Antibody Drug Conjugates for Cancer Therapy: From Metallodrugs to Nature-Inspired Payloads
Abstract
1. Introduction
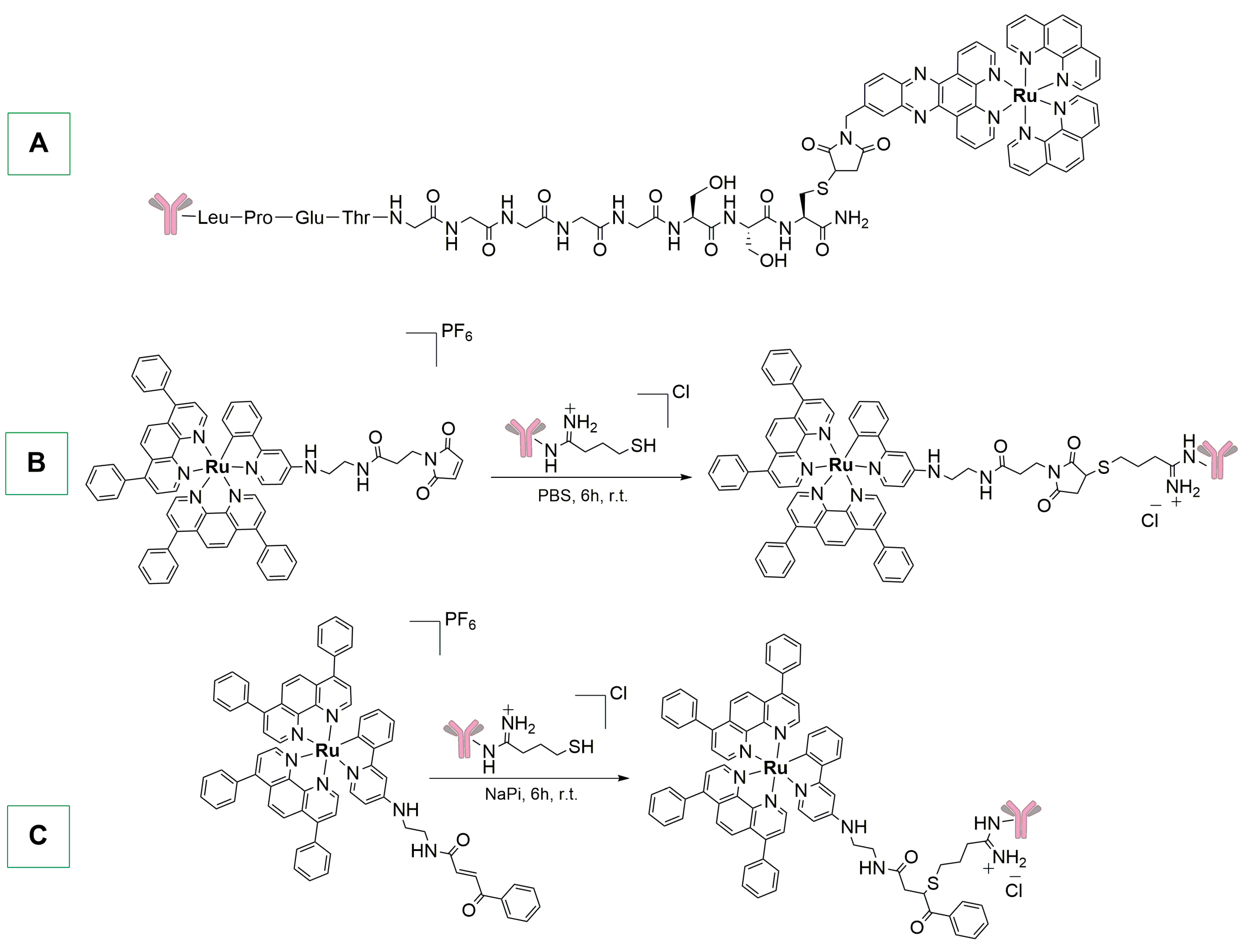
2. Photosynthetic Approaches to Metal-Based ADCs
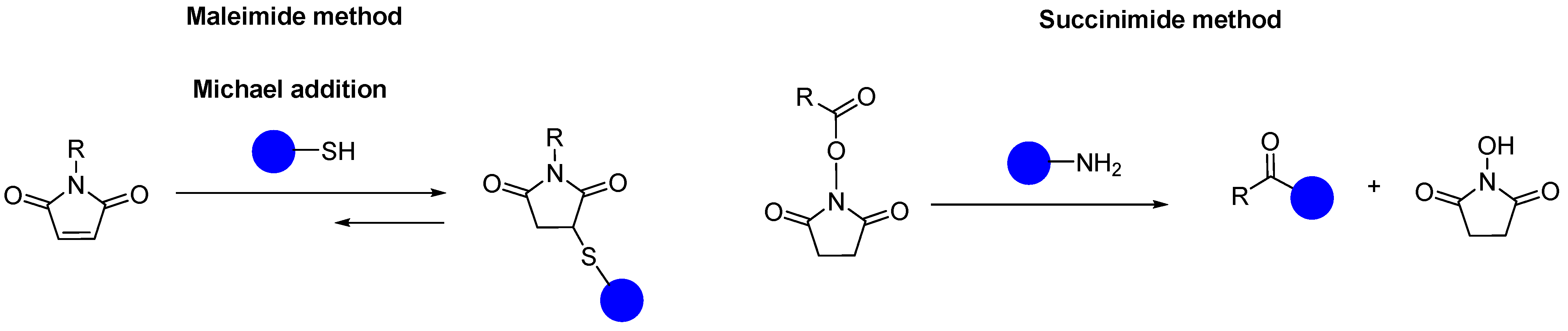
3. Maleimide, Succinimide, and Other Linkers for Metal-Based ADCs
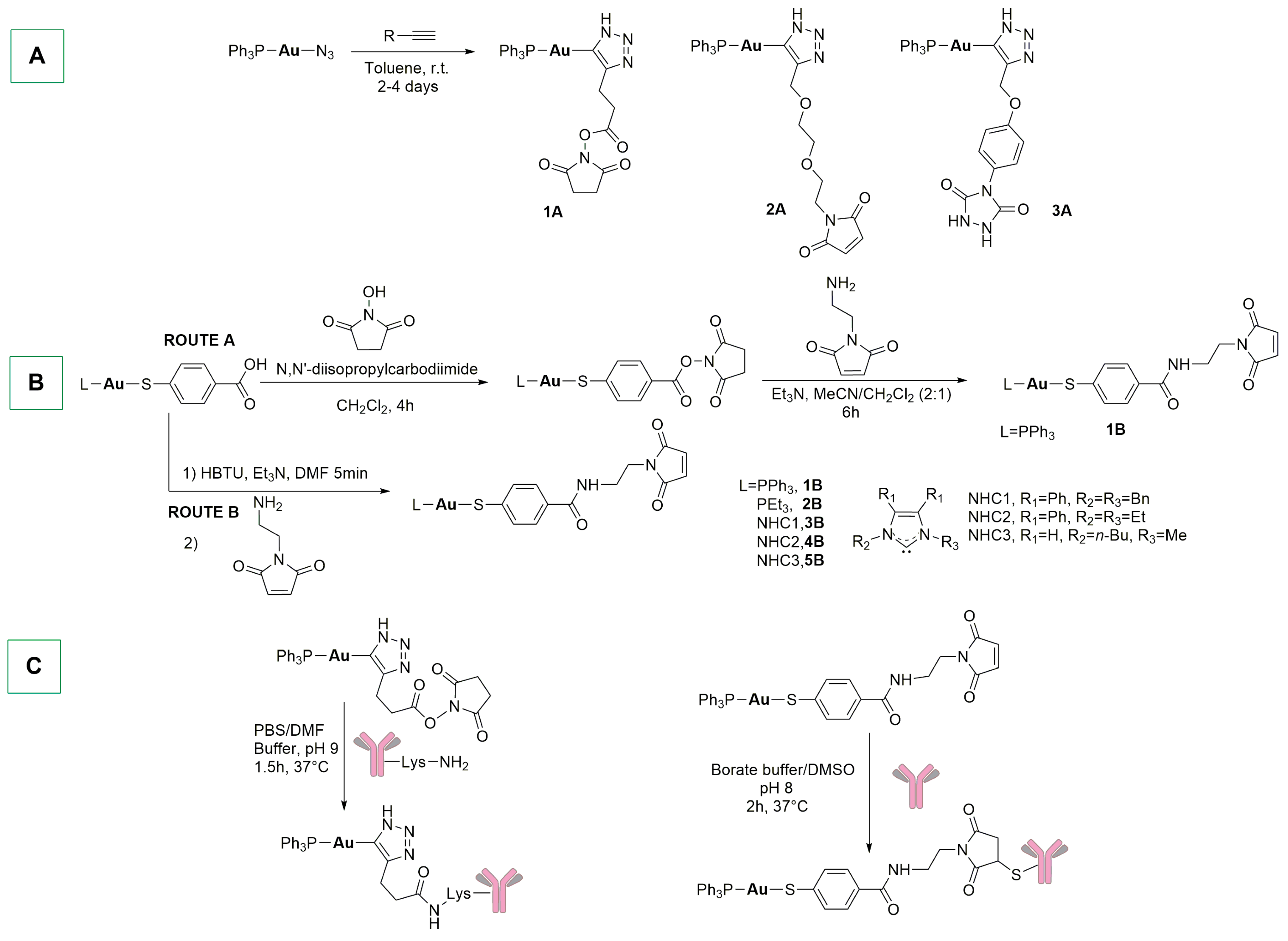
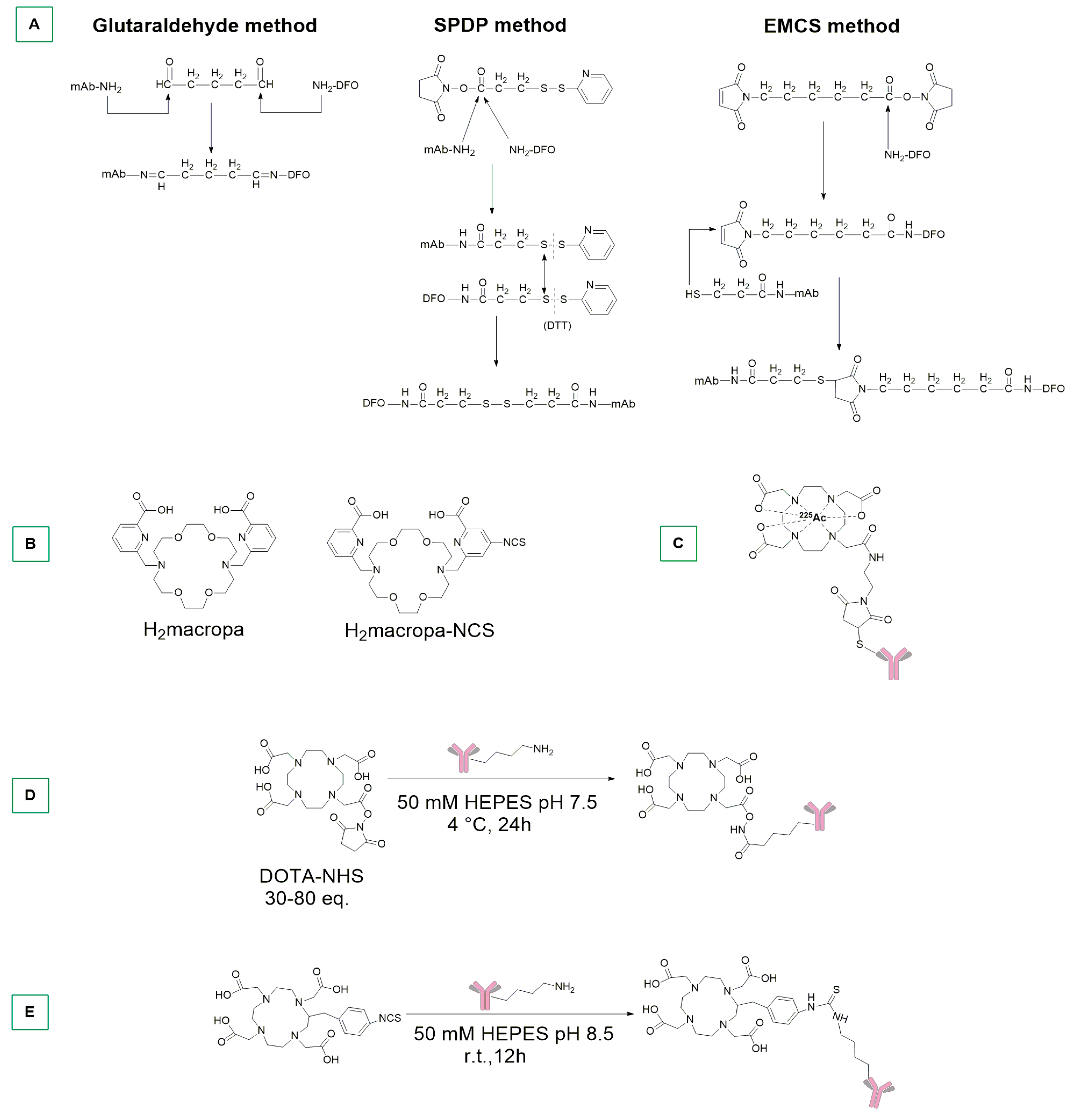
4. Metal-Based ADCs with Direct Metal–Antibody Conjugation and Stochastic Conjugations with Radiometals
5. ADCs Bearing Natural or Nature-Inspired Organic Payloads
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dumontet, C.; Reichert, J.M.; Senter, P.D.; Lambert, J.M.; Beck, A. Antibody–Drug Conjugates Come of Age in Oncology. Nat. Rev. Drug Discov. 2023, 22, 641–661. [Google Scholar] [CrossRef] [PubMed]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody–Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the Potential of Antibody–Drug Conjugates for Cancer Therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, N.K.; Dwiwedi, P.; Charan, J.; Kaur, R.; Sidhu, P.; Chugh, V.K. Monoclonal Antibodies: A Review. Curr. Clin. Pharmacol. 2018, 13, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Tsao, L.-C.; Force, J.; Hartman, Z.C. Mechanisms of Therapeutic Antitumor Monoclonal Antibodies. Cancer Res. 2021, 81, 4641–4651. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xiao, D.; Xie, F.; Liu, L.; Wang, Y.; Fan, S.; Zhou, X.; Li, S. Antibody–Drug Conjugates: Recent Advances in Linker Chemistry. Acta Pharm. Sin. B 2021, 11, 3889–3907. [Google Scholar] [CrossRef] [PubMed]
- Sheyi, R.; De La Torre, B.G.; Albericio, F. Linkers: An Assurance for Controlled Delivery of Antibody-Drug Conjugate. Pharmaceutics 2022, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Baah, S.; Laws, M.; Rahman, K.M. Antibody–Drug Conjugates—A Tutorial Review. Molecules 2021, 26, 2943. [Google Scholar] [CrossRef] [PubMed]
- Conilh, L.; Sadilkova, L.; Viricel, W.; Dumontet, C. Payload Diversification: A Key Step in the Development of Antibody–Drug Conjugates. J. Hematol. Oncol. 2023, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Masters, J.C.; Nickens, D.J.; Xuan, D.; Shazer, R.L.; Amantea, M. Clinical Toxicity of Antibody Drug Conjugates: A Meta-Analysis of Payloads. Investig. New Drugs 2018, 36, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Gou, L.; Li, W.; Wang, Y. Antibody–Drug Conjugates: Recent Advances in Payloads. Acta Pharm. Sin. B 2023, 13, 4025–4059. [Google Scholar] [CrossRef] [PubMed]
- Boni, V.; Sharma, M.R.; Patnaik, A. The Resurgence of Antibody Drug Conjugates in Cancer Therapeutics: Novel Targets and Payloads. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, e58–e74. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Polyak, K. Tumor Heterogeneity: Causes and Consequences. Biochim. Biophys. Acta BBA Rev. Cancer 2010, 1805, 105–117. [Google Scholar] [CrossRef]
- Alizadeh, A.A.; Aranda, V.; Bardelli, A.; Blanpain, C.; Bock, C.; Borowski, C.; Caldas, C.; Califano, A.; Doherty, M.; Elsner, M.; et al. Toward Understanding and Exploiting Tumor Heterogeneity. Nat. Med. 2015, 21, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Turashvili, G.; Brogi, E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef] [PubMed]
- Brady, L.; Kriner, M.; Coleman, I.; Morrissey, C.; Roudier, M.; True, L.D.; Gulati, R.; Plymate, S.R.; Zhou, Z.; Birditt, B.; et al. Inter- and Intra-Tumor Heterogeneity of Metastatic Prostate Cancer Determined by Digital Spatial Gene Expression Profiling. Nat. Commun. 2021, 12, 1426. [Google Scholar] [CrossRef] [PubMed]
- Proietto, M.; Crippa, M.; Damiani, C.; Pasquale, V.; Sacco, E.; Vanoni, M.; Gilardi, M. Tumor Heterogeneity: Preclinical Models, Emerging Technologies, and Future Applications. Front. Oncol. 2023, 13, 1164535. [Google Scholar] [CrossRef] [PubMed]
- Tsuchikama, K.; Anami, Y.; Ha, S.Y.Y.; Yamazaki, C.M. Exploring the next Generation of Antibody–Drug Conjugates. Nat. Rev. Clin. Oncol. 2024, 21, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Davodabadi, F.; Sajjadi, S.F.; Sarhadi, M.; Mirghasemi, S.; Nadali Hezaveh, M.; Khosravi, S.; Kamali Andani, M.; Cordani, M.; Basiri, M.; Ghavami, S. Cancer Chemotherapy Resistance: Mechanisms and Recent Breakthrough in Targeted Drug Delivery. Eur. J. Pharmacol. 2023, 958, 176013. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Li, Z.; He, Z.; Qiu, J.; Zhou, S. Molecular Mechanisms for Tumour Resistance to Chemotherapy. Clin. Exp. Pharmacol. Physiol. 2016, 43, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Pokhriyal, R.; Hariprasad, R.; Kumar, L.; Hariprasad, G. Chemotherapy Resistance in Advanced Ovarian Cancer Patients. Biomark. Cancer 2019, 11, 1179299X1986081. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.S.B. A Patent Review on FDA-Approved Antibody-Drug Conjugates, Their Linkers and Drug Payloads. ChemMedChem 2022, 17, e202200032. [Google Scholar] [CrossRef] [PubMed]
- Wedam, S.; Fashoyin-Aje, L.; Gao, X.; Bloomquist, E.; Tang, S.; Sridhara, R.; Goldberg, K.B.; King-Kallimanis, B.L.; Theoret, M.R.; Ibrahim, A.; et al. FDA Approval Summary: Ado-Trastuzumab Emtansine for the Adjuvant Treatment of HER2-Positive Early Breast Cancer. Clin. Cancer Res. 2020, 26, 4180–4185. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Brentuximab Vedotin: A Review in CD30-Positive Hodgkin Lymphoma. Drugs 2017, 77, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Weinstock, C.; Zhang, L.; Charlab, R.; Dorff, S.E.; Gong, Y.; Hsu, V.; Li, F.; Ricks, T.K.; Song, P.; et al. FDA Approval Summary: Enfortumab Vedotin for Locally Advanced or Metastatic Urothelial Carcinoma. Clin. Cancer Res. 2021, 27, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Abdollahpour-Alitappeh, M.; Lotfinia, M.; Gharibi, T.; Mardaneh, J.; Farhadihosseinabadi, B.; Larki, P.; Faghfourian, B.; Sepehr, K.S.; Abbaszadeh-Goudarzi, K.; Abbaszadeh-Goudarzi, G.; et al. Antibody–Drug Conjugates (ADCs) for Cancer Therapy: Strategies, Challenges, and Successes. J. Cell. Physiol. 2019, 234, 5628–5642. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lippard, S.J. Cellular Processing of Platinum Anticancer Drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L. The Resurgence of Platinum-Based Cancer Chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Alassadi, S.; Pisani, M.J.; Wheate, N.J. A Chemical Perspective on the Clinical Use of Platinum-Based Anticancer Drugs. Dalton Trans. 2022, 51, 10835–10846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-Based Drugs for Cancer Therapy and Anti-Tumor Strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D. Platinum(IV) Anticancer Agents; Are We En Route to the Holy Grail or to a Dead End? J. Inorg. Biochem. 2021, 217, 111353. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, C.Y.; Nam, T.-G. Ruthenium Complexes as Anticancer Agents: A Brief History and Perspectives. Drug Des. Devel. Ther. 2020, 14, 5375–5392. [Google Scholar] [CrossRef] [PubMed]
- Süss-Fink, G. Areneruthenium Complexes as Anticancer Agents. Dalton Trans. 2010, 39, 1673–1688. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I. Ruthenium Complexes as Anticancer Agents. Curr. Med. Chem. 2006, 13, 1085–1107. [Google Scholar] [CrossRef] [PubMed]
- Eichenberger, L.S.; Patra, M.; Holland, J.P. Photoactive Chelates for Radiolabelling Proteins. Chem. Commun. 2019, 55, 2257–2260. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Alcántar, G.; Picchetti, P.; Casini, A. Gold Complexes in Anticancer Therapy: From New Design Principles to Particle-Based Delivery Systems. Angew. Chem. 2023, 135, e202218000. [Google Scholar] [CrossRef]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent Advances in Gold–NHC Complexes with Biological Properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Collado, A.; Gómez-Suárez, A.; Martin, A.R.; Slawin, A.M.Z.; Nolan, S.P. Straightforward Synthesis of [Au(NHC)X] (NHC = N-Heterocyclic Carbene, X = Cl, Br, I) Complexes. Chem. Commun. 2013, 49, 5541. [Google Scholar] [CrossRef] [PubMed]
- Tzouras, N.V.; Scattolin, T.; Gobbo, A.; Bhandary, S.; Rizzolio, F.; Cavarzerani, E.; Canzonieri, V.; Van Hecke, K.; Vougioukalakis, G.C.; Cazin, C.S.J.; et al. A Green Synthesis of Carbene-Metal-Amides (CMAs) and Carboline-Derived CMAs with Potent in Vitro and Ex Vivo Anticancer Activity. ChemMedChem 2022, 17, e202200135. [Google Scholar] [CrossRef] [PubMed]
- Scattolin, T.; Tonon, G.; Botter, E.; Guillet, S.G.; Tzouras, N.V.; Nolan, S.P. Gold(I)- N -Heterocyclic Carbene Synthons in Organometallic Synthesis. Chem. Eur. J. 2023, 29, e202301961. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Gasser, G. The Medicinal Chemistry of Ferrocene and Its Derivatives. Nat. Rev. Chem. 2017, 1, 0066. [Google Scholar] [CrossRef]
- Braga, S.S.; Silva, A.M.S. A New Age for Iron: Antitumoral Ferrocenes. Organometallics 2013, 32, 5626–5639. [Google Scholar] [CrossRef]
- Ornelas, C. Application of Ferrocene and Its Derivatives in Cancer Research. New J. Chem. 2011, 35, 1973. [Google Scholar] [CrossRef]
- Van Staveren, D.R.; Metzler-Nolte, N. Bioorganometallic Chemistry of Ferrocene. Chem. Rev. 2004, 104, 5931–5986. [Google Scholar] [CrossRef] [PubMed]
- Scattolin, T.; Voloshkin, V.A.; Visentin, F.; Nolan, S.P. A Critical Review of Palladium Organometallic Anticancer Agents. Cell Rep. Phys. Sci. 2021, 2, 100446. [Google Scholar] [CrossRef]
- Scattolin, T.; Bortolamiol, E.; Visentin, F.; Palazzolo, S.; Caligiuri, I.; Perin, T.; Canzonieri, V.; Demitri, N.; Rizzolio, F.; Togni, A. Palladium(II)-η3-Allyl Complexes Bearing N-Trifluoromethyl N-Heterocyclic Carbenes: A New Generation of Anticancer Agents That Restrain the Growth of High-Grade Serous Ovarian Cancer Tumoroids. Chem. Eur. J. 2020, 26, 11868–11876. [Google Scholar] [CrossRef] [PubMed]
- Scattolin, T.; Pessotto, I.; Cavarzerani, E.; Canzonieri, V.; Orian, L.; Demitri, N.; Schmidt, C.; Casini, A.; Bortolamiol, E.; Visentin, F.; et al. Indenyl and Allyl Palladate Complexes Bearing N-Heterocyclic Carbene Ligands: An Easily Accessible Class of New Anticancer Drug Candidates. Eur. J. Inorg. Chem. 2022, 2022, e202200103. [Google Scholar] [CrossRef]
- Scattolin, T.; Bortolamiol, E.; Caligiuri, I.; Rizzolio, F.; Demitri, N.; Visentin, F. Synthesis and Comparative Study of the Anticancer Activity of H3-Allyl Palladium(II) Complexes Bearing N-Heterocyclic Carbenes as Ancillary Ligands. Polyhedron 2020, 186, 114607. [Google Scholar] [CrossRef]
- Bortolamiol, E.; Fama, F.; Zhang, Z.; Demitri, N.; Cavallo, L.; Caligiuri, I.; Rizzolio, F.; Scattolin, T.; Visentin, F. Cationic Palladium(II)-Indenyl Complexes Bearing Phosphines as Ancillary Ligands: Synthesis, and Study of Indenyl Amination and Anticancer Activity. Dalton Trans. 2022, 51, 11135–11151. [Google Scholar] [CrossRef] [PubMed]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Muhammad, N.; Hanif, M.; Yang, P. Beyond Cisplatin: New Frontiers in Metallodrugs for Hard-to-Treat Triple Negative Breast Cancer. Coord. Chem. Rev. 2024, 499, 215507. [Google Scholar] [CrossRef]
- González-Ballesteros, M.M.; Mejía, C.; Ruiz-Azuara, L. Metallodrugs: An Approach against Invasion and Metastasis in Cancer Treatment. FEBS Open Bio 2022, 12, 880–899. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Lucaciu, R.L.; Hangan, A.C.; Sevastre, B.; Oprean, L.S. Metallo-Drugs in Cancer Therapy: Past, Present and Future. Molecules 2022, 27, 6485. [Google Scholar] [CrossRef] [PubMed]
- Temesgen, A.; Ananda Murthy, H.C.; Enyew, A.Z.; Revathi, R.; Venkatesha Perumal, R. Emerging Trends in Metal-based Anticancer Agents: Drug Design to Clinical Trials and Their Mechanism of Action. ChemistrySelect 2023, 8, e202302113. [Google Scholar] [CrossRef]
- Zhang, P.; Sadler, P.J. Advances in the Design of Organometallic Anticancer Complexes. J. Organomet. Chem. 2017, 839, 5–14. [Google Scholar] [CrossRef]
- Hartinger, C.G. A Multifaceted Approach towards Organometallic Anticancer Agent Development. J. Organomet. Chem. 2024, 1012, 123144. [Google Scholar] [CrossRef]
- Peng, K.; Zheng, Y.; Xia, W.; Mao, Z.-W. Organometallic Anti-Tumor Agents: Targeting from Biomolecules to Dynamic Bioprocesses. Chem. Soc. Rev. 2023, 52, 2790–2832. [Google Scholar] [CrossRef] [PubMed]
- Del Solar, V.; Contel, M. Metal-Based Antibody Drug Conjugates. Potential and Challenges in Their Application as Targeted Therapies in Cancer. J. Inorg. Biochem. 2019, 199, 110780. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Maldonado, W.; Narváez-Pita, X.; Carmona-Negrón, J.; Olivero-Verbel, J.; Meléndez, E. Steroid-Functionalized Titanocenes: Docking Studies with Estrogen Receptor Alpha. Inorganics 2016, 4, 38. [Google Scholar] [CrossRef]
- Zinser, C.M.; Nahra, F.; Brill, M.; Meadows, R.E.; Cordes, D.B.; Slawin, A.M.Z.; Nolan, S.P.; Cazin, C.S.J. A Simple Synthetic Entryway into Palladium Cross-Coupling Catalysis. Chem. Commun. 2017, 53, 7990–7993. [Google Scholar] [CrossRef] [PubMed]
- Bortolamiol, E.; Visentin, F.; Scattolin, T. Recent Advances in Bioconjugated Transition Metal Complexes for Cancer Therapy. Appl. Sci. 2023, 13, 5561. [Google Scholar] [CrossRef]
- Englinger, B.; Pirker, C.; Heffeter, P.; Terenzi, A.; Kowol, C.R.; Keppler, B.K.; Berger, W. Metal Drugs and the Anticancer Immune Response. Chem. Rev. 2019, 119, 1519–1624. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Shen, B.; Rader, C. Challenges and Opportunities to Develop Enediyne Natural Products as Payloads for Antibody-Drug Conjugates. Antib. Ther. 2021, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Jin, Y.; Song, M.; Zhao, Y.; Zhang, H. When Natural Compounds Meet Nanotechnology: Nature-Inspired Nanomedicines for Cancer Immunotherapy. Pharmaceutics 2022, 14, 1589. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Wu, J.; Tian, M.; Zhang, S.; Li, Z.; Shi, L. Comprehensive Review on the Elaboration of Payloads Derived from Natural Products for Antibody-Drug Conjugates. Eur. J. Med. Chem. 2024, 268, 116233. [Google Scholar] [CrossRef] [PubMed]
- Krall, N.; Da Cruz, F.P.; Boutureira, O.; Bernardes, G.J.L. Site-Selective Protein-Modification Chemistry for Basic Biology and Drug Development. Nat. Chem. 2016, 8, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.L.; Hackenberger, C.P.R. Modern Ligation Methods to Access Natural and Modified Proteins. Chimia 2018, 72, 802. [Google Scholar] [CrossRef] [PubMed]
- Boutureira, O.; Bernardes, G.J.L. Advances in Chemical Protein Modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef] [PubMed]
- Guillou, A.; Earley, D.F.; Patra, M.; Holland, J.P. Light-Induced Synthesis of Protein Conjugates and Its Application in Photoradiosynthesis of 89Zr-Radiolabeled Monoclonal Antibodies. Nat. Protoc. 2020, 15, 3579–3594. [Google Scholar] [CrossRef] [PubMed]
- Perk, L.R.; Vosjan, M.J.W.D.; Visser, G.W.M.; Budde, M.; Jurek, P.; Kiefer, G.E.; Van Dongen, G.A.M.S. P-Isothiocyanatobenzyl-Desferrioxamine: A New Bifunctional Chelate for Facile Radiolabeling of Monoclonal Antibodies with Zirconium-89 for Immuno-PET Imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.P.; Gut, M.; Klingler, S.; Fay, R.; Guillou, A. Photochemical Reactions in the Synthesis of Protein–Drug Conjugates. Chem. Eur. J. 2020, 26, 33–48. [Google Scholar] [CrossRef] [PubMed]
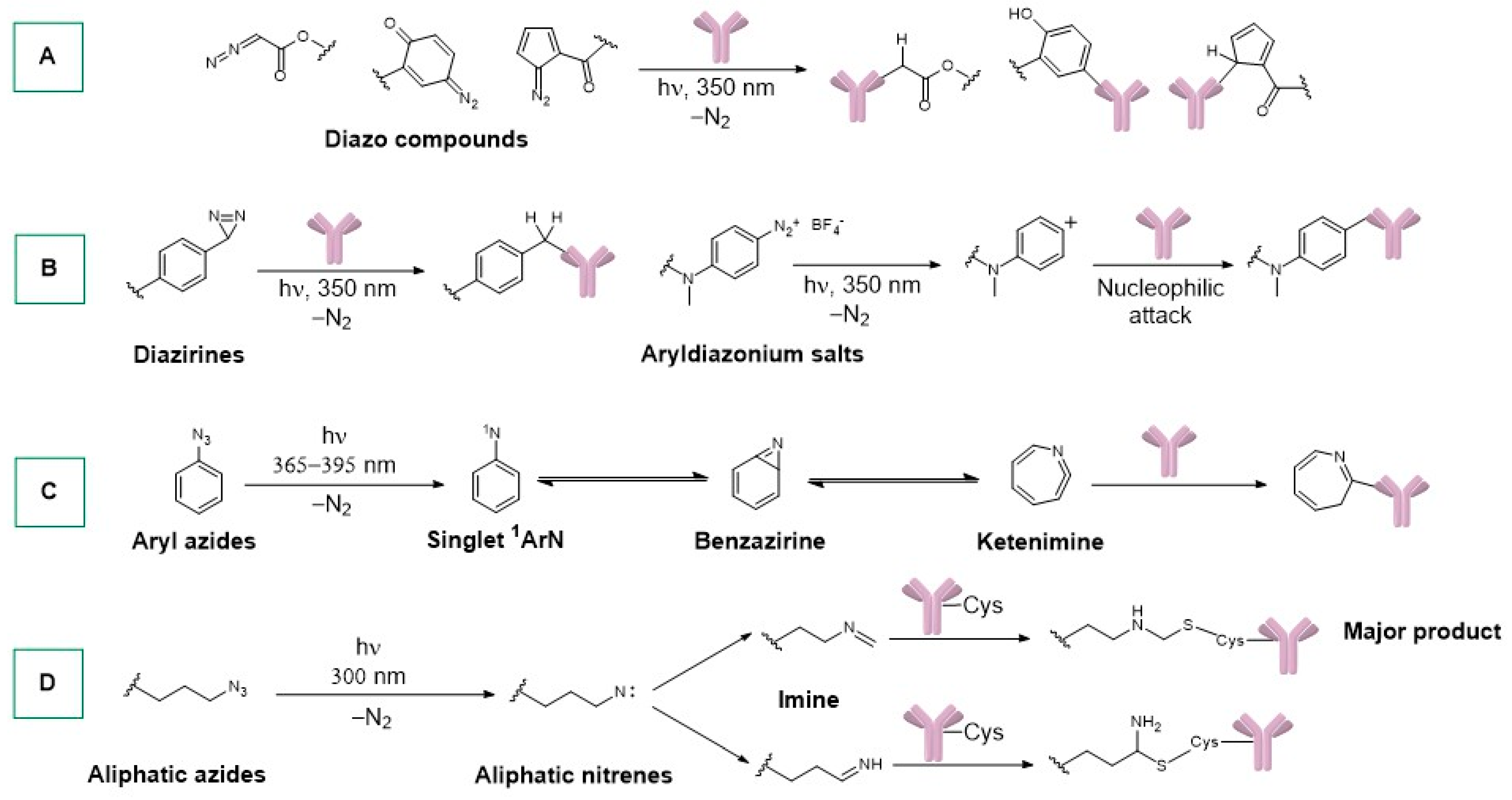
- Smith, R.A.G.; Knowles, J.R. Aryldiazirines. Potential Reagents for Photolabeling of Biological Receptor Sites. J. Am. Chem. Soc. 1973, 95, 5072–5073. [Google Scholar] [CrossRef] [PubMed]
- Doering, W.v.E.; Odum, R.A. Ring Enlargement in the Photolysis of Phenyl Azide. Tetrahedron 1966, 22, 81–93. [Google Scholar] [CrossRef]
- Gritsan, N.P.; Zhu, Z.; Hadad, C.M.; Platz, M.S. Laser Flash Photolysis and Computational Study of Singlet Phenylnitrene. J. Am. Chem. Soc. 1999, 121, 1202–1207. [Google Scholar] [CrossRef]
- Bou-Hamdan, F.R.; Lévesque, F.; O’Brien, A.G.; Seeberger, P.H. Continuous Flow Photolysis of Aryl Azides: Preparation of 3 H -Azepinones. Beilstein J. Org. Chem. 2011, 7, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Rousselot, P.; Mappus, E.; Blachère, T.; De Ravel, M.R.; Grenot, C.; Tonnelle, C.; Cuilleron, C.Y. Specific Photoaffinity Labeling of Tyr-49 on the Light Chain in the Steroid-Combining Site of a Mouse Monoclonal Anti-Estradiol Antibody Using Two Epimeric 6α- and 6β-(5-Azido-2-Nitrobenzoyl)Amidoestradiol Photoreagents. Biochemistry 1997, 36, 7860–7868. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, W.; Schreiber, C.; Scheefers, H. Lipids with Photosensitive Groups as Chemical Probes for the Structural Analysis of Biological Membranes. On the Localization of the G- and M-Protein of Vesicular Stomatitis Virus. Hoppe-Seyler’s Z. Für Physiol. Chem. 1978, 359, 923–932. [Google Scholar] [CrossRef] [PubMed]
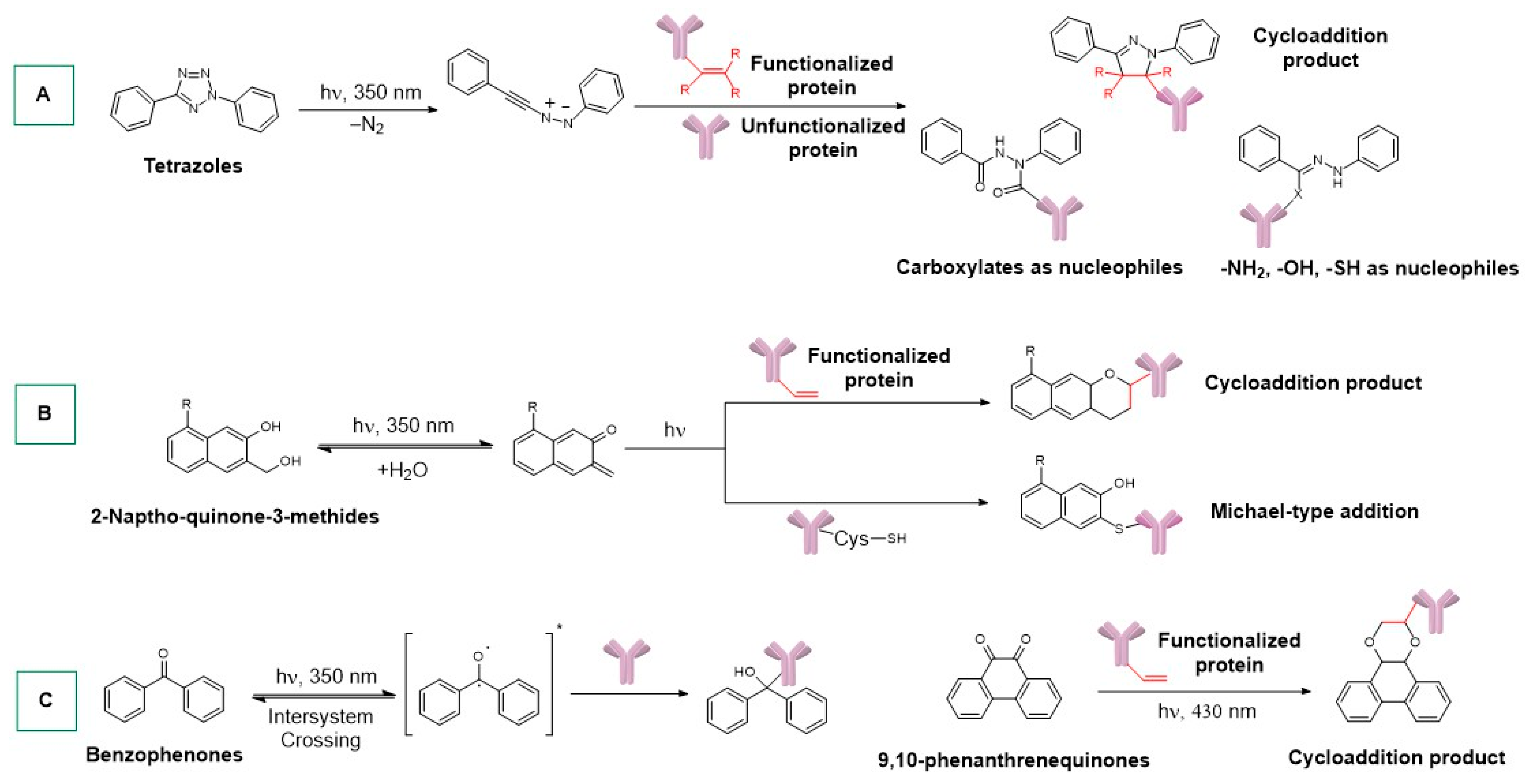
- Huisgen, R.; Sustmann, R.; Wallbillich, G. 1.3-Dipolare Cycloadditionen, XXIX. Orientierungsphänomene Bei Der Anlagerung von Nitriliminen an α.Β-ungesättigte Carbonester, Vinyläther Und Enamine. Chem. Ber. 1967, 100, 1786–1801. [Google Scholar] [CrossRef]
- Li, J.; Kong, H.; Huang, L.; Cheng, B.; Qin, K.; Zheng, M.; Yan, Z.; Zhang, Y. Visible Light-Initiated Bioorthogonal Photoclick Cycloaddition. J. Am. Chem. Soc. 2018, 140, 14542–14546. [Google Scholar] [CrossRef] [PubMed]
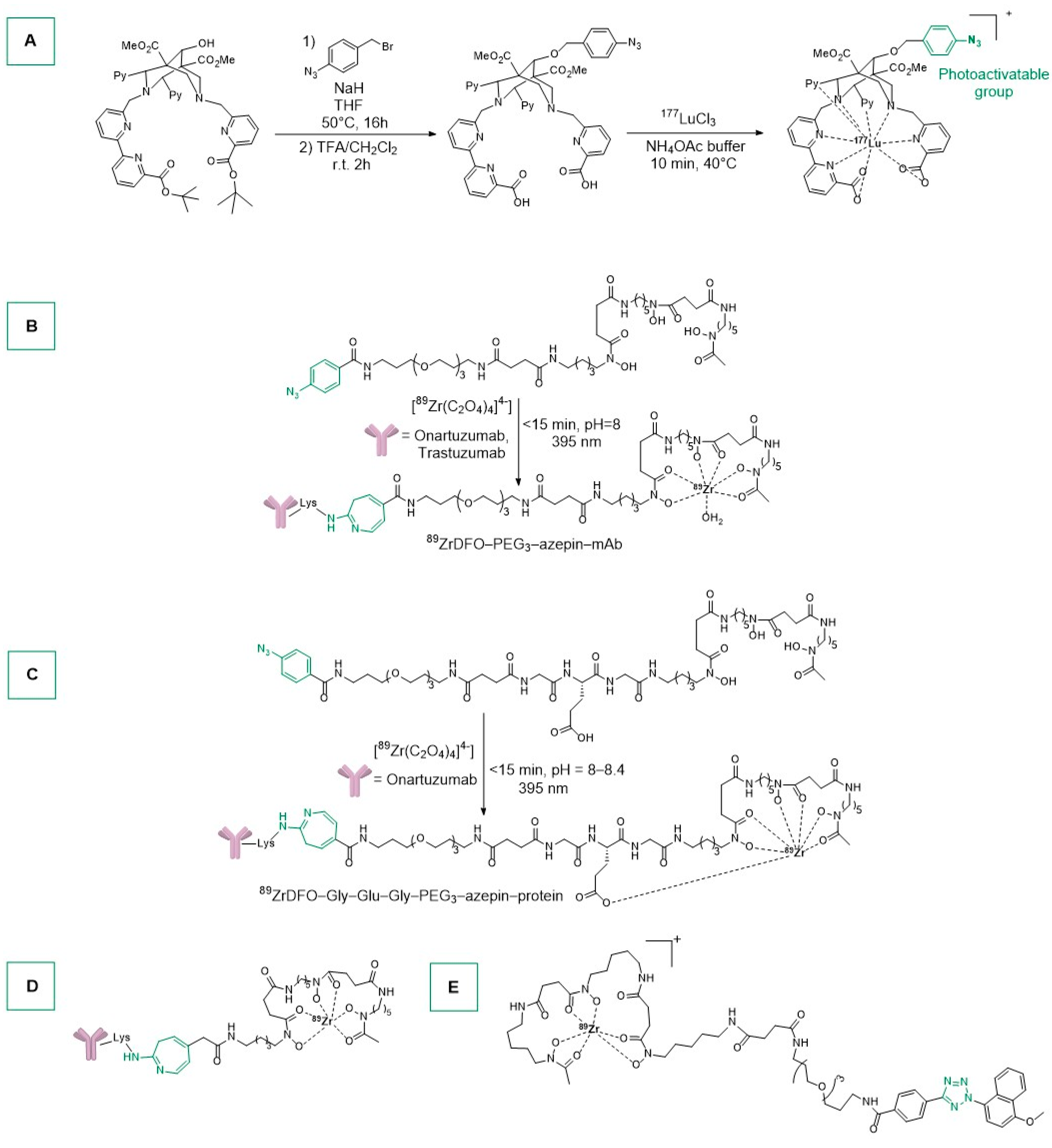
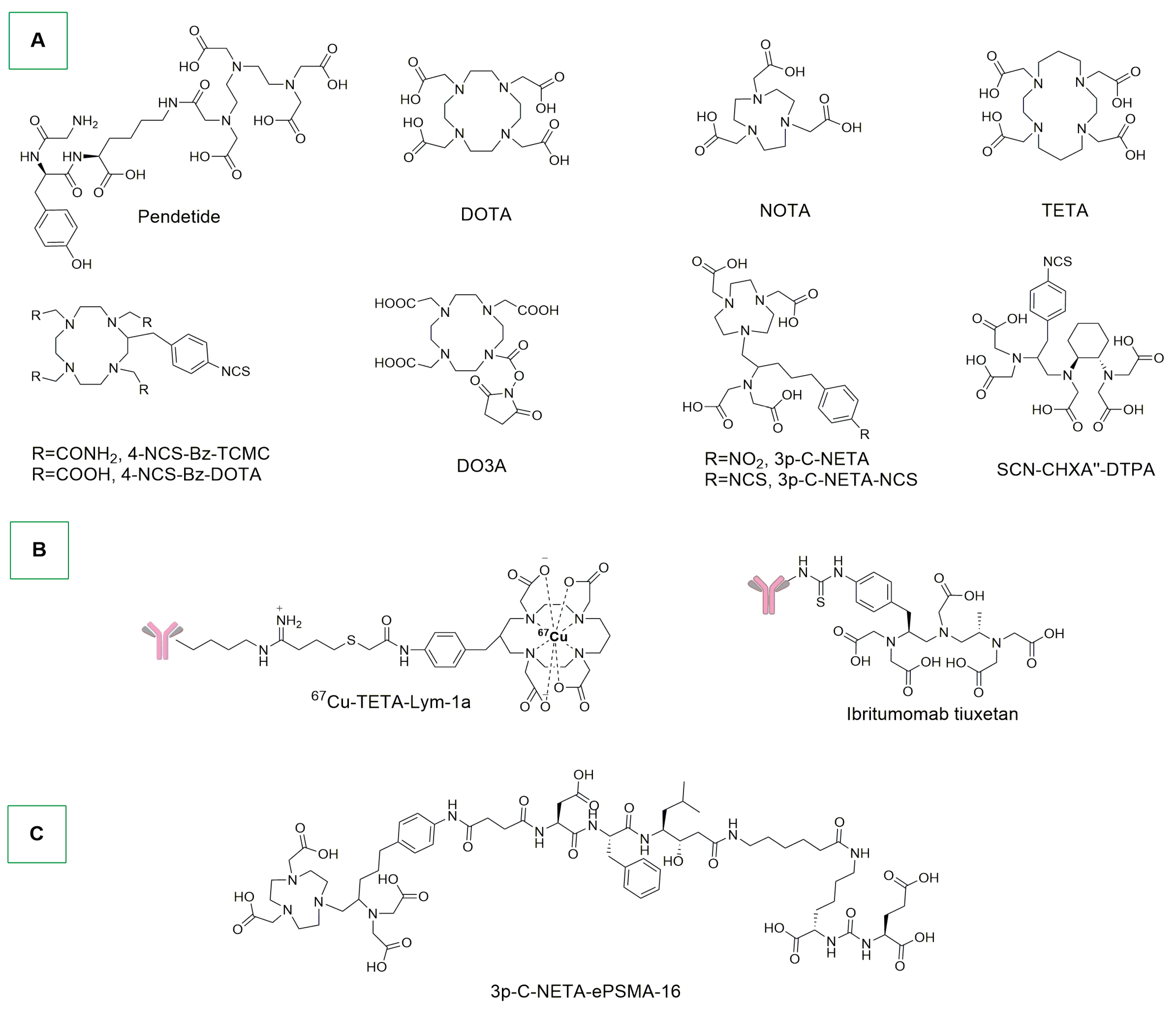
- Boros, E.; Holland, J.P. Chemical Aspects of Metal Ion Chelation in the Synthesis and Application Antibody-based Radiotracers. J. Label. Compd. Radiopharm. 2018, 61, 652–671. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, P.A.; Klingler, S.; Nolff, M.; Holland, J.P. Radiolabelled 177 Lu-Bispidine-Trastuzumab for Targeting Human Epidermal Growth Factor Receptor 2 Positive Cancers. Chem. Eur. J. 2024, 30, e202303805. [Google Scholar] [CrossRef] [PubMed]
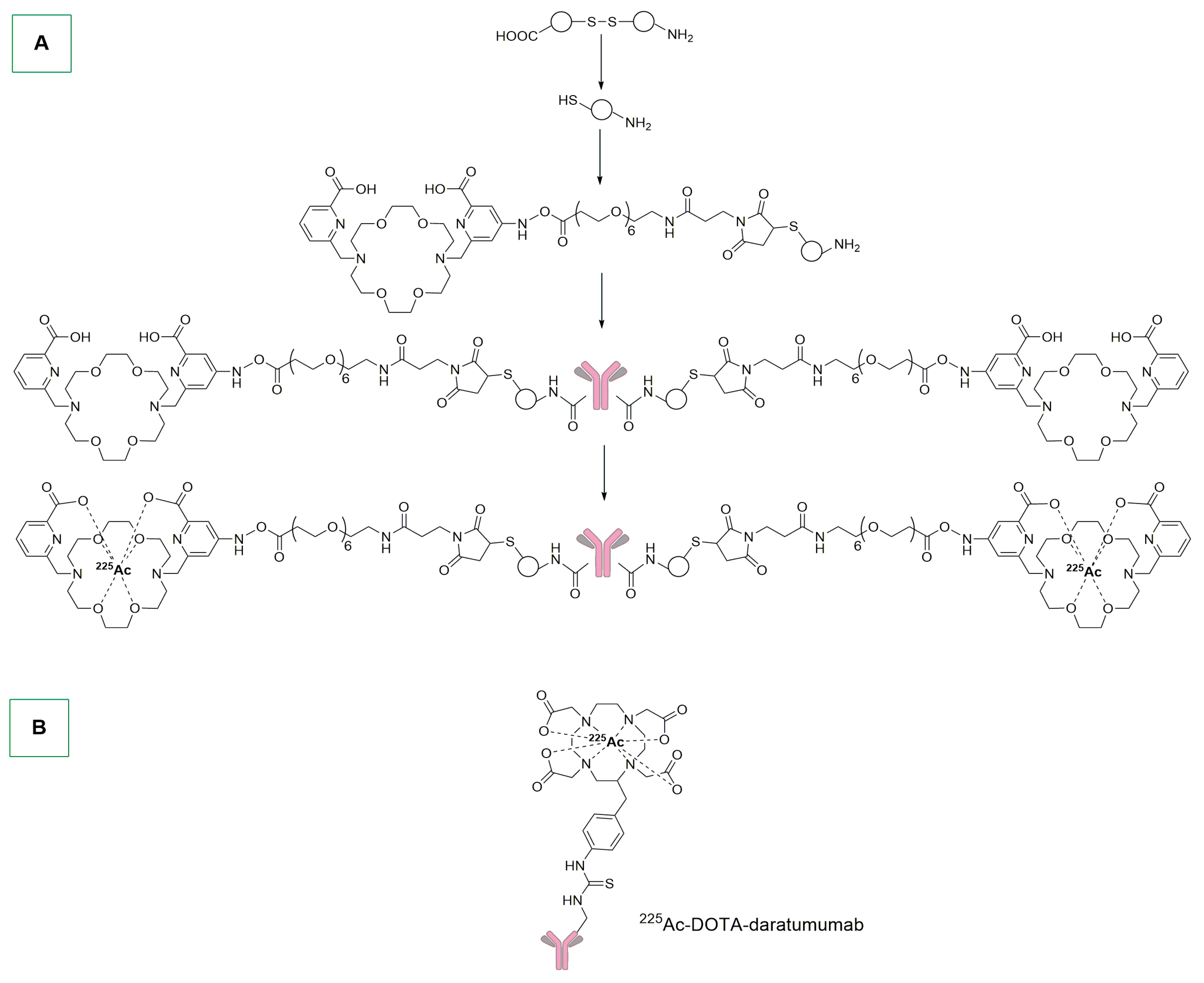
- Cieslik, P.; Kubeil, M.; Zarschler, K.; Ullrich, M.; Brandt, F.; Anger, K.; Wadepohl, H.; Kopka, K.; Bachmann, M.; Pietzsch, J.; et al. Toward Personalized Medicine: One Chelator for Imaging and Therapy with Lutetium-177 and Actinium-225. J. Am. Chem. Soc. 2022, 144, 21555–21567. [Google Scholar] [CrossRef]
- Cieslik, P.; Comba, P.; Dittmar, B.; Ndiaye, D.; Tóth, É.; Velmurugan, G.; Wadepohl, H. Exceptional Manganese(II) Stability and Manganese(II)/Zinc(II) Selectivity with Rigid Polydentate Ligands. Angew. Chem. Int. Ed. 2022, 61, e202115580. [Google Scholar] [CrossRef] [PubMed]
- Comba, P.; Jermilova, U.; Orvig, C.; Patrick, B.O.; Ramogida, C.F.; Rück, K.; Schneider, C.; Starke, M. Octadentate Picolinic Acid-Based Bispidine Ligand for Radiometal Ions. Chem. Eur. J. 2017, 23, 15945–15956. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, D.; Remelli, M. Deferoxamine B: A Natural, Excellent and Versatile Metal Chelator. Molecules 2021, 26, 3255. [Google Scholar] [CrossRef] [PubMed]
- Codd, R.; Richardson-Sanchez, T.; Telfer, T.J.; Gotsbacher, M.P. Advances in the Chemical Biology of Desferrioxamine B. ACS Chem. Biol. 2018, 13, 11–25. [Google Scholar] [CrossRef]
- Guillou, A.; Earley, D.F.; Holland, J.P. Light-Activated Protein Conjugation and 89Zr-Radiolabelling with Water-Soluble Desferrioxamine Derivatives. Chem. Eur. J. 2020, 26, 7185–7189. [Google Scholar] [CrossRef]
- Merchant, M.; Ma, X.; Maun, H.R.; Zheng, Z.; Peng, J.; Romero, M.; Huang, A.; Yang, N.; Nishimura, M.; Greve, J.; et al. Monovalent Antibody Design and Mechanism of Action of Onartuzumab, a MET Antagonist with Anti-Tumor Activity as a Therapeutic Agent. Proc. Natl. Acad. Sci. USA 2013, 110, E2987–E2996. [Google Scholar] [CrossRef]
- Guillou, A.; Ouadi, A.; Holland, J.P. Heptadentate Chelates for 89 Zr-Radiolabelling of Monoclonal Antibodies. Inorg. Chem. Front. 2022, 9, 3071–3081. [Google Scholar] [CrossRef] [PubMed]
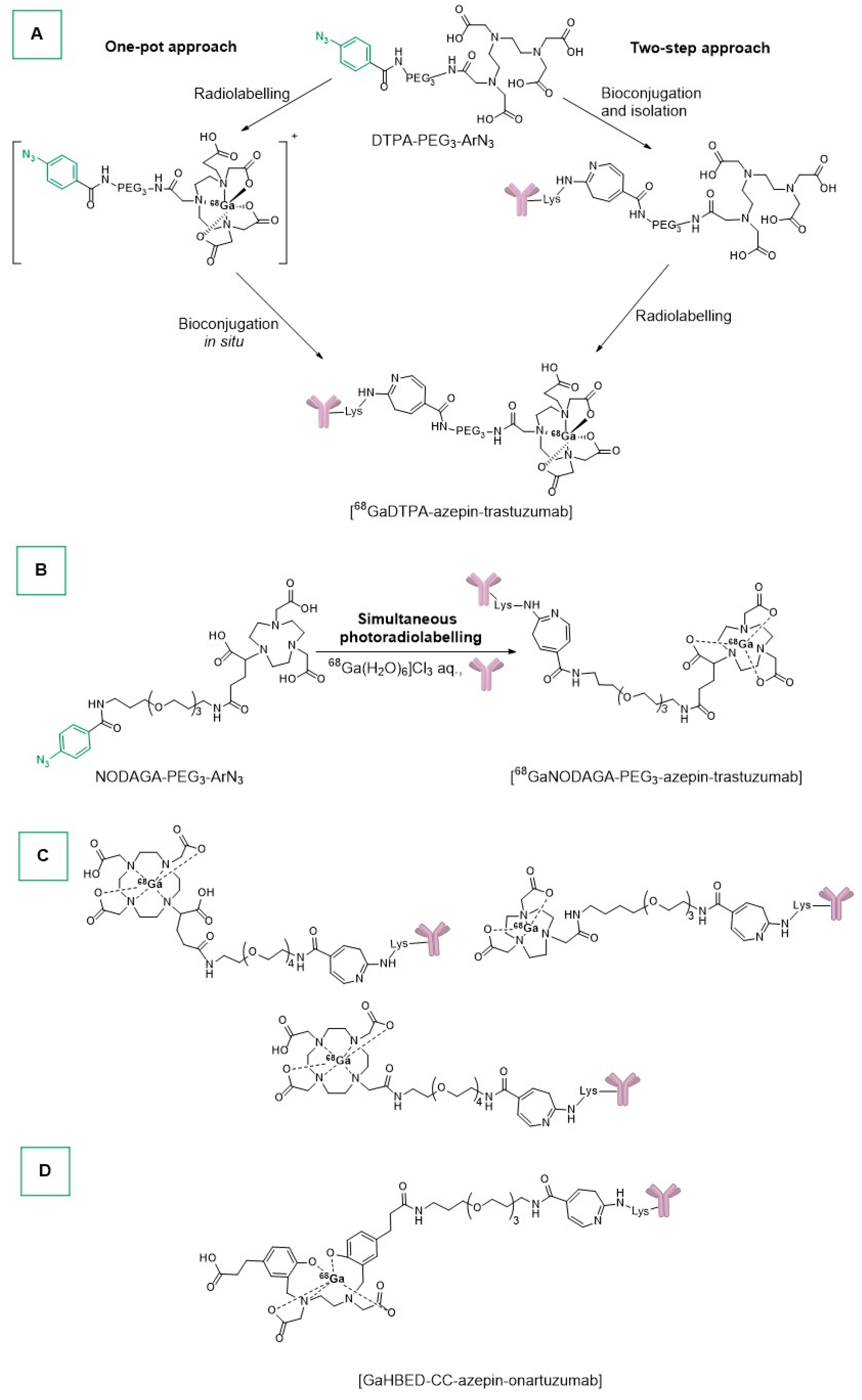
- Patra, M.; Klingler, S.; Eichenberger, L.S.; Holland, J.P. Simultaneous Photoradiochemical Labeling of Antibodies for Immuno-Positron Emission Tomography. iScience 2019, 13, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Fay, R.; Holland, J.P. Tuning Tetrazole Photochemistry for Protein Ligation and Molecular Imaging. Chem. Eur. J. 2021, 27, 4893–4897. [Google Scholar] [CrossRef] [PubMed]
- Gut, M.; Holland, J.P. Synthesis and Photochemical Studies on Gallium and Indium Complexes of DTPA-PEG 3-ArN3 for Radiolabeling Antibodies. Inorg. Chem. 2019, 58, 12302–12310. [Google Scholar] [CrossRef] [PubMed]
- Corman, M.L.; Galandiuk, S.; Block, G.E.; Prager, E.D.; Weiner, G.J.; Kahn, D.; Abdel-Nabi, H.; Mitchell, E.P.; Pascucci, V.L.; Maroli, A.N.; et al. Immunoscintigraphy With111 In-Satumomab Pendetide in Patients with Colorectal Adenocarcinoma: Performance and Impact on Clinical Management. Dis. Colon Rectum 1994, 37, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Eichenberger, L.S.; Fischer, G.; Holland, J.P. Photochemical Conjugation and One-Pot Radiolabelling of Antibodies for Immuno-PET. Angew. Chem. Int. Ed. 2019, 58, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Fay, R.; Gut, M.; Holland, J.P. Photoradiosynthesis of 68Ga-Labeled HBED-CC-Azepin-MetMAb for Immuno-PET of c-MET Receptors. Bioconjug. Chem. 2019, 30, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, J.M.J.M.; Faustino, H.; Trindade, A.; Gois, P.M.P. Bioconjugation with Maleimides: A Useful Tool for Chemical Biology. Chem. Eur. J. 2019, 25, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Barré, A.; Ţînţaş, M.-L.; Levacher, V.; Papamicaël, C.; Gembus, V. An Overview of the Synthesis of Highly Versatile N-Hydroxysuccinimide Esters. Synthesis 2016, 49, 472–483. [Google Scholar] [CrossRef]
- Schulze, W.X.; Mann, M. A Novel Proteomic Screen for Peptide-Protein Interactions. J. Biol. Chem. 2004, 279, 10756–10764. [Google Scholar] [CrossRef] [PubMed]
- Abello, N.; Kerstjens, H.A.M.; Postma, D.S.; Bischoff, R. Selective Acylation of Primary Amines in Peptides and Proteins. J. Proteome Res. 2007, 6, 4770–4776. [Google Scholar] [CrossRef] [PubMed]
- Matiadis, D.; Igglessi-Markopoulou, O. Design and Synthesis of Optically Active Esters of γ-Amino-β-oxo Acids as Precursors for the Synthesis of Tetramic Acids Derived from L-Serine, L-Tyrosine, and L-Threonine. Eur. J. Org. Chem. 2010, 2010, 5989–5995. [Google Scholar] [CrossRef]
- Jakobsche, C.E.; Parker, C.G.; Tao, R.N.; Kolesnikova, M.D.; Douglass, E.F.; Spiegel, D.A. Exploring Binding and Effector Functions of Natural Human Antibodies Using Synthetic Immunomodulators. ACS Chem. Biol. 2013, 8, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Curado, N.; Dewaele-Le Roi, G.; Poty, S.; Lewis, J.S.; Contel, M. Trastuzumab Gold-Conjugates: Synthetic Approach and in Vitro Evaluation of Anticancer Activities in Breast Cancer Cell Lines. Chem. Commun. 2019, 55, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Junutula, J.R.; Raab, H.; Clark, S.; Bhakta, S.; Leipold, D.D.; Weir, S.; Chen, Y.; Simpson, M.; Tsai, S.P.; Dennis, M.S.; et al. Site-Specific Conjugation of a Cytotoxic Drug to an Antibody Improves the Therapeutic Index. Nat. Biotechnol. 2008, 26, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Saeed, H.K.; Del Solar, V.; López-Hernández, J.E.; Michel, A.; Mathew, J.; Lewis, J.S.; Contel, M. Shifting the Antibody–Drug Conjugate Paradigm: A Trastuzumab-Gold-Based Conjugate Demonstrates High Efficacy against Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer Mouse Model. ACS Pharmacol. Transl. Sci. 2023, 6, 1972–1986. [Google Scholar] [CrossRef] [PubMed]
- Karges, J.; Jakubaszek, M.; Mari, C.; Zarschler, K.; Goud, B.; Stephan, H.; Gasser, G. Synthesis and Characterization of an Epidermal Growth Factor Receptor-Selective RuII Polypyridyl–Nanobody Conjugate as a Photosensitizer for Photodynamic Therapy. ChemBioChem 2020, 21, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alonso, M.; Gandioso, A.; Thibaudeau, C.; Qin, X.; Arnoux, P.; Demeubayeva, N.; Guérineau, V.; Frochot, C.; Jung, A.C.; Gaiddon, C.; et al. A Novel Near-IR Absorbing Ruthenium(II) Complex as Photosensitizer for Photodynamic Therapy and Its Cetuximab Bioconjugates. ChemBioChem 2023, 24, e202300203. [Google Scholar] [CrossRef]
- Martínez-Alonso, M.; Gasser, G. Ruthenium Polypyridyl Complex-Containing Bioconjugates. Coord. Chem. Rev. 2021, 434, 213736. [Google Scholar] [CrossRef]
- Machado, J.F.; Correia, J.D.G.; Morais, T.S. Emerging Molecular Receptors for the Specific-Target Delivery of Ruthenium and Gold Complexes into Cancer Cells. Molecules 2021, 26, 3153. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, M.; Endo, K.; Kunimatsu, M.; Sakahara, H.; Nakashima, T.; Kawamura, Y.; Watanabe, Y.; Saga, T.; Konishi, J.; Yamamuro, T. 67Ga-Labeled Antibodies for Immunoscintigraphy and Evaluation of Tumor Targeting of Drug-Antibody Conjugates in Mice. Cancer Res. 1988, 48, 1189–1194. [Google Scholar] [PubMed]
- Ferrier, M.G.; Batista, E.R.; Berg, J.M.; Birnbaum, E.R.; Cross, J.N.; Engle, J.W.; La Pierre, H.S.; Kozimor, S.A.; Lezama Pacheco, J.S.; Stein, B.W.; et al. Spectroscopic and Computational Investigation of Actinium Coordination Chemistry. Nat. Commun. 2016, 7, 12312. [Google Scholar] [CrossRef] [PubMed]
- Thiele, N.A.; Brown, V.; Kelly, J.M.; Amor-Coarasa, A.; Jermilova, U.; MacMillan, S.N.; Nikolopoulou, A.; Ponnala, S.; Ramogida, C.F.; Robertson, A.K.H.; et al. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem. Int. Ed. 2017, 56, 14712–14717. [Google Scholar] [CrossRef] [PubMed]
- Deal, K.A.; Davis, I.A.; Mirzadeh, S.; Kennel, S.J.; Brechbiel, M.W. Improved in Vivo Stability of Actinium-225 Macrocyclic Complexes. J. Med. Chem. 1999, 42, 2988–2992. [Google Scholar] [CrossRef] [PubMed]
- Lakes, A.L.; An, D.D.; Gauny, S.S.; Ansoborlo, C.; Liang, B.H.; Rees, J.A.; McKnight, K.D.; Karsunky, H.; Abergel, R.J. Evaluating 225Ac and 177Lu Radioimmunoconjugates against Antibody–Drug Conjugates for Small-Cell Lung Cancer. Mol. Pharm. 2020, 17, 4270–4279. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Kurihara, H.; Yonemori, K.; Tsuda, H.; Suzuki, J.; Kono, Y.; Honda, N.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; et al. 64Cu-DOTA-Trastuzumab PET Imaging in Patients with HER2-Positive Breast Cancer. J. Nucl. Med. 2013, 54, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Maguire, W.F.; McDevitt, M.R.; Smith-Jones, P.M.; Scheinberg, D.A. Efficient 1-Step Radiolabeling of Monoclonal Antibodies to High Specific Activity with 225Ac for α-Particle Radioimmunotherapy of Cancer. J. Nucl. Med. 2014, 55, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Labão-Almeida, C.; Sayers, C.; Dada, O.; Tacke, M.; Bernardes, G.J.L. Synthesis and Biological Evaluation of Homogeneous Thiol-Linked NHC*-Au-Albumin and -Trastuzumab Bioconjugates. Chem. Eur. J. 2018, 24, 12250–12253. [Google Scholar] [CrossRef] [PubMed]
- Merkul, E.; Sijbrandi, N.J.; Aydin, I.; Muns, J.A.; Peters, R.J.R.W.; Laarhoven, P.; Houthoff, H.-J.; Van Dongen, G.A.M.S. A Successful Search for New, Efficient, and Silver-Free Manufacturing Processes for Key Platinum(II) Intermediates Applied in Antibody–Drug Conjugate (ADC) Production. Green Chem. 2020, 22, 2203–2212. [Google Scholar] [CrossRef]
- Merkul, E.; Sijbrandi, N.J.; Muns, J.A.; Aydin, I.; Adamzek, K.; Houthoff, H.-J.; Nijmeijer, B.; Van Dongen, G.A.M.S. First Platinum(II)-Based Metal-Organic Linker Technology (Lx®) for a Plug-and-Play Development of Antibody-Drug Conjugates (ADCs). Expert Opin. Drug Deliv. 2019, 16, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Merkul, E.; Muns, J.A.; Sijbrandi, N.J.; Houthoff, H.; Nijmeijer, B.; Van Rheenen, G.; Reedijk, J.; Van Dongen, G.A.M.S. An Efficient Conjugation Approach for Coupling Drugs to Native Antibodies via the PtII Linker Lx for Improved Manufacturability of Antibody–Drug Conjugates. Angew. Chem. Int. Ed. 2021, 60, 3008–3015. [Google Scholar] [CrossRef] [PubMed]
- Muns, J.A.; Montserrat, V.; Houthoff, H.-J.; Codée-van Der Schilden, K.; Zwaagstra, O.; Sijbrandi, N.J.; Merkul, E.; Van Dongen, G.A.M.S. In Vivo Characterization of Platinum(II)-Based Linker Technology for the Development of Antibody–Drug Conjugates: Taking Advantage of Dual Labeling with 195mPt and 89Zr. J. Nucl. Med. 2018, 59, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Sijbrandi, N.J.; Merkul, E.; Muns, J.A.; Waalboer, D.C.J.; Adamzek, K.; Bolijn, M.; Montserrat, V.; Somsen, G.W.; Haselberg, R.; Steverink, P.J.G.M.; et al. A Novel Platinum(II)–Based Bifunctional ADC Linker Benchmarked Using 89Zr-Desferal and Auristatin F–Conjugated Trastuzumab. Cancer Res. 2017, 77, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.P.; Dillman, R.O.; DeNardo, S.J.; DeNardo, G.L.; Beauregard, J.; Hagan, P.L.; Amox, D.G.; Clutter, M.L.; Burnett, K.G.; Rulot, C.M. Breast Cancer Imaging with In-111 Human IgM Monoclonal Antibodies: Preliminary Studies. Radiology 1988, 167, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Manyak, M.J. Indium-111 Capromab Pendetide in the Management of Recurrent Prostate Cancer. Expert Rev. Anticancer Ther. 2008, 8, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Polascik, T.J.; Manyak, M.J.; Haseman, M.K.; Gurganus, R.T.; Rogers, B.; Maguire, R.T.; Partin, A.W. Comparison of Clinical Staging Algorithms and111Indium-Capromab Pendetide Immunoscintigraphy in the Prediction of Lymph Node Involvement in High Risk Prostate Carcinoma Patients. Cancer 1999, 85, 1586–1592. [Google Scholar] [CrossRef]
- Philpott, G.W.; Schwarz, S.W.; Anderson, C.J.; Dehdashti, F.; Connett, J.M.; Zinn, K.R.; Meares, C.F.; Cutler, P.D.; Welch, M.J.; Siegel, B.A. RadioimmunoPET: Detection of Colorectal Carcinoma with Positron-Emitting Copper-64-Labeled Monoclonal Antibody. J. Nucl. Med. 1995, 36, 1818–1824. [Google Scholar] [PubMed]
- Kukis, D.L.; DeNardo, G.L.; DeNardo, S.J.; Mirick, G.R.; Miers, L.A.; Greiner, D.P.; Meares, C.F. Effect of the Extent of Chelate Substitution on the Immunoreactivity and Biodistribution of 2IT-BAT-Lym-1 Immunoconjugates. Cancer Res. 1995, 55, 878–884. [Google Scholar] [PubMed]
- O’Donnell, R.T.; DeNardo, G.L.; Kukis, D.L.; Lamborn, K.R.; Shen, S.; Yuan, A.; Goldstein, D.S.; Carr, C.E.; Mirick, G.R.; DeNardo, S.J. A Clinical Trial of Radioimmunotherapy with 67Cu-2IT-BAT-Lym-1 for Non-Hodgkin’s Lymphoma. J. Nucl. Med. 1999, 40, 2014–2020. [Google Scholar] [PubMed]
- DeNardo, S.J.; DeNardo, G.L.; Kukis, D.L.; Shen, S.; Kroger, L.A.; DeNardo, D.A.; Goldstein, D.S.; Mirick, G.R.; Salako, Q.; Mausner, L.F.; et al. 67Cu-2IT-BAT-Lym-1 Pharmacokinetics, Radiation Dosimetry, Toxicity and Tumor Regression in Patients with Lymphoma. J. Nucl. Med. 1999, 40, 302–310. [Google Scholar] [PubMed]
- Mortimer, J.E.; Bading, J.R.; Park, J.M.; Frankel, P.H.; Carroll, M.I.; Tran, T.T.; Poku, E.K.; Rockne, R.C.; Raubitschek, A.A.; Shively, J.E.; et al. Tumor Uptake of 64 Cu-DOTA-Trastuzumab in Patients with Metastatic Breast Cancer. J. Nucl. Med. 2018, 59, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Hamada, A.; Yoshida, M.; Shimma, S.; Hashimoto, J.; Yonemori, K.; Tani, H.; Miyakita, Y.; Kanayama, Y.; Wada, Y.; et al. 64Cu-DOTA-Trastuzumab PET Imaging and HER2 Specificity of Brain Metastases in HER2-Positive Breast Cancer Patients. EJNMMI Res. 2015, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Sun, X.; Cao, Q.; Courter, D.; Koong, A.; Le, Q.-T.; Gambhir, S.S.; Chen, X. Cetuximab-Based Immunotherapy and Radioimmunotherapy of Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2010, 16, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Earley, D.F.; Esteban Flores, J.; Guillou, A.; Holland, J.P. Photoactivatable Bis(Thiosemicarbazone) Derivatives for Copper-64 Radiotracer Synthesis. Dalton Trans. 2022, 51, 5041–5052. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M.; Schlom, J.; Gansow, O.A.; Brechbiel, M.W.; Mirzadeh, S.; Pippin, C.G.; Milenic, D.E.; Colcher, D. Comparative Biodistribution Studies of DTPA-Derivative Bifunctional Chelates for Radiometal Labeled Monoclonal Antibodies. Int. J. Rad. Appl. Instrum. B 1991, 18, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Witzig, T.E.; Gordon, L.I.; Cabanillas, F.; Czuczman, M.S.; Emmanouilides, C.; Joyce, R.; Pohlman, B.L.; Bartlett, N.L.; Wiseman, G.A.; Padre, N.; et al. Randomized Controlled Trial of Yttrium-90–Labeled Ibritumomab Tiuxetan Radioimmunotherapy Versus Rituximab Immunotherapy for Patients with Relapsed or Refractory Low-Grade, Follicular, or Transformed B-Cell Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2002, 20, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; Ballard, B.; Jiang, Z.; Revskaya, E.; Sisay, N.; Miller, W.H.; Cutler, C.S.; Dadachova, E.; Francesconi, L.C. 166Ho and 90Y Labeled 6D2 Monoclonal Antibody for Targeted Radiotherapy of Melanoma: Comparison with 188Re Radiolabel. Nucl. Med. Biol. 2014, 41, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Dadachova, E.; Revskaya, E.; Sesay, M.A.; Damania, H.; Boucher, R.; Sellers, R.S.; Howell, R.C.; Burns, L.; Thornton, G.B.; Natarajan, A.; et al. Pre-Clinical Evaluation and Efficacy Studies of a Melanin-Binding IgM Antibody Labeled with 188Re against Experimental Human Metastatic Melanoma in Nude Mice. Cancer Biol. Ther. 2008, 7, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Lindén, O.; Hindorf, C.; Cavallin-Ståhl, E.; Wegener, W.A.; Goldenberg, D.M.; Horne, H.; Ohlsson, T.; Stenberg, L.; Strand, S.-E.; Tennvall, J. Dose-Fractionated Radioimmunotherapy in Non-Hodgkin’s Lymphoma Using DOTA-Conjugated, 90Y-Radiolabeled, Humanized Anti-CD22 Monoclonal Antibody, Epratuzumab. Clin. Cancer Res. 2005, 11, 5215–5222. [Google Scholar] [CrossRef]
- Kang, C.S.; Sun, X.; Jia, F.; Song, H.A.; Chen, Y.; Lewis, M.; Chong, H.-S. Synthesis and Preclinical Evaluation of Bifunctional Ligands for Improved Chelation Chemistry of 90 Y and 177 Lu for Targeted Radioimmunotherapy. Bioconjug. Chem. 2012, 23, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Song, H.A.; Kang, C.S.; Baidoo, K.E.; Milenic, D.E.; Chen, Y.; Dai, A.; Brechbiel, M.W.; Chong, H.-S. Efficient Bifunctional Decadentate Ligand 3p- C -DEPA for Targeted α-Radioimmunotherapy Applications. Bioconjug. Chem. 2011, 22, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Murce, E.; Ahenkorah, S.; Beekman, S.; Handula, M.; Stuurman, D.; De Ridder, C.; Cleeren, F.; Seimbille, Y. Radiochemical and Biological Evaluation of 3p-C-NETA-ePSMA-16, a Promising PSMA-Targeting Agent for Radiotheranostics. Pharmaceuticals 2023, 16, 882. [Google Scholar] [CrossRef] [PubMed]
- Chappell, L.L.; Dadachova, E.; Milenic, D.E.; Garmestani, K.; Wu, C.; Brechbiel, M.W. Synthesis, Characterization, and Evaluation of a Novel Bifunctional Chelating Agent for the Lead Isotopes 203Pb and 212Pb. Nucl. Med. Biol. 2000, 27, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Meredith, R.F.; Torgue, J.; Azure, M.T.; Shen, S.; Saddekni, S.; Banaga, E.; Carlise, R.; Bunch, P.; Yoder, D.; Alvarez, R. Pharmacokinetics and Imaging of 212Pb-TCMC-Trastuzumab after Intraperitoneal Administration in Ovarian Cancer Patients. Cancer Biother. Radiopharm. 2014, 29, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Meredith, R.F.; Torgue, J.J.; Rozgaja, T.A.; Banaga, E.P.; Bunch, P.W.; Alvarez, R.D.; Straughn, J.M.; Dobelbower, M.C.; Lowy, A.M. Safety and Outcome Measures of First-in-Human Intraperitoneal α Radioimmunotherapy with 212Pb-TCMC-Trastuzumab. Am. J. Clin. Oncol. 2018, 41, 716–721. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, M.R.; Barendswaard, E.; Ma, D.; Lai, L.; Curcio, M.J.; Sgouros, G.; Ballangrud, A.M.; Yang, W.H.; Finn, R.D.; Pellegrini, V.; et al. An Alpha-Particle Emitting Antibody ([213Bi]J591) for Radioimmunotherapy of Prostate Cancer. Cancer Res. 2000, 60, 6095–6100. [Google Scholar] [PubMed]
- Nikula, T.K.; McDevitt, M.R.; Finn, R.D.; Wu, C.; Kozak, R.W.; Garmestani, K.; Brechbiel, M.W.; Curcio, M.J.; Pippin, C.G.; Tiffany-Jones, L.; et al. Alpha-Emitting Bismuth Cyclohexylbenzyl DTPA Constructs of Recombinant Humanized Anti-CD33 Antibodies: Pharmacokinetics, Bioactivity, Toxicity and Chemistry. J. Nucl. Med. 1999, 40, 166–176. [Google Scholar] [PubMed]
- Sgouros, G.; Ballangrud, A.M.; Jurcic, J.G.; McDevitt, M.R.; Humm, J.L.; Erdi, Y.E.; Mehta, B.M.; Finn, R.D.; Larson, S.M.; Scheinberg, D.A. Pharmacokinetics and Dosimetry of an Alpha-Particle Emitter Labeled Antibody: 213Bi-HuM195 (Anti-CD33) in Patients with Leukemia. J. Nucl. Med. 1999, 40, 1935–1946. [Google Scholar] [PubMed]
- Sudo, H.; Tsuji, A.B.; Sugyo, A.; Harada, Y.; Nagayama, S.; Katagiri, T.; Nakamura, Y.; Higashi, T. Head-to-head Comparison of Three Chelates Reveals DOTAGA Promising for 225Ac Labeling of anti-FZD10 Antibody OTSA101. Cancer Sci. 2023, 114, 4677–4690. [Google Scholar] [CrossRef] [PubMed]
- Bidkar, A.P.; Wang, S.; Bobba, K.N.; Chan, E.; Bidlingmaier, S.; Egusa, E.A.; Peter, R.; Ali, U.; Meher, N.; Wadhwa, A.; et al. Treatment of Prostate Cancer with CD46-Targeted 225Ac Alpha Particle Radioimmunotherapy. Clin. Cancer Res. 2023, 29, 1916–1928. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, T.L.; McDevitt, M.R.; Carrasquillo, J.A.; Pandit-Taskar, N.; Frattini, M.G.; Maslak, P.G.; Park, J.H.; Douer, D.; Cicic, D.; Larson, S.M.; et al. Treatment of Patients with Acute Myeloid Leukemia with the Targeted Alpha-Particle Nanogenerator Actinium-225-Lintuzumab. Clin. Cancer Res. 2022, 28, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Solomon, V.R.; Barreto, K.; Bernhard, W.; Alizadeh, E.; Causey, P.; Perron, R.; Gendron, D.; Alam, M.K.; Carr, A.; Geyer, C.R.; et al. Nimotuzumab Site-Specifically Labeled with 89Zr and 225Ac Using SpyTag/SpyCatcher for PET Imaging and Alpha Particle Radioimmunotherapy of Epidermal Growth Factor Receptor Positive Cancers. Cancers 2020, 12, 3449. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide Tag Forming a Rapid Covalent Bond to a Protein, through Engineering a Bacterial Adhesin. Proc. Natl. Acad. Sci. USA 2012, 109, E690–E697. [Google Scholar] [CrossRef] [PubMed]
- Dawicki, W.; Allen, K.J.H.; Jiao, R.; Malo, M.E.; Helal, M.; Berger, M.S.; Ludwig, D.L.; Dadachova, E. Daratumumab- 225Actinium Conjugate Demonstrates Greatly Enhanced Antitumor Activity against Experimental Multiple Myeloma Tumors. OncoImmunology 2019, 8, 1607673. [Google Scholar] [CrossRef]
- Newman, D.J. Natural Product Based Antibody Drug Conjugates: Clinical Status as of November 9, 2020. J. Nat. Prod. 2021, 84, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Koehn, F.E. Natural Product Cytotoxins as Payloads for Antibody Drug Conjugates. In Natural Products and Cancer Drug Discovery; Koehn, F.E., Ed.; Springer: New York, NY, USA, 2013; pp. 97–119. ISBN 978-1-4614-4653-8. [Google Scholar]
- Gromek, S.; Balunas, M. Natural Products as Exquisitely Potent Cytotoxic Payloads for Antibody- Drug Conjugates. Curr. Top. Med. Chem. 2015, 14, 2822–2834. [Google Scholar] [CrossRef] [PubMed]
- Puthenveetil, S.; Loganzo, F.; He, H.; Dirico, K.; Green, M.; Teske, J.; Musto, S.; Clark, T.; Rago, B.; Koehn, F.; et al. Natural Product Splicing Inhibitors: A New Class of Antibody–Drug Conjugate (ADC) Payloads. Bioconjug. Chem. 2016, 27, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.-P.; Koehn, F.E.; Abraham, R.T. The Antibody-Drug Conjugate: An Enabling Modality for Natural Product-Based Cancer Therapeutics. Nat. Prod. Rep. 2013, 30, 625. [Google Scholar] [CrossRef] [PubMed]
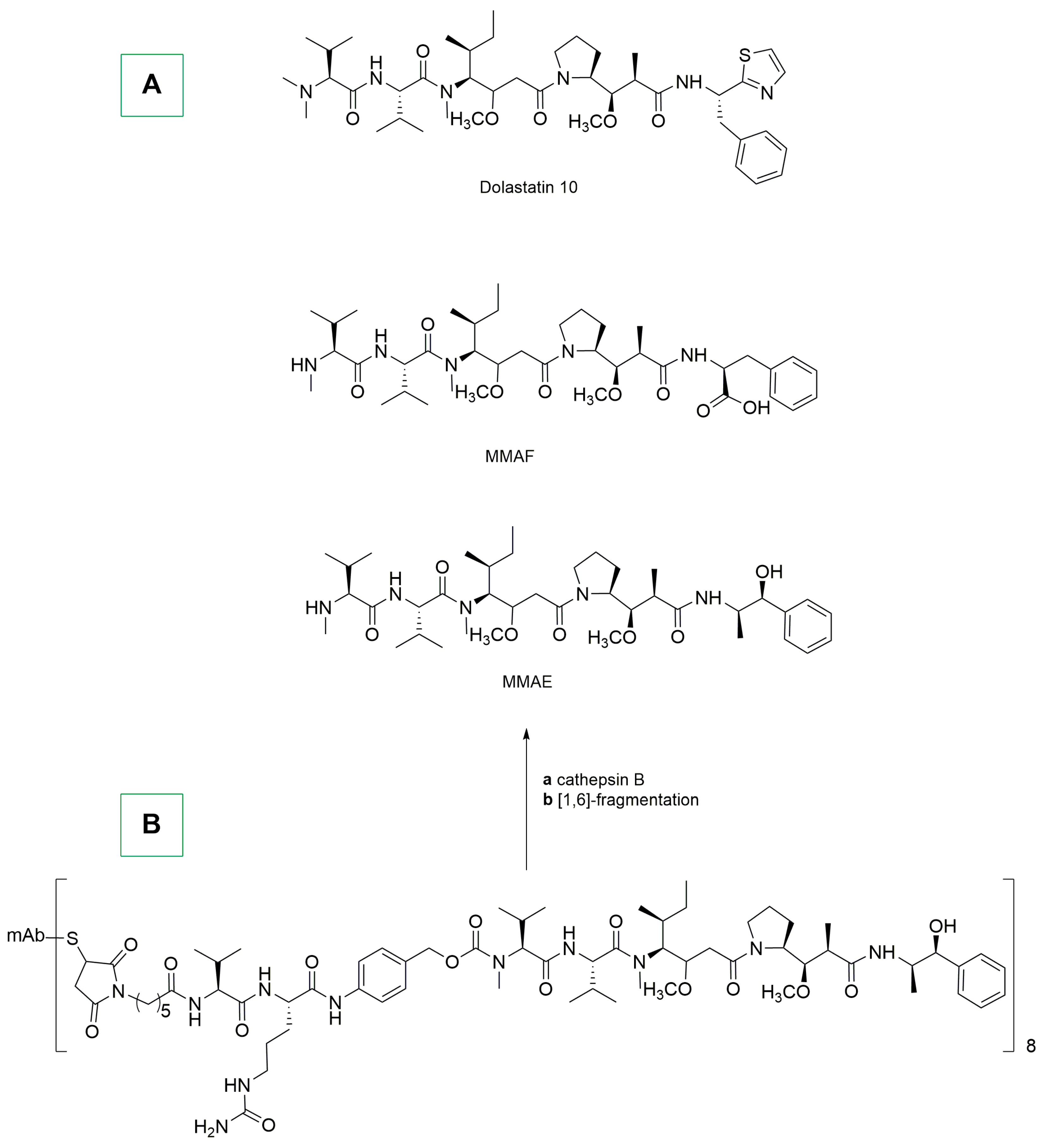
- Bai, R.; Petit, G.R.; Hamel, E. Dolastatin 10, a Powerful Cytostatic Peptide Derived from a Marine Animal. Biochem. Pharmacol. 1990, 39, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B. Discovery and Development of Dolastatin 10-Derived Antibody Drug Conjugate Anticancer Drugs. J. Nat. Prod. 2022, 85, 666–687. [Google Scholar] [CrossRef]
- Waight, A.B.; Bargsten, K.; Doronina, S.; Steinmetz, M.O.; Sussman, D.; Prota, A.E. Structural Basis of Microtubule Destabilization by Potent Auristatin Anti-Mitotics. PLoS ONE 2016, 11, e0160890. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Benz, F.W.; Wu, Y.; Wang, Q.; Chen, Y.; Chen, X.; Li, H.; Zhang, Y.; Zhang, R.; Yang, J. Structural Insights into the Pharmacophore of Vinca Domain Inhibitors of Microtubules. Mol. Pharmacol. 2016, 89, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Guo, Y.; Huang, H.; Li, W.; Ke, X.; Feng, J.; Xu, W.; Miao, H.; Kinley, J.; Song, G.; et al. Phase II Single-Arm Study of Brentuximab Vedotin in Chinese Patients with Relapsed/Refractory Classical Hodgkin Lymphoma or Systemic Anaplastic Large Cell Lymphoma. Expert Rev. Hematol. 2021, 14, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Doronina, S.O.; Toki, B.E.; Torgov, M.Y.; Mendelsohn, B.A.; Cerveny, C.G.; Chace, D.F.; DeBlanc, R.L.; Gearing, R.P.; Bovee, T.D.; Siegall, C.B.; et al. Development of Potent Monoclonal Antibody Auristatin Conjugates for Cancer Therapy. Nat. Biotechnol. 2003, 21, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Cheng-Sánchez, I.; Moya-Utrera, F.; Porras-Alcalá, C.; López-Romero, J.M.; Sarabia, F. Antibody-Drug Conjugates Containing Payloads from Marine Origin. Mar. Drugs 2022, 20, 494. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Polatuzumab Vedotin: First Global Approval. Drugs 2019, 79, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Herrera, A.F.; Flowers, C.R.; Kamdar, M.K.; McMillan, A.; Hertzberg, M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2020, 38, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab Vedotin Antibody–Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Concin, N.; Vergote, I.; De Bono, J.S.; Slomovitz, B.M.; Drew, Y.; Arkenau, H.-T.; Machiels, J.-P.; Spicer, J.F.; Jones, R.; et al. Tisotumab Vedotin in Previously Treated Recurrent or Metastatic Cervical Cancer. Clin. Cancer Res. 2020, 26, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Lorusso, D.; Gennigens, C.; González-Martín, A.; Randall, L.; Cibula, D.; Lund, B.; Woelber, L.; Pignata, S.; Forget, F.; et al. Efficacy and Safety of Tisotumab Vedotin in Previously Treated Recurrent or Metastatic Cervical Cancer (innovaTV 204/GOG-3023/ENGOT-Cx6): A Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol. 2021, 22, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; McCurdy, A.; Fu, M.; Sutherland, H.J.; Louzada, M.L.; Chu, M.P.; White, D.J.; Mian, H.S.; Kotb, R.; Othman, I.; et al. Belantamab Mafodotin in Combination with Pomalidomide and Dexamethasone Demonstrates Durable Responses in Triple Class Exposed/Refractory Multiple Myeloma. Blood 2022, 140, 7306–7307. [Google Scholar] [CrossRef]
- Deeks, E.D. Disitamab Vedotin: First Approval. Drugs 2021, 81, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Wang, L.; He, Z.; Shi, Y.; Luo, H.; Han, W.; Yao, X.; Shi, B.; Liu, J.; Hu, C.; et al. Efficacy and Safety of Disitamab Vedotin in Patients With Human Epidermal Growth Factor Receptor 2–Positive Locally Advanced or Metastatic Urothelial Carcinoma: A Combined Analysis of Two Phase II Clinical Trials. J. Clin. Oncol. 2024, 42, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, S.; Shan, X.; Wang, L.; Ma, J.; Huang, M.; Dong, L.; Chen, F. Preclinical Safety Profile of Disitamab Vedotin: A Novel Anti-HER2 Antibody Conjugated with MMAE. Toxicol. Lett. 2020, 324, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Jin, X.; Wang, L.; Wang, H.; Yan, Z.; Wang, G.; Liang, B.; Huang, F.; Luo, Y.; Chen, T.; et al. Efficacy of Disitamab Vedotin in Treating HER2 2+/FISH- Gastric Cancer. OncoTargets Ther. 2022, 15, 267–275. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, J.; Wang, A.; Wei, J.; Peng, Z.; Wang, X.; Zhou, J.; Qi, C.; Liu, D.; Li, J.; et al. Disitamab Vedotin (RC48) plus Toripalimab for HER2-Expressing Advanced Gastric or Gastroesophageal Junction and Other Solid Tumours: A Multicentre, Open Label, Dose Escalation and Expansion Phase 1 Trial. eClinicalMedicine 2024, 68, 102415. [Google Scholar] [CrossRef] [PubMed]
- Aicher, T.D.; Buszek, K.R.; Fang, F.G.; Forsyth, C.J.; Jung, S.H.; Kishi, Y.; Matelich, M.C.; Scola, P.M.; Spero, D.M.; Yoon, S.K. Total Synthesis of Halichondrin B and Norhalichondrin B. J. Am. Chem. Soc. 1992, 114, 3162–3164. [Google Scholar] [CrossRef]
- Huyck, T.K.; Gradishar, W.; Manuguid, F.; Kirkpatrick, P. Eribulin Mesylate. Nat. Rev. Drug Discov. 2011, 10, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, J.; Tanaka, K.; Majumder, U.; Milinichik, A.Z.; Verdi, A.C.; Maddage, C.J.; Rybinski, K.A.; Fernando, S.; Fernando, D.; et al. MORAb-202, an Antibody–Drug Conjugate Utilizing Humanized Anti-Human FRα Farletuzumab and the Microtubule-Targeting Agent Eribulin, Has Potent Antitumor Activity. Mol. Cancer Ther. 2018, 17, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Fujiwara, Y.; Yonemori, K.; Koyama, T.; Sato, J.; Tamura, K.; Shimomura, A.; Ikezawa, H.; Nomoto, M.; Furuuchi, K.; et al. First-in-Human Phase 1 Study of MORAb-202, an Antibody–Drug Conjugate Comprising Farletuzumab Linked to Eribulin Mesylate, in Patients with Folate Receptor-α–Positive Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3905–3915. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yao, L.; Yao, A. The Recent Developments of ADCs with the Tubulysins as the Payloads. Mini-Rev. Med. Chem. 2023, 23, 1797–1805. [Google Scholar] [CrossRef]
- Hamilton, J.Z.; Pires, T.A.; Mitchell, J.A.; Cochran, J.H.; Emmerton, K.K.; Zaval, M.; Stone, I.J.; Anderson, M.E.; Jin, S.; Waight, A.B.; et al. Improving Antibody-Tubulysin Conjugates through Linker Chemistry and Site-Specific Conjugation. ChemMedChem 2021, 16, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Cong, Q.; Dervin, D.; Stevens, A.; Vemuri, K.; Huber, M.; Juliano, J.; Cuison, S.; Sung, J.; Passmore, D.; et al. Synthesis and Biological Evaluation of a Carbamate-Containing Tubulysin Antibody–Drug Conjugate. Bioconjug. Chem. 2020, 31, 2350–2361. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S. Tubulysins as Antibody–Drug Conjugate (ADC) Payloads. In Cytotoxic Payloads for Antibody—Drug Conjugates; Thurston, D.E., Jackson, P.J.M., Eds.; The Royal Society of Chemistry: London, UK, 2019; pp. 380–397. ISBN 978-1-78801-077-1. [Google Scholar]
- Nicolaou, K.C.; Pan, S.; Pulukuri, K.K.; Ye, Q.; Rigol, S.; Erande, R.D.; Vourloumis, D.; Nocek, B.P.; Munneke, S.; Lyssikatos, J.; et al. Design, Synthesis, and Biological Evaluation of Tubulysin Analogues, Linker-Drugs, and Antibody–Drug Conjugates, Insights into Structure–Activity Relationships, and Tubulysin–Tubulin Binding Derived from X-ray Crystallographic Analysis. J. Org. Chem. 2021, 86, 3377–3421. [Google Scholar] [CrossRef] [PubMed]
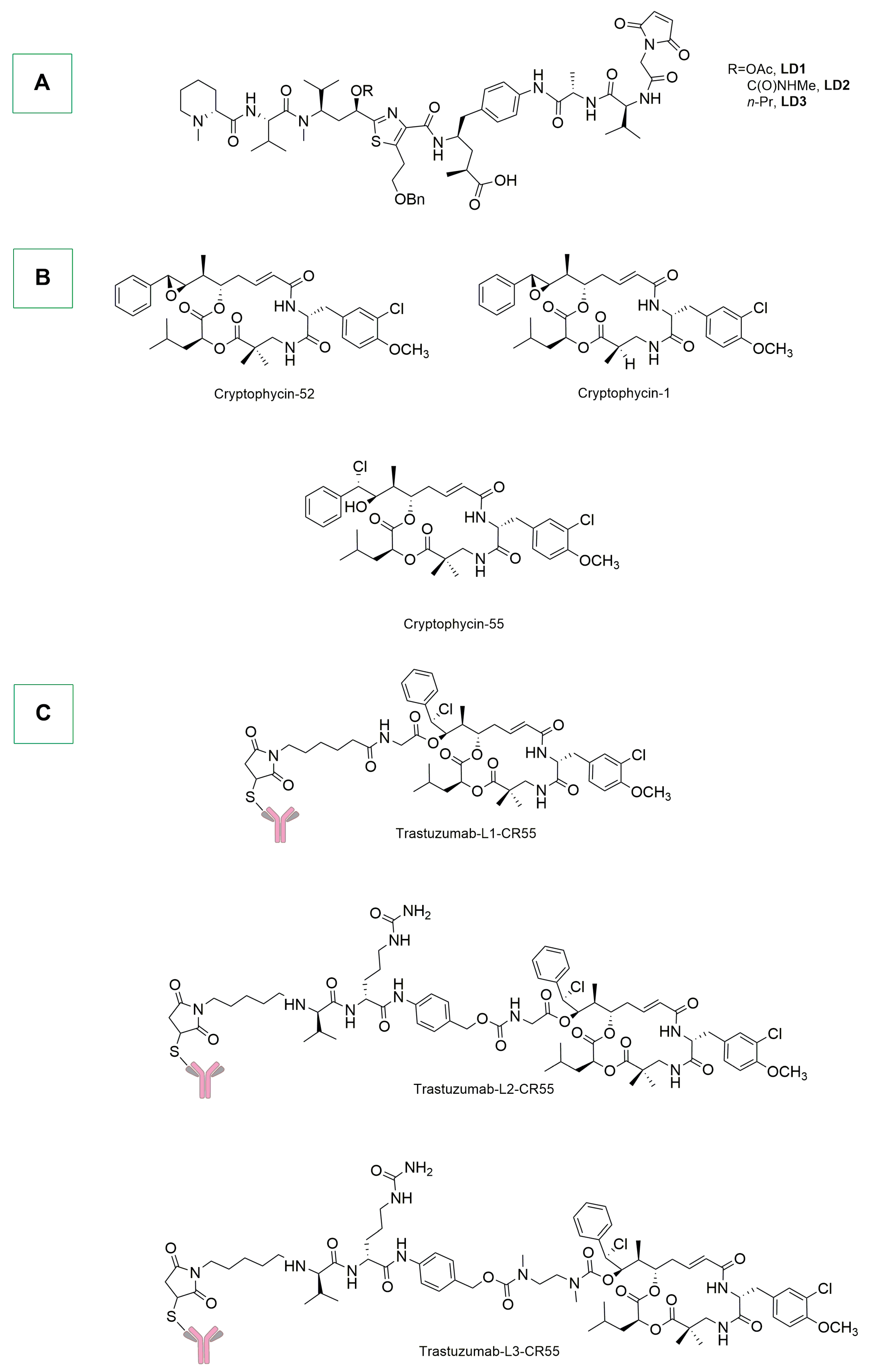
- Shih, C.; Teicher, B. Cryptophycins: A Novel Class of Potent Antimitotic Antitumor Depsipeptides. Curr. Pharm. Des. 2001, 7, 1259–1276. [Google Scholar] [CrossRef]
- Aesoy, R.; Herfindal, L. Cyanobacterial Anticancer Compounds in Clinical Use: Lessons from the Dolastatins and Cryptophycins. In The Pharmacological Potential of Cyanobacteria; Elsevier: Amsterdam, The Netherlands, 2022; pp. 55–79. ISBN 978-0-12-821491-6. [Google Scholar]
- Lai, Q.; Wu, M.; Wang, R.; Lai, W.; Tao, Y.; Lu, Y.; Wang, Y.; Yu, L.; Zhang, R.; Peng, Y.; et al. Cryptophycin-55/52 Based Antibody-Drug Conjugates: Synthesis, Efficacy, and Mode of Action Studies. Eur. J. Med. Chem. 2020, 199, 112364. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Liu, W.; Nonaka, K. Enediyne Natural Products: Biosynthesis and Prospect Towards Engineering Novel Antitumor Agents. Curr. Med. Chem. 2003, 10, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Basak, A.; Mandal, S.; Bag, S.S. Chelation-Controlled Bergman Cyclization: Synthesis and Reactivity of Enediynyl Ligands. Chem. Rev. 2003, 103, 4077–4094. [Google Scholar] [CrossRef]
- Li, X.; Zhou, M.; Qi, J.; Han, Y. Efficacy and Safety of Inotuzumab Ozogamicin (CMC-544) for the Treatment of Relapsed/Refractory Acute Lymphoblastic Leukemia and Non-Hodgkin Lymphoma: A Systematic Review and Meta-Analysis. Clin. Lymphoma Myeloma Leuk. 2021, 21, e227–e247. [Google Scholar] [CrossRef]
- Senapati, J.; Short, N.; Alvarado, Y.; Burger, J.A.; Jain, N.; Konopleva, M.; Ravandi, F.; DiNardo, C.D.; Masarova, L.; Sasaki, K.; et al. A Phase II Study of Inotuzumab Ozogamicin for the Treatment of Measurable Residual Disease-Positive B-Cell Acute Lymphoblastic Leukemia. Blood 2022, 140, 3253–3255. [Google Scholar] [CrossRef]
- Stelljes, M.; Alakel, N.; Wäsch, R.; Scholl, S.; Nachtkamp, K.; Rank, A.; Haenel, M.; Spriewald, B.; Hanoun, M.; Martin, S.; et al. Inotuzumab Ozogamicin Induction Followed By Standard Chemotherapy Yields High Remission Rates and Promising Survival in Older (>55 Years) Patients with De Novo B-Lymphoblastic Leukemia (GMALL-Initial1 Trial). Blood 2022, 140, 510–512. [Google Scholar] [CrossRef]
- Aujla, A.; Aujla, R.; Liu, D. Inotuzumab Ozogamicin in Clinical Development for Acute Lymphoblastic Leukemia and Non-Hodgkin Lymphoma. Biomark. Res. 2019, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- DeAngelo, D.J.; Advani, A.S.; Marks, D.I.; Stelljes, M.; Liedtke, M.; Stock, W.; Gökbuget, N.; Jabbour, E.; Merchant, A.; Wang, T.; et al. Inotuzumab Ozogamicin for Relapsed/Refractory Acute Lymphoblastic Leukemia: Outcomes by Disease Burden. Blood Cancer J. 2020, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Inotuzumab Ozogamicin: First Global Approval. Drugs 2017, 77, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
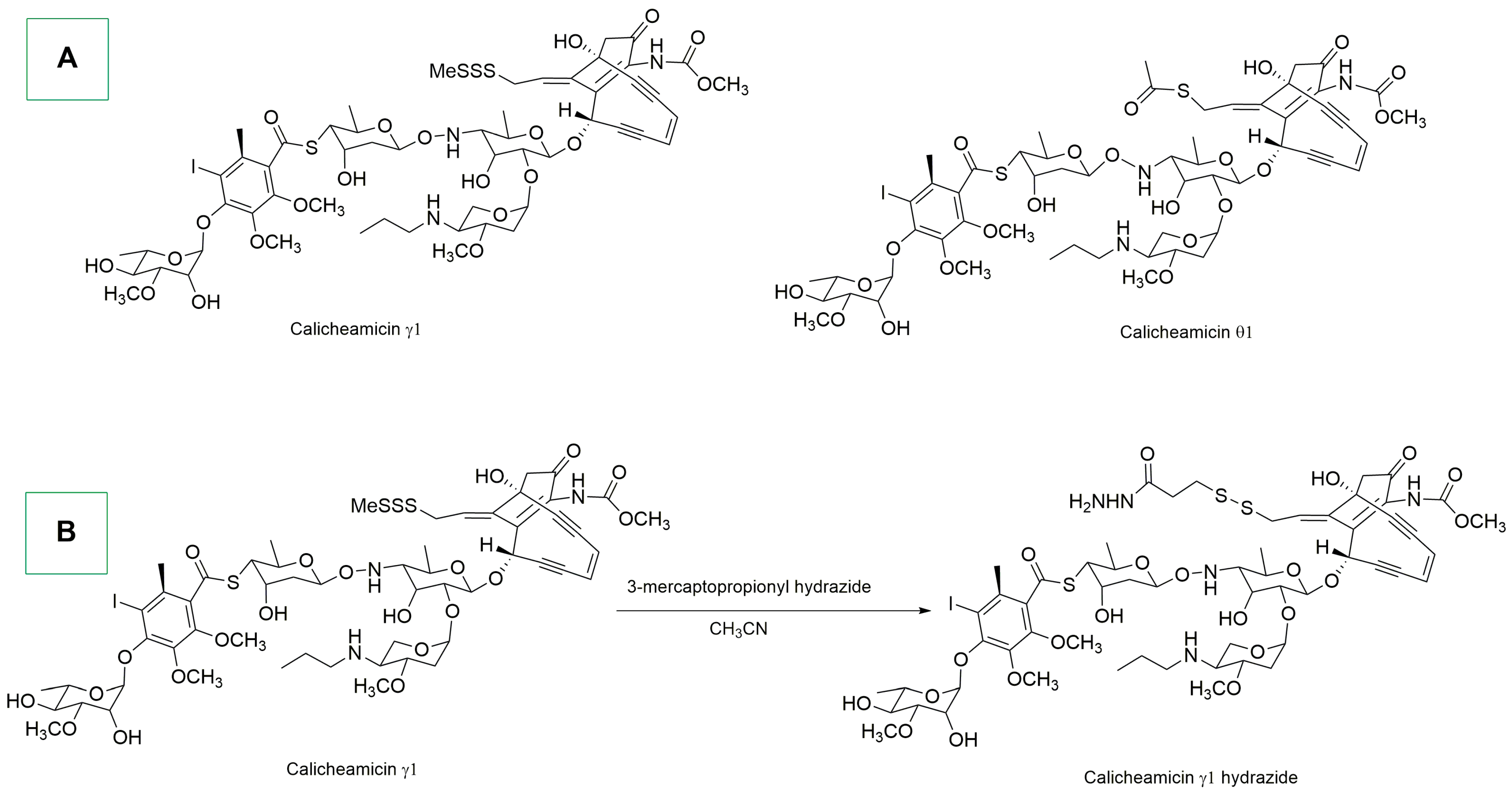
- Hinman, L.M.; Hamann, P.R.; Wallace, R.; Menendez, A.T.; Durr, F.E.; Upeslacis, J. Preparation and Characterization of Monoclonal Antibody Conjugates of the Calicheamicins: A Novel and Potent Family of Antitumor Antibiotics. Cancer Res. 1993, 53, 3336–3342. [Google Scholar] [PubMed]
- Kantarjian, H.M.; Stock, W.; Cassaday, R.D.; DeAngelo, D.J.; Jabbour, E.; O’Brien, S.M.; Stelljes, M.; Wang, T.; Paccagnella, M.L.; Nguyen, K.; et al. Inotuzumab Ozogamicin for Relapsed/Refractory Acute Lymphoblastic Leukemia in the INO-VATE Trial: CD22 Pharmacodynamics, Efficacy, and Safety by Baseline CD22. Clin. Cancer Res. 2021, 27, 2742–2754. [Google Scholar] [CrossRef] [PubMed]
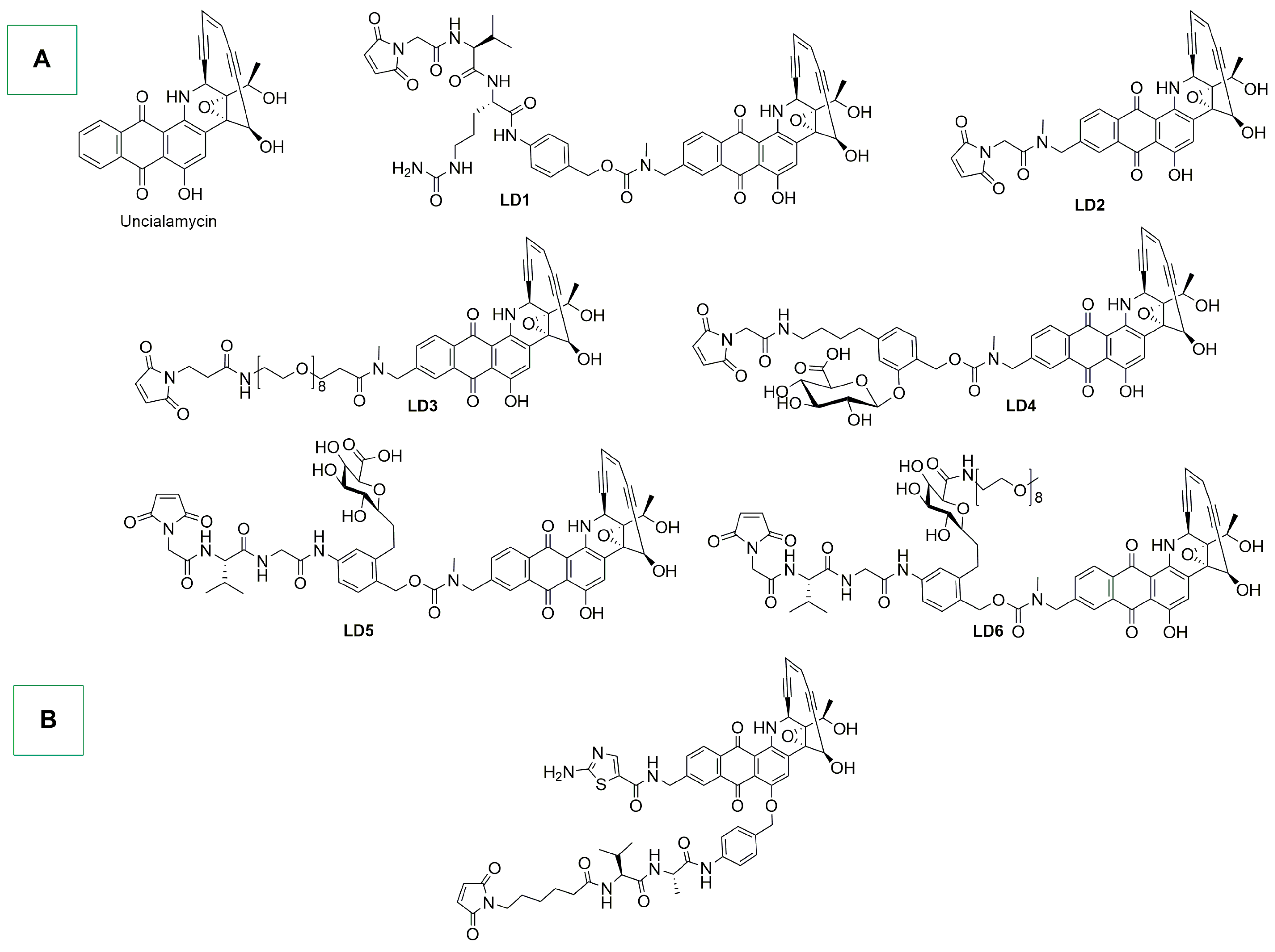
- Nicolaou, K.C.; Rigol, S.; Pitsinos, E.N.; Das, D.; Lu, Y.; Rout, S.; Schammel, A.W.; Holte, D.; Lin, B.; Gu, C.; et al. Uncialamycin-Based Antibody–Drug Conjugates: Unique Enediyne ADCs Exhibiting Bystander Killing Effect. Proc. Natl. Acad. Sci. USA 2021, 118, e2107042118. [Google Scholar] [CrossRef] [PubMed]
- Poudel, Y.B.; Rao, C.; Kotapati, S.; Deshpande, M.; Thevanayagam, L.; Pan, C.; Cardarelli, J.; Chowdari, N.; Kaspady, M.; Samikannu, R.; et al. Design, Synthesis and Biological Evaluation of Phenol-Linked Uncialamycin Antibody-Drug Conjugates. Bioorg. Med. Chem. Lett. 2020, 30, 126782. [Google Scholar] [CrossRef] [PubMed]
- Chowdari, N.S.; Pan, C.; Rao, C.; Langley, D.R.; Sivaprakasam, P.; Sufi, B.; Derwin, D.; Wang, Y.; Kwok, E.; Passmore, D.; et al. Uncialamycin as a Novel Payload for Antibody Drug Conjugate (ADC) Based Targeted Cancer Therapy. Bioorg. Med. Chem. Lett. 2019, 29, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Hartley, J.A. The Development of Pyrrolobenzodiazepines as Antitumour Agents. Expert Opin. Investig. Drugs 2011, 20, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Leimgruber, W.; Stefanović, V.; Schenker, F.; Karr, A.; Berger, J. Isolation and Characterization of Anthramycin, a New Antitumor Antibiotic. J. Am. Chem. Soc. 1965, 87, 5791–5793. [Google Scholar] [CrossRef] [PubMed]
- Brazhnikova, M.G.; Konstantinova, N.V.; Mesentsev, A.S. Sibiromycin: Isolation and Characterization. J. Antibiot. 1972, 25, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Kohsaka, M.; Tamura, G.; Imanaka, H.; Sakai, H. Studies on Tomaymycin, a New Antibiotic. I Isolation and Properties of Tomaymyin. J. Antibiot. 1972, 25, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Mantaj, J.; Jackson, P.J.M.; Rahman, K.M.; Thurston, D.E. From Anthramycin to Pyrrolobenzodiazepine (PBD)-Containing Antibody–Drug Conjugates (ADCs). Angew. Chem. Int. Ed. 2017, 56, 462–488. [Google Scholar] [CrossRef] [PubMed]
- Hartley, J.A. Antibody-Drug Conjugates (ADCs) Delivering Pyrrolobenzodiazepine (PBD) Dimers for Cancer Therapy. Expert Opin. Biol. Ther. 2021, 21, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Zhao, S.; Lai, Q.; Zhou, W.; Wu, M.; Jiang, X.; Wang, X.; Peng, Y.; Wei, X.; Ouyang, L.; et al. Design, Synthesis, and Bioevaluation of a Novel Hybrid Molecular Pyrrolobenzodiazepine–Anthracenecarboxyimide as a Payload for Antibody–Drug Conjugate. J. Med. Chem. 2022, 65, 11679–11702. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.D.; Yurkovetskiy, A.V.; Yin, M.; Bodyak, N.D.; Gumerov, D.R.; Tang, S.; Kelleher, E.; Jones, B.D.; Protopopova, M.; Qin, L.; et al. Discovery of Novel Polyamide-Pyrrolobenzodiazepine Hybrids for Antibody-Drug Conjugates. Bioorg. Med. Chem. Lett. 2022, 72, 128876. [Google Scholar] [CrossRef] [PubMed]
- Tiberghien, A.C.; Parker, J.S. Discovery and Chemical Development of Tesirine: An Antitumor Pyrrolobenzodiazepine Antibody-Drug Conjugate Drug-Linker. In ACS Symposium Series; Pesti, J.A., Abdel-Magid, A.F., Vaidyanathan, R., Eds.; American Chemical Society: Washington, DC, USA, 2020; Volume 1369, pp. 215–252. ISBN 978-0-8412-9864-4. [Google Scholar]
- Min, B.; Jin, J.; Kim, H.; Her, N.-G.; Park, C.; Kim, D.; Yang, J.; Hwang, J.; Kim, E.; Choi, M.; et al. cIRCR201-dPBD, a Novel Pyrrolobenzodiazepine Dimer-Containing Site-Specific Antibody–Drug Conjugate Targeting c-Met Overexpression Tumors. ACS Omega 2020, 5, 25798–25809. [Google Scholar] [CrossRef] [PubMed]
- Zou, N.; Han, A. Application of Pyrrolobenzodiazepines in Antibody Drug Conjugates. In Contemporary Accounts in Drug Discovery and Development; Huang, X., Aslanian, R.G., Tang, W.H., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 293–339. ISBN 978-1-119-62771-5. [Google Scholar]
- Zammarchi, F.; Havenith, K.E.; Chivers, S.; Hogg, P.; Bertelli, F.; Tyrer, P.; Janghra, N.; Reinert, H.W.; Hartley, J.A.; Van Berkel, P.H. Preclinical Development of ADCT-601, a Novel Pyrrolobenzodiazepine Dimer-Based Antibody–Drug Conjugate Targeting AXL-Expressing Cancers. Mol. Cancer Ther. 2022, 21, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Gregson, S.J.; Pugh, K.; Patel, N.; Afif-Rider, S.; Vijayakrishnan, B.; Santos, K.; Riedl, J.; Hutchinson, I.; Kang, G.-D.; Chooi, K.P.; et al. Efficacy, Tolerability, and Pharmacokinetic Studies of Antibody–Drug Conjugates Containing a Low-Potency Pyrrolobenzodiazepine Dimer. Mol. Cancer Ther. 2022, 21, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
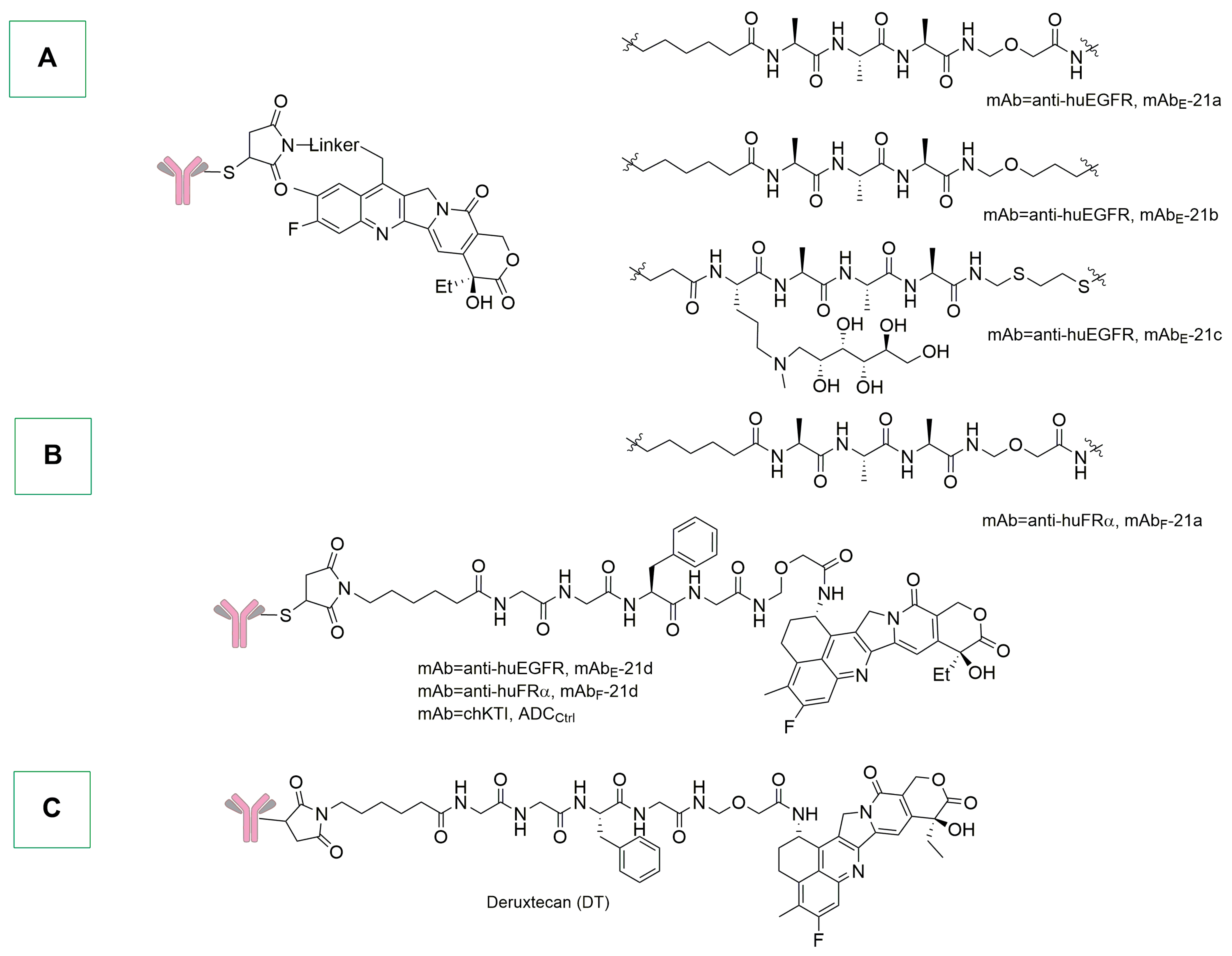
- Wall, M.E.; Wani, M.C.; Cook, C.E.; Palmer, K.H.; McPhail, A.T.; Sim, G.A. Plant Antitumor Agents. I. The Isolation and Structure of Camptothecin, a Novel Alkaloidal Leukemia and Tumor Inhibitor from Camptotheca Acuminata 1,2. J. Am. Chem. Soc. 1966, 88, 3888–3890. [Google Scholar] [CrossRef]
- Shamma, M.; Georgiev, V.S. Camptothecin. J. Pharm. Sci. 1974, 63, 163–183. [Google Scholar] [CrossRef] [PubMed]
- Lyski, R.D.; Bou, L.B.; Lau, U.Y.; Meyer, D.W.; Cochran, J.H.; Okeley, N.M.; Emmerton, K.K.; Zapata, F.; Simmons, J.K.; Trueblood, E.S.; et al. Development of Novel Antibody–Camptothecin Conjugates. Mol. Cancer Ther. 2021, 20, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Veale, K.H.; Qiu, Q.; Sinkevicius, K.W.; Maloney, E.K.; Costoplus, J.A.; Lau, J.; Evans, H.L.; Setiady, Y.; Ab, O.; et al. Synthesis and Evaluation of Camptothecin Antibody–Drug Conjugates. ACS Med. Chem. Lett. 2019, 10, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab Deruxtecan (DS-8201) in Patients with HER2-Expressing Metastatic Colorectal Cancer (DESTINY-CRC01): A Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2 -Mutant Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Trastuzumab Deruxtecan: First Approval. Drugs 2020, 80, 501–508. [Google Scholar] [CrossRef] [PubMed]
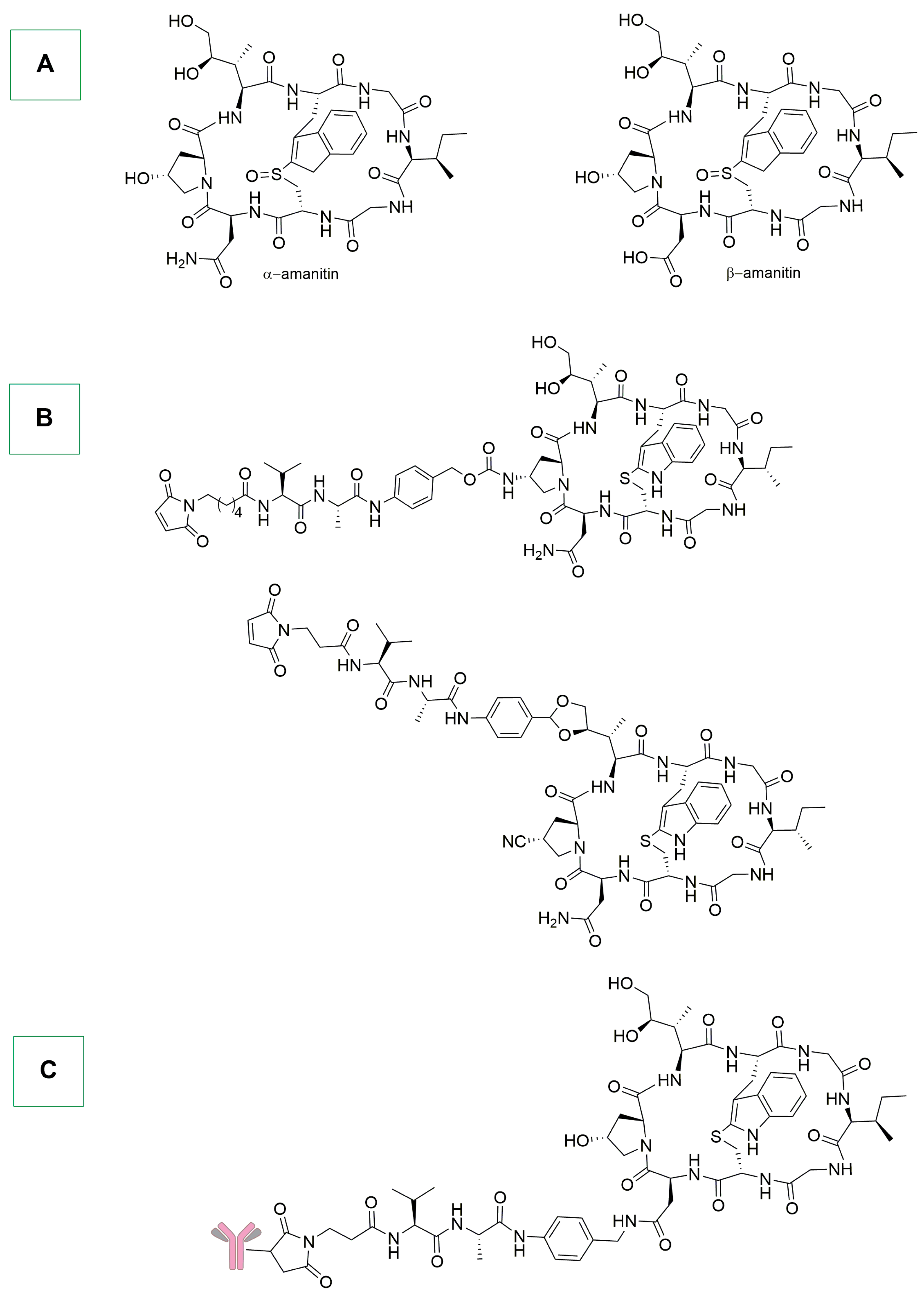
- Barbosa, I.; Domingues, C.; Ramos, F.; Barbosa, R.M. Analytical Methods for Amatoxins: A Comprehensive Review. J. Pharm. Biomed. Anal. 2023, 232, 115421. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.; Xue, J.; Lou, X.; Shao, R.; Liu, Y.; Chen, G. Transforming Toxins into Treatments: The Revolutionary Role of α-Amanitin in Cancer Therapy. Arch. Toxicol. 2024, 98, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Matinkhoo, K.; Wong, A.A.W.L.; Hambira, C.M.; Kato, B.; Wei, C.; Müller, C.; Hechler, T.; Braun, A.; Gallo, F.; Pahl, A.; et al. Design, Synthesis, and Biochemical Evaluation of Alpha-Amanitin Derivatives Containing Analogs of the Trans-Hydroxyproline Residue for Potential Use in Antibody-Drug Conjugates. Chem. Eur. J. 2021, 27, 10282–10292. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Vazquez, V.; Ko, J.; Breunig, C.; Baumann, A.; Giesen, N.; Pálfi, A.; Müller, C.; Lutz, C.; Hechler, T.; Kulke, M.; et al. HDP-101, an Anti-BCMA Antibody–Drug Conjugate, Safely Delivers Amanitin to Induce Cell Death in Proliferating and Resting Multiple Myeloma Cells. Mol. Cancer Ther. 2021, 20, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Pearse, B.R.; McDonough, S.M.; Proctor, J.L.; Panwar, R.; Sarma, G.N.; Kien, L.; Dushime, J.; Adams, H.L.; Hyzy, S.L.; Brooks, M.; et al. A CD117-Amanitin Antibody Drug Conjugate (ADC) Effectively Depletes Human and Non-Human Primate Hematopoietic Stem and Progenitor Cells (HSPCs): Targeted Non-Genotoxic Conditioning for Bone Marrow Transplant. Biol. Blood Marrow Transplant. 2019, 25, S29–S30. [Google Scholar] [CrossRef][Green Version]
- Gallo, F.; Korsak, B.; Müller, C.; Hechler, T.; Yanakieva, D.; Avrutina, O.; Kolmar, H.; Pahl, A. Enhancing the Pharmacokinetics and Antitumor Activity of an α-Amanitin-Based Small-Molecule Drug Conjugate via Conjugation with an Fc Domain. J. Med. Chem. 2021, 64, 4117–4129. [Google Scholar] [CrossRef] [PubMed]
- Almaliti, J.; Miller, B.; Pietraszkiewicz, H.; Glukhov, E.; Naman, C.B.; Kline, T.; Hanson, J.; Li, X.; Zhou, S.; Valeriote, F.A.; et al. Exploration of the Carmaphycins as Payloads in Antibody Drug Conjugate Anticancer Agents. Eur. J. Med. Chem. 2019, 161, 416–432. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonon, G.; Rizzolio, F.; Visentin, F.; Scattolin, T. Antibody Drug Conjugates for Cancer Therapy: From Metallodrugs to Nature-Inspired Payloads. Int. J. Mol. Sci. 2024, 25, 8651. https://doi.org/10.3390/ijms25168651
Tonon G, Rizzolio F, Visentin F, Scattolin T. Antibody Drug Conjugates for Cancer Therapy: From Metallodrugs to Nature-Inspired Payloads. International Journal of Molecular Sciences. 2024; 25(16):8651. https://doi.org/10.3390/ijms25168651
Chicago/Turabian StyleTonon, Giovanni, Flavio Rizzolio, Fabiano Visentin, and Thomas Scattolin. 2024. "Antibody Drug Conjugates for Cancer Therapy: From Metallodrugs to Nature-Inspired Payloads" International Journal of Molecular Sciences 25, no. 16: 8651. https://doi.org/10.3390/ijms25168651
APA StyleTonon, G., Rizzolio, F., Visentin, F., & Scattolin, T. (2024). Antibody Drug Conjugates for Cancer Therapy: From Metallodrugs to Nature-Inspired Payloads. International Journal of Molecular Sciences, 25(16), 8651. https://doi.org/10.3390/ijms25168651







