Abstract
The combination of high and low LET radionuclides has been tested in several patient studies to improve treatment response. Radionuclide mixtures can also be released in nuclear power plant accidents or nuclear bomb deployment. This study investigated the DNA damage response and DNA double-strand break (DSB) repair in peripheral blood mononuclear cells (PBMCs) after internal exposure of blood samples of 10 healthy volunteers to either no radiation (baseline) or different radionuclide mixtures of the α- and β-emitters [223Ra]RaCl2 and [177Lu]LuCl3, i.e., 25 mGy/75 mGy, 50 mGy/50 mGy and 75 mGy/25 mGy, respectively. DSB foci and γ-H2AX α-track enumeration directly after 1 h of exposure or after 4 h or 24 h of repair revealed that radiation-induced foci (RIF) and α-track induction in 100 cells was similar for mixed α/β and pure internal α- or β-irradiation, as were the repair rates for all radiation qualities. In contrast, the fraction of unrepaired RIF (Qβ) in PBMCs after mixed α/β-irradiation (50% 223Ra & 50% 177Lu: Qβ = 0.23 ± 0.10) was significantly elevated relative to pure β-irradiation (50 mGy: Qβ, pure = 0.06 ± 0.02), with a similar trend being noted for all mixtures. This α-dose-dependent increase in persistent foci likely relates to the formation of complex DNA damage that remains difficult to repair.
1. Introduction
For therapeutic applications in nuclear medicine mostly β-emitting radiopharmaceuticals are currently administered to treat cancer while there is an increasing use of radiopharmaceuticals labelled with α-emitting radionuclides [1,2]. The approval of the first and only α-emitter [223Ra]RaCl2 (Xofigo®, Bayer, Germany) [3] resulted in unprecedented levels of interest and investment in other α-radionuclides, such as 227Th, 225Ac, 213Bi, 212Pb/212Bi and 211At, which are currently undergoing clinical trials for the treatment of a wide range of cancer types [4,5]. In particular, prostate-specific membrane antigen (PSMA) ligands labelled with 225Ac have shown remarkable therapeutic efficacy with complete radiological response in patients with metastatic prostate cancer, even when 177Lu-labelled analogues had failed [6,7]. These are promising treatments which take advantage of the high linear energy transfer (LET) and the relative biological effectiveness (RBE) of α-particles [8], which are known to induce lethal DNA damage in the targeted cancer cells whilst limiting the damage to nearby healthy cells, as compared to β-emitting radionuclides [9].
Furthermore, a growing number of publications address the use of combined α- and β-emitters to enhance the efficacy of radionuclide therapy. For example, Khreish et al. describe the first patients treated with tandem therapy of [225Ac]Ac-PSMA-617 and [177Lu]Lu-PSMA-617 [10]. Other studies and an ongoing clinical trial [11,12] highlight the growing interest in combining different forms of radiation for therapeutic purposes [7,13,14,15]. As the hematopoietic system is one of the organs at risk during radionuclide therapy [16,17], it is of interest to study the effects of internal mixed irradiation with α- and β-emitters.
In addition, nuclear power plant accidents or nuclear detonations are known to release various short-and long-lived α-, β- and γ-emitting radionuclide mixtures, including radionuclides like 60Co, 90Sr, 131I, 137Cs, 192Ir and 241Am [18,19]. The release of such radionuclide mixtures bears the danger of incorporation into exposed humans and other organisms through inhalation or wounds [20,21]. It is thus of interest to learn more about the effects of internal irradiation with radionuclide mixtures [20], especially with respect to DNA damages and biodosimetry applications.
Ionizing radiation causes damages to various biological molecules in the hit cells, of which cellular DNA double-strand breaks (DSB) are of particular importance as their misrepair may lead to mutational changes or cell death, especially at high acute doses [22,23,24]. The DNA damage response deals with the detection and repair of DSBs and involves a succession of detector, signaling and repair molecules [25]. Among the latter, DSB-dependent phosphorylation of histone H2AX (then termed γ-H2AX) [26] and the effector molecule 53BP1 [27] have proven useful as focal markers of DSBs in cell nuclei, leading to their widespread application in biodosimetry, e.g., [28,29,30,31]. Especially, peripheral blood mononuclear cells (PBMCs) are easily obtained and have thus been increasingly used to assess DNA damage response in various research applications as well as prostate cancer patients treated with α- or β-particle emitting radiopharmaceuticals [28,32,33]. So far, the DSB damage response in patients following radiopharmaceutical therapy with mixed types of internal irradiations remains unexplored, leaving a lack of information on the combined effects after internal α- and β-irradiation of PBMCs in vivo. Therefore, the aim of our study was to investigate the induction and repair of DNA damage after simultaneous internal ex vivo irradiation of PBMCs with a combination of an α- and a β-emitter in an absorbed dose range comparable to patient treatments [28,32,33,34].
2. Results
2.1. Dosimetry
Absorbed doses of the blood samples of 10 healthy donors were calculated using the measured activity values and the published dose coefficients for internal blood irradiation in a test tube for one hour [35], as described in more detail in the Materials and Methods, Section 4. The total absorbed dose reported is the sum of the absorbed dose delivered by the α-particles (α-dose) and the absorbed dose by β-particle irradiation (β-dose). The combination of the α-dose and the β-dose was set to reach a total absorbed dose of 100 mGy (total absorbed dose = α-dose + β-dose). Hereafter, we refer to 223Ra and 177Lu when [223Ra]RaCl2 and [177Lu]LuCl3 were used in the solutions.
One hour of internal irradiation with the different α/β mixing ratios resulted in mean α-doses in the PBMCs of the ten volunteers of 25.3 ± 0.7 mGy, 50.6 ± 1.8 mGy and 76.5 ± 2.5 mGy, and the corresponding mean β-doses of 75.7 ± 3.1 mGy, 51.3 ± 2.7 mGy and 26.7 ± 1.2 mGy for the different α/β-mixing ratios were achieved (Table 1; Section 4.1).

Table 1.
Ratios of the different α-doses and β-doses to the blood with corresponding average number of α-tracks in 100 cells and the average number of RIF per cell for the time points d: direct fixation after irradiation, 4 h: 4 h after irradiation and 24 h: 24 h after irradiation. In each case the mean value (including minimum and maximum value) is given.
2.2. RIF Induction
The induction of DSB-indicating foci (γ-H2AX + 53BP1 co-localizing foci) was determined in PBMCs for 25%, 50% and 75% mixtures of the α-emitter 223Ra and the β-emitter 177Lu (Table 1; see Section 4.1), with the radionuclides mixed such that the total nominal absorbed dose after 1 h of ex vivo internal irradiation of blood samples was 100 mGy. This absorbed dose to the blood was chosen as it reflects a value seen in radionuclide therapies [28,32,33,34]. The internal ex vivo irradiation of blood with the different 223Ra/177Lu radionuclide mixtures induced, directly after 1 h of irradiation, on average, 0.7 ± 0.2 (25% β-dose) to 1.0 ± 0.3 (75% β-dose) radiation-induced DSB foci (RIF) per cell (Table 1; see Section 4.2). All foci and RIF values were normally distributed.
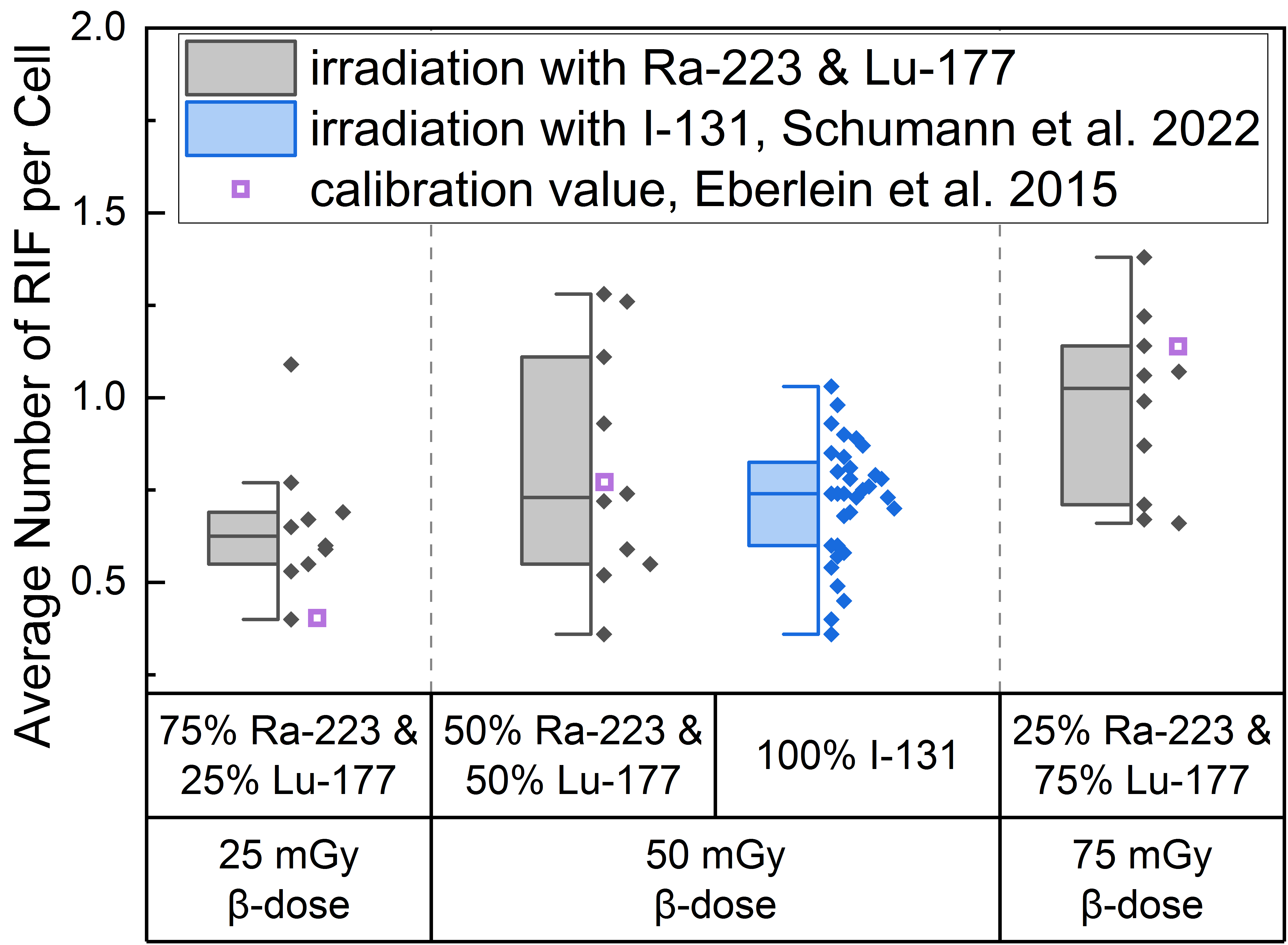
The average number of RIF per cell values induced by the different α/β-mixing ratios were similar to values obtained with pure 131I β-irradiation (average RIF per cell value of 0.72 ± 0.16 for 50 mGy, Schumann et al. [36]) directly after irradiation (time point d), as shown in the boxplot of Figure 1. In addition, an average RIF value for an internal pure low LET irradiation were calculated for each β-dose using the β-emitter calibration curve of Eberlein et al. [37] and is shown in Figure 1 as a purple open square symbol to indicate any differences between the mixed α/β-irradiation and the pure β-irradiation.
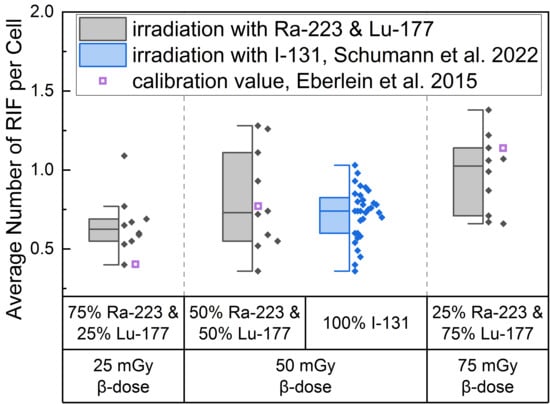
Figure 1.
Boxplot of average number of RIF per cell directly after 1 h irradiation for the different α/β-mixing ratios as well as the pure 131I irradiation [36]. In the mixed irradiations there were β-doses of 25 mGy, 50 mGy and 75 mGy, while the α-dose added up to a total absorbed dose of 100 mGy in each mixture. The average number of RIF per cell obtained by mixed irradiation is displayed in grey, the pure 131I irradiated (Schumann et al. [36]) in blue and the values taken from the calibration curve by Eberlein et al. [37] as purple open square. Significant differences were only noted for the average number of RIF per cell of 75% 223Ra and 25% 177Lu compared to 25% 223Ra and 75% 177Lu (paired sample t-test, p < 0.05). The average number of RIF per cell of 50% 223Ra and 50% 177Lu in comparison to the pure 50 mGy 131I irradiation were similar (two-sample independent t-test with Welch correction, p > 0.05).
This approach revealed that the calculated average value for 25 mGy pure β-irradiation was at the lower end of the RIF data points after mixed α/β-irradiation (Figure 1). For 50 mGy it was similar to the average of the mixed α/β-irradiation RIF values and for 75 mGy the RIF average of the pure irradiation was at the upper end of the mixed data range. The comparison of the mixed samples of 50 mGy β-dose with the 50 mGy pure 131I irradiation [36] revealed similar RIF values with less scatter in the RIF values obtained by pure β-irradiation (Figure 1, center). The values determined using the β-calibration curve (purple open squares) were within the range of the mixed irradiation.
A significant increase in the average number of DBS foci per cell as a function of the β-dose was observed for all d-samples obtained directly after irradiation relative to the pre-exposure baseline average number of foci per cell. When comparing the RIF values of the d-samples of the different α/β-mixing ratio values, a significant difference was found between the group of 25% 223Ra and 75% 177Lu and 75% 223Ra and 25% 177Lu, reflecting the sufficiently large increase in the β-dose between these two points.
2.3. α-Track Induction
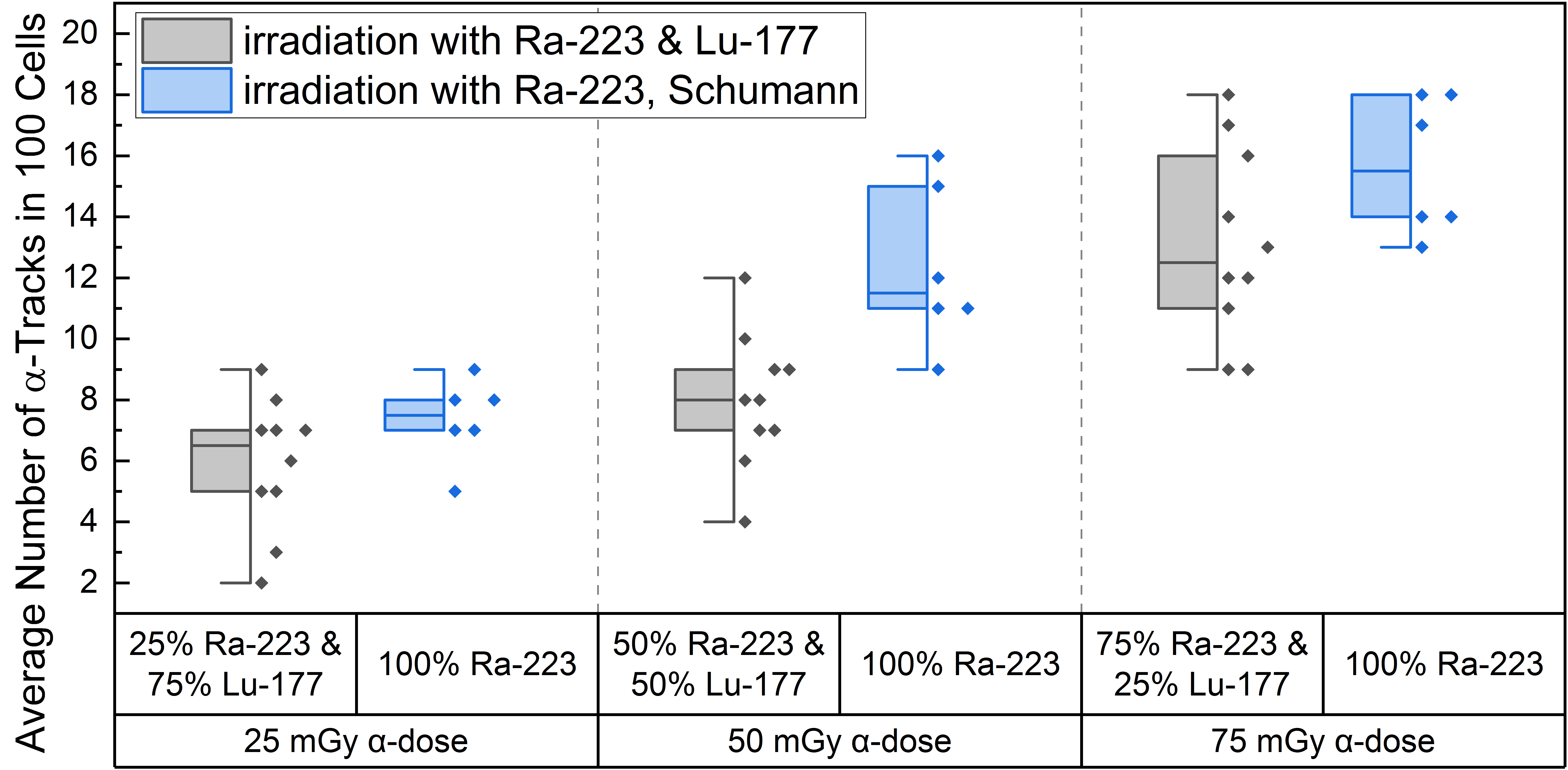
High LET irradiation induces, in particle traversed nuclei, so-called γ-H2AX-positive α-tracks [38,39,40] that partly co-localize with 53BP1 [41,42]. Internal α/β-irradiation of PBMCs with the different radionuclide mixtures induced an average number of γ-H2AX-positive α-tracks in 100 cells ranging from 5.9 ± 2.2 (25% α-dose) to 13.1 ± 3.1 (75% α-dose), as shown in Table 1. The comparison of the induced number of α-tracks in 100 cells for each mixing ratio at the time point d directly after 1 h of internal irradiation with the pure α-irradiation data of Schumann et al. [38] (Figure 2) revealed statistical difference for the 50 mGy α-dose pure 223Ra irradiation and the 50 mGy α-dose obtained by the mixed 50% 223Ra and 50% 177Lu irradiation. Similar values were obtained for the 25 mGy and 75 mGy α-doses in the mixtures and pure α-irradiation (Figure 2), with the 75% α-dose likely delivering enough α-particles to reach a similar value to pure α-irradiation and the former being too low yielding only rare events that fail to deliver a statistical difference.
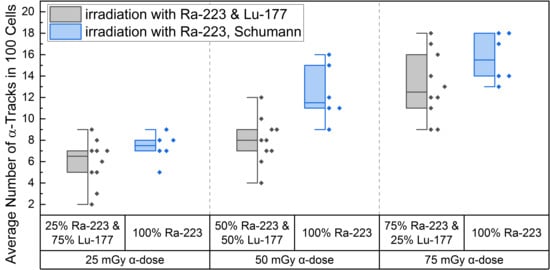
Figure 2.
Boxplot of the number of α-tracks in 100 cells directly after irradiation for the different α/β-mixing ratios as well as the pure 223Ra irradiation. In the mixed irradiations there were α-doses of 25 mGy, 50 mGy and 75 mGy, while the β-dose added up to a total absorbed dose of 100 mGy in each mixture. The number of α-tracks in 100 cells obtained by mixed irradiation is displayed in grey, the pure 223Ra irradiated number of α-tracks in 100 cells by Schumann et al. [38] are shown in blue. The number of α-tracks in 100 cells of 25% 223Ra and 75% 177Lu compared to 50% 223Ra and 50% 177Lu showed no significant differences (paired sample t-test, p > 0.05). Only the number of α-tracks in 100 cells of 75% 223Ra and 25% 177Lu compared to 25% 223Ra and 75% 177Lu and 75% 223Ra and 25% 177Lu compared to 50% 223Ra and 50% 177Lu were significantly different (paired sample t-test, p < 0.05). Comparing the α-track values of pure 223Ra irradiation for an α-dose of 25 mGy, 50 mGy and 75 mGy with the values obtained by different α/β-mixing ratios, only the 50% 223Ra and 50% 177Lu (50 mGy α-dose) compared to the obtained α-track values with pure 50 mGy α-dose irradiation were significantly different (two-sample independent t-test, p < 0.05).
A significant increase in α-tracks in 100 cells was observed for all irradiated d-samples compared to the pre-exposure baseline α-track values. As the number of α-tracks in 100 cells for the directly irradiated samples are normally distributed, the t-test was used for comparison. Comparing the α-track values in 100 cells of the different mixtures, revealed that all were significantly different among each other except between 25% 223Ra and 75% 177Lu and 50% 223Ra and 50% 177Lu, possibly because of a wider range of data points in the mixed irradiation and/or an insufficient increase in the α-dose contribution. The latter is supported by the fact that an increase from 25 mGy to 75 mGy α-dose led to a significant increase in α-track frequency after mixed irradiation (Figure 2).
2.4. DNA Damage Repair of Focal DSB Damage: RIF
An analysis of the repair rates “Rβ” (see Equation (1) in Section 4.3) for the RIF per cell values over time revealed an Rβ value of (0.25 ± 0.12) h−1 for the combination 75% 223Ra and 25% 177Lu, (0.29 ± 0.13) h−1 for an equal mixture of 50% 223Ra and 50% 177Lu and (0.31 ± 0.09) h−1 for the combination of 25% 223Ra and 75% 177Lu. Within the margin of error, the repair rates between the different α/β-mixing ratios are similar. The values of the fractions of unrepaired RIF “Qβ” (see Equation (1)) were (0.35 ± 0.10) h−1 for 75% 223Ra and 25% 177Lu, (0.23 ± 0.10) h−1 for 50% 223Ra and 50% 177Lu and (0.14 ± 0.07) h−1 for 25% 223Ra and 75% 177Lu. The Qβ values increased with an increasing fraction of 223Ra, suggesting an accumulation of unrepaired RIF at a higher proportion of 223Ra at late time points (see below). Table 2 shows the fit parameters of the pooled data points of the different α/β-mixtures.

Table 2.
Fit parameters of the RIF DNA damage repair model and percentage of persisting RIF per cell as a function of the repair time. The fits were performed without weighting.
When comparing the mixed irradiation induced average RIF values per cell between different time points (d, 4 h and 24 h), a significant difference is observed between time points d and 4 h (paired sample t-test, β-doses 25 mGy: p < 0.01, β-doses 50 mGy: p < 0.01) and d and 24 h (paired sample t-test, β-doses 25 mGy: p < 0.01, β-doses 50 mGy: p < 0.01) for the β-doses of 25 mGy and 50 mGy.
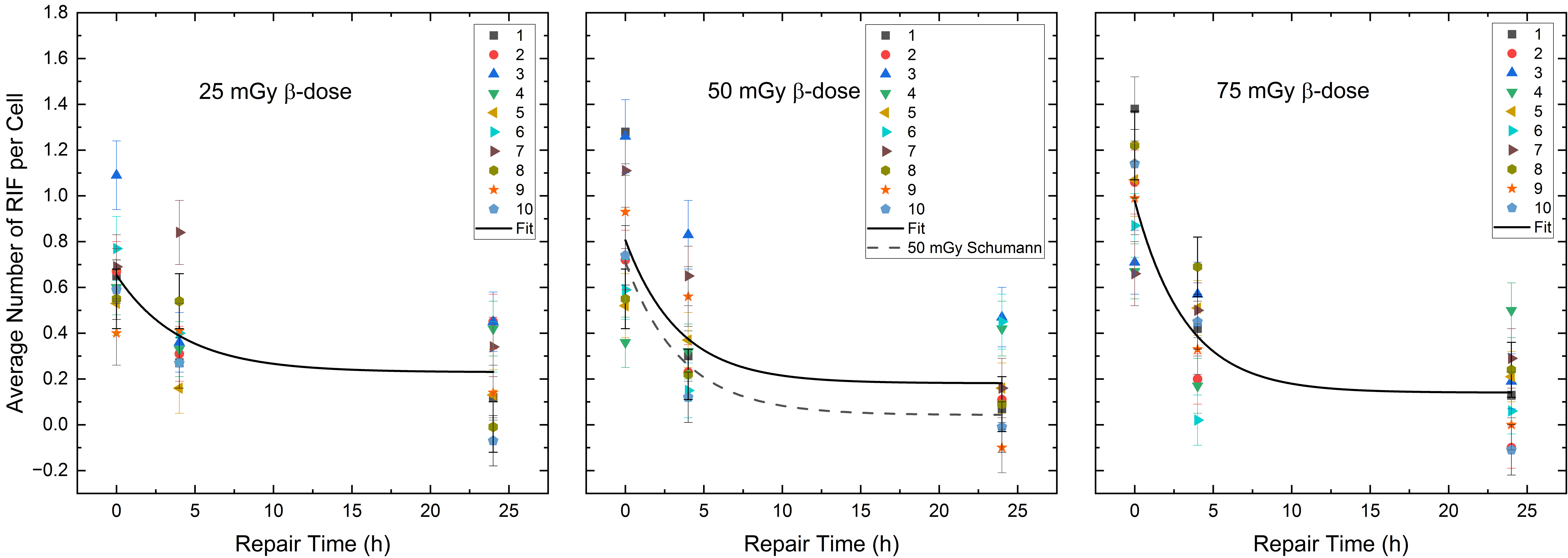
A decreasing trend was observed in the mean RIF values per cell between 4 h and 24 h for the β-doses of 25 mGy and 50 mGy, with no significant differences in the mean RIF values per cell between 4 h and 24 h (paired sample t-test, β-doses 25 mGy: p = 0.07, β-doses 50 mGy: p = 0.05), suggesting slowed or impaired DNA repair. For the β-dose of 75 mGy, the reduction in RIF per cell values was significant for all time points (paired sample t-test, d vs. 4 h: p < 0.01, 4 h vs. 24 h: p < 0.01, d vs. 24 h: p < 0.01), indicating a DNA repair-induced decrease in the RIF per cell values between 4 h and 24 h. Figure 3 shows the monoexponential fits (Equation (1) in Section 4.3) of the pooled RIF data for different α/β-mixing ratios compared to the repair fit published by Schumann et al. [36] at 50 mGy absorbed β-dose.
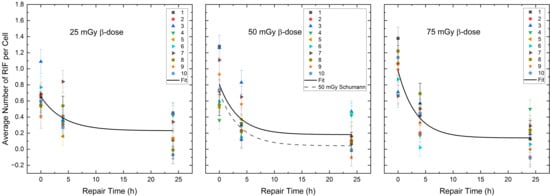
Figure 3.
Average number of RIF per cell (counted in 100 cells per sample) for each volunteer as a function of the repair time for the β-dose of 25 mGy, 50 mGy and 75 mGy in the radionuclide mixtures. The black curves represent the population-based fits according to Equation (1) of Section 4.3. The black dotted line shows the repair fit by Schumann et al. [36] at the absorbed dose of 50 mGy after pure β-irradiation.
A comparison of the RIF per cell values between the combined 50% 177Lu and 50% 223Ra irradiation and the pure 50 mGy β-irradiation, published by Schumann et al. [36] directly, 4 h and 24 h after irradiation revealed insignificant differences. However, the difference in the mean RIF per cell values at 24 h in both exposure groups (50% 177Lu and 50% 223Ra—24 h vs. pure 50 mGy β-irradiation—24 h) were close to significance (two-sample independent t-test with Welch correction, p = 0.06). Examination of the monoexponential fit parameters confirms this difference, as the Qβ value of the 50% 223Ra and 50% 177Lu group differs within the margin of error from the Qβ, pure value reported by Schumann et al. [36] with pure β-irradiation.
2.5. DNA Damage Repair of α-Tracks
To study the γ-H2AX α-induced DNA damage tracks [38,42] by mixed irradiation, we determined the α-track values over time which revealed the repair rates Rα for α-track values in 100 cells in the different radionuclide mixtures of (0.26 ± 0.05) h−1 for the 75% 223Ra and 25% 177Lu, (0.16 ± 0.05) h−1 for the 50% 223Ra and 50% 177Lu and (0.22 ± 0.07) h−1 for the 25% 223Ra and 75% 177Lu. The fractions of unrepaired α-tracks Qα were similar for all mixtures, i.e., (0.05 ± 0.06) h−1 for the 75% 223Ra and 25% 177Lu, (0.10 ± 0.09) h−1 for the 50% 223Ra and 50% 177Lu and (0.10 ± 0.09) h−1 for the 25% 223Ra and 75% 177Lu. Table 3 provides the fit parameters of the pooled data points of the different α/β-mixing ratios.

Table 3.
Fit parameters of the α-track DNA damage repair model and percentage of persisting α-tracks in 100 cells as a function of the repair time. The fits were performed without weighting.
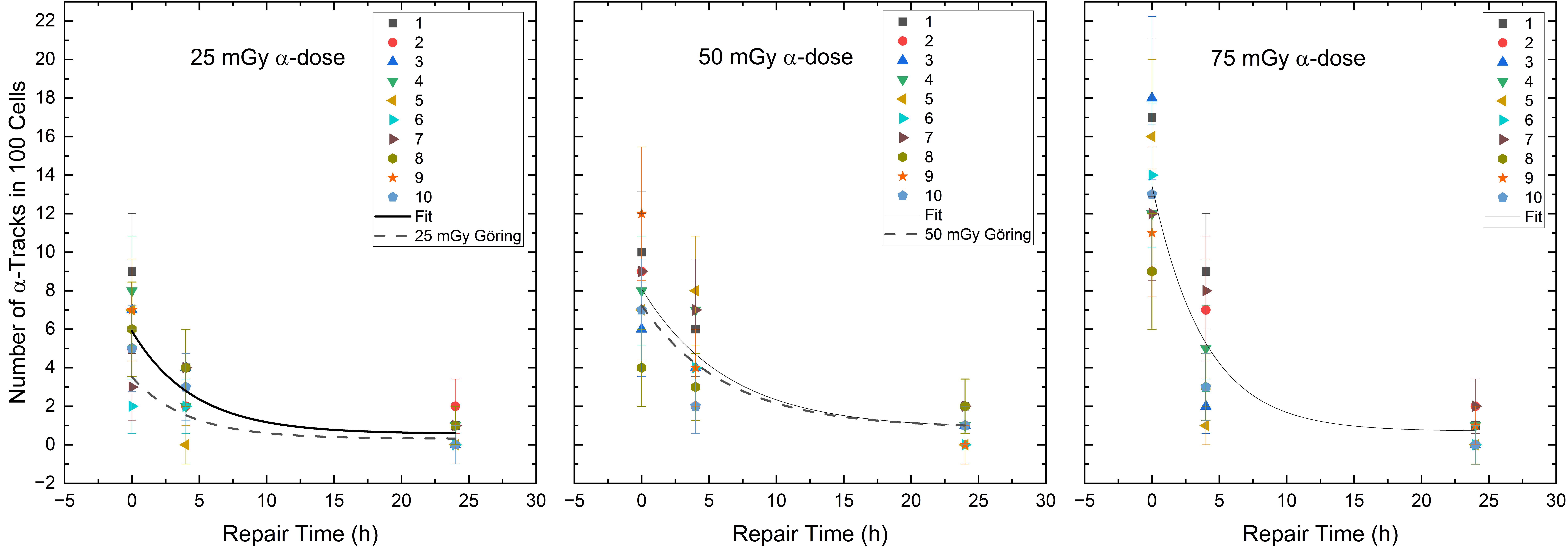
Comparing the RIF values at the time points d, 4 h and 24 h of the different α/β-mixing ratios, significant differences were obtained among time points d vs. 4 h, d vs. 24 h and 4 h vs. 24 h for the α-doses of 25 mGy (Wilcoxon-signed-rank test, d vs. 4 h: p < 0.01, d vs. 24 h: p < 0.01 and 4 h vs. 24 h: p < 0.01), 50 mGy (Wilcoxon-signed-rank test, d vs. 4 h: p < 0.01, d vs. 24 h: p < 0.01 and 4 h vs. 24 h: p < 0.01) and 75 mGy (Wilcoxon-signed-rank test, d vs. 4 h: p < 0.01, d vs. 24 h: p < 0.01 and 4 h vs. 24 h: p < 0.01). To test the difference in repair between mixed and pure α-irradiation, the d, 4 h and 24 h α-track numbers of the different α/β-mixing ratios were compared with the numbers of induced α-tracks after the pure 223Ra irradiation of Göring et al. [39]. There were no significant differences between mixed and pure α-irradiation for the 4 h and 24 h α-track numbers per 100 cells. The pooled α-track data for the different α/β-mixing ratios compared to the repair fits of the pure α-irradiation [39] are shown in Figure 4.
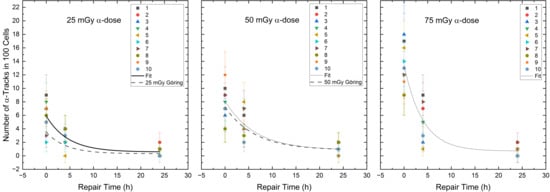
Figure 4.
Number of the α-tracks in 100 cells for each volunteer as a function of the repair time for the α-doses of 25 mGy, 50 mGy and 75 mGy in the radionuclide mixtures. The black curves represent the population-based fits according to Equation (1) (see Section 4.3). The dashed line shows the repair fit by Göring et al. [39] for the absorbed doses of 25 mGy and 50 mGy after pure α-irradiation.
3. Discussion
This study investigated the internal irradiation-induced DNA damage and DSB repair in peripheral blood mononuclear cells (PBMCs) exposed to different mixtures of the α-emitter 223Ra and the β-emitter 177Lu, a scenario that may occur in nuclear power plant accidents, after atomic bomb blasts or in modern nuclear medicine therapies. Here, we observed that there was a similar induction of DSB foci by pure β-irradiation [36,37] and mixed α/β-irradiation at a total absorbed dose of 100 mGy. Staaf et al. [43] irradiated VH10 fibroblast cells with external mixed beams and determined large (likely α-tracks perpendicular to the viewing plane) and small isolated γ-H2AX foci at α-doses of 0.13–0.32 Gy and X-ray doses of 0.27–0.8 Gy in a mixture of 25% α-dose to 75% β-dose. If expected values for the X-ray dose of 75 mGy are extrapolated using the linear fit from Staaf et al. [43], obtained from Figure 2A [43], a value of 1.7 RIF per cell is obtained for a total absorbed dose of 100 mGy. Although their value is slightly higher compared to our mean value of 1.0 RIF per cell for a β-dose of 75 mGy, these RIF values are in the same range, taking into account the differences in irradiation, cell line and analysis of γ-H2AX DSB foci only by Staaf et al. [43]. It appears that their results in terms of small γ-H2AX DSB IRIF values are comparable to our γ-H2AX+53BP1 RIF values after internal mixed low and high LET-irradiation in the 100 mGy dose range. A similar induction of small DSB foci in different cell types likely relates to low LET-induced dispersed DSBs that are subject to fast NHEJ-mediated repair [44]. This possibility is supported by the fact that in the low dose range DSB repair after low LET β-irradiation is completed after 24 h [36].
In addition to the study by Staaf et al. [43], there are other studies, e.g., [45,46,47,48], that have used the γ-H2AX and/or 53BP1 assay for DSB-equivalent damage enumeration after mixed irradiation. However, it is difficult to compare these studies with ours because of differences in irradiation design (e.g., external irradiation with simultaneous or sequential irradiation), evaluation of the different parameters (e.g., large foci, small foci, α-tracks, etc.) and choice of cell line (e.g., human osteosarcoma (U2OS), human breast cancer (MDA-MB-231), BEAS-2B, SVEC4-10EHR1 cell lines). Furthermore, these studies reported only total absorbed doses for the different external mixed beam exposures, which in combination with a different irradiation geometry makes the distinction between DSB damage induced by α- or by β-irradiation in these studies difficult.
To investigate whether α/β-mixed beam or pure β-irradiated PBMCs display different RIF numbers at equal dose, the data from internal mixed α/β-irradiation were compared to those of β-irradiation only (Eberlein et al. [37], Schumann et al. [36]). The mean of the average number of RIF per cell after mixed α/β-irradiation with a nominal β-dose of 25 mGy, 50 mGy and 75 mGy was 0.65 ± 0.18, 0.81 ± 0.32 and 0.98 ± 0.25. This is in good agreement, within the respective uncertainties, with the mean average number of RIF per cell of 0.72 ± 0.16 (50 mGy) noted after pure 131I β-irradiation by Schumann et al. [36], and the expected value of 0.77 ± 0.03 RIF per cell (50 mGy), 1.14 ± 0.03 (75 mGy) calculated from the β-dose-response curve by Eberlein et al. [37] (see Figure 1). The comparison of the 25 mGy β-induced RIF number per cell between mixed α/β-irradiation in this study (0.65 ± 0.18 RIF per cell) and the pure β-irradiation (0.40 ± 0.03 RIF per cell) by Eberlein et al. [37] revealed a significant difference. This difference may reflect low numbers of foci counted at low absorbed doses and dose rates, with the calculation of the RIF value being strongly affected by the uncertainty of the baseline value. The difference in the average number of RIF per cell value for the absorbed dose of 25 mGy β-irradiation compared to the median number of RIF per cell value of mixed α/β-irradiation (75% 223Ra and 25% 177Lu) might also be attributed to the scatter between the data sets of the volunteers, as well as between the samples observed at low absorbed doses. Furthermore, the mixed α/β-irradiation data show considerable intra-individual dispersion of RIF values, which may be due to the combination of α- and β-irradiation, DNA repair fidelity [49], as well as other factors such as age, sex [50] and pre-existing health conditions of the subjects [49].
To investigate the α-track numbers induced by the high LET component in PBMCs in mixed α/β-irradiation and α-irradiation only, the α-tracks observed in 100 cells after mixed α/β-irradiation were compared with the α-track frequency observed after pure α-irradiation by Schumann et al. [38]. For the 25 mGy and 75 mGy absorbed α-doses, this analysis failed to reveal a significant difference between the α-track frequency after mixed α/β-irradiation and the 25 mGy and 75 mGy pure α-irradiation [38]. A significant difference was reached for the 50 mGy α-dose when comparing of pure α -induced γ-H2AX track frequencies [38] with that of the mixed α/β-irradiation. Overall, the mean values for the α-tracks in 100 cells irradiated with different α/β-mixing ratios tend to be lower than the mean values for α-tracks obtained with pure α-irradiation [38], indicating a higher occupancy of the damage response machinery [51,52] in the presence of additional β-irradiation.
As far as the induction of α-tracks and thus the maximum number of α-track N0-values of the repair fits are concerned, they are very close to the values obtained with pure internal 223Ra irradiation [38,39]. However, there are differences between the studies, which may be due to the different cell enumeration approaches. In the study by Göring et al. [39], 500 cells per sample were analysed, whereas Schumann et al. [38] analysed only 100 cells per sample (as in the present study). In addition, a slightly different dose range and minor difference in the staining may partly explain the differences in the measured induction of α-tracks in these studies.
Four hours of DNA repair in the cultured cells induced a significant reduction in the RIF per cell and α-track values compared to the directly fixed PBMCs at all absorbed doses, indicating progress of DSB repair as demonstrated by Löbrich et al. for the reduction in low LET-induced RIF in lymphocytes after diagnostic CT scans [53] and by Göring et al. [39] for the reduction of induced α-track numbers in PBMCs.
After 24 h, RIF values decreased to 35–14% of the initial values, indicating DSB repair removing 65–86% of the initially induced focal DSB damage. Comparing the RIF values of mixed α/β-irradiation to pure β-irradiation of the same absorbed dose [36] at 4 h and 24 h reveals a tendency towards a higher average RIF value remaining at later time points after mixed α/β-irradiation. It is notable that samples with a higher proportion of 177Lu show fewer remaining DNA DBS foci after 24 h relative to samples with a higher proportion of 223Ra. This is also reflected by the increased Qβ values after mixed irradiation compared to the Qβ,pure value (Q = 0.06 ± 0.02) of the 50 mGy ex vivo of 131I β-irradiation of Schumann et al. [36]. This observation can likely be attributed to the more complex DNA damage elicited by the high LET α-emitter, or by the possibility that partial repair of DSBs in the α-tracks convert initial α-track-containing cells to foci-containing cells (due to complex DNA damage) in cell samples 24 h after mixed α/β-irradiation. It has been observed that high LET-induced α-tracks in human and Chinese hamster cells, over time, lose the typical α-track appearance, leading to cells without ordered tracks and focal DNA damage at late time points [40], suggesting that mixed α/β-irradiation-induced α-tracks in PBMCs will also transform into foci with the progress of DSB repair time and the associated γ-H2AX dephosphorylation or dispersal [54].
Staaf et al. [43] measured DNA DSB repair up to 24 h after external mixed beam irradiation and observed no differences between α-, X-ray or mixed irradiation [43]. In contrast, Antonelli et al. [55] showed that 2.5% (γ) and 27.3% (α) of the radiation-induced γ-H2AX foci 24 h after irradiation of primary human foreskin fibroblasts persisted after external γ- or α-irradiation at an absorbed dose of 0.5 Gy for γ and α, respectively. Ugenskiene et al. [56], irradiated human skin fibroblasts with X-rays (0.1 Gy) or 3He particles (three 3He particles per cell nucleus ≈ 0.5 Gy) and found a residual γ-H2AX foci fraction of 2% after X-ray and 33% after 3He exposure after 24 h [56]. The data of the aforementioned publications agree well with our residual fractions of RIF per cell in PBMCs 24 h after mixed α/β-irradiation with increasing α-doses, i.e., 14% residual damage after 25 mGy 223Ra/75 mGy 177Lu, 23% residual damage after 50 mGy 223Ra/50 mGy 177Lu and 35% residual damage after 75 mGy 223Ra/25 mGy 177Lu. These observations indicate that even at lower doses of α-irradiation, a higher number of RIF per cell remains after 24 h of repair. Since this effect appears to depend on an increasing α contribution with high LET to the absorbed dose in the mixed α/β-irradiation, it is likely that incomplete repair converts parts of the α-track into foci with growing repair time, as has been noted for α-irradiated Hela and fibroblast cells by Aten et al. [40].
Our study showed a reduction in α-track frequency to 5–12% at 24 h post irradiation indicating the repair of up to 88% of the α-track damage, being in good agreement with a previous repair study after internal 223Ra irradiation [39]. While the 24 h repair after mixed irradiation appears to eliminate a similar number of α-tracks after mixed α/β- or pure internal α-irradiation, the proportion of unrepaired DSB foci increases in proportion to the increase in α-dose.
The repair rates Rβ of the number of RIF per cell were 0.25 ± 0.12 h−1 for 25 mGy β-dose, 0.29 ± 0.13 h−1 for 50 mGy β-dose and 0.31 ± 0.09 h−1 for 75 mGy β-dose. These values are well within the error of the repair rate Rβ,pure of 0.28 ± 0.03 h−1 for the 50 mGy pure internal 131I low LET irradiation from Schumann et al. [36] and the calculated values from the data of Löbrich et al. (23 patients, Figure 5 of [53]) of 0.29 h−1 and 0.35 h−1 for lymphocytes irradiated externally with X-rays with a resulting absorbed dose of 20 mGy and 100 mGy. Horn et al. [57] reported a repair rate of 0.35 h−1 for absorbed doses ≥ 0.5 Gy (data of 21 healthy donors, external X-rays). For different internal β-doses in the range of 26.7 to 75.7 mGy, we found no differences in the repair rates. Nevertheless, we observed a tendency for an increasing repair rate with increasing β-doses, whereas a high α-dose seems to be associated with a slowing of the repair rate, as α-tracks are likely converted into few persistent foci. This agrees with Göring et al. [39], who observed a decreasing repair rate with increasing α-dose after pure internal irradiation with 223Ra. We could only partially confirm these effects with mixed α/β-irradiation in the low dose range studied, as a reduction in the α-repair rates Rα from 0.22 ± 0.07 h−1 to 0.16 ± 0.05 h−1 was observed for the α-doses of 25 mGy and 50 mGy. However, within the respective uncertainties, the values appear similar. In addition, we obsorbed a repair rate Rα for α-tracks of 0.26 ± 0.05 h−1 for an α-dose of 75 mGy for the mixed α/β-irradiation. Thus, there remains the possibility that the DSB repair rate induced by the α-component is influenced by the absorbed dose and/or by the radiation quality (high vs. low LET). Future research has to determine whether parts of the DDR may suffer from saturation [58] in mixed radiation scenarios.
4. Materials and Methods
4.1. Internal Irradiation, Preparation of Blood Samples and Blood Dosimetry
Blood samples were obtained from 10 healthy volunteers whose ages ranged from 26 to 65 years. Li heparin blood collection tubes (S-Monovette®, Sarstedt AG & Co. KG, Nürnbrecht, Germany) were used to collect about 30 mL of blood per volunteer. Each blood sample was divided into three aliquots of 7 mL for the irradiation experiments, and one non-irradiated aliquot of 8 mL served as a non-irradiated baseline. The three 7 mL aliquots were each diluted with 1 mL of an isotonic NaCl (0.9%, B. Braun Melsungen AG, Melsungen, Germany) solution containing different ratios of [223Ra]RaCl2 (Xofigo®, Bayer GmbH, Leverkusen, Germany) and [177Lu]LuCl3 (EndolucinBeta, ITM Medical Isotopes GmbH, Garching, Germany) to achieve a total absorbed dose to the blood of 100 mGy after 1 h of irradiation. The total absorbed dose is the sum of the absorbed dose delivered by the α-particles (α-dose) and the absorbed dose by β-particle irradiation, including the very low contribution of photon emission (β-dose). The combination of the α-dose and the β-dose was set to reach a total absorbed dose of 100 mGy (total absorbed dose = α-dose + β-dose). Hereafter, we refer to 223Ra and 177Lu when [223Ra]RaCl2 and [177Lu]LuCl3 were used in the solutions. The effects of the following α/β mixing ratios of the α-dose and β-dose were analysed: 25% 223Ra and 75% 177Lu, 50% 223Ra and 50% 177Lu and 75% 223Ra and 25% 177Lu (for the corresponding α-doses and β-doses and the nominal activity concentrations see Table 4).

Table 4.
Overview of different nominal activity concentrations with corresponding α- and β-doses for various α/β-mixing ratios.
Each blood mixture was incubated for one hour on a roller mixer at 37 °C in an 8 mL vial with a screw cap (No. 60.542.007, Sarstedt AG & Co. KG, Nürnbrecht, Germany) to ensure uniform irradiation of the blood samples by 223Ra and 177Lu. Immediately after the incubation the sample-specific activity concentration was determined by pipetting 0.8 mL of the blood mixture into a round bottom tube (No. 55.1579.002, Sarstedt AG & Co. KG, Nürnbrecht, Germany), which was measured in a calibrated, high-purity germanium detector (Canberra GmbH, Rüsselsheim, Germany). The activity of each sample was determined by analysing the γ-emission lines of 177Lu at 208 keV and 211Bi from the decay of 223Ra at 351 keV. The activity concentration was decay-corrected to the start time of irradiation.
The calculation of the absorbed dose to the blood was performed using the measured activity values and the published dose coefficients for an irradiation duration of one hour by Salas-Ramirez et al. [35]. These dose coefficients were converted into S-values to account for the exact duration of the sample irradiation. This results into converted S-values for 223Ra of 4.003x10−9 Gy·s−1·Bq−1 mL (α-dose), electron and photon part of 223Ra of 1.165x10−10 Gy·s−1·Bq−1 mL (for the calculation of 223Ra β-dose) and for 177Lu of 2.226x10−11·Gy·s−1·Bq−1 mL (for the calculation of 177Lu β-dose). The α-dose and β-dose for each sample were calculated by multiplying these S-values by the individual activity concentrations and the time-integrated activity coefficient of the respective radionuclide taking into account the actual irradiation duration and physical decay.
The samples were mixed with an equal volume of phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS) and then layered onto 15 mL Lymphoprep™ (STEMCELL™ Technologies, Germany GmbH, Cologne, Germany), which was already filled in the SepMate™-50 tube (STEMCELL™ Technologies, Germany GmbH, Cologne, Germany). This tube was then centrifuged to separate the PBMCs from the rest of the blood and from the remaining radioactive solution. The isolated PBMCs of each sample were divided into three parts and washed twice with PBS. One of the three parts was fixed directly with 70% ice-cold ethanol (resulting in sample “d” = 0 h) to study the DNA damage induction. The other two parts were cultured in RPMI medium (containing HEPES; Gibco® by Thermo Fisher Scientific, Langensebold, Germany) for 4 h and 24 h, washed and then fixed with ethanol. The fixed cells were stored at −20 °C before being sent to the Bundeswehr Institute of Radiobiology in Munich, Germany, for immunofluorescence staining and assessment of DNA damage.
4.2. Immunofluorescent Staining and Evaluation of DNA Damage
The fixed PBMCs underwent immunofluorescence staining with the primary antibodies against γ-H2AX (Mouse anti-γ-H2AX, Merck, Darmstadt, Germany) and 53BP1 (rab-a-53BP1, Abcam, Cambridge, UK) and were detected with secondary goat anti-mouse-Alexa488 and donkey anti-rabbit-Cy3 antibodies (both Jackson Laboratories, Bar Harbor, ME, USA), as described previously in detail elsewhere [59]. The mixture of the α-emitter and β-emitter elicited DSB damage as the α-particle induced γ-H2AX-postive DSB damage tracks in hit nuclei, so called “α-tracks” [38], and co-localized γ-H2AX + 53BP1 DSB foci [37]. These damage qualities were microscopically enumerated in 100 cells of each sample by an experienced investigator (H.S.).
The number of α-tracks observed in 100 cells and the average number of radiation-induced foci (RIF) per cell were used to analyse the data. For the calculation of the average number of RIF per cell, the average DSB foci values were determined in 100 cells of all baseline and irradiated cell samples. The average number of RIF per cell was calculated as the difference between the average number of foci per cell of the irradiated samples at d, 4 h and 24 h after irradiation and the corresponding baseline value of the respective non-irradiated sample from each time point. The corresponding baseline value of the respective non-irradiated sample from each time point were obtained at 0–d, 0–4 h, 0–24 h.
4.3. Modelling of DNA Damage Repair
A monoexponential fit was used to describe the time-dependent repair of the average number of RIF per cell and the number of α-tracks in 100 cells. Lobachevsky et al. already described this model and used it for ex vivo irradiation of PBMC in radiotherapy [60]. In the publication by Schumann et al. it was used to describe the ex vivo RIF reduction over time after internal irradiation with 131I [36]. The same model was used to describe the repair of the α-tracks in 223Ra exposed PBMCs by Göring et al. [39]. In our study the influence of the different emitters during mixed irradiation on the repair is investigated for α-tracks and RIF.
with N0,β being the maximum number of RIF per cell or N0,α the maximum number of α-tracks in 100 cells, and Qβ being the fraction of unrepaired RIF per cell or Qα the fraction of unrepaired α-tracks in 100 cells (i.e., the residual damage), while Rβ denotes the repair rate of the RIF per cell in h−1 or Rα, the repair rate of the α-tracks in 100 cells in h−1.
4.4. Statistical Analysis
OriginPro 2023 (Origin Lab Corporation, Northampton, MA, USA) was used for statistical analysis and plotting. The Shapiro–Wilk test was used to test for normal distribution. A two-sample F-test of variance was used to test whether the groups had the same homogeneity of variance. In the case of a normal distribution of the groups and no homogeneity of variance, a Welch correction was applied in a two-sample independent t-test. The two-sample independent t-test (for normally distributed data) and the Mann–Whitney U-test (for non-normally distributed data) were used to compare the data set of the mixed α/β-irradiation with the pure irradiation. The paired samples t-test (for normally distributed data) and the Wilcoxon-signed-rank test (for non-normally distributed data) were used to compare the data set between the different time points (d, 4 h and 24 h) and the different α/β-mixing ratios. Results were considered statistically significant at p < 0.05. A monoexponential fit without weighting was used to plot the time course of the data.
5. Conclusions
The results of this study show that for a total absorbed dose of 100 mGy the induction of DNA double-strand breaks after irradiation of PBMCs with mixtures of α- and β-emitters, and our statistical analysis revealed no systematic deviation of the results to those of pure α- or β-irradiation for different mixing ratios. In this dose range there was no dependency of the α-dose on the repair of α-tracks. However, an increasing fraction of unrepaired radiation-induced DSB foci correlated with increasing α-dose, suggesting an impact of a high LET contribution to the formation of complex DSBs that are difficult to repair.
Author Contributions
U.E., H.S., I.S., S.S. and M.L. designed the study and developed the methodology. I.S. performed the irradiation and processing of the blood samples and the calculations of the absorbed doses. H.S. and J.M. performed γ-H2AX + 53BP1 staining and foci analysis. M.P. and A.K.B. provided resources. I.S., U.E., H.S. and M.L. analysed and interpreted the data and wrote the manuscript. All authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work received funding by a contract research project for the Bundeswehr Medical Service to U.E. (Research grant number: E/U2AD/KA367/KF554). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Institutional Review Board Statement
The study protocol was presented to the local ethics committee of the Medical Faculty of the University Würzburg (UKW) and approved. All procedures performed in this study involving human participants were in accordance with the ethical standards of the ethics committee (Az. 174/19) and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Data Availability Statement
The data sets generated and analyzed in the course of the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank all volunteers for their participation in this study. For technical assistance we thank Sarah Schoof and Alberto Meca Zapata (Munich) and Gabriele Riehl and Susanne Hirsch (Würzburg).
Conflicts of Interest
M.L. has received institutional grants from IPSEN Pharma, Nordic Nanovector, Novartis and Pentixapharm. A.K.B. has received institutional grants and personal fees from Novartis, AAA, Takeda, Siemens, Janssen, Novartis, Eisai, Eli Lilly and Pfizer. U.E. has received an institutional grant from Novartis. All others have no conflicts of interest to declare.
References
- Song, H.; Sgouros, G. Alpha and Beta Radiation for Theragnostics. PET Clin. 2024, 19, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Marcu, L.; Bezak, E.; Allen, B.J. Global comparison of targeted alpha vs targeted beta therapy for cancer: In vitro, in vivo and clinical trials. Crit. Rev. Oncol./Hematol. 2018, 123, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. Drugmakers go nuclear, continuing push into radiopharmaceuticals. Nat. Biotechnol. 2021, 39, 647–649. [Google Scholar] [CrossRef]
- Albertsson, P.; Bäck, T.; Bergmark, K.; Hallqvist, A.; Johansson, M.; Aneheim, E.; Lindegren, S.; Timperanza, C.; Smerud, K.; Palm, S. Astatine-211 based radionuclide therapy: Current clinical trial landscape. Front. Med. 2022, 9, 1076210. [Google Scholar] [CrossRef]
- Sathekge, M.M.; Lawal, I.O.; Bal, C.; Bruchertseifer, F.; Ballal, S.; Cardaci, G.; Davis, C.; Eiber, M.; Hekimsoy, T.; Knoesen, O.; et al. Actinium-225-PSMA radioligand therapy of metastatic castration-resistant prostate cancer (WARMTH Act): A multicentre, retrospective study. Lancet Oncol. 2024, 25, 175–183. [Google Scholar] [CrossRef]
- Rosar, F.; Hau, F.; Bartholomä, M.; Maus, S.; Stemler, T.; Linxweiler, J.; Ezziddin, S.; Khreish, F. Molecular imaging and biochemical response assessment after a single cycle of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy in mCRPC patients who have progressed on [177Lu]Lu-PSMA-617 monotherapy. Theranostics 2021, 11, 4050–4060. [Google Scholar] [CrossRef] [PubMed]
- Raju, M.R.; Jett, J.H. RBE and OER Variations of Mixtures of Plutonium Alpha Particles and X-Rays for Damage to Human Kidney Cells (T-1). Radiat. Res. 1974, 60, 473–481. [Google Scholar] [CrossRef]
- Sgouros, G.; Hobbs, R.; Josefsson, A. Dosimetry and Radiobiology of Alpha-Particle Emitting Radionuclides. Curr. Radiopharm. 2018, 11, 209–214. [Google Scholar] [CrossRef]
- Khreish, F.; Ebert, N.; Ries, M.; Maus, S.; Rosar, F.; Bohnenberger, H.; Stemler, T.; Saar, M.; Bartholoma, M.; Ezziddin, S. 225Ac-PSMA-617/177Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: Pilot experience. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 721–728. [Google Scholar] [CrossRef]
- Kostos, L.; Buteau, J.P.; Yeung, T.; Iulio, J.D.; Xie, J.; Cardin, A.; Chin, K.Y.; Emmerson, B.; Owen, K.L.; Parker, B.S.; et al. AlphaBet: Combination of Radium-223 and [177Lu]Lu-PSMA-I&T in men with metastatic castration-resistant prostate cancer (clinical trial protocol). Front. Med. 2022, 9, 1059122. [Google Scholar] [CrossRef]
- Peter MacCallum Cancer Centre. A.R.P. Combination of Radium-223 and Lutetium-177 PSMA-I&T in Men with Metastatic Castration-Resistant Prostate Cancer. Available online: https://clinicaltrials.gov/study/NCT05383079 (accessed on 12 February 2024).
- Meyer, C.; Stuparu, A.; Lueckerath, K.; Calais, J.; Czernin, J.; Slavik, R.; Dahlbom, M. Tandem Isotope Therapy with 225Ac- and 177Lu-PSMA-617 in a Murine Model of Prostate Cancer. J. Nucl. Med. 2023, 64, 1772–1778. [Google Scholar] [CrossRef]
- Rosar, F.; Krause, J.; Bartholomä, M.; Maus, S.; Stemler, T.; Hierlmeier, I.; Linxweiler, J.; Ezziddin, S.; Khreish, F. Efficacy and Safety of [225Ac]Ac-PSMA-617 Augmented [177Lu]Lu-PSMA-617 Radioligand Therapy in Patients with Highly Advanced mCRPC with Poor Prognosis. Pharmaceutics 2021, 13, 722. [Google Scholar] [CrossRef] [PubMed]
- Delker, A.; Schleske, M.; Liubchenko, G.; Berg, I.; Zacherl, M.J.; Brendel, M.; Gildehaus, F.J.; Rumiantcev, M.; Resch, S.; Hürkamp, K.; et al. Biodistribution and dosimetry for combined [177Lu]Lu-PSMA-I&T/[225Ac]Ac-PSMA-I&T therapy using multi-isotope quantitative SPECT imaging. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Walrand, S.; Jamar, F. Renal and Red Marrow Dosimetry in Peptide Receptor Radionuclide Therapy: 20 Years of History and Ahead. Int. J. Mol. Sci. 2021, 22, 8326. [Google Scholar] [CrossRef]
- Parlani, M.; Boccalatte, F.; Yeaton, A.; Wang, F.; Zhang, J.; Aifantis, I.; Dondossola, E. (223)Ra Induces Transient Functional Bone Marrow Toxicity. J. Nucl. Med. 2022, 63, 1544–1550. [Google Scholar] [CrossRef]
- Brambilla, S.; Nelson, M.A.; Brown, M.J. Dirty bomb source term characterization and downwind dispersion: Review of experimental evidence. J. Environ. Radioact. 2023, 263, 107166. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H. Rays as weapons. Eur. J. Radiol. 2007, 63, 167–177. [Google Scholar] [CrossRef]
- Simon, S.L.; Bouville, A.; Beck, H.L.; Anspaugh, L.R.; Thiessen, K.M.; Hoffman, F.O.; Shinkarev, S. Dose Estimation for Exposure to Radioactive Fallout from Nuclear Detonations. Health Phys. 2022, 122, 1–20. [Google Scholar] [CrossRef]
- Romanov, S.A.; Efimov, A.V.; Aladova, E.E.; Suslova, K.G.; Kuznetsova, I.S.; Sokolova, A.B.; Khokhryakov, V.V.; Sypko, S.A.; Ishunina, M.V.; Khokhryakov, V.F. Plutonium production and particles incorporation into the human body. J. Environ. Radioact. 2020, 211, 106073. [Google Scholar] [CrossRef]
- Yeager, M.; Machiela, M.J.; Kothiyal, P.; Dean, M.; Bodelon, C.; Suman, S.; Wang, M.; Mirabello, L.; Nelson, C.W.; Zhou, W.; et al. Lack of transgenerational effects of ionizing radiation exposure from the Chernobyl accident. Science 2021, 372, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Adewoye, A.B.; Lindsay, S.J.; Dubrova, Y.E.; Hurles, M.E. The genome-wide effects of ionizing radiation on mutation induction in the mammalian germline. Nat. Commun. 2015, 6, 6684. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.J. The Production of Mutations by X-Rays. Proc. Natl. Acad. Sci. USA 1928, 14, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Mladenova, V.; Mladenov, E.; Stuschke, M.; Iliakis, G. DNA Damage Clustering after Ionizing Radiation and Consequences in the Processing of Chromatin Breaks. Molecules 2022, 27, 1540. [Google Scholar] [CrossRef] [PubMed]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA Double-stranded Breaks Induce Histone H2AX Phosphorylation on Serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef]
- Panier, S.; Boulton, S.J. Double-strand break repair: 53BP1 comes into focus. Nat. Rev. Mol. Cell Biol. 2014, 15, 7–18. [Google Scholar] [CrossRef]
- Schumann, S.; Scherthan, H.; Lapa, C.; Serfling, S.; Muhtadi, R.; Lassmann, M.; Eberlein, U. DNA damage in blood leucocytes of prostate cancer patients during therapy with 177Lu-PSMA. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Nikitaki, Z.; Nikolov, V.; Mavragani, I.V.; Mladenov, E.; Mangelis, A.; Laskaratou, D.A.; Fragkoulis, G.I.; Hellweg, C.E.; Martin, O.A.; Emfietzoglou, D.; et al. Measurement of complex DNA damage induction and repair in human cellular systems after exposure to ionizing radiations of varying linear energy transfer (LET). Free Radic. Res. 2016, 50, S64–S78. [Google Scholar] [CrossRef]
- Marková, E.; Torudd, J.; Belyaev, I. Long time persistence of residual 53BP1/γ-H2AX foci in human lymphocytes in relationship to apoptosis, chromatin condensation and biological dosimetry. Int. J. Radiat. Biol. 2011, 87, 736–745. [Google Scholar] [CrossRef]
- Port, M.; Barquinero, J.F.; Endesfelder, D.; Moquet, J.; Oestreicher, U.; Terzoudi, G.; Trompier, F.; Vral, A.; Abe, Y.; Ainsbury, L.; et al. RENEB Inter-Laboratory Comparison 2021: Inter-Assay Comparison of Eight Dosimetry Assays. Radiat. Res. 2023, 199, 535–555. [Google Scholar] [CrossRef]
- Schumann, S.; Eberlein, U.; Lapa, C.; Müller, J.; Serfling, S.; Lassmann, M.; Scherthan, H. alpha-Particle-induced DNA damage tracks in peripheral blood mononuclear cells of [223Ra]RaCl2-treated prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2761–2770. [Google Scholar] [CrossRef]
- Widjaja, L.; Werner, R.A.; Krischke, E.; Christiansen, H.; Bengel, F.M.; Bogdanova, N.; Derlin, T. Individual radiosensitivity reflected by γ-H2AX and 53BP1 foci predicts outcome in PSMA-targeted radioligand therapy. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 602–612. [Google Scholar] [CrossRef]
- Eberlein, U.; Scherthan, H.; Bluemel, C.; Peper, M.; Lapa, C.; Buck, A.K.; Port, M.; Lassmann, M. DNA Damage in Peripheral Blood Lymphocytes of Thyroid Cancer Patients After Radioiodine Therapy. J. Nucl. Med. 2016, 57, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Salas-Ramirez, M.; Lassmann, M.; Eberlein, U. GATE/Geant4-based dosimetry for ex vivo in solution irradiation of blood with radionuclides. Z. Für Med. Phys. 2023, 33, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Schumann, S.; Scherthan, H.; Pfestroff, K.; Schoof, S.; Pfestroff, A.; Hartrampf, P.; Hasenauer, N.; Buck, A.K.; Luster, M.; Port, M.; et al. DNA damage and repair in peripheral blood mononuclear cells after internal ex vivo irradiation of patient blood with 131I. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1447–1455. [Google Scholar] [CrossRef]
- Eberlein, U.; Peper, M.; Fernandez, M.; Lassmann, M.; Scherthan, H. Calibration of the gamma-H2AX DNA double strand break focus assay for internal radiation exposure of blood lymphocytes. PLoS ONE 2015, 10, e0123174. [Google Scholar] [CrossRef]
- Schumann, S.; Eberlein, U.; Muhtadi, R.; Lassmann, M.; Scherthan, H. DNA damage in leukocytes after internal ex-vivo irradiation of blood with the alpha-emitter Ra-223. Sci. Rep. 2018, 8, 2286. [Google Scholar] [CrossRef] [PubMed]
- Göring, L.; Schumann, S.; Müller, J.; Buck, A.K.; Port, M.; Lassmann, M.; Scherthan, H.; Eberlein, U. Repair of alpha-particle-induced DNA damage in peripheral blood mononuclear cells after internal ex vivo irradiation with 223Ra. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3981–3988. [Google Scholar] [CrossRef]
- Aten, J.A.; Stap, J.; Krawczyk, P.; van Oven, C.V.; Hoebe, R.; Essers, J.; Kanaar, R. Dynamics of DNA Double-Strand Breaks Revealed by Clustering of Damaged Chromosome Domains. Science 2004, 303, 92–95. [Google Scholar] [CrossRef]
- Scherthan, H.; Lee, J.H.; Maus, E.; Schumann, S.; Muhtadi, R.; Chojowski, R.; Port, M.; Lassmann, M.; Bestvater, F.; Hausmann, M. Nanostructure of Clustered DNA Damage in Leukocytes after In-Solution Irradiation with the Alpha Emitter Ra-223. Cancers 2019, 11, 1877. [Google Scholar] [CrossRef]
- Ding, D.; Zhang, Y.; Wang, J.; Wang, X.; Fan, D.; He, L.; Zhang, X.; Gao, Y.; Li, Q.; Chen, H. γ-H2AX/53BP1/pKAP-1 foci and their linear tracks induced by in vitro exposure to radon and its progeny in human peripheral blood lymphocytes. Sci. Rep. 2016, 6, 38295. [Google Scholar] [CrossRef] [PubMed]
- Staaf, E.; Brehwens, K.; Haghdoost, S.; Czub, J.; Wojcik, A. Gamma-H2AX foci in cells exposed to a mixed beam of X-rays and alpha particles. Genome Integr. 2012, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Iliakis, G.; Wang, H.; Perrault, A.R.; Boecker, W.; Rosidi, B.; Windhofer, F.; Wu, W.; Guan, J.; Terzoudi, G.; Pantelias, G. Mechanisms of DNA double strand break repair and chromosome aberration formation. Cytogenet. Genome Res. 2004, 104, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Sollazzo, A.; Brzozowska, B.; Cheng, L.; Lundholm, L.; Haghdoost, S.; Scherthan, H.; Wojcik, A. Alpha Particles and X Rays Interact in Inducing DNA Damage in U2OS Cells. Radiat. Res. 2017, 188, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Sollazzo, A.; Brzozowska, B.; Cheng, L.; Lundholm, L.; Scherthan, H.; Wojcik, A. Live Dynamics of 53BP1 Foci Following Simultaneous Induction of Clustered and Dispersed DNA Damage in U2OS Cells. Int. J. Mol. Sci. 2018, 19, 519. [Google Scholar] [CrossRef]
- Akuwudike, P.; Lopez-Riego, M.; Ginter, J.; Cheng, L.; Wieczorek, A.; Zycienska, K.; Lysek-Gladysinska, M.; Wojcik, A.; Brzozowska, B.; Lundholm, L. Mechanistic insights from high resolution DNA damage analysis to understand mixed radiation exposure. DNA Repair 2023, 130, 103554. [Google Scholar] [CrossRef]
- Lee, U.-S.; Lee, D.-H.; Kim, E.-H. Characterization of γ-H2AX foci formation under alpha particle and X-ray exposures for dose estimation. Sci. Rep. 2022, 12, 3761. [Google Scholar] [CrossRef] [PubMed]
- Rall-Scharpf, M.; Friedl, T.W.P.; Biechonski, S.; Denkinger, M.; Milyavsky, M.; Wiesmüller, L. Sex-specific differences in DNA double-strand break repair of cycling human lymphocytes during aging. Aging 2021, 13, 21066–21089. [Google Scholar] [CrossRef]
- Garm, C.; Moreno-Villanueva, M.; Bürkle, A.; Petersen, I.; Bohr, V.A.; Christensen, K.; Stevnsner, T. Age and gender effects on DNA strand break repair in peripheral blood mononuclear cells. Aging Cell 2013, 12, 58–66. [Google Scholar] [CrossRef]
- Horn, S.; Brady, D.; Prise, K. Alpha particles induce pan-nuclear phosphorylation of H2AX in primary human lymphocytes mediated through ATM. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2015, 1853, 2199–2206. [Google Scholar] [CrossRef]
- Lorat, Y.; Brunner, C.U.; Schanz, S.; Jakob, B.; Taucher-Scholz, G.; Rube, C.E. Nanoscale analysis of clustered DNA damage after high-LET irradiation by quantitative electron microscopy—The heavy burden to repair. DNA Repair 2015, 28, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Löbrich, M.; Rief, N.; Kühne, M.; Heckmann, M.; Fleckenstein, J.; Rübe, C.; Uder, M. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc. Natl. Acad. Sci. USA 2005, 102, 8984–8989. [Google Scholar] [CrossRef]
- Bouquet, F.; Muller, C.; Salles, B. The Loss of γH2AX Signal is a Marker of DNA Double Strand Breaks Repair Only at Low Levels of DNA Damage. Cell Cycle 2006, 5, 1116–1122. [Google Scholar] [CrossRef]
- Antonelli, F.; Campa, A.; Esposito, G.; Giardullo, P.; Belli, M.; Dini, V.; Meschini, S.; Simone, G.; Sorrentino, E.; Gerardi, S.; et al. Induction and Repair of DNA DSB as Revealed by H2AX Phosphorylation Foci in Human Fibroblasts Exposed to Low- and High-LET Radiation: Relationship with Early and Delayed Reproductive Cell Death. Radiat. Res. 2015, 183, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Ugenskiene, R.; Prise, K.; Folkard, M.; Lekki, J.; Stachura, Z.; Zazula, M.; Stachura, J. Dose response and kinetics of foci disappearance following exposure to high- and low-LET ionizing radiation. Int. J. Radiat. Biol. 2009, 85, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Barnard, S.; Rothkamm, K. Gamma-H2AX-Based Dose Estimation for Whole and Partial Body Radiation Exposure. PLoS ONE 2011, 6, e25113. [Google Scholar] [CrossRef] [PubMed]
- Greubel, C.; Hable, V.; Drexler, G.A.; Hauptner, A.; Dietzel, S.; Strickfaden, H.; Baur, I.; Krücken, R.; Cremer, T.; Dollinger, G.; et al. Competition effect in DNA damage response. Radiat. Environ. Biophys. 2008, 47, 423–429. [Google Scholar] [CrossRef]
- Scherthan, H.; Wagner, S.-Q.; Grundhöfer, J.; Matejka, N.; Müller, J.; Müller, S.; Rudigkeit, S.; Sammer, M.; Schoof, S.; Port, M.; et al. Planar Proton Minibeam Irradiation Elicits Spatially Confined DNA Damage in a Human Epidermis Model. Cancers 2022, 14, 1545. [Google Scholar] [CrossRef]
- Lobachevsky, P.; Leong, T.; Daly, P.; Smith, J.; Best, N.; Tomaszewski, J.; Thompson, E.R.; Li, N.; Campbell, I.G.; Martin, R.F.; et al. Compromized DNA repair as a basis for identification of cancer radiotherapy patients with extreme radiosensitivity. Cancer Lett. 2016, 383, 212–219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).