High-Fat Feeding Alters Circulating Triglyceride Composition: Roles of FFA Desaturation and ω-3 Fatty Acid Availability
Abstract
:1. Introduction
2. Results
2.1. Effects of Diet on Energy Intake, Weight Gain, and Metabolic Parameters
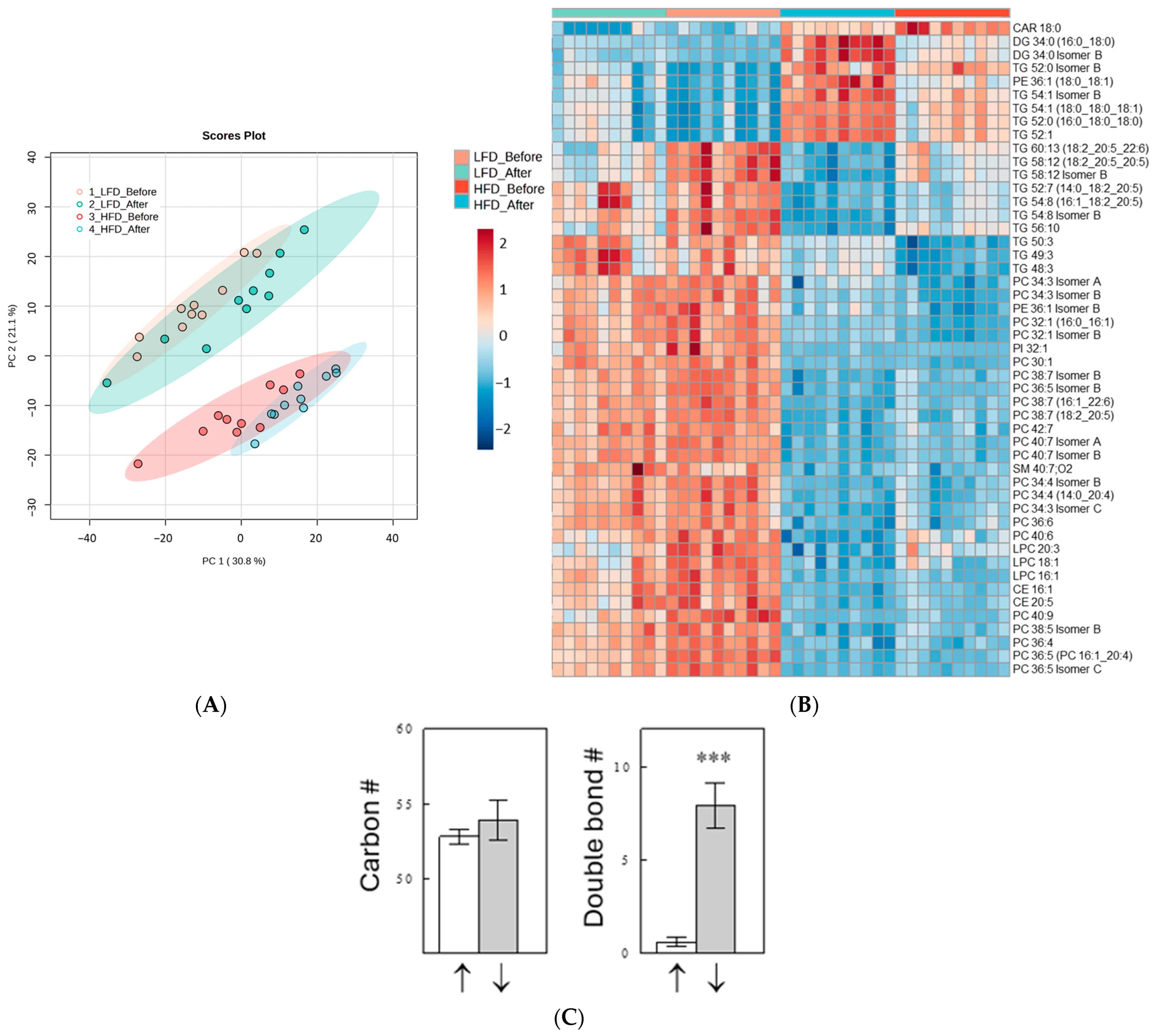
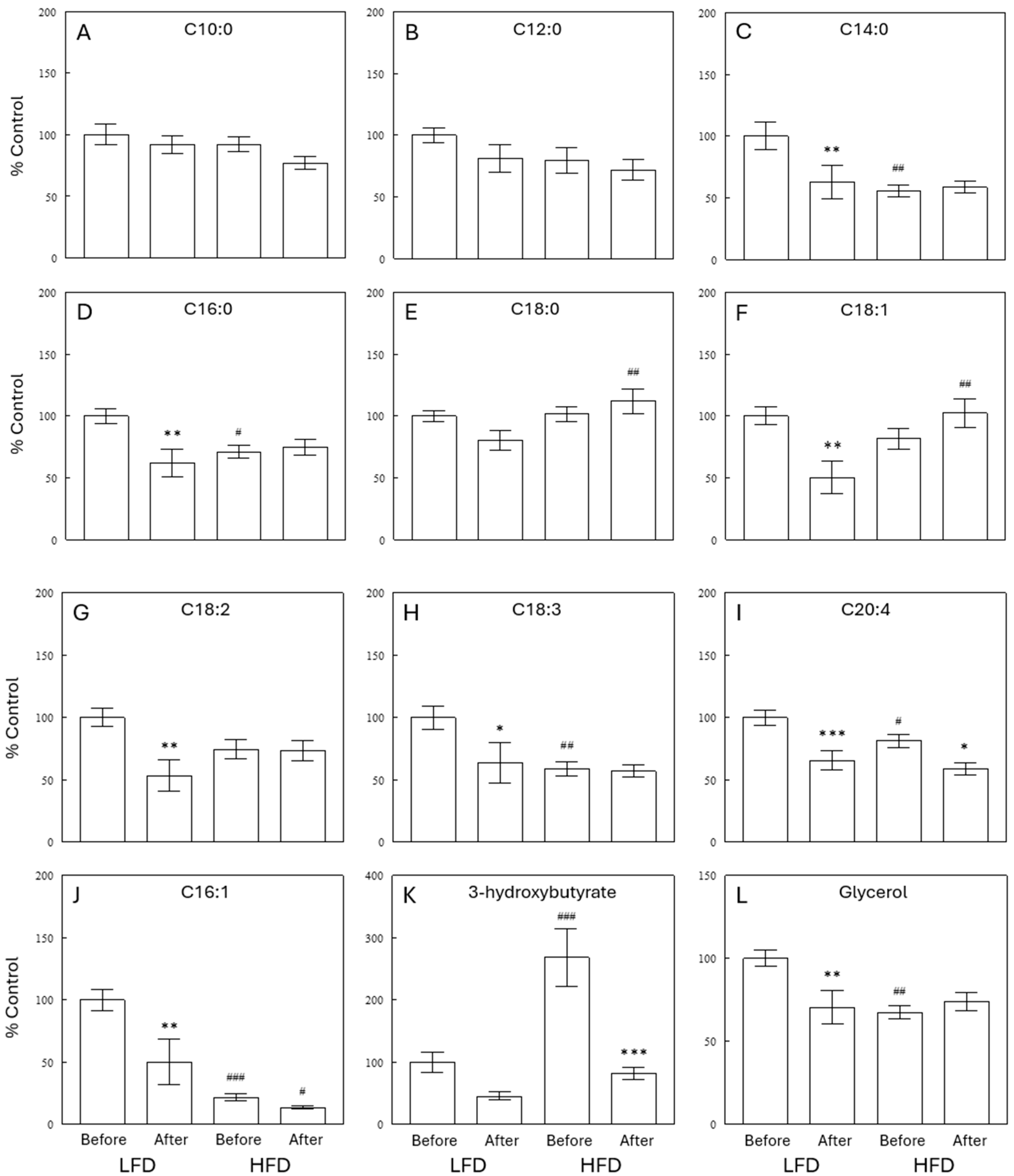
2.2. Effects of Diet and Meal on Plasma Lipid Profiles
2.3. Effects of a Meal on Plasma Lipid Profiles
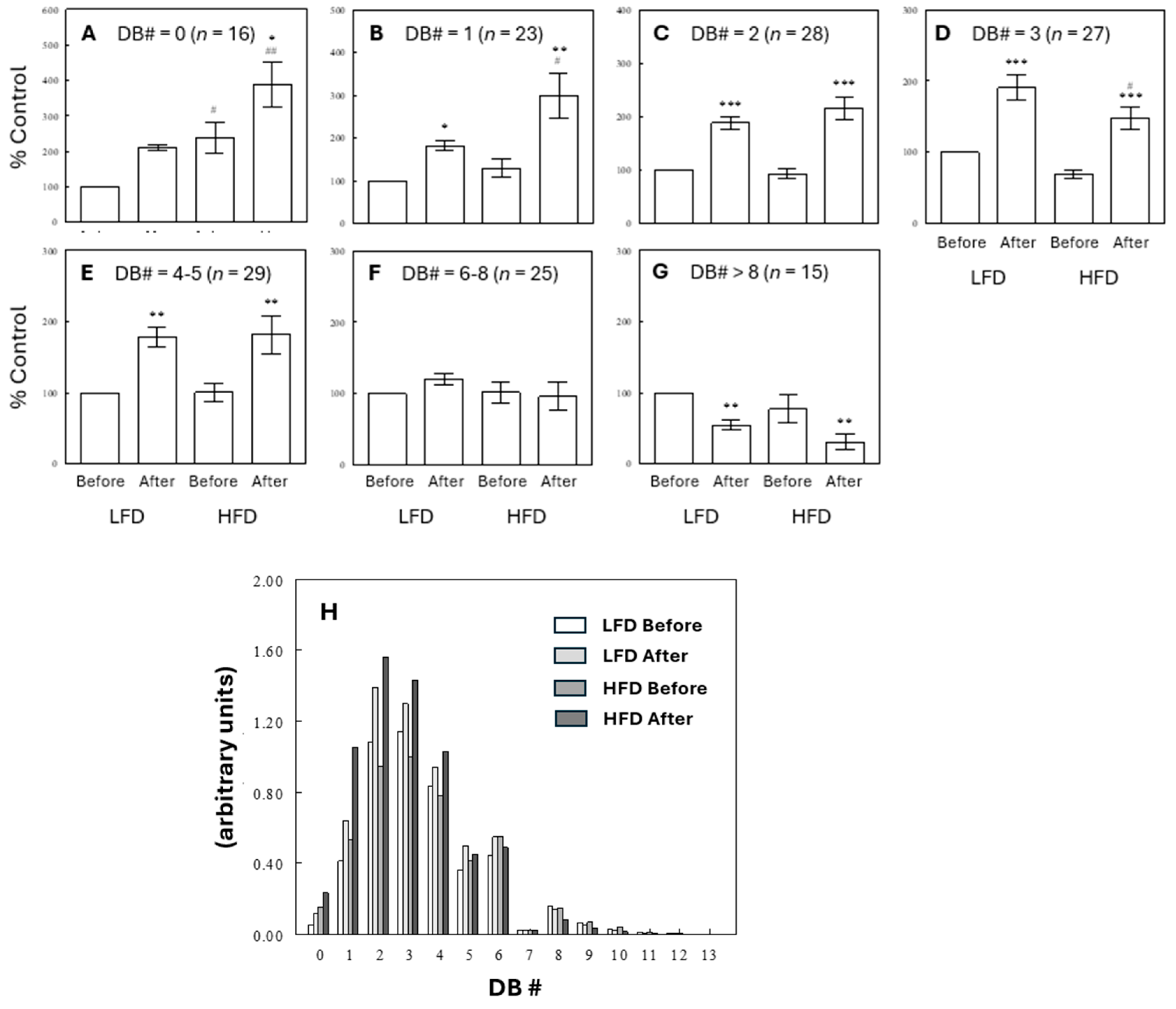
2.4. Effects of Diet and Meal on Plasma TG Profiles
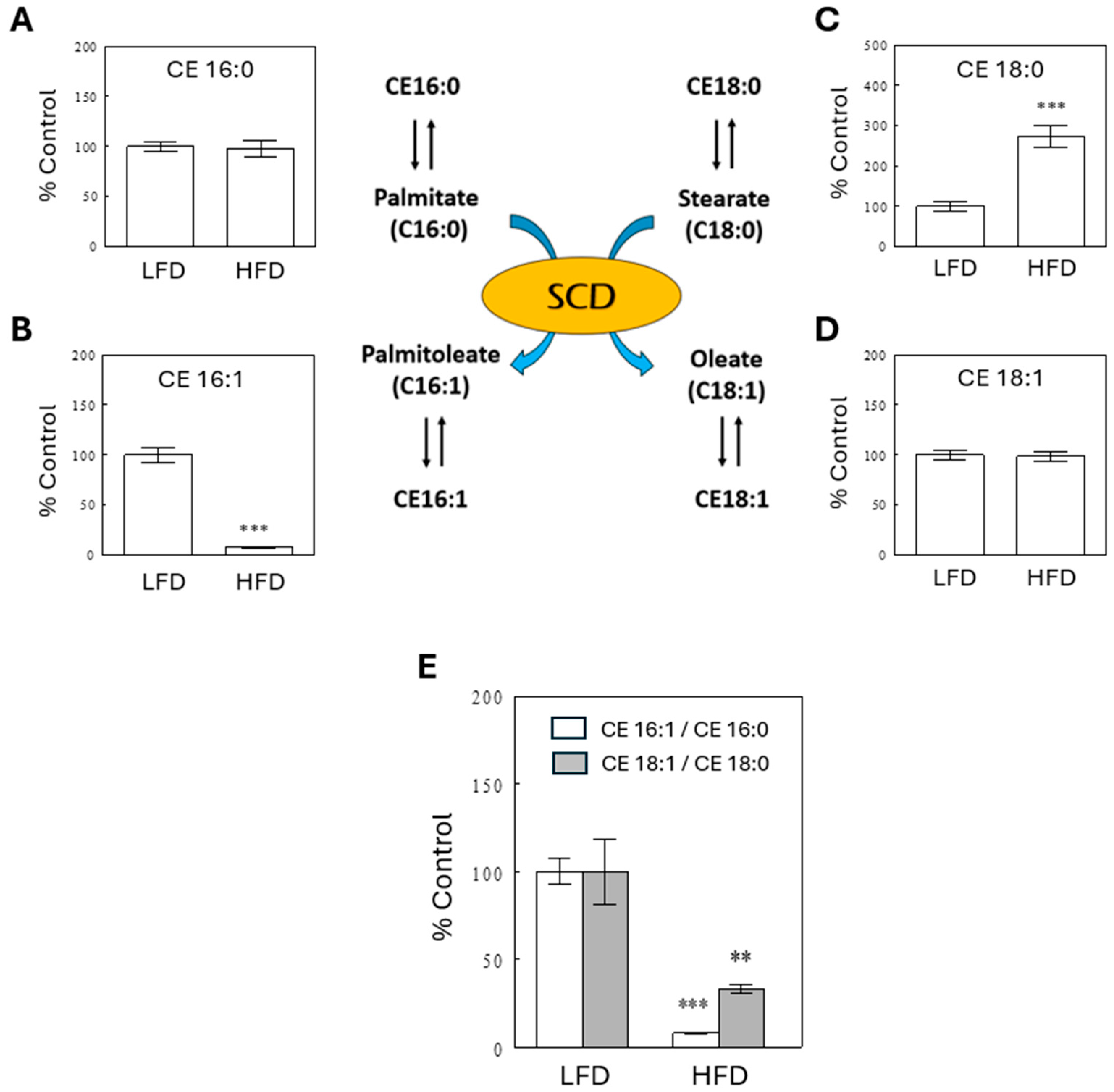
2.5. Decreased SCD Activity Indices in the HFD Group
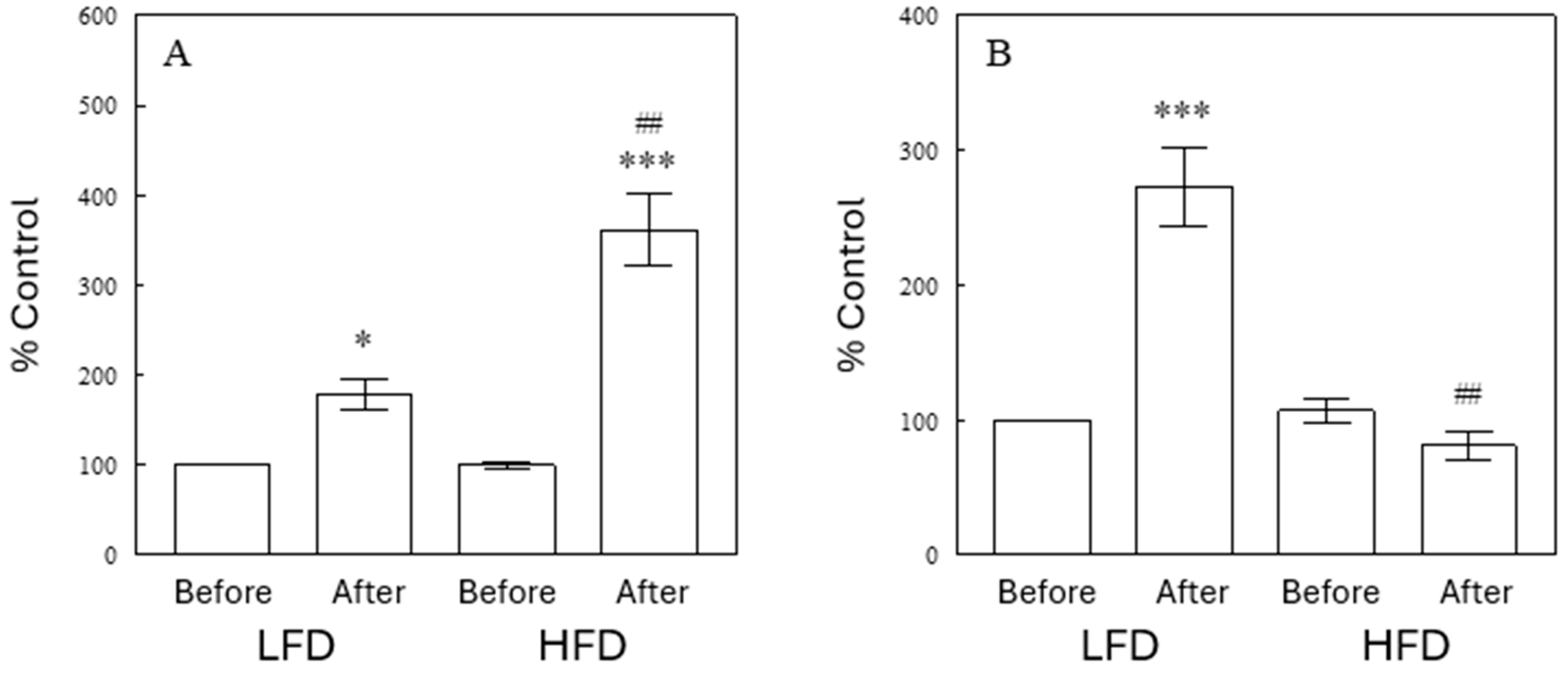
2.6. Depletion of Circulating ω-3 FFAs by HFD
2.7. Effects of Diet and Meal on Oxidized TGs and Ether TGs
2.8. Lipids and Markers of Lipid Metabolism Detected by GC-TOF MS
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Chromatographic and Mass Spectrometric Conditions for Lipidomics Analysis
4.3. LC-MS Data Processing Using MS-DIAL
4.4. Chemical Enrichment Analysis
4.5. GC-TOF MS Analysis for Primary Metabolism
4.6. Other Assays
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lewis, G.F.; Hegele, R.A. Effective, disease-modifying, clinical approaches to patients with mild-to-moderate hypertriglyceridaemia. Lancet Diabetes Endocrinol. 2022, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.P.; Cheng, S.; Larson, M.G.; Walford, G.A.; Lewis, G.D.; McCabe, E.; Yang, E.; Farrell, L.; Fox, C.S.; O’Donnell, C.J.; et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J. Clin. Investig. 2011, 121, 1402. [Google Scholar] [CrossRef] [PubMed]
- Folz, J.; Oh, Y.T.; Blaženović, I.; Richey, J.; Fiehn, O.; Youn, J.H. Interaction of gut microbiota and high-sodium, low-potassium diet in altering plasma triglyceride profiles revealed by lipidomics analysis. Mol. Nutr. Food Res. 2019, 63, e1900752. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.; Seppänen-Laakso, T.; Yetukuri, L.; Agren, J.; Kolehmainen, M.; Laaksonen, D.E.; Ruskeepää, A.L.; Gylling, H.; Uusitupa, M.; Oresic, M.; et al. Triacylglycerol fatty acid composition in diet-induced weight loss in subjects with abnormal glucose metabolism—The GENOBIN study. PLoS ONE 2008, 3, e2630. [Google Scholar] [CrossRef] [PubMed]
- Wali, J.A.; Jarzebska, N.; Raubenheimer, D.; Simpson, S.J.; Rodionov, R.N.; O’Sullivan, J.F. Cardio-metabolic effects of high-fat diets and their underlying mechanisms-a narrative review. Nutrients 2020, 12, 1505. [Google Scholar] [CrossRef] [PubMed]
- McDonough, A.A.; Veiras, L.C.; Guevara, C.A.; Ralph, D.L. Cardiovascular benefits associated with higher dietary K+ vs. lower dietary Na+: Evidence from population and mechanistic studies. Am. J. Physiol.-Endo Metab. 2017, 312, E348. [Google Scholar] [CrossRef] [PubMed]
- Aaron, K.J.; Sanders, P.W. Role of dietary salt and potassium intake in cardiovascular health and disease: A review of the evidence. Mayo Clin. Proc. 2013, 88, 987. [Google Scholar] [CrossRef]
- Olga, L.; van Diepen, J.A.; Bobeldijk-Pastorova, I.; Gross, G.; Prentice, P.M.; Snowden, S.G.; Furse, S.; Kooistra, T.; Hughes, I.A.; Schoemaker, M.H.; et al. Lipid ratios representing SCD1, FADS1, and FADS2 activities as candidate biomarkers of early growth and adiposity. EBioMedicine 2021, 63, 103198. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Virk, R.; Van Dyke, T.E. Potential mechanisms by which hydroxyeicosapentaenoic acids regulate glucose homeostasis in obesity. Adv. Nutr. 2022, 13, 2316. [Google Scholar] [CrossRef]
- Jiang, S.; Han, S.; Wang, D.W. The involvement of soluble epoxide hydrolase in the development of cardiovascular diseases through epoxyeicosatrienoic acids. Front. Pharmacol. 2024, 15, 1358256. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.; Soto-Alarcón, S.A.; Orellana, P.; Espinosa, A.; Campos, C.; López-Arana, S.; Rincón, M.A.; Illesca, P.; Valenzuela, R.; Videla, L.A. Suppression of high-fat diet-induced obesity-associated liver mitochondrial dysfunction by docosahexaenoic acid and hydroxytyrosol co-administration. Dig. Liver Dis. 2020, 52, 895. [Google Scholar] [CrossRef]
- Illesca, P.; Valenzuela, R.; Espinosa, A.; Echeverría, F.; Soto-Alarcon, S.; Campos, C.; Rodriguez, A.; Vargas, R.; Magrone, T.; Videla, L.A. Protective effects of eicosapentaenoic acid plus hydroxytyrosol supplementation against white adipose tissue abnormalities in mice fed a high-fat diet. Molecules 2020, 25, 4433. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Espinosa, A.; González-Mañán, D.; D’Espessailles, A.; Fernández, V.; Videla, L.A.; Tapia, G. N-3 long-chain polyunsaturated fatty acid supplementation significantly reduces liver oxidative stress in high fat induced steatosis. PLoS ONE 2012, 7, e46400. [Google Scholar] [CrossRef]
- Chu, K.Y.; Mellet, N.; Thai, L.M.; Meikle, P.J.; Biden, T.J. Short-term inhibition of autophagy benefits pancreatic β-cells by augmenting ether lipids and peroxisomal function, and by countering depletion of n-3 polyunsaturated fatty acids after fat-feeding. Mol. Metab. 2020, 40, 101023. [Google Scholar] [CrossRef] [PubMed]
- Matikainen, N.; Adiels, M.; Söderlund, S.; Stennabb, S.; Ahola, T.; Hakkarainen, A.; Borén, J.; Taskinen, M.R. Hepatic lipogenesis and a marker of hepatic lipid oxidation, predict postprandial responses of triglyceride-rich lipoproteins. Obesity (Silver Spring) 2014, 22, 1854. [Google Scholar] [CrossRef] [PubMed]
- Biddinger, S.B.; Almind, K.; Miyazaki, M.; Kokkotou, E.; Ntambi, J.M.; Ronald, K. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes 2005, 54, 1314. [Google Scholar] [CrossRef]
- Ampong, I.; John Ikwuobe, O.; Brown, J.E.P.; Bailey, C.J.; Gao, D.; Gutierrez-Merino, J.; Griffiths, H.R. Odd chain fatty acid metabolism in mice after a high fat diet. Int. J. Biochem. Cell Biol. 2022, 143, 106135. [Google Scholar] [CrossRef]
- Gianotti, T.F.; Burgueño, A.; Mansilla, N.G.; Pirola, C.J.; Sookoian, S. Fatty liver is associated with transcriptional downregulation of stearoyl-CoA desaturase and impaired protein dimerization. PLoS ONE 2013, 8, e76912. [Google Scholar]
- Warensjo, E.; Riserus, U.; Gustafsson, I.B.; Mohsen, R.; Cederholm, T.; Vessby, B. Effects of saturated and unsaturated fatty acids on estimated desaturase activities during a controlled dietary intervention. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 683. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Miyazaki, M.; Stoehr, J.P.; Lan, H.; Kendziorski, C.M.; Yandell, B.S.; Song, Y.; Cohen, P.; Friedman, J.M.; Attie, A.D. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 2002, 99, 11482. [Google Scholar] [CrossRef]
- Miyazaki, M.; Kim, Y.C.; Gray-Keller, M.P.; Attie, A.D.; Ntambi, J.M. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J. Biol. Chem. 2000, 275, 30132. [Google Scholar] [CrossRef]
- Miyazaki, M.; Flowers, M.T.; Sampath, H.; Chu, K.; Otzelberger, C.; Liu, X.; Ntambi, J.M. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007, 6, 484. [Google Scholar] [CrossRef]
- Miyazaki, M.; Sampath, H.; Liu, X.; Flowers, M.T.; Chu, K.; Dobrzyn, A.; Ntambi, J.M. Stearoyl-CoA desaturase-1 deficiency attenuates obesity and insulin resistance in leptin-resistant obese mice. Biochem. Biophys. Res. Commun. 2009, 380, 818. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Gerhold, K.; Mayers, J.R.; Wiest, M.M.; Watkins, S.M.; Hotamisligil, G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008, 134, 933. [Google Scholar] [CrossRef]
- Stefan, N.; Kantartzis, K.; Celebi, N.; Staiger, H.; Machann, J.; Schick, F.; Cegan, A.; Elcnerova, M.; Schleicher, E.; Fritsche, A.; et al. Circulating palmitoleate strongly and independently predicts insulin sensitivity in humans. Diabetes Care 2010, 33, 405. [Google Scholar] [CrossRef]
- Fabbrini, E.; Magkos, F.; Su, X.; Abumrad, N.A.; Nejedly, N.; Coughlin, C.C.; Okunade, A.L.; Patterson, B.W.; Klein, S. Insulin sensitivity is not associated with palmitoleate availability in obese humans. J. Lipid. Res. 2011, 52, 808. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.M.; Sakr, H.F. Simvastatin and vitamin E effects on cardiac and hepatic oxidative stress in rats fed on high fat diet. J. Physiol. Biochem. 2013, 69, 737. [Google Scholar] [CrossRef]
- Sarna, L.K.; Wang, N.W.P.; Hwang, S.; Siow, Y.L.; Karmin, O. Folic acid supplementation attenuates high fat diet induced hepatic oxidative stress via regulation of NADPH oxidase. Can. J. Physiol. Pharmacol. 2012, 90, 155. [Google Scholar] [CrossRef]
- Dean, J.M.; Lodhi, I.J. Structural and functional roles of ether lipids. Protein Cell 2018, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Goodenowe, D.B.; Haroon, J.; Kling, M.A.; Zielinski, M.; Mahdavi, K.; Habelhah, B.; Shtilkind, L.; Jordan, S. Targeted Plasmalogen Supplementation: Effects on Blood Plasmalogens, Oxidative Stress Biomarkers, Cognition, and Mobility in Cognitively Impaired Persons. Front. Cell Dev. Biol. 2022, 10, 864842. [Google Scholar] [CrossRef]
- Engelmann, B. Plasmalogens: Targets for oxidants and major lipophilic antioxidants. Biochem. Soc. Trans. 2004, 32, 147. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandusse, S.; McWalter, K.; Te Brinke, H.; IJlst, L.; Mooijer, P.M.; Ruiter, J.P.N.; van Lint, A.E.M.; Pras-Raves, M.; Wever, E.; Millan, F.; et al. An autosomal dominant neurological disorder caused by de novo variants in FAR1 resulting in uncontrolled synthesis of ether lipids. Genet. Med. 2021, 23, 740. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Cao, L.-P.; Du, J.-L.; He, Q.; Gu, Z.-Y.; Jeney, G.; Xu, P.; Yin, G.-J. Effects of high-fat diet on antioxidative status, apoptosis and inflammation in liver of tilapia (Oreochromis niloticus) via Nrf2, TLRs and JNK pathways. Fish Shellfish Immunol. 2020, 104, 391. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Jiang, T.; Bao, Y.-Y.; Chen, G.-J.; Chen, L.; Zhang, Q.; Lu, Y.-X. Fenofibrate improves high-fat diet-induced and palmitate-induced endoplasmic reticulum stress and inflammation in skeletal muscle. Life Sci. 2016, 157, 158. [Google Scholar] [CrossRef] [PubMed]
- Cajka, T.; Smilowitz, J.T.; Fiehn, O. Validating quantitative untargeted lipidomics across nine liquid chromatography-high-resolution mass spectrometry platforms. Anal. Chem. 2017, 89, 12360. [Google Scholar] [CrossRef] [PubMed]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137. [Google Scholar] [CrossRef]
- Koelmel, J.P.; Kroeger, N.M.; Gill, E.L.; Ulmer, C.Z.; Bowden, J.A.; Patterson, R.E.; Yost, R.A.; Garrett, T.J. Expanding lipidome coverage using LC-MS/MS data-dependent acquisition with automated exclusion list generation. J. Am. Soc. Mass Spectrom. 2017, 28, 908. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Ikeda, K.; Takahashi, M.; Satoh, A.; Mori, Y.; Uchino, H.; Okahashi, N.; Yamada, Y.; Tada, I.; Bonini, P.; et al. A lipidome atlas in MS-DIAL 4. Nat. Biotechnol. 2020, 38, 1159. [Google Scholar] [CrossRef]
- Defelice, B.C.; Mehta, S.S.; Samra, S.; Wancewicz, B.; Fahrmann, J.F.; Fiehn, O. Mass Spectral Feature List Optimizer (MS-FLO): A tool to minimize false positive peak reports in untargeted liquid chromatography-mass spectroscopy (LC-MS) data processing. Anal. Chem. 2017, 89, 3250. [Google Scholar] [CrossRef]
- Barupal, D.K.; Fiehn, O. Chemical Similarity Enrichment Analysis (ChemRICH) as alternative to biochemical pathway mapping for metabolomic data sets. Sci. Rep. 2017, 7, 14567. [Google Scholar] [CrossRef]
- Barupal, D.K.; Zhang, Y.; Shen, T.; Fan, S.; Roberts, B.S.; Fitzgerald, P.; Wancewicz, B.; Valdiviez, L.; Wohlgemuth, G.; Byram, G.; et al. A comprehensive plasma metabolomics dataset for a cohort of mouse knockouts within the international mouse phenotyping consortium. Metabolites 2019, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Skogerson, K.; Wohlgemuth, G.; Barupal, D.K.; Fiehn, O. The volatile compound BinBase mass spectral database. BMC Bioinform. 2011, 12, 321. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486. [Google Scholar] [CrossRef]


| LFD (10% Fat) | HFD (60% Fat) | |
|---|---|---|
| Food composition (g/kg) | ||
| Casein | 210 | 265 |
| Lard | 20 | 310 |
| Soybean oil | 20 | 30 |
| Corn starch | 465 | 0 |
| Maltodextrin | 100 | 160 |
| Sucrose | 90 | 90 |
| Body weight and energy intake | ||
| Initial body weight (g) | 213 ± 2 | 210 ± 2 |
| Final body weight (g) | 316 ± 3 | 323 ± 4 |
| Energy intake (Kcal/day) week 1 | 77.2 ± 1.3 | 88.7 ± 1.5 * |
| week 2 | 74.8 ± 1.3 | 78.1 ± 1.3 |
| Fold Change | p Value | FDR | |
|---|---|---|---|
| FA 16:1 (palmitoleic acid) | 0.29 | 0.00001 | 0.00011 |
| FA 20:5 (eicosapentaenoic acid) | 0.30 | 0.00004 | 0.00027 |
| FA 22:6 (docosahexaenoic acid) | 0.57 | 0.00389 | 0.01152 |
| FA 18:3 (γ-linolenic acid) | 0.56 | 0.00520 | 0.01497 |
| FA 14:1 (physeteric acid) | 0.60 | 0.00893 | 0.02376 |
| FA 16:2 | 0.74 | 0.02881 | 0.06344 |
| FA 20:3 (homo-gamma-linolenic acid) | 0.71 | 0.05243 | 0.10558 |
| FA 24:1 | 0.89 | 0.07526 | 0.14067 |
| FA 20:4 b | 1.34 | 0.12301 | 0.21000 |
| FA 18:2 | 0.79 | 0.16549 | 0.26751 |
| FA 26:1 | 0.86 | 0.16549 | 0.26751 |
| FA 20:2 | 1.22 | 0.21756 | 0.33250 |
| FA 20:2 (eicosadienoic acid) | 1.22 | 0.21756 | 0.33250 |
| FA 20:4 | 0.84 | 0.21756 | 0.33250 |
| FA 13:0 (tridecylic acid) | 0.93 | 0.39305 | 0.51721 |
| FA 15:1 | 0.85 | 0.39305 | 0.51721 |
| FA 17:1 | 0.85 | 0.39305 | 0.51721 |
| FA 19:1 | 1.16 | 0.43587 | 0.55648 |
| FA 18:1 | 0.93 | 0.63053 | 0.73427 |
| FA 22:2 (docosadienoic acid) | 0.95 | 0.68421 | 0.77572 |
| FA 22:4 | 1.07 | 0.68421 | 0.77572 |
| FA 20:1 (eicosenoic acid) | 1.12 | 0.91180 | 0.93357 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, T.; Oh, Y.; Jeong, S.; Cho, S.; Fiehn, O.; Youn, J.H. High-Fat Feeding Alters Circulating Triglyceride Composition: Roles of FFA Desaturation and ω-3 Fatty Acid Availability. Int. J. Mol. Sci. 2024, 25, 8810. https://doi.org/10.3390/ijms25168810
Shen T, Oh Y, Jeong S, Cho S, Fiehn O, Youn JH. High-Fat Feeding Alters Circulating Triglyceride Composition: Roles of FFA Desaturation and ω-3 Fatty Acid Availability. International Journal of Molecular Sciences. 2024; 25(16):8810. https://doi.org/10.3390/ijms25168810
Chicago/Turabian StyleShen, Tong, Youngtaek Oh, Shinwu Jeong, Suengmok Cho, Oliver Fiehn, and Jang H. Youn. 2024. "High-Fat Feeding Alters Circulating Triglyceride Composition: Roles of FFA Desaturation and ω-3 Fatty Acid Availability" International Journal of Molecular Sciences 25, no. 16: 8810. https://doi.org/10.3390/ijms25168810






