Involvement of Expression of miR33-5p and ABCA1 in Human Peripheral Blood Mononuclear Cells in Coronary Artery Disease
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Population
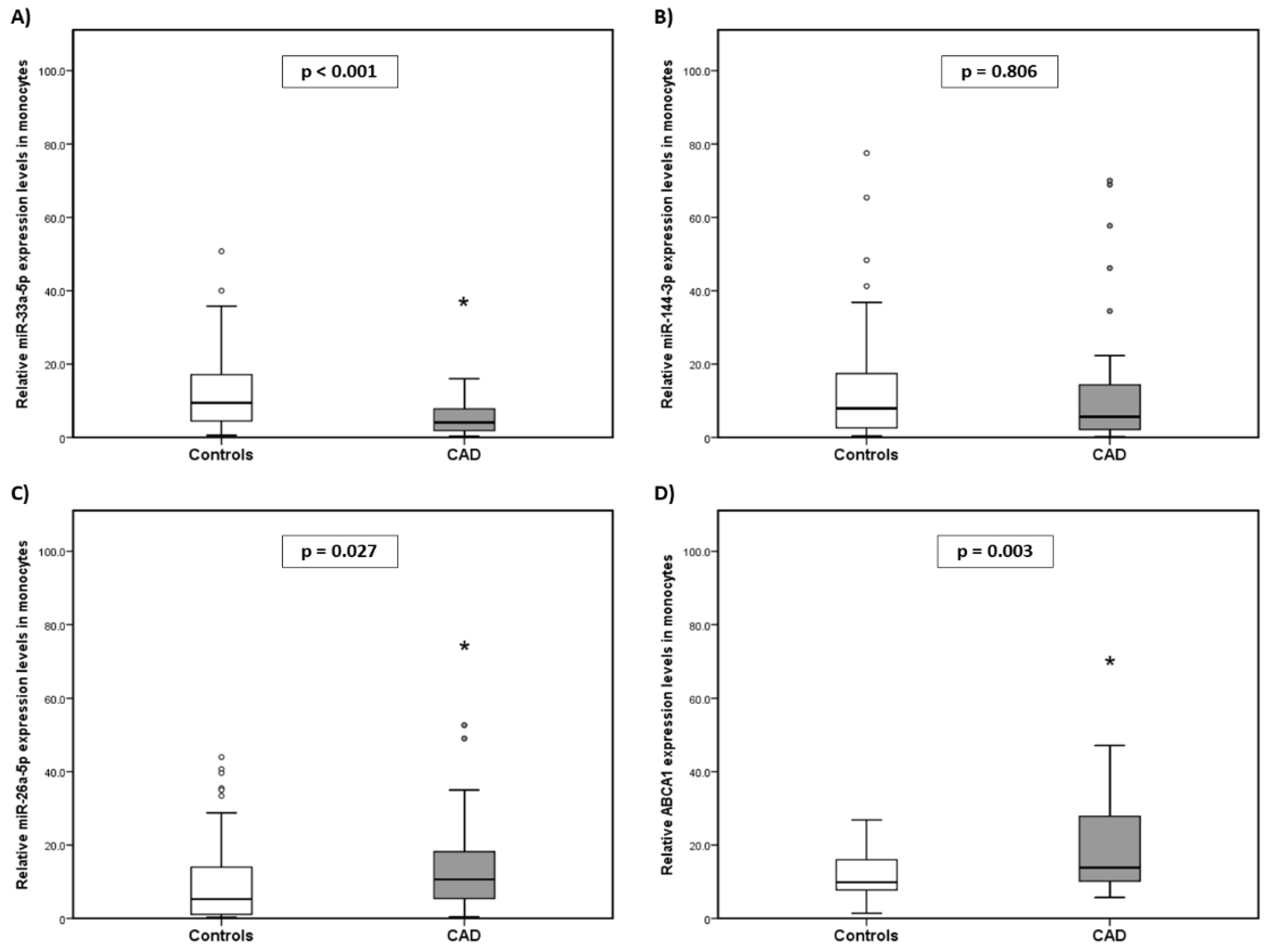
2.2. Expression Levels of miR-33a-5p, miR-144-3p, miR-26a-5p, and ABCA1 in the Monocytes of the Subjects
2.3. Association of Clinical Parameters and miR/ABCA1 Expression with the Risk of Developing CAD
2.4. miR/ABCA1 Expression with the Use of Drugs
2.5. miR-33a-5p Expression Cut-Off Points
2.6. Efflux Cholesterol in CAD
2.7. Relationship between miRs/ABCA1 and Efflux Cholesterol
3. Discussion
Limitations
4. Materials and Methods
4.1. Patient Population
4.2. Arterial Intima-Media Measurement
4.3. Blood and Plasma Samples
4.4. Laboratory Analysis
4.5. Isolation of PBMCs and RNA Extraction
4.6. miR Reverse Transcription and Quantitative PCR
4.7. mRNA Reverse Transcription and Quantitative PCR
4.8. Cholesterol Efflux Capacity
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Słomka, A.; Piekuś, A.; Kowalewski, M.; Pawliszak, W.; Anisimowicz, L.; Żekanowska, E. Assessment of the Procoagulant Activity of Microparticles and the Protein Z System in Patients Undergoing Off-Pump Coronary Artery Bypass Surgery. Angiology 2018, 69, 347–357. [Google Scholar] [CrossRef]
- Mirzaei, H.; Ferns, G.A.; Avan, A.; Mobarhan, M.G. Cytokines and MicroRNA in Coronary Artery Disease. Adv. Clin. Chem. 2017, 82, 47–70. [Google Scholar] [CrossRef]
- Velázquez, M.O.; Barinagarrementería, A.F.S.; Rubio, G.A.F.; Verdejo, J.; Méndez, B.M.A.; Violante, R.; Pavía, A.; Alvarado-Ruiz, R.; Lara, E.A. Morbidity and mortality by ischemic heart disease and stroke in Mexico. Arch. Cardiol. Mex. 2007, 77, 31–39. [Google Scholar]
- Badimón, L.; Martínez-González, J. Disfunción endotelial. Rev. Española Cardiol. Supl. 2006, 6, 21A–30A. [Google Scholar] [CrossRef]
- Pou, J.; Rebollo, A.; Alegret, M. El monocito/macrófago como diana terapéutica en la aterosclerosis. Clín. Investig. Arterioscler. 2007, 19, 92–108. [Google Scholar] [CrossRef]
- Ono, K.; Horie, T.; Nishino, T.; Baba, O.; Kuwabara, Y.; Kimura, T. MicroRNAs and high-density lipoprotein cholesterol metabolism. Int. Heart J. 2015, 56, 365–371. [Google Scholar] [CrossRef]
- Torres-Paz, Y.E.; Gamboa, R.; Fuentevilla-Álvarez, G.; Soto, M.E.; González-Moyotl, N.; Martínez-Alvarado, R.; Torres-Tamayo, M.; Ramírez-Marroquín, E.S.; Vásquez-Jiménez, X.; Sainz-Escarrega, V.; et al. Overexpression of microRNA-21-5p and microRNA-221-5p in Monocytes Increases the Risk of Developing Coronary Artery Disease. Int. J. Mol. Sci. 2023, 24, 8641. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Matute, J.; Rotllan, N.; Aranda, J.F.; Fernández-Hernando, C. MicroRNAs and atherosclerosis. Curr. Atheroscler. Rep. 2013, 15, 322. [Google Scholar] [CrossRef]
- Yang, Z.; Cappello, T.; Wang, L. Emerging role of microRNAs in lipid metabolism. Acta Pharm. Sin. B 2015, 5, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Ono, K.; Horiguchi, M.; Nishi, H.; Nakamura, T.; Nagao, K.; Kinoshita, M.; Kuwabara, Y.; Marusawa, H.; Iwanaga, Y.; et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 17321–17326. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Horie, T.; Nishino, T.; Baba, O.; Kuwabara, Y.; Yokode, M.; Kita, T.; Kimura, T. MicroRNA-33a/b in lipid metabolism—Novel “thrifty” models. Circ. J. 2015, 79, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Ono, K. Functions of microRNA-33a/b and microRNA therapeutics. J. Cardiol. 2016, 67, 28–33. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, J.; Xie, J.; Wei, W.; Chen, M.; Zhao, X. MiR-26 controls LXR-dependent cholesterol efflux by targeting ABCA1 and ARL7. FEBS Lett. 2012, 586, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, C.M.; Rotllan, N.; Vlassov, A.V.; Dávalos, A.; Li, M.; Goedeke, L.; Aranda, J.F.; Cirera-Salinas, D.; Araldi, E.; Salerno, A.; et al. Control of cholesterol metabolism and plasma high-density lipoprotein levels by microRNA-144. Circ. Res. 2013, 112, 1592–1601. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.W.; Hu, Y.R.; Zhao, J.Y.; Li, S.F.; Ma, X.; Wu, S.G.; Lu, J.B.; Qiu, Y.R.; Sha, Y.H.; Wang, Y.C.; et al. An agomir of miR-144-3p accelerates plaque formation through impairing reverse cholesterol transport and promoting pro-inflammatory cytokine production. PLoS ONE 2014, 9, e94997. [Google Scholar] [CrossRef] [PubMed]
- Singaraja, R.R.; Fievet, C.; Castro, G.; James, E.R.; Hennuyer, N.; Clee, S.M.; Bissada, N.; Choy, J.C.; Fruchart, J.C.; McManus, B.M.; et al. Increased ABCA1 activity protects against atherosclerosis. J. Clin. Investig. 2002, 110, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Rayner, K.J.; Sheedy, F.J.; Esau, C.C.; Hussain, F.N.; Temel, R.E.; Parathath, S.; van Gils, J.M.; Rayner, A.J.; Chang, A.N.; Suarez, Y.; et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J. Clin. Investig. 2011, 121, 2921–2931. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar Vallim, T.Q.; Tarling, E.J.; Kim, T.; Civelek, M.; Baldán, Á.; Esau, C.; Edwards, P.A. MicroRNA-144 regulates hepatic ATP binding cassette transporter A1 and plasma high-density lipoprotein after activation of the nuclear receptor farnesoid X receptor. Circ. Res. 2013, 112, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.J.F.; Biessen, E.A.L.; Hohl, M.; Weber, C.; van der Vorst, E.P.C.; Santovito, D. Small Things Matter: Relevance of MicroRNAs in Cardiovascular Disease. Front. Physiol. 2020, 11, 793. [Google Scholar] [CrossRef] [PubMed]
- Hromadnikova, I.; Kotlabova, K.; Hympanova, L.; Krofta, L. Cardiovascular and cerebrovascular disease associated microRNAs are dysregulated in placental tissues affected with gestational hypertension, preeclampsia and intrauterine growth restriction. PLoS ONE 2015, 10, e0138383. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, T.; Hirata, R.D.C.; Hirata, M.H.; Cerda, Á.; Salazar, L.A. Statins differentially modulate microRNAs expression in peripheral cells of hyperlipidemic subjects: A pilot study. Eur. J. Pharm. Sci. 2018, 117, 55–61. [Google Scholar] [CrossRef]
- Argmann, C.A.; Edwards, J.Y.; Sawyez, C.G.; O’Neil, C.H.; Hegele, R.A.; Pickering, J.G.; Huff, M.W. Regulation of macrophage cholesterol efflux through hydroxymethylglutaryl-CoA reductase inhibition: A role for RhoA in ABCA1-mediated cholesterol efflux. J. Biol. Chem. 2005, 280, 22212–22221. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Ren, J.; Song, J.; Zhang, F.; Zhang, J.; Lee, C.; Li, S.; Geng, Q.; Cao, C.; et al. Effects of statin on circulating microRNAome and predicted function regulatory network in patients with unstable angina. BMC Med. Genom. 2015, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cao, C.; Chen, H.; Song, J.; Lee, C.; Zhang, J.; Zhang, F.; Geng, Q.; Li, Z.; Li, J. Atheroprotective effects of statins in patients with unstable angina by regulating the blood-borne microRNA network. Mol. Med. Rep. 2017, 16, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Cerda, A.; Hirata, M.H.; Hirata, R.D. Molecular mechanisms underlying statin effects on genes involved in the reverse cholesterol transport. Drug Metabol. Drug Interact. 2012, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H. Rho-kinase as a novel therapeutic target in treatment of cardiovascular diseases. J. Cardiovasc. Pharmacol. 2002, 39, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, C.P.; Baffic, J.; Lam, M.H.; Lund, E.G.; Adams, A.; Fu, X.; Hayes, N.; Jones, A.B.; Macnaul, K.L.; Ondeyka, J.; et al. A potent synthetic LXR agonist is more effective than cholesterol loading at inducing ABCA1 mRNA and stimulating cholesterol efflux. J. Biol. Chem. 2012, 277, 10021–10027. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Demler, O.V.; Adelman, S.J.; Collins, H.L.; Glynn, R.J.; Ridker, P.M.; Rader, D.J.; Mora, S. Cholesterol efflux capacity, high-density lipoprotein particle number, and incident cardiovascular events: An analysis from the JUPITER trial (justification for the use of statins in prevention: An intervention trial evaluating rosuvastatin). Circulation 2017, 135, 2494–2504. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Tang, W.H.; Mosior, M.K.; Huang, Y.; Wu, Y.; Matter, W.; Gao, V.; Schmitt, D.; Didonato, J.A.; Fisher, E.A.; et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1696–1705. [Google Scholar] [CrossRef]
- Saleheen, D.; Scott, R.; Javad, S.; Zhao, W.; Rodrigues, A.; Picataggi, A.; Lukmanova, D.; Mucksavage, M.L.; Luben, R.; Billheimer, J.; et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: A prospective case-control study. Lancet Diabetes Endocrinol. 2015, 3, 507–513. [Google Scholar] [CrossRef]
- Rye, K.-A.; Barter, P.J. Cardioprotective functions of HDLs. J. Lipid Res. 2014, 55, 168–179. [Google Scholar] [CrossRef]
- Du, X.M.; Kim, M.J.; Hou, L.; Le Goff, W.; Chapman, M.J.; Van Eck, M.; Curtiss, L.K.; Burnett, J.R.; Cartland, S.P.; Quinn, C.M.; et al. HDL Particle Size is a Critical Determinant of ABCA1-Mediated Macrophage Cellular Cholesterol Export. Circ. Res. 2015, 116, 1133–1142. [Google Scholar] [CrossRef]
- Wang, X.; Collins, H.L.; Ranalletta, M.; Fuki, I.V.; Billheimer, J.T.; Rothblat, G.H.; Tall, A.R.; Rader, D.J. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Investig. 2007, 117, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- De Wood, M.A.; Spores, J.; Notske, R.; Mouser, L.T.; Burroughs, R.; Golden, M.S.; Lang, H.T. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N. Engl. J. Med. 1980, 303, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Xiang, C. Design and implementation of an AUDIT trail in compliance with US regulations. Clin. Trials. 2011, 8, 624–633. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levi, R.I.; Fredrickson, D.S. Estimation of concentration of low-density lipoproteins cholesterol in plasma without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- De Long, D.A.; De Long, E.R.; Weed, P.D. A comparison of methods for the estimation of plasma low- and very low-density lipoprotein cholesterol. The Lipid Research Clinics Prevalence Study. J. Am. Med. Assoc. 1986, 256, 2372–2377. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCq method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Miyakawa, Y.; Kagaya, K.; Watanabe, K.; Fukazawa, Y. Characteristics of macrophage activation by gamma interferon for tumor cytotoxicity in peritoneal macrophages and macrophage cell line J774.1. Microbiol. Immunol. 1989, 33, 1027–1038. [Google Scholar] [CrossRef]

| Variable | Controls (n = 50) | CAD (n = 50) | p |
|---|---|---|---|
| Age (years) | 48.67 ± 6.47 | 62.45 ± 13.00 | <0.001 |
| Sex % M/W | 60.7%/39.3% | 86.0%/14.0% | 0.002 |
| BMI (kg/m2) | 27.75 ± 3.78 | 26.54 ± 4.00 | 0.968 |
| Total cholesterol (mg/dL) | 171.06 ± 24.47 | 134.50 ± 47.05 | <0.001 |
| HDL-C (mg/dL) | 45.80 ± 14.75 | 31.07 ± 8.23 | <0.001 |
| LDL-C (mg/dL) | 102.86 ± 23.17 | 88.43 ± 51.00 | 0.069 |
| Triglycerides (mg/dL) | 131.48 ± 49.86 | 140.43 ± 60.77 | 0.411 |
| Statins % | 0% | 62.0% | <0.001 |
| Glucose (mg/dL) | 93.76 ± 7.82 | 117.83 ± 43.23 | <0.001 |
| Diabetes % | 0% | 41.0% | <0.001 |
| Hypoglycemic agents % | 0% | 20.0% | <0.001 |
| SBP (mmHg) | 112.43 ± 9.12 | 126.31 ± 20.76 | <0.001 |
| DBP (mmHg) | 70.43 ± 6.01 | 79.59 ± 12.66 | <0.001 |
| Hypertension % | 0% | 64.0% | <0.001 |
| Antihypertensive % | 0% | 48.0% | <0.001 |
| Smoking % | 9.5% | 12.0% | 0.532 |
| Alcoholism % | 0% | 2.0% | <0.001 |
| cIMT | 0.6026 | - | - |
| χ2 | OR | CI 95% | p | |
|---|---|---|---|---|
| Age | 6.177 | 1.088 | 1.018–1.163 | 0.013 |
| HDL-C (mg/dL) | 8.863 | 1.180 | 1.058–1.314 | 0.003 |
| SBP (mmHg) | 5.951 | 1.071 | 1.014–1.133 | 0.015 |
| miR-33a-5p | 4.139 | 0.135 | 0.020–0.929 | 0.042 |
| miR-26a-5p | 3.149 | 3.105 | 0.888–10.853 | 0.076 |
| ABCA1 | 10.975 | 0.060 | 0.011–0.318 | 0.001 |
| Controls | CAD Non-Medicated Group with Atorvastatin | p1 | CAD Medicated Group with Atorvastatin | p2 | p3 | |
| n = 50 | n = 19 | n = 31 | ||||
| miR-33a-5p | 9.43 (0.61–50.75) | 3.44 (1.20–15.63) | 0.001 | 5.06 (0.31–15.98) | <0.001 | 0.169 |
| miR-26a-5p | 5.26 (0.23–43.95) | 11.51 (1.13–19.46) | 0.131 | 8.70 (0.41–52.66) | 0.117 | 0.876 |
| miR-144-3p | 7.95 (0.41–77.53) | 5.15 (0.21–57.70) | 0.354 | 9.07 (0.50–70.00) | 0.557 | 0.284 |
| ABCA1 | 9.84 (1.44–26.85) | 11.26 (6.49–14.36) | 0.812 | 18.35 (6.58–47.08) | 0.001 | 0.011 |
| Controls | CAD non-medicated group with metformin | p1 | CAD medicated group with metformin | p2 | p3 | |
| n = 50 | n = 40 | n = 10 | ||||
| miR-33a-5p | 9.42 (0.61–50.75) | 5.04 (0.30–15.98) | 0.001 | 3.90 (0.84–12.60) | 0.001 | 0.767 |
| miR-26a-5p | 5.26 (0.23–43.95) | 9.75 (1.07–52.66) | 0.029 | 4.91 (1.13–34.96) | 0.851 | 0.092 |
| miR-144-3p | 7.95 (0.41–77.53) | 5.58 (0.50–68.93) | 0.726 | 6.03 (0.21–70.00) | 0.931 | 0.840 |
| ABCA1 | 14.04 (0.74–78.72) | 7.98 (0.91–33.15) | 0.036 | 4.26 (2.63–21.63) | 0.050 | 0.089 |
| χ2 | OR | IC 95% | p | |
|---|---|---|---|---|
| Cholesterol efflux | 5.71 | 1.52 | 1.13–2.77 | 0.017 |
| χ2 | OR | IC 95% | p | |
|---|---|---|---|---|
| Statins | 4.555 | 2.659 | 1.083–6.528 | 0.033 |
| Controls | CAD | CAD Non-Medicated Group with Atorvastatin | CAD Medicated Group with Atorvastatin | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | R | p | |
| miR-33a-5p | −0.357 | 0.019 | 0.005 | 0.976 | −0.022 | 0.947 | −0.157 | 0.444 |
| miR-26a-5p | −0.592 | <0.001 | −0.047 | 0.758 | −0.190 | 0.555 | 0.093 | 0.643 |
| miR-144-3p | −0.625 | <0.001 | −0.107 | 0.505 | −0.124 | 0.701 | −0.162 | 0.471 |
| ABCA1 | 0.316 | 0.042 | −0.008 | 0.962 | 0.362 | 0.248 | −0.016 | 0.941 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Paz, Y.E.; Gamboa, R.; Fuentevilla-Álvarez, G.; Cardoso-Saldaña, G.; Martínez-Alvarado, R.; Soto, M.E.; Huesca-Gómez, C. Involvement of Expression of miR33-5p and ABCA1 in Human Peripheral Blood Mononuclear Cells in Coronary Artery Disease. Int. J. Mol. Sci. 2024, 25, 8605. https://doi.org/10.3390/ijms25168605
Torres-Paz YE, Gamboa R, Fuentevilla-Álvarez G, Cardoso-Saldaña G, Martínez-Alvarado R, Soto ME, Huesca-Gómez C. Involvement of Expression of miR33-5p and ABCA1 in Human Peripheral Blood Mononuclear Cells in Coronary Artery Disease. International Journal of Molecular Sciences. 2024; 25(16):8605. https://doi.org/10.3390/ijms25168605
Chicago/Turabian StyleTorres-Paz, Yazmín Estela, Ricardo Gamboa, Giovanny Fuentevilla-Álvarez, Guillermo Cardoso-Saldaña, Rocío Martínez-Alvarado, María Elena Soto, and Claudia Huesca-Gómez. 2024. "Involvement of Expression of miR33-5p and ABCA1 in Human Peripheral Blood Mononuclear Cells in Coronary Artery Disease" International Journal of Molecular Sciences 25, no. 16: 8605. https://doi.org/10.3390/ijms25168605
APA StyleTorres-Paz, Y. E., Gamboa, R., Fuentevilla-Álvarez, G., Cardoso-Saldaña, G., Martínez-Alvarado, R., Soto, M. E., & Huesca-Gómez, C. (2024). Involvement of Expression of miR33-5p and ABCA1 in Human Peripheral Blood Mononuclear Cells in Coronary Artery Disease. International Journal of Molecular Sciences, 25(16), 8605. https://doi.org/10.3390/ijms25168605






