Intrapleural Fibrinolytic Interventions for Retained Hemothoraces in Rabbits
Abstract
1. Introduction
2. Results
2.1. Experimental Design
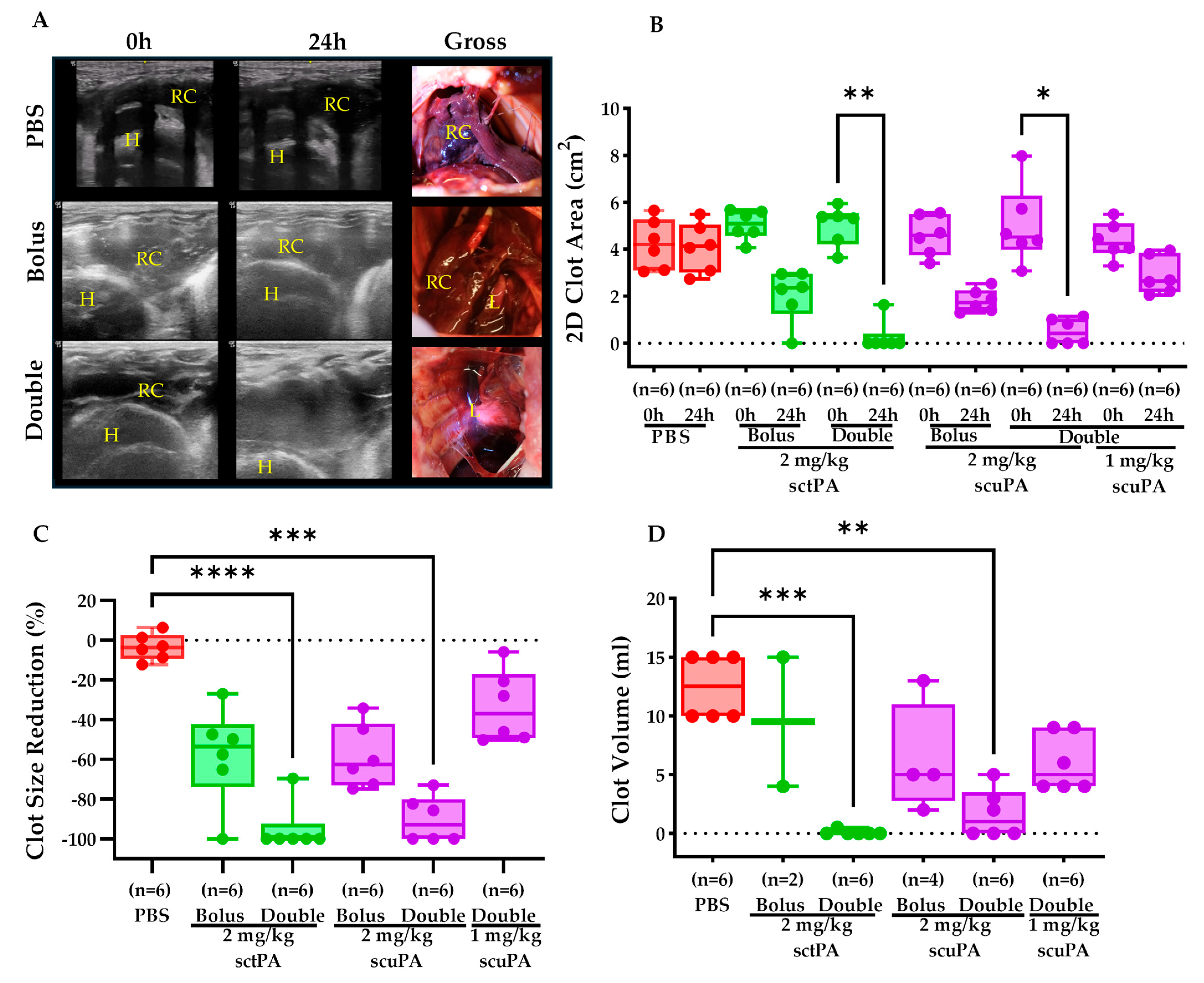
2.2. IPFT Successfully Reduced Clot Retention
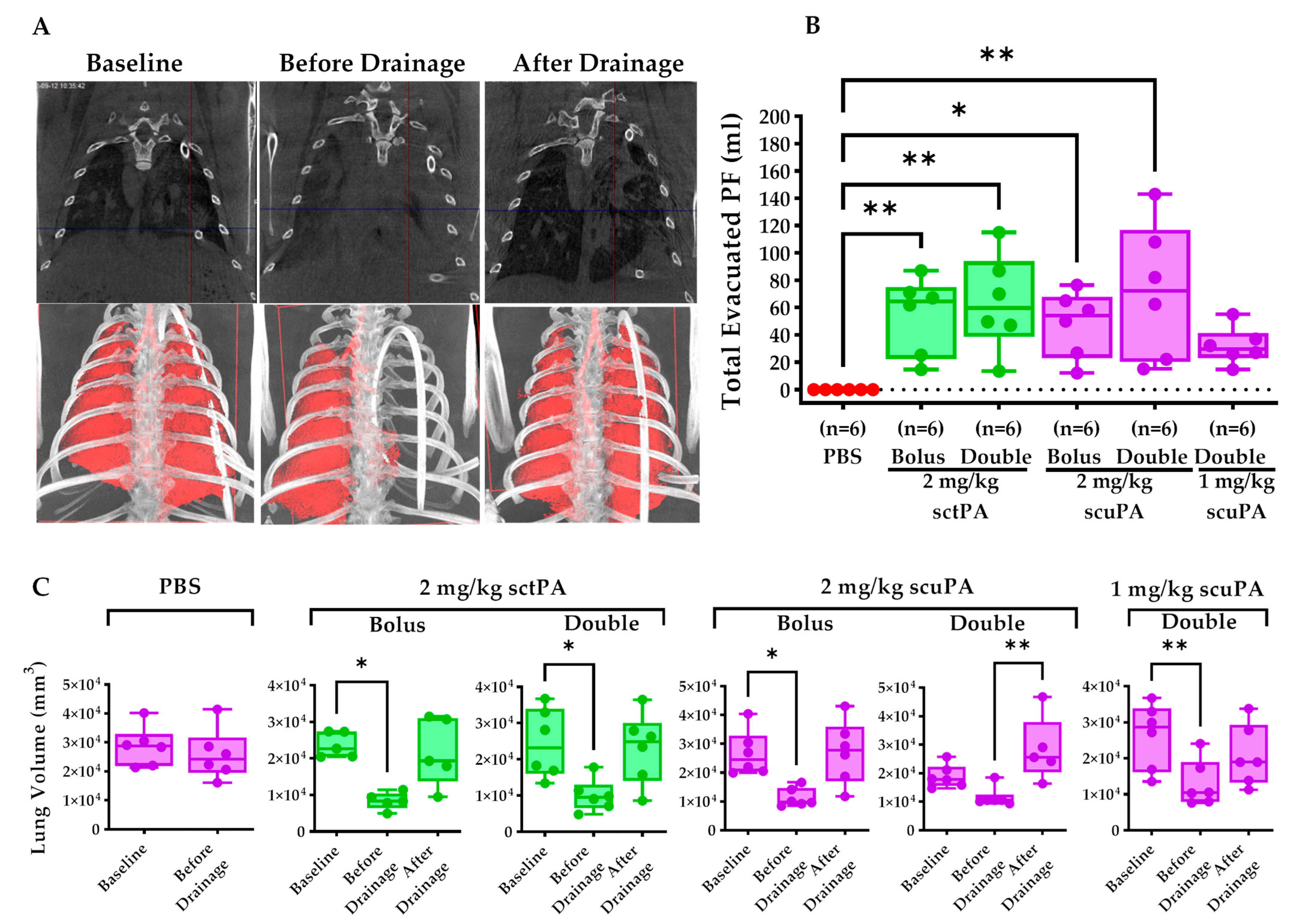
2.3. IPFT Restored Pleural Drainage and Mitigate Lung Restriction
2.4. Pleural Thickening Was Unchanged in Rabbits Receiving IPFT
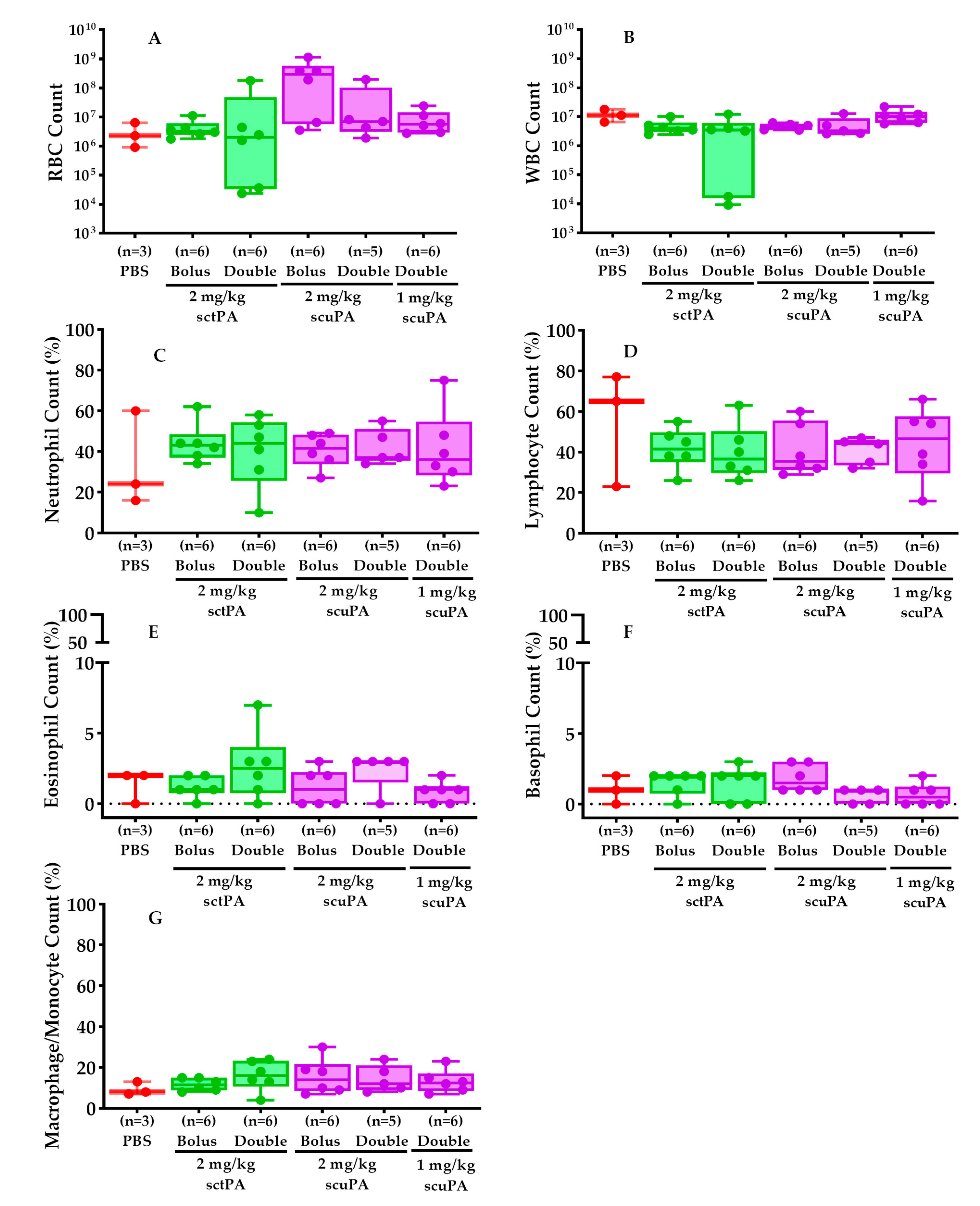
2.5. PF Myeloid Cell Counts Were Comparable in RH Rabbits Treated with IPFT
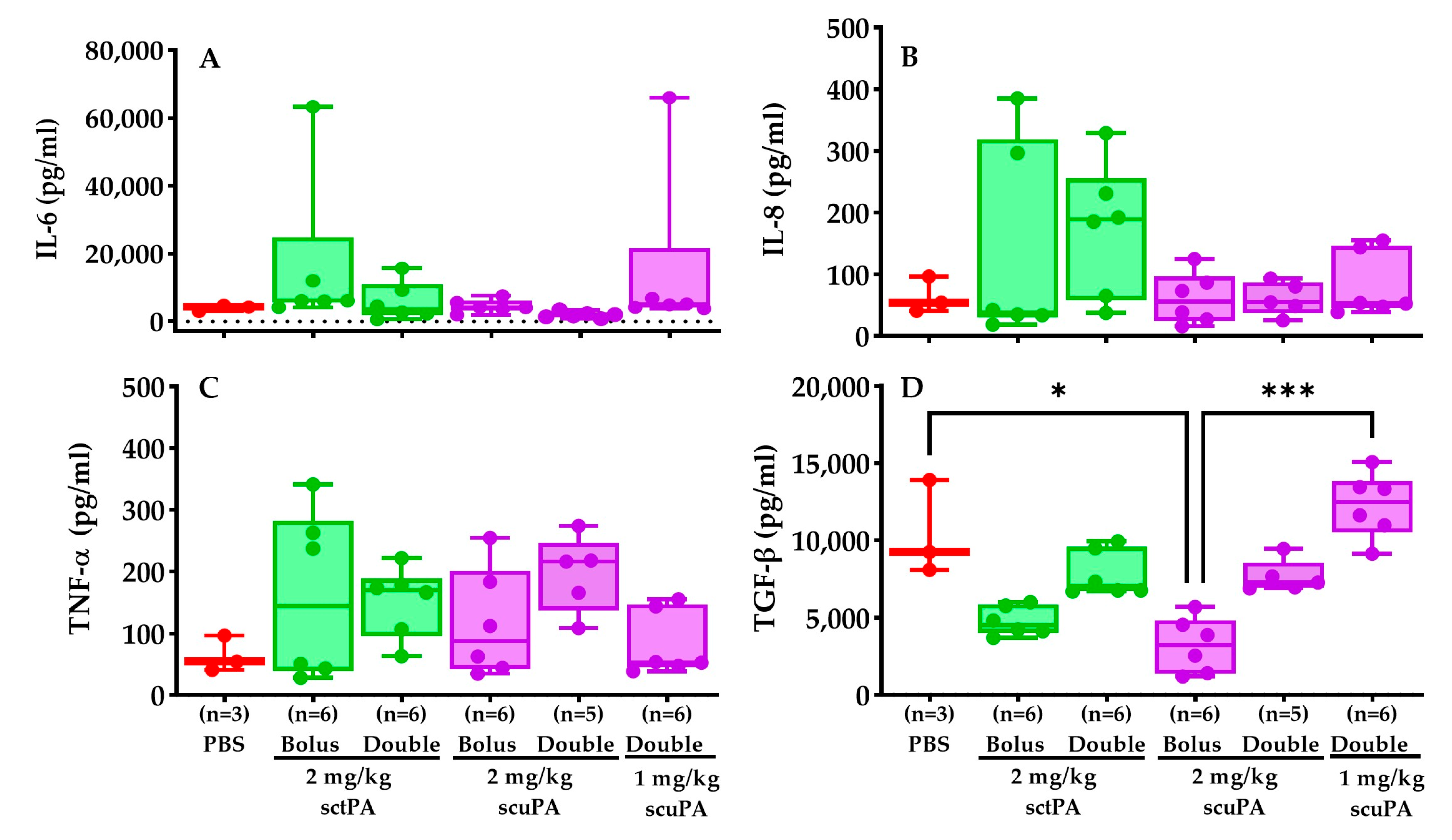
2.6. Inflammatory Profile of RH PFs after IPFT Treatment
2.7. Intrapleural Levels of Plasminogen Activators (tPA and uPA) and PAI-1 during IPFT of RH Model
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Non-Survival Blood Collection
4.3. Chest (Thoracostomy) Tube Placement, Retention, and Maintenance
4.4. RH Injury Induction and Evaluation
4.5. Gross and Histological Evaluation of Retained Hemothorax Injury
4.6. IPFT Dosing Regimen
4.7. Cytological and Biochemical Markers in RH PFs
4.8. Anesthesia
4.9. Post-Procedure Monitoring, Nursing, and Support
4.10. Humane Endpoints
4.11. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tillett, W.S.; Sherry, S. The effect in patients of streptococcal fibrinolysin (streptokinase) and streptococcal desoxyribonuclease on fibrinous, purulent and sanguinous pleural exudations. J. Clin. Investig. 1949, 28, 173–190. [Google Scholar] [CrossRef]
- Tillett, W.S.; Sherry, S.; Read, C.T. The use of streptokinase-streptodornase in the treatment of postpneumonic empyema. J. Thorac. Surg. 1951, 21, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, J.; Idell, S.; Norwood, S.; Cook, A. Hemothorax: A Review of the Literature. Clin. Pulm. Med. 2020, 27, 1–12. [Google Scholar] [CrossRef]
- Holsen, M.R.; Tameron, A.M.; Evans, D.C.; Thompson, M. Intrapleural Tissue Plasminogen Activator for Traumatic Retained Hemothorax. Ann. Pharmacother. 2019, 53, 1060–1066. [Google Scholar] [CrossRef]
- Hendriksen, B.S.; Kuroki, M.T.; Armen, S.B.; Reed, M.F.; Taylor, M.D.; Hollenbeak, C.S. Lytic Therapy for Retained Traumatic Hemothorax: A Systematic Review and Meta-analysis. Chest 2019, 155, 805–815. [Google Scholar] [CrossRef]
- Huang, D.; Zhao, D.; Zhou, Y.; Liu, H.; Chen, X. Intrapleural Fibrinolytic Therapy for Residual Coagulated Hemothorax After Lung Surgery. World J. Surg. 2016, 40, 1121–1128. [Google Scholar] [CrossRef]
- Boersma, W.G.; Stigt, J.A.; Smit, H.J. Treatment of haemothorax. Respir. Med. 2010, 104, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Broderick, S.R. Hemothorax: Etiology, diagnosis, and management. Thorac. Surg. Clin. 2013, 23, 89–96. [Google Scholar] [CrossRef] [PubMed]
- DuBose, J.; Inaba, K.; Demetriades, D.; Scalea, T.M.; O’Connor, J.; Menaker, J.; Morales, C.; Konstantinidis, A.; Shiflett, A.; Copwood, B. Management of post-traumatic retained hemothorax: A prospective, observational, multicenter AAST study. J. Trauma Acute Care Surg. 2012, 72, 11–22. [Google Scholar] [CrossRef]
- Komissarov, A.A.; Rahman, N.; Lee, Y.C.G.; Florova, G.; Shetty, S.; Idell, R.; Ikebe, M.; Das, K.; Tucker, T.A.; Idell, S. Fibrin turnover and pleural organization: Bench to bedside. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L757–L768. [Google Scholar] [CrossRef]
- Idell, S.; Rahman, N.M. Intrapleural Fibrinolytic Therapy for Empyema and Pleural Loculation: Knowns and Unknowns. Ann. Am. Thorac. Soc. 2018, 15, 515–517. [Google Scholar] [CrossRef] [PubMed]
- De Vera, C.J.; Emerine, R.L.; Girard, R.A.; Sarva, K.; Jacob, J.; Azghani, A.O.; Florence, J.M.; Cook, A.; Norwood, S.; Singh, K.P.; et al. A Novel Rabbit Model of Retained Hemothorax with Pleural Organization. Int. J. Mol. Sci. 2023, 25, 470. [Google Scholar] [CrossRef]
- Tucker, T.; Idell, S. Plasminogen-plasmin system in the pathogenesis and treatment of lung and pleural injury. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers: Leipzig, Germany, 2013; Volume 39, pp. 373–381. [Google Scholar] [CrossRef]
- Beckert, L.; Brockway, B.; Simpson, G.; Southcott, A.M.; Lee, Y.C.G.; Rahman, N.; Light, R.W.; Shoemaker, S.; Gillies, J.; Komissarov, A.A.; et al. Phase 1 trial of intrapleural LTI-01; single chain urokinase in complicated parapneumonic effusions or empyema. JCI Insight 2019, 5, 127470. [Google Scholar] [CrossRef]
- Florova, G.; De Vera, C.J.; Emerine, R.L.; Girard, R.A.; Azghani, A.O.; Sarva, K.; Jacob, J.; Morris, D.E.; Chamiso, M.; Idell, S.; et al. Targeting the PAI-1 Mechanism with a Small Peptide Increases the Efficacy of Alteplase in a Rabbit Model of Chronic Empyema. Pharmaceutics 2023, 15, 1498. [Google Scholar] [CrossRef] [PubMed]
- Sasse, S.; Nguyen, T.K.; Mulligan, M.; Wang, N.S.; Mahutte, C.K.; Light, R.W. The effects of early chest tube placement on empyema resolution. Chest 1997, 111, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Sasse, S.; Nguyen, T.; Teixeira, L.R.; Light, R. The utility of daily therapeutic thoracentesis for the treatment of early empyema. Chest 1999, 116, 1703–1708. [Google Scholar] [CrossRef]
- Teixeira, L.R.; Sasse, S.A.; Villarino, M.A.; Nguyen, T.; Mulligan, M.E.; Light, R.W. Antibiotic levels in empyemic pleural fluid. Chest 2000, 117, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Light, R.W.; Nguyen, T.; Mulligan, M.E.; Sasse, S.A. The in vitro efficacy of varidase versus streptokinase or urokinase for liquefying thick purulent exudative material from loculated empyema. Lung 2000, 178, 13–18. [Google Scholar] [CrossRef]
- Kunz, C.R.; Jadus, M.R.; Kukes, G.D.; Kramer, F.; Nguyen, V.N.; Sasse, S.A. Intrapleural injection of transforming growth factor-beta antibody inhibits pleural fibrosis in empyema. Chest 2004, 126, 1636–1644. [Google Scholar] [CrossRef]
- Dikensoy, O.; Zhu, Z.; Na, M.J.; Liao, H.; Donnelly, E.; Light, R.W. Intrapleural heparin or heparin combined with human recombinant DNase is not effective in the treatment of empyema in a rabbit model. Respirology 2006, 11, 755–760. [Google Scholar] [CrossRef]
- Zhu, Z.; Hawthorne, M.L.; Guo, Y.; Drake, W.; Bilaceroglu, S.; Misra, H.L.; Light, R.W. Tissue plasminogen activator combined with human recombinant deoxyribonuclease is effective therapy for empyema in a rabbit model. Chest 2006, 129, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Idell, S.; Jun, N.M.; Liao, H.; Gazar, A.E.; Drake, W.; Lane, K.B.; Koenig, K.; Komissarov, A.; Tucker, T.; Light, R.W. Single-chain urokinase in empyema induced by Pasturella multocida. Exp. Lung Res. 2009, 35, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Komissarov, A.A.; Florova, G.; Azghani, A.O.; Buchanan, A.; Boren, J.; Allen, T.; Rahman, N.M.; Koenig, K.; Chamiso, M.; Karandashova, S.; et al. Dose dependency of outcomes of intrapleural fibrinolytic therapy in new rabbit empyema models. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L389–L399. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Humphries, P.; Ekpo, O.E.; Smit, E.; van der Merwe, C.F. Comparative ultrastructural analyses of mouse, rabbit, and human platelets and fibrin networks. Microsc. Res. Tech. 2007, 70, 823–827. [Google Scholar] [CrossRef]
- Rahman, N.M.; Maskell, N.A.; West, A.; Teoh, R.; Arnold, A.; Mackinlay, C.; Peckham, D.; Davies, C.W.; Ali, N.; Kinnear, W.; et al. Intrapleural Use of Tissue Plasminogen Activator and DNase in Pleural Infection. N. Engl. J. Med. 2011, 365, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Florova, G.; Girard, R.A.; Azghani, A.O.; Sarva, K.; Buchanan, A.; Karandashova, S.; DeVera, C.J.; Morris, D.; Chamiso, M.; Koenig, K.; et al. Precision targeting of the plasminogen activator inhibitor-1 mechanism increases efficacy of fibrinolytic therapy in empyema. Physiol. Rep. 2021, 9, e14861. [Google Scholar] [CrossRef] [PubMed]
- Florova, G.; Azghani, A.; Karandashova, S.; Schaefer, C.; Koenig, K.; Stewart-Evans, K.; Declerck, P.J.; Idell, S.; Komissarov, A.A. Targeting of plasminogen activator inhibitor 1 improves fibrinolytic therapy for tetracycline-induced pleural injury in rabbits. Am. J. Respir. Cell Mol. Biol. 2015, 52, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Florova, G.; Azghani, A.O.; Karandashova, S.; Schaefer, C.; Yarovoi, S.V.; Declerck, P.J.; Cines, D.B.; Idell, S.; Komissarov, A.A. Targeting plasminogen activator inhibitor-1 in tetracycline-induced pleural injury in rabbits. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L54–L68. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Porcel, J.M.; Madronero, A.B.; Galindo, C. Hemothorax following administration of intrapleural alteplase. Respiration 2006, 73, 715. [Google Scholar] [CrossRef]
- Agarwal, R.; Aggarwal, A.N.; Gupta, D. Intrapleural fibrinolysis in clotted haemothorax. Singap. Med. J. 2006, 47, 984–986. [Google Scholar]
- Basile, A.; Boullosa-Seoane, E.; Dominguez, V.L.; Certo, A.; Mundo, E.; Garcia-Medina, J.; Casal-Rivas, M. Intrapleural fibrinolysis in the management of empyemas and haemothoraces. Our experience. Radiol. Med. 2003, 105, 12–16. [Google Scholar] [PubMed]
- Ben-Or, S.; Feins, R.H.; Veeramachaneni, N.K.; Haithcock, B.E. Effectiveness and risks associated with intrapleural alteplase by means of tube thoracostomy. Ann. Thorac. Surg. 2011, 91, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Jerjes-Sánchez, C.; Ramirez-Rivera, A.; Elizalde, J.J.; Delgado, R.; Cicero, R.; Ibarra-Perez, C.; Arroliga, A.C.; Padua, A.; Portales, A.; Villarreal, A.; et al. Intrapleural fibrinolysis with streptokinase as an adjunctive treatment in hemothorax and empyema: A Multicenter Trial. Chest 1996, 109, 1514–1519. [Google Scholar] [CrossRef]
- Skeete, D.A.; Rutherford, E.J.; Schlidt, S.A.; Abrams, J.E.; Parker, L.A.; Rich, P.B. Intrapleural tissue plasminogen activator for complicated pleural effusions. J. Trauma 2004, 57, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Yasumitsu, T.; Nakagawa, K.; Shiono, S.; Fukuhara, K. Intrapleural streptokinase-streptodornase in the treatment of empyema and hemothorax. Kyobu Geka Jpn. J. Thorac. Surg. 2002, 55, 1115–1119. [Google Scholar]
- Heimes, J.; Copeland, H.; Lulla, A.; Duldulao, M.; Bahjri, K.; Zaheer, S.; Wallen, J.M. The use of thrombolytics in the management of complex pleural fluid collections. J. Thorac. Dis. 2017, 9, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rathi, V.; Rattan, A.; Chaudhary, S.; Agarwal, N. VATS versus intrapleural streptokinase: A prospective, randomized, controlled clinical trial for optimum treatment of post-traumatic Residual Hemothorax. Injury 2015, 46, 1749–1752. [Google Scholar] [CrossRef] [PubMed]
- Cangir, A.K.; Yuksel, C.; Dakak, M.; Ozgencil, E.; Genc, O.; Akay, H. Use of intrapleural streptokinase in experimental minimal clotted hemothorax. Eur. J. Cardiothorac. Surg. 2005, 27, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, E.H.; Richardson, J.D. Thoracoscopy in the management of hemothorax and retained blood after trauma. Curr. Opin. Pulm. Med. 1998, 4, 243–246. [Google Scholar] [CrossRef]
- Lin, H.L.; Huang, W.Y.; Yang, C.; Chou, S.M.; Chiang, H.I.; Kuo, L.C.; Lin, T.Y.; Chou, Y.P. How early should VATS be performed for retained haemothorax in blunt chest trauma? Injury 2014, 45, 1359–1364. [Google Scholar] [CrossRef]
- MacLeod, J.B.; Ustin, J.S.; Kim, J.T.; Lewis, F.; Rozycki, G.S.; Feliciano, D.V. The Epidemiology of Traumatic Hemothorax in a Level I Trauma Center: Case for Early Video-assisted Thoracoscopic Surgery. Eur. J. Trauma Emerg. Surg. 2010, 36, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Billeter, A.T.; Druen, D.; Franklin, G.A.; Smith, J.W.; Wrightson, W.; Richardson, J.D. Video-assisted thoracoscopy as an important tool for trauma surgeons: A systematic review. Langenbecks Arch. Surg. 2013, 398, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.P.; Lin, H.L.; Wu, T.C. Video-assisted thoracoscopic surgery for retained hemothorax in blunt chest trauma. Curr. Opin. Pulm. Med. 2015, 21, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Inci, I.; Ozcelik, C.; Ulku, R.; Tuna, A.; Eren, N. Intrapleural fibrinolytic treatment of traumatic clotted hemothorax. Chest 1998, 114, 160–165. [Google Scholar] [CrossRef] [PubMed]



| Investigational Drugs | Number of Rabbits Received Investigational Drugs | Number of Rabbits Used as Donors in the Experiment |
|---|---|---|
| PBS | 9 − 3 * = 6 | 11 |
| sctPA Bolus (2 mg/kg) | 7 − 1 * = 6 | 8 |
| sctPA Double (2 mg/kg) | 6 | 7 |
| scuPA Bolus (2 mg/kg) | 6 | 7 |
| scuPA Double (2 mg/kg) | 7 − 2 * = 5 | 9 |
| scuPA Double (1 mg/kg) | 6 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vera, C.J.; Jacob, J.; Sarva, K.; Christudas, S.; Emerine, R.L.; Florence, J.M.; Akiode, O.; Gorthy, T.V.; Tucker, T.A.; Singh, K.P.; et al. Intrapleural Fibrinolytic Interventions for Retained Hemothoraces in Rabbits. Int. J. Mol. Sci. 2024, 25, 8778. https://doi.org/10.3390/ijms25168778
De Vera CJ, Jacob J, Sarva K, Christudas S, Emerine RL, Florence JM, Akiode O, Gorthy TV, Tucker TA, Singh KP, et al. Intrapleural Fibrinolytic Interventions for Retained Hemothoraces in Rabbits. International Journal of Molecular Sciences. 2024; 25(16):8778. https://doi.org/10.3390/ijms25168778
Chicago/Turabian StyleDe Vera, Christian J., Jincy Jacob, Krishna Sarva, Sunil Christudas, Rebekah L. Emerine, Jon M. Florence, Oluwaseyi Akiode, Tanvi V. Gorthy, Torry A. Tucker, Karan P. Singh, and et al. 2024. "Intrapleural Fibrinolytic Interventions for Retained Hemothoraces in Rabbits" International Journal of Molecular Sciences 25, no. 16: 8778. https://doi.org/10.3390/ijms25168778
APA StyleDe Vera, C. J., Jacob, J., Sarva, K., Christudas, S., Emerine, R. L., Florence, J. M., Akiode, O., Gorthy, T. V., Tucker, T. A., Singh, K. P., Azghani, A. O., Komissarov, A. A., Florova, G., & Idell, S. (2024). Intrapleural Fibrinolytic Interventions for Retained Hemothoraces in Rabbits. International Journal of Molecular Sciences, 25(16), 8778. https://doi.org/10.3390/ijms25168778






