Brain Region-Specific Expression Levels of Synuclein Genes in an Acid Sphingomyelinase Knockout Mouse Model: Correlation with Depression-/Anxiety-Like Behavior and Locomotor Activity in the Absence of Genotypic Variation
Abstract
:1. Introduction
2. Results
2.1. Brain Region-Specific Variation of Synuclein Expression in the Absende of an ASM Genotype Effect
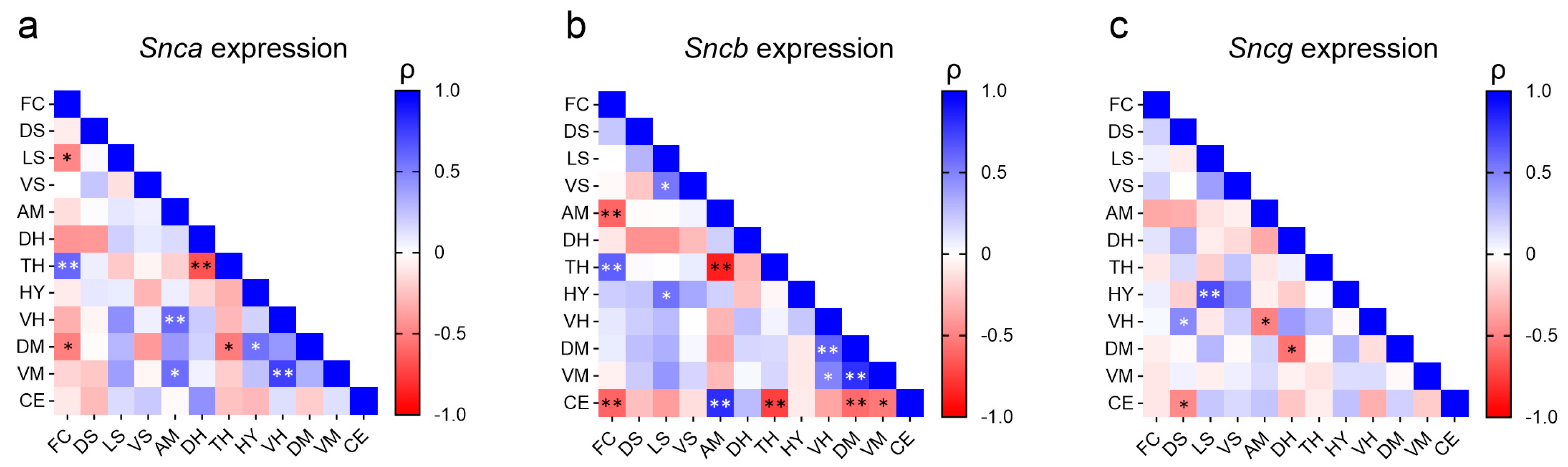
2.2. Correlation of Synuclein Expression across Brain Regions
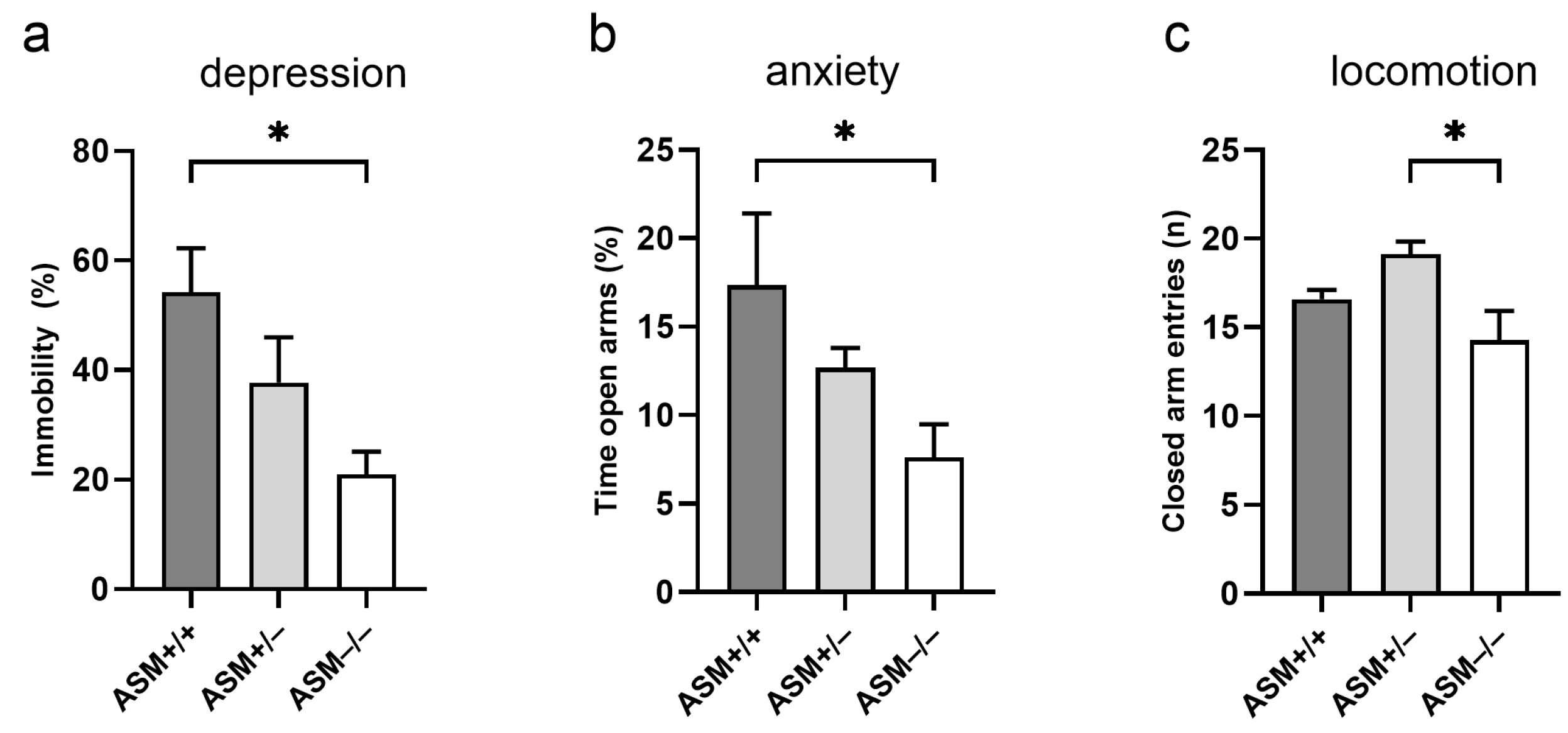
2.3. Behavioral Phenotype of ASM-Deficient and Wild-Type Mice
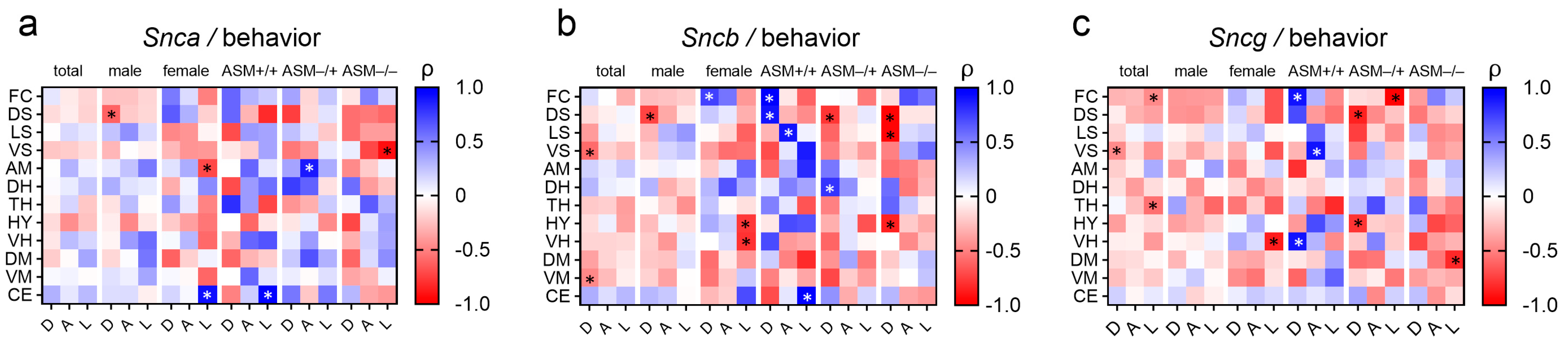
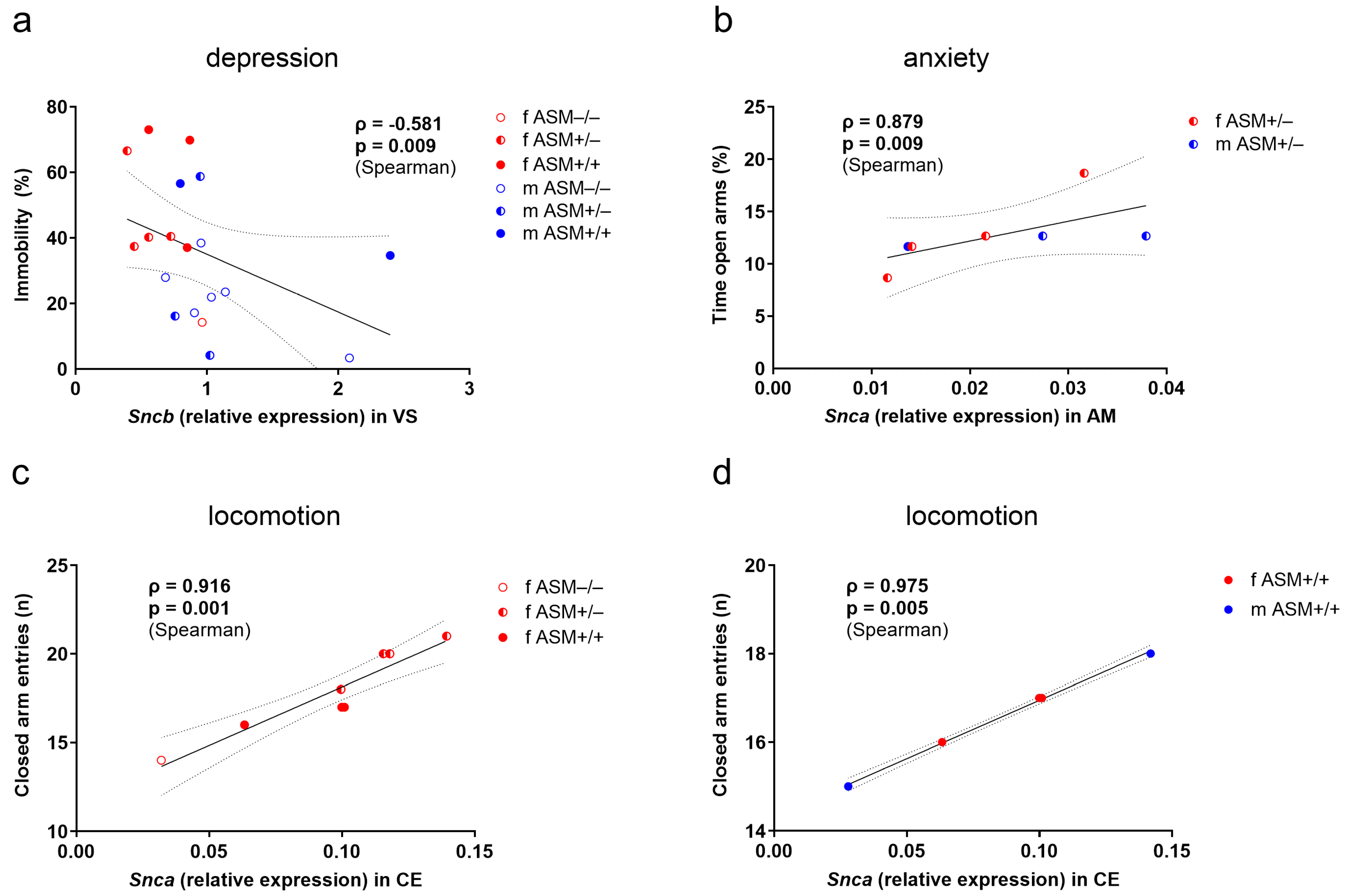
2.4. Associations between Synuclein Gene Expression and Behavior
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Overview
4.3. Elevated Plus-Maze (EPM) Test
4.4. Forced Swim Test (FST)
4.5. Extraction of RNA and Synthesis of cDNA
4.6. Quantitative PCR Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giussani, P.; Prinetti, A.; Tringali, C. The role of Sphingolipids in myelination and myelin stability and their involvement in childhood and adult demyelinating disorders. J. Neurochem. 2021, 156, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, S.Y.; Bae, Y.-S. Functional roles of sphingolipids in immunity and their implication in disease. Exp. Mol. Med. 2023, 55, 1110–1130. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Mühle, C.; Reichel, M.; Gulbins, E.; Kornhuber, J. Sphingolipids in Psychiatric Disorders and Pain Syndromes. In Sphingolipids in Disease; Gulbins, E., Petrache, I., Eds.; Springer: Vienna, Austria, 2013; pp. 431–456. [Google Scholar]
- Mühle, C.; Weinland, C.; Gulbins, E.; Lenz, B.; Kornhuber, J. Peripheral Acid Sphingomyelinase Activity Is Associated with Biomarkers and Phenotypes of Alcohol Use and Dependence in Patients and Healthy Controls. Int. J. Mol. Sci. 2018, 19, 4028. [Google Scholar] [CrossRef] [PubMed]
- Muhle, C.; Wagner, C.J.; Farber, K.; Richter-Schmidinger, T.; Gulbins, E.; Lenz, B.; Kornhuber, J. Secretory Acid Sphingomyelinase in the Serum of Medicated Patients Predicts the Prospective Course of Depression. J. Clin. Med. 2019, 8, 846. [Google Scholar] [CrossRef] [PubMed]
- Kornhuber, J.; Rhein, C.; Müller, C.P.; Mühle, C. Secretory sphingomyelinase in health and disease. Biol. Chem. 2015, 396, 707–736. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.-x.; Zhao, Y.-t.; Sun, Y.-x.; Xu, A.-h. Acid sphingomyelinase mediates ferroptosis induced by high glucose via autophagic degradation of GPX4 in type 2 diabetic osteoporosis. Mol. Med. 2023, 29, 125. [Google Scholar] [CrossRef] [PubMed]
- Mir, I.H.; Thirunavukkarasu, C. The relevance of acid sphingomyelinase as a potential target for therapeutic intervention in hepatic disorders: Current scenario and anticipated trends. Arch. Toxicol. 2023, 97, 2069–2087. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Jin, S.; Tan, F.; Xu, Y.; Lu, Y.; Wu, T. Physiological functions and therapeutic applications of neutral sphingomyelinase and acid sphingomyelinase. Biomed. Pharmacother. 2021, 139, 111610. [Google Scholar] [CrossRef]
- Zoicas, I.; Kornhuber, J. Acid Sphingomyelinase Is a Modulator of Contextual Fear. Int. J. Mol. Sci. 2022, 23, 3398. [Google Scholar] [CrossRef]
- Choi, B.J.; Park, K.H.; Park, M.H.; Huang, E.J.; Kim, S.H.; Bae, J.S.; Jin, H.K. Acid sphingomyelinase inhibition improves motor behavioral deficits and neuronal loss in an amyotrophic lateral sclerosis mouse model. BMB Rep. 2022, 55, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, K.; Erlich, S.; Perl, D.P.; Ferlinz, K.; Bisgaier, C.L.; Sandhoff, K.; Desnick, R.J.; Stewart, C.L.; Schuchman, E.H. Acid sphingomyelinase deficient mice: A model of types A and B Niemann–Pick disease. Nat. Genet. 1995, 10, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, K.; Fallahi, J.; Razban, V.; Sirat, R.Z.; Varasteh, M.; Tarhriz, V. Overview of clinical, molecular, and therapeutic features of Niemann-Pick disease (types A, B, and C): Focus on therapeutic approaches. Cell Biochem. Funct. 2024, 42, e4028. [Google Scholar] [CrossRef] [PubMed]
- Gulbins, E.; Palmada, M.; Reichel, M.; Lüth, A.; Böhmer, C.; Amato, D.; Müller, C.P.; Tischbirek, C.H.; Groemer, T.W.; Tabatabai, G.; et al. Acid sphingomyelinase–ceramide system mediates effects of antidepressant drugs. Nat. Med. 2013, 19, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, P.; Conte, C.; Albi, E. The Multiple Roles of Sphingomyelin in Parkinson’s Disease. Biomolecules 2021, 11, 1311. [Google Scholar] [CrossRef] [PubMed]
- Dagan, E.; Schlesinger, I.; Ayoub, M.; Mory, A.; Nassar, M.; Kurolap, A.; Peretz-Aharon, J.; Gershoni-Baruch, R. The contribution of Niemann-Pick SMPD1 mutations to Parkinson disease in Ashkenazi Jews. Parkinsonism Relat. Disord. 2015, 21, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.Y.; Yang, J.; Wang, H.; Zhang, S.Y.; Yang, Z.H.; Luo, H.Y.; Li, F.; Shi, M.; Liu, Y.T.; Zhuang, Z.P.; et al. SMPD1 variants in Chinese Han patients with sporadic Parkinson’s disease. Parkinsonism Relat. Disord. 2017, 34, 59–61. [Google Scholar] [CrossRef]
- Usenko, T.S.; Senkevich, K.A.; Bezrukova, A.I.; Baydakova, G.V.; Basharova, K.S.; Zhuravlev, A.S.; Gracheva, E.V.; Kudrevatykh, A.V.; Miliukhina, I.V.; Krasakov, I.V.; et al. Impaired Sphingolipid Hydrolase Activities in Dementia with Lewy Bodies and Multiple System Atrophy. Mol. Neurobiol. 2022, 59, 2277–2287. [Google Scholar] [CrossRef]
- Alcalay, R.N.; Mallett, V.; Vanderperre, B.; Tavassoly, O.; Dauvilliers, Y.; Wu, R.Y.J.; Ruskey, J.A.; Leblond, C.S.; Ambalavanan, A.; Laurent, S.B.; et al. SMPD1 mutations, activity, and alpha-synuclein accumulation in Parkinson’s disease. Mov. Disord. 2019, 34, 526–535. [Google Scholar] [CrossRef]
- Goedert, M.; Jakes, R.; Spillantini, M.G. The Synucleinopathies: Twenty Years On. J. Parkinsons Dis. 2017, 7, S51–S69. [Google Scholar] [CrossRef]
- Lavedan, C. The synuclein family. Genome Res. 1998, 8, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Marín, I. Emergence of the Synucleins. Biology 2023, 12, 1053. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Brás, I.C.; Outeiro, T.F. Alpha-Synuclein: Mechanisms of Release and Pathology Progression in Synucleinopathies. Cells 2021, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Barba, L.; Paolini Paoletti, F.; Bellomo, G.; Gaetani, L.; Halbgebauer, S.; Oeckl, P.; Otto, M.; Parnetti, L. Alpha and Beta Synucleins: From Pathophysiology to Clinical Application as Biomarkers. Mov. Disord. 2022, 37, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Bordoni, B. Physiology, Synuclein; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Carnazza, K.E.; Komer, L.E.; Xie, Y.X.; Pineda, A.; Briano, J.A.; Gao, V.; Na, Y.; Ramlall, T.; Buchman, V.L.; Eliezer, D.; et al. Synaptic vesicle binding of α-synuclein is modulated by β- and γ-synucleins. Cell Rep. 2022, 39, 110675. [Google Scholar] [CrossRef] [PubMed]
- Surguchov, A.; Surguchev, A. Synucleins: New Data on Misfolding, Aggregation and Role in Diseases. Biomedicines 2022, 10, 3241. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.R.; Rivers, R.C.; Christodoulou, J.C.; Vendruscolo, M.; Dobson, C.M. A relationship between the transient structure in the monomeric state and the aggregation propensities of α-synuclein and β-synuclein. Biochemistry 2014, 53, 7170–7183. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, J.; Carver, J.A. β-Synuclein: An Enigmatic Protein with Diverse Functionality. Biomolecules 2022, 12, 142. [Google Scholar] [CrossRef]
- Chandra, S.; Fornai, F.; Kwon, H.B.; Yazdani, U.; Atasoy, D.; Liu, X.; Hammer, R.E.; Battaglia, G.; German, D.C.; Castillo, P.E.; et al. Double-knockout mice for alpha- and beta-synucleins: Effect on synaptic functions. Proc. Natl. Acad. Sci. USA 2004, 101, 14966–14971. [Google Scholar] [CrossRef]
- George, J.M. The synucleins. Genome Biol. 2001, 3, reviews3002.1. [Google Scholar] [CrossRef] [PubMed]
- Surguchov, A. γ-Synuclein as a Cancer Biomarker: Viewpoint and New Approaches. Oncomedicine 2016, 1, 1–3. [Google Scholar] [CrossRef]
- Pons, M.L.; Loftus, N.; Vialaret, J.; Moreau, S.; Lehmann, S.; Hirtz, C. Proteomics Challenges for the Assessment of Synuclein Proteoforms as Clinical Biomarkers in Parkinson’s Disease. Front. Aging Neurosci. 2022, 14, 818606. [Google Scholar] [CrossRef] [PubMed]
- Mühle, C.; Kornhuber, J. Assay to measure sphingomyelinase and ceramidase activities efficiently and safely. J. Chromatogr. A 2017, 1481, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Muhle, C.; Huttner, H.B.; Walter, S.; Reichel, M.; Canneva, F.; Lewczuk, P.; Gulbins, E.; Kornhuber, J. Characterization of acid sphingomyelinase activity in human cerebrospinal fluid. PLoS ONE 2013, 8, e62912. [Google Scholar] [CrossRef] [PubMed]
- Dadgar-Kiani, E.; Bieri, G.; Melki, R.; Gitler, A.D.; Lee, J.H. Mesoscale connections and gene expression empower whole-brain modeling of α-synuclein spread, aggregation, and decay dynamics. Cell Rep. 2022, 41, 111631. [Google Scholar] [CrossRef] [PubMed]
- Geertsma, H.M.; Fisk, Z.A.; Sauline, L.; Prigent, A.; Kurgat, K.; Callaghan, S.M.; Arenkiel, B.R.; Mollenhauer, B.; Schlossmacher, M.G.; Stadelmann, C.; et al. A topographical atlas of α-synuclein dosage and cell type-specific expression in adult mouse brain and peripheral organs. NPJ Park. Dis. 2024, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Kalinichenko, L.S.; Mühle, C.; Eulenburg, V.; Praetner, M.; Reichel, M.; Gulbins, E.; Kornhuber, J.; Müller, C.P. Enhanced Alcohol Preference and Anxiolytic Alcohol Effects in Niemann-Pick Disease Model in Mice. Front. Neurol. 2019, 10, 731. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A. Animal models of anxiety: Do I need multiple tests? Trends Pharmacol. Sci. 2008, 29, 493–498. [Google Scholar] [CrossRef]
- Ramos, A.; Mellerin, Y.; Mormède, P.; Chaouloff, F. A genetic and multifactorial analysis of anxiety-related behaviours in Lewis and SHR intercrosses. Behav. Brain Res. 1998, 96, 195–205. [Google Scholar] [CrossRef]
- Hinojosa, F.R.; Spricigo, L., Jr.; Izídio, G.S.; Brüske, G.R.; Lopes, D.M.; Ramos, A. Evaluation of two genetic animal models in behavioral tests of anxiety and depression. Behav. Brain Res. 2006, 168, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, R.; Strazielle, C. Relations between open-field, elevated plus-maze, and emergence tests as displayed by C57/BL6J and BALB/c mice. J. Neurosci. Methods 2008, 171, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Zoicas, I.; Huber, S.E.; Kalinichenko, L.S.; Gulbins, E.; Müller, C.P.; Kornhuber, J. Ceramides affect alcohol consumption and depressive-like and anxiety-like behavior in a brain region- and ceramide species-specific way in male mice. Addict. Biol. 2020, 25, e12847. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Tanimura, A.; Graves, S.M.; Shen, W.; Surmeier, D.J. Striatal synapses, circuits, and Parkinson’s disease. Curr. Opin. Neurobiol. 2018, 48, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, K.; Ng, R.; Scott, H.; Kodavaganti, S.; Smyda, G.; Diwadkar, V.; Phillips, M. Ventral Striatum Functional Connectivity during Rewards and Losses and Symptomatology in Depressed Patients. Biol. Psychol. 2017, 123, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Balleine, B.W.; Delgado, M.R.; Hikosaka, O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007, 27, 8161–8165. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Lu, Y.; Pan, B.X.; Zhang, W.H. New Insights into the Pivotal Role of the Amygdala in Inflammation-Related Depression and Anxiety Disorder. Int. J. Mol. Sci. 2022, 23, 1076. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, J.A.; Venheim, E.R.; Padival, M. Chronic stress causes amygdala hyperexcitability in rodents. Biol. Psychiatry 2010, 67, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Nagaraja, C.; Daniels, S.; Fisk, Z.A.; Dvorak, R.; Meyerdirk, L.; Steiner, J.A.; Escobar Galvis, M.L.; Henderson, M.X.; Rousseaux, M.W.C.; et al. Synaptic location is a determinant of the detrimental effects of α-synuclein pathology to glutamatergic transmission in the basolateral amygdala. eLife 2022, 11, e78055. [Google Scholar] [CrossRef]
- Lai, T.T.; Gericke, B.; Feja, M.; Conoscenti, M.; Zelikowsky, M.; Richter, F. Anxiety in synucleinopathies: Neuronal circuitry, underlying pathomechanisms and current therapeutic strategies. NPJ Parkinsons Dis. 2023, 9, 97. [Google Scholar] [CrossRef]
- Darmohray, D.M.; Jacobs, J.R.; Marques, H.G.; Carey, M.R. Spatial and Temporal Locomotor Learning in Mouse Cerebellum. Neuron 2019, 102, 217–231.e214. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Sillitoe, R.V.; Silva, C.; Martina, M.; Sekerkova, G. α-Synuclein expression in the mouse cerebellum is restricted to VGluT1 excitatory terminals and is enriched in unipolar brush cells. Cerebellum 2015, 14, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, S.L.; Wassouf, Z.; Lazaro, D.F.; Xylaki, M.; Gladish, N.; Lin, D.T.S.; MacIsaac, J.; Ramadori, K.; Hentrich, T.; Schulze-Hentrich, J.M.; et al. Alpha-synuclein overexpression induces epigenomic dysregulation of glutamate signaling and locomotor pathways. Human. Mol. Genet. 2022, 31, 3694–3714. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Tehranian, R.; Dietrich, P.; Stefanis, L.; Perez, R.G. Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J. Cell Sci. 2005, 118, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- Zoicas, I.; Schumacher, F.; Kleuser, B.; Reichel, M.; Gulbins, E.; Fejtova, A.; Kornhuber, J.; Rhein, C. The Forebrain-Specific Overexpression of Acid Sphingomyelinase Induces Depressive-Like Symptoms in Mice. Cells 2020, 9, 1244. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chiang, Y.P.; He, M.; Zhang, K.; Zheng, J.; Wu, W.; Cai, J.; Chen, Y.; Chen, G.; Chen, Y.; et al. Effect of liver total sphingomyelin synthase deficiency on plasma lipid metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158898. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.R.; Dong, J.B.; Li, Y.; Wu, M.P. Sphingomyelin synthase 2 over-expression induces expression of aortic inflammatory biomarkers and decreases circulating EPCs in ApoE KO mice. Life Sci. 2012, 90, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Muhle, C.; Bilbao Canalejas, R.D.; Kornhuber, J. Sphingomyelin Synthases in Neuropsychiatric Health and Disease. Neurosignals 2019, 27, 54–76. [Google Scholar] [CrossRef]
- Naser, E.; Kadow, S.; Schumacher, F.; Mohamed, Z.H.; Kappe, C.; Hessler, G.; Pollmeier, B.; Kleuser, B.; Arenz, C.; Becker, K.A.; et al. Characterization of the small molecule ARC39, a direct and specific inhibitor of acid sphingomyelinase in vitro. J. Lipid Res. 2020, 61, 896–910. [Google Scholar] [CrossRef]
- Lu, M.H.; Ji, W.L.; Xu, D.E.; Yao, P.P.; Zhao, X.Y.; Wang, Z.T.; Fang, L.P.; Huang, R.; Lan, L.J.; Chen, J.B.; et al. Inhibition of sphingomyelin synthase 1 ameliorates alzheimer-like pathology in APP/PS1 transgenic mice through promoting lysosomal degradation of BACE1. Exp. Neurol. 2019, 311, 67–79. [Google Scholar] [CrossRef]
- Adachi, R.; Ogawa, K.; Matsumoto, S.I.; Satou, T.; Tanaka, Y.; Sakamoto, J.; Nakahata, T.; Okamoto, R.; Kamaura, M.; Kawamoto, T. Discovery and characterization of selective human sphingomyelin synthase 2 inhibitors. Eur. J. Med. Chem. 2017, 136, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Brazdis, R.M.; von Zimmermann, C.; Lenz, B.; Kornhuber, J.; Muhle, C. Peripheral Upregulation of Parkinson’s Disease-Associated Genes Encoding alpha-Synuclein, beta-Glucocerebrosidase, and Ceramide Glucosyltransferase in Major Depression. Int. J. Mol. Sci. 2024, 25, 3219. [Google Scholar] [CrossRef] [PubMed]
- Toth, I.; Neumann, I.D.; Slattery, D.A. Social Fear Conditioning: A Novel and Specific Animal Model to Study Social Anxiety Disorder. Neuropsychopharmacology 2012, 37, 1433–1443. [Google Scholar] [CrossRef] [PubMed]

| Forward | Reverse | Product | |
|---|---|---|---|
| Snca | 5′-GGCTGAGAAGACCAAAGAGC-3′ | 5′-GGCATGTCTTCCAGGATTCC-3′ | 186 bp |
| Sncb | 5′-GAGAAAACCAAGGAGCAGGC-3′ | 5′-ATCAGAGGCTCAATCAGGGG-3′ | 167 bp |
| Sncg | 5′-GACCAAGGAGCAGGCCAAT-3′ | 5′-TTTGGCTTCTTGGTCCTGTG-3′ | 157 bp |
| Ppia | 5′-TTCCAGGATTCATGTGCCAG-3′ | 5′-CCATCCAGCCATTCAGTCTT-3′ | 202 bp |
| Hprt | 5′-TCATTATGCCGAGGATTTGGA-3′ | 5′-GCCTCCCATCTCCTTCATGA-3′ | 100 bp |
| Gusb | 5′-CGGTTGTGATGTGGTCTGTG-3′ | 5′-CTTTGGTGTGGGTGATCAGC-3′ | 90 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brazdis, R.-M.; Zoicas, I.; Kornhuber, J.; Mühle, C. Brain Region-Specific Expression Levels of Synuclein Genes in an Acid Sphingomyelinase Knockout Mouse Model: Correlation with Depression-/Anxiety-Like Behavior and Locomotor Activity in the Absence of Genotypic Variation. Int. J. Mol. Sci. 2024, 25, 8685. https://doi.org/10.3390/ijms25168685
Brazdis R-M, Zoicas I, Kornhuber J, Mühle C. Brain Region-Specific Expression Levels of Synuclein Genes in an Acid Sphingomyelinase Knockout Mouse Model: Correlation with Depression-/Anxiety-Like Behavior and Locomotor Activity in the Absence of Genotypic Variation. International Journal of Molecular Sciences. 2024; 25(16):8685. https://doi.org/10.3390/ijms25168685
Chicago/Turabian StyleBrazdis, Razvan-Marius, Iulia Zoicas, Johannes Kornhuber, and Christiane Mühle. 2024. "Brain Region-Specific Expression Levels of Synuclein Genes in an Acid Sphingomyelinase Knockout Mouse Model: Correlation with Depression-/Anxiety-Like Behavior and Locomotor Activity in the Absence of Genotypic Variation" International Journal of Molecular Sciences 25, no. 16: 8685. https://doi.org/10.3390/ijms25168685






