Abstract
Recent studies have highlighted the therapeutic potential of stem cells for various diseases. However, unlike other tissues, brain tissue has a specific structure, consisting of synapses. These synapses not only transmit but also process and refine information. Therefore, synaptic regeneration plays a key role in therapy of neurodegenerative disorders. Neurexins (NRXNs) and neuroligins (NLGNs) are synaptic cell adhesion molecules that connect pre- and postsynaptic neurons at synapses, mediate trans-synaptic signaling, and shape neural network properties by specifying synaptic functions. In this study, we investigated the synaptic regeneration effect of human neural stem cells (NSCs) overexpressing NRXNs (F3.NRXN) and NLGNs (F3.NLGN) in a spinal cord injury model. Overexpression of NRXNs and NLGNs in the neural stem cells upregulated the expression of synaptophysin, PSD95, VAMP2, and synapsin, which are synaptic markers. The BMS scores indicated that the transplantation of F3.NRXN and F3.NLGN enhanced the recovery of locomotor function in adult rodents following spinal cord injury. Transplanted F3.NRXN and F3.NLGN differentiated into neurons and formed a synapse with the host cells in the spinal cord injury mouse model. In addition, F3.NRXN and F3.NLGN cells restored growth factors (GFs) and neurotrophic factors (NFs) and induced the proliferation of host cells. This study suggested that NSCs overexpressing NRXNs and NLGNs could be candidates for cell therapy in spinal cord injuries by facilitating synaptic regeneration.
1. Introduction
Spinal cord injury (SCI) induces transient or permanent motor, sensory, or autonomic function interruption. The number of patients with SCI is reported to be more than 250,000 in the United States, with more than 11,000 new cases added annually [1]. SCI research has greatly expanded in recent years, but the mechanisms for functional recovery are not well-understood [2]. However, many researchers have tried to find effective therapies for SCI [3], including stem cell therapy [4,5].
Since stem cells were first identified and characterized, they have been used in therapy for various diseases such as SCI [4,5], traumatic brain injury [6], and neurodegenerative diseases [7]. Since they can self-renew and differentiate into any cell type [8], stem cells and neural progenitor cells are good candidates for SCI [9,10]. Although neurons in the adult central nervous system (CNS) have a low regenerative capacity due to a lack of growth signals [11], after the transplantation of stem cells, the neurons are activated by the upregulation of growth and neurotrophic factors derived from stem cells [12]. However, stem cell transplantation is still not effective in aiding recovery from SCI.
Unlike other tissues, the nervous system comprises specific structures called synapses [13]. SCI, in addition to causing a loss of neuronal cells, alters neuronal connectivity, leading to axonal and synaptic losses [14]. Since synapses transfer and process neural information [13], synaptic regeneration is one of the main goals of SCI therapy [15]. Synapses are connected by neurexins (NRXNs) at the presynaptic neurons and neuroligins (NLGNs) at the postsynaptic neurons [16]. NRXNs and NLGNs are synaptic cell adhesion molecules that form synapses in the pre- and postsynaptic neurons, mediate synaptic signaling, and shape neural network properties by specifying functions [17,18]. Indeed, the synaptic plasticity of these neurons is not related to cognitive functions but rather motor functions [16,19], and decreases after SCI [2]. Therefore, we hypothesized that transplantation of stem cells overexpressing NRXNs or NLGNs into the damaged site would promote SCI recovery by upregulating growth/neurotrophic factors and promoting synapse regeneration.
In this study, we established human neural stem cells (hNSCs) overexpressing NRXNs (F3.NRXN) or NLGNs (F3.NLGN). We then analyzed the expression of other synaptic proteins such as synaptophysin, PSD95, VAMP2, and synapsin in cell and animal models. We investigated the effects of F3.NRXN and F3.NLGN in an SCI mouse model induced by complete transection.
2. Results
2.1. Construction of F3.NLGN and F3.NRXN Cells
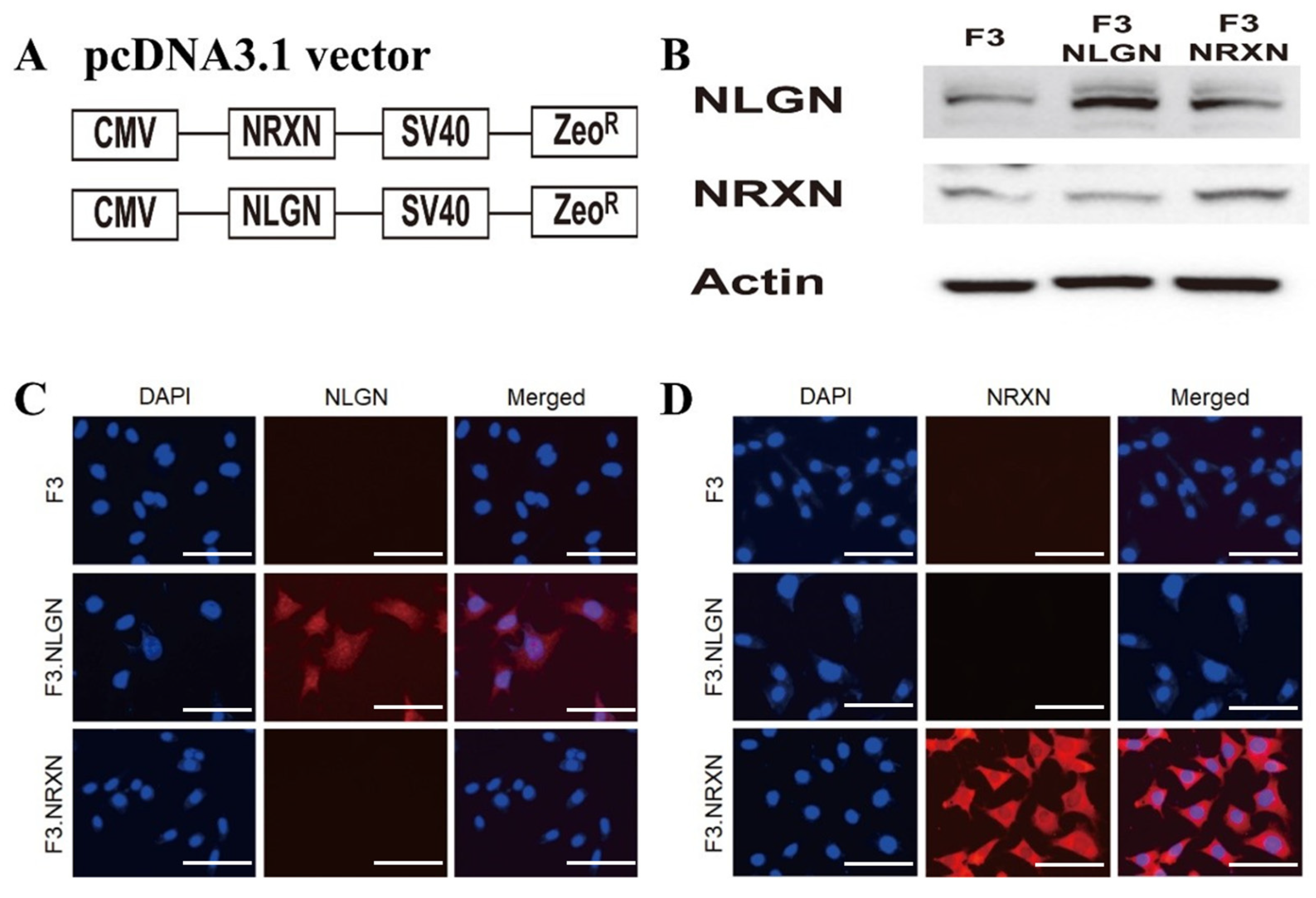
The construction of the pcDNA3.1_NLGN and NRXN vectors is shown in Figure 1A. Western blotting analysis confirmed the overexpression of NLGN and NRXN proteins in F3 human NSCs (Figure 1B). Immunocytochemistry also demonstrated strong expression of NLGN and NRXN proteins in F3.NLGN and F3.NRXN cells, respectively (Figure 1C,D).
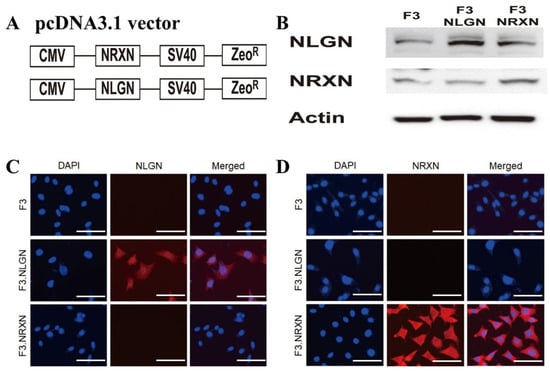
Figure 1.
Construction of F3.NLGN and F3.NRXN cells. (A) F3.NLGN and F3.NRXN cells were constructed via the transfection of F3 human neural stem cells with a plasmid vector encoding either human NLGNs or NRXNs. (B–D) Expression of NLGNs and NRXNs in F3.NLGN and F3.NRXN cells assessed via (B) Western blotting and (C,D) immunohistochemistry. Scale bar = 30 μm
2.2. Expression of Synaptic Markers in F3.NLGN and F3.NRXN Cells
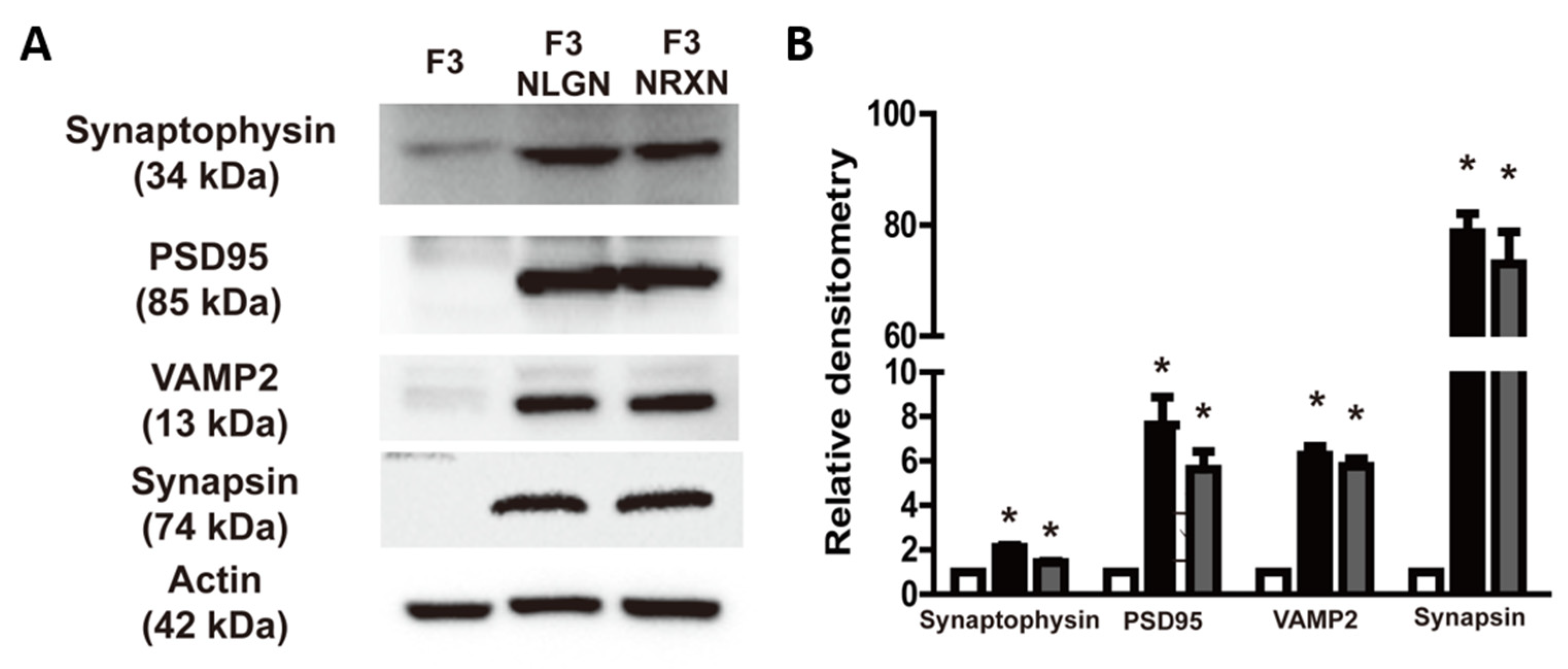
The expression of synaptic markers such as synaptophysin, PSD95, VAMP2, and synapsin was analyzed to confirm whether they were affected by the overexpression of NLGNs and NRXNs. As shown in Figure 2, the expression of these markers was enhanced in F3.NLGN and F3.NRXN cells compared with F3 cells. Interestingly, there was no significant difference between F3.NLGN and F3.NRXN cells.
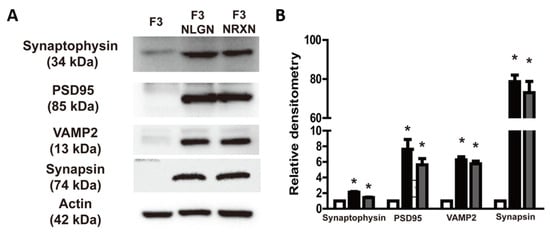
Figure 2.
Expression of synaptic markers (synaptophysin, PSD95, VAMP2, and synapsin) in F3.NLGN and F3.NRXN cells. (A) Expression of synaptic markers assessed by means of Western blotting. (B) Band densities normalized to actin. Densitometric analysis of the Western blot was performed using ImageJ 1.54g. n = 3 per treatment group. * Significantly different from F3 cells (p < 0.05).
2.3. Effects of F3.NLGN and F3.NRXN Cell Transplantation on Locomotor Function in SCI Mice
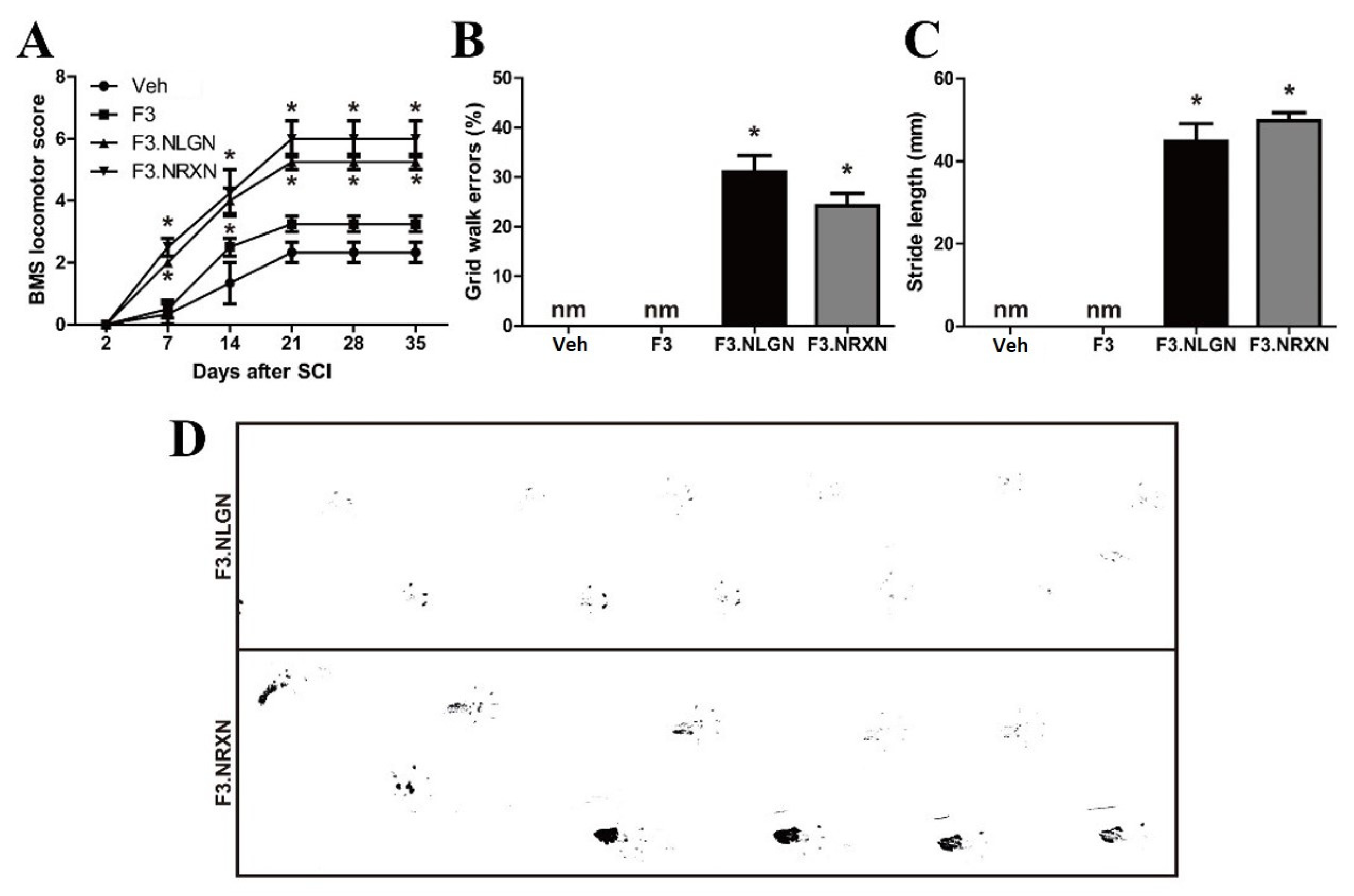
We evaluated functional recovery in SCI mice by measuring the standardized BMS locomotor scores, grid walk, and footprints of the hind paw. Two days after complete transection, mice showed no observable or slight extensive hindlimb movement (BMS: 0–1), and there were no differences among the four groups (Figure 3A). The recovery reached a stable level approximately 3 weeks after lesioning, and mice generally had some coordination and hind paw rotation when making initial contact with the testing surface and on lift-off. Interestingly, the F3.NLGN and F3.NRXN groups showed a significant increase in BMS scores compared to the vehicle group. However, in mice transplanted with F3 cells, the BMS score increased due to stem cell transplantation compared to the vehicle group, but not significantly. We also performed a grid-walking test by evaluating the incidence of the hindlimbs slipping below the grid plane at 5 weeks after SCI. The F3.NLGN and F3.NRXN groups could walk after stem cell transplantation on the grid, and the percentage of missteps was less than 40% (Figure 3B). However, the vehicle and F3 groups could not walk properly and dragged their hind legs, making it impossible to measure the grid walk error. On the same day, the stride length was measured by inking the feet of mice (Figure 3C,D), recording the gait, and measuring the distance between the hindlimb footprints.
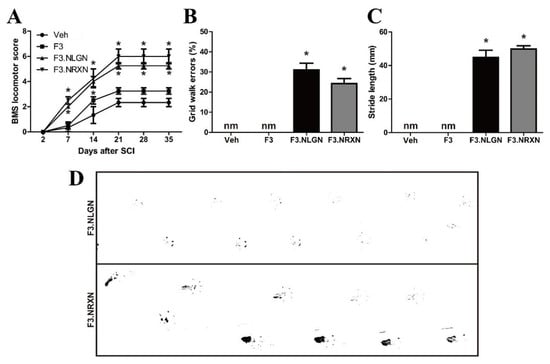
Figure 3.
Improvement in locomotor recovery after F3.NLGN and F3.NRXN transplantation in SCI mice. (A) Locomotor BMS scores following T7 complete transection SCI. (B) Grid walk errors in mice 5 weeks after SCI. (C) Stride length of the hindlimbs at 5 weeks after injury in F3.NLGN and F3.NRXN groups. This value could not be measured in the vehicle and F3 groups because they crawled. (D) Representative footprints of the hindlimbs in the F3.NLGN and F3.NRXN groups. Data are expressed as mean ± standard deviation. n = 10. * Significantly different from the vehicle control (p < 0.05). nm, not measured; BMS, Basso Motor Scale; SCI, spinal cord injury.
Similarly, the stride length could not be measured in the vehicle and F3 groups, and was ≥40 mm in the F3.NLGN and F3.NRXN groups, showing a significant difference.
2.4. Effects of F3.NLGN and F3.NRXN Cell Transplantation on Synaptic Regeneration in SCI Mice
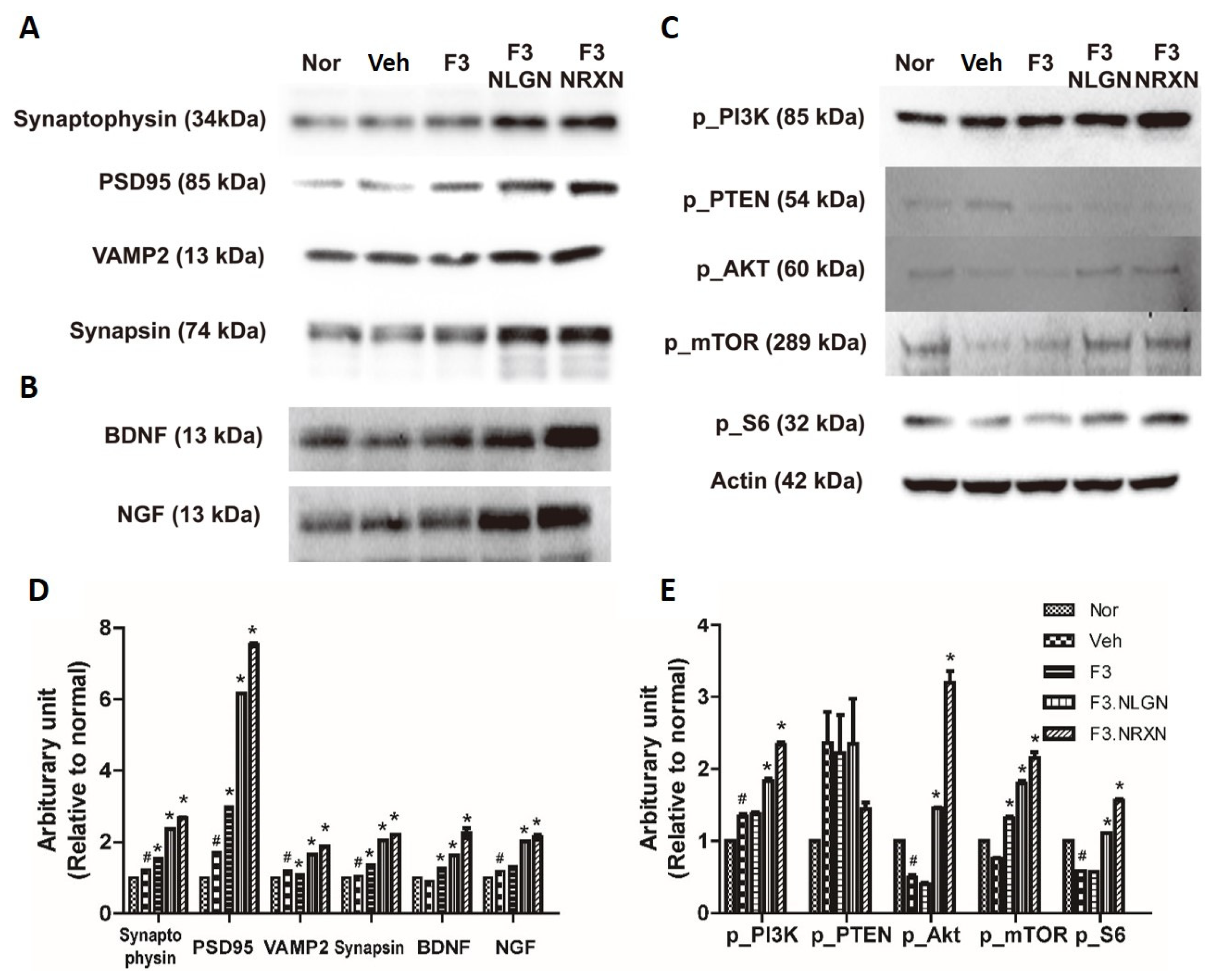
Since the F3.NLGN and F3.NRXN groups had improved locomotor function compared to the F3 and vehicle groups, we assumed that transplantation of F3.NLGN and F3.NRXN cells increased synaptic regeneration and cell proliferation after SCI. We tested this hypothesis by measuring spinal cord synaptic markers and PTEN-related signaling proteins. As shown in Figure 4A, all synapse regeneration markers significantly increased in the F3.NLGN and F3.NRXN groups compared to the vehicle group. In addition, the transplantation of F3.NLGN and F3.NRXN cells significantly increased the expression of BDNF and NGF. Likewise, transplantation of F3.NLGN and F3.NRXN enhanced the levels of p_PI3K, p_AKT, p_mTOR, and p_S6 in the spinal cord, but reduced that of p_PTEN.
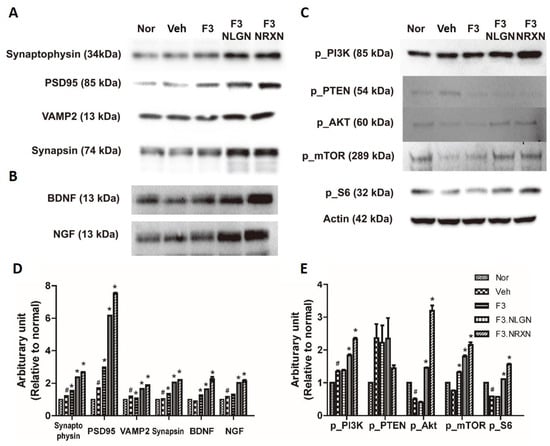
Figure 4.
Protein expression after F3.NLGN and F3.NRXN transplantation at the lesion site (2 mm rostral and caudal to injury center) in SCI mice. (A) Expression of synaptic markers. (B) Expression of BDNF and NGF. (C) Expression of the PI3K/PTEN/mTOR signaling pathway after F3.NLGN and F3.NRXN transplantation. Protein expression is analyzed by means of Western blotting. (D,E) Band densities normalized to actin. Densitometric analysis of the Western blot was performed using ImageJ. n = 10 per treatment group. # Significantly different from the normal control (p < 0.05). * Significantly different from the vehicle control (p < 0.05). SCI, spinal cord injury; BDNF, brain-derived neurotrophic factor; NGF, nerve growth factor.
2.5. Effects of F3.NLGN and F3.NRXN Cell Transplantation on VAMP2 and Neurofilament Expression in SCI Mice
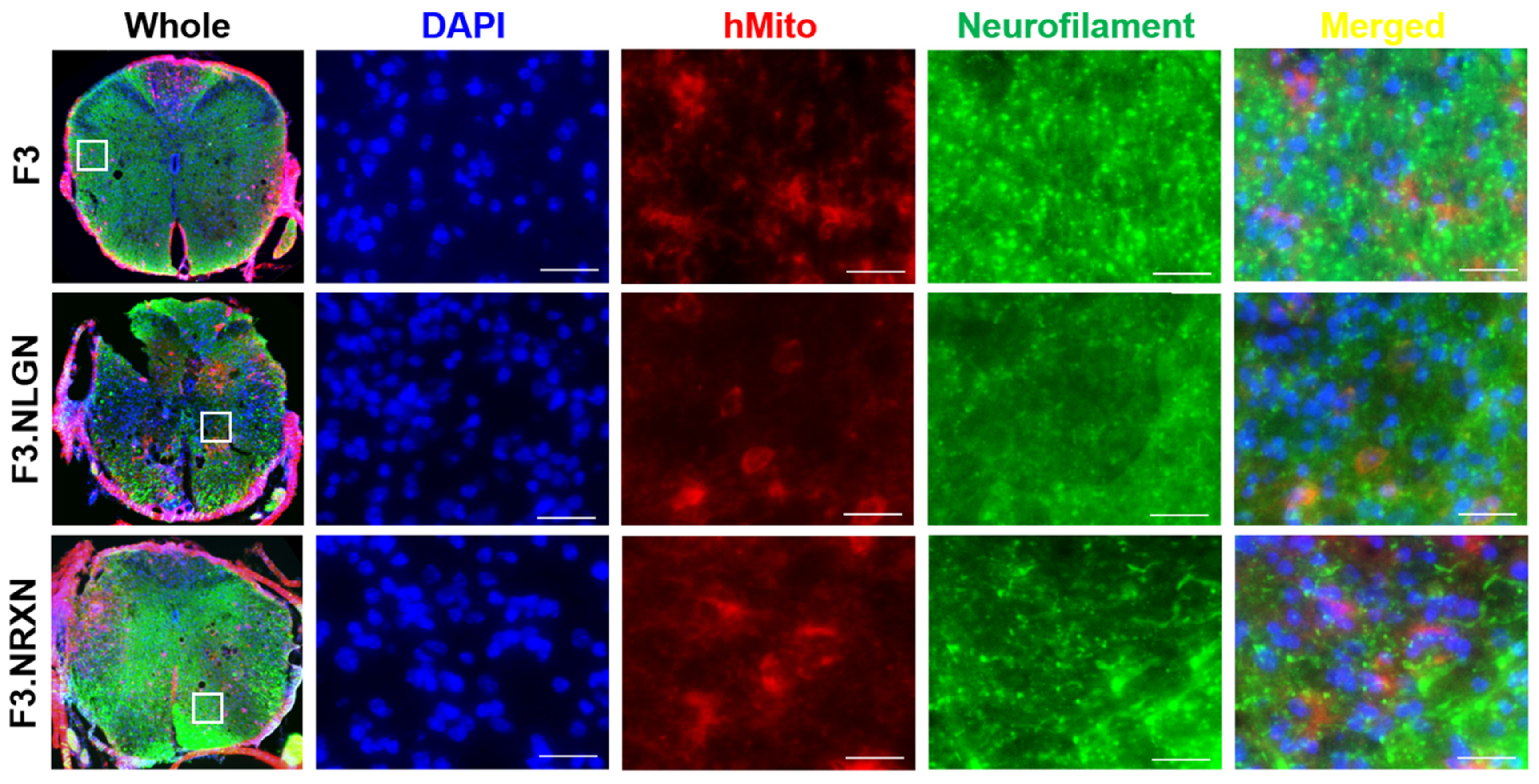
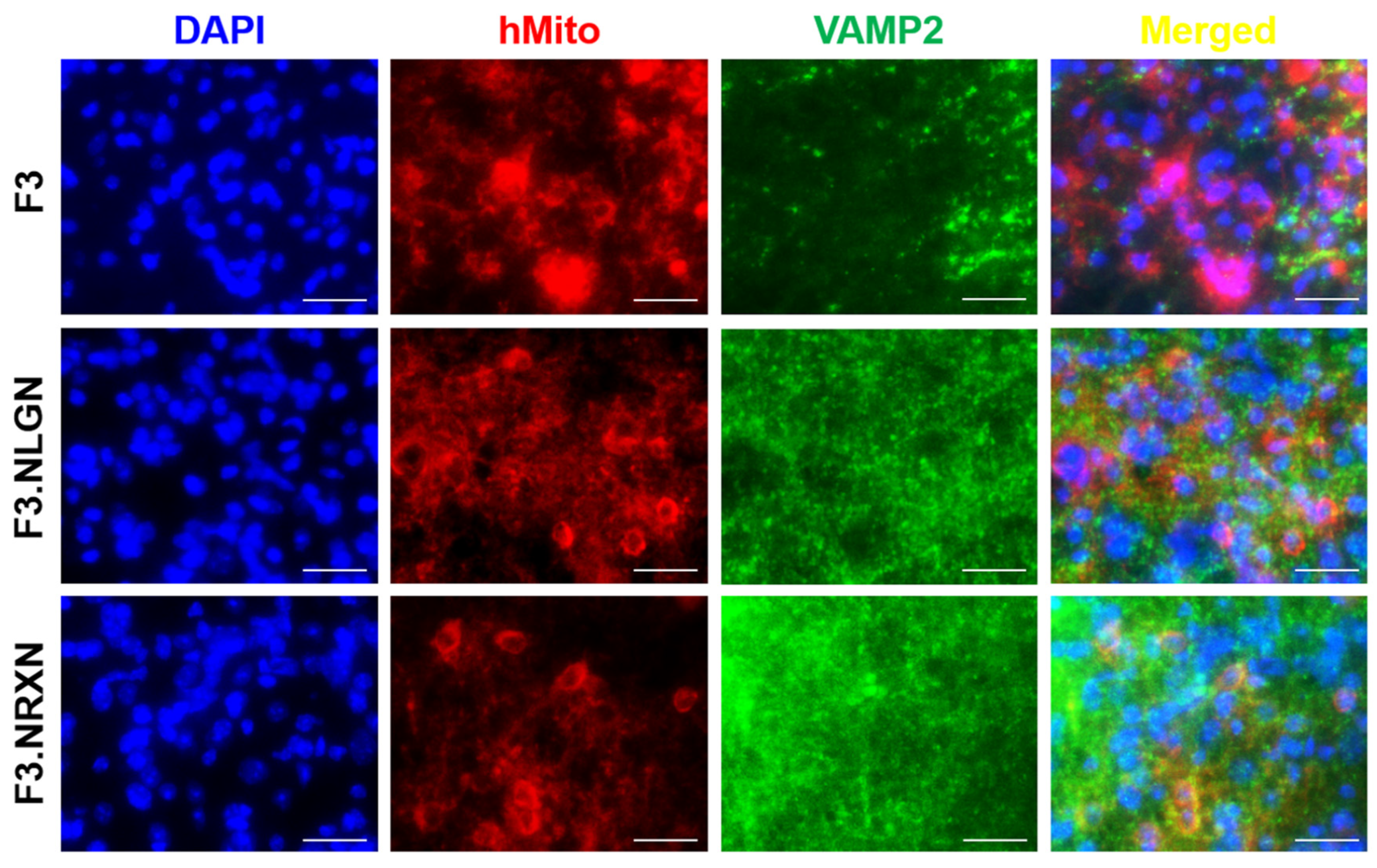
After double immunostaining, F3.NLGN and F3.NRXN cells were checked at the injury site. As shown in Figure 5, transplanted F3, F3.NLGN, and F3.NRXN cells were observed in the injury site and had differentiated into neurons according to immunostaining results for neurofilament. F3.NLGN and F3.NRXN cells were positive for VAMP2 (Figure 6). Interestingly, F3 cells were also observed in the injury site but were negative for VAMP2.
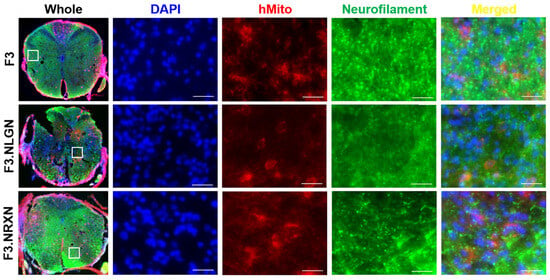
Figure 5.
Differentiation of transplanted F3.NLGN and F3.NRXN cells into neurons in SCI mice. hMito (red color) was used as a stem cell marker. Neurofilament (green color) was used as a neuronal marker. DAPI (blue color) was used as a counterstain for the nucleus. Scale bar = 50 μm. SCI, spinal cord injury.
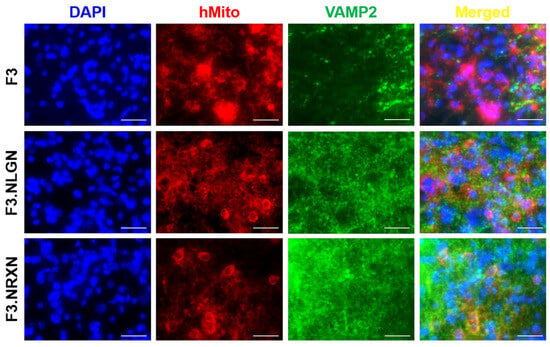
Figure 6.
Expression of VAMP2 in transplanted F3.NLGN and F3.NRXN cells in SCI mice. hMito (red color) was used as a stem cell marker. VAMP2 (green color) was used as a synaptic marker. DAPI (blue color) was used as a counterstain for the nucleus. Scale bar = 50 μm. SCI, spinal cord injury.
3. Discussion
The utility of stem cells in the treatment and recovery after an SCI has been widely researched [4,5]. Stem cells are attracting attention as prospective treatment options for some disorders, including SCI. Stem cells can replace damaged cells and can differentiate on their own [8]. Additionally, various growth factors produced by stem cells accelerate tissue regeneration [12,20]. Since neurons have a minimal capacity for regeneration and secrete few neurotrophic substances, it is difficult to repair injured neurons [21,22]. Therefore, the major goals of therapeutic techniques, including techniques involving stem cells, are to replace damaged cells and supply growth factors that assist in recovery. In animal models of Alzheimer’s disease, stroke, and SCI, we have previously shown that the transplantation of hNSCs expressing a variety of functional genes involving growth factors helps to maintain host neurons and recover function [7,23,24].
SCI causes direct damage to axons and neuronal cell bodies [22,25]. As a consequence of the injured neuron’s death, synaptic connections are destroyed, resulting in functional loss below the point of injury [26,27]. Restoration of function by means of synapse reconstruction is expected to be a viable therapeutic approach [28]. In this study, we examined the treatment and recovery of SCI, focusing on synapse reconstruction by transplanting stem cells overexpressing NRXN and NLGN proteins involved in connecting neurons at the synapse. NLGNs and NRXNs are essential for synaptic structure and function in the synaptic cleft [16]. Indeed, their expression is increased during synaptogenesis in the cerebellum [29]. NLGNs expressed in non-neuronal cells trigger presynaptic development in contacting axons [30], and regulating their expression influences synaptic markers such as VAMP2, PSD95, and synaptophysin [31,32]. Similarly, this study confirmed that overexpression of NLGNs or NRXNs increased synaptophysin, PSD95, VAMP2, and synapsin levels in NSCs.
In addition, it is well known that SCI causes synaptic changes in the neuronal circuitry within the spinal cord at higher levels over the weeks and months following injury [2,22,33]. As shown in the results, levels of synaptic markers were decreased after SCI, and the mice lost locomotor function. Transplantation of F3 cells increased the BMS score; however, normal gait was impossible in these mice, and they resorted to dragging their hind legs. In comparison, transplantation of F3.NLGN and F3.NRXN cells increased in the BMS, and the gait of these animals improved compared with the SCI animal model. Regarding the immunohistochemistry for neurofilament and VAMP2, the transplanted F3 cells were positive for neurofilament but not VAMP2; in contrast, F3.NLGN and F3.NRXN cells were positive for both neurofilament and VAMP2. After the transplanted NSCs were replaced and differentiated into neurons [34], NSCs overexpressing proteins related to synaptic formation formed synapses with host neurons. Stem cells may be able to differentiate and replace damaged cells after transplantation. However, if they do not form a circuit with host cells, their effect may be insignificant; in contrast, if they form a circuit with host cells like F3.NLGN or F3.NRXN, their function may be significantly restored. In addition to forming circuits with host cells, neurotrophic factors, including NGF and BDNF, are known to play an important role in promoting functional recovery after SCI through either neural protection or neuron regeneration [35,36]. Previous studies have reported that the spontaneous provision of neurotrophic factors through stem cell transplantation can improve recovery from SCI [37]. In this study, NSCs were transplanted in mice to help them recover from SCI. This led to an increase in the expression of neurotrophic factors compared to the vehicle group, resulting in more activation of signaling pathways for cell proliferation. The transplanted F3.NLGN or F3.NRXN cells secreted more BDNF and NGF than F3 cells, and the signaling pathway was activated significantly compared to these cells. Many signaling pathways, including the PI3K/AKT/mTOR pathway, are involved in SCI [38]. As an important intracellular signaling pathway, the PI3K/AKT/mTOR pathway is a good candidate for molecular SCI therapy [39]. The activation of the PI3K/AKT/mTOR pathway is known to regulate cell proliferation, differentiation, and physiological and pathological conditions [40,41]. Many studies have already been conducted targeting this pathway in the treatment of CNS diseases such as stroke [23,42], and the possibility of treatment using its activation in SCI has been suggested [38,39]. Stem cells release BDNF and NGF when transplanted, activating the PI3K/AKT/mTOR signaling pathway and restoring neuronal function.
The mechanism of synaptic remodeling remains unclear owing to the complexity of the nervous system. Interestingly, the degree of recovery, such as locomotor activity and synapse formation, was better in the F3.NRXN group than in the F3.NLGN group. Depending on the characteristics of signal transmission in one direction [43], when neurons are damaged and degenerated due to injury, they do not receive signals below the site of injury, resulting in functional loss [44,45,46]. Although the potential of synaptogenesis is maintained caudal to the lesion [33,47], it requires adequate presynaptic input. Accordingly, recovery of damage caused by SCI is thought to occur more quickly when proteins involved in synapse formation are expressed in presynaptic neurons.
This study investigated the transplantation of F3.NLGN and F3.NRXN cells in SCI mice, which positively impacted motor function. In these mice, the PI3K/AKT/mTOR signaling pathway was activated by BDNF and NGF secreted from transplanted stem cells. NFs promote the differentiation of transplanted stem cells into neurons at the lesion site. The transplantation of stem cells overexpressing functional genes related to synapse formation increased the rate of motor function recovery in SCI mice by promoting synapse formation. This study suggests that hNSC-based therapy using overexpressed NRXN rather than NLGN can effectively restore motor function after SCI, as the degree of recovery was higher in presynaptic neurons.
4. Materials and Methods
4.1. Construction of F3, F3.NLGN, and F3.NRXN Cells
For the construction of F3.NLGN and F3.NRXN cells, we used HB1.F3 (F3), which is an immortalized human NSC line previously established from primary cultures of a 15-week gestational human fetal brain via infection with a retroviral vector encoding the v-myc oncogene [23]. The full length of NLGN (GQ489206.1) and NRXN (AB035356.1) cDNA was ligated into multiple cloning sites of the pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA) to generate F3 overexpressing NLGNs and NRXNs. The recombinant plasmids were transformed into DH5α bacteria (Real Biotech Corporation, Banqiao City, Taiwan) and cultured in Luria–Bertani (LB) broth containing 50 μg/mL ampicillin at 37 °C. After inoculation with a single colony in LB medium, plasmid DNA was extracted with a midiprep kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. To achieve stable overexpression of NLGNs and NRXNs, F3 cells were plated in 6-well plates before transfection with recombinant plasmid DNA using Lipofectamine 2000 (Invitrogen). Stably transfected colonies were selected using the zeocin (2.0 μg/mL) resistance method (Invitrogen). Western blotting and immunohistochemistry confirmed NLGN and NRXN protein overexpression in each cell.
4.2. Cell Culture
F3, F3.NLGN, and F3.NRXN cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Biowest, Nuaillé, Cholet, France) containing antibiotics (100 IU/mL penicillin and 100 μg/mL streptomycin) and 10% heat-inactivated fetal bovine serum (Biowest) at 37 °C in a 5% CO2/95% air atmosphere. In all experiments, cells were grown until more than 90% confluence was achieved and were subjected to no more than 20 passages.
4.3. Animal Model and NSC Transplantation
Eight-week-old male C57BL mice (n = 10/group) were purchased from Daehan Biolink (Eumseong, Republic of Korea). Mice were housed in an environmentally controlled room with a constant temperature (23 ± 3 °C) and relative humidity (50% ± 10%) and a 12 h light/dark cycle and were fed standard rodent chow and purified water ad libitum. After anesthetizing mice with ketamine and rumpun, we approached them dorsally and damaged the T7 area completely using microscissors. After surgery, ampicillin (0.1 mg/kg) and tramadol (0.3 mL/kg) in phosphate-buffered saline (PBS) were injected subcutaneously twice daily in the morning and evening, and urination was performed. Two days after the SCI, F3, F3.NLGN, and F3.NRXN cells were dissolved in an appropriate volume of saline (1 × 106 cells/mouse), intrathecal injection was administered in each mouse. All experimental procedures were approved and carried out according to the Institutional Animal Care and Use Committee of the Korea National University of Education (#KNUE-201908-002-04).
4.4. Behavioral Test
The Basso Mouse Scale (BMS) score was measured to evaluate hindlimb motor function [48]. BMS measurements were performed 2, 7, 14, 21, 28, and 35 d after surgery. The BMS scores were evaluated while mice walked in an open field and re-evaluated from digital video documents. Grid walk performance and footprint analyses were carried out 35 d after SCI. The grid walk errors were counted from videotapes played slowly (4 separate trials per test) and averaged across different trials. The stride length on each side and stride width between the two sides of the prints were measured and calculated using multiple steps. For footprint analysis, walking patterns of mouse hind paws were recorded with ink during continuous locomotion across a 50 cm runway.
4.5. Western Blot Analysis
Western blotting was performed to analyze protein expression after F3.NLGN and F3.NRXN transplantation. Thirty-five days after SCI, mice were perfused through the heart with cold PBS, and fresh spinal cord tissue containing the injury site (2 mm rostral to and 2 mm caudal to the injury site) was obtained. Collected tissue samples were homogenized in radioimmunoprecipitation cell lysis buffer (Thermo Fisher Scientific, Waltham, MA, USA) with protease inhibitor (Sigma-Aldrich, St. Louis, MO, USA) and phosphatase inhibitors (Sigma-Aldrich). After centrifugation at 15,000 rpm at 4 °C for 15 min, total protein in the supernatant was obtained and quantified using the BCA protein assay kit (Pierce, Rockford, IL, USA). Samples containing the same amount of protein were prepared for Western blots using antibodies against brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), synaptic markers, proliferation markers, and actin. As synaptic markers, antibodies specific for synaptophysin, PSD95, VAMP2, and synapsin were used. Phosphorylated PI3K (p_PI3K), PTEN (p_PTEN), Akt (p_Akt), mTOR (p_mTOR), and S6 (p_S6) were identified as proliferation markers. The list of antibodies is shown in Supplementary Table S1.
4.6. Immunohistochemistry
The mouse spinal cord (n = 2/group), including the NSC transplantation site, was fixed in 4% paraformaldehyde for 24 h, followed by cryoprotection in 30% sucrose–PBS solution for 72 h. The spinal cord was cut into 30 µm slices and immunostained for human mitochondria (hMito; for human cells) as a stem cell marker. VAMP2 was immunostained as a synaptic marker to confirm synaptic formation. For immunohistochemical staining of hMito and VAMP2, spinal cord cryosections were washed in PBS, including 0.01% Tween 20 (PBS-T), and treated with 3% hydrogen peroxide for 10 min to block endoperoxidase activity. After PBS-T washing and blocking with 5% BSA for 30 min, the sections were incubated with primary antibodies overnight at 4 °C, followed by incubation with secondary antibodies conjugated with Alexa Fluor-488 or -594 (1:300, Invitrogen) for 2 h at room temperature. The sections were stained with 4′,6-diamino-2-phenylindole (DAPI, Sigma-Aldrich) to confirm cellular nuclei. All samples were evaluated immediately after staining and photographed using a fluorescence microscope (EVOS FL Auto2 Cell imaging system; Thermo Fisher Scientific).
4.7. Statistical Analysis
Statistical comparisons between the groups were performed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. All analyses were conducted using SPSS for Windows software (version 12.0; IBM, Armonk, NY, USA). Statistical significance was set at p < 0.05. All data are expressed as mean ± standard deviation.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms25168744/s1.
Author Contributions
Conceptualization, E.-J.Y. and D.P.; animal experiments, J.J., Y.C. and N.K.; formal analysis, J.J., Y.C. and H.L.; establishment of cells, J.J., E.-J.Y. and D.P.; writing—original draft preparation, J.J. and Y.C.; writing—review and editing, E.-J.Y. and D.P.; visualization, J.J. and Y.C.; supervision, D.P.; project administration, D.P.; funding acquisition, D.P. All authors have read and agreed to the published version of the manuscript.
Funding
The Basic Science Research Program supported this research through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2018R1D1A3B07043733).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of the Korea National University of Education (#KNUE-201908-002-04).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article or the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jackson, A.B.; Dijkers, M.; Devivo, M.J.; Poczatek, R.B. A demographic profile of new traumatic spinal cord injuries: Change and stability over 30 years. Arch. Phys. Med. Rehabil. 2004, 85, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Darian-Smith, C. Synaptic plasticity, neurogenesis, and functional recovery after spinal cord injury. Neuroscientist 2009, 15, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Kim, H.J.; Ehsanipour, A.; Bierman, R.D.; Kaarela, O.; Xue, C.; Khademhosseini, A.; Seidlits, S.K. Regenerative Therapies for Spinal Cord Injury. Tissue Eng. Part. B Rev. 2019, 25, 471–491. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhu, J.; Yang, R.; Wang, H.; Li, Y.; Fu, C. Mesenchymal stem cells in the treatment of spinal cord injury: Mechanisms, current advances and future challenges. Front. Immunol. 2023, 14, 1141601. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Wang, M.; Zhang, B.; Wang, X.; Wanyan, P. Clinical translation of stem cell therapy for spinal cord injury still premature: Results from a single-arm meta-analysis based on 62 clinical trials. BMC Med. 2022, 20, 284. [Google Scholar] [CrossRef] [PubMed]
- Adugna, D.G.; Aragie, H.; Kibret, A.A.; Belay, D.G. Therapeutic Application of Stem Cells in the Repair of Traumatic Brain Injury. Stem Cells Cloning 2022, 15, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Choi, E.K.; Cho, T.H.; Joo, S.S.; Kim, Y.B. Human Neural Stem Cells Encoding ChAT Gene Restore Cognitive Function via Acetylcholine Synthesis, Abeta Elimination, and Neuroregeneration in APPswe/PS1dE9 Mice. Int. J. Mol. Sci. 2020, 21, 3958. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.Z. Mechanisms that mediate stem cell self-renewal and differentiation. J. Cell Biochem. 2008, 103, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.W. Advancing Spinal Cord Injury Treatment through Stem Cell Therapy: A Comprehensive Review of Cell Types, Challenges, and Emerging Technologies in Regenerative Medicine. Int. J. Mol. Sci. 2023, 24, 14349. [Google Scholar] [CrossRef]
- Fischer, I.; Dulin, J.N.; Lane, M.A. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat. Rev. Neurosci. 2020, 21, 366–383. [Google Scholar] [CrossRef]
- Mahar, M.; Cavalli, V. Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci. 2018, 19, 323–337. [Google Scholar] [CrossRef]
- Yoon, E.J.; Choi, Y.; Park, D. Improvement of Cognitive Function in Ovariectomized Rats by Human Neural Stem Cells Overexpressing Choline Acetyltransferase via Secretion of NGF and BDNF. Int. J. Mol. Sci. 2022, 23, 5560. [Google Scholar] [CrossRef] [PubMed]
- Caire, M.J.; Reddy, V.; Varacallo, M. Physiology, Synapse. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef]
- Punjani, N.; Deska-Gauthier, D.; Hachem, L.D.; Abramian, M.; Fehlings, M.G. Neuroplasticity and regeneration after spinal cord injury. N. Am. Spine Soc. J. 2023, 15, 100235. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 2008, 455, 903–911. [Google Scholar] [CrossRef]
- Craig, A.M.; Kang, Y. Neurexin-neuroligin signaling in synapse development. Curr. Opin. Neurobiol. 2007, 17, 43–52. [Google Scholar] [CrossRef]
- Dean, C.; Dresbach, T. Neuroligins and neurexins: Linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006, 29, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Martin, J.H. Boosting corticospinal system synaptic plasticity to recover motor functions. Neural Regen. Res. 2023, 18, 2182–2183. [Google Scholar] [CrossRef]
- Nie, W.B.; Zhang, D.; Wang, L.S. Growth Factor Gene-Modified Mesenchymal Stem Cells in Tissue Regeneration. Drug Des. Devel Ther. 2020, 14, 1241–1256. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, P.G.; Chen, H.; Wang, D.Y. Neuroregeneration and plasticity: A review of the physiological mechanisms for achieving functional recovery postinjury. Mil. Med. Res. 2020, 7, 30. [Google Scholar] [CrossRef]
- Hu, X.; Xu, W.; Ren, Y.; Wang, Z.; He, X.; Huang, R.; Ma, B.; Zhao, J.; Zhu, R.; Cheng, L. Spinal cord injury: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.J.; Choi, Y.; Kim, T.M.; Choi, E.K.; Kim, Y.B.; Park, D. The Neuroprotective Effects of Exosomes Derived from TSG101-Overexpressing Human Neural Stem Cells in a Stroke Model. Int. J. Mol. Sci. 2022, 23, 9532. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.H.; Kim, B.G.; Kim, E.J.; Lee, S.I.; Joo, I.S.; Suh-Kim, H.; Sohn, S.; Kim, S.U. Transplantation of human neural stem cells transduced with Olig2 transcription factor improves locomotor recovery and enhances myelination in the white matter of rat spinal cord following contusive injury. BMC Neurosci. 2009, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Zarruk, J.G.; Ghasemlou, N. Inflammatory pathways in spinal cord injury. Int. Rev. Neurobiol. 2012, 106, 127–152. [Google Scholar] [CrossRef] [PubMed]
- Gorman, A.M. Neuronal cell death in neurodegenerative diseases: Recurring themes around protein handling. J. Cell Mol. Med. 2008, 12, 2263–2280. [Google Scholar] [CrossRef] [PubMed]
- Rossano, S.; Toyonaga, T.; Bini, J.; Nabulsi, N.; Ropchan, J.; Cai, Z.; Huang, Y.; Carson, R.E. Feasibility of imaging synaptic density in the human spinal cord using [(11)C]UCB-J PET. EJNMMI Phys. 2022, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Aljovic, A.; Jacobi, A.; Marcantoni, M.; Kagerer, F.; Loy, K.; Kendirli, A.; Brautigam, J.; Fabbio, L.; Van Steenbergen, V.; Plesniar, K.; et al. Synaptogenic gene therapy with FGF22 improves circuit plasticity and functional recovery following spinal cord injury. EMBO Mol. Med. 2023, 15, e16111. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yamagata, A.; Imai, A.; Kim, J.; Izumi, H.; Nakashima, S.; Shiroshima, T.; Maeda, A.; Iwasawa-Okamoto, S.; Azechi, K.; et al. Canonical versus non-canonical transsynaptic signaling of neuroligin 3 tunes development of sociality in mice. Nat. Commun. 2021, 12, 1848. [Google Scholar] [CrossRef] [PubMed]
- Scheiffele, P.; Fan, J.; Choih, J.; Fetter, R.; Serafini, T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 2000, 101, 657–669. [Google Scholar] [CrossRef]
- Wittenmayer, N.; Korber, C.; Liu, H.; Kremer, T.; Varoqueaux, F.; Chapman, E.R.; Brose, N.; Kuner, T.; Dresbach, T. Postsynaptic Neuroligin1 regulates presynaptic maturation. Proc. Natl. Acad. Sci. USA 2009, 106, 13564–13569. [Google Scholar] [CrossRef]
- Lleo, A.; Nunez-Llaves, R.; Alcolea, D.; Chiva, C.; Balateu-Panos, D.; Colom-Cadena, M.; Gomez-Giro, G.; Munoz, L.; Querol-Vilaseca, M.; Pegueroles, J.; et al. Changes in Synaptic Proteins Precede Neurodegeneration Markers in Preclinical Alzheimer’s Disease Cerebrospinal Fluid. Mol. Cell Proteomics 2019, 18, 546–560. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Kubota, K.; Kobayakawa, K.; Saito, T.; Hara, M.; Kijima, K.; Maeda, T.; Katoh, H.; Ohkawa, Y.; Nakashima, Y.; et al. Pathological changes of distal motor neurons after complete spinal cord injury. Mol. Brain 2019, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Tropel, P.; Platet, N.; Platel, J.C.; Noel, D.; Albrieux, M.; Benabid, A.L.; Berger, F. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells 2006, 24, 2868–2876. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gu, S.; Gan, J.; Tian, Y.; Zhang, F.; Zhao, H.; Lei, D. Neural Stem Cells Overexpressing Nerve Growth Factor Improve Functional Recovery in Rats Following Spinal Cord Injury via Modulating Microenvironment and Enhancing Endogenous Neurogenesis. Front. Cell Neurosci. 2021, 15, 773375. [Google Scholar] [CrossRef] [PubMed]
- Garraway, S.M.; Huie, J.R. Spinal Plasticity and Behavior: BDNF-Induced Neuromodulation in Uninjured and Injured Spinal Cord. Neural Plast. 2016, 2016, 9857201. [Google Scholar] [CrossRef] [PubMed]
- Deznabi, N.; Hosseini, S.; Rajabi, M. Neurotrophic factors-based therapeutic strategies in the spinal cord injury: An overview of recent preclinical studies in rodent models. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 63. [Google Scholar] [CrossRef]
- Ohtake, Y.; Park, D.; Abdul-Muneer, P.M.; Li, H.; Xu, B.; Sharma, K.; Smith, G.M.; Selzer, M.E.; Li, S. The effect of systemic PTEN antagonist peptides on axon growth and functional recovery after spinal cord injury. Biomaterials 2014, 35, 4610–4626. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.L.; Yin, W.C.; Zhong, Y.C.; Luo, J.Q.; Liu, L.L.; Liu, W.Y.; Zhao, K. The role of PI3K/Akt signalling pathway in spinal cord injury. Biomed. Pharmacother. 2022, 156, 113881. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Na, L.; Li, Y.; Chen, L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020, 10, 54. [Google Scholar] [CrossRef]
- Roy, T.; Boateng, S.T.; Uddin, M.B.; Banang-Mbeumi, S.; Yadav, R.K.; Bock, C.R.; Folahan, J.T.; Siwe-Noundou, X.; Walker, A.L.; King, J.A.; et al. The PI3K-Akt-mTOR and Associated Signaling Pathways as Molecular Drivers of Immune-Mediated Inflammatory Skin Diseases: Update on Therapeutic Strategy Using Natural and Synthetic Compounds. Cells 2023, 12, 1671. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, Q.; Li, Y.; Li, R.; Feng, J.; Chen, W.; Ahmed, W.; Soufiany, I.; Huang, S.; Long, J.; et al. The PI3K/AKT Pathway-The Potential Key Mechanisms of Traditional Chinese Medicine for Stroke. Front. Med. 2022, 9, 900809. [Google Scholar] [CrossRef] [PubMed]
- Hormuzdi, S.G.; Filippov, M.A.; Mitropoulou, G.; Monyer, H.; Bruzzone, R. Electrical synapses: A dynamic signaling system that shapes the activity of neuronal networks. Biochim. Biophys. Acta 2004, 1662, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.J.; Rotterman, T.M.; Akhter, E.T.; Lane, A.R.; English, A.W.; Cope, T.C. Synaptic Plasticity on Motoneurons After Axotomy: A Necessary Change in Paradigm. Front. Mol. Neurosci. 2020, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.S.; Coleman, M.P.; Menon, D.K. Traumatic Axonal Injury: Mechanisms and Translational Opportunities. Trends Neurosci. 2016, 39, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Menorca, R.M.; Fussell, T.S.; Elfar, J.C. Nerve physiology: Mechanisms of injury and recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Nagendran, T.; Larsen, R.S.; Bigler, R.L.; Frost, S.B.; Philpot, B.D.; Nudo, R.J.; Taylor, A.M. Distal axotomy enhances retrograde presynaptic excitability onto injured pyramidal neurons via trans-synaptic signaling. Nat. Commun. 2017, 8, 625. [Google Scholar] [CrossRef]
- Basso, D.M.; Fisher, L.C.; Anderson, A.J.; Jakeman, L.B.; McTigue, D.M.; Popovich, P.G. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 2006, 23, 635–659. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).