Adipose Tissue: A Novel Target of the Incretin Axis? A Paradigm Shift in Obesity-Linked Insulin Resistance
Abstract
1. The Remodeling of Adipose Tissue
2. The Incretin Hormones
3. Expression of GLP-1 and GIP Receptors in Animal and Human Tissues
4. Molecular Mechanisms of GLP-1 and GIP
5. GLP-1 and GIP Actions in Liver and Skeletal Muscle
6. Expression and Activity of GLP-1 Receptors in Adipose Tissue
6.1. White Adipose Tissue and GLP-1R Expression in Adipocytes and Stroma Vascular Fraction: A Depot-Dependent Difference?
6.2. Sex Differences in Response to GLP-1 RA Therapy in Type 2 Diabetes and Obesity: Prefential Effects in Subcutaneous vs. Visceral Fat Depot
6.2.1. Glucose Control
6.2.2. Weight Loss
6.2.3. Effects on Subcutaneous vs. Visceral Fat Depot
6.3. Brown and Bright Adipose Tissue: Other Potential Targets of GLP-1?
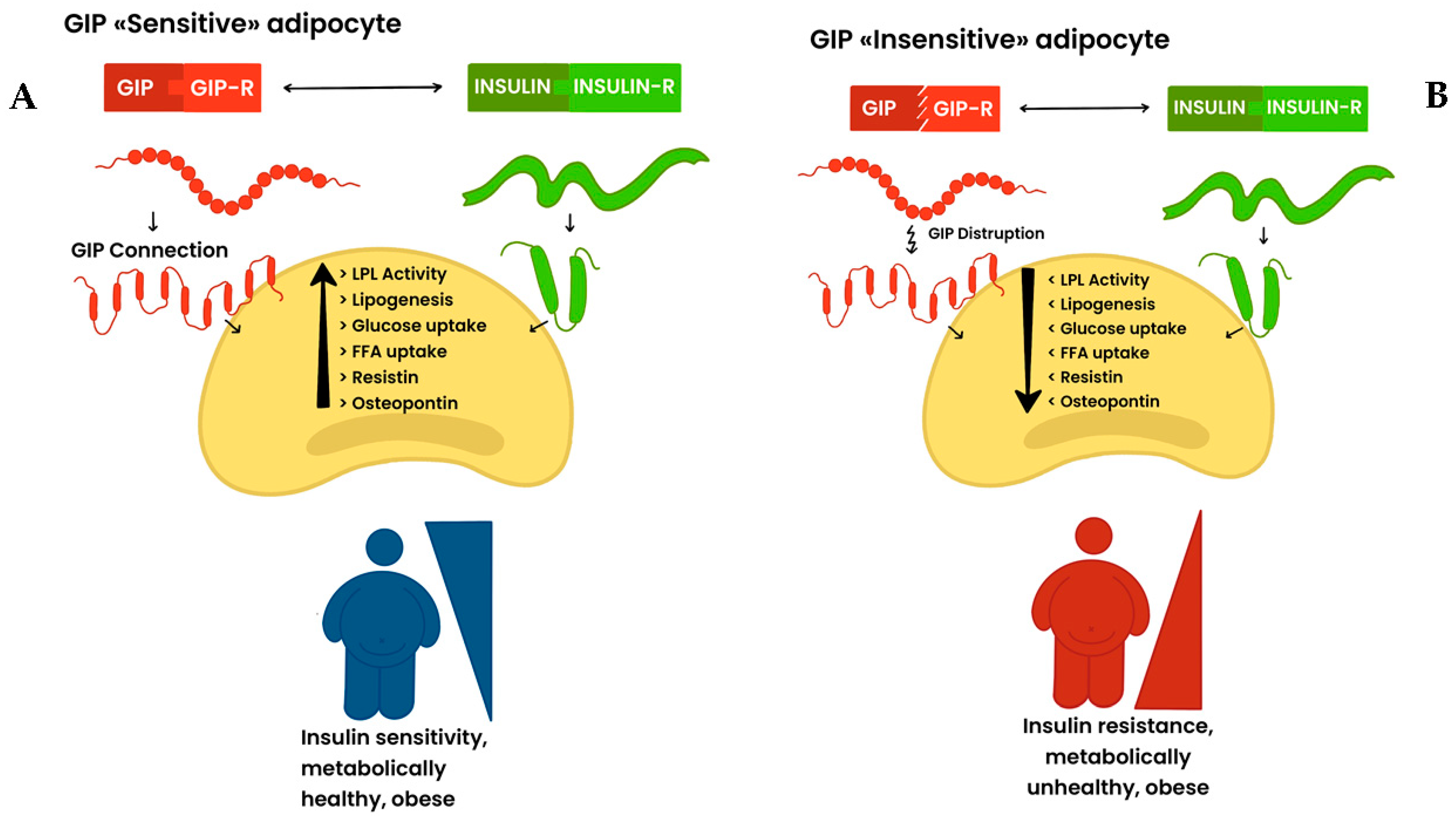
7. GIP and GIP Receptors: Novel Actors in Adipose Tissue (Patho)Physiopathology?
8. The Fine-Tuning of GLP-1/GLP1-R and GIP/GIP-R Axis in Fat: How to Reconcile This Challenging Puzzle?
9. Incretin-Based Therapy and Cancer Risk: Fact or Fake?
10. Concluding Remarks and Open Questions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cypess, A.M. Reassessing human adipose tissue. N. Engl. J. Med. 2022, 386, 768–779. [Google Scholar] [CrossRef] [PubMed]
- De Fano, M.; Bartolini, D.; Tortoioli, C.; Vermigli, C.; Malara, M.; Galli, F.; Murdolo, G. Adipose tissue plasticity in response to pathophsyiological cues: A connecting link between obesity and its associated comorbidities. Int. J. Mol. Sci. 2022, 23, 5511. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Danforth, E., Jr. Failure of adipocyte differentiation causes type II diabetes mellitus? Nat. Genet. 2000, 26, 13. [Google Scholar] [CrossRef]
- Sethi, J.K. Activatin’ human adipose progenitors in obesity. Diabetes 2010, 59, 2354–2357. [Google Scholar] [CrossRef]
- Sun, K.; Christine, M.; Kusminski, M.; Scherer, P. Adipose tissue remodelling in obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef]
- Hammarstedt, A.; Gogg, S.; Hedjazifar, S.; Nerstedt, A.; Smith, U. Impaired adipogenesis and dysfunctional adipose tissue in human hypetrophic obesity. Phisyiol. Rev. 2018, 98, 1911–1941. [Google Scholar] [CrossRef]
- Arner, E.; Westermark, P.O.; Spalding, K.L.; Britton, T.; Ryden, M.; Frisen, J.; Bernard, S.; Arner, P. Adipocye turnover: Relevance to human adipose tissue morphology. Diabetes 2010, 59, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Spalding, K.L.; Arner, E.; Westmermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Naslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Arner, P.; Arner, E.; Hmmarstedt, A.; Smith, U. Genetic predisposition for type 2 diabetes, but not for overweight/obesity, is associated with a restricted adipogenesis. PLoS ONE 2011, 6, e18284. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Pfeiffer, A.F.H. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes Obes. Metab. 2021, 23 (Suppl. 3), 5–29. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; El-Ouaghlidi, A.; Gabrys, B.; Hücking, K.; Holst, J.J.; Deacon, C.F.; Gallwitz, B.; Schmidt, W.E.; Meier, J.J. Secretion of incretin hormones (GIP and GLP-1) and incretin effect after oral glucose in first-degree relatives of patients with type 2 diabetes. Regul. Pept. 2004, 122, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Gallwitz, B.; Siepmann, N.; Holst, J.J.; Deacon, C.F.; Schmidt, W.E.; Nauck, M.A. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion n healthy human subjects ay euglycaemia. Diabetologia 2003, 46, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Heimesaat, M.M.; Ørskov, C.; Holst, J.J.; Ebert, R.; Creutzfeldt, W. Preserved incretin activity of glucagon-like peptide 1 (7–36 amide) but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Investig. 1993, 91, 301–307. [Google Scholar] [CrossRef]
- Nauck, M.A.; Stöckmann, F.; Ebert, R.; Creutzfeldy, W. Reduced incretin effect in Type 2 (non-insulin dependent) diabetes. Diabetologia 1986, 29, 46–54. [Google Scholar] [CrossRef]
- Bagger, J.I.; Knop, F.K.; Lund, A.; Vestergaard, H.; Holst, J.J.; Vilsbøll, T. Impaired regulation of the incretin effect in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2011, 96, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Knop, F.K.; Vilsbøll, T.; Højberg, P.V.; Larsen, S.; Madsbad, M.; Vølund, A.; Holst, J.J.; Krarup, T. Reduced incretin effect in type 2 diabetes: Cause or consequence of the diabetic state? Diabetes 2007, 56, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Vilsbøll, T.; Krarup, T.; Madsbad, S.; Holst, J.J. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia 2002, 45, 1111–1119. [Google Scholar] [CrossRef]
- Iqbal, J.; Wu, H.X.; Hu, N.; Zhou, Y.H.; Li, L.; Xiao, F.; Wang, T.; Jiang, H.L.; Xu, S.N.; Huang, B.L.; et al. Effect of glucagon-like peptide-1 receptor agonists on body weight in adults with obesity without diabetes mellitus-a systematic review and meta-analysis of randomized control trials. Obes. Rev. 2022, 23, e13435. [Google Scholar] [CrossRef]
- Kittah, E.; Camilleri, M.; Jensen, M.D.; Vella, A. A pilot study examining the effects of GLP-1 receptor blockage using exendin-(9,39) on gastric emptying and caloric intake in subjects with and without bariatric surgery. Metab. Syndr. Relat. Disord. 2020, 18, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Flint, A.; Jones, K.L.; Hindsberger, C.; Rasmussen, M.F.; Kapitza, C.; Doran, S.; Jax, T.; Zdravkovic, M.; Champan, I.M. Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res. Clin. Pract. 2012, 97, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in treatment of type 2 diabetes—State-of-the-art. Mol Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, M.A.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; D’Alessio, D.A. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regarding glycaemic control and body weight reduction. Cardiovasc. Diabetol. 2022, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- McLean, B.A.; Wong, C.K.; Campbell, J.E.; Hodson, D.J.; Trapp, S.; Drucker, D.J. Revisiting the complexity of GLP-1 action from sites of synthesis to receptor activation. Endocr. Rev. 2021, 42, 101–132. [Google Scholar] [CrossRef]
- Heimbürger, S.M.; Bergmann, N.C.; Augustin, R.; Gasbjerg, L.S.; Christensen, M.B.; Knop, F.K. Glucose-dependent insulinotropic polypeptide (GIP) and cardiovascular disease. Peptides 2020, 125, 170–174. [Google Scholar] [CrossRef]
- Muskiet, M.H.A.; Tonneijck, L.; Smits, M.M.; van Baar, M.J.B.; Kramer, M.H.H.; Hoorn, E.J.; Joles, J.A.; van Raalte, D.H. GLP-1 and the kidney: From physiology to pharmacology and outcomes in diabetes. Nat. Rev. Nephrol. 2017, 13, 605–628. [Google Scholar] [CrossRef]
- Secher, A.; Jelsing, J.; Baquero, A.F.; Hecksher-Sørensen, J.; Cowley, M.A.; Dalbøge, L.S.; Hansen Gm Grove, K.L.; Pyke, C.; Raun, K.; Schäffer, L.; et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Investig. 2014, 124, 4473–4488. [Google Scholar] [CrossRef]
- Gabery, S.; Salinas, C.G.; Paulsen, S.J.; Anhfelt-Rønne, J.; Alanentalo, T.; Baquero, A.F.; Buckley, S.T.; Farkas, E.; Fekete, C.; Frederiksen, K.S.; et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020, 5, e133429. [Google Scholar] [CrossRef]
- Mejer, J.J.; Gallwitz, B.; Salmen, S.; Goetze, O.; Holst, J.J.; Schmidt, W.E.; Nauck, M.A. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2003, 88, 2719–2725. [Google Scholar] [CrossRef]
- Thazhath, S.S.; Marathe, C.S.; Wu, T.; Chang, J.; Khoo, J.; Kuo, P.; Checklin, H.L.; Bound, M.J.; Rigda, R.S.; Crouch, B.; et al. The glucagon-like peptide 1 receptor agonist exenatide inhibits small intestinal motility, flow, transit, and absorption of glucose in healthy subjects and patients with type 2 diabetes: A randomized controlled trial. Diabetes 2016, 65, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Heppner, K.M.; Kirigiti, M.; Secher, A.; Paulsen, S.J.; Buckingham, R.; Pyke, C.; Knudsen, L.B.; Vrang, N.; Grove, K.L. Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology 2015, 156, 255–267. [Google Scholar] [CrossRef]
- Caltabiano, R.; Condorelli, D.; Panza, S.; Boitani, C.; Musso, N.; Jezek, D.; Memeo, L.; Colarossi, M.; Rago, V.; Mularoni, V.; et al. Glucagon-like peptide-1 receptor is expressed in human and rodent testis. Andrology 2020, 8, 1935–1945. [Google Scholar] [CrossRef]
- Zhang, E.; Xu, F.; Liang, H.; Yan, J.; Xu, H.; Li, Z.; Wen, X.; Weng, J. GLP-1 receptor agonis exenatide attenuates the detrimental effects of obesity on inflammatory profile in testis and sperm quality in mice. Am. J. Reprod. Immunol. 2015, 74, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Ma, D.; Gao, X.; Wang, J.; Li, R.; Liu, Z.; Wang, T.; Wang, S.; Liu, J.; Liu, X. Liraglutide ameliorates erectile dysfunction via regulating oxidative stress, the RhoA/ROCK pathway and autophagy in diabetes mellitus. Front. Pharmacol. 2020, 11, 1257. [Google Scholar] [CrossRef] [PubMed]
- Marzook, A.; Tomas, A.; Jones, B. The interplay of glucagon-like peptide-1 receptor trafficking and signalling in pancreatic beta cells. Front. Endocrinol. 2021, 12, 678055. [Google Scholar] [CrossRef] [PubMed]
- Khoo, S.; Griffen, S.C.; Xia, Y.; Baer, R.J.; German, M.S.; Cobb, M.H. Regulation of insulin gene transcription by ERK1 and ERK2 in pancreatic β cells. J. Biol. Chem. 2003, 278, 32969–32977. [Google Scholar] [CrossRef] [PubMed]
- Quoyer, J.; Longuet, C.; Broca, C.; Linck, N.; Costes, S.; Varin, E.; Bockaert, J.; Bertrand, G. Dalle SGLP-1 mediates antiapoptotic effect by phosphorylating Bad through a β-arrestin 1-mediated ERK1/2 activation in pancreatic β-cells. J. Biol. Chem. 2010, 285, 1989–2002. [Google Scholar] [CrossRef]
- Dyachok, O.; Isakov, Y.; Sågetorp, J.; Tengholm, A. Oscillations of cyclic AMP in hormonestimulated insulin-secreting β-cells. Nature 2006, 439, 349–352. [Google Scholar] [CrossRef]
- Wooten, D.; Miller, L.J.; Koole, C.; Christopoulos, A.; Sexton, P.M. Allostery and biased agonism at class B G protein-coupled receptors. Chem. Rev. 2017, 117, 111–138. [Google Scholar] [CrossRef] [PubMed]
- Ehses, J.A.; Pelech, S.L.; Pederson, R.A.; McIntosh, C.H.S. Glucose-dependent insulinotropic polypeptide activates the Raf-Mek1/2-ERK1/2 module via a cyclic AMP/cAMP-dependent protein kinase/Rap1-mediated pathway. J. Biol. Chem. 2002, 277, 37008–37097. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Fu, Y.; Lin, H.Y.; Cordonier, E.L.; Mo, Q.; Gao, Y.; Yao, T.; Naylor, J.; Howard, V.; Saito, K.; et al. Gut-dervide GIP activates central Rap1 to impair neural leptin sensitivity during overnutrition. J. Clin. Investig. 2019, 129, 3786–3791. [Google Scholar] [CrossRef] [PubMed]
- Prigeon, R.L.; Quddusi, S.; Paty, B.; D’Alessio, D.A. Suppression of glucose production by GLP-1 independent of islet hormones: A novel extrapancreatic effect. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E701–E707. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, D.A.; D’Alessio, D.A. Physiology of Proglucagon Peptides: Role of Glucagon and GLP-1 in Health and Disease. Physiol. Rev. 2015, 95, 513–548. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, S.; Zvibel, I.; Shnell, M.; Shlomai, A.; Chepurko, E.; Halpern, Z.; Barzilai, N.; Oren, R.; Fishman, S. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J. Hepatol. 2011, 54, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Svegliati-Baroni, G.; Saccomanno, S.; Rychlicki, C.; Agostinelli, L.; De Minicis, S.; Candelaresi, C.; Faraci, G.; Pacetti, D.; Vivarelli, M.; Nicolini, D.; et al. Glucagon-like peptide-1 receptoractivation stimulates hepatic lipid oxidation and restores hepatic signalling alterationinduced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011, 31, 1285–1297. [Google Scholar] [CrossRef]
- Ding, X.; Saxena, N.K.; Lin, S.; Gupta, N.A.; Gupta, N.; Anania, F.A. Exendin-4, a glucagon-likeprotein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 2006, 43, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.A.; Mells, J.; Dunham, R.M.; Grakoui, A.; Handy, J.; Saxena, N.K.; Anania, F.A. Gluca-gon-like peptide-1 receptor is present on human hepatocytes and has a direct role indecreasing hepatic steatosis in vitro by modulating elements of the insulin signalingpathway. Hepatology 2010, 51, 1584–1592. [Google Scholar] [CrossRef]
- Thondam, S.K.; Cuthbertson, D.J.; Wilding, J.P.H. The influence of Glucose-dependent Insulinotropic Polypeptide (GIP) on human adipose tissue and fat metabolism: Implications for obesity, type 2 diabetes and Non-Alcoholic Fatty Liver Disease (NAFLD). Peptides 2020, 125, 170208. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Pacini, G.; De Michieli, F.; Cassader, M. Prolonged satured fat-induced, glucose-dependent insulinotropic polypeptide elevation is associated with adipokine imbalance and liver injury in nonalcoholic steatohepatitis: Dysregulated enteroadipocyte axis as a novel feature of fatty liver. Am. J. Clin. Nutr. 2009, 89, 558–567. [Google Scholar] [CrossRef]
- Pfeiffer, A.F.H.; Keyhani-Nejad, F. High glycemic inder metabolic damage—A pivotal role of GIP and GLP-1. Trends Endocrinol. Metab. 2018, 29, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Rudovich, N.N.; Weickert, M.O.; Machann, J.; Pfeiffer, A.F.H. Combination of acarbose and ezetimibe prevents non-alcoholic fatty liver disease: A break of intestinal insulin resistance? J. Hepatol. 2010, 52, 952–953. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, M.; Li, T.; Dong, N.; Yi, L.; Zhang, Q.; Mi, M. GLP-1 regulates exercise endurance and skeletal muscle remodeling via GLP-1R/AMPK pathway. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119300. [Google Scholar] [CrossRef]
- O’Harte, F.P.; Gray, A.M.; Flatt, P.R. Gastric inhibitory polypeptide and effects of glycation on glucose transport and metabolism in isolated mouse abdominal muscle. J. Endocrinol. 1998, 156, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Snook, L.A.; Nelson, E.M.; Dyck, D.J.; Wright, D.C.; Holloway, G.P. Glucose-dependent insulinotropic polypeptide directly induces glucose transport in rat skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, 295–303. [Google Scholar] [CrossRef]
- Vendrell, J.; El Bekay, R.; Peral, B.; García-Fuentes, E.; Megia, A.; Macias-Gonzalez, M.; Fernández Real, J.; Jimenez-Gomez, Y.; Escoté, X.; Pachón, G.; et al. Study of the potential association of adipose tissue GLP-1 receptor with obesity and insulin resistance. Endocrinology 2011, 152, 4072–4079. [Google Scholar] [CrossRef]
- Ruiz-Grande, C.; Alarcón, C.; Mérida, E.; Valverde, I. Lipolytic action of glucagon-like peptides in isolated rad adipocytes. Peptides 1992, 13, 13–16. [Google Scholar] [CrossRef]
- Perea, A.; Vinambres, C.; Clemente, F.; Villanueva-Penacarillo, M.L.; Valverde, I. GLP-1 (7-36) amide: Effects on glucose transport and metabolism in rat adipose tissue. Horm. Metab. Res. 1997, 29, 417–421. [Google Scholar] [CrossRef]
- Villanuela-Penacarillo, M.L.; Márquez, L.; González, N.; Díaz-Miguel, M.; Valverde, I. Effect of GLP-1 on lipid metabolism in human adipocytes. Horm. Metab. Res. 2001, 33, 73–77. [Google Scholar] [CrossRef] [PubMed]
- El Bekay, R.; Coín-Aragüez, L.; Fernández-García, D.; Oliva-Olivera, W.; Bernal-López, R.; Clemente-Postigo, M.; Delgado-Lista, J.; Diaz-Ruiz, A.; Guzman-Ruiz, R.; Vázquez-Martínez, R.; et al. Effects of glucagon-like peptide-1 on the differentiation and metabolism of human adipocytes. Br. J. Pharmacol. 2016, 173, 1820–1834. [Google Scholar] [CrossRef] [PubMed]
- Challa, T.D.; Beaton, N.; Arnold, M.; Rudofsky, G.; Langhans, W.; Wolfrum, C. Regulation of adipocyte formation by GLP-1/GLP-1R signalign. J. Bio Chem. 2012, 287, 6421–6430. [Google Scholar] [CrossRef]
- Yang, J.; Ren, J.; Song, J.; Liu, F.; Wu, C.; Wang, X.; Gong, L.; Li, W.; Xiao, F.; Yan, F.; et al. Glucagon-like peptide 1 regulates adipogenesis in 3T3-L1 preadipocytes. Int. J. Mol. Med. 2013, 31, 1429–1435. [Google Scholar] [CrossRef]
- Gao, H.; Wang, X.; Zhang, Z.; Yang, Y.; Yang, J.; Li, X.; Ning, G. GLP-1 amplifies insulin signaling by up-regulation of IRbeta, IRS-1 and Glut4 IN 3T3-L1 adipocytes. Endocrine 2007, 32, 90–95. [Google Scholar] [CrossRef]
- Hoffstedt, J.; Arner, E.; Wahrenberg, H.; Andersson, D.P.; Qvisth, V.; Löfgren, P.; Rydén, M.; Thörne, A.; Wirén, M.; Palmér, M.; et al. Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia 2010, 53, 2496–2503. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; MacCallum, P.R. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J. Cardiometab. Syndr. 2009, 4, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Ejarque, M.; Guerrero-Pérez, F.; de la Morena, N.; Casajoana, A.; Virgili, N.; López-Urdiales, R.; Maymó-Masip, E.; Gebelli, J.P.; Garcia Ruiz de Gordjuela, A.; Perez-Maraver, M.; et al. Role of adipose tissue GL-1R expression in metabolic improvement after bariatric surgery in patients with type 2 diabetes. Sci. Rep. 2019, 9, 6274. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Camarena, V.; Sant, D.W.; Wang, G. Human epicardial fat expresses glucagon-like peptide 1 and 2 receptors genes. Horm. Metab. Res. 2017, 49, 625–630. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Wajchenberg, B.L. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr. Rev. 2000, 21, 697–738. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Park, M.S.; Choung, J.S.; Kim, S.S.; Oh, H.H.; Choi, C.S.; Ha, S.Y.; Kang, Y.; Kim, Y.; Jun, H.S. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 2012, 55, 2456–2468. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.R.; Kimura, K.; Ishida, M.; Sugisawa, H.; Kishikawa, A.; Shima, K.; Ogawa, S.; Qi, J.; Kitaura, H. The glucagon-like peptide-1 receptor agonist Exendin-4 inhibits lipopolysaccharide-induced osteoclast formation and bone resorption via inhibition of TNF-α expression in macrophages. J. Immuol. Res. 2018, 13, 5783639. [Google Scholar] [CrossRef] [PubMed]
- Krasner, N.M.; Ido, Y.; Ruderma, N.B.; Cacicedo, J.M. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS ONE 2014, 9, e97554. [Google Scholar] [CrossRef] [PubMed]
- Rentzeperi, E.; Pegiou, S.; Koufakis, T.; Grammatiki, M.; Kotsa, K. Sex differences in response to treatment with Glucagon-like peptide 1 receptor agonists: Opportunities for a tailored approach to diabetes and obesity care. J. Pers. Med. 2022, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Milani, I.; Guarisco, G.; Chinucci, M.; Gaita, C.; Leonetti, F.; Capoccia, D. Sex-differences in response to treatment with liraglutide 3.0 mg. J. Clin. Med. 2024, 13, 3369. [Google Scholar] [CrossRef] [PubMed]
- Piccini, S.; Favecchio, G.; Morenghi, E.; Mazziotti, G.; Lania, A.G.A.; Mirani, M. Real-world sex differences in type 2 diabetes patients treated with GLP-1 receptor agonists. Diabetes Res. Clin. Pract. 2024, 212, 111689. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.; Vicennati, V.; Gambineri, A.; Pagotto, U. Sex-dependent role of glucocorticoids and andogens in the pathopshyiology of human obesity. Int. J. Obes. 2008, 32, 1764–1779. [Google Scholar] [CrossRef]
- Roche, M.M.; Wang, P.P. Sex diffences in all-cause and cardiovascular mortality, hospitalization for individuals with and without diabetes, and patients with diabetes diagnosed early and late. Diabetes Care 2013, 36, 2582–2590. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.; Vlajnic, A.; Knutsen, P.G.; Recklein, C.; Rimler, M.; Fisher, S.J. Effect of gender on treatment outcomes in type 2 diabetes mellitus. Diabetes Res. Clin. Pr. 2013, 102, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Kim, A.; Lee, S.J.; Kim, J.Y.; Kim, J.H.; Lee, W.J.; Lee, B.W. Characteristics of dapagliflozin responders: A longitudinal, prospective, nationwide dapagliflozin surveillance study in Korea. Diabetes Ther. 2018, 9, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Campesi, I.; Seghieri, G.; Franconi, F. Type 2 diabetic woman are not small type 2 diabetic men: Sex-and-gender differences in antidiabetic drugs. Curr. Opin. Pharmacol. 2021, 60, 40–45. [Google Scholar] [CrossRef]
- Gallwitz, B.; Dagogo-Jack, S.; Thieu, V.; Garcia-Perez, L.E.; Pavo, I.; Yu, M.; Robertson, K.E.; Zhang, N.; Giorgino, F. Effect of once-weekly dulaglutide on glycated haemoglobin (Hba1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes Obes. Metab. 2018, 20, 409–418. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, H.; Wei, W.; Fang, T. Gender-related different effects of a combined therapy of Exenatide and Metformin on overweight or obesity patients with type 2 diabetes mellitus. J. Diabetes Its Complicat. 2016, 30, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Durden, E.; Lenhart, G.; Lopez-Gonzalez, L.; Hammer, M.; Langer, J. Predictors of glycemic control and diabetes-related costs among type 2 diabetes patients initiating therapy with liraglutide in the United States. J. Med. Econ. 2016, 19, 403–413. [Google Scholar] [CrossRef]
- Anichini, R.; Cosimi, S.; Di Carlo, A.; Orsini, P.; De Bellis, A.; Seghieri, G.; Franconi, F.; Baccetti, F. Gender difference in response predictors after 1-year exenatide therapy twice daily in type 2 diabetic patients: A real world experience. Diabetes Metab. Syndr. Obes. 2013, 6, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, R.V.; Petri, K.C.; Jacobsen, L.V.; Jensen, C.B. Liraglutide 3.0 mg for weight management: A population pharmacokinetic analysis. Clin. Pharmacokinet. 2016, 55, 1413–1422. [Google Scholar] [CrossRef]
- Weiss, T.; Carr, R.D.; Pal, S.; Yang, L.; Sawhney, B.; Boggs, R.; Rajpathak, S.; Iglay, K. Real-World adherence and discontinuations of glucagon-like peptide-1 receptor agonists therapy in type 2 diabetes mellitus patients in the United States. Patient Prefer. Adherence 2020, 14, 2337–2345. [Google Scholar] [CrossRef]
- Richard, J.E.; Anderberg, R.H.; López-Ferreras, L.; Olandersson, K.; Skibicka, K.P. Sex and estrogens alter the action of glucagon-like peptide-1 on reward. Biol. Sex Differ. 2016, 7, 6. [Google Scholar] [CrossRef]
- Gong, E.J.; Garrel, D.R.; Calloway, D.H. Menstrual cycle and voluntary food intake. Am. J. Clin. Nutr. 1989, 49, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, Q.; Zhang, H.; Zhang, Y.; Yang, G.; Ban, B.; Li, Y.; Zhang, M. The effects of glucagon-like peptide-1 receptor agonists on adipose tissues in patients with type 2 diabetes: A meta-analysis of randomised controlled trials. PLoS ONE 2022, 17, e0270899. [Google Scholar] [CrossRef]
- Xu, F.; Lin, B.; Zheng, X.; Chen, Z.; Cao, H.; Xu, H.; Liang, H.; Weng, J. GLP-1 receptor agonist promotes brown remodelling in mouse white adipose tissue through SIRT1. Diabetologia 2016, 59, 1059–1069. [Google Scholar] [CrossRef]
- Hropot, T.; Herman, R.; Janez, A.; Lezaic, L.; Jensterle, M. Brown adipose tissue: A new potential target for glucagon-like peptide 1 receptor agonists in the treatment of obesity. Int. J. Mol. Sci. 2023, 24, 8592. [Google Scholar] [CrossRef] [PubMed]
- Kurylowicz, A.; Puzianowska-Kuznicka, M. Induction of adipose tissue browning as a strategy to combat obesity. Int. J. Mol. Sci. 2020, 21, 6241. [Google Scholar] [CrossRef]
- Oliveira, F.C.B.; Bauer, E.J.; Ribeiro, C.M.; Pereira, S.A.; Beserra, B.T.S.; Wajner, S.M.; Maia, A.L.; Neves, F.A.R.; Coelho, M.S.; Amato, A.A. Liraglutide activates type 2 deiodinase and enhances β3-adrenergic-induced thermogenesis in mouse adipose tissue. Front. Endocrinol. 2022, 12, 803363. [Google Scholar] [CrossRef]
- Gutierrez, A.D.; Gao, Z.; Hamidi, V.; Andre, K.B.S.; Riggs, K.; Ruscheinksy, M.; Wang, H.; Yu, Y.; Miller, C.; Miller, C., 3rd; et al. Anti-diabetic effects of GLP1 analogs are mediated by thermogenic interleukin-6 signaling in adipocytes. Cell Rep. Med. 2022, 3, 100813. [Google Scholar] [CrossRef] [PubMed]
- van Eyk, H.J.; Paiman, E.H.M.; Bizino, M.B.; Ijzermans, S.L.; Kleiburg, F.; Boers, T.G.W.; Rappel, E.J.; Burakiewicz, J.; Kan, H.E.; Smit, J.W.A.; et al. Liraglutide decreases energy expenditure and does not affect the fat fraction of supraclavicular brown adipose tissue in patients with type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 616–624. [Google Scholar] [CrossRef]
- Janssen, L.G.M.; Nahon, K.J.; Bracké, K.F.M.; van den Broek, D.; Smit, R.; Sardjoe Mishre, A.S.D.; Koorneef, L.L.; Martinez-Tellez, B.; Burakiewicz, J.; Kan, H.E.; et al. Twelve weeks of exenatide treatment increases [18F] fluorodeoxyglucose uptake by brown adipose tissue without affecting oxidative resting energy expenditure in nondiabetic males. Metabolism 2020, 106, 154167. [Google Scholar] [CrossRef]
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villaroya, F.; Serrano, M.; Fernø, J.; Salvador, J.; Escalada, J.; et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014, 63, 3346–3358. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Hirsch, J.; Gallagher, D.A.; Leibel, R.L. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am. J. Clin. Nutr. 2008, 88, 906–912. [Google Scholar] [CrossRef]
- Hauner, H.; Glatting, G.; Kaminska, D.; Pfeiffer, E.F. Effects of gastric inhibitory polypeptide on glucose and lipid metabolism of isolated rat adipocytes. Ann. Nutr. Metab. 1988, 32, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Yip, R.G.; Boylan, M.O.; Kieffer, T.J.; Wolfe, M.M. Functional GIP receptor sare present on adipocytes. Endocrinology 1998, 139, 4004–4007. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Fujimoto, W.Y.; Brunzell, J.D. Gastric inhibitory polypeptide enhanced lipoprotein lipase activity in cultured preadipocytes. Diabetes 1979, 28, 1141–1142. [Google Scholar] [CrossRef] [PubMed]
- Getty-Kaushik, L.; Song, D.H.; Boylan, M.O.; Corkey, B.E.; Wolfe, M.M. Glucose-dependent insulinotropic polypeptide modulates adipocyte lipolysis and reesterification. Obesity 2006, 14, 1124–1131. [Google Scholar] [CrossRef]
- Song, D.H.; Getty-Kaushik, L.; Tseng, E.; Simon, J.; Corkey, B.E.; Wolfe, M.M. Glucose-dependent insulinotropic polypeptide enhances adipocyte development and glucose uptake in part through Akt activation. Gastroenterology 2007, 133, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Grisouard, J.; Sauter, N.S.; Herzog-Radimerski, T.; Dembinski, K.; Peterli, R.; Frey, D.M.; Zulewski, H.; Keller, U.; Müller, B.; et al. Glucose-dependent insulinotropic polypeptide induces cytokine expression, lipolysis, and insulin resistance in human adipocytes. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1–E13. [Google Scholar] [CrossRef] [PubMed]
- Samms, R.J.; Coghlan, M.P.; Sloop, K.W. How may GIP enhance the therapeutic efficacy of GLP-1? Trends Endocrinol. Metab. 2020, 31, 410–421. [Google Scholar] [CrossRef]
- Irwin, N.; Gault, V.A.; O’Harte, F.P.M.; Flatt, P.R. Blockade of gastric inhibitory polypeptide (GIP) action as a novel means of countering insulin resistance in the treatment of obesity-diabetes. Peptides 2020, 125, 170203. [Google Scholar] [CrossRef]
- Gasbjerg, L.S.; Gabe, M.B.N.; Hartmann, B.; Christensen, M.B.; Knop, F.K.; Holst, J.J.; Rosenkilde, M.M. Glucose-dependent insulinotropic polypeptide (GIP) receptor antagonists as anti-diabetic agents. Peptides 2018, 100, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, K.; Yamada, Y.; Ban, N.; Ihara, Y.; Tsukiyama, K.; Zhou, H.; Fujimoto, S.; Oku, A.; Tsuda, K.; Toyokuni, S.; et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat. Med. 2002, 8, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, B.; Capozzi, M.E.; Nui, J.; Hannou, S.A.; Finan, B.; Naylor, J.; Ravn, P.; D’Alessio, D.; Campbell, J.E. Pharmacological antagonism of the incretin system protects against diet-induced obesity. Mol. Metab. 2020, 32, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Asmar, M.; Simonsen, L.; Madsbad, S.; Stallknecht, B.; Holst, J.J.; Bulow, J. Glucose-dependent insulinotropic polypeptide may enhance fatty acid re-esterification in subcutaneous abdominal adipose tissue in lean humans. Diabetes 2010, 59, 2160–2163. [Google Scholar] [CrossRef] [PubMed]
- Asmar, M.; Simonsen, L.; Asmar, A.; Holst, J.J.; Dela, F.; Bulow, J. Insulin plays a permissive role for the vasoactive effect of GIP regulating adipose tissue metabolism in humans. J. Clin. Endocrinol. Metab. 2016, 101, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Gogebakan, O.; Osterhoff, M.A.; Schuler, R.; Pivovarova, O.; Kruse, M.; Seltmann, A.C.; Mosig, A.S.; Rudovich, N.; Nauck, M.; Pfeiffer, A.F.H. GIP increases adipose tissue expression and blood levels of MCP-1 in humans and links high energy diets to inflammation: A randomised trial. Diabetologia 2015, 58, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Ceperuelo-Mallafré, V.; Duran, X.; Pachón, G.; Roche, K.; Garrido-Sànchez, L.; Vilarrasa, N.; Tinahones, F.J.; Vicente, V.; Pujol, J.; Vendrell, J.; et al. Disruption of GIP/GIPR axis in human adipose tissue is linked to obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2014, 99, E908–E919. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Ma, R.C.; Chan, J.C.; Xu, H.; Xu, G. Glucose-dependent insulinotropic peptide impairs insulin signaling via inducing adipocyte inflammation in glucose-dependent insulinotropic peptide receptor-overexpressing adipocytes. FASEB J. 2012, 26, 2388–2393. [Google Scholar] [CrossRef] [PubMed]
- Kloting, N.; Fasshauer, M.; Dietrich, A.; Kovacs, P.; Schön, M.R.; Kern, M.; Stumvoll, M.; Blüher, M. Insulin-sensitive obesity. Am. J. Physiol. (Endocrinol. Metab.) 2010, 299, E506–E515. [Google Scholar] [CrossRef]
- Wernstedt Asterholm, I.; Tao, C.; Morley, T.S.; Wang, Q.A.; Delgado-Lopez, F.; Wang, Z.V.; Scherer, P.E. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014, 20, 103–118. [Google Scholar] [CrossRef]
- Mantelmacher, F.D.; Zvibel, I.; Cohen, K.; Epshtein, A.; Pasmanik-Chor, M.; Vogl, T.; Kuperman, Y.; Weiss, S.; Drucker, D.J.; Varol, C.; et al. GIP regulates inflammation and body weight by restraining myeloid-cell-derived S100A8/A9. Nat. Metab. 2019, 1, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Mantelmacher, F.D.; Fishman, S.; Cohen, K.; Chor, M.S.; Yamada, Y.; Zvibel, I.; Varol, C. Glucose-dependent insulinotropic polypeptide receptor deficiency leads to impaire dbone marrow hematopoiesis. J. Immunol. 2017, 198, 3089–3098. [Google Scholar] [CrossRef]
- Kim, S.J.; Nian, C.; Karunakaran, S.; Clee, S.M.; Isales, C.M.; McIntosh, C.H. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS ONE 2012, 7, e40156. [Google Scholar] [CrossRef] [PubMed]
- Goralska, J.; Razny, U.; Polus, A.; Stancel-Mozwillo, J.; Chojnacka, M.; Gruca, A.; Zdzienicka, A.; Dembinska-Kiec, A.; Kiec-Wilk, B.; Solnica, B.; et al. Pro-inflammatory gene expression profile in obese adults with high plasma GIP levels. Int. J. Obes. 2018, 42, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Ahlqvist, E.; Osmark, P.; Kuulasmaa, T.; Pilgaard, K.; Omar, B.; Brøns, C.; Kotova, O.; Zetterqvst, A.V.; Stancáková, A.; Jonsson, A.; et al. Link between GIP and osteopontin in adipose tissue and insulin resistance. Diabetes 2013, 62, 2088–2094. [Google Scholar] [CrossRef]
- Finan, B.; Müller, T.D.; Clemmensen, C.; Perez-Tilve, D.; DiMarchi, R.D.; Tschöp, M.H. Reapprisal of GIP pharmacology for metabolic disease. Trends Endocrinol. Metab. 2016, 22, 359–376. [Google Scholar] [CrossRef]
- Knudsen, L.B.; Madsen, L.W.; Andersen, S.; Almholt, K.; de Boer, A.S.; Drucker, D.J.; Gotfrdsen, C.; Egerod, F.L.; Hegelund, A.C.; Jacobsen, H.; et al. Glucagon-like peptide-1 receptor agonisys activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010, 15, 1473–1486. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Jensen, T.J.; Rosenkilde, C.; Calanna, S.; LEADER Publication Committee on behalf of the LEADER Trial Investigators. Neoplasms reported with liraglutide or placebo in people with type 2 diabetes: Results from the LEADER randomized trial. Diabetes Care 2018, 41, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.; Betal-Marques, F.; Macedo, A.F. A meta-analysis of serious adverse events reported with exenatide and liraglutide: Acute pancreatitis and cancer. Diabetes Res. Clin. Pract. 2012, 98, 271–284. [Google Scholar] [CrossRef]
- Dore, D.D.; Seeger, J.D.; Chan, K.A. Incidence of health insurance claims for thyroid neoplasm and pancreatic malignancy in association with exenatide: Signal refinement using active safety surveillance. Ther. Adv. Drug Saf. 2012, 3, 157–164. [Google Scholar] [CrossRef]
- Liang, C.; Bertoia, M.L.; Ding, Y.; Clifford, C.R.; Qiao, Q.; Gagne, J.J.; Dore, D.D. Exenatide use and incidence of pancreatic and thyroid cancer: A retrospective cohort study. Diabetes Obes. Metab. 2019, 21, 1037–1042. [Google Scholar] [CrossRef]
- Bezin, J.; Gouverneur, A.; Pénichon, M.; Mathieu, C.; Garrel, R.; Hillaire-Buys, D.; Pariente, A.; Faillie, J.L. GLP-1 receptor agonists and the risk of thyroid cancer. Diabetes Care 2023, 46, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Nreu, B.; Scatena, A.; Cresci, B.; Andreozzi, F.; Sesti, G.; Mannucci, E. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): Data from randomized controlled trials. Diabetes Obes. Metab. 2017, 19, 1233–1241. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Kaelber, D.C.; Xu, R.; Berger, N.A. GLP-1 receptor agonists and colorectal cancer risk in drug-naïve patients with type 2 diabetes, with and without overweight/obesity. JAMA Oncol. 2024, 10, 256–258. [Google Scholar] [CrossRef]
- Thondam, S.K.; Daousi, C.; Wilding, J.P.; Holst, J.J.; Ameen, G.I.; Yang, C.; Whitmore, C.; Mora, S.; Cuthbertson, D.J. Glucose-dependent insulinotropic polypeptide promotes lipid deposition in subcutaneous adipocytes in obese type 2 diabetes patients: A maladaptive response. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E224–E233. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Kaplan, L.M.; Frías, J.P.; Wu, Q.; Du, Y.; Gurbuz, S.; Coskun, T.; Haupt, A.; Milicevic, Z.; Hartman, M.L.; et al. Triple-Hormone-Receptor agonist Retatutride for obesity—A phase 2 trial. N. Engl. J. Med. 2023, 389, 514–526. [Google Scholar] [CrossRef]

| Effects of GLP-1 | Tissues | Effects of GIP |
|---|---|---|
| ↑↑ Insulin secretion ↓ Glucagon secretion | Pancreas | ↑ ↑ Insulin secretion ↑ Glucagon secretion |
| ↑ Glucose uptake, glycogen ↓ Hepatic glucose production ↓ Liver fat | Liver (indirect effects) | ↑ Glucose uptake, glycogen |
| ↑ Heart rate | Heart | ↑ Heart rate |
| ↑ Excretion of sodium | Kidney | No prominent direct effect |
| ↓ ↓ Caloric intake Effects of anti-apoptosis and synaptic plasticity | Brain | ↓ Caloric intake Effects of anti-apoptosis and synaptic plasticity (??) |
| ↓ Gastric emptying | Stomach | No prominent effect |
| ↓ Intestine motility | Gut | No prominent effect |
| ↓ Inflammation | Testicle | No prominent effect |
| ↑ Remodeling | Bone | ↑ ↑ Remodeling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Fano, M.; Malara, M.; Vermigli, C.; Murdolo, G. Adipose Tissue: A Novel Target of the Incretin Axis? A Paradigm Shift in Obesity-Linked Insulin Resistance. Int. J. Mol. Sci. 2024, 25, 8650. https://doi.org/10.3390/ijms25168650
De Fano M, Malara M, Vermigli C, Murdolo G. Adipose Tissue: A Novel Target of the Incretin Axis? A Paradigm Shift in Obesity-Linked Insulin Resistance. International Journal of Molecular Sciences. 2024; 25(16):8650. https://doi.org/10.3390/ijms25168650
Chicago/Turabian StyleDe Fano, Michelantonio, Massimo Malara, Cristiana Vermigli, and Giuseppe Murdolo. 2024. "Adipose Tissue: A Novel Target of the Incretin Axis? A Paradigm Shift in Obesity-Linked Insulin Resistance" International Journal of Molecular Sciences 25, no. 16: 8650. https://doi.org/10.3390/ijms25168650
APA StyleDe Fano, M., Malara, M., Vermigli, C., & Murdolo, G. (2024). Adipose Tissue: A Novel Target of the Incretin Axis? A Paradigm Shift in Obesity-Linked Insulin Resistance. International Journal of Molecular Sciences, 25(16), 8650. https://doi.org/10.3390/ijms25168650





