Effects of Ginsenoside Rb1 on the Crosstalk between Intestinal Stem Cells and Microbiota in a Simulated Weightlessness Mouse Model
Abstract
:1. Introduction
2. Results
2.1. Rb1 Enhanced Intestinal Barrier Function in HU Mice
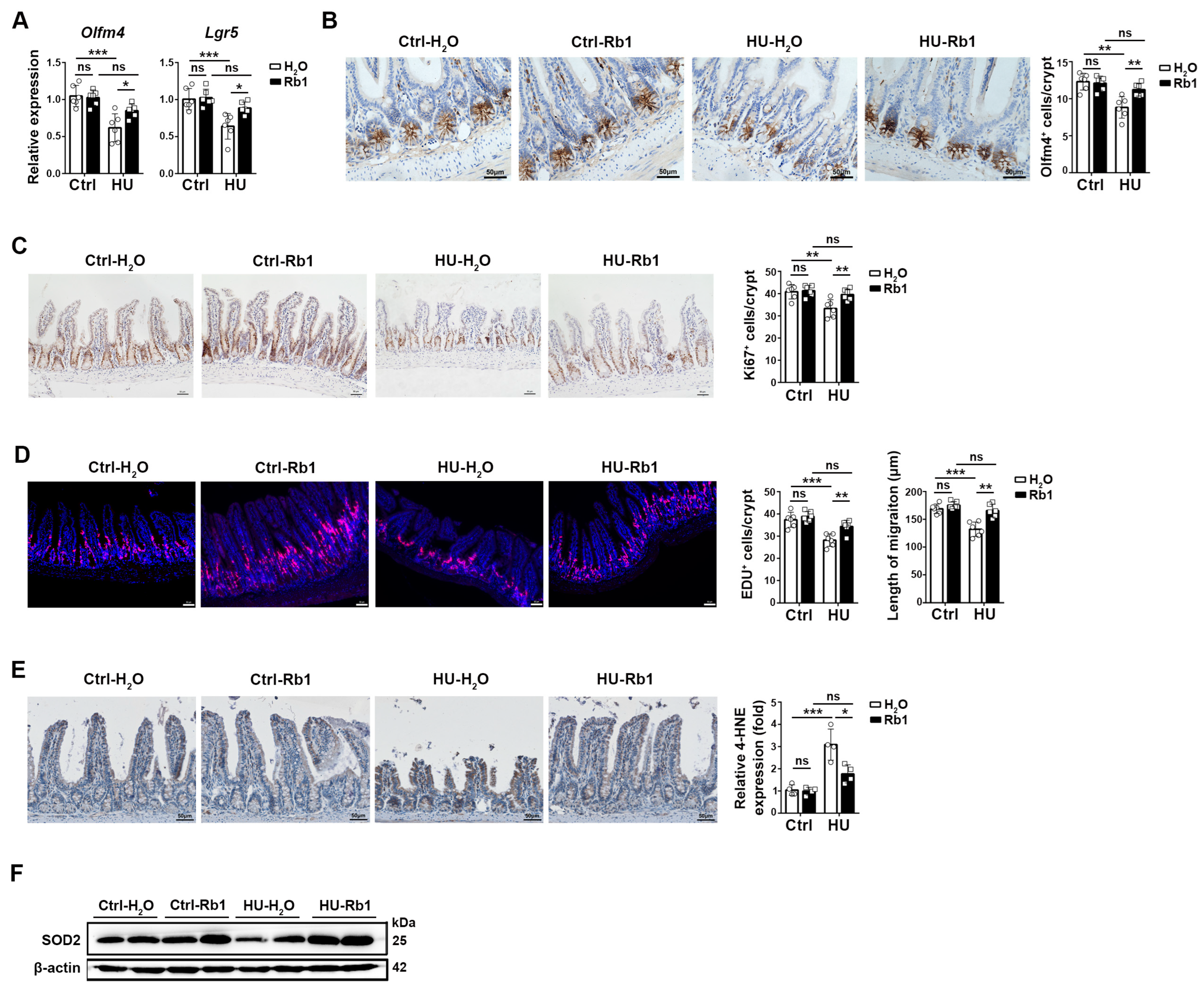
2.2. Rb1 Promoted ISC Homeostasis in HU Mice
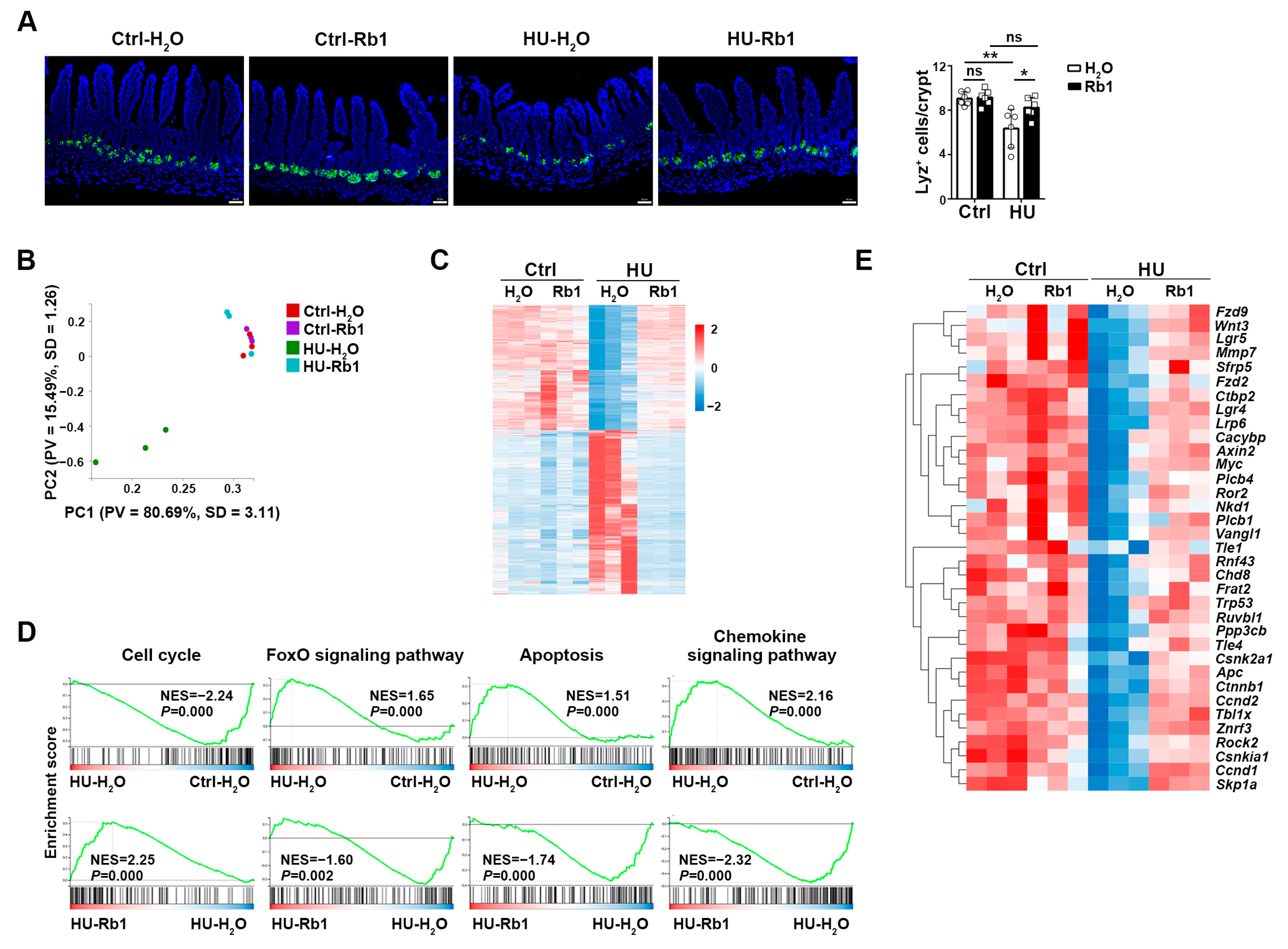
2.3. Rb1 Improved the Niche Function of ISCs
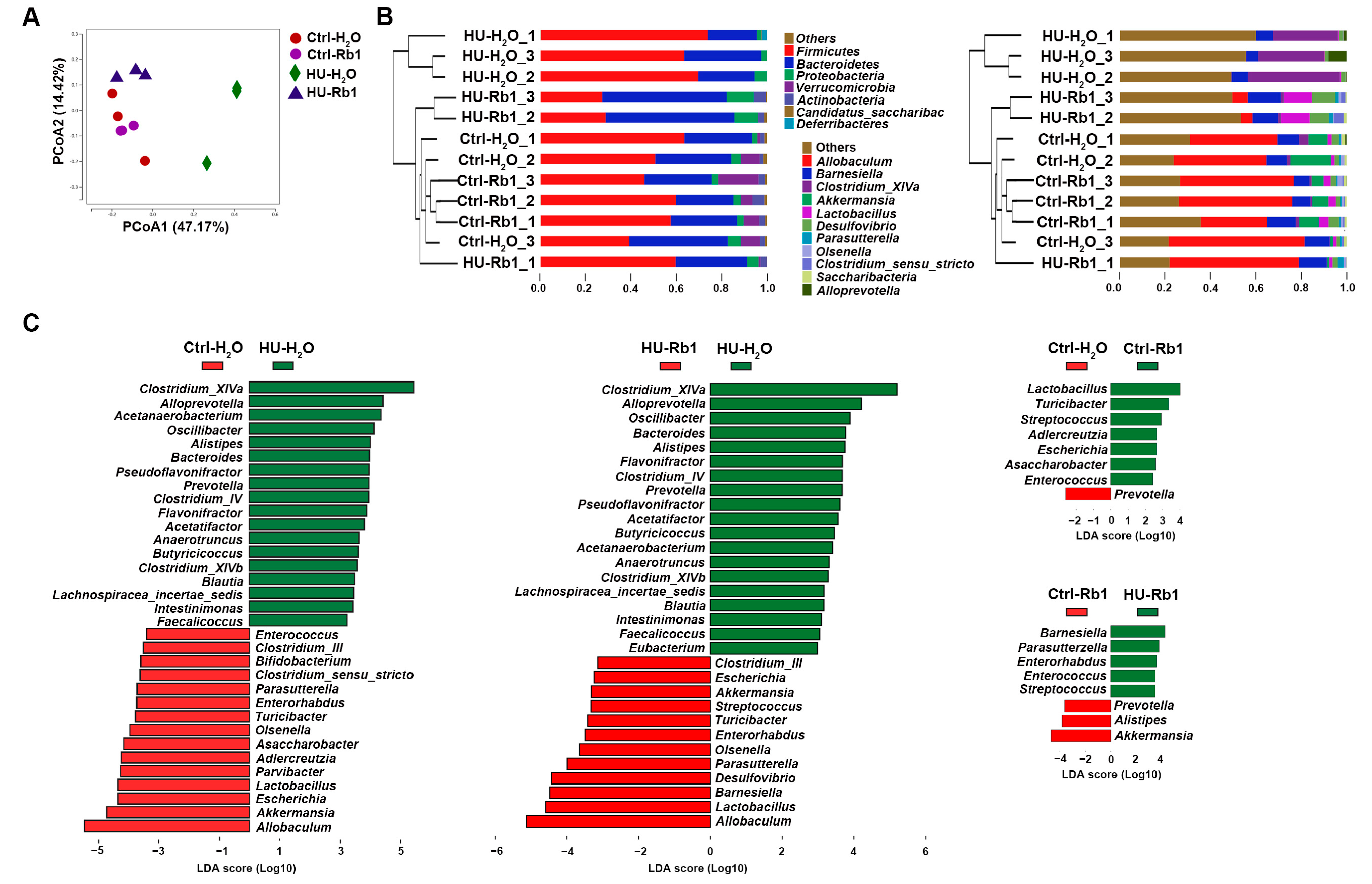
2.4. Rb1 Attenuated Gut Dysbiosis in HU Mice
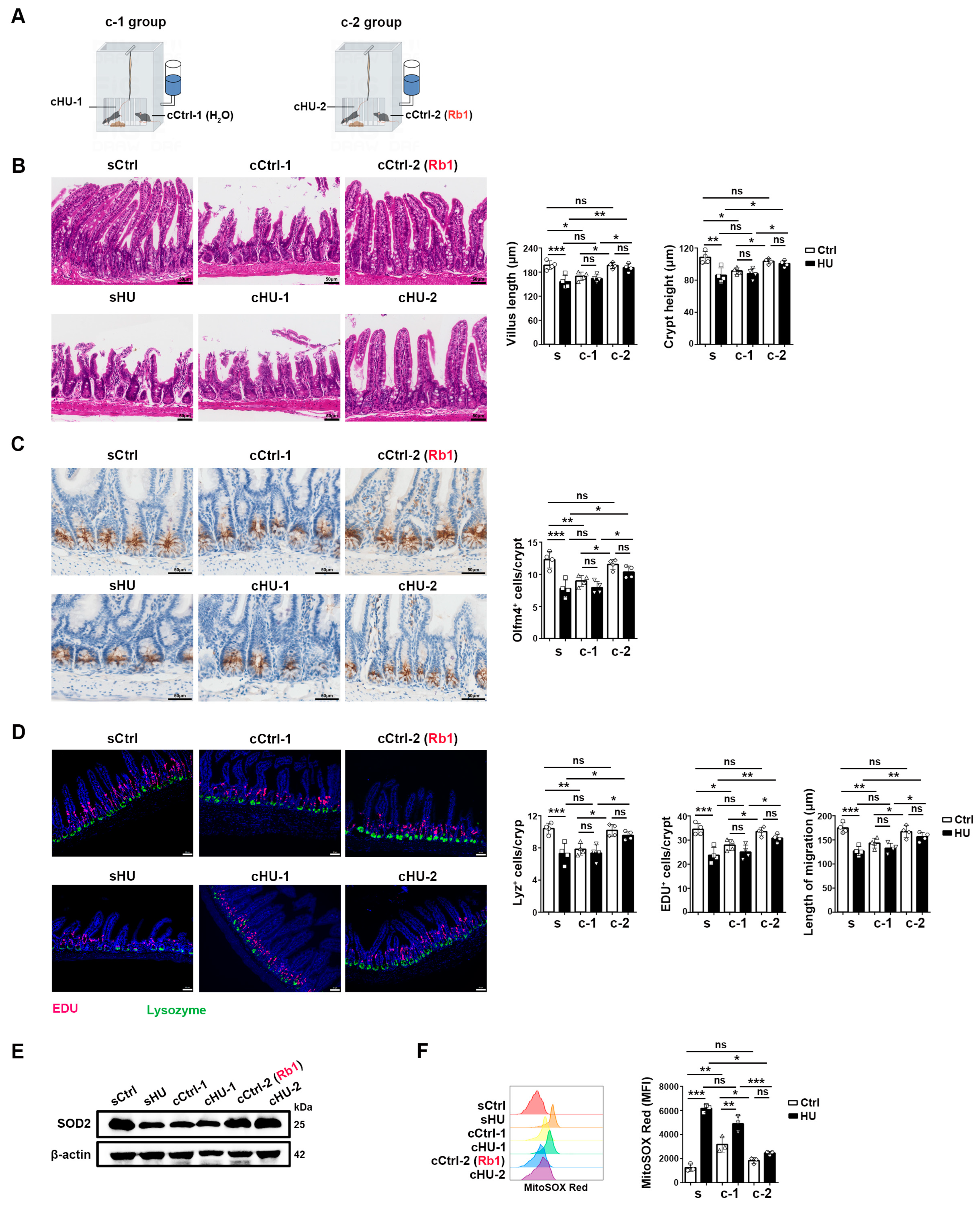
2.5. Rb1 Protected HU-Induced ISC Injury via Microbiota and/or Microbiota-Associated Metabolites
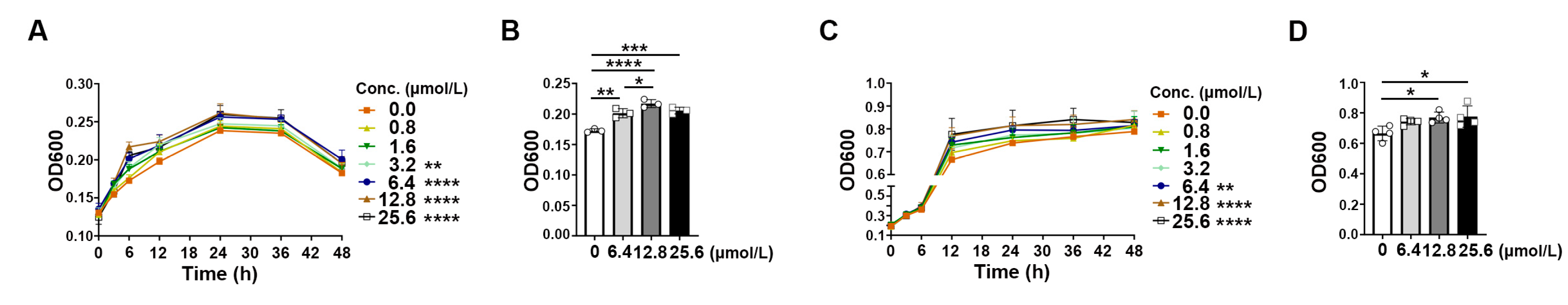
2.6. Rb1 Derivative Promoted the Growth of Akkermansia and Bifidobacterium
3. Discussion
4. Materials and Methods
4.1. Humane Care of Animals
4.2. Hindlimb Unloading and Rb1 Treatment
4.3. Co-Housing Experimental Design
4.4. Histology and Immunohistochemistry
4.5. Enrichment of Small Intestinal Epithelial Cells
4.6. ELISA
4.7. Flow Cytometry
4.8. RNA Isolation and Real-Time PCR
4.9. RNA Sequencing and Analysis
4.10. Western Blotting
4.11. 16s rRNA Sequencing and Data Analysis
4.12. Bacterial Growth and Measurements
4.13. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014, 15, 19–33. [Google Scholar] [CrossRef] [PubMed]
- van der Flier, L.G.; Clevers, H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Hageman, J.H.; Heinz, M.C.; Kretzschmar, K.; van der Vaart, J.; Clevers, H.; Snippert, H.J. Intestinal Regeneration: Regulation by the Microenvironment. Dev. Cell 2020, 54, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E. Single Lgr5 stem cells build crypt-villus structures without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, T.; Dong, Q.; Nice, E.C.; Huang, C.; Wei, Y. Redox homeostasis: The linchpin in stem cell self-renewal and differentiation. Cell Death Dis. 2013, 4, e537. [Google Scholar] [CrossRef] [PubMed]
- Hochmuth, C.E.; Biteau, B.; Bohmann, D.; Jasper, H. Redox Regulation by Keap1 and Nrf2 Controls Intestinal Stem Cell Proliferation in. Cell Stem Cell 2011, 8, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Pral, L.P.; Fachi, J.L.; Corrêa, R.O.; Colonna, M.; Vinolo, M.A.R. Hypoxia and HIF-1 as key regulators of gut microbiota and host interactions. Trends Immunol. 2021, 42, 604–621. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.P.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.O.; Lundgren, P.; Nath, K.; Thaiss, C.A. Metabolic control by the microbiome. Genome Med. 2022, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, H.R.; Moeller, C.L.; Phillips, R.W.; Smirnov, K.L. Proliferation of jejunal mucosal cells in rats flown in space. J. Appl. Physiol. 1992, 73, 148S–150S. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.A.; Bykov, E.G.; Il’in, E.A.; Pashkov, A.N. Tissue-specific reaction of the mucous coat of herbals’ small gut under the influence of spaceflight factors on board biosat “Foton M3”. Aviakosm. Ekolog. Med. 2011, 45, 25–30. [Google Scholar] [PubMed]
- Shubich, M.G.; Goriacheva, L.L.; Dudetskii, V.I.; Lutsenko, N.M.; Mogil’naia, G.M. Histochemical study of the digestive organs of rats after a flight on “Kosmos-605”. Kosm. Biol. Aviakosm. Med. 1977, 11, 40–44. [Google Scholar] [PubMed]
- Zhou, Y.; Ni, H.; Li, M.; Sanzari, J.K.; Diffenderfer, E.S.; Lin, L.; Kennedy, A.R.; Weissman, D. Effect of solar particle event radiation and hindlimb suspension on gastrointestinal tract bacterial translocation and immune activation. PLoS ONE 2012, 7, e44329. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Y.; He, J.; Li, P.; Jin, R.; Wang, K.; Xu, X.; Hao, J.; Zhang, Y.; Liu, H.; et al. Intestinal microbiota contributes to colonic epithelial changes in simulated microgravity mouse model. FASEB J. 2017, 31, 3695–3709. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, W.; Shi, J.; Wang, J.; Hao, J.; Pang, X.; Huang, X.; Chen, X.; Li, Y.; Jin, R.; et al. Intestinal microbiota contributes to altered glucose metabolism in simulated microgravity mouse model. FASEB J. 2019, 33, 10140–10151. [Google Scholar] [CrossRef] [PubMed]
- Chopra, V.; Fadl, A.A.; Sha, J.; Chopra, S.; Galindo, C.L.; Chopra, A.K. Alterations in the virulence potential of enteric pathogens and bacterial-host cell interactions under simulated microgravity conditions. J. Toxicol. Environ. Health A 2006, 69, 1345–1370. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, S.; Zhong, C.; Liu, L.; Wang, G.; Huang, X.; Yang, X.; Yang, H.; Li, L. The influence of simulated weightlessness on the composition and function of gut microbiota and bile acid metabolism products. Life Sci. Space Res. 2024, 41, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Globus, R.K.; Morey-Holton, E. Hindlimb unloading: Rodent analog for microgravity. J. Appl. Physiol. 2016, 120, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Qaisar, R.; Khan, N.A.; Alharbi, A.M.; Alfahemi, H.; Elmoselhi, A. Effect of Microgravity on the Gut Microbiota Bacterial Composition in a Hindlimb Unloading Model. Life 2022, 12, 1865. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Mungroo, M.R.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. The Use of Gut Microbial Modulation Strategies as Interventional Strategies for Ageing. Microorganisms 2022, 10, 1869. [Google Scholar] [CrossRef]
- Han, Y.J.; Shao, D.Y.; Han, C.C.; Huang, Q.S.; Zhao, W. Response of human gut microbiota under simulated microgravity. Appl. Microbiol. Biot. 2022, 106, 5221–5231. [Google Scholar] [CrossRef] [PubMed]
- Shama, S.; Ranade, A.V.; Qaisar, R.; Khan, N.A.; Tauseef, I.; Elmoselhi, A.; Siddiqui, R. Enhancing microbial diversity as well as multi-organ health in hind-limb unloaded mice. Life Sci. Space Res. 2024, 40, 62–71. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Yu, Y.; He, L.; Xiao, X.; Li, P.C. Ginsenoside Rb1 Deters Cell Proliferation, Induces Apoptosis, Alleviates Oxidative Stress, and Antimetastasis in Oral Squamous Carcinoma Cells. In Applied Biochemistry and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Lin, Z.A.; Xie, R.; Zhong, C.; Huang, J.; Shi, P.; Yao, H. Recent progress (2015–2020) in the investigation of the pharmacological effects and mechanisms of ginsenoside Rb1, a main active ingredient in Panax ginseng Meyer. J. Ginseng Res. 2022, 46, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Li, X.; Wang, Y.; Mu, P.; Chen, C.; Huang, P.; Liu, D. Ginsenoside Rb1 attenuates intestinal ischemia/reperfusion-induced inflammation and oxidative stress via activation of the PI3K/Akt/Nrf2 signaling pathway. Mol. Med. Rep. 2019, 19, 3633–3641. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.L.; Chen, L.; Hu, Q.; Yang, Y.; Tong, F.; Li, K.; Lin, T.; Nie, Y.; Rong, H.; Yu, S.; et al. Ginsenoside Rb1 improves intestinal aging via regulating the expression of sirtuins in the intestinal epithelium and modulating the gut microbiota of mice. Front. Pharmacol. 2022, 13, 991597. [Google Scholar] [CrossRef] [PubMed]
- Toyokawa, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Inoue, K.; Ushiroda, C.; Kashiwagi, S.; Nakano, T.; Hotta, Y.; Tanaka, M.; et al. Ginsenoside Rb1 promotes intestinal epithelial wound healing through extracellular signal-regulated kinase and Rho signaling. J. Gastroen. Hepatol. 2019, 34, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, P.; Chen, X.; Yan, J.; Yao, J.; Yu, Z.; Chen, X. Ginsenoside Rb1 protects the intestinal mucosal barrier following peritoneal air exposure. Exp. Ther. Med. 2016, 12, 2563–2567. [Google Scholar] [CrossRef] [PubMed]
- Medawar, E.; Haange, S.-B.; Rolle-Kampczyk, U.; Engelmann, B.; Dietrich, A.; Thieleking, R.; Wiegank, C.; Fries, C.; Horstmann, A.; Villringer, A.; et al. Gut microbiota link dietary fiber intake and short-chain fatty acid metabolism with eating behavior. Transl. Psychiatry 2021, 11, 500. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cao, L.; Zou, W.; Yu, R.; Shen, W. Panax notoginseng saponins inhibits NLRP3 inflammasome-mediated pyroptosis by downregulating lncRNA-ANRIL in cardiorenal syndrome type 4. Chin. Med. 2023, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.X.; Zhu, Y.; Song, S.X.; Bu, Q.Y.; You, X.Y.; Zou, H.; Zhao, G.P. Ginsenoside Rb1, Compound K and 20(S)-Protopanaxadiol Attenuate High-Fat Diet-Induced Hyperlipidemia in Rats via Modulation of Gut Microbiota and Bile Acid Metabolism. Molecules 2024, 29, 1108. [Google Scholar] [CrossRef]
- Zou, H.; Zhang, M.; Zhu, X.; Zhu, L.; Chen, S.; Luo, M.; Xie, Q.; Chen, Y.; Zhang, K.; Bu, Q.; et al. Ginsenoside Rb1 Improves Metabolic Disorder in High-Fat Diet-Induced Obese Mice Associated with Modulation of Gut Microbiota. Front. Microbiol. 2022, 13, 826487. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, B.; Xu, Y.; Wang, Y.; He, Z.; Cai, X.; Qin, Y.; Ye, J.; Yang, Y.; Shen, J.; et al. Geniposide protects against neurotoxicity in mouse models of rotenone-induced Parkinson’s disease involving the mTOR and Nrf2 pathways. J. Ethnopharmacol. 2024, 318, 116914. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Hong, Y.; Zhu, J.; Zhang, Y.; Zhang, J.; Ding, C.; Che, Y.; Wang, G.; Jiang, A.; et al. Ginsenosides retard atherogenesis via remodelling host-microbiome metabolic homeostasis. Br. J. Pharmacol. 2024, 181, 1768–1792. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Jeon, J.Y.; Yang, W.S.; Kim, C.H.; Eom, D.W. Combined Amelioration of Ginsenoside (Rg1, Rb1 and Rg3)-enriched Korean Red Ginseng and Probiotic on Non-alcoholic Liver Disease. Curr. Pharm. Biotechnol. 2019, 20, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.B.; Wang, H.; Yi, R.; Lou, J.; Wen, S.; Hu, Z. Gut microbiome and metabolome in aneurysm rat with hypertension after ginsenoside Rb1 treatment. Front. Pharmacol. 2023, 14, 1287711. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Shen, J.; Li, H.; Zheng, X.; Kang, D.; Xu, Y. Ginsenoside Rb1 exerts neuroprotective effects through regulation of abundance and GABA receptor expression. J. Ginseng Res. 2020, 44, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Hugon, P.; Dufour, J.-C.; Colson, P.; Fournier, P.-E.; Sallah, K.; Raoult, D. A comprehensive repertoire of prokaryotic species identified in human beings. Lancet Infect. Dis. 2015, 15, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Hati, S.; Patel, M.; Mishra, B.K.; Das, S. Short-chain fatty acid and vitamin production potentials of isolated from fermented foods of Khasi Tribes, Meghalaya, India. Ann. Microbiol. 2019, 69, 1191–1199. [Google Scholar] [CrossRef]
- Hou, Q.H.; Ye, L.; Liu, H.; Huang, L.; Yang, Q.; Turner, J.R.; Yu, Q. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018, 25, 1657–1670. [Google Scholar] [CrossRef]
- Ghotaslou, R.; Nabizadeh, E.; Memar, M.Y.; Law, W.M.H.; Ozma, M.A.; Abdi, M.; Yekani, M.; Kadkhoda, H.; Hosseinpour, R.; Bafadam, S.; et al. The metabolic, protective, and immune functions of Akkermansia muciniphila. Microbiol. Res. 2023, 266, 127245. [Google Scholar] [CrossRef]
- Abdulqadir, R.; Engers, J.; Al-Sadi, R. Role of Bifidobacterium in Modulating the Intestinal Epithelial Tight Junction Barrier: Current Knowledge and Perspectives. Curr. Dev. Nutr. 2023, 7, 102026. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, R.M.; Ahsan, M.Z.; Akhtar, U.; Ahmad, K.A.; Ali, U.; Deng, M.-Y.; Li, X.-Y.; Wang, Y.-X. Ginsenoside Rb1, a principal effective ingredient of Panax notoginseng, produces pain antihypersensitivity by spinal microglial dynorphin A expression. Neurosci. Res. 2023, 188, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Chapes, S.K.; Mastro, A.M.; Sonnenfeld, G.; Berry, W.D. Antiorthostatic suspension as a model for the effects of spaceflight on the immune system. J. Leukoc. Biol. 1993, 54, 227–235. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, B.; Wang, J.; Wang, K.; Hao, J.; Han, J.-Y.; Jin, R.; Ge, Q. Effects of Ginsenoside Rb1 on the Crosstalk between Intestinal Stem Cells and Microbiota in a Simulated Weightlessness Mouse Model. Int. J. Mol. Sci. 2024, 25, 8769. https://doi.org/10.3390/ijms25168769
Zong B, Wang J, Wang K, Hao J, Han J-Y, Jin R, Ge Q. Effects of Ginsenoside Rb1 on the Crosstalk between Intestinal Stem Cells and Microbiota in a Simulated Weightlessness Mouse Model. International Journal of Molecular Sciences. 2024; 25(16):8769. https://doi.org/10.3390/ijms25168769
Chicago/Turabian StyleZong, Beibei, Jingyi Wang, Kai Wang, Jie Hao, Jing-Yan Han, Rong Jin, and Qing Ge. 2024. "Effects of Ginsenoside Rb1 on the Crosstalk between Intestinal Stem Cells and Microbiota in a Simulated Weightlessness Mouse Model" International Journal of Molecular Sciences 25, no. 16: 8769. https://doi.org/10.3390/ijms25168769




