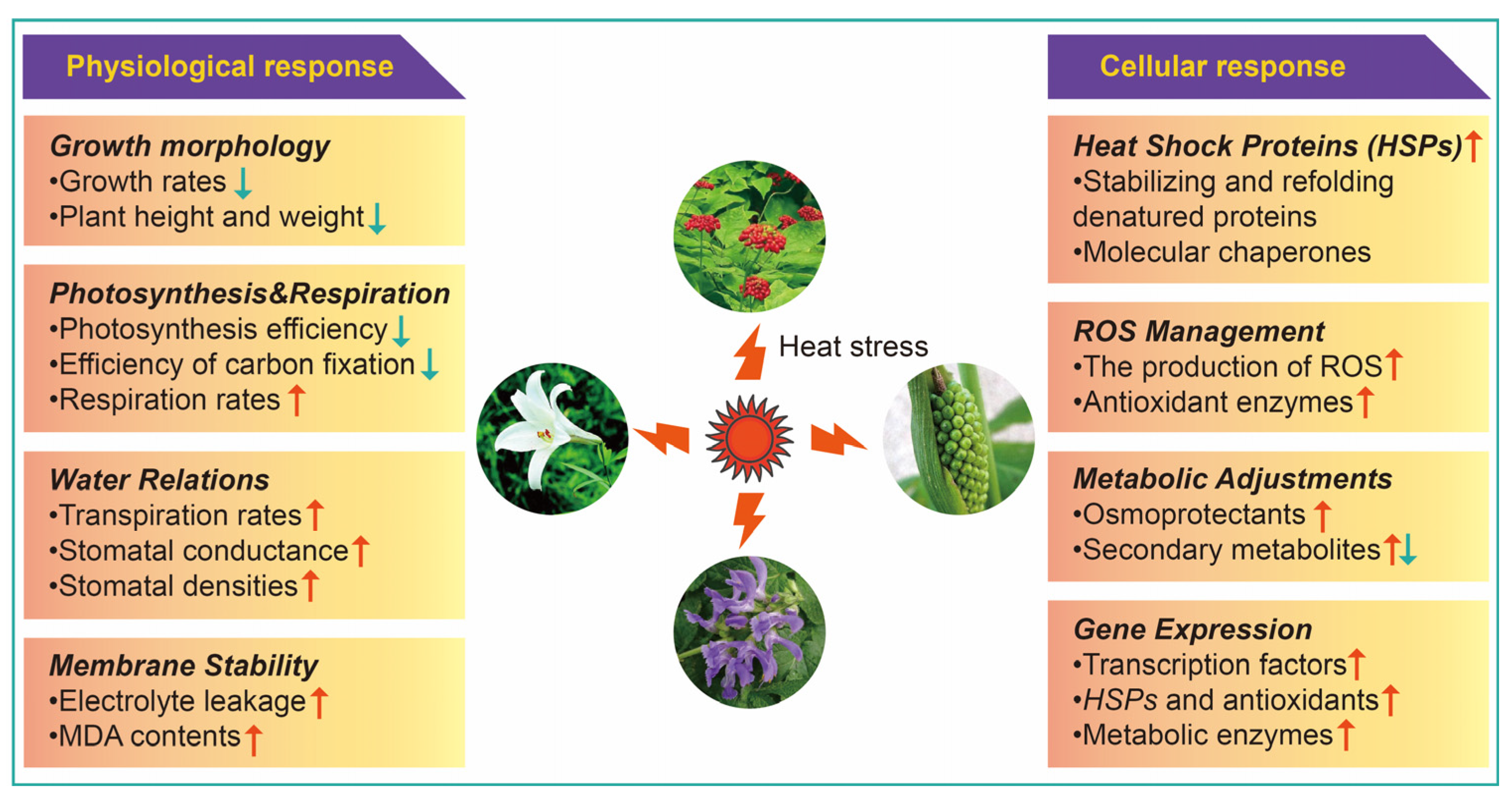
2. Physiological Response of Medicinal Plants to HS
2.1. Growth Morphology
2.2. Photosynthesis and Respiration
2.3. Water Relations
2.4. Membrane Sustainability
3. Cellular Response of Medicinal Plants to HS
3.1. Heat Shock Proteins (HSPs)
3.2. Reactive Oxygen Species (ROS) Management
3.3. Metabolic Adjustments
3.4. Gene Expression
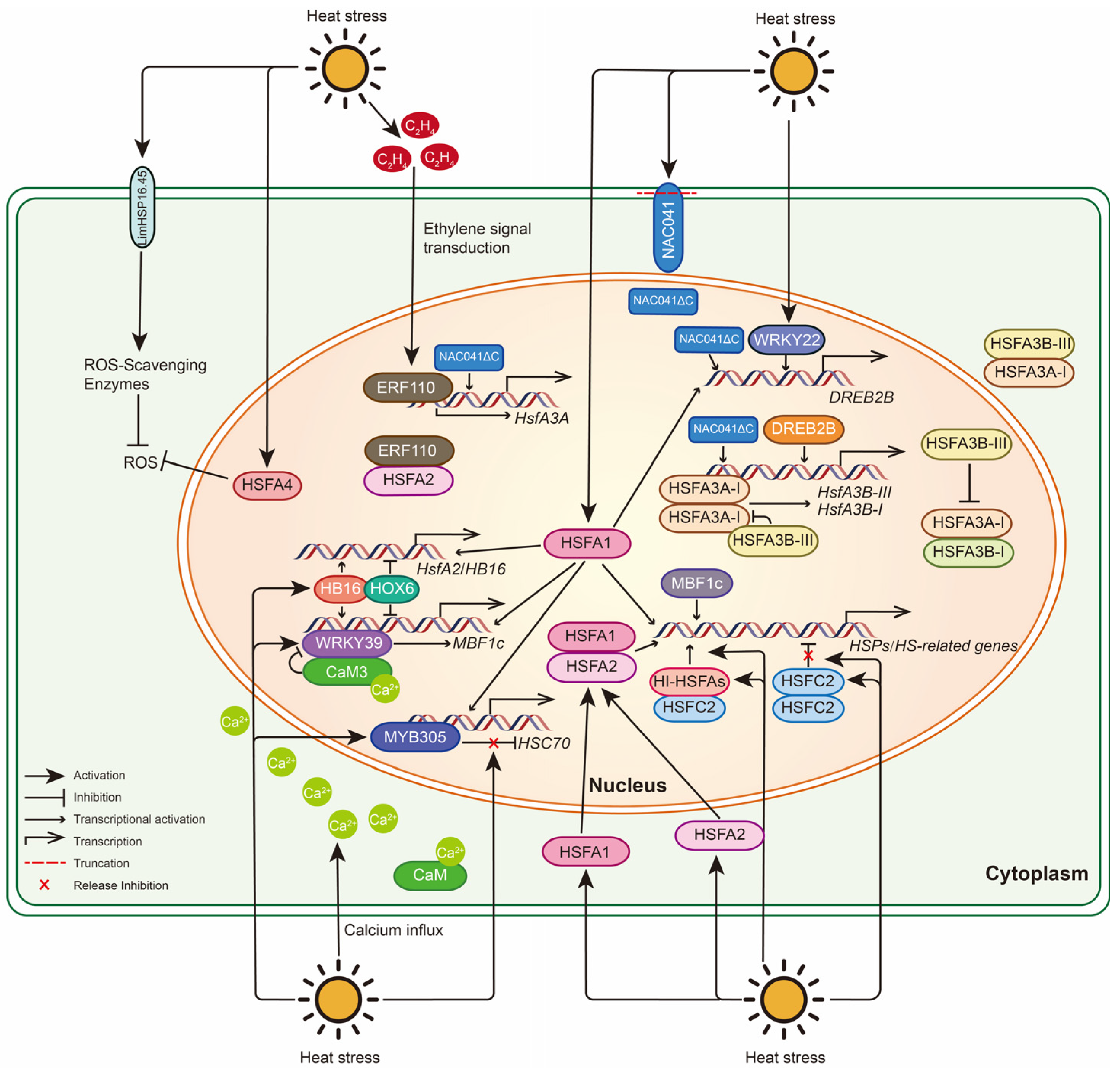
4. Molecular Mechanisms of Medicinal Plants for HS
4.1. Molecular Responses of Lily to HS
4.2. Molecular Responses of Other Medicinal Plants to HS
5. Exogenous Regulation of Enhancing Thermotolerance in Medicinal Plants
6. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmadi-Lahijani, M.J.; Moori, S. Current Status of Medicinal Plants in Perspective of Environmental Challenges and Global Climate Changes. In Environmental Challenges and Medicinal Plants: Sustainable Production Solutions under Adverse Conditions; Aftab, T., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–28. [Google Scholar]
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100255. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant Responses to Heat Stress: Physiology, Transcription, Noncoding RNAs, and Epigenetics. Int. J. Mol. Sci. 2020, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Alhaithloul, H.A.; Soliman, M.H.; Ameta, K.L.; El-Esawi, M.A.; Elkelish, A. Changes in Ecophysiology, Osmolytes, and Secondary Metabolites of the Medicinal Plants of Mentha piperita and Catharanthus roseus Subjected to Drought and Heat Stress. Biomolecules 2020, 10, 43. [Google Scholar] [CrossRef]
- Guo, X.-r.; Yang, L.; Yu, J.-h.; Tang, Z.-h.; Zu, Y.-g. Alkaloid variations in Catharanthus roseus seedlings treated by different temperatures in short term and long term. J. For. Res. 2007, 18, 313–315. [Google Scholar] [CrossRef]
- Kan, Y.; Mu, X.-R.; Gao, J.; Lin, H.-X.; Lin, Y. The molecular basis of heat stress responses in plants. Mol. Plant 2023, 16, 1612–1634. [Google Scholar] [CrossRef] [PubMed]
- Andrási, N.; Pettkó-Szandtner, A.; Szabados, L. Diversity of plant heat shock factors: Regulation, interactions, and functions. J. Exp. Bot. 2020, 72, 1558–1575. [Google Scholar] [CrossRef] [PubMed]
- Haydari, M.; Maresca, V.; Rigano, D.; Taleei, A.; Shahnejat-Bushehri, A.A.; Hadian, J.; Sorbo, S.; Guida, M.; Manna, C.; Piscopo, M.; et al. Salicylic Acid and Melatonin Alleviate the Effects of Heat Stress on Essential Oil Composition and Antioxidant Enzyme Activity in Mentha x piperita and Mentha arvensis L. Antioxidants 2019, 8, 547. [Google Scholar] [CrossRef]
- Lin, K.H.; Lin, T.Y.; Wu, C.W.; Chang, Y.S. Protective Effects of Salicylic Acid and Calcium Chloride on Sage Plants (Salvia officinalis L. and Salvia elegans Vahl) under High-Temperature Stress. Plants 2021, 10, 2110. [Google Scholar] [CrossRef]
- Cao, X.; Yi, J.; Wu, Z.; Luo, X.; Zhong, X.H.; Wu, J.; Khan, M.A.; Zhao, Y.; Yi, M.F. Involvement of Ca2+ and CaM3 in Regulation of Thermotolerance in Lily (Lilium longiflorum). Plant Mol. Biol. Rep. 2013, 31, 1293–1304. [Google Scholar] [CrossRef]
- Qin, L.; Li, C.Y.; Li, D.B.; Wang, J.Y.; Yang, L.; Qu, A.L.; Wu, Q.F. Physiological, Metabolic and Transcriptional Responses of Basil (Ocimum basilicum Linn. var. pilosum (Willd.) Benth.) to Heat Stress. Agronomy 2022, 12, 1434. [Google Scholar]
- Li, Y.S.; Lin, K.H.; Wu, C.W.; Chang, Y.S. Effects of temperatures on growth, physiological, and antioxidant characteristics in Houttuynia cordata. Not. Bot. Horti Agrobot. 2021, 49, 12536. [Google Scholar] [CrossRef]
- Jin, R.; Wang, Y.; Liu, R.; Gou, J.; Chan, Z. Physiological and Metabolic Changes of Purslane (Portulaca oleracea L.) in Response to Drought, Heat, and Combined Stresses. Front. Plant Sci. 2016, 6, 1123. [Google Scholar] [CrossRef]
- Guo, J.J.; Zhang, R.Y.; Cheng, S.Q.; Fu, Z.Q.; Jia, P.; Luan, H.A.; Zhang, X.M.; Qi, G.H.; Guo, S.P. Physiological and transcriptomic analysis reveal the crucial factors in heat stress response of red raspberry ‘Polka’ seedlings. Front. Plant Sci. 2023, 14, 1233448. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Wang, J.L.; Chen, J.L.; Zhang, X.Y.; Fang, X.; Li, X.W. Physiological and transcriptional responses to heat stress in a typical phenotype of Pinellia ternata. Chin. J. Nat. Med. 2023, 21, 243–252. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Fan, Y.; Posch, B.C.; Garcia, A.; Coast, O.; Atkin, O.K. Responses of leaf respiration to heatwaves. Plant Cell Environ. 2021, 44, 2090–2101. [Google Scholar] [CrossRef]
- Sadok, W.; Lopez, J.R.; Smith, K.P. Transpiration increases under high-temperature stress: Potential mechanisms, trade-offs and prospects for crop resilience in a warming world. Plant Cell Environ. 2021, 44, 2102–2116. [Google Scholar] [CrossRef]
- Pelletier, V.; Pepin, S.; Gallichand, J.; Caron, J. Reducing cranberry heat stress and midday depression with evaporative cooling. Sci. Hortic. 2016, 198, 445–453. [Google Scholar] [CrossRef]
- Pandey, V.; Tiwari, D.C.; Dhyani, V.; Bhatt, I.D.; Rawal, R.S.; Nandi, S.K. Physiological and metabolic changes in two Himalayan medicinal herbs under drought, heat and combined stresses. Physiol. Mol. Biol. Plants 2021, 27, 1523–1538. [Google Scholar] [CrossRef]
- Qian, R.; Hu, Q.; Ma, X.; Zhang, X.; Ye, Y.; Liu, H.; Gao, H.; Zheng, J. Comparative transcriptome analysis of heat stress responses of Clematis lanuginosa and Clematis crassifolia. BMC Plant Biol. 2022, 22, 138. [Google Scholar] [CrossRef]
- Nazdar, T.; Tehranifar, A.; Nezami, A.; Nemati, H.; Samiei, L. Physiological and anatomical responses of calendula (Calendula officinalis L.) cultivars to heat-stress duration. J. Hortic. Sci. Biotech. 2018, 94, 400–411. [Google Scholar] [CrossRef]
- Asgary, S.; Karimi, R.; Pour, P.M.; Heydarpour, F.; Mostafaei, S.; Farzaei, M.H.; Moradi, S.; Aneva, I.Y. Is Consumption of Pomegranate Supplementation Effective on Oxidative Stress Biomarkers Including MDA, ox-LDL, POX 1, GPX, TAC, and TBRAS? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Prob. Cardiol. 2023, 48, 101198. [Google Scholar] [CrossRef] [PubMed]
- Wassie, M.; Zhang, W.; Zhang, Q.; Ji, K.; Cao, L.; Chen, L. Exogenous salicylic acid ameliorates heat stress-induced damages and improves growth and photosynthetic efficiency in alfalfa (Medicago sativa L.). Ecotoxicol. Environ. Saf. 2020, 191, 110206. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.H.; Zhu, G.S.; Guo, Q.S.; Zhu, Z.B.; Wang, C.L.; Liu, Z.Y. A Comparative Proteomic Analysis of Pinellia ternata Leaves Exposed to Heat Stress. Int. J. Mol. Sci. 2013, 14, 20614–20634. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.G.; Wassie, M.; Zhou, W.X.; Wang, H.; You, J.W.; Ma, G.J.; Zhang, M.D. Transcriptomic Analysis Provides Novel Insights into the Heat Stress-Induced Response in Codonopsis tangshen. Life 2023, 13, 168. [Google Scholar] [CrossRef]
- Al-Whaibi, M.H. Plant heat-shock proteins: A mini review. J. King Saud. Univ. Sci. 2011, 23, 139–150. [Google Scholar] [CrossRef]
- Khan, S.; Jabeen, R.; Deeba, F.; Waheed, U.; Khanum, P.; Iqbal, N. Heat Shock Proteins: Classification, Functions and Expressions in Plants during Environmental Stresses. J. Biores Manag. 2021, 8, 85–97. [Google Scholar] [CrossRef]
- Howlader, J.; Park, J.I.; Robin, A.H.K.; Sumi, K.R.; Nou, I.S. Identification, Characterization and Expression Profiling of Stress-Related Genes in Easter Lily (Lilium formolongi). Genes 2017, 8, 172. [Google Scholar] [CrossRef]
- Mu, C.J.; Wang, S.B.; Zhang, S.J.; Pan, J.J.; Chen, N.; Li, X.F.; Wang, Z.Y.; Liu, H. Small heat shock protein LimHSP16.45 protects pollen mother cells and tapetal cells against extreme temperatures during late zygotene to pachytene stages of meiotic prophase I in David Lily. Plant Cell Rep. 2011, 30, 1981–1989. [Google Scholar] [CrossRef]
- Zhou, Y.Z.; Wang, Y.; Xu, F.X.; Song, C.X.; Yang, X.; Zhang, Z.; Yi, M.F.; Ma, N.; Zhou, X.F.; He, J.N. Small HSPs play an important role in crosstalk between HSF-HSP and ROS pathways in heat stress response through transcriptomic analysis in lilies (Lilium longiflorum). BMC Plant Biol. 2022, 22, 202. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Choi, W.G.; Miller, G.; Wallace, I.; Harper, J.; Mittler, R.; Gilroy, S. Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. Plant J. 2017, 90, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef]
- Zhong, F.R.; Ke, W.J.; Li, Y.R.; Chen, X.Y.; Zhou, T.; Xu, B.J.; Qi, L.M.; Yan, Z.Y.; Ma, Y.T. Comprehensive analysis of the complete mitochondrial genomes of three Coptis species (C. chinensis, C. deltoidea and C. omeiensis): The important medicinal plants in China. Front. Plant Sci. 2023, 14, 1166420. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011, 6, 1720–1731. [Google Scholar]
- Kumar, D.; Punetha, A.; Suryavanshi, P.; Padalıa, R.; Kt, V. Environmental Abiotic Stress and Secondary Metabolites Production in Medicinal Plants: A Review. J. Agric. Sci. 2022, 28, 351–362. [Google Scholar]
- Wani, S.H.; Kapoor, N.; Mahajan, R. Metabolic Responses of Medicinal Plants to Global Warming, Temperature and Heat Stress. In Medicinal Plants and Environmental Challenges; Ghorbanpour, M., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 69–80. [Google Scholar]
- Mohammadi, H.; Hazrati, S.; Ghorbanpour, M. Tolerance mechanisms of medicinal plants to abiotic stresses. In Plant Life Under Changing Environment; Tripathi, D.K., Pratap Singh, V., Chauhan, D.K., Sharma, S., Prasad, S.M., Dubey, N.K., Ramawat, N., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 663–679. [Google Scholar]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejeryte, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Qin, X.Y.; Qin, B.B.; He, W.; Chen, Y.; Yin, Y.; Cao, Y.L.; An, W.; Mu, Z.X.; Qin, K. Metabolomic and Transcriptomic Analyses of Lycium barbarum L. under Heat Stress. Sustainability 2022, 14, 12617. [Google Scholar] [CrossRef]
- Kumar, R.; Joshi, R.; Kumari, M.; Thakur, R.; Kumar, D.; Kumar, S. Elevated CO2 and temperature influence key proteins and metabolites associated with photosynthesis, antioxidant and carbon metabolism in Picrorhiza kurroa. J. Proteom. 2020, 219, 103755. [Google Scholar] [CrossRef] [PubMed]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Network of Plant Heat Stress Response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Yang, L.P.; Gao, H.H.; Wenji, X.; Li, Q.; Li, H.Q.; Gao, J. Comparative transcriptome analysis reveals heat stress-responsive genes and their signalling pathways in lilies (Lilium longiflorum vs. Lilium distichum). PLoS ONE 2020, 15, e0239605. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Hemmati, H.; Gupta, D.; Basu, C. Molecular Physiology of Heat Stress Responses in Plants. In Elucidation of Abiotic Stress Signaling in Plants: Functional Genomics Perspectives; Pandey, G.K., Ed.; Springer: New York, NY, USA, 2015; pp. 109–142. [Google Scholar]
- Gong, B.H.; Yi, J.; Wu, J.; Sui, J.J.; Khan, M.A.; Wu, Z.; Zhong, X.H.; Seng, S.S.; He, J.N.; Yi, M.F. LlHSFA1, a novel heat stress transcription factor in lily (Lilium longiflorum), can interact with LlHSFA2 and enhance the thermotolerance of transgenic Arabidopsis thaliana. Plant Cell Rep. 2014, 33, 1519–1533. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, J.H.; Zhang, S.; Zhang, B.; Zhao, Q.C.; Li, G.Q.; Yang, X.; Wang, C.P.; He, J.N.; Yi, M.F. A Canonical DREB2-Type Transcription Factor in Lily Is Post-translationally Regulated and Mediates Heat Stress Response. Front. Plant Sci. 2018, 9, 243. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, T.; Cao, X.; Zhang, D.H.; Teng, N.J. Lily WRKY factor LlWRKY22 promotes thermotolerance through autoactivation and activation of LlDREB2B. Hortic. Res. 2022, 9, uhac186. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, T.; Xiang, J.; Teng, R.D.; Zhang, D.H.; Teng, N.J. A lily membrane-associated NAC transcription factor LlNAC014 is involved in thermotolerance via activation of the DREB2-HSFA3 module. J. Exp. Bot. 2023, 74, 945–963. [Google Scholar] [CrossRef]
- Xin, H.B.; Zhang, H.; Chen, L.; Li, X.X.; Lian, Q.L.; Yuan, X.; Hu, X.Y.; Cao, L.; He, X.L.; Yi, M.F. Cloning and characterization of HsfA2 from Lily (Lilium longiflorum). Plant Cell Rep. 2010, 29, 875–885. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.Z.; Wang, R.; Xu, F.X.; Tong, S.; Song, C.X.; Shao, Y.A.; Yi, M.F.; He, J.N. Ethylene Response Factor LlERF110 Mediates Heat Stress Response via Regulation of LlHsfA3A Expression and Interaction with LlHsfA2 in Lilies (Lilium longiflorum). Int. J. Mol. Sci. 2022, 23, 16135. [Google Scholar] [CrossRef]
- Wu, Z.; Li, T.; Zhang, D.H.; Teng, N.J. Lily HD-Zip I Transcription Factor LlHB16 Promotes Thermotolerance by Activating LlHSFA2 and LlMBF1c. Plant Cell Physiol. 2022, 63, 1729–1744. [Google Scholar] [CrossRef]
- Wu, Z.; Li, T.; Zhang, Y.Y.; Zhang, D.H.; Teng, N.J. HD-Zip I protein LlHOX6 antagonizes homeobox protein LlHB16 to attenuate basal thermotolerance in lily. Plant Physiol. 2024, 194, 1870–1888. [Google Scholar] [CrossRef]
- Suzuki, N.; Sejima, H.; Tam, R.; Schlauch, K.; Mittler, R. Identification of the MBF1 heat-response regulon of Arabidopsis thaliana. Plant J. 2011, 66, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Bajad, S.; Shuman, J.; Shulaev, V.; Mittler, R. The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana. J. Biol. Chem. 2008, 283, 9269–9275. [Google Scholar] [CrossRef]
- Ding, L.P.; Wu, Z.; Teng, R.D.; Xu, S.J.; Cao, X.; Yuan, G.Z.; Zhang, D.H.; Teng, N.J. LlWRKY39 is involved in thermotolerance by activating LlMBF1c and interacting with LlCaM3 in lily (Lilium longiflorum). Hortic. Res. 2021, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liang, J.H.; Wang, C.P.; Zhao, X.; Zhong, X.H.; Cao, X.; Li, G.Q.; He, J.N.; Yi, M.F. Overexpression of lily HsfA3s in Arabidopsis confers increased thermotolerance and salt sensitivity via alterations in proline catabolism. J. Exp. Bot. 2018, 69, 2005–2021. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liang, J.H.; Wang, C.P.; Ding, L.P.; Zhao, X.; Cao, X.; Xu, S.J.; Teng, N.J.; Yi, M.F. Alternative Splicing Provides a Mechanism to Regulate LlHSFA3 Function in Response to Heat Stress in Lily. Plant Physiol. 2019, 181, 1651–1667. [Google Scholar] [CrossRef]
- Wu, Z.; Li, T.; Ding, L.P.; Wang, C.P.; Teng, R.D.; Xu, S.J.; Cao, X.; Teng, N.J. Lily LlHSFC2 coordinates with HSFAs to balance heat stress response and improve thermotolerance. New Phytol. 2024, 241, 2124–2142. [Google Scholar] [CrossRef]
- Medina, E.; Kim, S.-H.; Yun, M.; Choi, W.-G. Recapitulation of the Function and Role of ROS Generated in Response to Heat Stress in Plants. Plants 2021, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.P.; Zhou, Y.Z.; Yang, X.; Zhang, B.; Xu, F.X.; Wang, Y.; Song, C.X.; Yi, M.F.; Ma, N.; Zhou, X.F.; et al. The Heat Stress Transcription Factor LlHsfA4 Enhanced Basic Thermotolerance through Regulating ROS Metabolism in Lilies (Lilium Longiflorum). Int. J. Mol. Sci. 2022, 23, 572. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.J.; Zhang, S.J.; Yu, G.Z.; Chen, N.; Li, X.F.; Liu, H. Overexpression of Small Heat Shock Protein LimHSP16.45 in Arabidopsis Enhances Tolerance to Abiotic Stresses. PLoS ONE 2013, 8, e82264. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, T.; Liu, X.Y.; Yuan, G.Z.; Hou, H.Z.; Teng, N.J. A novel R2R3-MYB transcription factor LlMYB305 from Lilium longiflorum plays a positive role in thermotolerance via activating heat-protective genes. Environ. Exp. Bot. 2021, 184, 104399. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Zhu, G.S.; Guo, Q.S.; Zhu, Z.B.; Wang, C.L.; Liu, Z.Y. Cloning and expression of a new cytoplasmic small heat shock protein gene from pinellia ternata. Acta Physiol. Plant 2018, 40, 44. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, Z.Y.; Huang, Y.; Xu, J.J.; Liu, Z.; Xiang, Z.M.; Zhao, F.L.; Xue, J.P.; Xue, T.; Duan, Y.B. Functional characterization of the Pinellia ternata cytoplasmic class II small heat shock protein gene PtsHSP17.2 via promoter analysis and overexpression in tobacco. Plant Physiol. Biochem. 2022, 177, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.G.; Liu, Q.; Zhai, Y.F.; Zhao, L.M.; Zhu, J.J.; Zhang, X.D.; Jia, Q.J.; Liang, Z.S.; Wang, D.K. Genome-wide analysis of the HSP20 gene family and its response to heat and drought stress in Coix (Coix lacryma-jobi L.). BMC Genom. 2023, 24, 478. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zou, P.J.; Yan, Z.Y.; Chen, X. Identification, classification, and expression profile analysis of heat shock transcription factor gene family in Salvia miltiorrhiza. PeerJ 2022, 10, 14464. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Mishra, M.; Akhtar, N.; Gupta, P.; Mishra, P.; Tuli, R. Sterol glycosyltransferases-identification of members of gene family and their role in stress in Withania somnifera. Mol. Biol. Rep. 2012, 39, 9755–9764. [Google Scholar] [CrossRef]
- Wang, D.H.; Yao, W.; Song, Y.; Liu, W.C.; Wang, Z.Z. Molecular characterization and expression of three galactinol synthase genes that confer stress tolerance in Salvia miltiorrhiza. J. Plant Physiol. 2012, 169, 1838–1848. [Google Scholar] [CrossRef]
- Wang, X.F.; Su, J.; Yang, N.; Zhang, H.; Cao, X.Y.; Kang, J.F. Functional Characterization of Selected Universal Stress Protein from Salvia miltiorrhiza (SmUSP) in Escherichia coli. Genes 2017, 8, 224. [Google Scholar] [CrossRef]
- Zhang, T.T.; Xu, Y.; Ding, Y.D.; Yu, W.A.; Wang, J.; Lai, H.G.; Zhou, Y. Identification and Expression Analysis of WRKY Gene Family in Response to Abiotic Stress in Dendrobium catenatum. Front. Genet. 2022, 13, 800019. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Peng, W.Y.; Rong, J.B.; Zhang, M.Y.; Jia, W.H.; Lei, X.J.; Wang, Y.P. Molecular analysis of the 14-3-3 genes in Panax ginseng and their responses to heat stress. PeerJ 2023, 11, e15331. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hasanuzzaman, M.; Ahamed, K.U.; Hakeem, K.R.; Ozturk, M.; Fujita, M. Plant Responses and Tolerance to High Temperature Stress: Role of Exogenous Phytoprotectants. In Crop Production and Global Environmental Issues; Hakeem, K.R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 385–435. [Google Scholar]
- Cingoz, G.S.; Gurel, E. Effects of salicylic acid on thermotolerance and cardenolide accumulation under high temperature stress in Digitalis trojana Ivanina. Plant Physiol. Biochem. 2016, 105, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Garoosi, M.K.; Sanjarian, F.; Chaichi, M. The role of γ-aminobutyric acid and salicylic acid in heat stress tolerance under salinity conditions in Origanum vulgare L. PLoS ONE 2023, 18, e0288169. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.J.; Zhang, M.D.; Xu, J.L.; Zhou, W.X.; Cao, L.W. Transcriptomic analysis of short-term heat stress response in Pinellia ternata provided novel insights into the improved thermotolerance by spermidine and melatonin. Ecotoxicol. Environ. Saf. 2020, 202, 110877. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Rong, X.; Li, X.; Ma, Y.; Cheng, H.; Sheng, J.; Huang, L.; Jin, S. Transcriptome and Flavonoid Compounds Metabolome Analyses Reveal the Mechanisms of Heat Stress in Rhododendron with Exogenously Applied Calcium. Agron. J. 2024, 14, 1282. [Google Scholar] [CrossRef]
- Cao, X.; Sui, J.J.; Li, H.Y.; Yue, W.X.; Liu, T.; Hou, D.; Liang, J.H.; Wu, Z. Enhancing heat stress tolerance in Lanzhou lily (Lilium davidii var. unicolor) with Trichokonins isolated from Trichoderma longibrachiatum SMF2. Front. Plant Sci. 2023, 14, 1182977. [Google Scholar] [CrossRef]
- Liu, H.; Su, Y.; Fan, Y.; Zuo, D.; Xu, J.; Liu, Y.; Mei, X.; Huang, H.; Yang, M.; Zhu, S. Exogenous leucine alleviates heat stress and improves saponin synthesis in Panax notoginseng by improving antioxidant capacity and maintaining metabolic homeostasis. Front. Plant Sci. 2023, 14, 1175878. [Google Scholar] [CrossRef]
- Yang, H.; Fang, R.; Luo, L.; Yang, W.; Huang, Q.; Yang, C.; Hui, W.; Gong, W.; Wang, J. Uncovering the mechanisms of salicylic acid-mediated abiotic stress tolerance in horticultural crops. Front. Plant Sci. 2023, 14, 1226041. [Google Scholar] [CrossRef]
- Ahmad, F.; Singh, A.; Kamal, A. Salicylic Acid–Mediated Defense Mechanisms to Abiotic Stress Tolerance. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 355–369. [Google Scholar]
- Sangwan, S.; Shameem, N.; Yashveer, S.; Tanwar, H.; Parray, J.A.; Jatav, H.S.; Sharma, S.; Punia, H.; Sayyed, R.Z.; Almalki, W.H.; et al. Role of Salicylic Acid in Combating Heat Stress in Plants: Insights into Modulation of Vital Processes. Front. Biosci. 2022, 27, 310. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, L.; Liu, F.; Du, L. Spermidine-Regulated Biosynthesis of Heat-Stable Antifungal Factor (HSAF) in Lysobacter enzymogenes OH11. Front. Microbiol. 2018, 9, 2984. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Back, K. Melatonin synthesis in rice seedlings in vivo is enhanced at high temperatures and under dark conditions due to increased serotonin N-acetyltransferase and N-acetylserotonin methyltransferase activities. J. Pineal Res. 2014, 56, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.V.; Pereira Júnior, A.A.; Medeiros, D.B.; Daloso, D.M.; Pham, P.A.; Barros, K.A.; Engqvist, M.K.M.; Florian, A.; Krahnert, I.; Maurino, V.G.; et al. The influence of alternative pathways of respiration that utilize branched-chain amino acids following water shortage in Arabidopsis. Plant Cell Environ. 2016, 39, 1304–1319. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-Y.; Shen, Q.-T.; Xie, S.-T.; Chen, X.-L.; Sun, C.-Y.; Zhang, Y.-Z. Broad-spectrum antimicrobial activity and high stability of Trichokonins from Trichoderma koningii SMF2 against plant pathogens. FEMS Microbiol. Lett. 2006, 260, 119–125. [Google Scholar]
- Shaffique, S.; Khan, M.A.; Wani, S.H.; Pande, A.; Imran, M.; Kang, S.-M.; Rahim, W.; Khan, S.A.; Bhatta, D.; Kwon, E.-H.; et al. A Review on the Role of Endophytes and Plant Growth Promoting Rhizobacteria in Mitigating Heat Stress in Plants. Microorganisms 2022, 10, 1286. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, K.; Das, D.; Dey, S.; Singh, P. Microbe-mediated alleviation of heat stress in plant: Current trends and applications. In Mitigation of Plant Abiotic Stress by Microorganisms; Santoyo, G., Kumar, A., Aamir, M., Uthandi, S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 129–147. [Google Scholar]
- Lin, W.; Ye, Q.; Liang, J.; Tang, X.; Shi, J.; Liu, L.; Duan, X.; Li, X.; Wu, P.; Liu, Y.; et al. Response mechanism of interaction between Rhododendron hainanense and microorganisms to heat stress. Ind. Crops Prod. 2023, 199, 116764. [Google Scholar] [CrossRef]
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 2019, 14, 517–522. [Google Scholar] [CrossRef]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nano-Biotechnology in Agriculture: Use of Nanomaterials to Promote Plant Growth and Stress Tolerance. J. Agric. Food. Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Zhang, S.; Zhou, Y.; Xiao, X.; Cui, P.; Xu, B.; Zhao, Q.; Kong, S.; Dai, Y. Emerging biotechnology applications in natural product and synthetic pharmaceutical analyses. Acta Pharm. Sin. B 2022, 12, 4075–4097. [Google Scholar] [CrossRef]
- Canter, P.H.; Thomas, H.; Ernst, E. Bringing medicinal plants into cultivation: Opportunities and challenges for biotechnology. Trends Biotechnol. 2005, 23, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jia, M.; Guo, J. Functional Genome of Medicinal Plants. In Molecular Pharmacognosy; Huang, L.-Q., Ed.; Springer: Singapore, 2019; pp. 191–234. [Google Scholar]
| Species | Applied Technical Measures | Mode of Application | Mechanism of Heat Resistance | Reference |
|---|---|---|---|---|
| Digitalis trojana Ivanina | 150 mM Salicylic acid | Culture medium | Induced synthesis of antioxidants and cardenolides | [78] |
| Mentha-piperita L. (Mitcham variety) and Mentha arvensis L. (var. piperascens) | 30 M Melatonin, 4 mM Salicylic acid | Foliar spray | Increased antioxidant enzyme activity | [8] |
| Origanum vulgare L. | 1 mM Salicylic acid | Foliar spray | A rise in the activity of superoxide dismutase and the levels of total phenol and hydrogen peroxide | [79] |
| Salvia officinalis L. and Salvia elegans Vahl | 100 μM Salicylic acid and 5 mM CaCl2 | Soil watering | Increasing values of soil–plant analysis development, normalized difference vegetation index, and the maximal quantum yield of photosystem II photochemistry | [9] |
| Pinellia ternata | 100 μM Spermidine, 100 μM Melatonin | Foliar spray | Up-regulation of heat-responsive genes | [80] |
| Rhododendron × pulchrum | 10 mM CaCl2 | Foliar spray | Inducing the production of flavonoid compounds to regulate the antioxidant system | [81] |
| Lilium longiflorum | 20 mM CaCl2 | Apical treatment | LlCaM3 is a major component in Ca2+-CaM HS signaling pathway in lily and might be in the upstream of HSF | [10] |
| Lilium longiflorum | 2 ppm Ethylene | Seedling treatment | LlERF110 mediates HS response via regulation of LlHsfA3A expression and interaction with LlHsfA2 | [55] |
| Lilium davidii var. unicolor | 0.5, 1, 2, 4, or 8 mg/L Trichokonins | Root treatment | Inducing the HSF-HSP pathway and LzHsfA2a-1 likely plays a key role in acquisition of TKs-induced thermotolerance | [82] |
| Panax notoginseng | 3 and 5 mM leucine | Foliar spray | Enhanced the antioxidant capacity, carbohydrate metabolism, and TCA cycle metabolites | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Bao, Y.; Yang, Y.; Zhao, Q.; Li, R. Research Progress on Heat Stress Response Mechanism and Control Measures in Medicinal Plants. Int. J. Mol. Sci. 2024, 25, 8600. https://doi.org/10.3390/ijms25168600
Zhu Z, Bao Y, Yang Y, Zhao Q, Li R. Research Progress on Heat Stress Response Mechanism and Control Measures in Medicinal Plants. International Journal of Molecular Sciences. 2024; 25(16):8600. https://doi.org/10.3390/ijms25168600
Chicago/Turabian StyleZhu, Ziwei, Ying Bao, Yixi Yang, Qi Zhao, and Rui Li. 2024. "Research Progress on Heat Stress Response Mechanism and Control Measures in Medicinal Plants" International Journal of Molecular Sciences 25, no. 16: 8600. https://doi.org/10.3390/ijms25168600
APA StyleZhu, Z., Bao, Y., Yang, Y., Zhao, Q., & Li, R. (2024). Research Progress on Heat Stress Response Mechanism and Control Measures in Medicinal Plants. International Journal of Molecular Sciences, 25(16), 8600. https://doi.org/10.3390/ijms25168600






