Investigating the Regulation of Ribosomal Protein S6 Kinase 1 by CoAlation
Abstract
1. Introduction
2. Results
2.1. p70S6K1 Is CoAlated at Cys217 in HEK293/Pank1β Cells Treated with Diamide
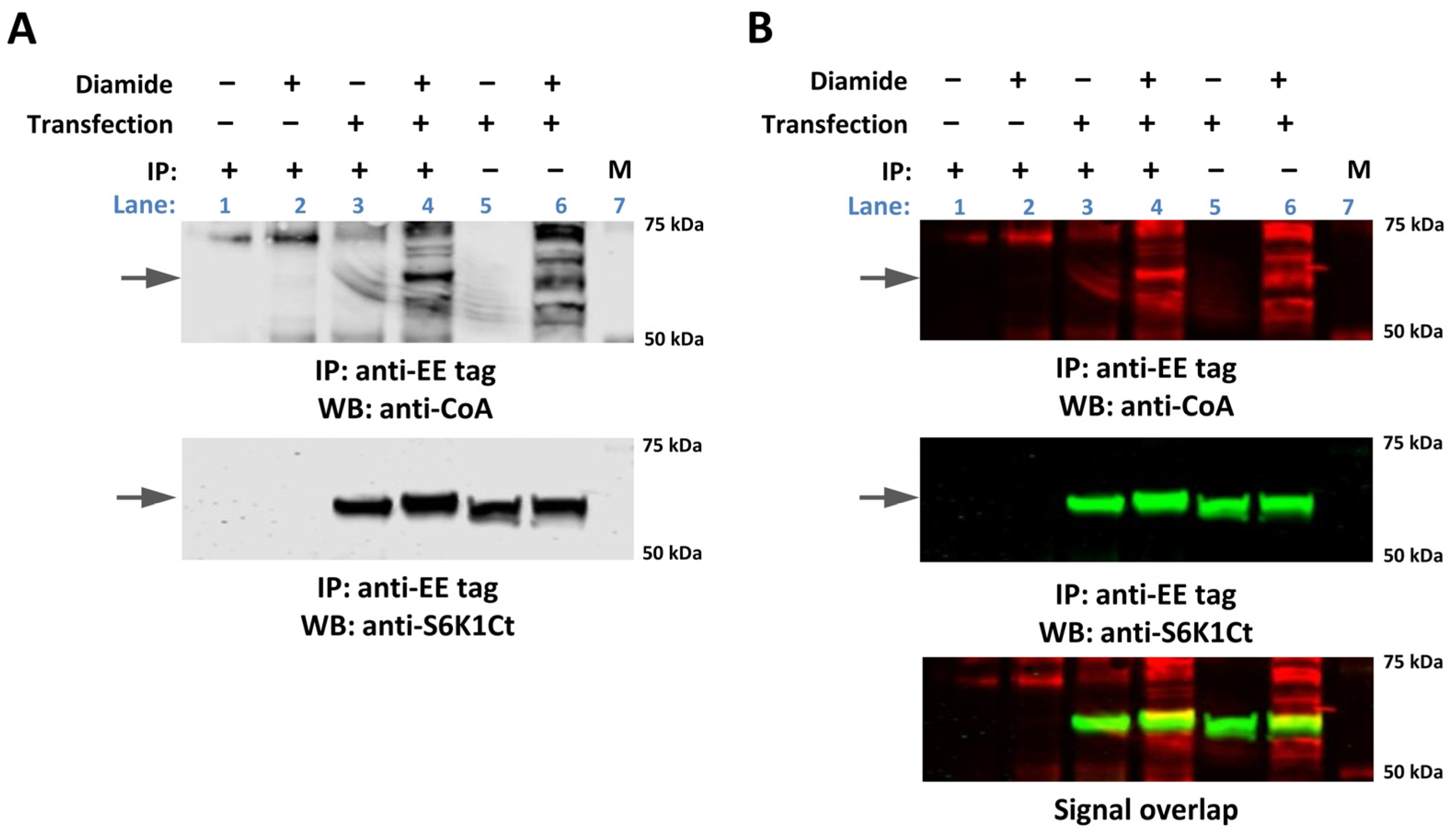
2.2. Transiently Overexpressed EE-p70S6K1 Is CoAlated in HEK293/Pank1β Cells Exposed to Oxidative Stress
2.3. In Vitro CoAlation of Recombinant p70S6K1
2.4. In Vitro Kinase Activity of CoAlated Recombinant p70S6K1
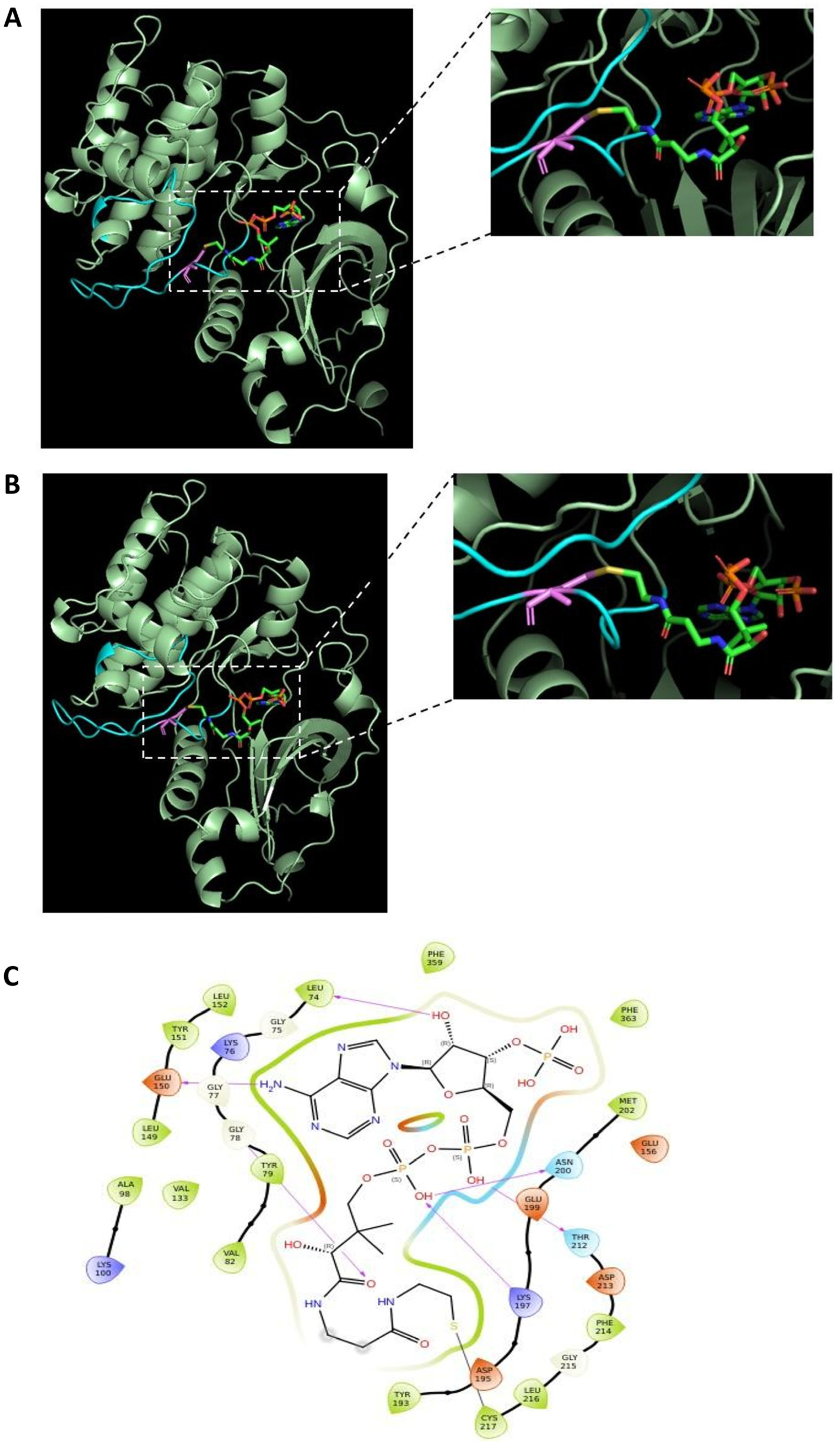
2.5. Molecular Docking of CoA in the Crystal Structure of p70S6K1
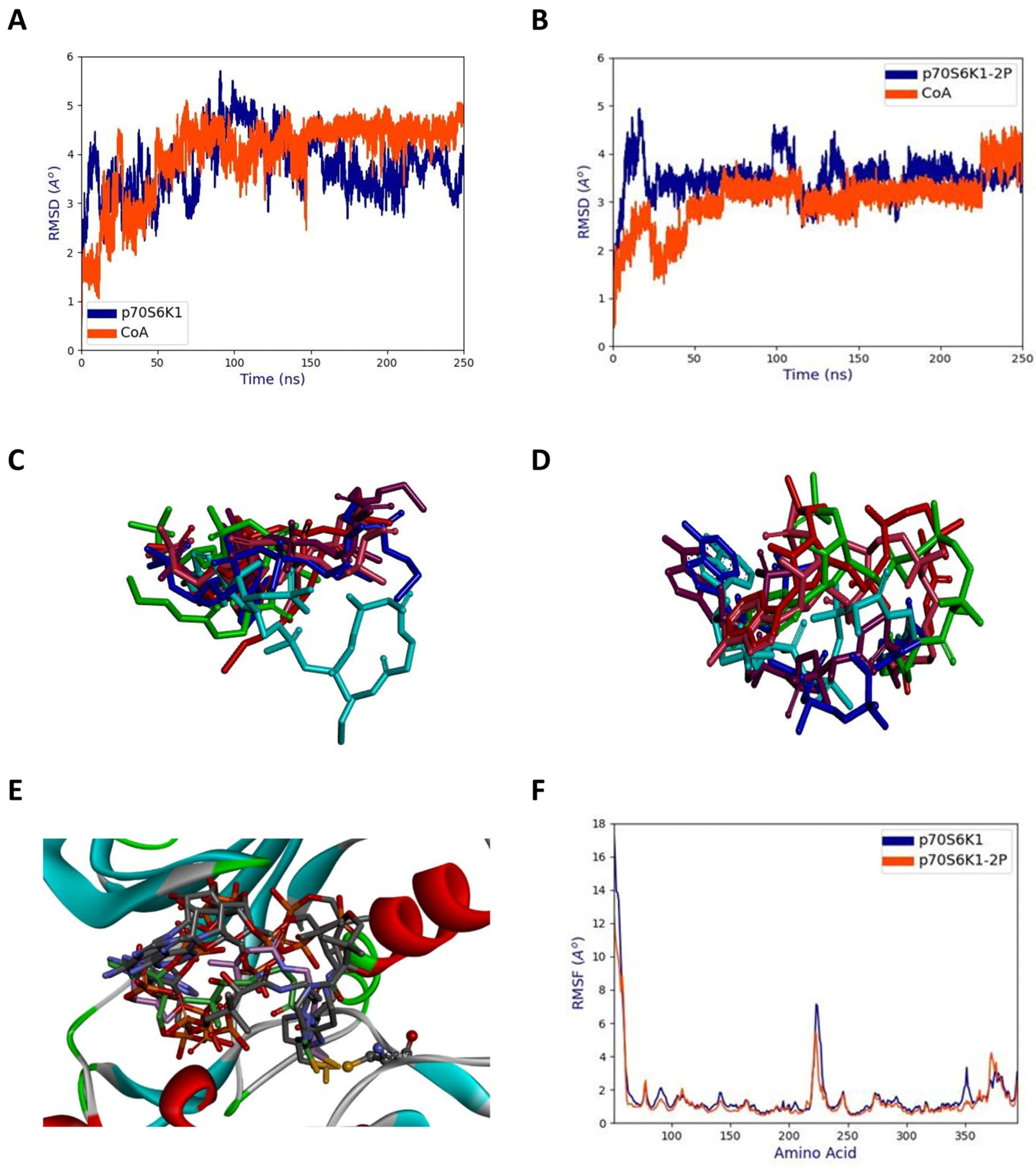
2.6. Molecular Dynamics Simulations of the Complex
3. Discussion and Conclusions
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Mammalian Cell Culture
4.3. Mass Spectrometry and Data Processing
4.4. Transient Transfection and Immunoprecipitation
4.5. Western Blot Analysis
4.6. Insect Cell Culture
4.7. Sf9 Cells Infection and Expression of Recombinant His-actp70S6K1
4.8. Purification of the Constitutively Active Recombinant His-actS6K1 Protein
4.9. In Vitro CoAlation Assay
4.10. In Vitro Kinase Assay
4.11. Molecular Docking
4.12. Molecular Dynamics Simulations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonni, A.; Brunet, A.; West, A.E.; Datta, S.R.; Takasu, M.A.; Greenberg, M.E. Cell Survival Promoted by the Ras-MAPK Signaling Pathway by Transcription-Dependent and -Independent Mechanisms. Science 1999, 286, 1358–1362. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, B.; Ekim, B.; Fingar, D.C. Regulation and Function of Ribosomal Protein S6 Kinase (S6K) within mTOR Signalling Networks. Biochem. J. 2012, 441, 1–21. [Google Scholar] [CrossRef]
- Shima, H. Disruption of the P70s6k/P85s6k Gene Reveals a Small Mouse Phenotype and a New Functional S6 Kinase. EMBO J. 1998, 17, 6649–6659. [Google Scholar] [CrossRef] [PubMed]
- Lee-Fruman, K.K.; Kuo, C.J.; Lippincott, J.; Terada, N.; Blenis, J. Characterization of S6K2, a Novel Kinase Homologous to S6K1. Oncogene 1999, 18, 5108–5114. [Google Scholar] [CrossRef]
- Koh, H.; Jee, K.; Lee, B.; Kim, J.; Kim, D.; Yun, Y.-H.; Kim, J.W.; Choi, H.-S.; Chung, J. Cloning and Characterization of a Nuclear S6 Kinase, S6 Kinase-Related Kinase (SRK); A Novel Nuclear Target of Akt. Oncogene 1999, 18, 5115–5119. [Google Scholar] [CrossRef]
- Saitoh, M.; Ten Dijke, P.; Miyazono, K.; Ichijo, H. Cloning and Characterization of p70S6KβDefines a Novel Family of P70 S6 Kinases. Biochem. Biophys. Res. Commun. 1998, 253, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Zaiets, I.V.; Holiar, V.V.; Sivchenko, A.S.; Smialkovska, V.V.; Filonenko, V.V. P60-S6K1 Represents a Novel Kinase Active Isoform with the Mode of Regulation Distinct from P70/P85-S6K1 Isoforms. Ukr. Biochem. J. 2019, 91, 17–25. [Google Scholar] [CrossRef]
- Rosner, M.; Hengstschläger, M. Nucleocytoplasmic Localization of P70 S6K1, but Not of Its Isoforms P85 and P31, Is Regulated by TSC2/mTOR. Oncogene 2011, 30, 4509–4522. [Google Scholar] [CrossRef]
- Leroux, A.E.; Schulze, J.O.; Biondi, R.M. AGC Kinases, Mechanisms of Regulation and Innovative Drug Development. Semin. Cancer Biol. 2018, 48, 1–17. [Google Scholar] [CrossRef]
- Ruvinsky, I.; Katz, M.; Dreazen, A.; Gielchinsky, Y.; Saada, A.; Freedman, N.; Mishani, E.; Zimmerman, G.; Kasir, J.; Meyuhas, O. Mice Deficient in Ribosomal Protein S6 Phosphorylation Suffer from Muscle Weakness That Reflects a Growth Defect and Energy Deficit. PLoS ONE 2009, 4, e5618. [Google Scholar] [CrossRef] [PubMed]
- Pende, M.; Kozma, S.C.; Jaquet, M.; Oorschot, V.; Burcelin, R.; Le Marchand-Brustel, Y.; Klumperman, J.; Thorens, B.; Thomas, G. Hypoinsulinaemia, Glucose Intolerance and Diminished β-Cell Size in S6K1-Deficient Mice. Nature 2000, 408, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Selman, C.; Tullet, J.M.A.; Wieser, D.; Irvine, E.; Lingard, S.J.; Choudhury, A.I.; Claret, M.; Al-Qassab, H.; Carmignac, D.; Ramadani, F.; et al. Ribosomal Protein S6 Kinase 1 Signaling Regulates Mammalian Life Span. Science 2009, 326, 140–144. [Google Scholar] [CrossRef]
- Wu, X.; Xie, W.; Xie, W.; Wei, W.; Guo, J. Beyond Controlling Cell Size: Functional Analyses of S6K in Tumorigenesis. Cell Death Dis. 2022, 13, 646. [Google Scholar] [CrossRef] [PubMed]
- Ben-Hur, V.; Denichenko, P.; Siegfried, Z.; Maimon, A.; Krainer, A.; Davidson, B.; Karni, R. S6K1 Alternative Splicing Modulates Its Oncogenic Activity and Regulates mTORC1. Cell Rep. 2013, 3, 103–115. [Google Scholar] [CrossRef]
- Artemenko, M.; Zhong, S.S.W.; To, S.K.Y.; Wong, A.S.T. P70 S6 Kinase as a Therapeutic Target in Cancers: More than Just an mTOR Effector. Cancer Lett. 2022, 535, 215593. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, S. Research Progress of 70 kDa Ribosomal Protein S6 Kinase (P70S6K) Inhibitors as Effective Therapeutic Tools for Obesity, Type II Diabetes and Cancer. Curr. Med. Chem. 2020, 27, 4699–4719. [Google Scholar] [CrossRef]
- Leonardi, R.; Zhang, Y.; Rock, C.; Jackowski, S. Coenzyme A: Back in Action. Prog. Lipid Res. 2005, 44, 125–153. [Google Scholar] [CrossRef]
- Barritt, S.A.; DuBois-Coyne, S.E.; Dibble, C.C. Coenzyme A Biosynthesis: Mechanisms of Regulation, Function and Disease. Nat. Metab. 2024, 6, 1008–1023. [Google Scholar] [CrossRef]
- Choudhary, C.; Weinert, B.T.; Nishida, Y.; Verdin, E.; Mann, M. The Growing Landscape of Lysine Acetylation Links Metabolism and Cell Signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 536–550. [Google Scholar] [CrossRef]
- Filonenko, V.; Gout, I. Discovery and Functional Characterisation of Protein CoAlation and the Antioxidant Function of Coenzyme, A. BBA Adv. 2023, 3, 100075. [Google Scholar] [CrossRef] [PubMed]
- Nemazanyy, I.; Panasyuk, G.; Zhyvoloup, A.; Panayotou, G.; Gout, I.T.; Filonenko, V. Specific Interaction between S6K1 and CoA Synthase: A Potential Link between the mTOR/S6K Pathway, CoA Biosynthesis and Energy Metabolism. FEBS Lett. 2004, 578, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Van Laer, K.; Hamilton, C.J.; Messens, J. Low-Molecular-Weight Thiols in Thiol–Disulfide Exchange. Antioxid. Redox Signal. 2013, 18, 1642–1653. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Peak-Chew, S.Y.; Newell, C.; Miller-Aidoo, S.; Mangal, S.; Zhyvoloup, A.; Baković, J.; Malanchuk, O.; Pereira, G.C.; Kotiadis, V.; et al. Protein CoAlation: A Redox-Regulated Protein Modification by Coenzyme A in Mammalian Cells. Biochem. J. 2017, 474, 2489–2508. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Zhyvoloup, A.; Baković, J.; Thomas, N.; Yu, B.Y.K.; Das, S.; Orengo, C.; Newell, C.; Ward, J.; Saladino, G.; et al. Protein CoAlation and Antioxidant Function of Coenzyme A in Prokaryotic Cells. Biochem. J. 2018, 475, 1909–1937. [Google Scholar] [CrossRef] [PubMed]
- Malanchuk, O.M.; Panasyuk, G.G.; Serbin, N.M.; Gout, I.T.; Filonenko, V.V. Generation and Characterization of Monoclonal Antibodies Specific to Coenzyme A. Biopolym. Cell 2015, 31, 187–192. [Google Scholar] [CrossRef]
- Tossounian, M.-A.; Baczynska, M.; Dalton, W.; Newell, C.; Ma, Y.; Das, S.; Semelak, J.A.; Estrin, D.A.; Filonenko, V.; Trujillo, M.; et al. Profiling the Site of Protein CoAlation and Coenzyme A Stabilization Interactions. Antioxidants 2022, 11, 1362. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Byrne, D.P.; Burgess, S.G.; Bormann, J.; Baković, J.; Huang, Y.; Zhyvoloup, A.; Yu, B.Y.K.; Peak-Chew, S.; Tran, T.; et al. Covalent Aurora A Regulation by the Metabolic Integrator Coenzyme A. Redox Biol. 2020, 28, 101318. [Google Scholar] [CrossRef]
- Rock, C.O.; Calder, R.B.; Karim, M.A.; Jackowski, S. Pantothenate Kinase Regulation of the Intracellular Concentration of Coenzyme, A. J. Biol. Chem. 2000, 275, 1377–1383. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, C.; Wang, F.; Qu, F.; Ding, J. Crystal Structures of S6K1 Provide Insights into the Regulation Mechanism of S6K1 by the Hydrophobic Motif. Biochem. J. 2013, 454, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Zhao, B.; Lombard, D.B.; Fingar, D.C.; Inoki, K. Cross-Talk between Sirtuin and Mammalian Target of Rapamycin Complex 1 (mTORC1) Signaling in the Regulation of S6 Kinase 1 (S6K1) Phosphorylation. J. Biol. Chem. 2014, 289, 13132–13141. [Google Scholar] [CrossRef]
- Wang, M.-L.; Panasyuk, G.; Gwalter, J.; Nemazanyy, I.; Fenton, T.; Filonenko, V.; Gout, I. Regulation of Ribosomal Protein S6 Kinases by Ubiquitination. Biochem. Biophys. Res. Commun. 2008, 369, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Gwalter, J.; Wang, M.-L.; Gout, I. The Ubiquitination of Ribosomal S6 Kinases Is Independent from the Mitogen-Induced Phosphorylation/Activation of the Kinase. Int. J. Biochem. Cell Biol. 2009, 41, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Luan, H.H.; Zhang, B.; Zhang, K.; Nam, J.H.; Li, Z.; Fu, M.; Munk, A.; Zhang, D.; et al. OGT Suppresses S6K1-Mediated Macrophage Inflammation and Metabolic Disturbance. Proc. Natl. Acad. Sci. USA 2020, 117, 16616–16625. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Ihara, Y.; Nakamura, H.; Yodoi, J.; Sumikawa, K.; Kondo, T. Glutaredoxin Exerts an Antiapoptotic Effect by Regulating the Redox State of Akt. J. Biol. Chem. 2003, 278, 50226–50233. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Begley, M.; Morgenstern, K.A.; Gu, Y.; Rose, P.; Zhao, H.; Zhu, X. Crystal Structure of an Inactive Akt2 Kinase Domain. Structure 2003, 11, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Beullens, M.; Vancauwenbergh, S.; Morrice, N.; Derua, R.; Ceulemans, H.; Waelkens, E.; Bollen, M. Substrate Specificity and Activity Regulation of Protein Kinase MELK. J. Biol. Chem. 2005, 280, 40003–40011. [Google Scholar] [CrossRef]
- Bendzunas, G.N.; Byrne, D.P.; Shrestha, S.; Daly, L.A.; Oswald, S.O.; Katiyar, S.; Venkat, A.; Yeung, W.; Eyers, C.E.; Eyers, P.A.; et al. Redox Regulation of Brain Selective Kinases BRSK1/2: Implications for Dynamic Control of the Eukaryotic AMPK Family through Cys-Based Mechanisms. bioRxiv 2023. [Google Scholar] [CrossRef]
- Yu, B.Y.K.; Tossounian, M.-A.; Hristov, S.D.; Lawrence, R.; Arora, P.; Tsuchiya, Y.; Peak-Chew, S.Y.; Filonenko, V.; Oxenford, S.; Angell, R.; et al. Regulation of Metastasis Suppressor NME1 by a Key Metabolic Cofactor Coenzyme A. Redox Biol. 2021, 44, 101978. [Google Scholar] [CrossRef]
- Tossounian, M.-A.; Hristov, S.D.; Semelak, J.A.; Yu, B.Y.K.; Baczynska, M.; Zhao, Y.; Estrin, D.A.; Trujillo, M.; Filonenko, V.; Gouge, J.; et al. A Unique Mode of Coenzyme A Binding to the Nucleotide Binding Pocket of Human Metastasis Suppressor NME1. Int. J. Mol. Sci. 2023, 24, 9359. [Google Scholar] [CrossRef] [PubMed]
- Savinska, L.O.; Kijamova, R.G.; Pogrebnoy, P.V.; Ovcharenko, G.V.; Gout, I.T.; Filonenko, V.V. Comparative Characterization of S6 Kinase α and β Isoforms Expression in Mammalian Tissues. Biopolym. Cell 2001, 17, 374–379. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant Enables High Peptide Identification Rates, Individualized p.p.b.-Range Mass Accuracies and Proteome-Wide Protein Quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Bernstein, F.C.; Koetzle, T.F.; Williams, G.J.B.; Meyer, E.F.; Brice, M.D.; Rodgers, J.R.; Kennard, O.; Shimanouchi, T.; Tasumi, M. The Protein Data Bank: A Computer-Based Archival File for Macromolecular Structures. J. Mol. Biol. 1977, 112, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Ben-Shalom, I.Y.; Lin, C.; Kurtzman, T.; Walker, R.C.; Gilson, M.K. Simulating Water Exchange to Buried Binding Sites. J. Chem. Theory Comput. 2019, 15, 2684–2691. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Homeyer, N.; Horn, A.H.C.; Lanig, H.; Sticht, H. AMBER Force-Field Parameters for Phosphorylated Amino Acids in Different Protonation States: Phosphoserine, Phosphothreonine, Phosphotyrosine, and Phosphohistidine. J. Mol. Model. 2006, 12, 281–289. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic Atom Type and Bond Type Perception in Molecular Mechanical Calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef]
- Gordon, J.C.; Myers, J.B.; Folta, T.; Shoja, V.; Heath, L.S.; Onufriev, A. H++: A Server for Estimating pKas and Adding Missing Hydrogens to Macromolecules. Nucleic Acids Res. 2005, 33, W368–W371. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Andersen, H.C. Molecular Dynamics Simulations at Constant Pressure and/or Temperature. J. Chem. Phys. 1980, 72, 2384–2393. [Google Scholar] [CrossRef]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J.C. Numerical Integration of the Cartesian Equations of Motion of a System with Constraints: Molecular Dynamics of n-Alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Cheatham, T.E.I.; Miller, J.L.; Fox, T.; Darden, T.A.; Kollman, P.A. Molecular Dynamics Simulations on Solvated Biomolecular Systems: The Particle Mesh Ewald Method Leads to Stable Trajectories of DNA, RNA, and Proteins. J. Am. Chem. Soc. 1995, 117, 4193–4194. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malanchuk, O.; Bdzhola, A.; Palchevskyi, S.; Bdzhola, V.; Chai, P.; Pardo, O.E.; Seckl, M.J.; Banerjee, A.; Peak-Chew, S.Y.; Skehel, M.; et al. Investigating the Regulation of Ribosomal Protein S6 Kinase 1 by CoAlation. Int. J. Mol. Sci. 2024, 25, 8747. https://doi.org/10.3390/ijms25168747
Malanchuk O, Bdzhola A, Palchevskyi S, Bdzhola V, Chai P, Pardo OE, Seckl MJ, Banerjee A, Peak-Chew SY, Skehel M, et al. Investigating the Regulation of Ribosomal Protein S6 Kinase 1 by CoAlation. International Journal of Molecular Sciences. 2024; 25(16):8747. https://doi.org/10.3390/ijms25168747
Chicago/Turabian StyleMalanchuk, Oksana, Anna Bdzhola, Sergii Palchevskyi, Volodymyr Bdzhola, Peng Chai, Olivier E. Pardo, Michael J. Seckl, Adrija Banerjee, Sew Yeu Peak-Chew, Mark Skehel, and et al. 2024. "Investigating the Regulation of Ribosomal Protein S6 Kinase 1 by CoAlation" International Journal of Molecular Sciences 25, no. 16: 8747. https://doi.org/10.3390/ijms25168747
APA StyleMalanchuk, O., Bdzhola, A., Palchevskyi, S., Bdzhola, V., Chai, P., Pardo, O. E., Seckl, M. J., Banerjee, A., Peak-Chew, S. Y., Skehel, M., Guruprasad, L., Zhyvoloup, A., Gout, I., & Filonenko, V. (2024). Investigating the Regulation of Ribosomal Protein S6 Kinase 1 by CoAlation. International Journal of Molecular Sciences, 25(16), 8747. https://doi.org/10.3390/ijms25168747







