Design of Novel Membranes for the Efficient Separation of Bee Alarm Pheromones in Portable Membrane Inlet Mass Spectrometric Systems
Abstract
1. Introduction
2. Results and Discussion
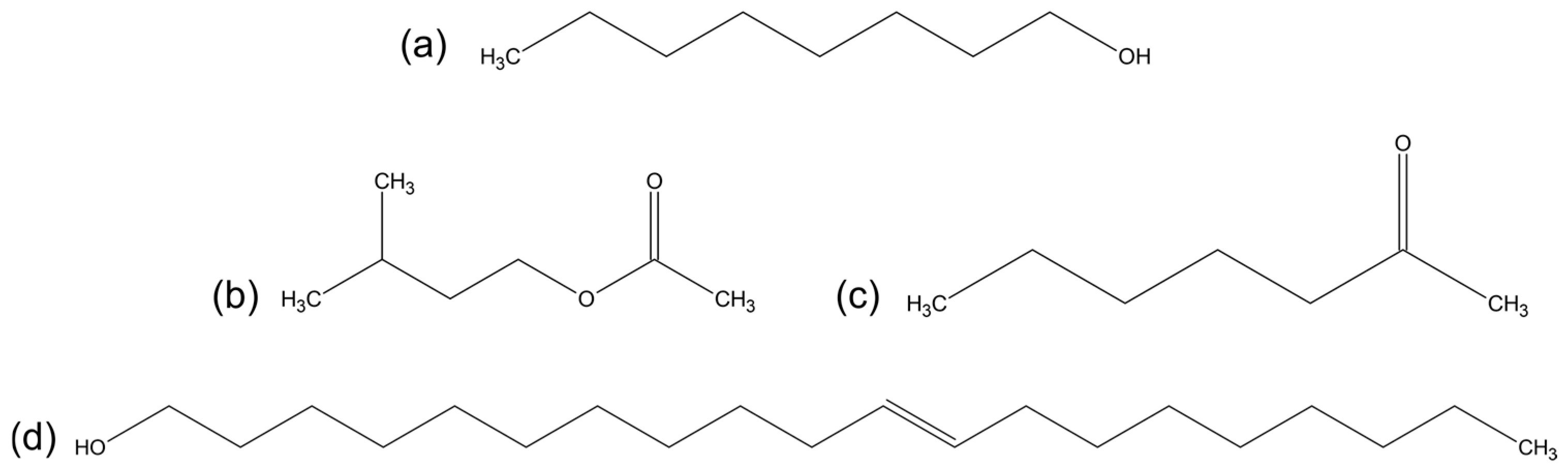
2.1. Molecular Structures and Computational Workflow
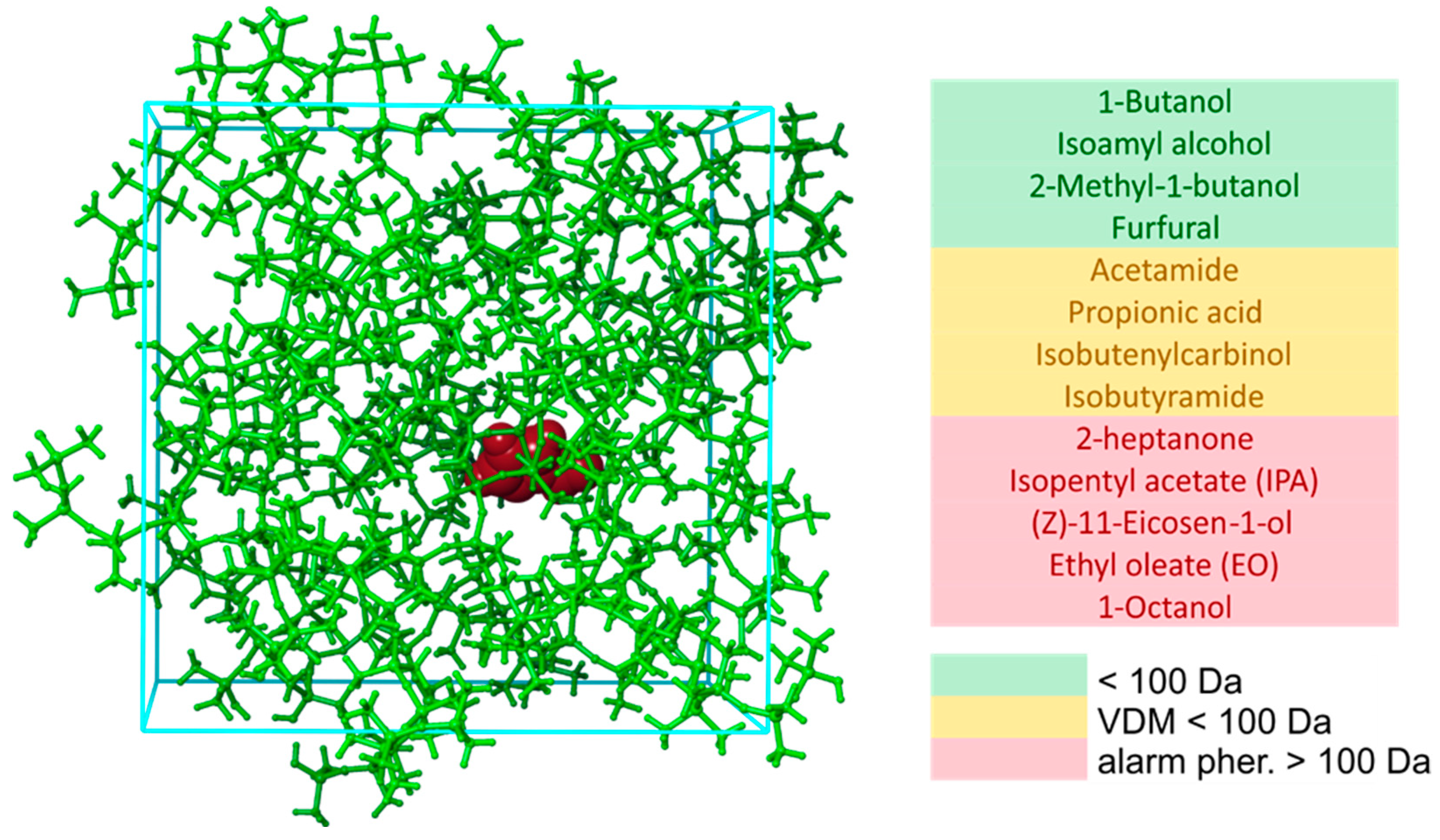
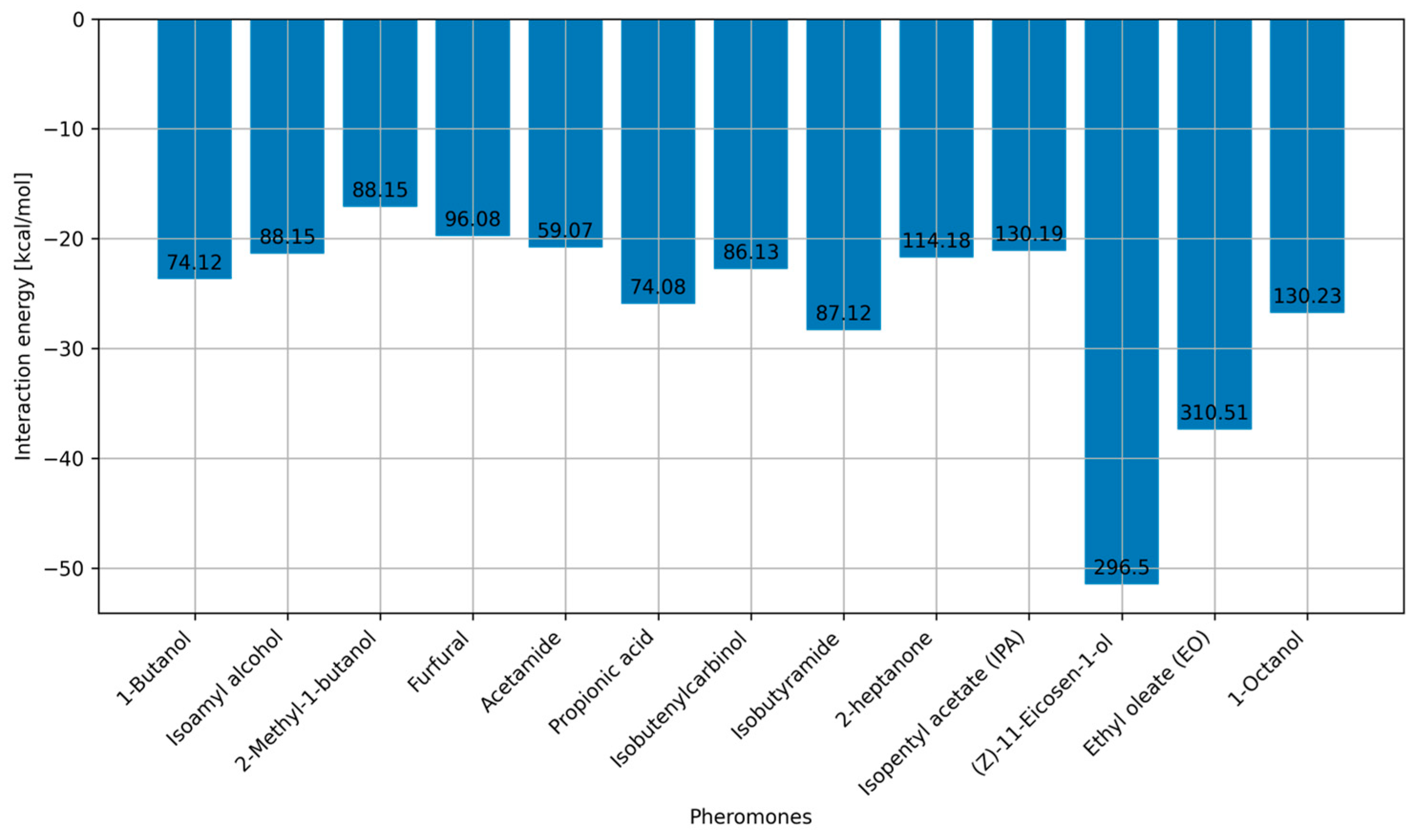
2.2. Interaction Energies via MD Simulations
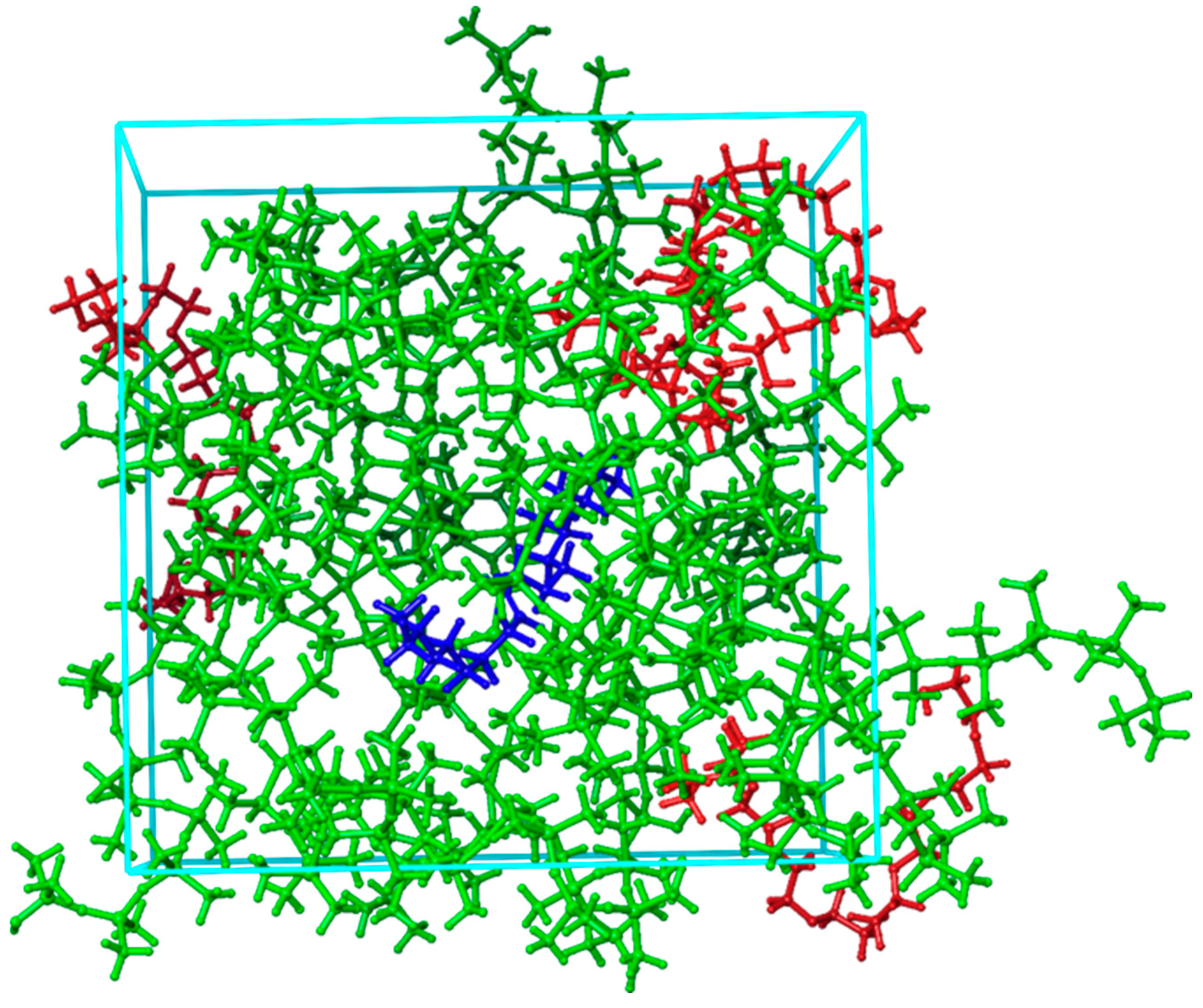
2.3. Influence of Temperature and PEG Blending to Interaction Energies
2.4. Binding Energies via First-Principles Calculations
2.5. Noncovalent Interactions
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Agudo, M.; González-Cabrera, J.; Picó, Y.; Calatayud-Vernich, P.; Urbaneja, A.; Dicke, M.; Tena, A. Neonicotinoids in Excretion Product of Phloem-Feeding Insects Kill Beneficial Insects. Proc. Natl. Acad. Sci. USA 2019, 116, 16817–16822. [Google Scholar] [CrossRef] [PubMed]
- Brodschneider, R.; Crailsheim, K. Nutrition and Health in Honey Bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Bikaun, J.M.; Bates, T.; Bollen, M.; Flematti, G.R.; Melonek, J.; Praveen, P.; Grassl, J. Volatile Biomarkers for Non-Invasive Detection of American Foulbrood, a Threat to Honey Bee Pollination Services. Sci. Total Environ. 2022, 845, 157123. [Google Scholar] [CrossRef] [PubMed]
- Slessor, K.N.; Winston, M.L.; Le Conte, Y. Pheromone Communication in the Honeybee (Apis mellifera L.). J. Chem. Ecol. 2005, 31, 2731–2745. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.E. Pheromones in Action. BioScience 1999, 49, 154–156. [Google Scholar] [CrossRef][Green Version]
- Laurencelle, N.M. Determination of Components of the Honey Bee Queen (Apis mellifera L.) Mandibular Gland Pheromone as Fluorescent Pyrenyl Ester Derivatives. Available online: https://summit.sfu.ca/item/6765 (accessed on 15 June 2024).
- Abd El-Wahed, A.A.; Farag, M.A.; Eraqi, W.A.; Mersal, G.A.M.; Zhao, C.; Khalifa, S.A.M.; El-Seedi, H.R. Unravelling the Beehive Air Volatiles Profile as Analysed via Solid-Phase Microextraction (SPME) and Chemometrics. J. King Saud Univ.-Sci. 2021, 33, 101449. [Google Scholar] [CrossRef]
- Jakšić, M.; Mihajlović, A.; Vujić, D.; Giannoukos, S.; Brkić, B. Membrane Inlet Mass Spectrometry Method for Food Intake Impact Assessment on Specific Volatile Organic Compounds in Exhaled Breath. Anal. Bioanal. Chem. 2022, 414, 6077–6091. [Google Scholar] [CrossRef] [PubMed]
- Krogh, E.T.; Gill, C.G. Membrane Introduction Mass Spectrometry (MIMS): A Versatile Tool for Direct, Real-Time Chemical Measurements. J. Mass Spectrom. 2014, 49, 1205–1213. [Google Scholar] [CrossRef]
- Ketola, R.A.; Kotiaho, T.; Cisper, M.E.; Allen, T.M. Environmental Applications of Membrane Introduction Mass Spectrometry. J. Mass Spectrom. 2002, 37, 457–476. [Google Scholar] [CrossRef]
- Ilić, D.; Brkić, B.; Sekulić, M.T. Biomonitoring: Developing a Beehive Air Volatiles Profile as an Indicator of Environmental Contamination Using a Sustainable In-Field Technique. Sustainability 2024, 16, 1713. [Google Scholar] [CrossRef]
- Wong, G.K.S.; Ng, S.J.; Webster, R.D. Quantitative Analysis of Atmospheric Volatile Organic Pollutants by Thermal Desorption Gas Chromatography Mass Spectrometry. Anal. Methods 2012, 5, 219–230. [Google Scholar] [CrossRef]
- Xu, X.; Stee, L.L.P.; Williams, J.; Beens, J.; Adahchour, M.; Vreuls, R.J.J.; Brinkman, U.A.; Lelieveld, J. Comprehensive Two-Dimensional Gas Chromatography (GC × GC) Measurements of Volatile Organic Compounds in the Atmosphere. Atmos. Chem. Phys. 2003, 3, 665–682. [Google Scholar] [CrossRef]
- Aleksić, M.; Simeon, A.; Vujić, D.; Giannoukos, S.; Brkić, B. Food and Lifestyle Impact on Breath VOCs Using Portable Mass Spectrometer—Pilot Study across European Countries. J. Breath Res. 2023, 17, 046004. [Google Scholar] [CrossRef]
- Mazurek, A.H.; Szeleszczuk, Ł.; Pisklak, D.M. Periodic DFT Calculations—Review of Applications in the Pharmaceutical Sciences. Pharmaceutics 2020, 12, 415. [Google Scholar] [CrossRef] [PubMed]
- Makkar, P.; Nath Ghosh, N. A Review on the Use of DFT for the Prediction of the Properties of Nanomaterials. RSC Adv. 2021, 11, 27897–27924. [Google Scholar] [CrossRef] [PubMed]
- Schleder, G.R.; Padilha, A.C.M.; Acosta, C.M.; Costa, M.; Fazzio, A. From DFT to Machine Learning: Recent Approaches to Materials Science—A Review. J. Phys. Mater. 2019, 2, 032001. [Google Scholar] [CrossRef]
- Burke, K.; Wagner, L.O. DFT in a Nutshell. Int. J. Quantum Chem. 2013, 113, 96–101. [Google Scholar] [CrossRef]
- Hansson, T.; Oostenbrink, C.; van Gunsteren, W. Molecular Dynamics Simulations. Curr. Opin. Struct. Biol. 2002, 12, 190–196. [Google Scholar] [CrossRef]
- Hospital, A.; Goñi, J.R.; Orozco, M.; Gelpí, J.L. Molecular Dynamics Simulations: Advances and Applications. Adv. Appl. Bioinform. Chem. 2015, 8, 37–47. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.; Dixit, A.R. A Review on the Mechanical and Thermal Properties of Graphene and Graphene-Based Polymer Nanocomposites: Understanding of Modelling and MD Simulation. Mol. Simul. 2020, 46, 136–154. [Google Scholar] [CrossRef]
- Krishna, S.; Sreedhar, I.; Patel, C.M. Molecular Dynamics Simulation of Polyamide-Based Materials—A Review. Comput. Mater. Sci. 2021, 200, 110853. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef]
- Sumithra, M.; Sundaraganesan, N.; Rajesh, R.; Vetrivelan, V.; Ilangovan, V.; Javed, S.; Muthu, S. Electron Acceptor, Excitation Energies, Oscillatory Strength, Spectroscopic and Solvent Effects on 5-Amino-4,6-Dichloro-2-(Propylthio) Pyrimidine—Anticancer Agent. Chem. Phys. Impact 2023, 6, 100145. [Google Scholar] [CrossRef]
- Sumithra, M.; Sundaraganesan, N.; Venkata Prasad, K.; Rajesh, R.; Vetrivelan, V.; Ilangovan, V.; Irfan, A.; Muthu, S. Effect of Green Solvents Physical, Chemical, Biological and Bonding Nature on 5-Acetyl-Thiophene-2-Carboxylic Acid by DFT and TD-DFT Approach—An Antiviral Agent. J. Indian Chem. Soc. 2023, 100, 100867. [Google Scholar] [CrossRef]
- Al-Otaibi, J.S.; Mary, Y.S.; Mary, Y.S.; Thomas, R. Evidence of Cluster Formation of Pyrrole with Mixed Silver Metal Clusters, Agx-My (x = 4,5, y = 2/1 and M = Au/Ni/Cu) Using DFT/SERS Analysis. Comput. Theor. Chem. 2022, 1208, 113569. [Google Scholar] [CrossRef]
- Haruna, K.; Kumar, V.S.; Armaković, S.J.; Armaković, S.; Mary, Y.S.; Thomas, R.; Popoola, S.A.; Almohammedi, A.R.; Roxy, M.S.; Al-Saadi, A.A. Spectral Characterization, Thermochemical Studies, Periodic SAPT Calculations and Detailed Quantum Mechanical Profiling Various Physico-Chemical Properties of 3,4-Dichlorodiuron. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117580. [Google Scholar] [CrossRef]
- Bielenica, A.; Beegum, S.; Mary, Y.S.; Mary, Y.S.; Thomas, R.; Armaković, S.; Armaković, S.J.; Madeddu, S.; Struga, M.; Van Alsenoy, C. Experimental and Computational Analysis of 1-(4-Chloro-3-Nitrophenyl)-3-(3,4-Dichlorophenyl) Thiourea. J. Mol. Struct. 2020, 1205, 127587. [Google Scholar] [CrossRef]
- Pooventhiran, T.; Thomas, R.; Bhattacharyya, U.; Sowrirajan, S.; Irfan, A.; Rao, D.J. Structural Aspects, Reactivity Analysis, Wavefunction Based Properties, Cluster Formation with Helicene and Subsequent Detection from Surface Enhancement in Raman Spectra of Triclabendazole Studies Using First Principle Simulations. Vietnam J. Chem. 2021, 59, 887–901. [Google Scholar] [CrossRef]
- Armaković, S.; Vujić, Đ.; Brkić, B. A Computational Study of Polydimethylsiloxane Derivatives as a Semi-Permeable Membrane for in-Field Identification of Naphthenic Acids in Water Using Portable Mass Spectrometry. J. Mol. Liq. 2022, 351, 118657. [Google Scholar] [CrossRef]
- Apaolaza, A.; Richard, D.; Tejerina, M.R. Experimental and Ab Initio Study of the Structural and Optical Properties of ZnO Coatings: Performance of the DFT+ U Approach. Process. Appl. Ceram. 2020, 14, 362–371. [Google Scholar] [CrossRef]
- Janani, S.; Rajagopal, H.; Sakthivel, S.; Aayisha, S.; Raja, M.; Irfan, A.; Javed, S.; Muthu, S. Molecular Structure, Electronic Properties, ESP Map (Polar Aprotic and Polar Protic Solvents), and Topology Investigations on 1-(tert-Butoxycarbonyl)-3-Piperidinecarboxylic Acid- Anticancer Therapeutic Agent. J. Mol. Struct. 2022, 1268, 133696. [Google Scholar] [CrossRef]
- Muthu, S.; Uma Maheswari, J. Quantum Mechanical Study and Spectroscopic (FT-IR, FT-Raman, 13C, 1H, UV) Study, First Order Hyperpolarizability, NBO Analysis, HOMO and LUMO Analysis of 4-[(4-Aminobenzene) Sulfonyl] Aniline by Ab Initio HF and Density Functional Method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 92, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Tonel, M.Z.; González-Durruthy, M.; Zanella, I.; Fagan, S.B. Interactions of Graphene Derivatives with Glutamate-Neurotransmitter: A Parallel First Principles—Docking Investigation. J. Mol. Graph. Model. 2019, 88, 121–127. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.V.; Zanella, I.; Bulhões, L.O.S.; Fagan, S.B. Adsorption of 17 β- Estradiol in Graphene Oxide through the Competing Methanol Co-Solvent: Experimental and Computational Analysis. J. Mol. Liq. 2021, 321, 114738. [Google Scholar] [CrossRef]
- da Bruckmann S., F.; Zuchetto, T.; Ledur, C.M.; dos Santos, C.L.; da Silva, W.L.; Binotto Fagan, S.; Zanella da Silva, I.; Bohn Rhoden, C.R. Methylphenidate Adsorption onto Graphene Derivatives: Theory and Experiment. New J. Chem. 2022, 46, 4283–4291. [Google Scholar] [CrossRef]
- Murthy, P.K.; Valverde, C.; Suneetha, V.; Armaković, S.; Armaković, S.J.; Rani, N.U.; Naidu, N.V. An Analysis of Structural and Spectroscopic Signatures, the Reactivity Study of Synthetized 4,6-Dichloro-2-(Methylsulfonyl)Pyrimidine: A Potential Third-Order Nonlinear Optical Material. J. Mol. Struct. 2019, 1186, 263–275. [Google Scholar] [CrossRef]
- Murthy, P.K.; Sheena Mary, Y.; Shyma Mary, Y.; Panicker, C.Y.; Suneetha, V.; Armaković, S.; Armaković, S.J.; Van Alsenoy, C.; Suchetan, P.A. Synthesis, Crystal Structure Analysis, Spectral Investigations, DFT Computations and Molecular Dynamics and Docking Study of 4-Benzyl-5-Oxomorpholine-3-Carbamide, a Potential Bioactive Agent. J. Mol. Struct. 2017, 1134, 25–39. [Google Scholar] [CrossRef]
- Stepulane, A.; Rajasekharan, A.K.; Andersson, M. Multifunctional Surface Modification of PDMS for Antibacterial Contact Killing and Drug-Delivery of Polar, Nonpolar, and Amphiphilic Drugs. ACS Appl. Bio Mater. 2022, 5, 5289–5301. [Google Scholar] [CrossRef]
- Diachun, N.A.; Marcus, A.H.; Hussey, D.M.; Fayer, M.D. Dynamics in Polydimethylsiloxane: The Effect of Solute Polarity. J. Am. Chem. Soc. 1994, 116, 1027–1032. [Google Scholar] [CrossRef]
- Asandulesa, M.; Musteata, V.E.; Bele, A.; Dascalu, M.; Bronnikov, S.; Racles, C. Molecular Dynamics of Polysiloxane Polar-Nonpolar Co-Networks and Blends Studied by Dielectric Relaxation Spectroscopy. Polymer 2018, 149, 73–84. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, P.; Yang, X.; Wang, X. Novel PEG Functionalized Graphene Nanosheets: Enhancement of Dispersibility and Thermal Stability. Nanoscale 2011, 3, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Mongin, C.; Golden, J.H.; Castellano, F.N. Liquid PEG Polymers Containing Antioxidants: A Versatile Platform for Studying Oxygen-Sensitive Photochemical Processes. ACS Appl. Mater. Interfaces 2016, 8, 24038–24048. [Google Scholar] [CrossRef]
- Bowers, K.J.; Chow, D.E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. In Proceedings of the SC’06: Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, Tampa, FL, USA, 11–17 November 2006; p. 43. [Google Scholar]
- Bochevarov, A.D.; Harder, E.; Hughes, T.F.; Greenwood, J.R.; Braden, D.A.; Philipp, D.M.; Rinaldo, D.; Halls, M.D.; Zhang, J.; Friesner, R.A. Jaguar: A High-performance Quantum Chemistry Software Program with Strengths in Life and Materials Sciences. Int. J. Quantum Chem. 2013, 113, 2110–2142. [Google Scholar] [CrossRef]
- Jacobson, L.D.; Bochevarov, A.D.; Watson, M.A.; Hughes, T.F.; Rinaldo, D.; Ehrlich, S.; Steinbrecher, T.B.; Vaitheeswaran, S.; Philipp, D.M.; Halls, M.D. Automated Transition State Search and Its Application to Diverse Types of Organic Reactions. J. Chem. Theory Comput. 2017, 13, 5780–5797. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2021-4: Desmond Molecular Dynamics System, D.E. Shaw Research, New York, NY, 2021. Maestro-Desmond Interoperability Tools; Schrödinger: New York, NY, USA, 2021.
- Schrödinger Release 2021-4: Jaguar; Schrödinger, LLC: New York, NY, USA, 2021.
- Jorgensen, W.L.; Tirado-Rives, J. The OPLS [Optimized Potentials for Liquid Simulations] Potential Functions for Proteins, Energy Minimizations for Crystals of Cyclic Peptides and Crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Shivakumar, D.; Williams, J.; Wu, Y.; Damm, W.; Shelley, J.; Sherman, W. Prediction of Absolute Solvation Free Energies Using Molecular Dynamics Free Energy Perturbation and the OPLS Force Field. J. Chem. Theory Comput. 2010, 6, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef]
- Ehlert, S.; Stahn, M.; Spicher, S.; Grimme, S. Robust and Efficient Implicit Solvation Model for Fast Semiempirical Methods. J. Chem. Theory Comput. 2021, 17, 4250–4261. [Google Scholar] [CrossRef]
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended Tight-Binding Quantum Chemistry Methods. WIREs Comput. Mol. Sci. 2021, 11, e1493. [Google Scholar] [CrossRef]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A Robust and Accurate Tight-Binding Quantum Chemical Method for Structures, Vibrational Frequencies, and Noncovalent Interactions of Large Molecular Systems Parametrized for All Spd-Block Elements (Z = 1–86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate Spin-Dependent Electron Liquid Correlation Energies for Local Spin Density Calculations: A Critical Analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Valero, R.; Costa, R.; de P. R. Moreira, I.; Truhlar, D.G.; Illas, F. Performance of the M06 Family of Exchange-Correlation Functionals for Predicting Magnetic Coupling in Organic and Inorganic Molecules. J. Chem. Phys. 2008, 128, 114103. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, D.; Perpète, E.A.; Ciofini, I.; Adamo, C.; Valero, R.; Zhao, Y.; Truhlar, D.G. On the Performances of the M06 Family of Density Functionals for Electronic Excitation Energies. J. Chem. Theory Comput. 2010, 6, 2071–2085. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Armaković, S.; Armaković, S.J. Atomistica.Online—Web Application for Generating Input Files for ORCA Molecular Modelling Package Made with the Anvil Platform. Mol. Simul. 2023, 49, 117–123. [Google Scholar] [CrossRef]
- Armaković, S.; Armaković, S.J. Online and Desktop Graphical User Interfaces for Xtb Programme from Atomistica.Online Platform. Mol. Simul. 2024, 50, 560–570. [Google Scholar] [CrossRef]
- Cao, Y.; Halls, M.D.; Vadicherla, T.R.; Friesner, R.A. Pseudospectral Implementations of Long-Range Corrected Density Functional Theory. J. Comput. Chem. 2021, 42, 2089–2102. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Balduf, T.; Beachy, M.D.; Bennett, M.C.; Bochevarov, A.D.; Chien, A.; Dub, P.A.; Dyall, K.G.; Furness, J.W.; Halls, M.D.; et al. Quantum Chemical Package Jaguar: A Survey of Recent Developments and Unique Features. J. Chem. Phys. 2024, 161, 052502. [Google Scholar] [CrossRef]
- Cao, Y.; Hughes, T.; Giesen, D.; Halls, M.D.; Goldberg, A.; Vadicherla, T.R.; Sastry, M.; Patel, B.; Sherman, W.; Weisman, A.L.; et al. Highly Efficient Implementation of Pseudospectral Time-Dependent Density-Functional Theory for the Calculation of Excitation Energies of Large Molecules. J. Comput. Chem. 2016, 37, 1425–1441. [Google Scholar] [CrossRef]


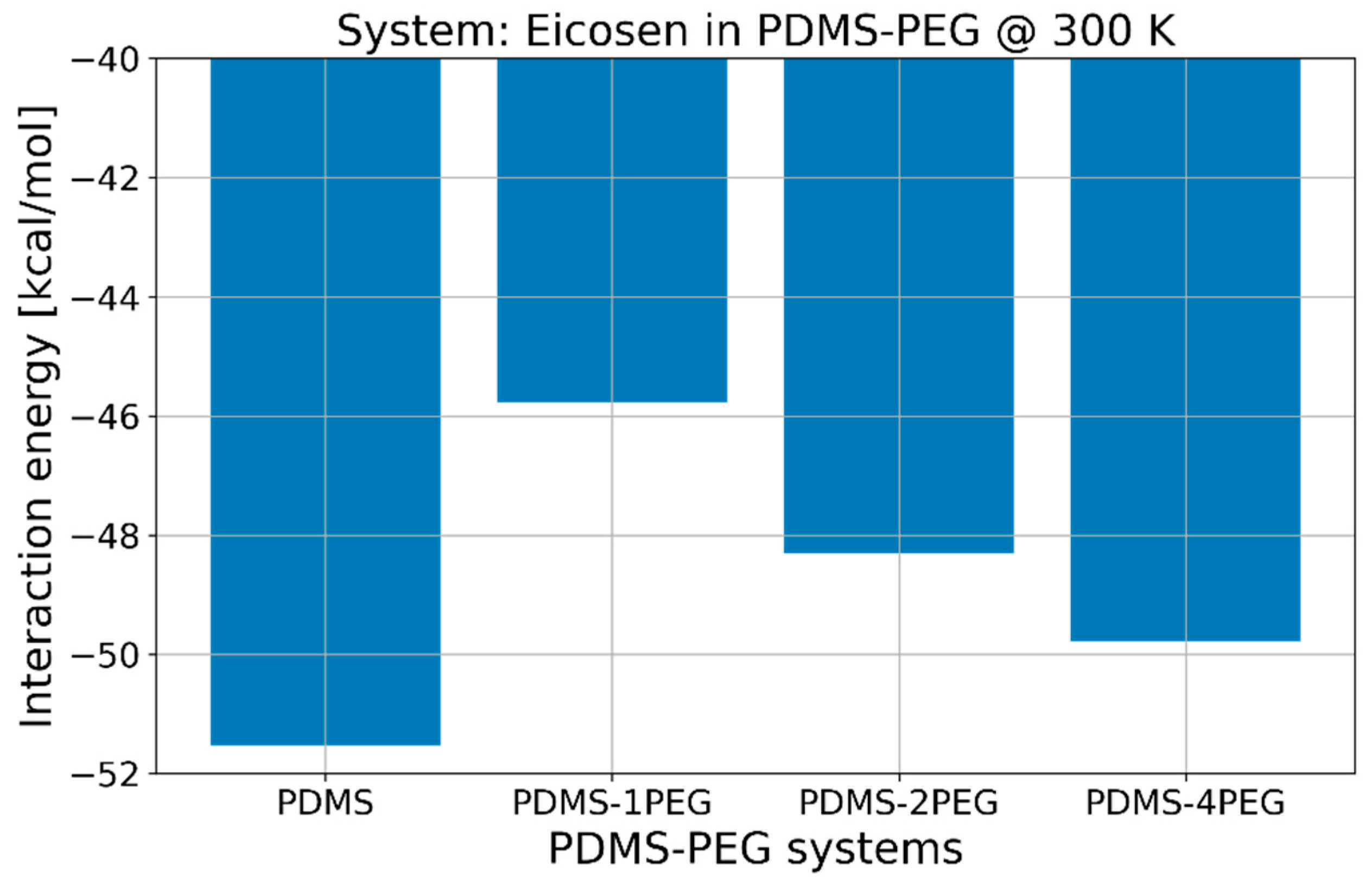
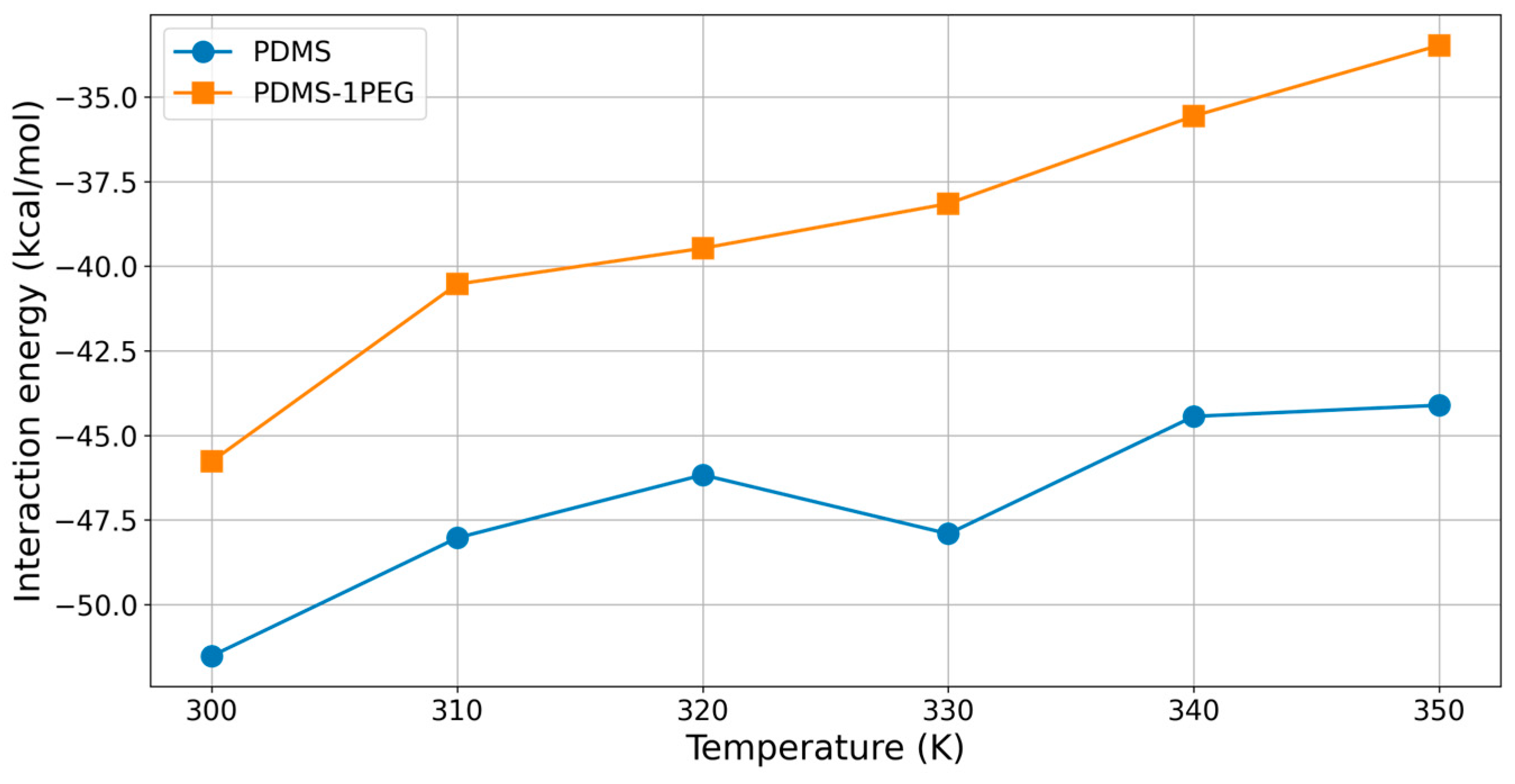
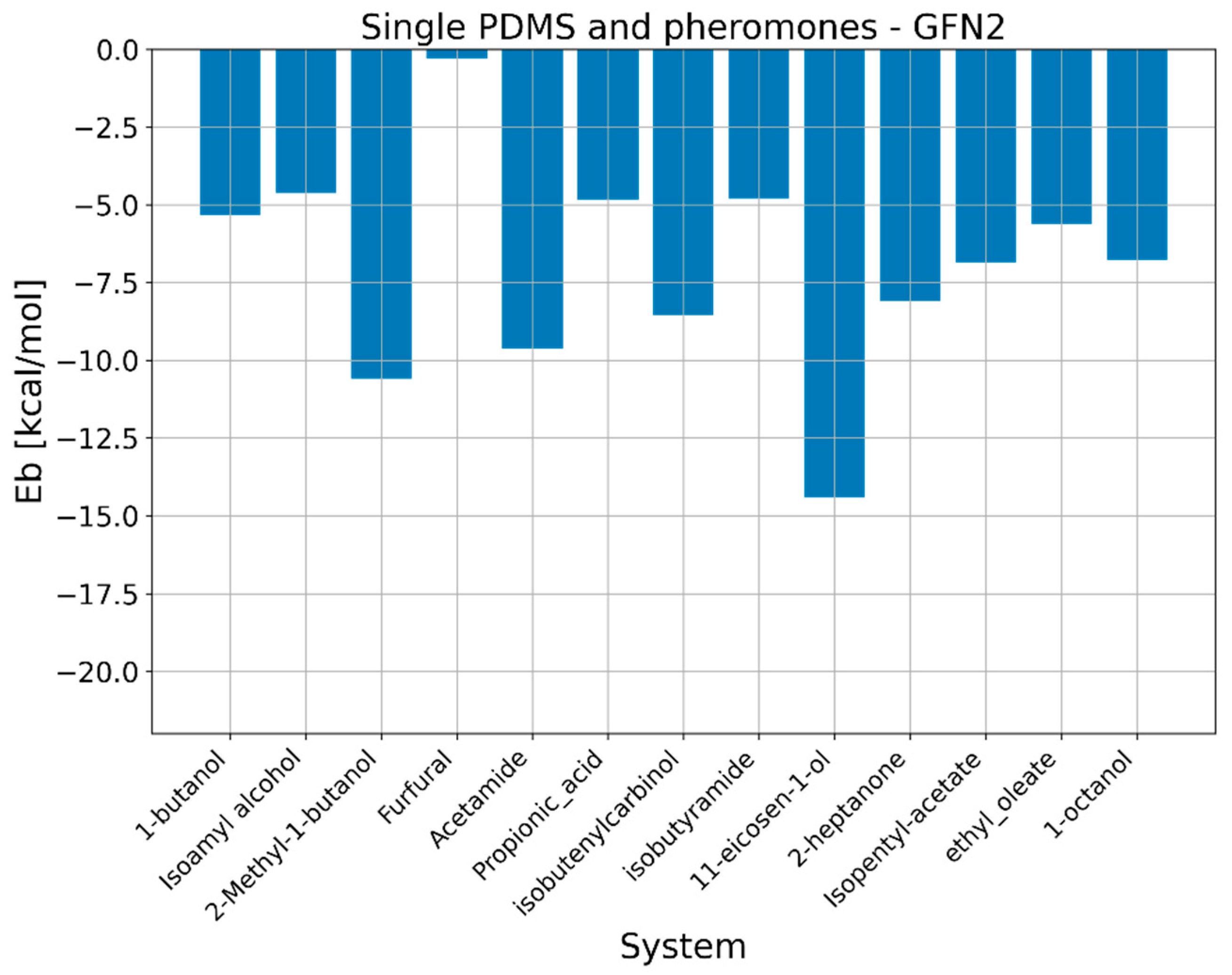


| Temperature [K] | Eint [kcal/mol] |
|---|---|
| 300 | −51.52 |
| 310 | −48.03 |
| 320 | −46.17 |
| 330 | −47.91 |
| 340 | −44.44 |
| 350 | −44.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armaković, S.; Ilić, D.; Brkić, B. Design of Novel Membranes for the Efficient Separation of Bee Alarm Pheromones in Portable Membrane Inlet Mass Spectrometric Systems. Int. J. Mol. Sci. 2024, 25, 8599. https://doi.org/10.3390/ijms25168599
Armaković S, Ilić D, Brkić B. Design of Novel Membranes for the Efficient Separation of Bee Alarm Pheromones in Portable Membrane Inlet Mass Spectrometric Systems. International Journal of Molecular Sciences. 2024; 25(16):8599. https://doi.org/10.3390/ijms25168599
Chicago/Turabian StyleArmaković, Stevan, Daria Ilić, and Boris Brkić. 2024. "Design of Novel Membranes for the Efficient Separation of Bee Alarm Pheromones in Portable Membrane Inlet Mass Spectrometric Systems" International Journal of Molecular Sciences 25, no. 16: 8599. https://doi.org/10.3390/ijms25168599
APA StyleArmaković, S., Ilić, D., & Brkić, B. (2024). Design of Novel Membranes for the Efficient Separation of Bee Alarm Pheromones in Portable Membrane Inlet Mass Spectrometric Systems. International Journal of Molecular Sciences, 25(16), 8599. https://doi.org/10.3390/ijms25168599







