Overexpression of the β-Subunit of Acid Ceramidase in the Epidermis of Mice Provokes Atopic Dermatitis-like Skin Symptoms
Abstract
:1. Introduction
2. Results
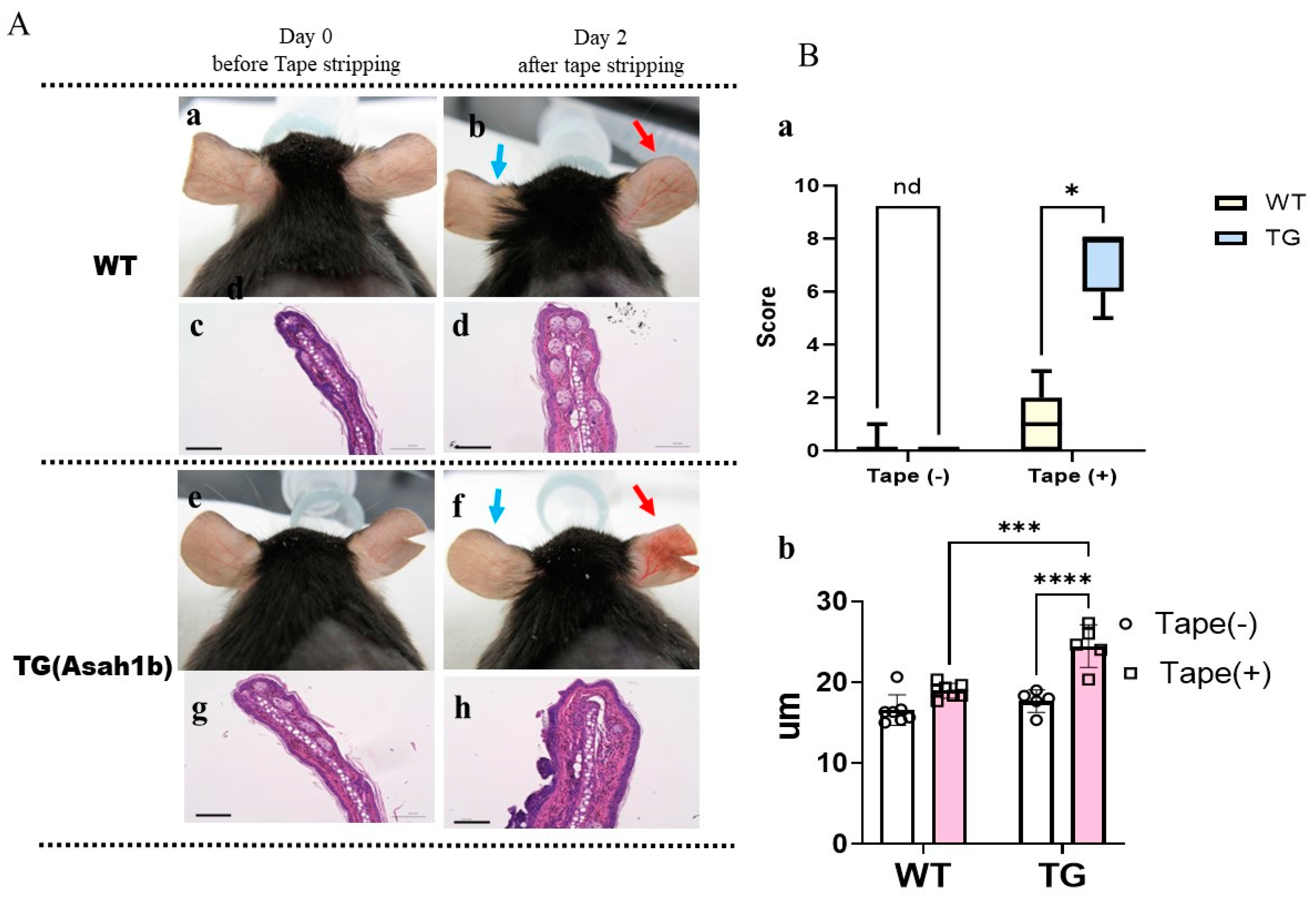
2.1. Overexpression of ASAH1b in the Upper Epidermis of the Ear Skin of TG Mice Facilities the Susceptibility to Tape-Stripping
2.2. Overexpression of ASAH1b in the Upper Epidermis Does Not Elicit Barrier-Disruption under Normal Conditions
2.3. Overexpression of ASAH1b in the Upper Epidermis Causes the Spontaneous Development of Skin Reactions in TG Mice That Resemble AD after Hair Removal Using a Depilatory Cream Containing Glycolic Acid
2.4. Overexpression of ASAH1b in the Upper Epidermis Facilities the Susceptibility against Tape-Stripping in the Dorsal Skin of Mice
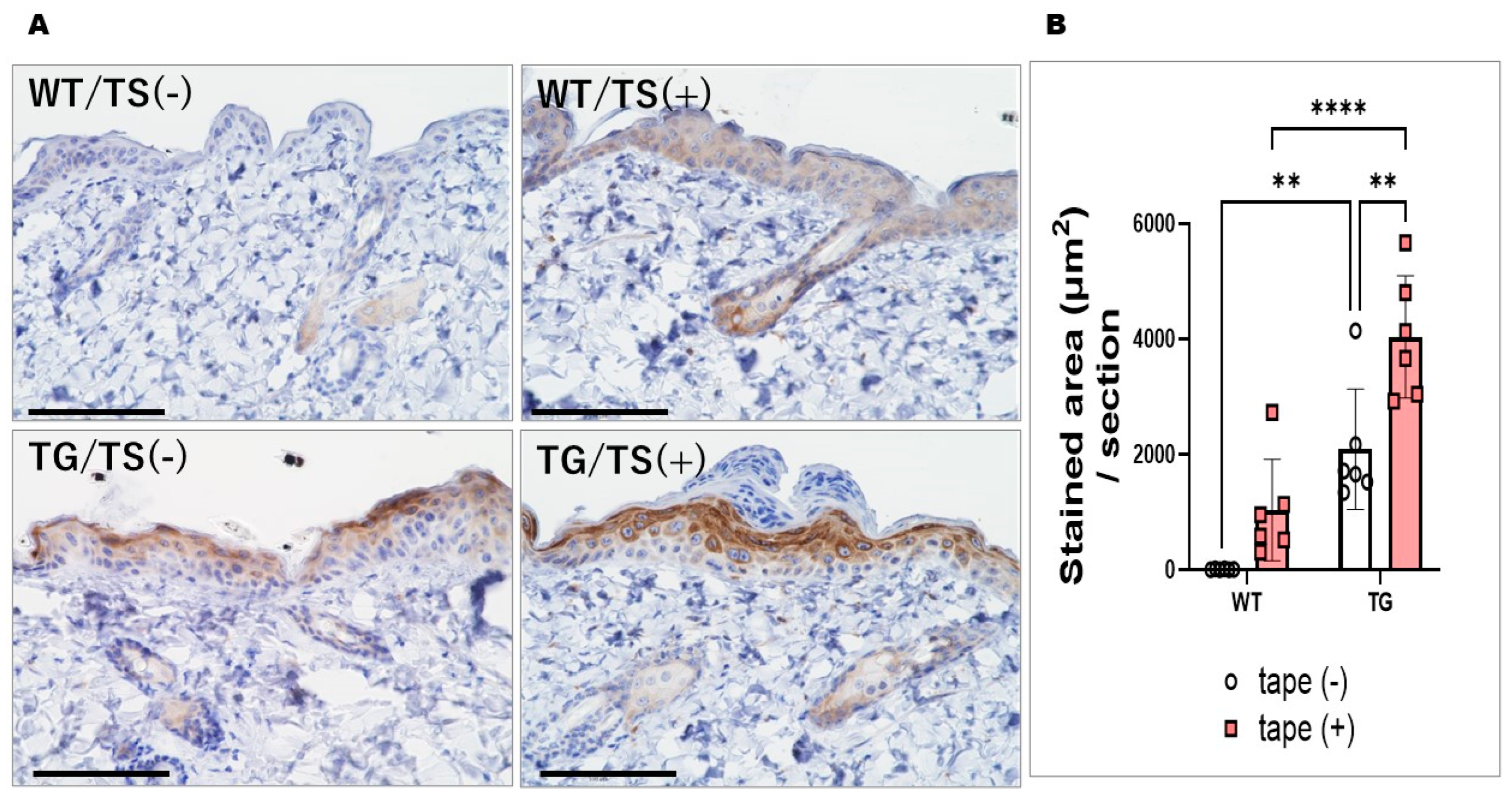
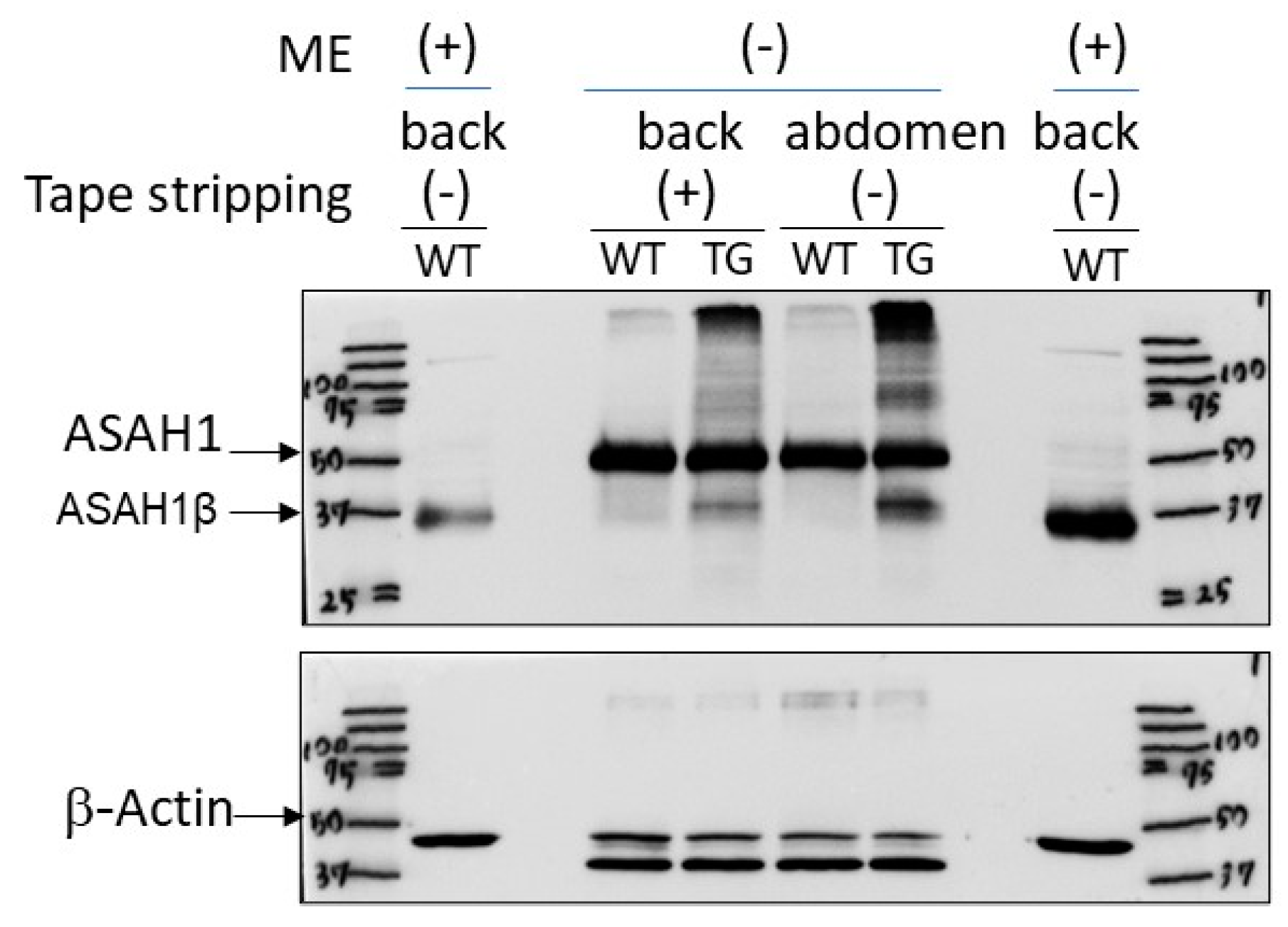
2.5. Immunostaining with an Anti-ASAH1b Antibody and an Anti-Ceramide Antibody before and after Tape-Stripping and Western Blotting of ASAH1b Protein in the Upper Epidermis of TG Mice Overexpressing ASAH1b
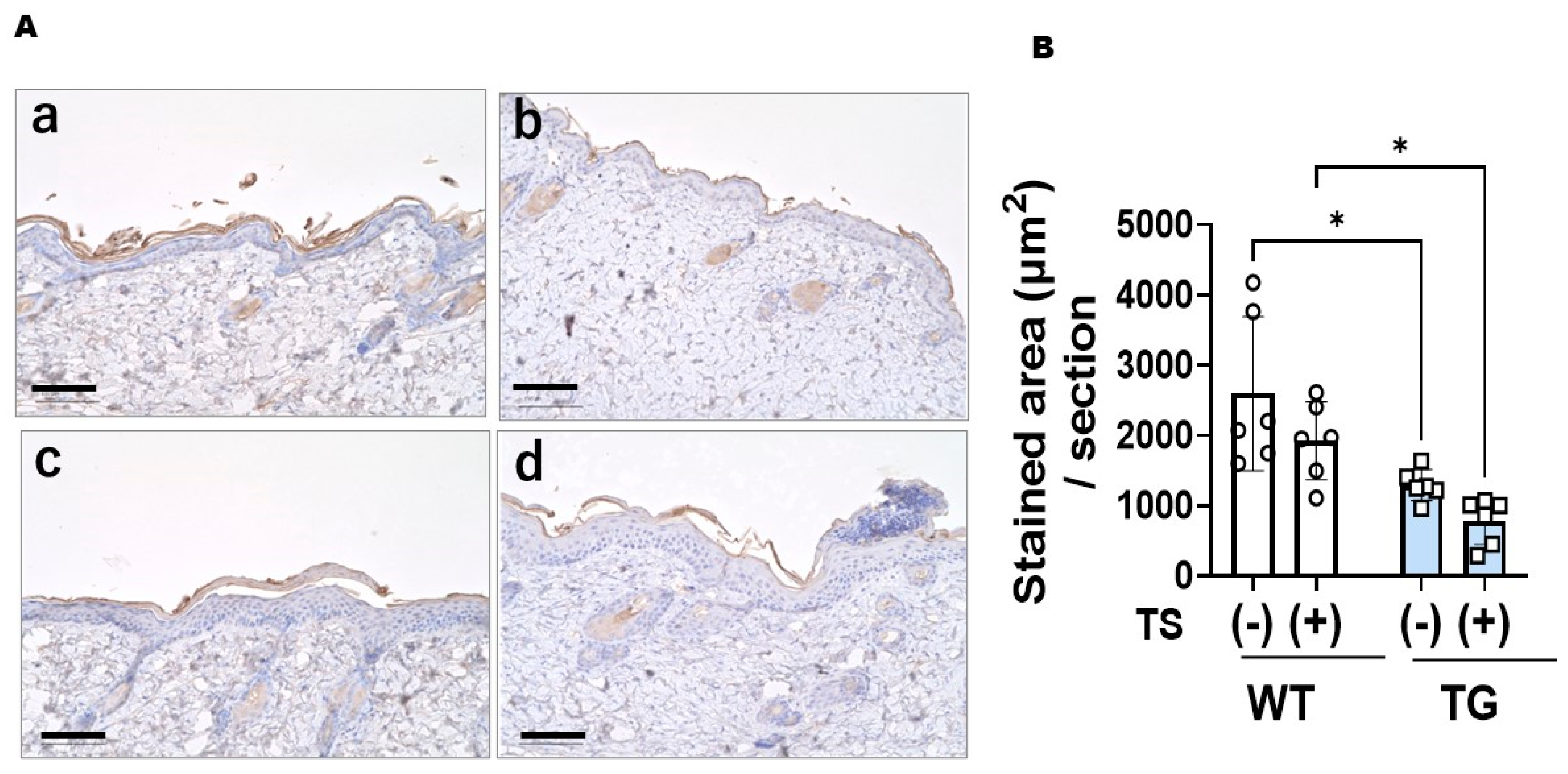
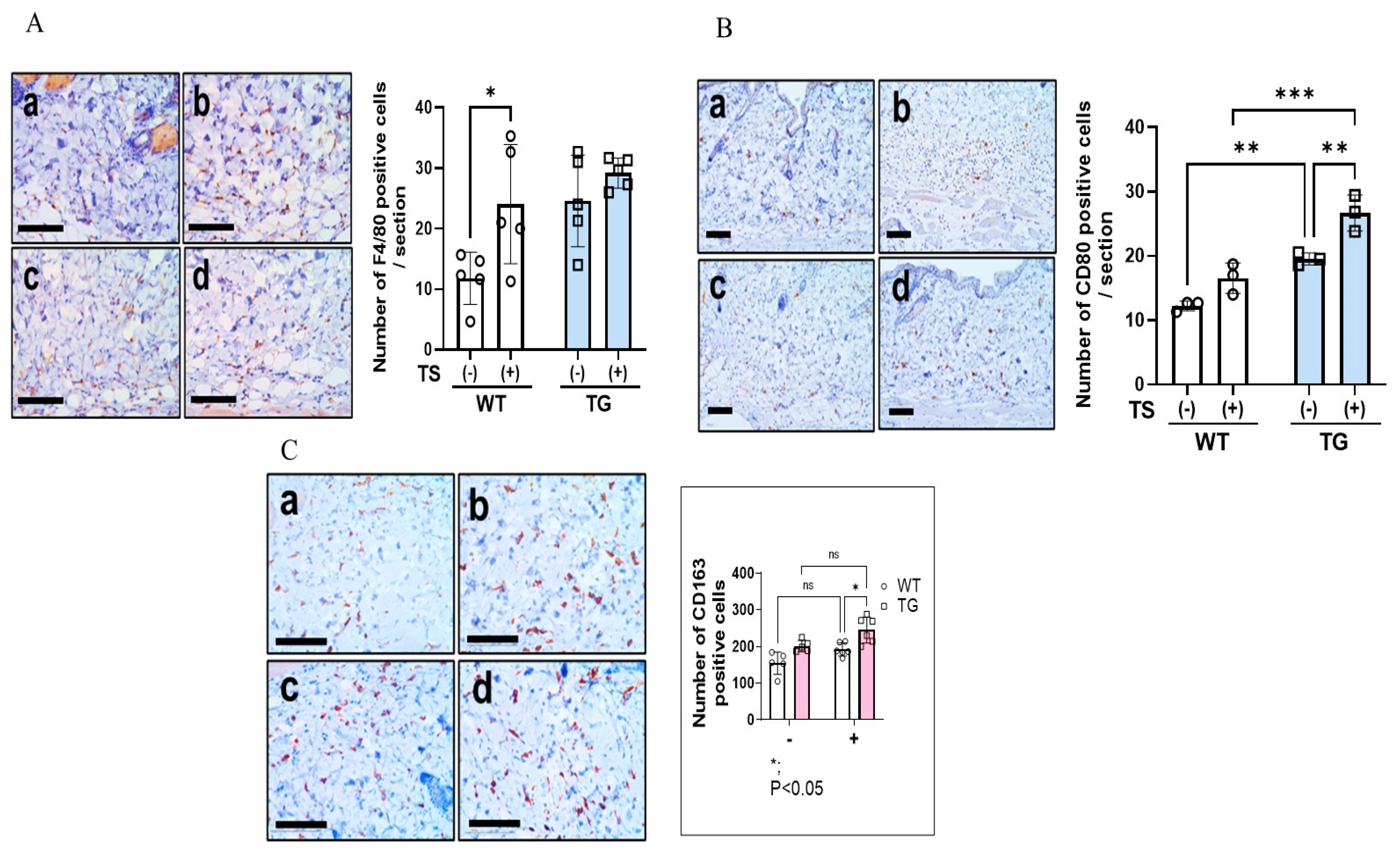
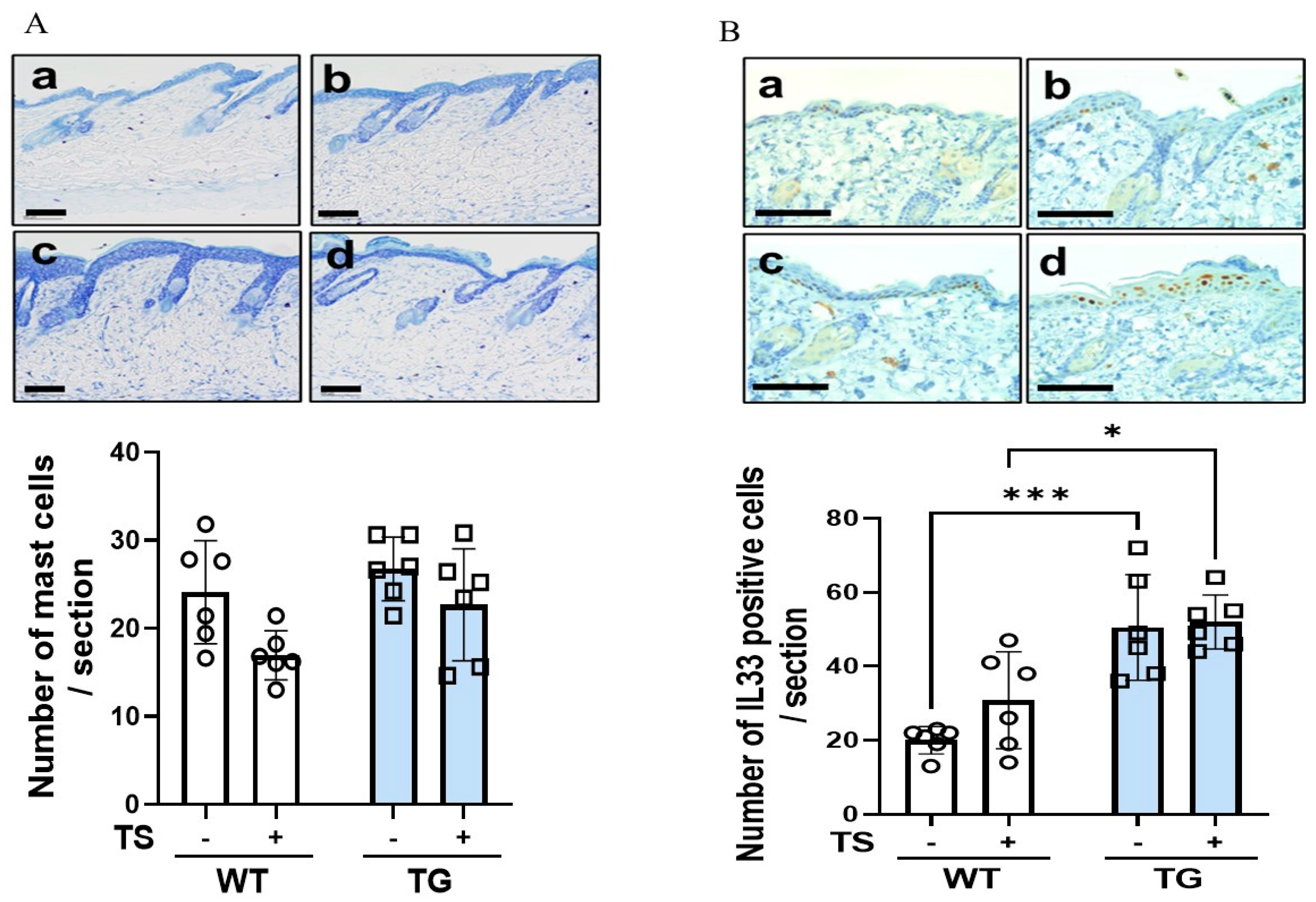
2.6. Immunostaining of Macrophages Using Various Macrophage Markers, Mast Cells and IL-33 in the Dorsal Skin of TG and WT Mice before and after Tape-Stripping
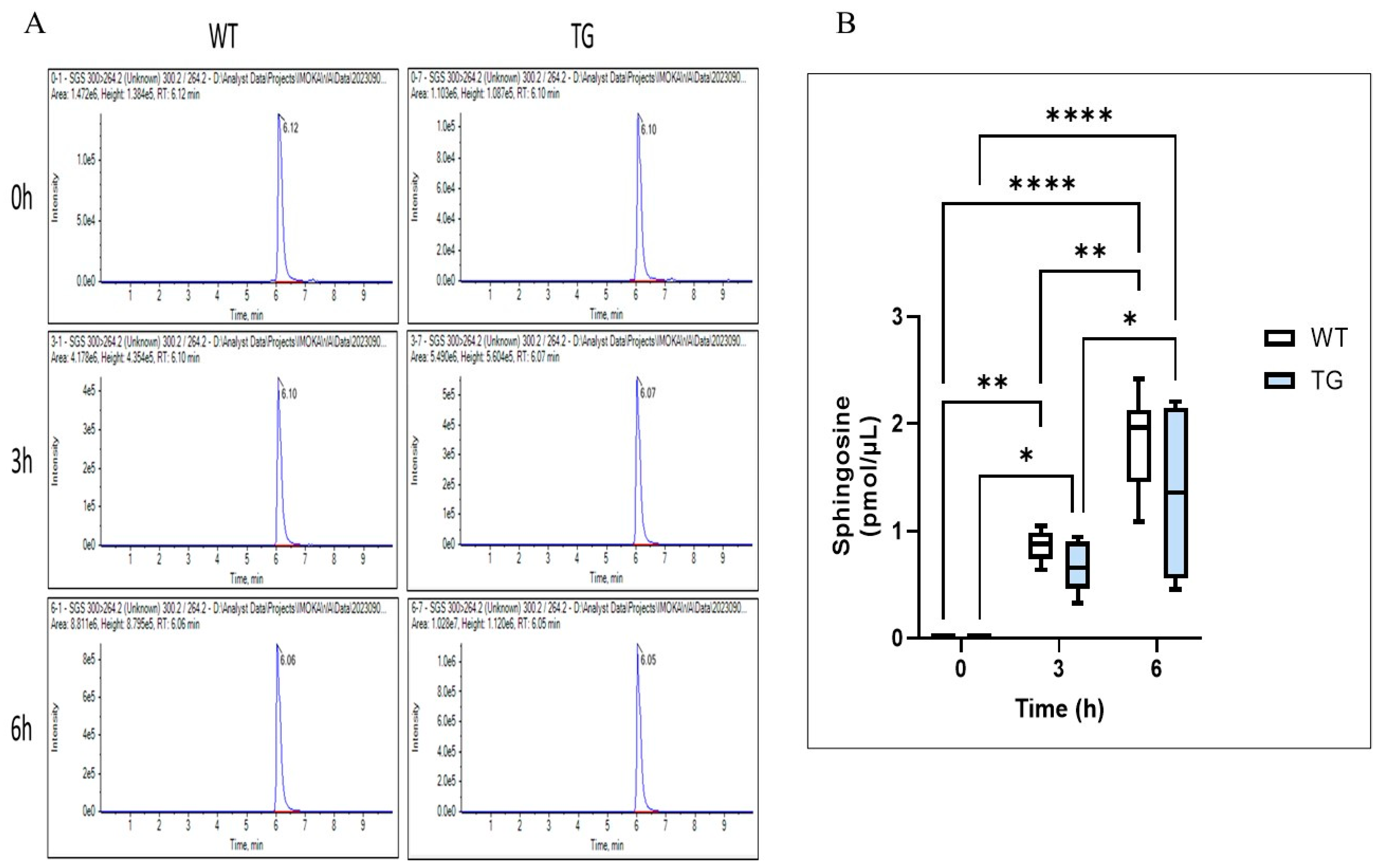
2.7. Effects of Overexpressing ASAH1b on the Activity of SGDase and ASAH1 in the Epidermis
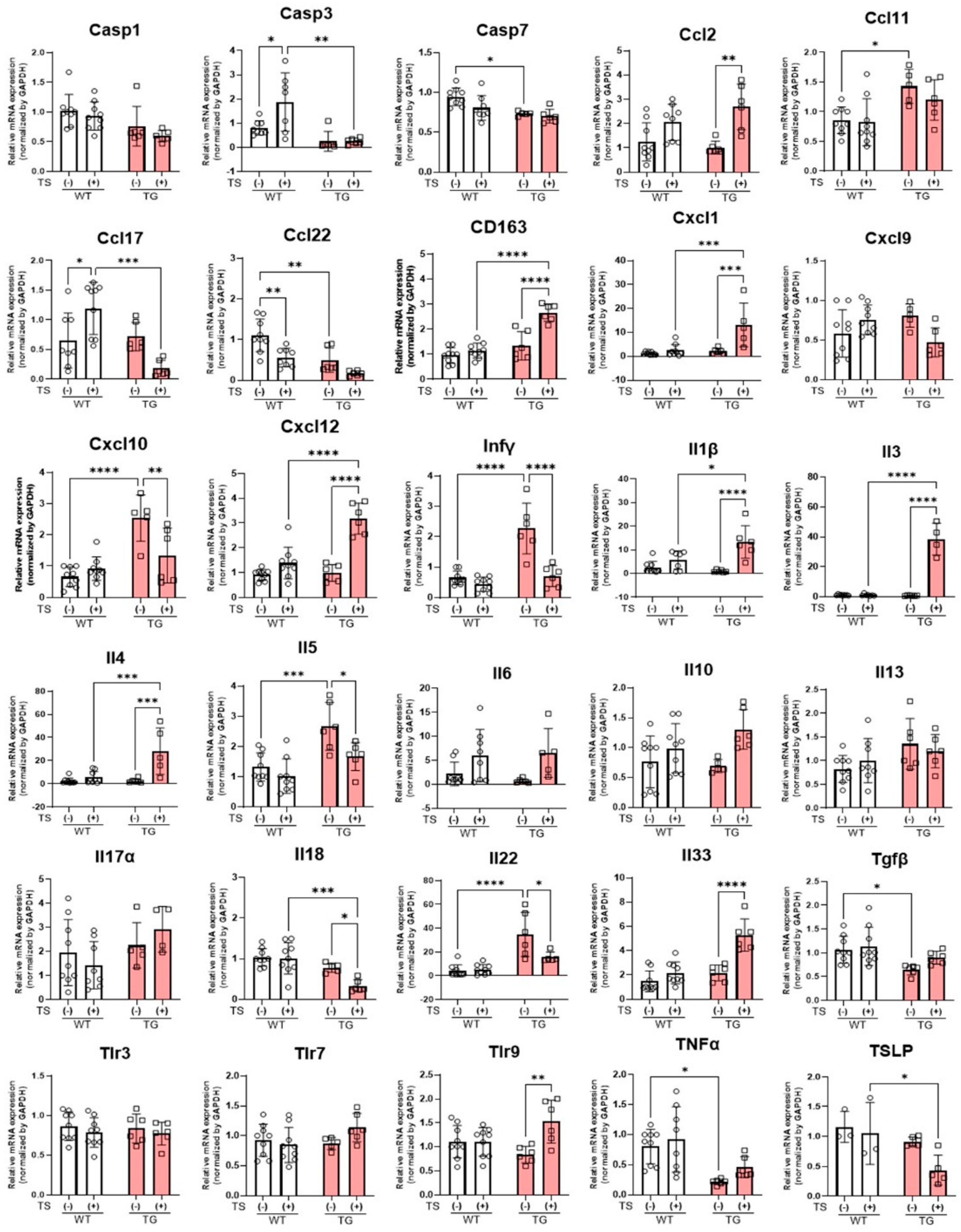
2.8. mRNA Expression Levels of Inflammatory Cytokines and Chemokines in the Skin of TG and WT Mice before and at Day 2 after Tape-Stripping
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Construction of Expression Vectors
4.3. Production of ASAH1b-Overexpressing TG Mice
4.4. Skin Eruption Scoring
4.5. Measurement of Skin Barrier Function
4.6. Tape-Stripping
4.7. Histological Observation
4.8. Immunohistochemical Staining
4.9. Real-Time Polymerase Chain Reaction (RT-PCR)
4.10. Western Blotting Analysis
4.11. Assays for SM Deacylase and ASAH1 Activities
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S. Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef] [PubMed]
- Schuler, C.F.t.; Billi, A.C.; Maverakis, E.; Tsoi, L.C.; Gudjonsson, J.E. Novel insights into atopic dermatitis. J. Allergy Clin. Immunol. 2023, 151, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Imokawa, G. Cutting Edge of the Pathogenesis of Atopic Dermatitis: Sphingomyelin Deacylase, the Enzyme Involved in Its Ceramide Deficiency, Plays a Pivotal Role. Int. J. Mol. Sci. 2021, 22, 1613. [Google Scholar] [CrossRef]
- Czarnowicki, T.; Krueger, J.G.; Guttman-Yassky, E. Novel concepts of prevention and treatment of atopic dermatitis through barrier and immune manipulations with implications for the atopic march. J. Allergy Clin. Immunol. 2017, 139, 1723–1734. [Google Scholar] [CrossRef]
- Leung, D.Y.; Bieber, T. Atopic dermatitis. Lancet 2003, 361, 151–160. [Google Scholar] [CrossRef]
- Peng, W.; Novak, N. Pathogenesis of atopic dermatitis. Clin. Exp. Allergy 2015, 45, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Rehbinder, E.M.; Winger, A.J.; Landrø, L.; Asarnoj, A.; Berents, T.L.; Carlsen, K.H.; Hedlin, G.; Jonassen, C.M.; Nordlund, B.; Sandvik, L.; et al. Dry skin and skin barrier in early infancy. Br. J. Dermatol. 2019, 181, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y. Extrinsic and intrinsic types of atopic dermatitis. J. Dermatol. Sci. 2010, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y. Atopic dermatitis: New insights and opportunities for therapeutic intervention. J. Allergy Clin. Immunol. 2000, 105, 860–876. [Google Scholar] [CrossRef] [PubMed]
- Scalabrin, D.M.; Bavbek, S.; Perzanowski, M.S.; Wilson, B.B.; Platts-Mills, T.A.; Wheatley, L.M. Use of specific IgE in assessing the relevance of fungal and dust mite allergens to atopic dermatitis: A comparison with asthmatic and nonasthmatic control subjects. J. Allergy Clin. Immunol. 1999, 104, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Kapp, A. The role of eosinophils in the pathogenesis of atopic dermatitis—Eosinophil granule proteins as markers of disease activity. Allergy 1993, 48, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Abe, A.; Jin, K.; Higaki, Y.; Kawashima, M.; Hidano, A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin? J. Investig. Dermatol. 1991, 96, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, A.; Wertz, P.; Giannetti, A.; Seidenari, S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm. Venereol. 1998, 78, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Takahashi, A.; Bito, K.; Draelos, Z.; Imokawa, G. Treatment with Synthetic Pseudoceramide Improves Atopic Skin, Switching the Ceramide Profile to a Healthy Skin Phenotype. J. Investig. Dermatol. 2020, 140, 1762–1770.e8. [Google Scholar] [CrossRef]
- Kusuda, S.; Cui, C.Y.; Takahashi, M.; Tezuka, T. Localization of sphingomyelinase in lesional skin of atopic dermatitis patients. J. Investig. Dermatol. 1998, 111, 733–738. [Google Scholar] [CrossRef]
- Danso, M.; Boiten, W.; van Drongelen, V.; Gmelig Meijling, K.; Gooris, G.; El Ghalbzouri, A.; Absalah, S.; Vreeken, R.; Kezic, S.; van Smeden, J.; et al. Altered expression of epidermal lipid bio-synthesis enzymes in atopic dermatitis skin is accompanied by changes in stratum corneum lipid composition. J. Dermatol. Sci. 2017, 88, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.M.; Fölster-Holst, R.; Baranowsky, A.; Schunck, M.; Winoto-Morbach, S.; Neumann, C.; Schütze, S.; Proksch, E. Impaired sphingomyelinase activity and epidermal differentiation in atopic dermatitis. J. Investig. Dermatol. 2004, 122, 1423–1431. [Google Scholar] [CrossRef]
- Teranishi, Y.; Kuwahara, H.; Ueda, M.; Takemura, T.; Kusumoto, M.; Nakamura, K.; Sakai, J.; Kimura, T.; Furutani, Y.; Kawashima, M.; et al. Sphingomyelin Deacylase, the Enzyme Involved in the Pathogenesis of Atopic Dermatitis, Is Identical to the beta-Subunit of Acid Ceramidase. Int. J. Mol. Sci. 2020, 21, 8789. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, K.; Hara, J.; Okamoto, R.; Kawashima, M.; Imokawa, G. The skin of atopic dermatitis patients contains a novel enzyme, glucosylceramide sphingomyelin deacylase, which cleaves the N-acyl linkage of sphingomyelin and glucosylceramide. Biochem. J. 2000, 350 Pt 3, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Kiekens, R.C.; Thepen, T.; Oosting, A.J.; Bihari, I.C.; Van De Winkel, J.G.; Bruijnzeel-Koomen, C.A.; Knol, E.F. Heterogeneity within tissue-specific macrophage and dendritic cell populations during cutaneous inflammation in atopic dermatitis. Br. J. Dermatol. 2001, 145, 957–965. [Google Scholar] [CrossRef]
- Kasraie, S.; Werfel, T. Role of macrophages in the pathogenesis of atopic dermatitis. Mediat. Inflamm. 2013, 2013, 942375. [Google Scholar] [CrossRef] [PubMed]
- Dickel, H.; Gambichler, T.; Kamphowe, J.; Altmeyer, P.; Skrygan, M. Standardized tape stripping prior to patch testing induces upregulation of Hsp90, Hsp70, IL-33, TNF-α and IL-8/CXCL8 mRNA: New insights into the involvement of ‘alarmins’. Contact Dermat. 2010, 63, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Bhan, A.K.; Schneeberger, E.E.; Geha, R.S. Characterization of the mononuclear cell infiltrate in atopic dermatitis using monoclonal antibodies. J. Allergy Clin. Immunol. 1983, 71 Pt 1, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y. Atopic dermatitis: The skin as a window into the pathogenesis of chronic allergic diseases. J. Allergy Clin. Immunol. 1995, 96, 302–318, quiz 319. [Google Scholar] [CrossRef]
- Hristodorov, D.; Mladenov, R.; von Felbert, V.; Huhn, M.; Fischer, R.; Barth, S.; Thepen, T. Targeting CD64 mediates elimination of M1 but not M2 macrophages in vitro and in cutaneous inflammation in mice and patient biopsies. MAbs 2015, 7, 853–862. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Hansson, M.; Silverpil, E.; Lindén, A.; Glader, P. Interleukin-22 produced by alveolar macrophages during activation of the innate immune response. Inflamm. Res. 2013, 62, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Balato, A.; Raimondo, A.; Balato, N.; Ayala, F.; Lembo, S. Interleukin-33: Increasing role in dermatological conditions. Arch. Dermatol. Res. 2016, 308, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Dajnoki, Z.; Béke, G.; Mócsai, G.; Kapitány, A.; Gáspár, K.; Hajdu, K.; Emri, G.; Nagy, B.; Kovács, I.; Beke, L.; et al. Immune-mediated Skin Inflammation is Similar in Severe Atopic Dermatitis Patients With or Without Filaggrin Mutation. Acta Derm. Venereol. 2016, 96, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y. Interleukin-33 in atopic dermatitis. J. Dermatol. Sci. 2019, 96, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.M.; Romero, M.R.; Watt, F.M. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell 1995, 83, 957–968. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Wang, X.; Zhang, L.; Hu, M.; Le, Y.; Chen, L.; Zheng, J. Topical emollient prevents the development of atopic dermatitis and atopic march in mice. Exp. Dermatol. 2023, 32, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Zhu, Z.; Liu, X.; Chen, B.; Yin, H.; Gu, C.; Fang, X.; Zhu, R.; Yu, T.; Mi, W.; et al. A dysregulated sebum-microbial metabolite-IL-33 axis initiates skin inflammation in atopic dermatitis. J. Exp. Med. 2022, 219, e20212397. [Google Scholar] [CrossRef]
- Vinaik, R.; Abdullahi, A.; Barayan, D.; Jeschke, M.G. NLRP3 inflammasome activity is required for wound healing after burns. Transl. Res. 2020, 217, 47–60. [Google Scholar] [CrossRef]
- Cho, B.S.; Kim, J.O.; Ha, D.H.; Yi, Y.W. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res. Ther. 2018, 9, 187. [Google Scholar] [CrossRef]
- Li, Y.; Sakamoto, M.; Matsuno, K.; Sawaragi, E.; Zhao, Q.; Dong, H.; Nakano, T.; Yamanaka, H.; Tsuge, I.; Tabata, Y.; et al. Potential of gelatin hydrogel nonwoven fabrics (Genocel) as a skin substitute in a diabetic mouse skin defect model. Regen. Ther. 2024, 27, 482–487. [Google Scholar] [CrossRef]




| Symbol | Gene Name | Sequence (5′ > 3′) | |
|---|---|---|---|
| Casp1 | caspase 1 | F | TGCCTGGTCTTGTGACTTGGA |
| R | CCTATCAGCAGTGGGCATCTGTA | ||
| Casp3 | caspase 3 | F | AGCCATGGGCACATCTTCAG |
| R | TGGTAACTTGGACATCATCCACAC | ||
| Casp7 | caspase 7 | F | AAGCCACTGCCTGAGATGGAA |
| R | GGAATTATAAGGCGCTGGTGGA | ||
| Ccl2 | chemokine (C-C motif) ligand 2 | F | AGCAGCAGGTGTCCCAAAGA |
| R | GTGCTGAAGACCTTAGGGCAGA | ||
| Ccl11 | chemokine (C-C motif) ligand 11 | F | CAGATGCACCCTGAAAGCCATA |
| R | TGCTTTGTGGCATCCTGGAC | ||
| Ccl17 | chemokine (C-C motif) ligand 17 | F | CCGAGAGTGCTGCCTGGATTA |
| R | AGCTTGCCCTGGACAGTCAGA | ||
| CCL22 | chemokine (C-C motif) ligand 22 | F | CTGACGAGGACACATAACATCATGG |
| R | CTTCACTAAACGTGATGGCAGAGG | ||
| CD163 | CD163 antigen | F | GCCAAACCGTGGAGTCACAG |
| R | GGACCAATAGAATGGCTCCACAA | ||
| Cxcl1 | chemokine (C-X-C motif) ligand 1 | F | TGCACCCAAACCGAAGTC |
| R | GTCAGAAGCCAGCGTTCACC | ||
| Cxcl9 | chemokine (C-X-C motif) ligand 9 | F | GAAGTGTGGACAGGGCCAAGTTA |
| R | GACGGAACTTCTGCCCAGAGAC | ||
| Cxcl10 | chemokine (C-X-C motif) ligand 10 | F | ATCCGGAATCTAAGACCATCAAGAA |
| R | GGACTAGCCATCCACTGGGTAAAG | ||
| CXCL12 | chemokine (C-X-C motif) ligand 12 | F | CAGAGCCAACGTCAAGCATC |
| R | TTAATTTCGGGTCAATGCACAC | ||
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase | F | TGTGTCCGTCGTGGATCTCTGA |
| R | TTGCTGTTGAAGTCGCAGGAG | ||
| Ifng | interferon gamma | F | CGGCACAGTCATTGAAAGCCTA |
| R | GTTGCTGATGGCCTGATTGTC | ||
| Il1b | interleukin 1 beta | F | TCCAGGATGAGGACATGAGCAC |
| R | GAACGTCACACACCAGCAGGTTA | ||
| Il3 | interleukin 3 | F | ACCGTTTAACCAGAACGTTGAATTG |
| R | TCCACGAATTTGGACAGGTTTACTC | ||
| Il4 | interleukin 4 | F | TCTCGAATGTACCAGGAGCCATATC |
| R | AGCACCTTGGAAGCCCTACAGA | ||
| Il5 | interleukin 5 | F | TCAGCTGTGTCTGGGCCACT |
| R | TTATGAGTAGGGACAGGAAGCCTCA | ||
| Il6 | interleukin 6 | F | CCACTTCACAAGTCGGAGGCTTA |
| R | CCAGTTTGGTAGCATCCATCATTTC | ||
| Il10 | interleukin 10 | F | GCCAGAGCCACATGCTCCTA |
| R | GATAAGGCTTGGCAACCCAAGTAA | ||
| Il13 | interleukin 13 | F | CAATTGCAATGCCATCTACAGGAC |
| R | CGAAACAGTTGCTTTGTGTAGCTGA | ||
| Il17α | interleukin 17 alpha | F | ACGCGCAAACATGAGTCCAG |
| R | AGGCTCAGCAGCAGCAACAG | ||
| Il18 | interleukin 18 | F | AAGACTCTTGCGTCAACTTCAAGGA |
| R | ATGCGGCCAAAGTTGTCTGATTC | ||
| Il22 | interleukin 22 | F | ACATCAGCCGTGACGACCAG |
| R | CGCTCAGACGCAAGCATTTC | ||
| Il33 | interleukin 33 | F | GATGAGATGTCTCGGCTGCTTG |
| R | AGCCGTTACGGATATGGTGGTC | ||
| Tgfb1 | transforming growth factor, beta 1 | F | GTGTGGAGCAACATGTGGAACTCTA |
| R | CGCTGAATCGAAAGCCCTGTA | ||
| TLR3 | toll-like receptor 3 | F | AAATCCTTGCGTTGCGAAGTG |
| R | TCAGTTGGGCGTTGTTCAAGA | ||
| TLR7 | toll-like receptor 7 | F | CTTTGCAACTGTGATGCTGTGTG |
| R | ACCTTTGTGTGCTCCTGGACCTA | ||
| TLR9 | toll-like receptor 9 | F | GAGACCCTGGTGTGGAACATC |
| R | ACTGCAGCCTGTACCAGGAG | ||
| TNFa | tumor necrosis factor | F | TATGGCCCAGACCCTCACA |
| R | GGAGTAGACAAGGTACAACCCATC | ||
| TSLP | thymic stromal lymphopoietin | F | AATGACCACTGCCCAGGCTA |
| R | TTGTGAGGTTTGATTCAGACAGATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sashikawa-Kimura, M.; Takada, M.; Hossain, M.R.; Tsuda, H.; Xie, X.; Komine, M.; Ohtsuki, M.; Imokawa, G. Overexpression of the β-Subunit of Acid Ceramidase in the Epidermis of Mice Provokes Atopic Dermatitis-like Skin Symptoms. Int. J. Mol. Sci. 2024, 25, 8737. https://doi.org/10.3390/ijms25168737
Sashikawa-Kimura M, Takada M, Hossain MR, Tsuda H, Xie X, Komine M, Ohtsuki M, Imokawa G. Overexpression of the β-Subunit of Acid Ceramidase in the Epidermis of Mice Provokes Atopic Dermatitis-like Skin Symptoms. International Journal of Molecular Sciences. 2024; 25(16):8737. https://doi.org/10.3390/ijms25168737
Chicago/Turabian StyleSashikawa-Kimura, Miho, Mariko Takada, Md Razib Hossain, Hidetoshi Tsuda, Xiaonan Xie, Mayumi Komine, Mamitaro Ohtsuki, and Genji Imokawa. 2024. "Overexpression of the β-Subunit of Acid Ceramidase in the Epidermis of Mice Provokes Atopic Dermatitis-like Skin Symptoms" International Journal of Molecular Sciences 25, no. 16: 8737. https://doi.org/10.3390/ijms25168737






