Transcriptomic Analysis of Vitrified–Warmed vs. Fresh Mouse Blastocysts: Cryo-Induced Physiological Mechanisms and Implantation Impact
Abstract
:1. Introduction
2. Results
2.1. Impact of Vitrified–Warmed Mouse Blastocysts on Implantation
2.2. Effect of Cryopreservation on Transcriptomic Profile
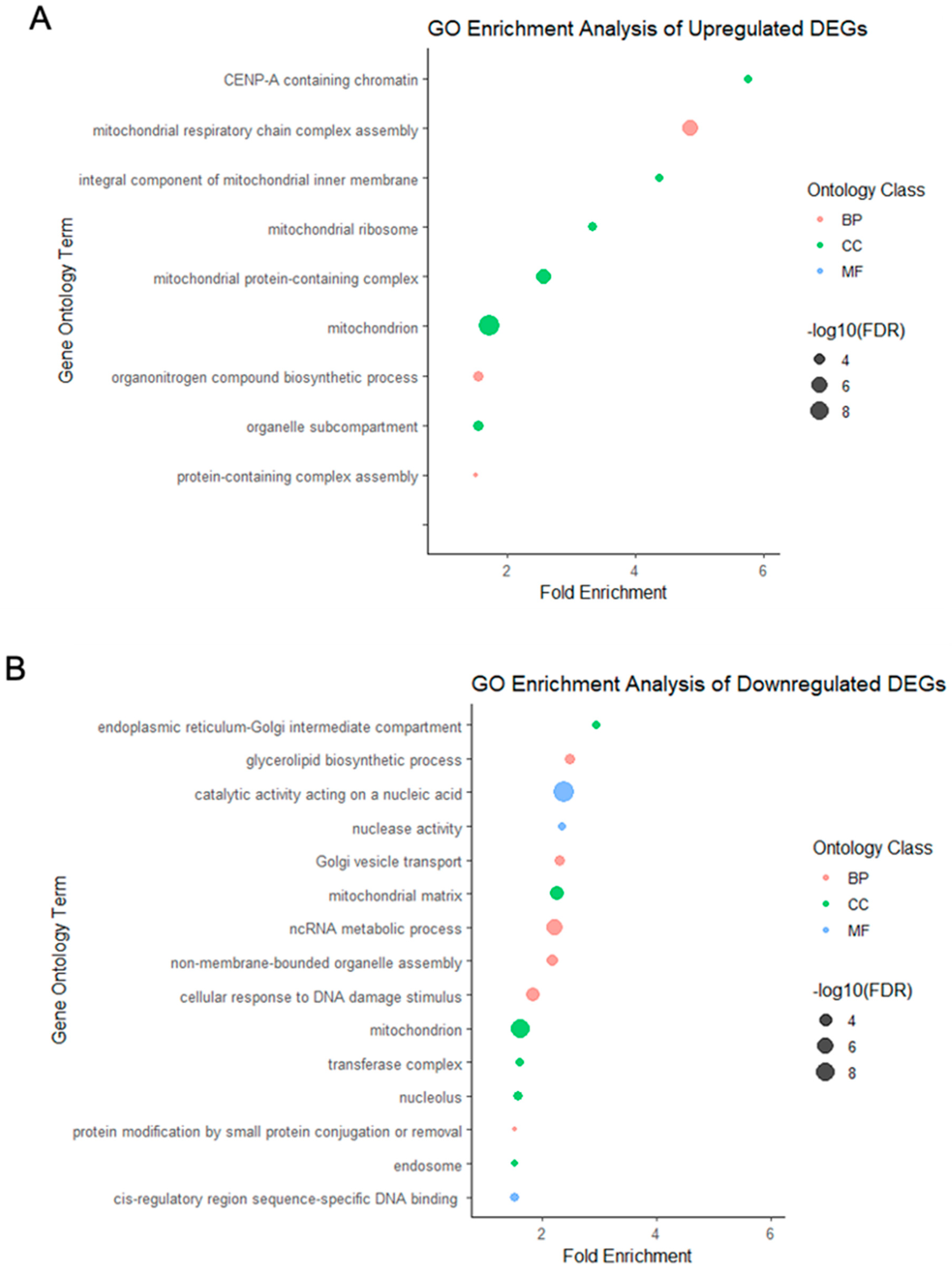
2.3. Gene Ontology Enrichment Analysis in Vitrified–Warmed and Fresh Preimplantation Embryos
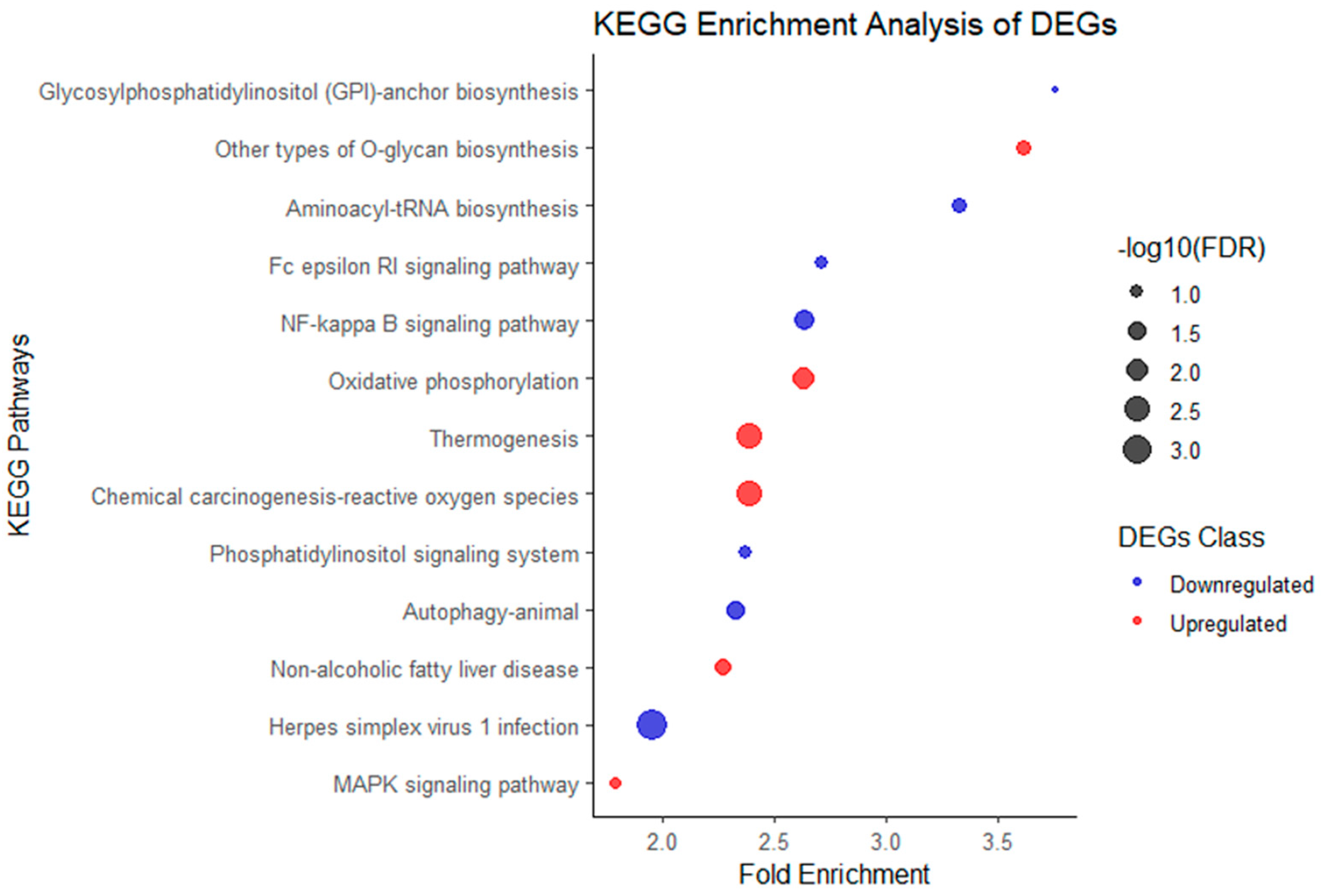
2.4. Kyoto Encyclopedia of Genes and Genomes Pathway Enrichment Analysis
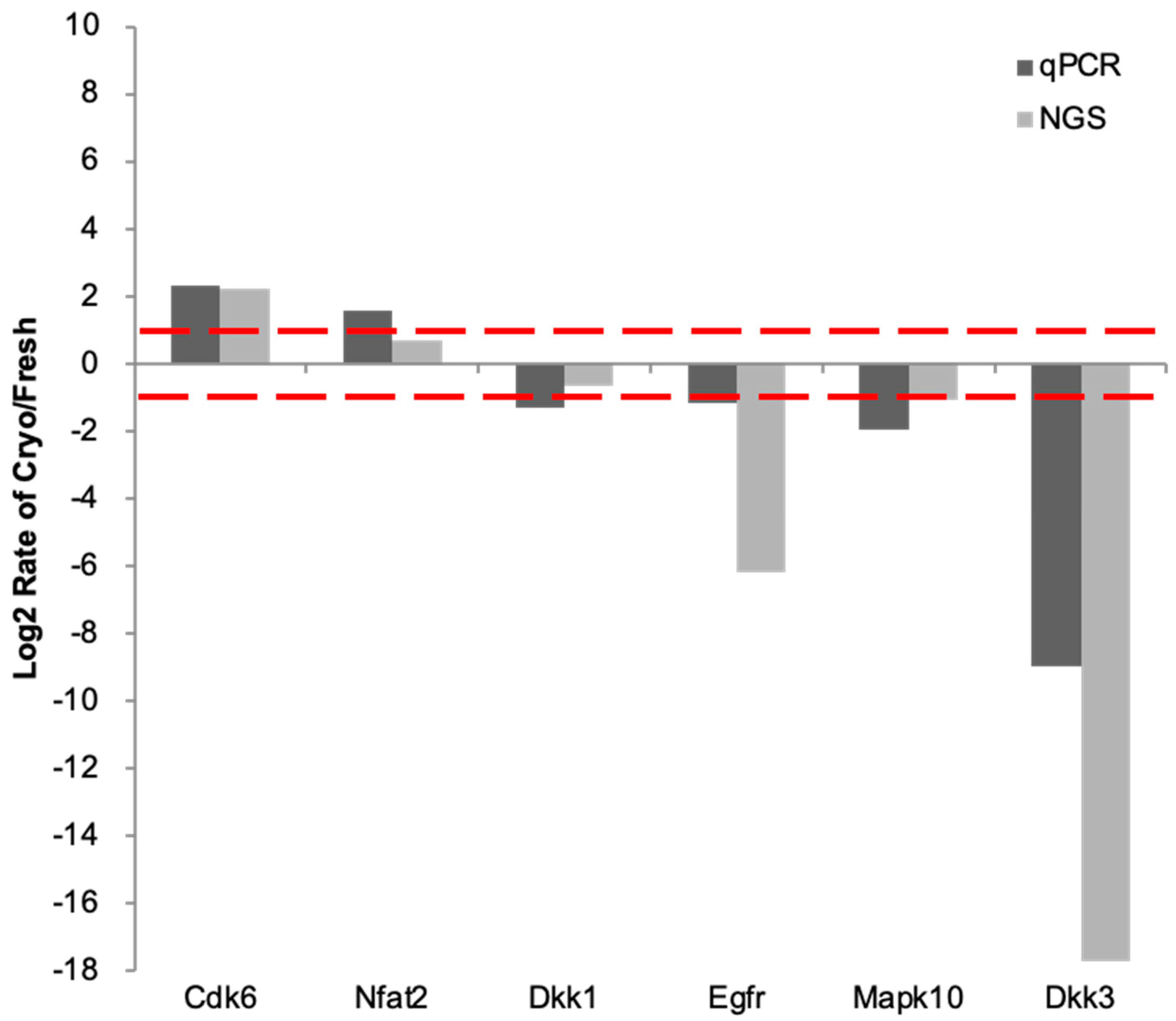
2.5. RT-qPCR Validation of Key Genes
2.6. Prediction of MicroRNA Involvement in Vitrified–Warmed Embryos
2.7. Identification and Functional Analysis of Validated MicroRNA Candidates in Vitrified–Warmed Embryos
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Preparation of Embryos
4.3. Embryo Vitrification and Warming
4.4. Embryo Transfer
4.5. RNA-Seq Analysis
4.6. Data Preprocessing
4.7. Real-Time RT-PCR
4.8. MicroRNA Expression Analysis in Vitrified–Warmed Blastocysts
4.9. Functional Annotation and Pathway Enrichment Analysis of Differentially Expressed Genes
4.10. MicroRNA Analysis and Functional Enrichment in Vitrified–Warmed Embryos
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abha, M.; Shilpi, P.; Ashalatha, S.; Mark, H.; Siladitya, B.; Siladitya, B. Obstetric and Perinatal Outcomes in Singleton Pregnancies Resulting from the Transfer of Frozen Thawed Versus Fresh Embryos Generated through in Vitro Fertilization Treatment: A Systematic Review and Meta-Analysis. Fertil. Steril. 2012, 98, 368–377. [Google Scholar] [CrossRef]
- Abha, M.; Shilpi, P.; Shilpi, P.; Shilpi, P.; Edwin Amalraj, R.; Ashalatha, S.; Mark, H.; Siladitya, B.; Siladitya, B. Is Frozen Embryo Transfer Better for Mothers and Babies? Can Cumulative Meta-Analysis Provide a Definitive Answer? Hum. Reprod. Update 2018, 24, 35–58. [Google Scholar] [CrossRef]
- Osamu, I.; Osamu, I.; Ryuichiro, A.; Akira, K.; Akira, K.; Atsuo, I.; Hidekazu, S.; Hidekazu, S.; Adamson, G.D.; Adamson, G.D. Impact of Frozen-Thawed Single-Blastocyst Transfer on Maternal and Neonatal Outcome: An Analysis of 277,042 Single-Embryo Transfer Cycles from 2008 to 2010 in Japan. Fertil. Steril. 2014, 101, 128–133. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, Y.; Hao, C.; Zhang, H.; Wei, D.; Zhang, Y.; Zhu, Y.; Deng, X.; Qi, X.; Li, H.; et al. Transfer of Fresh versus Frozen Embryos in Ovulatory Women. N. Engl. J. Med. 2018, 378, 126–136. [Google Scholar] [CrossRef]
- Sharov, A.A.; Piao, Y.; Matoba, R.; Dudekula, D.B.; Qian, Y.; VanBuren, V.; Falco, G.; Martin, P.R.; Stagg, C.A.; Bassey, U.C.; et al. Transcriptome Analysis of Mouse Stem Cells and Early Embryos. PLoS Biol. 2003, 1, E74. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, S.; Wang, Z. Role of Micrornas in Embryo Implantation. Reprod. Biol. Endocrinol. 2017, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Cuello, C.; Martinez, C.A.; Cambra, J.M.; Parrilla, I.; Rodriguez-Martinez, H.; Gil, M.A.; Martinez, E.A. Effects of Vitrification on the Blastocyst Gene Expression Profile in a Porcine Model. Int. J. Mol. Sci. 2021, 22, 1222. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.A.; Martinez, C.A.; Nohalez, A.; Sanchez-Osorio, J.; Vazquez, J.M.; Roca, J.; Parrilla, I.; Gil, M.A.; Cuello, C. Nonsurgical Deep Uterine Transfer of Vitrified, in Vivo-Derived, Porcine Embryos Is as Effective as the Default Surgical Approach. Sci. Rep. 2015, 5, 10587. [Google Scholar] [CrossRef] [PubMed]
- Vajta, G.; Nagy, Z.P. Are Programmable Freezers Still Needed in the Embryo Laboratory? Review on Vitrification. Reprod. Biomed. Online 2006, 12, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Kuwayama, M.; Vajta, G.; Kato, O.; Leibo, S.P. Highly Efficient Vitrification Method for Cryopreservation of Human Oocytes. Reprod. Biomed. Online 2005, 11, 300–308. [Google Scholar] [CrossRef]
- Reza, A.; Choi, Y.J.; Han, S.G.; Song, H.; Park, C.; Hong, K.; Kim, J.H. Roles of Micrornas in Mammalian Reproduction: From the Commitment of Germ Cells to Peri-Implantation Embryos. Biol. Rev. Camb. Philos. Soc. 2019, 94, 415–438. [Google Scholar] [CrossRef] [PubMed]
- Azizi, E.; Ghaffari Novin, M.; Naji, M.; Amidi, F.; Hosseinirad, H.; Shams Mofarahe, Z. Effect of Vitrification on Biogenesis Pathway and Expression of Development-Related Micrornas in Preimplantation Mouse Embryos. Cell Tissue Bank. 2021, 22, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Jung, D.; Yao, R. Shinygo: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstrale, M.; Laurila, E.; et al. Pgc-1alpha-Responsive Genes Involved in Oxidative Phosphorylation Are Coordinately Downregulated in Human Diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, J.; Cao, S.; Zhang, J.; Heng, B.C.; Huang, M.; Ling, X.; Duan, T.; Tong, G.Q. Vitrified-Warmed Blastocyst Transfer Cycles Yield Higher Pregnancy and Implantation Rates Compared with Fresh Blastocyst Transfer Cycles—Time for a New Embryo Transfer Strategy? Fertil. Steril. 2011, 95, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Bing, Y.Z.; Che, L.M.; Brown, E.O. Equivalent Pregnancy Rates Obtained from Vitrified and Fresh Blastocyst Transfer. Fertil. Steril. 2013, 100, S180. [Google Scholar] [CrossRef]
- Ku, P.Y.; Lee, R.K.; Lin, S.Y.; Lin, M.H.; Hwu, Y.M. Comparison of the Clinical Outcomes between Fresh Blastocyst and Vitrified-Thawed Blastocyst Transfer. J. Assist. Reprod. Genet. 2012, 29, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hao, H.; Du, W.; Zhu, H. Effect of Vitrification on the Microrna Transcriptome in Mouse Blastocysts. PLoS ONE 2015, 10, e0123451. [Google Scholar] [CrossRef] [PubMed]
- Cañón-Beltrán, K.; Giraldo-Giraldo, J.; Cajas, Y.N.; Beltrán-Breña, P.; Hidalgo, C.O.; Vásquez, N.; Leal, C.L.V.; Gutiérrez-Adán, A.; González, E.M.; Rizos, D. Inhibiting Diacylglycerol Acyltransferase-1 Reduces Lipid Biosynthesis in Bovine Blastocysts Produced In vitro. Theriogenology 2020, 158, 267–276. [Google Scholar] [CrossRef]
- Hara, T.; Kin, A.; Aoki, S.; Nakamura, S.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Resveratrol Enhances the Clearance of Mitochondrial Damage by Vitrification and Improves the Development of Vitrified-Warmed Bovine Embryos. PLoS ONE 2018, 13, e0204571. [Google Scholar] [CrossRef] [PubMed]
- Martino, N.A.; Dell’Aquila, M.E.; Cardone, R.A.; Somoskoi, B.; Lacalandra, G.M.; Cseh, S. Vitrification Preserves Chromatin Integrity, Bioenergy Potential and Oxidative Parameters in Mouse Embryos. Reprod. Biol. Endocrinol. 2013, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Kopeika, J.; Thornhill, A.; Khalaf, Y. The Effect of Cryopreservation on the Genome of Gametes and Embryos: Principles of Cryobiology and Critical Appraisal of the Evidence. Hum. Reprod. Update 2014, 21, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Chun, S.; Kim, D. Cold Exposure Lowers Energy Expenditure at the Cellular Level. Cell Biol. Int. 2013, 37, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Zhang, K.; Lin, F.; He, Q.; Wu, S.; Xu, Z.; Zhang, Y.; Quan, F. Effects of Micu1-Mediated Mitochondrial Calcium Uptake on Energy Metabolism and Quality of Vitrified-Thawed Mouse Metaphase II Oocytes. Int. J. Mol. Sci. 2022, 23, 8629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, L.; Yang, A.; Lin, J.; Tang, F.; Jin, S.; Wei, Z.; Li, J.; Jin, Y. Calcineurin-Nfat Signaling Critically Regulates Early Lineage Specification in Mouse Embryonic Stem Cells and Embryos. Cell Stem Cell 2011, 8, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Han, H.B.; Tian, X.Z.; Tan, D.X.; Wang, L.; Zhou, G.B.; Zhu, S.E.; Liu, G.S. Melatonin Promotes Embryonic Development and Reduces Reactive Oxygen Species in Vitrified Mouse 2-Cell Embryos. J. Pineal Res. 2012, 52, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Du, W.H.; Wang, D.; Hao, H.S.; Liu, Y.; Qin, T.; Zhu, H.B. Recovery of Mitochondrial Function and Endogenous Antioxidant Systems in Vitrified Bovine Oocytes During Extended in Vitro Culture. Mol. Reprod. Dev. 2011, 78, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Mognol, G.P.; Carneiro, F.R.; Robbs, B.K.; Faget, D.V.; Viola, J.P. Cell Cycle and Apoptosis Regulation by Nfat Transcription Factors: New Roles for an Old Player. Cell Death Dis. 2016, 7, e2199. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, K.; Heller, G.; Schneckenleithner, C.; Warsch, W.; Scheicher, R.; Ott, R.G.; Schäfer, M.; Fajmann, S.; Schlederer, M.; Schiefer, A.I.; et al. A Kinase-Independent Function of Cdk6 Links the Cell Cycle to Tumor Angiogenesis. Cancer Cell 2013, 24, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.M.; Havens, M.A.; Clipstone, N.A. Identification of Amino Acid Residues and Protein Kinases Involved in the Regulation of Nfatc Subcellular Localization. J. Biol. Chem. 2000, 275, 3543–3551. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.L.; Leonard, D.M.; Wolfe, S.A.; Liu, J.; Rivera, J.; Yang, M.; Leonard, R.T.; Johnson, J.P.S.; Kumar, P.; Liebmann, K.L.; et al. The Dkk3 Gene Encodes a Vital Intracellular Regulator of Cell Proliferation. PLoS ONE 2017, 12, e0181724. [Google Scholar] [CrossRef]
- De Smaele, E.; Zazzeroni, F.; Papa, S.; Nguyen, D.U.; Jin, R.; Jones, J.; Cong, R.; Franzoso, G. Induction of Gadd45beta by Nf-Kappab Downregulates Pro-Apoptotic Jnk Signalling. Nature 2001, 414, 308–313. [Google Scholar] [CrossRef]
- King, A.E.; Critchley, H.O.; Kelly, R.W. The Nf-Kappab Pathway in Human Endometrium and First Trimester Decidua. Mol. Hum. Reprod. 2001, 7, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Lecce, L.; Day, M.L.; Murphy, C.R. Focal Adhesion Kinase Localizes to Sites of Cell-to-Cell Contact in Vivo and Increases Apically in Rat Uterine Luminal Epithelium and the Blastocyst at the Time of Implantation. J. Morphol. 2012, 273, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.T.; Mainigi, M.A.; Word, R.A.; Kraus, W.L.; Mendelson, C.R. miR-200 Regulates Endometrial Development During Early Pregnancy. Mol. Endocrinol. 2016, 30, 977–987. [Google Scholar] [CrossRef]
- Shen, L.J.; He, J.L.; Yang, D.H.; Ding, Y.B.; Chen, X.M.; Geng, Y.Q.; Liu, S.J.; Liu, X.Q.; Wang, Y.X. Mmu-Microrna-200a Overexpression Leads to Implantation Defect by Targeting Phosphatase and Tensin Homolog in Mouse Uterus. Reprod. Sci. 2013, 20, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Berardi, E.; Pues, M.; Thorrez, L.; Sampaolesi, M. Mirnas in Esc Differentiation. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H931–H939. [Google Scholar] [CrossRef] [PubMed]
- Hasuwa, H.; Ueda, J.; Ikawa, M.; Okabe, M. Mir-200b and Mir-429 Function in Mouse Ovulation and Are Essential for Female Fertility. Science 2013, 341, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Foshay, K.M.; Gallicano, G.I. Mir-17 Family Mirnas Are Expressed During Early Mammalian Development and Regulate Stem Cell Differentiation. Dev. Biol. 2009, 326, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.N.; Tang, X.M.; Wang, X.Q.; Gao, J.; Li, N.; Wang, Y.Y.; Xia, H.F. MiR-93 Inhibits Trophoblast Cell Proliferation and Promotes Cell Apoptosis by Targeting BCL2L2 in Recurrent Spontaneous Abortion. Reprod. Sci. 2020, 27, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wei, L.; Lali, M.S.; Chen, Y.; Yu, J.; Feng, L. Mir-150-5p Mediates Extravillous Trophoblast Cell Migration and Angiogenesis Functions by Regulating Vegf and Mmp9. Placenta 2020, 93, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.L. Micrornas and the Immune Response. Trends Immunol. 2008, 29, 343–351. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, W.; Zhang, R.; Liu, P.; Ye, Y.; Yu, W.; Guo, X.; Yu, J. Cancer Exosome-Derived Mir-9 and Mir-181a Promote the Development of Early-Stage Mdscs Via Interfering with Socs3 and Pias3 Respectively in Breast Cancer. Oncogene 2020, 39, 4681–4694. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhao, Z.-L.; Zhao, W.-T.; Fan, Q.-R.; Wang, S.-C.; Li, J.; Zhang, Y.-Q.; Shi, J.-W.; Lin, X.-L.; Yang, S.; et al. Mir-9 Modulates the Expression of Interferon-Regulated Genes and Mhc Class I Molecules in Human Nasopharyngeal Carcinoma Cells. Biochem. Biophys. Res. Commun. 2013, 431, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Leucci, E.; Alya, Z.; Gregersen, L.; Klaus Thorleif, J.; Obad, S.; Bellan, C.; Leoncini, L.; Kauppinen, S.; Kauppinen, S.; Lund, A. Inhibition of Mir-9 De-Represses Hur and Dicer1 and Impairs Hodgkin Lymphoma Tumour Outgrowth in Vivo. Oncogene 2012, 31, 5081–5089. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.L.; Wissink, E.M.; Grimson, A.; Rudd, B.D. miR-150 Regulates Differentiation and Cytolytic Effector Function in CD8+ T cells. Sci. Rep. 2015, 5, 16399. [Google Scholar] [CrossRef] [PubMed]
- Ménoret, A.; Agliano, F.; Karginov, T.A.; Karlinsey, K.S.; Zhou, B.; Vella, A.T. Antigen-Specific Downregulation of Mir-150 in Cd4 T Cells Promotes Cell Survival. Front. Immunol. 2023, 14, 1102403. [Google Scholar] [CrossRef] [PubMed]
- Moles, R.; Bellon, M.; Nicot, C. Stat1: A Novel Target of Mir-150 and Mir-223 Is Involved in the Proliferation of Htlv-I-Transformed and Atl Cells. Neoplasia 2015, 17, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Z.; Cheng, W.C.; Chen, S.F.; Nieh, S.; O’Connor, C.; Liu, C.L.; Tsai, W.W.; Wu, C.J.; Martin, L.; Lin, Y.S.; et al. Mir-25/93 Mediates Hypoxia-Induced Immunosuppression by Repressing Cgas. Nat. Cell Biol. 2017, 19, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.; Trabulo, S.M.; Vallespinos, M.; Raj, D.; Kheir, T.B.; Lin, M.L.; Begum, J.; Baker, A.M.; Amgheib, A.; Saif, J.; et al. The Mir-25-93-106b Cluster Regulates Tumor Metastasis and Immune Evasion Via Modulation of Cxcl12 and Pd-L1. Oncotarget 2017, 8, 21609–21625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Zhang, K.; Liao, L.D.; Li, L.Y.; Du, Z.P.; Wu, B.L.; Wu, J.Y.; Xu, X.E.; Zeng, F.M.; Chen, B.; et al. Mir-200b Suppresses Invasiveness and Modulates the Cytoskeletal and Adhesive Machinery in Esophageal Squamous Cell Carcinoma Cells Via Targeting Kindlin-2. Carcinogenesis 2014, 35, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Ghisi, M.; Corradin, A.; Basso, K.; Frasson, C.; Serafin, V.; Mukherjee, S.; Mussolin, L.; Ruggero, K.; Bonanno, L.; Guffanti, A.; et al. Modulation of Microrna Expression in Human T-Cell Development: Targeting of Notch3 by Mir-150. Blood 2011, 117, 7053–7062. [Google Scholar] [CrossRef] [PubMed]
- Cuman, C.; Van Sinderen, M.; Gantier, M.P.; Rainczuk, K.; Sorby, K.; Rombauts, L.; Osianlis, T.; Dimitriadis, E. Human Blastocyst Secreted microRNA Regulate Endometrial Epithelial Cell Adhesion. EBioMedicine 2015, 2, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Delaloy, C.; Liu, L.; Lee, J.A.; Su, H.; Shen, F.; Yang, G.Y.; Young, W.L.; Ivey, K.N.; Gao, F.B. Microrna-9 Coordinates Proliferation and Migration of Human Embryonic Stem Cell-Derived Neural Progenitors. Cell Stem Cell 2010, 6, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Su, J.; Kong, W.; Fang, Z.; Li, Y.; Huang, Z.; Wen, J.; Wang, Y. Roles of miR-10a-5p and miR-103a-3p, Regulators of BDNF Expression in Follicular Fluid, in the Outcomes of IVF-ET. Front. Endocrinol. 2021, 12, 637384. [Google Scholar] [CrossRef] [PubMed]
- Goharitaban, S.; Abedelahi, A.; Hamdi, K.; Khazaei, M.; Esmaeilivand, M.; Niknafs, B. Role of Endometrial Micrornas in Repeated Implantation Failure (Mini-Review). Front. Cell Dev. Biol. 2022, 10, 936173. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Fu, Y.; Shen, L.; Quan, S. Microrna Signatures in Plasma and Plasma Exosome During Window of Implantation for Implantation Failure Following in-Vitro Fertilization and Embryo Transfer. Reprod. Biol. Endocrinol. 2021, 19, 180. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, W.; Zhang, L.; Ji, Y.; Qin, J.; Wang, L.; Wang, M.; Qi, L.; Xue, J.; Lv, B.; et al. Effect of Sperm Cryopreservation on miRNA Expression and Early Embryonic Development. Front. Cell Dev. Biol. 2021, 9, 749486. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, H.; Chen, M.; Ren, S.; Cheng, P.; Zhang, H. Mir-301b~Mir-130b—Pparγ Axis Underlies the Adipogenic Capacity of Mesenchymal Stem Cells with Different Tissue Origins. Sci. Rep. 2017, 7, 1160. [Google Scholar] [CrossRef] [PubMed]
- Ramalho-Carvalho, J.; Graça, I.; Gomez, A.; Oliveira, J.; Henrique, R.; Esteller, M.; Jerónimo, C. Downregulation of Mir-130b~301b Cluster Is Mediated by Aberrant Promoter Methylation and Impairs Cellular Senescence in Prostate Cancer. J. Hematol. Oncol. 2017, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.H.; Liu, J.Y.; Lee, T.H.; Huang, C.C.; Chen, C.I.; Huang, L.S.; Lee, M.S. Requirement of Leukemia Inhibitory Factor or Epidermal Growth Factor for Pre-Implantation Embryogenesis via JAK/STAT3 Signaling Pathways. PLoS ONE 2016, 11, e0153086. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P. Enhanced Results in Mouse and Human Embryo Culture Using a Modified Human Tubal Fluid Medium Lacking Glucose and Phosphate. J. Assist. Reprod. Genet. 1995, 12, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.C.; Huang, C.C.; Huang, L.S.; Chen, C.I.; Lee, M.S.; Liu, J.Y. Evaluation of Mouse Blastocyst Implantation Rate by Morphology Grading. Chin. J. Physiol. 2004, 47, 43–47. [Google Scholar] [PubMed]
- Gutnisky, C.; Alvarez, G.M.; Cetica, P.D.; Dalvit, G.C. Evaluation of the Cryotech Vitrification Kit for bovine embryos. Cryobiology 2013, 67, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and Quantifying Mammalian Transcriptomes by Rna-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Birger, C.; Thorvaldsdottir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (Msigdb) Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdottir, H.; Tamayo, P.; Mesirov, J.P. Molecular Signatures Database (Msigdb) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, X.; Wan, Y.; Zhang, S.; Zhao, Y.; Fan, R.; Cui, Q.; Zhou, Y. Tam 2.0: Tool for Microrna Set Analysis. Nucleic Acids Res. 2018, 46, W180–W185. [Google Scholar] [CrossRef] [PubMed]

| Number of Mice | Fresh Blastocyst | Vitrified–Warmed Blastocyst |
|---|---|---|
| No.1 | 3/6 | 3/6 |
| No.2 | 4/6 | 6/6 |
| No.3 | 2/6 | 5/6 |
| No.4 | 6/6 | 6/6 |
| No.5 | 2/6 | 5/6 |
| total | 17/30 (56.7%) | 25/30 (83.3%) |
| microRNA | p-Value of GSEA 1 | NES of GSEA 2 | miRCURY Log2 of Fold Change 3 |
|---|---|---|---|
| miR-200b-3p | 0.010 | −1.28 | −0.60 |
| miR-361-5p | 0.020 | −1.36 | −0.62 |
| miR-93-3p | 0.020 | −1.37 | −0.62 |
| miR-9-5p | 0.016 | −1.27 | −0.76 |
| miR-30e-3p | 0.007 | −1.31 | −0.79 |
| miR-214-3p | 0.009 | −1.39 | −0.81 |
| miR-148b-3p | 0.017 | −1.29 | −0.86 |
| miR-301b-3p | 0.027 | −1.25 | −1.00 |
| miR-103a-2-5p | 0.031 | −1.33 | −1.18 |
| miR-30d-3p | 0.006 | −1.30 | −1.25 |
| miR-130b-3p | 0.021 | −1.24 | −1.29 |
| miR-150-5p | 0.001 | −1.54 | −1.36 |
| Function | Bonferroni p-Value # | microRNA $ |
|---|---|---|
| Immune Response | 9.30 × 10−4 | mir-9, mir-103a, mir-150, mir-93, mir-200b |
| Embryonic Stem Cell Differentiation | 9.30 × 10−4 | mir-9, mir-103a, mir-130b |
| Nephrotoxicity | 2.40 × 10−3 | mir-30d, mir-130b, mir-30e, mir-200b |
| Regulation of Stem Cell | 6.54 × 10−3 | mir-9, mir-200b, mir-214, mir-93 |
| Cell Proliferation | 7.04 × 10−3 | mir-9, mir-200b, mir-93, mir-150 |
| Cell Cycle | 8.73 × 10−3 | mir-9, mir-103a, mir-200b, mir-150 |
| Aging | 3.35 × 10−2 | mir-9, mir-30d, mir-30e |
| Inflammation | 4.92 × 10−2 | mir-9, mir-150, mir-93, mir-130b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-Y.; Tsai, H.-N.; Cheng, E.-H.; Lee, T.-H.; Lin, P.-Y.; Lee, M.-S.; Lee, C.-I. Transcriptomic Analysis of Vitrified–Warmed vs. Fresh Mouse Blastocysts: Cryo-Induced Physiological Mechanisms and Implantation Impact. Int. J. Mol. Sci. 2024, 25, 8658. https://doi.org/10.3390/ijms25168658
Lee C-Y, Tsai H-N, Cheng E-H, Lee T-H, Lin P-Y, Lee M-S, Lee C-I. Transcriptomic Analysis of Vitrified–Warmed vs. Fresh Mouse Blastocysts: Cryo-Induced Physiological Mechanisms and Implantation Impact. International Journal of Molecular Sciences. 2024; 25(16):8658. https://doi.org/10.3390/ijms25168658
Chicago/Turabian StyleLee, Chi-Ying, Han-Ni Tsai, En-Hui Cheng, Tsung-Hsien Lee, Pin-Yao Lin, Maw-Sheng Lee, and Chun-I Lee. 2024. "Transcriptomic Analysis of Vitrified–Warmed vs. Fresh Mouse Blastocysts: Cryo-Induced Physiological Mechanisms and Implantation Impact" International Journal of Molecular Sciences 25, no. 16: 8658. https://doi.org/10.3390/ijms25168658
APA StyleLee, C.-Y., Tsai, H.-N., Cheng, E.-H., Lee, T.-H., Lin, P.-Y., Lee, M.-S., & Lee, C.-I. (2024). Transcriptomic Analysis of Vitrified–Warmed vs. Fresh Mouse Blastocysts: Cryo-Induced Physiological Mechanisms and Implantation Impact. International Journal of Molecular Sciences, 25(16), 8658. https://doi.org/10.3390/ijms25168658






