Impact of Smoking and Obesity on the Selected Peptide Hormones and Metabolic Parameters in the Blood of Women with Polycystic Ovary Syndrome—Preliminary Study
Abstract
1. Introduction
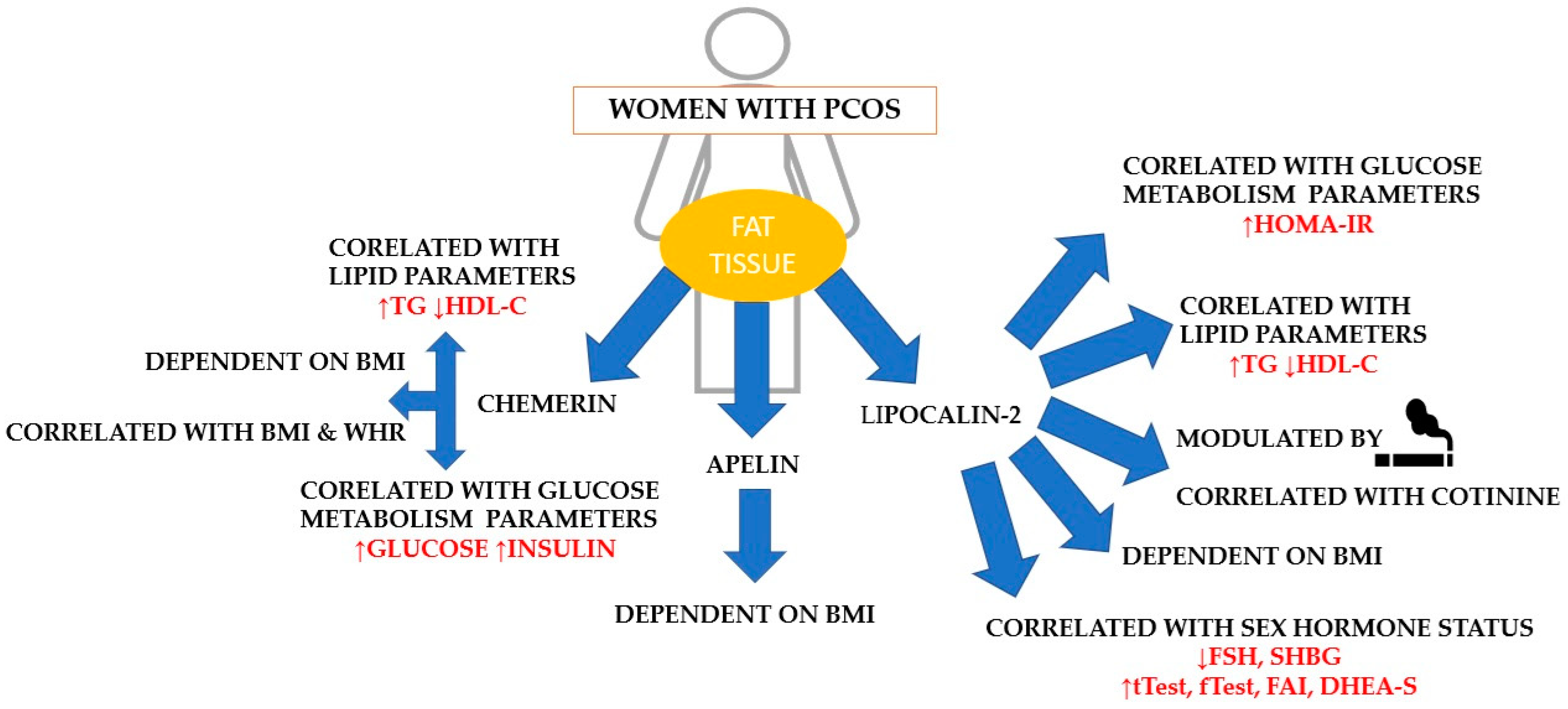
2. Results
3. Discussion
4. Materials and Methods
4.1. Diagnostic Parameters
4.2. Anthropometric Parameters, Acne, and Hirsutism Scale
- Clear—no inflammatory or non-inflammatory lesions.
- Almost clear—rare non-inflammatory lesions (≤one papule).
- Mild—some non-inflammatory lesions (≤few papules, no nodules).
- Moderate—many non-inflammatory lesions, some inflammatory lesions, no more than one small nodule.
- Severe—up to many non-inflammatory and inflammatory lesions, a few nodules.
4.3. Other Parameters
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nik Hazlina, N.H.; Norhayati, M.N.; Shaiful Bahari, I.; Nik Muhammad Arif, N.A. Worldwide Prevalence, Risk Factors and Psychological Impact of Infertility among Women: A Systematic Review and Meta-Analysis. BMJ Open 2022, 12, e057132. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.S.; Gupta, A.S. Causes and Prevalence of Factors Causing Infertility in a Public Health Facility. J. Hum. Reprod. Sci. 2019, 12, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, G.; Gainder, S.; Suri, V.; Sachdeva, N.; Chopra, S. Comparison of the Different PCOS Phenotypes Based on Clinical Metabolic, and Hormonal Profile, and Their Response to Clomiphene. Indian J. Endocrinol. Metab. 2019, 23, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, M.; Ramé, C.; Bertoldo, M.; Dupont, J. Adipokines and the Female Reproductive Tract. Int. J. Endocrinol. 2014, 2014, 232454. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S. Obesity and PCOS: Implications for Diagnosis and Treatment. Semin. Reprod. Med. 2012, 30, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Chudzicka-Strugała, I.; Gołębiewska, I.; Banaszewska, B.; Brudecki, G.; Zwoździak, B. The Role of Individually Selected Diets in Obese Women with PCOS—A Review. Nutrients 2022, 14, 4555. [Google Scholar] [CrossRef] [PubMed]
- Vrbikova, J.; Hainer, V. Obesity and Polycystic Ovary Syndrome. Obes. Facts 2009, 2, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Park, S.-Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef]
- Glueck, C.J.; Goldenberg, N. Characteristics of Obesity in Polycystic Ovary Syndrome: Etiology, Treatment, and Genetics. Metabolism 2019, 92, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Mattern, A.; Zellmann, T.; Beck-Sickinger, A.G. Processing, Signaling, and Physiological Function of Chemerin: Processing, Signaling, and Physiological Function of Chemerin. IUBMB Life 2014, 66, 19–26. [Google Scholar] [CrossRef]
- Dray, C.; Debard, C.; Jager, J.; Disse, E.; Daviaud, D.; Martin, P.; Attané, C.; Wanecq, E.; Guigné, C.; Bost, F.; et al. Apelin and APJ Regulation in Adipose Tissue and Skeletal Muscle of Type 2 Diabetic Mice and Humans. Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E1161–E1169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Foncea, R.; Deis, J.A.; Guo, H.; Bernlohr, D.A.; Chen, X. Lipocalin 2 Expression and Secretion Is Highly Regulated by Metabolic Stress, Cytokines, and Nutrients in Adipocytes. PLoS ONE 2014, 9, e96997. [Google Scholar] [CrossRef]
- Helfer, G.; Wu, Q.-F. Chemerin: A Multifaceted Adipokine Involved in Metabolic Disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Masri, B.; Daviaud, D.; Gesta, S.; Guigné, C.; Mazzucotelli, A.; Castan-Laurell, I.; Tack, I.; Knibiehler, B.; Carpéné, C.; et al. Apelin, a Newly Identified Adipokine up-Regulated by Insulin and Obesity. Endocrinology 2005, 146, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Mosialou, I.; Shikhel, S.; Luo, N.; Petropoulou, P.I.; Panitsas, K.; Bisikirska, B.; Rothman, N.J.; Tenta, R.; Cariou, B.; Wargny, M.; et al. Lipocalin-2 Counteracts Metabolic Dysregulation in Obesity and Diabetes. J. Exp. Med. 2020, 217, e20191261. [Google Scholar] [CrossRef]
- Ernst, M.C.; Sinal, C.J. Chemerin: At the Crossroads of Inflammation and Obesity. Trends Endocrinol. Metab. 2010, 21, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Jeon, S.; Jin, M.; Ock, J.; Kim, J.-H.; Lee, W.-H.; Suk, K. The Pivotal Role Played by Lipocalin-2 in Chronic Inflammatory Pain. Exp. Neurol. 2014, 254, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-L.; Ren, L.-R.; Sun, L.-F.; Huang, C.; Xiao, T.-X.; Wang, B.-B.; Chen, J.; Zabel, B.A.; Ren, P.; Zhang, J.V. The Role of GPR1 Signaling in Mice Corpus Luteum. J. Endocrinol. 2016, 230, 55–65. [Google Scholar] [CrossRef][Green Version]
- Reverchon, M.; Cornuau, M.; Ramé, C.; Guerif, F.; Royère, D.; Dupont, J. Chemerin Inhibits IGF-1-Induced Progesterone and Estradiol Secretion in Human Granulosa Cells. Hum. Reprod. 2012, 27, 1790–1800. [Google Scholar] [CrossRef] [PubMed]
- Dawid, M.; Mlyczynska, E.; Kurowska, P.; Sierpowski, M.; Rak, A. Apelin Decreased Placental Hormone Secretion by Human Trophoblast BeWo Cells via Apelin Receptor, Protein Kinase A and Extracellular Signal-Regulated Kinases 1/2 Activation. J. Physiol. Pharmacol. 2019, 70. [Google Scholar] [CrossRef]
- Krizanac, M.; Mass Sanchez, P.B.; Weiskirchen, R.; Schröder, S.K. Overview of the Expression Patterns and Roles of Lipocalin 2 in the Reproductive System. Front. Endocrinol. 2024, 15, 1365602. [Google Scholar] [CrossRef] [PubMed]
- Cesur, S.; Yucel, A.; Noyan, V.; Sagsoz, N. Plasma Lipocalin-2 Levels in Pregnancy. Acta Obstet. Gynecol. Scand. 2012, 91, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yang, Y.; Huang, C.; Ge, L.; Xue, L.; Xiao, Z.; Xiao, T.; Zhao, H.; Ren, P.; Zhang, J.V. Chemerin: A Functional Adipokine in Reproductive Health and Diseases. Biomedicines 2022, 10, 1910. [Google Scholar] [CrossRef]
- Jafari, A.; Rajabi, A.; Gholian-Aval, M.; Peyman, N.; Mahdizadeh, M.; Tehrani, H. National, Regional, and Global Prevalence of Cigarette Smoking among Women/Females in the General Population: A Systematic Review and Meta-Analysis. Environ. Health Prev. Med. 2021, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Dechanet, C.; Anahory, T.; Mathieu Daude, J.C.; Quantin, X.; Reyftmann, L.; Hamamah, S.; Hedon, B.; Dechaud, H. Effects of Cigarette Smoking on Reproduction. Hum. Reprod. Update 2011, 17, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Stillman, R.J.; Rosenberg, M.J.; Sachs, B.P. Smoking and Reproduction. Fertil. Steril. 1986, 46, 545–566. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.R.; Melo, M.A. Cigarette Smoking and Reproductive Function. Curr. Opin. Obstet. Gynecol. 2008, 20, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Bizoń, A.; Franik, G.; Niepsuj, J.; Czwojdzińska, M.; Leśniewski, M.; Nowak, A.; Szynkaruk-Matusiak, M.; Madej, P.; Piwowar, A. The Associations between Sex Hormones and Lipid Profiles in Serum of Women with Different Phenotypes of Polycystic Ovary Syndrome. J. Clin. Med. 2021, 10, 3941. [Google Scholar] [CrossRef] [PubMed]
- Franik, G.; Bizoń, A.; Włoch, S.; Pluta, D.; Blukacz, Ł.; Milnerowicz, H.; Madej, P. The Effect of Abdominal Obesity in Patients with Polycystic Ovary Syndrome on Metabolic Parameters. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4755–4761. [Google Scholar] [PubMed]
- Bizoń, A.; Płaczkowska, S.; Niepsuj, J.; Czwojdzińska, M.; Leśniewski, M.; Nowak, A.; Pluta, D.; Madej, P.; Piwowar, A.; Franik, G. Body Composition and Its Impact on the Hormonal Disturbances in Women with Polycystic Ovary Syndrome. Nutrients 2021, 13, 4217. [Google Scholar] [CrossRef] [PubMed]
- Niepsuj, J.; Franik, G.; Madej, P.; Piwowar, A.; Bizoń, A. Evaluation of Pro/Antioxidant Imbalance in Blood of Women with Polycystic Ovary Syndrome Based on Determination of Oxidized Low-Density Lipoproteins and Ferric Reducing Ability of Plasma Values. Biomedicines 2022, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Milnerowicz-Nabzdyk, E.; Bizoń, A. How Does Tobacco Smoke Influence the Morphometry of the Fetus and the Umbilical Cord?—Research on Pregnant Women with Intrauterine Growth Restriction Exposed to Tobacco Smoke. Reprod. Toxicol. 2015, 58, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Milnerowicz-Nabzdyk, E.; Bizoń, A.; Zimmer, M. How Does Tobacco Smoke Affect Fetal Growth Potential in the First Trimester of Pregnancy as Measured by Volume Parameters of the Fetus, Trophoblast, and Gestational Sac? Reprod. Sci. 2017, 24, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Bizoń, A.; Milnerowicz, H. The Effect of Passive and Active Exposure to Tobacco Smoke on Lipid Profile Parameters and the Activity of Certain Membrane Enzymes in the Blood of Women in the First Trimester of Pregnancy. Environ. Toxicol. Pharmacol. 2017, 53, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Bizoń, A.; Milnerowicz, H.; Kowalska-Piastun, K.; Milnerowicz-Nabzdyk, E. The Impact of Early Pregnancy and Exposure to Tobacco Smoke on Blood Antioxidant Status and Copper, Zinc, Cadmium Concentration-A Pilot Study. Antioxidants 2021, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol.-Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Guzel, E.C.; Celik, C.; Abali, R.; Kucukyalcin, V.; Celik, E.; Guzel, M.; Yilmaz, M. Omentin and Chemerin and Their Association with Obesity in Women with Polycystic Ovary Syndrome. Gynecol. Endocrinol. 2014, 30, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Q.; Zhang, L.; Wei, W.; Liu, L.; Li, B.; Zhang, L.; Zhang, Y.; Hui, Y.; Lei, Y. Circulating Chemerin Levels in Women with Polycystic Ovary Syndrome: A Meta-Analysis. Gynecol. Endocrinol. 2022, 38, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.D.A.; Nivet, A.-L.; Wang, Q.; Chen, Y.-A.; Leader, A.; Cheung, A.; Tzeng, C.-R.; Tsang, B.K. Polycystic Ovary Syndrome: Possible Involvement of Androgen-Induced, Chemerin-Mediated Ovarian Recruitment of Monocytes/Macrophages†. Biol. Reprod. 2018, 99, 838–852. [Google Scholar] [CrossRef]
- Wang, Q.; Kim, J.Y.; Xue, K.; Liu, J.; Leader, A.; Tsang, B.K. Chemerin, a Novel Regulator of Follicular Steroidogenesis and Its Potential Involvement in Polycystic Ovarian Syndrome. Endocrinology 2012, 153, 5600–5611. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Yang, H.I.; Cho, S.; Jung, J.A.; Jeon, Y.E.; Kim, H.Y.; Seo, S.K.; Lee, B.S. Serum Asymmetric Dimethylarginine, Apelin, and Tumor Necrosis Factor-α Levels in Non-Obese Women with Polycystic Ovary Syndrome. Steroids 2012, 77, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- ©Akal, E.; Ozkaya, M.; Engin-Ustun, Y.; Ustun, Y. Serum Lipocalin-2 as an Insulin Resistance Marker in Patients with Polycystic Ovary Syndrome. J. Endocrinol. Invest. 2011, 34, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Panidis, D.; Tziomalos, K.; Koiou, E.; Kandaraki, E.A.; Tsourdi, E.; Delkos, D.; Kalaitzakis, E.; Katsikis, I. The Effects of Obesity and Polycystic Ovary Syndrome on Serum Lipocalin-2 Levels: A Cross-Sectional Study. Reprod. Biol. Endocrinol. 2010, 8, 151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koiou, E.; Tziomalos, K.; Katsikis, I.; Kandaraki, E.A.; Kalaitzakis, E.; Delkos, D.; Vosnakis, C.; Panidis, D. Weight Loss Significantly Reduces Serum Lipocalin-2 Levels in Overweight and Obese Women with Polycystic Ovary Syndrome. Gynecol. Endocrinol. 2012, 28, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Halawa, M.R.; Ali Hendawy, L.M.; Makram, M.A. Lipocalin-2 Level in Patients with Polycystic Ovary Syndrome: Association with Insulin Resistance and Metformin Therapy. QJM Int. J. Med. 2024, 117, hcae070.318. [Google Scholar] [CrossRef]
- Daya, S. Follicle-Stimulating Hormone in Clinical Practice: An Update. Treat. Endocrinol. 2004, 3, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, Z.; Feng, W.; Long, S.; Mo, Z.-C. Sex Hormone-Binding Globulin and Polycystic Ovary Syndrome. Clin. Chim. Acta 2019, 499, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [CrossRef] [PubMed]
- Franik, G.; Bizoń, A.; Włoch, S.; Kowalczyk, K.; Biernacka-Bartnik, A.; Madej, P. Hormonal and Metabolic Aspects of Acne Vulgaris in Women with Polycystic Ovary Syndrome. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4411–4418. [Google Scholar] [CrossRef] [PubMed]
- Hatch, R.; Rosenfield, R.L.; Kim, M.H.; Tredway, D. Hirsutism: Implications, Etiology, and Management. Am. J. Obstet. Gynecol. 1981, 140, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Espinós, J.J.; Calaf, J.; Estadella, J.; Checa, M.A. Hirsutism Scoring in Polycystic Ovary Syndrome: Concordance between Clinicians’ and Patients’ Self-Scoring. Fertil. Steril. 2010, 94, 2815–2816. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Women with PCOS | Control Group | p Value |
|---|---|---|---|
| n = 88 | n = 28 | ||
| Age (years) | 26.40 ± 4.52 24.00 (21.00–27.00) | 25.72 ± 6.10 25.00 (21.00–31.00) | NS |
| BMI (kg/m2) | 25.63 ± 6.39 23.92 (20.75–30.46) | 26.89 ± 24.03 21.80 (18.34–26.70) | NS |
| WHR | 0.79 ± 0.09 0.77 (0.72–0.84) | 0.76 ± 0.08 0.73 (0.70–0.80) | NS |
| Cotinine (ng/mL) | 16.46 ± 24.08 0.41 (0.01–34.68) | 5.74 ± 14.35 0.41 (0.02–0.88) | NS |
| mF-G (0–36) | 5.59 ± 4.63 5.00 (1.00–9.00) | 4.22 ± 5.54 2.00 (0.00–7.00) | 0.033 |
| Acne (1–5) | 3.60 ± 12.47 2.00 (1.00–2.00) | 0.88 ± 0.87 1.00 (0.00–1.00) | 0.000 |
| Apelin (pg/mL) | 96.08 ± 454.00 32.57 (30.11–37.91) | 80.37 ± 202.78 33.84 (27.76–39.19) | NS |
| Chemerin (ng/L) | 128.7 ± 57.80 120.52 (79.51–156.80) | 117.48 ± 55.40 118.34 (78.31–150.83) | NS |
| Lipocalin-2 (ng/mL) | 217.35 ± 84.66 207.07 (153.42–276.68) | 189.41 ± 71.44 180.58 (151.77–213.51) | NS |
| Cholesterol (mg/dL) | 163.23 ± 30.36 159.50 (141.00–187.00) | 167.56 ± 32.87 163.00 (144.00–192.00) | NS |
| HDL-C (mg/dL) | 55.50 ± 12.34 54.65 (48.05–63.25) | 65.16 ± 14.25 63.85 (57.10–72.25) | 0.000 |
| LDL-C (mg/dL) | 87.00 ± 25.23 83.42 (67.06–104.92) | 87.31 ± 28.42 88.42 (73.51–103.47) | NS |
| Triglycerides (mg/dL) | 103.78 ± 57.60 83.60 (63.15–130.00) | 79.68 ± 30.77 70.80 (54.80–95.95) | NS |
| Glucose 0’ (mg/dL) | 85.04 ± 6.20 84.50 (80.50–89.05) | 81.64 ± 5.31 80.95 (78.40–86.20) | 0.015 |
| Insulin 0’ (mU/mL) | 9.03 ± 6.44 7.03 (5.16–11.30) | 5.27 ± 2.43 4.93 (3.55–7.10) | 0.000 |
| HOMA-IR | 1.99 ± 1.56 1.50 (1.08–2.40) | 1.07 ± 0.52 0.98 (0.68–1.44) | 0.000 |
| LH (lU/L) | 8.74 ± 6.44 7.18 (5.58–9.90) | 9.49 ± 17.66 5.42 (5.51–8.98) | NS |
| FSH (lU/L) | 6.16 ± 1.23 6.03 (5.45–6.93) | 6.09 ± 2.32 6.25 (5.21–7.33) | NS |
| DHEA-S (µg/mL) | 321.03 ± 142.64 290.00 (222.00–372.00) | 257.26 ± 132.31 227.00 (172.00–314.50) | 0.029 |
| SHBG (nmol/L) | 53.96 ± 33.25 47.35 (29.75–70.90) | 65.15 ± 32.88 59.98 (45.75–71.85) | 0.035 |
| tTest (ng/mL) | 1.40 ± 0.633 1.23 (0.95–1.78) | 1.26 ± 1.26 0.98 (0.65–1.26) | 0.011 |
| fTest (pg/mL) | 3.02 ± 2.66 2.02 (1.45–3.82) | 2.43 ± 2.56 1.45 (0.76–2.81) | 0.047 |
| AD (ng/mL) | 2.48 ± 0.98 2.19 (1.80–2.91) | 2.59 ± 2.70 1.87 (1.56–2.73) | NS |
| FAI | 3.91 ± 3.62 2.66 (1.65–4.40) | 2.33 ± 2.43 1.48 (0.88–2.43) | 0.003 |
| AMH (ng/mL) | 6.34 ± 3.50 5.92 (3.63–8.37) | 3.62 ± 2.80 2.95 (1.43–5.00) | 0.000 |
| Parameters | Women with PCOS | p Value | |
|---|---|---|---|
| Non-Smoking n = 57 | Smoking n = 31 | ||
| Age (years) | 24.15 ± 4.35 24.00 (21.00–27.00) | 24.83 ± 4.79 24.00 (21.00–28.00) | NS |
| BMI (kg/m2) | 25.17 ± 6.12 23.66 (20.42–29.49) | 26.71 ± 6.86 25.28 (21.01–30.49) | NS |
| WHR | 0.79 ± 0.10 0.78 (0.71–0.85) | 0.78 ± 0.07 0.76 (0.73–0.82) | NS |
| Cotinine (ng/mL) | 0.47 ± 0.24 0.38 (0.09–0.42) | 45.23 ± 18.08 49.45 (30.75–60.42) | 0.000 |
| mF-G (0–36) | 5.65 ± 4.93 4.00 (1.00–9.00) | 5.26 ± 4.04 5.00 (2.00–8.00) | NS |
| Acne (1–5) | 4.74 ± 15.37 2.00 (1.00–2.00) | 1.45 ± 1.03 2.00 (1.00–2.00) | NS |
| Apelin (pg/mL) | 33.59 ± 6.25 32.57 (30.11–36.46) | 36.35 ± 10.99 31.33 (30.11–40.59) | NS |
| Chemerin (ng/L) | 126.45 ± 60.22 119.74 (74.11–154.77) | 131.93 ± 53.05 126.01 (86.25–157.29) | NS |
| Lipocalin-2 (ng/mL) | 200.96 ± 82.40 199.08 (142.15–253.04) | 248.00 ± 80.00 237.73 (178.22–328.14) | 0.016 |
| Cholesterol (mg/dL) | 166.02 ± 31.26 161.00 (139.00–195.00) | 159.20 ± 28.70 157.50 (142.00–180.00) | NS |
| HDL-C (mg/dL) | 57.82 ± 11.86 57.40 (50.90–63.70) | 51.85 ± 12.71 49.70 (43.40–62.30) | 0.029 |
| LDL-C (mg/dL) | 89.04 ± 25.26 88.08 (67.20–105.20 | 83.67 ± 24.92 80.40 (66.92–103.00) | NS |
| Triglycerides (mg/dL) | 95.77 ± 52.93 81.40 (61.70–111.00) | 118.70 ± 62.69 91.10 (66.80–154.00) | 0.042 |
| Glucose 0’ (mg/dL) | 84.15 ± 5.21 83.80 (79.80–88.90) | 86.87 ± 7.49 86.65 (80.90–89.60) | NS |
| Insulin 0’ (mU/mL) | 8.51 ± 5.72 6.83 (4.70–10.00) | 9.91 ± 7.52 7.89 (5.47–12.00) | NS |
| HOMA-IR | 1.82 ± 1.27 1.48 (0.98–2.07) | 2.20 ± 1.20 1.60 (1.19–2.41) | NS |
| LH (lU/L) | 8.68 ± 7.25 7.15 (5.53–9.51) | 8.78 ± 4.56 7.26 (5.62–10.40) | NS |
| FSH (lU/L) | 6.16 ± 1.27 5.94 (5.45–7.11) | 6.16 ± 1.18 6.11 (5.35–6.67) | NS |
| DHEA-S (µg/mL) | 300.91 ± 120.80 285.00 (223.00–364.00) | 339.40 ± 174.94 293.00 (209.00–446.00) | NS |
| SHBG (nmol/L) | 58.10 ± 33.41 54.70 (32.90–73.00) | 46.12 ± 31.49 34.45 (26.10–55.50) | 0.023 |
| tTest (ng/mL) | 0.39 ± 0.165 0.34 (0.27–0.49) | 0.43 ± 0.21 0.37 (0.29–0.57) | NS |
| fTest (pg/mL) | 2.87 ± 2.44 2.02 (1.41–3.67) | 3.29 ± 3.04 2.14 (1.50–4.11) | NS |
| AD (ng/mL) | 2.51 ± 0.98 2.18 (1.77–3.16) | 2.40 ± 1.01 2.19 (1.79–2.78) | NS |
| FAI | 3.28 ± 2.95 2.32 (1.65–3.77) | 4.98 ± 4.39 3.79 (1.83–6.45) | 0.040 |
| AMH (ng/mL) | 6.66 ± 3.57 6.18 (3.76–8.99) | 5.73 ± 3.27 5.50 (3.43–7.65) | NS |
| Parameters | Women with PCOS | |||
|---|---|---|---|---|
| BMI < 25.0 n = 47 | BMI ≥ 25.0 n = 18 | BMI ≥ 30.0 n = 23 | p Value | |
| Age (years) | 24.13 ± 4.80 23.00 (21.00–27.00) 26.00 (23.00–29.00) | 23.61 ± 4.72 23.00 (20.00–25.00) | 25.61 ± 3.46 26.00 (23.00–29.00) | NS |
| BMI (kg/m2) | 20.90 ± 2.07 20.82 (19.96–22.04) | 26.52 ± 1.90 25.86 (25.15–28.55) | 34.59 ± 3.70 34.23 (31.25–37.18) | 0.000 |
| WHR | 0.75 ± 0.09 0.74 (0.71–0.78) | 0.79 ± 0.07 0.79 (0.76–0.83) | 0.85 ± 0.08 0.86 (0.82–0.91) | 0.000 |
| Cotinine (ng/mL) | 14.07 ± 23.13 041 (0.01–19.41) | 18.35 ± 25.13 0.41 (0.41–42.39) | 20.26 ± 26.22 0.41 (0.00–37.86) | NS |
| mF-G (0–36) | 5.63 ± 4.81 5.00 (1.00–9.00) | 5.78 ± 4.41 6.50 (1.00–9.00) | 5.77 ± 4.50 4.50 (2.00–9.00) | NS |
| Acne (1–5) | 5.28 ± 16.60 2.00 (1.00–2.00) | 1.11 ± 1.08 1.00 (0.00–1.08) | 1.77 ± 1.38 2.00 (1.00–2.00) | NS |
| Apelin (pg/mL) | 37.22 ± 9.07 35.13 (30.11–43.49) | 30.75 ± 4.84 30.11 (27.76–32.58) | 46.50 ± 65.99 31.33 (27.76–33.84) | 0.016 |
| Chemerin (ng/L) | 111.14 ± 40.93 106.60 (77.16–136.45) | 149.40 ± 69.85 132.54 (101.63–194.62) | 151.83 ± 66.98 154.76 (104.94–203.75) | 0.037 |
| Lipocalin-2 (ng/mL) | 199.34 ± 88.52 190.48 (141.19–244.58) | 235.72 ± 75.00 222.47 (186.15–272.71) | 245.72 ± 82.62 262.12 (199.37–299.40) | 0.023 |
| Cholesterol (mg/dL) | 158.78 ± 28.53 154.50 (137.00–180.00) | 159.44 ± 25.69 163.00 (144.00–178.00) | 176.78 ± 33.69 180.00 (143.00–201.00) | NS |
| HDL-C (mg/dL) | 60.81 ± 11.13 60.05 (52.90–67.20) | 53.64 ± 8.50 54.05 (47.80–62.00) | 46.40 ± 11.05 45.90 (38.90–51.50) | 0.000 |
| LDL-C (mg/dL) | 82.66 ± 23.58 81.57 (66.34–101.58) | 85.48 ± 21.71 86.46 (67.20–97.50) | 98.16 ± 27.86 99.90 (72.50–121.50) | NS |
| Triglycerides (mg/dL) | 76.79 ± 27.89 66.45 (55.80–91.50) | 101.61 ± 38.89 89.80 (76.50–110.00) | 161.09 ± 70.36 153.00 (92.60–211.00) | 0.000 |
| Glucose 0’ (mg/dL) | 83.52 ± 4.91 83.60 (79.60–86.70) | 84.61 ± 4.86 85.00 (80.90–87.50) | 88.48 ± 7.86 87.90 (82.20–92.00) | 0.025 |
| Insulin 0’ (mU/mL) | 5.90 ± 2.38 5.47 (4.18–7.23) | 8.36 ± 2.53 7.67 (6.14–10.00) | 15.91 ± 8.37 13.50 (11.20–18.70) | 0.000 |
| HOMA-IR | 1.22 ± 0.53 1.16 (0.81–0.53) | 1.75 ± 0.56 1.61 (1.32–2.07) | 3.67 ± 2.09 3.08 (2.41–3.95) | 0.000 |
| LH (lU/L) | 9.65 ± 7.73 7.73 (6.02–7.73) | 6.64 ± 2.50 6.17 (5.38–7.63) | 8.62 ± 5.04 7.27 (5.43–10.50) | NS |
| FSH (lU/L) | 6.43 ± 1.15 6.29 (5.71–7.53) | 5.91 ± 1.67 5.68 (4.56–7.53) | 5.92 ± 0.90 5.68 (5.35–6.23) | NS |
| DHEA-S (µg/mL) | 295.57 ± 127.51 274.50 (207.00–336.00) | 355.28 ± 116.63 303.00 (289.00–379.00) | 343.09 ± 173.88 298.00 (201.00–473.00) | NS |
| SHBG (nmol/L) | 68.84 ± 35.93 66.50 (45.30–80.70) | 43.07 ± 17.56 38.85 (29.60–56.00) | 32.07 ± 16.53 26.80 (20.90–44.40) | 0.000 |
| tTest (ng/mL) | 0.36 ± 0.18 0.34 (0.25–0.43) | 0.43 ± 0.17 0.38 (0.29–0.56) | 0.47 ± 0.21 0.45 (0.31–0.64) | 0.029 |
| fTest (pg/mL) | 2.23 ± 1.60 1.71 (1.23–2.61) | 2.57 ± 1.65 2.11 (1.30–3.49) | 5.07 ± 3.82 4.09 (2.02–6.77) | 0.003 |
| AD (ng/mL) | 2.52 ± 0.91 2.29 (1.85–3.03) | 2.31 ± 0.77 2.07 (1.79–2.57) | 2.56 ± 1.25 2.08 (1.65–3.07) | NS |
| FAI | 2.35 ± 1.94 1.96 (1.14–2.82) | 4.07 ± 2.96 3.36 (2.11–3.95) | 6.89 ± 4.61 5.49 (3.73–9.80) | 0.000 |
| AMH (ng/mL) | 6.55 ± 3.32 6.21 (3.87–9.34) | 4.97 ± 2.59 3.82 (3.19–7.39) | 6.60 ± 4.28 5.72 (4.33–7.57) | 0.000 |
| Parameters | Women with PCOS | ||
|---|---|---|---|
| WHR < 0.8 n = 54 | WHR ≥ 0.8 n = 34 | p Value | |
| Age (years) | 23.86 ± 4.83 23.00 (20.00–27.00) | 25.21 ± 3.93 25.00 (22.00–27.00) | NS |
| BMI (kg/m2) | 23.13 ± 4.75 21.72 (20.44–24.68) | 29.43 ± 6.73 30.46 (25.95–34.29) | 0.000 |
| WHR | 0.73 ± 0.04 0.74 (0.70–0.76) | 0.88 ± 0.80 0.86 (0.82–0.90) | 0.000 |
| Cotinine (ng/mL) | 17.30 ± 24.43 0.41 (0.02–37.86) | 15.21 ± 23.86 0.39 (0.08–24.94) | NS |
| mF-G (0–36) | 4.66 ± 4.59 4.00 (1.00–7.00) | 7.00 ± 4.39 8.00 (3.00–10.00) | NS |
| Acne (1–5) | 3.24 ± 10.71 2.00 (1.00–2.00) | 4.15 ± 14.92 2.00 (1.00–2.00) | NS |
| Apelin (pg/mL) | 36.05 ± 9.13 33.84 (30.11–37.81) | 42.30 ± 55.26 31.95 (27.76–35.13) | NS (p = 0.059) |
| Chemerin (ng/L) | 121.92 ± 50.62 117.82 (78.81–147.53) | 137.11 ± 66.76 122.09 (89.96–178.07) | NS |
| Lipocalin-2 (ng/mL) | 214.43 ± 88.77 206.39 (144.31–272.71) | 221.65 ± 79.35 213.81 (160.30–284.85) | NS |
| Cholesterol (mg/dL) | 158.94 ± 28.21 154.00 (137.00–178.00) | 169.53 ± 32.67 165.00 (146.00–195.00) | NS |
| HDL-C (mg/dL) | 59.62 ± 11.25 57.60 (51.30–66.50) | 49.45 ± 11.48 50.40 (41.10–58.10) | 0.000 |
| LDL-C (mg/dL) | 82.23 ± 24.36 79.00 (65.60–100.00) | 94.00 ± 25.21 90.84 (72.80–116.20) | 0.026 |
| Triglycerides (mg/dL) | 85.65 ± 37.19 77.05 (57.20–106.00) | 130.44 ± 71.14 111.00 (73.80–157.00) | 0.001 |
| Glucose 0’ (mg/dL) | 84.84 ± 5.11 84.35 (81.00–87.60) | 85.32 ± 7.61 84.65 (79.80–89.40) | NS |
| Insulin 0’ (mU/mL) | 6.52 ± 2.55 6.14 (4.56–7.38) | 12.72 ± 8.43 11.75 (6.89–16.00) | 0.000 |
| HOMA-IR | 1.37 ± 0.57 1.27 (0.93–1.58) | 2.84 ± 2.10 2.41 (1.60–3.40) | 0.000 |
| LH (lU/L) | 9.26 ± 7.61 7.15 (5.75–9.80) | 7.96 ± 4.13 7.21 (5.27–10.10) | NS |
| FSH (lU/L) | 6.40 ± 1.28 6.25 (5.64–7.59) | 5.81 ± 1.09 5.68 (5.31–6.31) | 0.011 |
| DHEA-S (µg/mL) | 301.24 ± 127.73 278.00 (222.00–323.00) | 350.15 ± 159.63 314.00 (218.00–446.00) | NS |
| SHBG (nmol/L) | 61.08 ± 26.60 57.10 (42.40–72.70) | 43.48 ± 40.02 29.90 (21.60–47.30) | 0.000 |
| tTest (ng/mL) | 0.36 ± 0.16 0.33 (0.25–0.43) | 0.46 ± 0.20 0.44 (0.32–0.62) | 0.003 |
| fTest (pg/mL) | 2.12 ± 1.50 1.71 (1.20–2.51) | 4.32 ± 3.38 3.49 (1.60–5.68) | 0.000 |
| AD (ng/mL) | 2.35 ± 0.81 2.18 (1.77–2.57) | 2.67 ± 1.18 2.46 (1.91–3.21) | NS |
| FAI | 2.53 ± 1.96 2.01 (1.43–2.82) | 5.97 ± 4.49 4.18 (3.33–8.26) | 0.000 |
| AMH (ng/mL) | 5.97 ± 3.34 5.67 (3.24–8.47) | 6.87 ± 3.70 6.58 (4.36–7.73) | NS |
| Correlated Parameters | Entire Group of Women with PCOS | ||
|---|---|---|---|
| Apelin [pg/mL] | Chemerin [ng/L] | Lipocalin-2 [ng/mL] | |
| Age (years) | NS | NS | NS |
| BMI (kg/m2) | r = 0.23; p < 0.035 | NS | |
| WHR | r = 0.26; p < 0.017 | NS | |
| Cotinine (ng/mL) | NS | r = 0.29; p < 0.007 | |
| mF-G (0–36) | NS | NS | |
| Acne (1–5) | NS | NS | |
| Cholesterol (mg/dL) | NS | NS | |
| HDL-C (mg/dL) | r = −0.24; p < 0.028 | r = −0.32; p < 0.003 | |
| LDL-C (mg/dL) | NS | NS | |
| Triglycerides (mg/dL) | r = 0.23; p < 0.036 | r = 0.37; p < 0.001 | |
| Glucose 0’ (mg/dL) | r = 0.27; p < 0.013 | NS | |
| Insulin 0’ (mU/mL) | r = 0.24; p < 0.029 | NS | |
| HOMA-IR | NS | r = 0.30; p < 0.006 | |
| LH (lU/L) | NS | NS | |
| FSH (lU/L) | NS | r = −0.26; p < 0.016 | |
| DHEA-S (µg/mL) | NS | r = 0.36; p < 0.001 | |
| SHBG (nmol/L) | NS | r = −0.43; p < 0.000 | |
| tTest (ng/mL) | NS | r = 0.32; p < 0.002 | |
| fTest (pg/mL) | NS | r = 0.33; p < 0.002 | |
| AD (ng/mL) | NS | NS | |
| FAI | NS | r = 0.44; p < 0.001 | |
| AMH (ng/mL) | NS | NS | |
| Correlated Parameters | Smoking Women with PCOS | ||
|---|---|---|---|
| Apelin [pg/mL] | Chemerin [ng/L] | Lipocalin-2 [ng/mL] | |
| Age (years) | NS | NS | NS |
| BMI (kg/m2) | r = −0.45; p < 0.012 | NS | NS |
| WHR | NS | r = 0.36; p < 0.048 | NS |
| Cotinine (ng/mL) | NS | NS | NS |
| mF-G (0–36) | NS | NS | r = −0.46; p < 0.009 |
| Acne (1–5) | NS | NS | NS |
| Cholesterol (mg/dL) | NS | r = 0.42; p < 0.019 | NS |
| HDL-C (mg/dL) | NS | r = −0.44; p < 0.013 | NS |
| LDL-C (mg/dL) | NS | r = 0.50; p < 0.004 | NS |
| Triglycerides (mg/dL) | NS | r = 0.45; p < 0.010 | NS |
| Glucose 0’ (mg/dL) | NS | r = 0.40; p < 0.026 | NS |
| Insulin 0’ (mU/mL) | r = −0.38; p < 0.035 | r = 0.38; p < 0.034 | NS |
| HOMA-IR | r = −0.39; p < 0.029 | r = 0.42; p < 0.019 | NS |
| LH (lU/L) | NS | NS | NS |
| FSH (lU/L) | NS | NS | r = −0.51; p < 0.003 |
| DHEA-S (µg/mL) | NS | NS | NS |
| SHBG (nmol/L) | r = 0.39; p < 0.028 | r = −0.39; p < 0.031 | r = −0.43; p < 0.017 |
| tTest (ng/mL) | NS | NS | NS |
| fTest (pg/mL) | NS | r = 0.40; p < 0.029 | NS |
| AD (ng/mL) | NS | NS | r = 0.40; p < 0.026 |
| FAI | NS | r = 0.36; p < 0.045 | NS |
| AMH (ng/mL) | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niepsuj, J.; Piwowar, A.; Franik, G.; Bizoń, A. Impact of Smoking and Obesity on the Selected Peptide Hormones and Metabolic Parameters in the Blood of Women with Polycystic Ovary Syndrome—Preliminary Study. Int. J. Mol. Sci. 2024, 25, 8713. https://doi.org/10.3390/ijms25168713
Niepsuj J, Piwowar A, Franik G, Bizoń A. Impact of Smoking and Obesity on the Selected Peptide Hormones and Metabolic Parameters in the Blood of Women with Polycystic Ovary Syndrome—Preliminary Study. International Journal of Molecular Sciences. 2024; 25(16):8713. https://doi.org/10.3390/ijms25168713
Chicago/Turabian StyleNiepsuj, Justyna, Agnieszka Piwowar, Grzegorz Franik, and Anna Bizoń. 2024. "Impact of Smoking and Obesity on the Selected Peptide Hormones and Metabolic Parameters in the Blood of Women with Polycystic Ovary Syndrome—Preliminary Study" International Journal of Molecular Sciences 25, no. 16: 8713. https://doi.org/10.3390/ijms25168713
APA StyleNiepsuj, J., Piwowar, A., Franik, G., & Bizoń, A. (2024). Impact of Smoking and Obesity on the Selected Peptide Hormones and Metabolic Parameters in the Blood of Women with Polycystic Ovary Syndrome—Preliminary Study. International Journal of Molecular Sciences, 25(16), 8713. https://doi.org/10.3390/ijms25168713







