Hypoxic Cardioprotection by New Antihypertensive Compounds in High Salt-Diet Hypertensive Rats: Glucose Transport Participation and Its Possible Pathway
Abstract
1. Introduction
2. Results
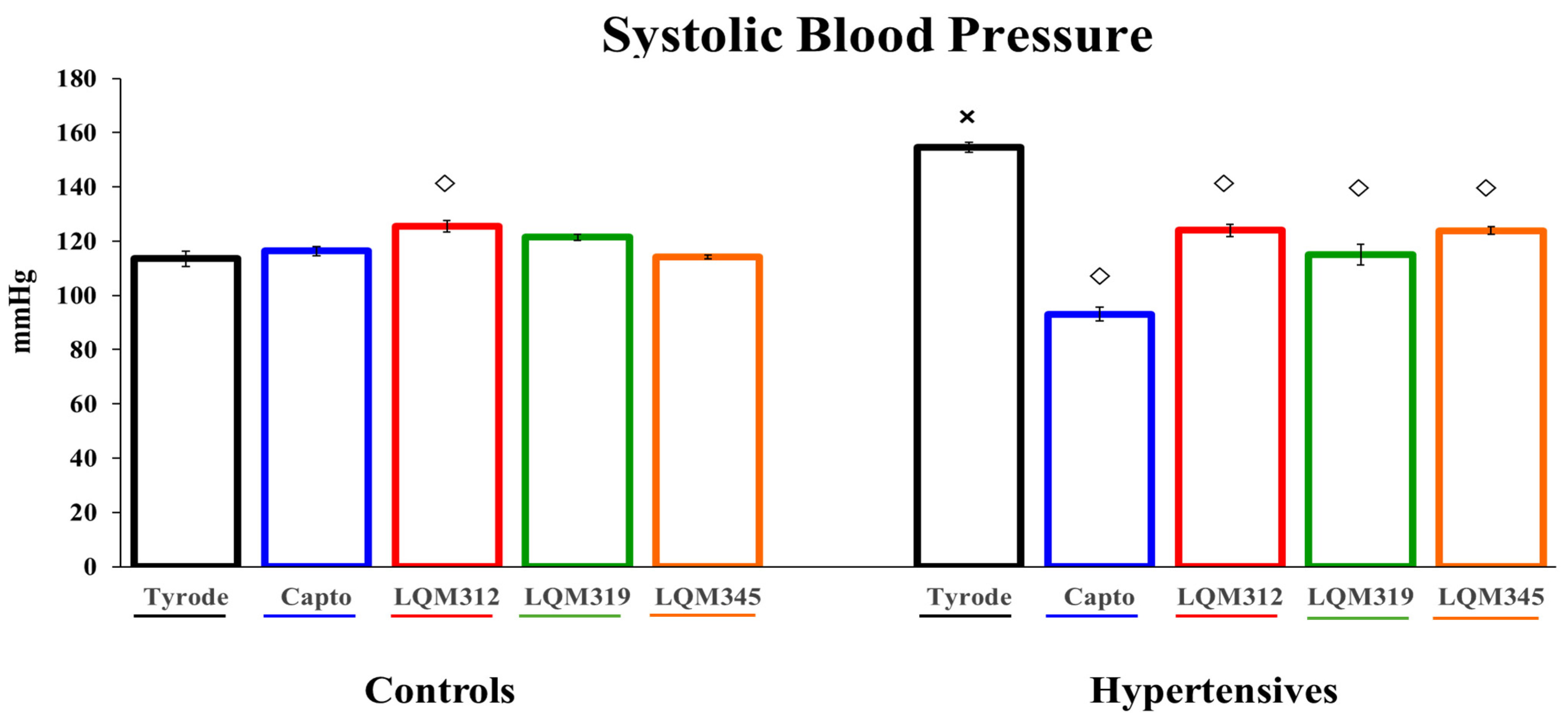
2.1. HP Model
2.2. Antihypertensive Effect of Captopril and LQMs
2.3. Cardiomyocyte Glucose Uptake
2.3.1. Controls
2.3.2. Hypertensives
2.4. Captopril Effect on Glucose Uptake
2.4.1. Controls
2.4.2. Hypertensives

2.5. The LQM312 Effect on Glucose Uptake
2.5.1. Controls
2.5.2. Hypertensives
2.6. The LQM319 Effect on Glucose Uptake
2.6.1. Controls
2.6.2. Hypertensives
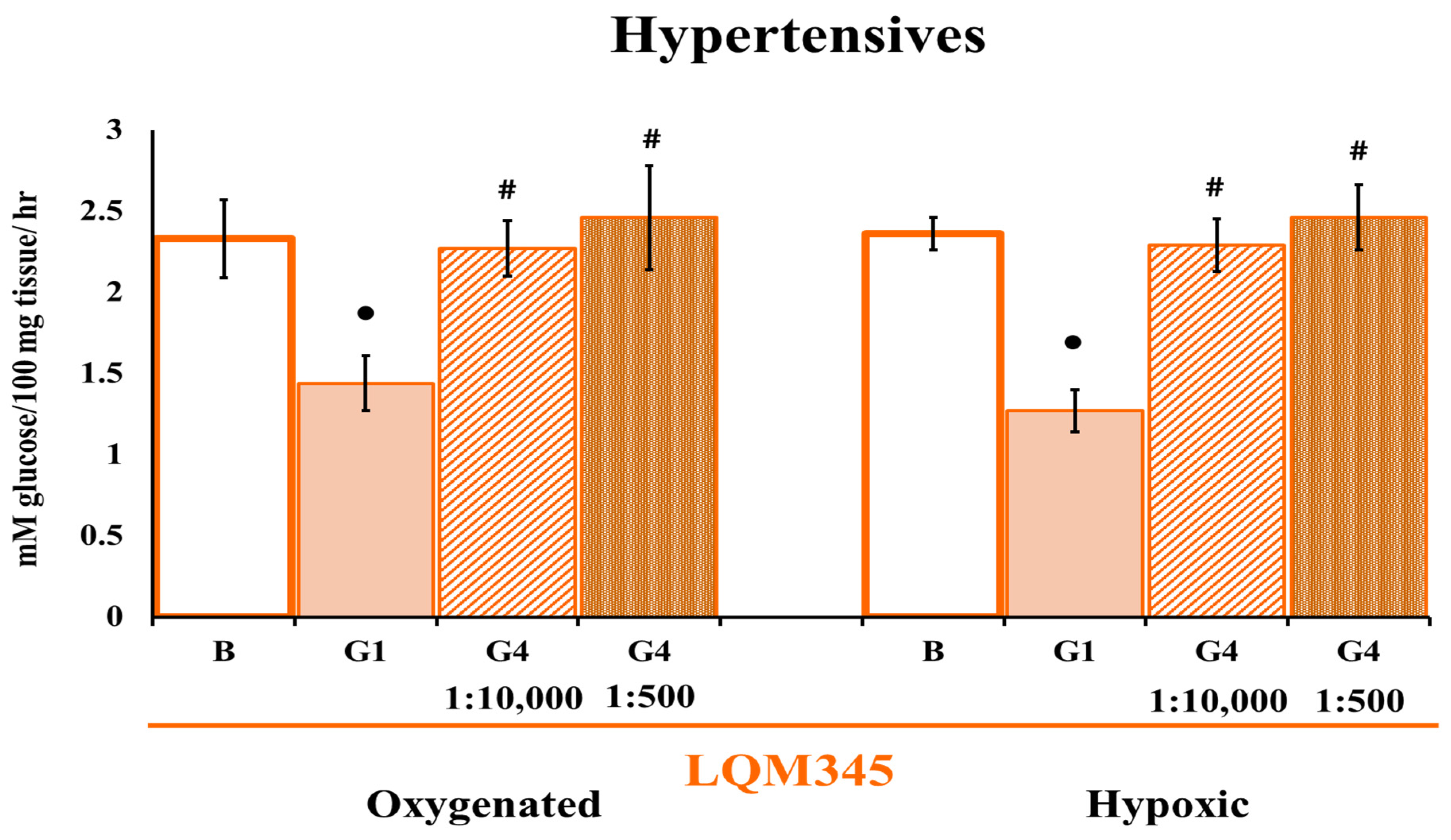
2.7. The LQM345 Effect on Glucose Uptake
2.7.1. Controls
2.7.2. Hypertensives
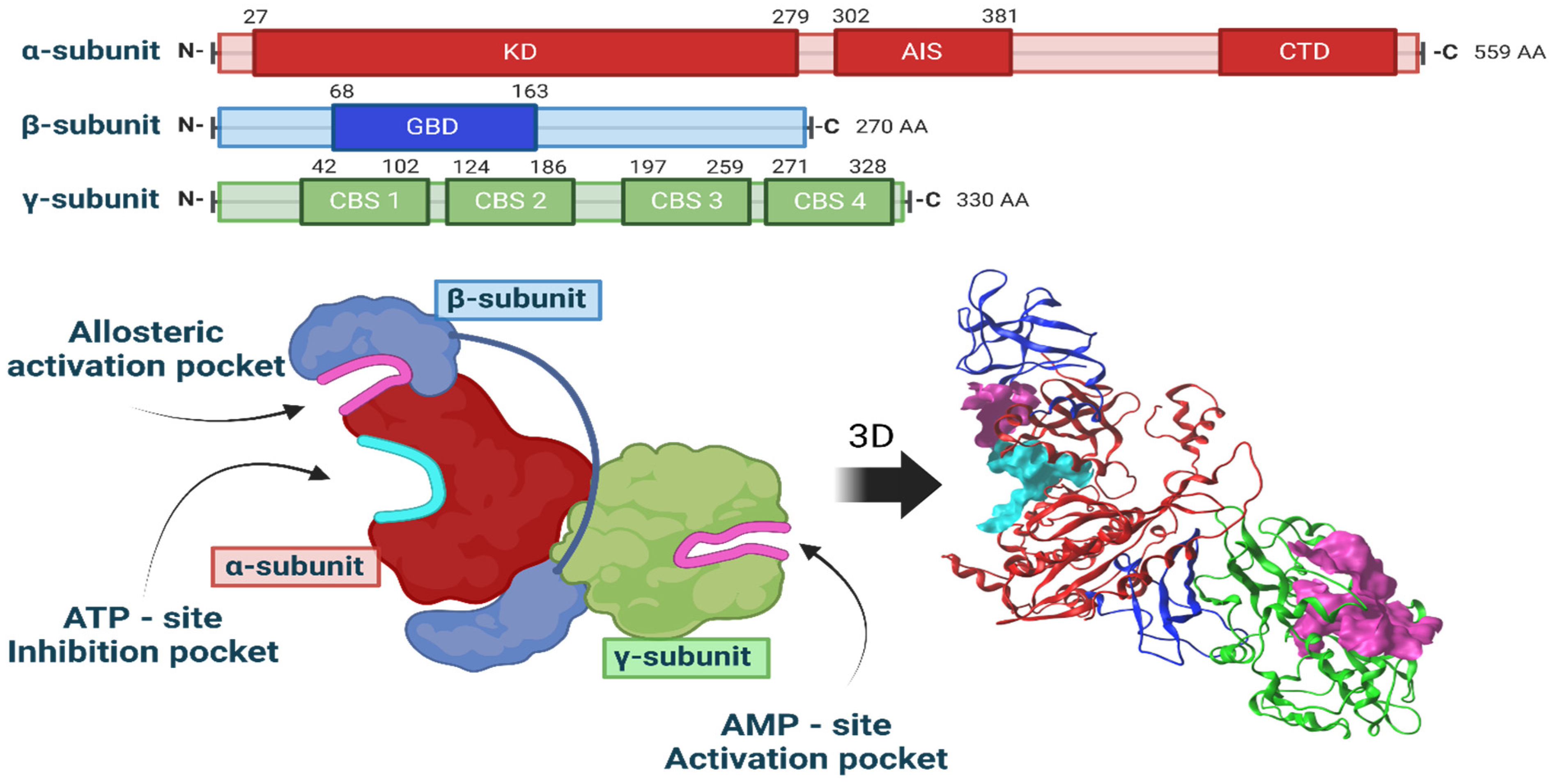
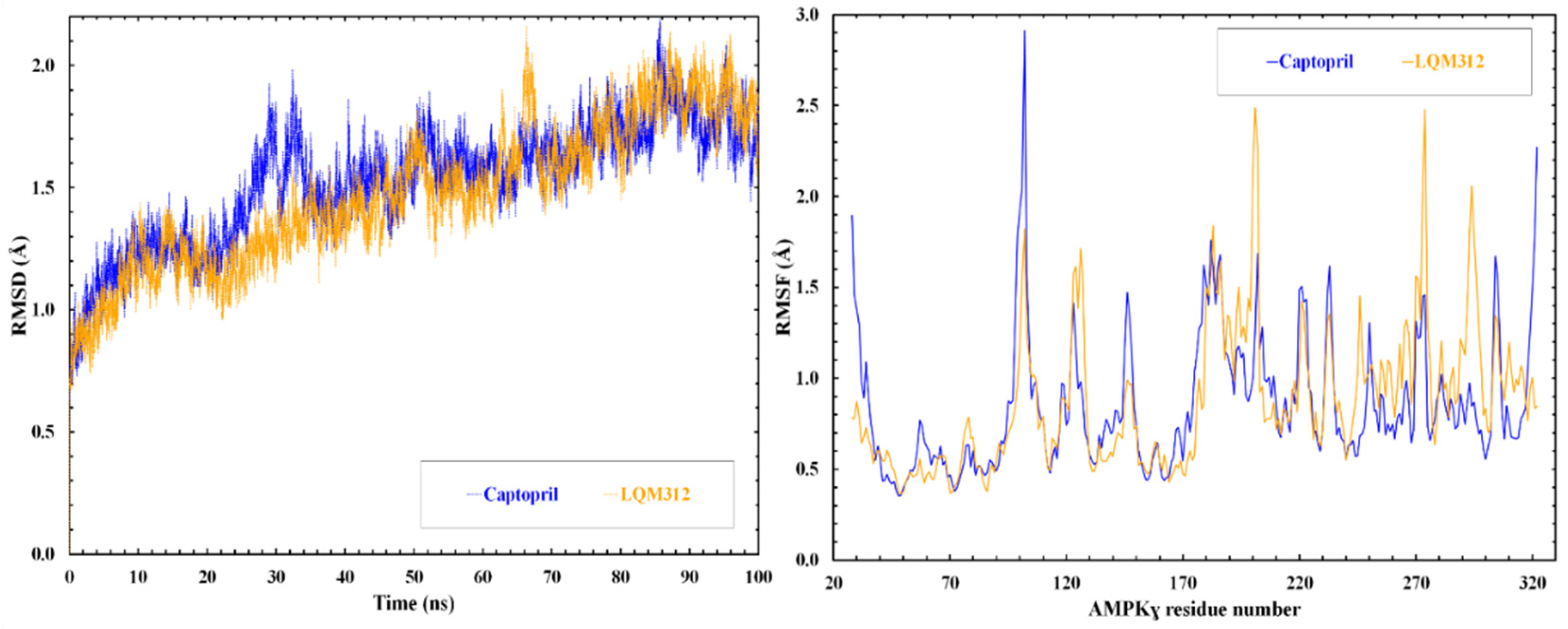
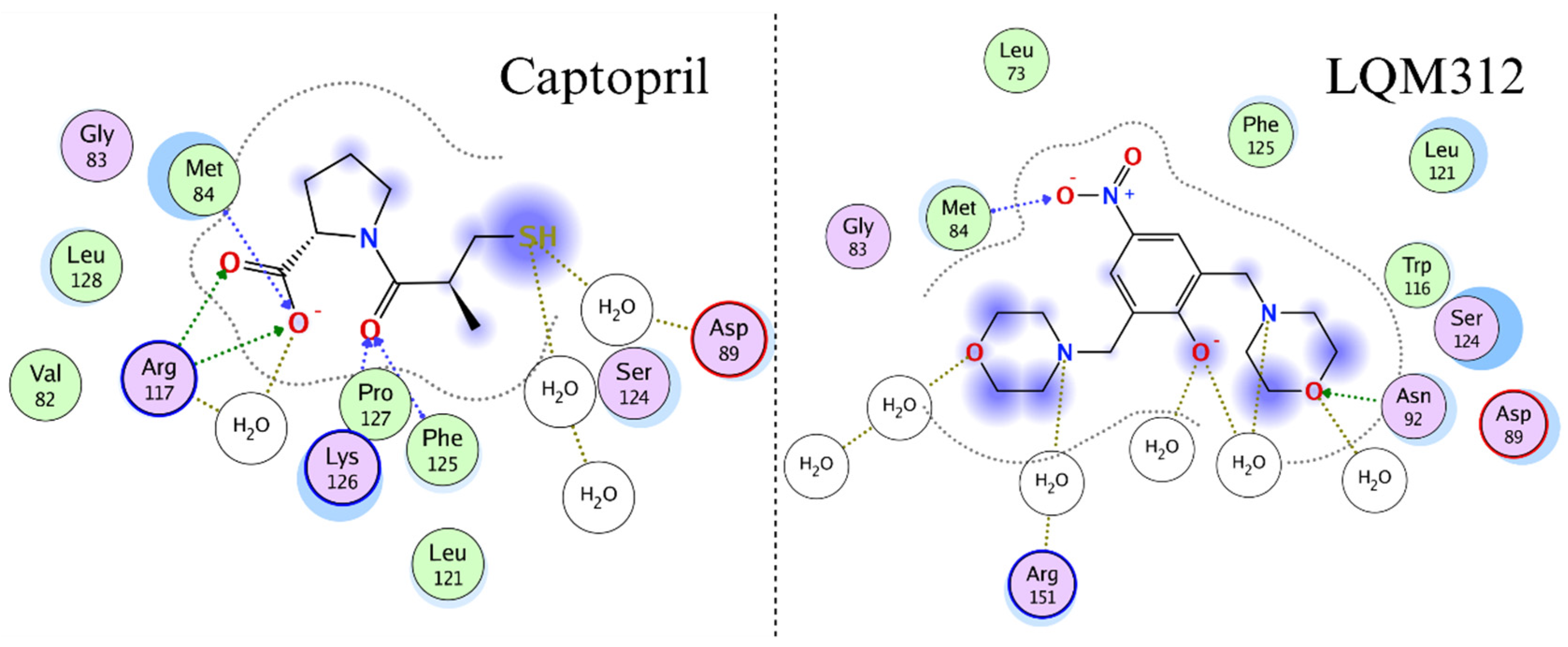
2.8. Computational Studies of Molecular Interactions
3. Discussion
4. Materials and Methods
4.1. Chemical Compounds and General Synthesis Methodology
- 2,6-bis(morpholinomethyl)-4-nitrophenol (LQM312). M.p. 108–110 °C. Yield 85%. Reaction time: 10 min. IR (cm−1; CHCl3 film) 3376, 3026, 2952, 1725, 1600. 1H-NMR (200 MHz; DMSO-d6; Me4Si, δH): 8.02 (2H, s), 4.7 (1H, s, OH), 374–3.72 (8H, m), 3.70 (4H, s), 2.58–253 (8H, m). 13C-NMR (δC): 163.29, 138.57, 124.13, 123.18, 65.97, 57.23, 52.26. FAB-MS (M + 1) 338 (7%). Calculated for C16H23N3O5. C 51.73%, H 6.78%, N 11.31%, O 12.92%, S 17.26%.
- 4-(tert-butyl)-2,6-bis(thiomorpholinomethyl)phenol (LQM319). IR (CHCl3 film) cm−1 3456 (OH), 3197 (Csp2-H Ar), 2886 (Csp3-H). 1H NMR (CDCl3) d: 10.33 (1H, s, OH), 7.18 (1H, dd, J = 8.4 Hz, 2.7 Hz), 6.94 (1H, d, J = 2.7 Hz), 6.74 (1H, d, J = 8.4 Hz), 3.70 (2H, s, Ar-CH2), 2.82 (4H, m, -S-CH2-), 2.71 (4H, m, -N-CH2-),1.27 (9H, CH3). 13C NMR (CDCl3) d: 155 (C), 141.8 (C), 125.60 (CH), 125.49 (CH), 119.77 (C), 115.47 (CH), 62.51 (Ar-CH2), 54.36 (-N-CH2-), 33.84 (C), 31.48 (CH3), 27.79 (-S-CH2-). FAB-MS m/z (M + 1) 266 (80%), 265 (100%),163 (45%).
- 4-nitro-2,6-bis(piperidin-1-ylmethyl)phenol (LQM345). Recrystallized from ethyl acetate yellow plates. mp 109–111 °C. 80% yield. IR (CHCl3 film) cm−1, 3450 (OH), 3150 (Csp2H Ar), 2903 (Csp3H). 1H NMR (CDCl3) δ ppm: 8.93 (1H, s, OH), 8.07 (2H, s), 3.81 (2H, s, Ar-CH2), 2.66 (4H, m), 1.72 (4H, m, CH2); 1.52 (2H, m, CH2). 13C NMR (CDCl3) δ ppm: 164.74 (C), 138.93 (C), 125.83 (CH), 121.44 (C), 57.91 (Ar-CH2), 53.34 (CH2), 24.84 (CH2), 23.37 (CH2). FAB-MS m/z (M + 1) 334 (35%), 240 (100%, M − C5H10N), 226 (23%).
4.2. High-Salt Diet
4.3. Biological Models
4.4. Evaluation of the High Blood Pressure Model
4.5. Captopril and LQM Stock Solutions
4.6. Isolation of Cardiac Myocytes
4.7. Experimental Procedure
- Basal conditions: Normal Tyrode.
- G1: Normal Tyrode with a goat polyclonal IgG anti-GLUT1 antibody directed to the extracellular domain (SC-1603, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:1000 in Tyrode.
- G4: Normal Tyrode with a goat polyclonal IgG GLUT4 antibody directed to the extracellular domain (SC-1606, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:10,000 in Tyrode.
- G4 for HP cells in LQM345 repetition experiments: Normal Tyrode with a goat polyclonal IgG GLUT4 antibody directed to the extracellular domain (SC-1606, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:500 in Tyrode.
4.8. Determination of Glucose Uptake
4.9. Molecular Docking and Molecular Dynamics Simulation
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kućmierz, J.; Frąk, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Molecular Interactions of Arterial Hypertension in Its Target Organs. Int. J. Mol. Sci. 2021, 22, 9696. [Google Scholar] [CrossRef] [PubMed]
- Rouette, J.; McDonald, E.G.; Schuster, T.; Brophy, J.M.; Azoulay, L. Treatment and prescribing trends of antihypertensive drugs in 2.7 million UK primary care patients over 31 years: A population-based cohort study. BMJ Open 2022, 12, e057510. [Google Scholar] [CrossRef]
- Reddy, P.; Dupree, L. Approach to Antihypertensive Therapy. Am. J. Ther. 2016, 23, e451–e473. [Google Scholar] [CrossRef]
- Cutler, J.A. High blood pressure and end-organ damage. J. Hypertens. Suppl. 1996, 14, S3–S6. [Google Scholar] [PubMed]
- Polak-Iwaniuk, A.; Harasim-Symbor, E.; Golaszewska, K.; Chabowski, A. How Hypertension Affects Heart Metabolism. Front. Physiol. 2019, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Klip, A.; McGraw, T.E.; James, D.E. Thirty sweet years of GLUT4. J. Biol. Chem. 2019, 294, 11369–11381. [Google Scholar] [CrossRef] [PubMed]
- Stockli, J.; Fazakerley, D.J.; James, D.E. GLUT4 exocytosis. J. Cell. Sci. 2011, 124 Pt 24, 4147–4159. [Google Scholar] [CrossRef]
- Carbó, R.; Rodríguez, E. Relevance of Sugar Transport across the Cell Membrane. Int. J. Mol. Sci. 2023, 24, 6085. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, R.E.; Wilkinson, K.A.; Craig, T.J. Insulin-dependent GLUT4 trafficking is not regulated by protein SUMOylation in L6 myocytes. Sci. Rep. 2019, 9, 6477. [Google Scholar] [CrossRef]
- Zhou, L.; Cryan, E.V.; D’Andrea, M.R.; Belkowski, S.; Conway, B.R.; Demarest, K.T. Human cardiomyocytes express high level of Na+/glucose transporter 1 (SGLT1). J. Cell. Biochem. 2003, 90, 339–346. [Google Scholar] [CrossRef]
- van Gerwen, J.; Shun-Shion, A.S.; Fazakerley, D.J. Insulin signalling and GLUT4 trafficking in insulin resistance. Biochem. Soc. Trans. 2023, 51, 1057–1069. [Google Scholar] [CrossRef]
- Till, M.; Kolter, T.; Eckel, J. Molecular mechanisms of contraction-induced translocation of GLUT4 in isolated cardiomyocytes. Am. J. Cardiol. 1997, 80, 85A–89A. [Google Scholar] [CrossRef] [PubMed]
- Pulinilkunnil, T.; Nagendran, J.; Dyck, J.R.B. Chapter 5: AMPK and Metabolic Remodeling in Cardiac Disease. In Translational Cardiology, Molecular and Translational Medicine; Patterson, C., Willis, M.S., Eds.; Springer Science + Business Media, LLC: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Sylow, L.; Kleinert, M.; Richter, E.A.; Jensen, T.E. Exercise-stimulated glucose uptake—Regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 2017, 13, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Rodelo, C.; Arellano-Plancarte, A.; Hernandez-Aranda, J.; Landa-Galvan, H.V.; Parra-Mercado, G.K.; Moreno-Licona, N.J.; Hernandez-Gonzalez, K.D.; Catt, K.J.; Villalobos-Molina, R.; Olivares-Reyes, J.A. Angiotensin II Inhibits Insulin Receptor Signaling in Adipose Cells. Int. J. Mol. Sci. 2022, 23, 6048. [Google Scholar] [CrossRef] [PubMed]
- Joyner, N.T.; Smoak, I.W. In Vivo Hyperglycemia and Its Effect on Glut-1 Expression in the Embryonic Heart. Birth Defects Res. (Part A) Clin. Mol. Teratol. 2004, 70, 438–448. [Google Scholar] [CrossRef]
- Mackenzie, R.W.A.; Watt, P. A Molecular and Whole-Body Insight of the Mechanisms Surrounding Glucose Disposal and Insulin Resistance with Hypoxic Treatment in Skeletal Muscle. J. Diabetes Res. 2016, 2016, 6934937. [Google Scholar] [CrossRef] [PubMed]
- Holman, G.D.; Kozka, I.J.; Clark, A.E.; Flower, C.J.; Saltis, J.; Habberfield, A.D.; Simpson, I.A.; Cushman, S.W. Cell surface labeling of glucose transporter isoform GLUT4 by bis-mannose photolabel. Correlation with stimulation of glucose transport in rat adipose cells by insulin and phorbol ester. J. Biol. Chem. 1990, 265, 18172–18179. [Google Scholar] [CrossRef] [PubMed]
- Siques, P.; Brito, J.; Flores, K.; Ordenes, S.; Arriaza, K.; Pena, E.; León-Velarde, F.; López de Pablo, Á.L.; Gonzalez, M.C.; Arribas, S. Long-Term Chronic Intermittent Hypobaric Hypoxia Induces Glucose Transporter (GLUT4) Translocation through AMP-Activated Protein Kinase (AMPK) in the Soleus Muscle in Lean Rats. Front. Physiol. 2018, 9, 799. [Google Scholar] [CrossRef]
- Evans, A.M.; Hardie, D.G. AMPK and the Need to Breathe and Feed: What’s the Matter with Oxygen? Int. J. Mol. Sci. 2020, 21, 3518. [Google Scholar] [CrossRef]
- Nagendran, J.; Waller, T.J.; Dyck, J.R.B. AMPK signalling, and the control of substrate use in the heart. Mol. Cell. Endocrinol. 2013, 366, 180–193. [Google Scholar] [CrossRef]
- Fischer, Y.; Thomas, J.; Sevilla, L.; Muñoz, P.; Becker, C.; Holman, G.; Kozka, I.J.; Palacín, M.; Testar, X.; Kammermeier, H.; et al. Insulin-induced Recruitment of Glucose Transporter 4 (GLUT4) and GLUT1 in Isolated Rat Cardiac Myocytes: Evidence of the Existence of Different Intracellular GLUT4 Vesicle Populations. J. Biol. Chem. 1997, 272, 7085–7092. [Google Scholar] [CrossRef]
- Trang-Nguyen, N.D.; Le, L.T. Targeted proteins for diabetes drug design. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 3001. [Google Scholar] [CrossRef]
- Kamil, S.; Sehested, T.S.G.; Houlind, K.; Lassen, J.F.; Gislason, G.H.; Dominguez, H. Trends in Use of Cardioprotective Medication in Peripheral Artery Disease: A Nationwide Study. J. Am. Heart Assoc. 2021, 10, e020333. [Google Scholar] [CrossRef]
- Matta-Herrera, G.J.; Ballestas-Alarcón, L.M.; Ramírez-Rincón, A. Agonistas de GLP-1 más inhibidores de SGLT2. ¿Efectos cardioprotectores aditivos? Med. Interna De México 2018, 34, 601–613. [Google Scholar] [CrossRef]
- Li, W.; Saud, S.M.; Young, M.R.; Chen, G.; Hua, B. Targeting AMPK for cancer prevention and treatment. Oncotarget 2015, 6, 7365–7378. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.Z.; Rong, P.; Huang, J.; Liu, J.; Cheng, J.H.; Chen, Y.H.; Qiu, Y.T.; Huang, W.L.; Peng, S.X. Antiarrhythmic activities of six indole derivatives of changrolin. Zhongguo Yao Li Xue Bao 1991, 12, 411–415. [Google Scholar] [PubMed]
- Chen, W.H.; Yang, D.; Wang, W.Y.; Zhang, J.; Wang, Y.P. Cellular electrophysiological effects of changrolin in isolated rat cardiac myocytes. Eur. J. Pharmacol. 2010, 647, 139–146. [Google Scholar] [CrossRef]
- Lin, M.L.; Liu, Y.F.; Lu, Y.Y.; Zhang, H.Q.; Zheng, W.M. [Studies on antiarrhythmics--synthesis of 2-[(alkylamino) methyl]-and 2,6-bis-[(alkylamino) methyl]-4-substituted amino phenols (author’s transl)]. Yao Xue Xue Bao 1981, 16, 757–761. [Google Scholar] [PubMed]
- Artico, M.; Mai, A.; Sbardella, G.; Massa, S.; Lampis, G.; Deidda, D.; Pompei, R. N-[4-(1,1’-biphenyl)methyl]-4-(4-thiomorpholinylmethyl) benzenamines as non-oxazolidinone analogues of antimycobacterial U-100480. Bioorg. Med. Chem. Lett. 1998, 8, 1493–1498. [Google Scholar] [CrossRef]
- Biava, M.; Fioravanti, R.; Porretta, G.C.; Deidda, D.; Maullu, C.; Pompei, R. New pyrrole derivatives as antimycobacterial agents’ analogs of BM212. Bioorg. Med. Chem. Lett. 1999, 9, 2983–2988. [Google Scholar] [CrossRef]
- Velazquez, A.M.; Martinez, L.; Abrego, V.; Balboa, M.A.; Torres, L.A.; Camacho, B.; Díaz-Barriga, S.; Romero, A.; López-Castañares, R.; Angeles, E. Synthesis and antihypertensive effects of new methylthiomorpholinphenol derivatives. Eur. J. Med. Chem. 2008, 43, 486–500. [Google Scholar] [CrossRef]
- Zuñiga, O.; Vázquez, V.H.; Velázquez, A.M.; Abrego, V.H.; Arceo, S.D.-B.; Ramírez, P.; Díaz, R.; Ángeles, E. Molecular Docking of 4-Tert-buthyl-bis-(2,6-thiomorpholin-4-ylmethyl)-1-phenol (LQM319) on Fas Receptor (CD95). J. Cancer Ther. 2013, 4, 176–181. [Google Scholar] [CrossRef][Green Version]
- Haworth, R.A.; Hunter, D.R.; Berkoff, H.A. The isolation of Ca2+-resistant myocytes from the adult rat. J. Mol. Cell. Cardiol. 1980, 12, 715–723. [Google Scholar] [CrossRef]
- Bustamante, J.O.; Watanabe, T.; McDonald, T.F. Single cells from adult mammalian heart: Isolation procedure and preliminary electrophysiological studies. Can. J. Physiol. Pharmacol. 1981, 59, 907–910. [Google Scholar] [CrossRef]
- Carbó, R.; Rodríguez, E. A glucose-insulin-potassium solution improves glucose intake in hypoxic cardiomyocytes by a differential expression of glucose transporters in a metabolic syndrome model. J. Biosci. 2019, 44, 19. [Google Scholar] [CrossRef]
- Carbó, R.; Nava, P.; Guarner, V. Effects of polarizing solution on glucose uptake of rat oxygenated or hypoxic ventricular myocytes. Clin. Exp. Pharmacol. Physiol. 2003, 30, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, M.F.; Cytochalasin, B. A natural photoaffinity ligand for labeling the human erythrocyte glucose transporter. J. Biol. Chem. 1982, 257, 7290–7293. [Google Scholar] [CrossRef] [PubMed]
- Bairwa, S.C.; Parajuli, N.; Dyck, J.R.B. The role of AMPK in cardiomyocyte health and survival. Biochim. Et Biophys. Acta 2016, 1862, 2199–2210. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Ramasamy, R.; Hwang, Y.C.; Whang, J.; Bergmann, S.R. Protection of ischemic hearts by high glucose is mediated, in part, by GLUT-4. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H290–H297. [Google Scholar] [CrossRef]
- Dodd, M.S.; Ball, D.R.; Schroeder, M.A.; Le Page, L.M.; Atherton, H.J.; Heather, L.C.; Seymour, A.M.; Ashrafian, H.; Watkins, H.; Clarke, K.; et al. In vivo alterations in cardiac metabolism and function in the spontaneously hypertensive rat heart. Cardiovasc. Res. 2012, 95, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Inaba, M.; Maruno, Y.; Morita, T.; Awata, T.; Oka, Y. Glucose intolerance in spontaneously hypertensive and Wistar-Kyoto rats: Enhanced gene expression and synthesis of skeletal muscle glucose transporter 4. Hypertens. Res. 1997, 20, 279–286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heather, L.C.; Pates, K.M.; Atherton, H.J.; Cole, M.A.; Ball, D.R.; Evans, R.D.; Glatz, J.F.; Luiken, J.J.; Griffin, J.L.; Clarke, K. Differential translocation of the fatty acid transporter, FAT/CD36, and the glucose transporter, GLUT4, coordinates changes in cardiac substrate metabolism during ischemia and reperfusion. Circ. Heart Fail. 2013, 6, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Imahashi, K.; Kusuoka, H.; Hashimoto, K.; Yoshioka, J.; Yamaguchi, H.; Nishimura, T. Intracellular sodium accumulation during ischemia as the substrate for reperfusion injury. Circ. Res. 1999, 84, 1401–1406. [Google Scholar] [CrossRef]
- Martinez, L.A.; Villalobos-Molina, R. Early and chronic Captopril or Losartan therapy reduces infarct size and avoids congestive heart failure after myocardial infarction in rats. Arch. Med. Res. 2003, 34, 357–361. [Google Scholar] [CrossRef]
- Penna, C.; Tullio, F.; Moro, F.; Folino, A.; Merlino, A.; Pagliaro, P. Effects of a protocol of ischemic postconditioning and/or Captopril in hearts of normotensive and hypertensive rats. Basic Res. Cardiol. 2010, 105, 181–192. [Google Scholar] [CrossRef]
- Vázquez-Valaldez, V.H.; Hernández, S.M.A.; Cedillo, I.C.; Velázquez Sanchez, A.M.; Guevara, M.S.; Castañares, R.L.; Ángeles, E. In Silico Elucidation of the Molecular Recognition of Phenol Derivative Compounds and Hippuryl-histidyl-leucine as an Artificial Substrate with the Experimental Target: Angiotensin-converting Enzyme. Lett. Drug Des. Discov. 2020, 17, 445–466. [Google Scholar] [CrossRef]
- Sakagami, H.; Makino, Y.; Mizumoto, K.; Isoe, T.; Takeda, Y.; Watanabe, J.; Fujita, Y.; Takiyama, Y.; Abiko, A.; Masakazu, H. Loss of HIF-1a impairs GLUT4 translocation and glucose uptake by the skeletal muscle cells. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1065–E1076. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, L.; Auquier, J.; Renguet, E.; Angé, M.; Cumps, J.; Horman, S.; Beauloye, C. Glucose transporters in cardiovascular system in health and disease. Pflügers Archiv. Eur. J. Physiol. 2020, 472, 1385–1399. [Google Scholar] [CrossRef]
- Webster, I.; Friedrich, S.O.; Lochner, A.; Huisamen, B. AMP kinase activation and glut4 translocation in isolated cardiomyocytes. Cardiovasc. J. Africa 2010, 21, 72–78. [Google Scholar] [PubMed]
- Islas-Martinez, J.M.; Rodriguez-Barrientos, D.; Galano, A.; Angeles, E.; Torres, L.A.; Olvera, F.; Ramírez-Silva, M.T.; Rojas-Hernández, A. Deprotonation mechanism of new antihypertensive piperidinylmethylphenols: A combined experimental and theoretical study. J. Phys. Chem. B 2009, 113, 11765–11774. [Google Scholar] [CrossRef]
- Vázquez-Valadez, V.H.; Carrillo, I.C.; Ramírez, N.C.; Velázquez-S., A.M.; Abrego, V.H.; López, R.C.; Angeles, E. Evaluation of the Inhibition of Angiotensin-converting Enzyme by New Thiomorpholine Compounds Using Capillary Zone Electrophoresis. J. Mex. Chem. Soc. 2018, 62, 75–85. [Google Scholar] [CrossRef]
- Shao, D.; Tian, R. Glucose Transporters in Cardiac Metabolism and Hypertrophy. Compr. Physiol. 2016, 6, 331–351. [Google Scholar] [CrossRef]
- Dyck, J.R.B.; Sossalla, S.; Hamdani, N.; Coronel, R.; Weber, N.C.; Light, P.E.; Zuurbier, C.J. Cardiac mechanisms of the beneficial effects of SGLT2 inhibitors in heart failure: Evidence for potential off-target effects. J. Mol. Cell. Cardiol. 2022, 167, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.K.; McGaffin, K.R.; Pastor-Soler, N.M.; Ahmad, F. SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc. Res. 2009, 84, 111–118. [Google Scholar] [CrossRef]
- Velázquez, A.M.; Torres, L.A.; Díaz, G.; Ramírez, A.; Hernández, R.; Santillán, H.; Martínez, L.; Martínez, I.; Díaz-Barriga, S.; Abrego, V.; et al. A novel one pot Mannich synthesis of methylpiperidin phenols, methylphenylmorfolin phenols and methyltiophenylmorpholin phenols solvent-free and using infrared light irradiation. Arch. Org. Chem. 2006, 2006, 150–161. [Google Scholar] [CrossRef]
- LabDiet. LabDiet 5001; PMI Nutrition International: Brentwood, MO, USA, 2024; Available online: https://www.labdiet.com/product/detail/5001-laboratory-rodent-diet (accessed on 15 April 2024).
- Flores-Chávez, P.L.; Infante-Vázquez, O.; Sánchez-Torres, G.; Martínez-Memíje, R.; Rodríguez-Rossini, G. A non-invasive method to record vital signs in rats. Vet. Mex. 2002, 33, 179–187. [Google Scholar]
- Allison, D.C.; Ridolpho, P. Use of a trypan blue assay to measure the deoxyribonucleic acid content and radioactive labeling of viable cells. J. Histochem. Cytochem. 1980, 28, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Trinder, P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J. Clin. Pathol. 1969, 22, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Horman, S.; Leclerc, J.; Lantier, L.; Foretz, M.; Billaud, M.; Giri, S.; Andreelli, F. AMPK inhibition in health and disease. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 276–295. [Google Scholar] [CrossRef]
- UniProt, C. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef] [PubMed]
- ULC CCG. Molecular Operating Environment (MOE); Chemical Computing Group ULC: Montreal, QC, Canada, 2022. [Google Scholar]
- Phillips, J.C.; Hardy, D.J.; Maia, J.D.C.; Stone, J.E.; Ribeiro, J.V.; Bernardi, R.C.; Buch, R.; Fiorin, G.; Hénin, J.; Jiang, W.; et al. Scalable Molecular Dynamics on CPU and GPU Architectures with NAMD. J. Chem. Phys. 2020, 153, 044130. [Google Scholar] [CrossRef] [PubMed]
- Communities CotE. Council Directive 86/609/EEC of 24 November 1986 on the Approximation of Laws, Regulations and Administrative Provisions of the Member States Regarding the Protection of Animals Used for Experimental and Other Scientific Purposes. Official Journal of the European Communities, 24 November 1986. [Google Scholar]


| Parameters | Control | HP |
|---|---|---|
| Weight (g) | 280 ± 4.6 | 303.0 ± 5.2 * |
| Systolic pressure (mmHg) | 113.5 ± 2.8 | 152.02 ± 1.5 * |
| Diastolic pressure (mmHg) | 85.6 ± 1.9 | 93.0 ± 3.2 * |
| Heart rate (beats/min) | 394.3 ± 0.1 | 393.14 ± 0.4 * |
| Food (g) | 22.5 ± 2.4 | 35.6 ± 5.2 * |
| Water (mL) | 34.4 ± 4.4 | 168.5 ± 27.2 * |
| Urine (mL) | 3.8 ± 0.5 | 19.2 ± 2.8 * |
| Ligand | AMP Site Score (γ-Subunit) kcal mol−1 | ATP Site Score (α-Subunit) kcal mol−1 |
|---|---|---|
| AMP | −4.91 ± 0.59 | −3.80 ± 0.41 |
| Captopril | −3.26 ± 0.65 | −2.68 ± 0.24 |
| LQM312 | −3.65 ± 0.27 | −3.51 ± 2.7 |
| LQM319 | * | −4.06 ± 0.65 |
| LQM345 | * | −4.41 ± 0.54 |
| LIGAND | LIPINSKI RO5 | |
|---|---|---|
| LQM 312 | 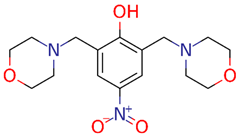 | MW: 337.37 g/mol cLogP(XLOGP3): 0.32 HA: 7 HD: 1 RB: 5 |
| LQM 319 |  | MW: 380.61 g/mol cLogP(XLOGP3): 3.76 HA: 3 HD: 1 RB: 5 |
| LQM 345 | 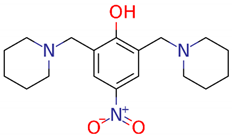 | MW: 333.43 g/mol cLogP(XLOGP3): 2.76 HA: 5 HD: 1 RB: 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Serda, M.A.; Alarcón-López, A.Y.; Vázquez-Valadez, V.H.; Briseño-Lugo, P.; Martínez-Soriano, P.A.; Leguízamo, V.; Torres, N.; González-Terán, R.; Cárdenas-Granados, L.A.; Sánchez Muñoz, F.; et al. Hypoxic Cardioprotection by New Antihypertensive Compounds in High Salt-Diet Hypertensive Rats: Glucose Transport Participation and Its Possible Pathway. Int. J. Mol. Sci. 2024, 25, 8812. https://doi.org/10.3390/ijms25168812
Hernández-Serda MA, Alarcón-López AY, Vázquez-Valadez VH, Briseño-Lugo P, Martínez-Soriano PA, Leguízamo V, Torres N, González-Terán R, Cárdenas-Granados LA, Sánchez Muñoz F, et al. Hypoxic Cardioprotection by New Antihypertensive Compounds in High Salt-Diet Hypertensive Rats: Glucose Transport Participation and Its Possible Pathway. International Journal of Molecular Sciences. 2024; 25(16):8812. https://doi.org/10.3390/ijms25168812
Chicago/Turabian StyleHernández-Serda, Manuel A., Aldo Y. Alarcón-López, Víctor H. Vázquez-Valadez, Paola Briseño-Lugo, Pablo A. Martínez-Soriano, Viridiana Leguízamo, Nalleli Torres, Rodrigo González-Terán, Luis A. Cárdenas-Granados, Fausto Sánchez Muñoz, and et al. 2024. "Hypoxic Cardioprotection by New Antihypertensive Compounds in High Salt-Diet Hypertensive Rats: Glucose Transport Participation and Its Possible Pathway" International Journal of Molecular Sciences 25, no. 16: 8812. https://doi.org/10.3390/ijms25168812
APA StyleHernández-Serda, M. A., Alarcón-López, A. Y., Vázquez-Valadez, V. H., Briseño-Lugo, P., Martínez-Soriano, P. A., Leguízamo, V., Torres, N., González-Terán, R., Cárdenas-Granados, L. A., Sánchez Muñoz, F., Rodríguez, E., Lerma, C., Zúñiga Muñoz, A. M., Ángeles, E., & Carbó, R. (2024). Hypoxic Cardioprotection by New Antihypertensive Compounds in High Salt-Diet Hypertensive Rats: Glucose Transport Participation and Its Possible Pathway. International Journal of Molecular Sciences, 25(16), 8812. https://doi.org/10.3390/ijms25168812









