Insights on the Mechanical Properties of SARS-CoV-2 Particles and the Effects of the Photosensitizer Hypericin
Abstract
1. Introduction
2. Results and Discussion
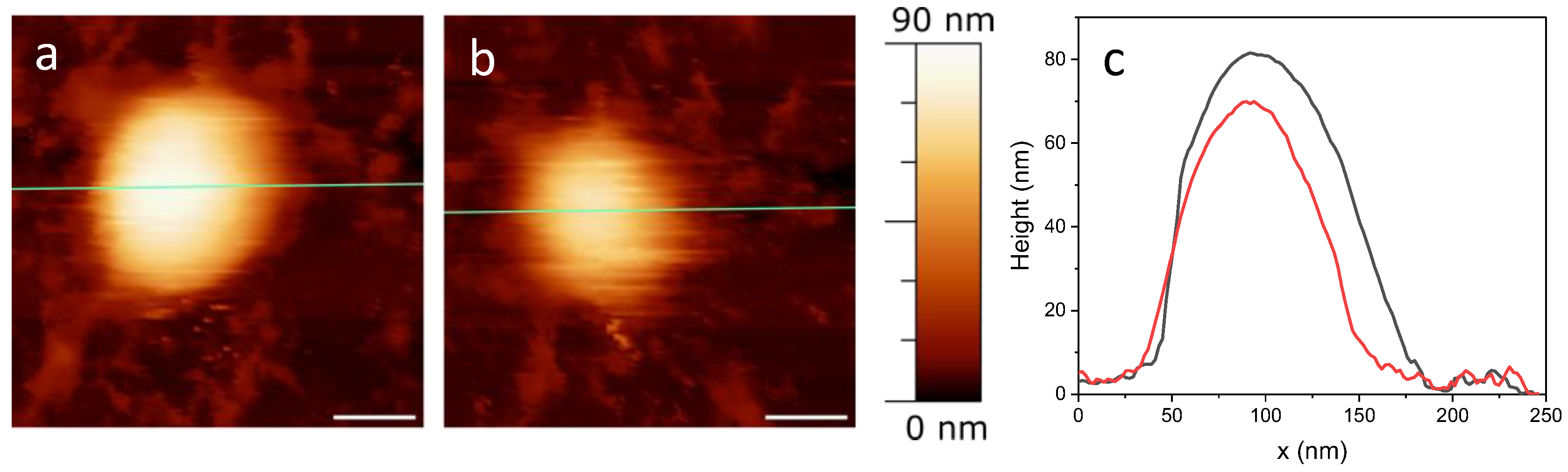
2.1. AFM Imaging
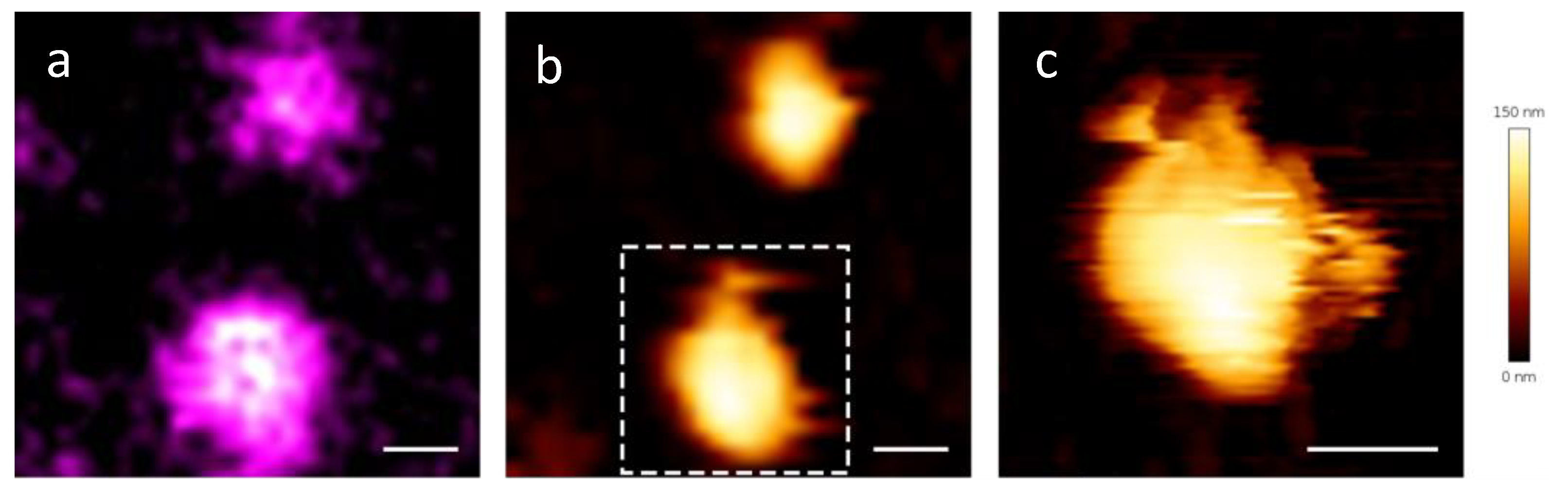
2.2. Correlative AFM-Fluorescence Microscopy
2.3. AFM Nanomechanical Properties
3. Materials and Methods
3.1. Imaging and Fatigue Experiments
3.1.1. Jumping Mode
3.1.2. QITM Mode
3.2. Nanomechanics
3.3. Correlative Microscopy
3.4. Virus Preparation and Inactivation
- Application of 40 μL of poly-L-lysine onto a freshly cleaved mica piece (0.5 cm × 0.5 cm) previously attached to a circular metallic support (diameter of 18 mm) for the measurements in Jumping mode. For the QITM and correlative measurements the viral suspension was deposited onto clean glass coverslips (diameter 25 mm).
- Waiting for 15 min, then rinse thoroughly with ultrapure MilliQ water three times. After each rinse, a nitrogen gas flow was used to dry the surface.
- To disperse any aggregate, the Eppendorf containing the viral particles was vortexed five times, with each vortexing lasting for 5 s.
- Placement of 50 μL of the vortexed particles onto the mica surface.
- After another 15 min, addiction of 20 μL of TRIS buffer (5 mM) mixed with NaCl (150 nM) at pH 7.4, to maintain appropriate osmotic pressure and pH levels.
- Following 15 min, three rinses with the same buffer to remove all unattached virions and debris, and addiction of the right amount of liquid for the measurement (usually 40–50 μL).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Modrow, S.; Falke, D.; Truyen, U.; Schätzl, H. (Eds.) Viruses with Single-Stranded, Positive-Sense RNA Genomes. In Molecular Virology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 185–349. [Google Scholar]
- Malik, Y.A. Properties of Coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020, 42, 3–11. [Google Scholar] [PubMed]
- Ke, Z.; Oton, J.; Qu, K.; Cortese, M.; Zila, V.; McKeane, L.; Nakane, T.; Zivanov, J.; Neufeldt, C.J.; Cerikan, B.; et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature 2020, 588, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.S. Virus entry: Molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2004, 2, 109–122. [Google Scholar] [CrossRef]
- Sieczkarski, S.B.; Whittaker, G.R. Viral entry. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2004; Volume 285. [Google Scholar] [CrossRef]
- Delcanale, P.; Uriati, E.; Mariangeli, M.; Mussini, A.; Moreno, A.; Lelli, D.; Cavanna, L.; Bianchini, P.; Diaspro, A.; Abbruzzetti, S.; et al. The Interaction of Hypericin with SARS-CoV-2 Reveals a Multimodal Antiviral Activity. ACS Appl. Mater. Interfaces 2022, 14, 14025–14032. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.B.; Lopez-Bazzocchf, I.; Towers, G.H.N. Antiviral activities of hypericin. Antivir. Res. 1991, 15, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.B.; Harris, L.; Towers, G.H.N. The importance of light in the anti-HIV effect of hypericin. Antivir. Res. 1993, 20, 173. [Google Scholar] [CrossRef] [PubMed]
- Lenard, J.; Rabson, A.; Vanderoef, R. Photodynamic inactivation of infectivity of human immunodeficiency virus and other enveloped viruses using hypericin and rose bengal: Inhibition of fusion and syncytia formation. Proc. Natl. Acad. Sci. USA 1993, 90, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.F.; Wu, M.H.; Cheng, B.H.; Chen, Y.W.; Shih, M.C. Lipid-mediated preferential localization of hypericin in lipid membranes. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Losi, A. Fluorescence and Time-Resolved Photoacoustics of Hypericin Inserted in Liposomes: Dependence on Pigment Concentration and Bilayer Phase. Photochem. Photobiol. 1997, 65, 791–801. [Google Scholar] [CrossRef]
- Eriksson, E.S.E.; dos Santos, D.J.V.A.; Guedes, R.C.; Eriksson, L.A. Properties and permeability of hypericin and brominated hypericin in lipid membranes. J. Chem. Theory Comput. 2009, 5, 3139–3149. [Google Scholar] [CrossRef]
- Bianchini, P.; Cozzolino, M.; Oneto, M.; Pesce, L.; Pennacchietti, F.; Tognolini, M.; Giorgio, C.; Nonell, S.; Cavanna, L.; Delcanale, P.; et al. Hypericin-Apomyoglobin: An Enhanced Photosensitizer Complex for the Treatment of Tumor Cells. Biomacromolecules 2019, 20, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Kubin, A.; Wierrani, F.; Burner, U.; Alth, G.; Grunberger, W. Hypericin—The Facts About a Controversial Agent. Curr. Pharm. Des. 2005, 11, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Huis in ‘t Veld, R.V.; Heuts, J.; Ma, S.; Cruz, L.J.; Ossendorp, F.A.; Jager, M.J. Current Challenges and Opportunities of Photodynamic Therapy against Cancer. Pharmaceutics 2023, 15, 330. [Google Scholar] [CrossRef] [PubMed]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Tanaka, M.; Vecchio, D.; Garcia-Diaz, M.; Chang, J.; Morimoto, Y.; Hamblin, M.R. Photodynamic therapy induces an immune response against a bacterial pathogen. Expert Rev. Clin. Immunol. 2012, 8, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Colacino, J.M.; Larsen, S.H.; Spitzer, W. Virucidal activity of hypericin against enveloped and non-enveloped DNA and RNA viruses. Antivir. Res. 1990, 13, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Zhang, Y.; Yao, Q.; Peng, Y.; Pan, Q.; Jiao, Q.; Ren, K.; Sun, F.; Zhang, Q.; Guo, R.; et al. Hypericin blocks the function of HSV-1 alkaline nuclease and suppresses viral replication. J. Ethnopharmacol. 2022, 296, 115524. [Google Scholar] [CrossRef] [PubMed]
- de Pablo, P.J.; Mateu, M.G. Mechanical properties of viruses. In Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2013; Volume 68. [Google Scholar] [CrossRef]
- Kol, N.; Gladnikoff, M.; Barlam, D.; Shneck, R.Z.; Rein, A.; Rousso, I. Mechanical properties of murine leukemia virus particles: Effect of maturation. Biophys. J. 2006, 91, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Kol, N.; Shi, Y.; Tsvitov, M.; Barlam, D.; Shneck, R.Z.; Kay, M.S.; Rousso, I. A stiffness switch in human immunodeficiency virus. Biophys. J. 2007, 92, 1777–1783. [Google Scholar] [CrossRef]
- Pang, H.B.; Hevroni, L.; Kol, N.; Eckert, D.M.; Tsvitov, M.; Kay, M.S.; Rousso, I. Virion stiffness regulates immature HIV-1 entry. Retrovirology 2013, 10, 4. [Google Scholar] [CrossRef]
- Kuznetsov, Y.G.; McPherson, A. Atomic Force Microscopy in Imaging of Viruses and Virus-Infected Cells. Microbiol. Mol. Biol. Rev. 2011, 75, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, C.; Carreira, A.; Schaap, I.A.T.; Serena, P.A.; Gómez-Herrero, J.; Mateu, M.G.; De Pablo, P.J. DNA-mediated anisotropic mechanical reinforcement of a virus. Proc. Natl. Acad. Sci. USA 2006, 103, 13706–13711. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Pérez, M.; Pascual, E.; Aznar, M.; Ionel, A.; Castón, J.R.; Luque, A.; Carrascosa, J.L.; Reguera, D.; De Pablo, P.J. The interplay between mechanics and stability of viral cages. Nanoscale 2014, 6, 2702–2709. [Google Scholar] [CrossRef] [PubMed]
- Roos, W.H.; Gertsman, I.; May, E.R.; Brooks, C.L.; Johnson, J.E.; Wuite, G.J.L. Mechanics of bacteriophage maturation. Proc. Natl. Acad. Sci. USA 2012, 109, 2342–2347. [Google Scholar] [CrossRef] [PubMed]
- De Pablo, P.J.; Colchero, J.; Gómez-Herrero, J.; Baró, A.M. Jumping mode scanning force microscopy. Appl. Phys. Lett. 1998, 73, 3300–3302. [Google Scholar] [CrossRef]
- Moreno-Herrero, F.; Colchero, J.; Gómez-Herrero, J.; Baró, A.M. Atomic force microscopy contact, tapping, and jumping modes for imaging biological samples in liquids. Phys. Rev. E 2004, 69, 031915. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Esteban, A.; Horcas, I.; Hernando-Pérez, M.; Ares, P.; Pérez-Berná, A.J.; San Martín, C.; Carrascosa, J.L.; De Pablo, P.J.; Gómez-Herrero, J. Minimizing tip-sample forces in jumping mode atomic force microscopy in liquid. Ultramicroscopy 2012, 114, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Chopinet, L.; Formosa, C.; Rols, M.P.; Duval, R.E.; Dague, E. Imaging living cells surface and quantifying its properties at high resolution using AFM in QI™ mode. Micron 2013, 48, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Lyonnais, S.; Hénaut, M.; Neyret, A.; Merida, P.; Cazevieille, C.; Gros, N.; Chable-Bessia, C.; Muriaux, D. Atomic force microscopy analysis of native infectious and inactivated SARS-CoV-2 virions. Sci. Rep. 2021, 11, 11885. [Google Scholar] [CrossRef]
- Cardoso-Lima, R.; Souza, P.F.N.; Guedes, M.I.F.; Santos-Oliveira, R.; Rebelo Alencar, L.M. SARS-CoV-2 Unrevealed: Ultrastructural and Nanomechanical Analysis. Langmuir 2021, 37, 10762–10769. [Google Scholar] [CrossRef]
- Cantero, M.; Carlero, D.; Chichón, F.J.; Martín-Benito, J.; De Pablo, P.J. Monitoring SARS-CoV-2 Surrogate TGEV Individual Virions Structure Survival under Harsh Physicochemical Environments. Cells 2022, 11, 1759. [Google Scholar] [CrossRef]
- Ortega-Esteban, A.; Pérez-Berná, A.J.; Menéndez-Conejero, R.; Flint, S.J.; San Martín, C.; De Pablo, P.J. Monitoring dynamics of human adenovirus disassembly induced by mechanical fatigue. Sci. Rep. 2013, 3, srep01434. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Madrid, F.; Martín-González, N.; Llauró, A.; Ortega-Esteban, A.; Hernando-Pérez, M.; Douglas, T.; Schaap, I.A.T.; De Pablo, P.J. Atomic force microscopy of virus shells. Biochem. Soc. Trans. 2017, 45, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.D.; Garcia, R. Determination of the Elastic Moduli of a Single Cell Cultured on a Rigid Support by Force Microscopy. Biophys. J. 2018, 114, 2923–2932. [Google Scholar] [CrossRef]
- Harke, B.; Chacko, J.V.; Haschke, H.; Canale, C.; Diaspro, A. A novel nanoscopic tool by combining AFM with STED microscopy. Opt. Nanoscopy 2012, 1, 3. [Google Scholar] [CrossRef]
- Chacko, J.V.; Zanacchi, F.C.; Diaspro, A. Probing cytoskeletal structures by coupling optical superresolution and AFM techniques for a correlative approach. Cytoskeleton 2013, 70, 729–740. [Google Scholar] [CrossRef]
- Monserrate, A.; Casado, S.; Flors, C. Correlative atomic force microscopy and localization-based super-resolution microscopy: Revealing labelling and image reconstruction artefacts. ChemPhysChem 2014, 15, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Diaspro, A.; Chacko, J.; Zanacchi, F.C.; Oropesa, R.; Dante, S.; Canale, C. Correlative nanoscopy: Super resolved fluorescence and atomic force microscopy towards nanoscale manipulation and multimodal investigations. Microsc. Microanal. 2015, 21, 2351–2352. [Google Scholar] [CrossRef][Green Version]
- Cosentino, M.; Canale, C.; Bianchini, P.; Diaspro, A. AFM-STED correlative nanoscopy reveals a dark side in fluorescence microscopy imaging. Sci. Adv. 2019, 5, eaav8062. [Google Scholar] [CrossRef]
- Jadavi, S.; Bianchini, P.; Cavalleri, O.; Dante, S.; Canale, C.; Diaspro, A. Correlative nanoscopy: A multimodal approach to molecular resolution. Microsc. Res. Tech. 2021, 84, 2472–2482. [Google Scholar] [CrossRef]
- Putman, C.A.J.; Van der Werf, K.O.; De Grooth, B.G.; Van Hulst, N.F.; Greve, J. Tapping mode atomic force microscopy in liquid. Appl. Phys. Lett. 1994, 64, 2454–2456. [Google Scholar] [CrossRef]
- Cappella, B.; Dietler, G. Force-distance curves by atomic force microscopy. Surf. Sci. Rep. 1999, 34, 1–104. [Google Scholar] [CrossRef]
- de Pablo, P.J. Atomic force microscopy of virus shells. Semin. Cell Dev. Biol. 2018, 73, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Cantero, M.; Cvirkaite-Krupovic, V.; Krupovic, M.; de Pablo, P.J. Mechanical tomography of an archaeal lemon-shaped virus reveals membrane-like fluidity of the capsid and liquid nucleoprotein cargo. Proc. Natl. Acad. Sci. USA 2023, 120, e2307717120. [Google Scholar] [CrossRef] [PubMed]
- Dufrêne, Y.F.; Boland, T.; Schneider, J.W.; Barger, W.R.; Lee, G.U. Characterization of the physical properties of model biomembranes at the nanometer scale with the atomic force microscope. Faraday Discuss. 1998, 111, 79–94; discussion 137–157. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manyes, S.; Sanz, F. Nanomechanics of lipid bilayers by force spectroscopy with AFM: A perspective. Biochim. Biophys. Acta (BBA) Biomembr. 2010, 1798, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Roos, W.H.; Bruinsma, R.; Wuite, G.J.L. Physical virology. Nat. Phys. 2010, 6, 733–743. [Google Scholar] [CrossRef]
- Roos, W.H.; Ivanovska, I.L.; Evilevitch, A.; Wuite, G.J.L. Viral capsids: Mechanical characteristics, genome packaging and delivery mechanisms. Cell. Mol. Life Sci. 2007, 64, 1484. [Google Scholar] [CrossRef] [PubMed]
- Mertens, J.; Bondia, P.; Allende-Ballestero, C.; Carrascosa, J.L.; Flors, C.; Castón, J.R. Mechanics of Virus-like Particles Labeled with Green Fluorescent Protein. Biophys. J. 2018, 115, 1561–1568. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 13705. [Google Scholar] [CrossRef]
- Hutter, J.L.; Bechhoefer, J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]

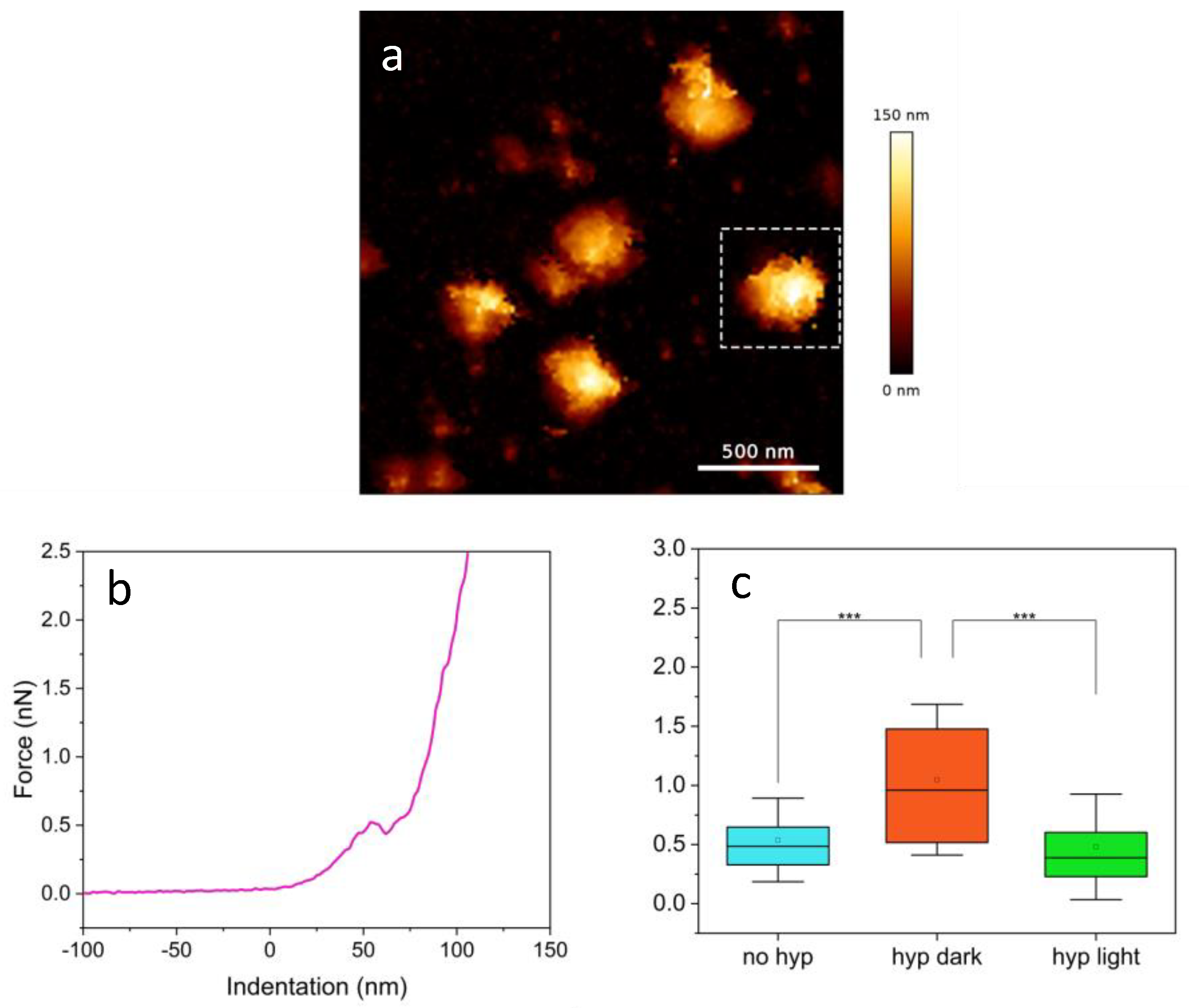
| [Hyp] | N° of Curves | Breakthrough Force |
|---|---|---|
| 0 μM | 34 | (0.54 ± 0.03) nN |
| 1 μM in dark | 71 | (1.05 ± 0.06) nN |
| 1 μM in light | 36 | (0.48 ± 0.04) nN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariangeli, M.; Moreno, A.; Delcanale, P.; Abbruzzetti, S.; Diaspro, A.; Viappiani, C.; Bianchini, P. Insights on the Mechanical Properties of SARS-CoV-2 Particles and the Effects of the Photosensitizer Hypericin. Int. J. Mol. Sci. 2024, 25, 8724. https://doi.org/10.3390/ijms25168724
Mariangeli M, Moreno A, Delcanale P, Abbruzzetti S, Diaspro A, Viappiani C, Bianchini P. Insights on the Mechanical Properties of SARS-CoV-2 Particles and the Effects of the Photosensitizer Hypericin. International Journal of Molecular Sciences. 2024; 25(16):8724. https://doi.org/10.3390/ijms25168724
Chicago/Turabian StyleMariangeli, Matteo, Ana Moreno, Pietro Delcanale, Stefania Abbruzzetti, Alberto Diaspro, Cristiano Viappiani, and Paolo Bianchini. 2024. "Insights on the Mechanical Properties of SARS-CoV-2 Particles and the Effects of the Photosensitizer Hypericin" International Journal of Molecular Sciences 25, no. 16: 8724. https://doi.org/10.3390/ijms25168724
APA StyleMariangeli, M., Moreno, A., Delcanale, P., Abbruzzetti, S., Diaspro, A., Viappiani, C., & Bianchini, P. (2024). Insights on the Mechanical Properties of SARS-CoV-2 Particles and the Effects of the Photosensitizer Hypericin. International Journal of Molecular Sciences, 25(16), 8724. https://doi.org/10.3390/ijms25168724







