Repetitive Sequence Stability in Embryonic Stem Cells
Abstract
:1. Introduction
2. Epigenetic Regulation of Repetitive Sequences in ESCs
3. Proteins Involved in DNA Replication Are Likely to Protect Repetitive Sequences from DNA Damage
4. Homologous Recombination (HR) Factors in Replication Fork Protection and Error-Free DNA Repair
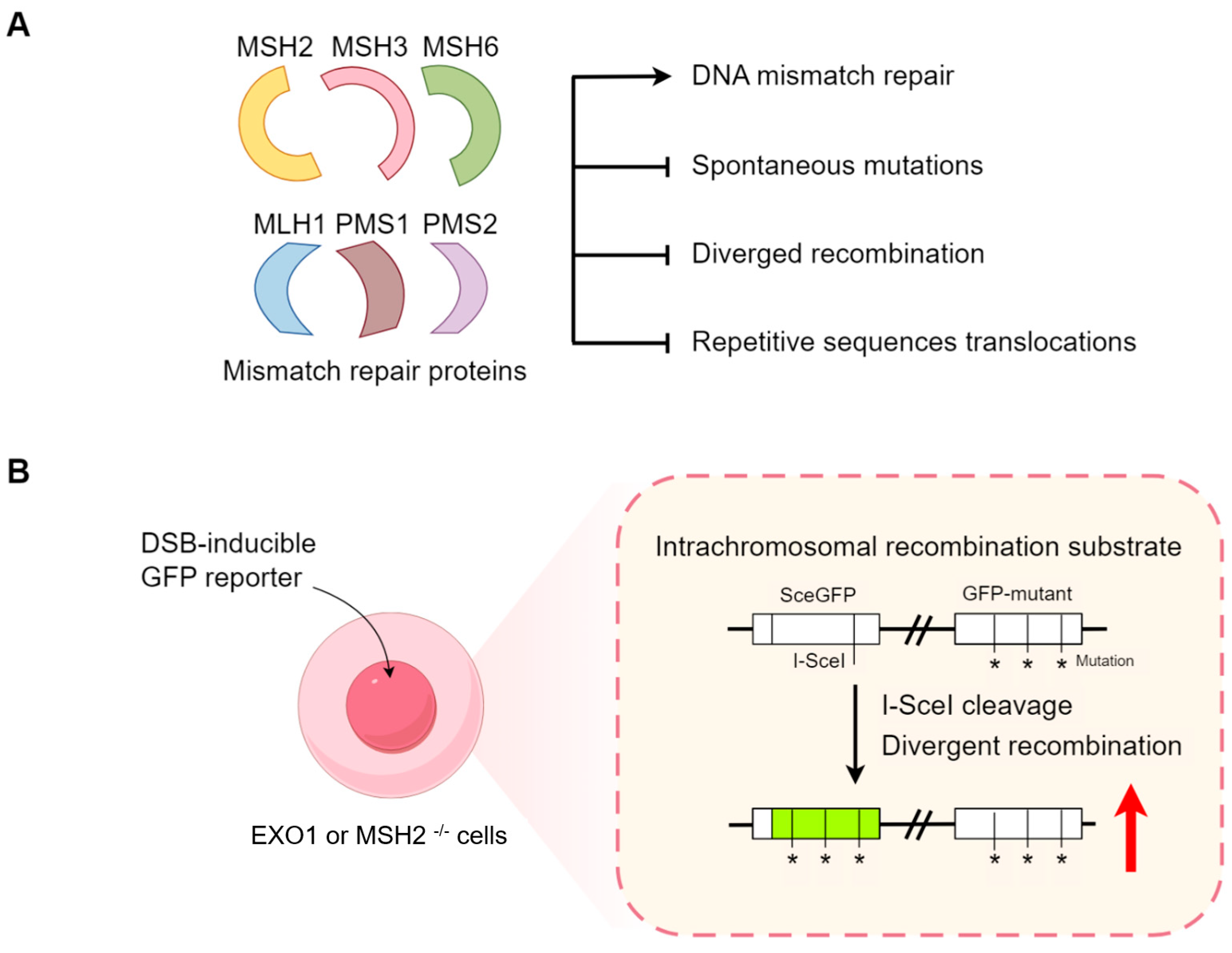
5. High Mismatch Repair (MMR) Inhibits Recombination between Diverged Sequences
6. LINE-1 Retrotransposon Drives DNA Damage and Transposition
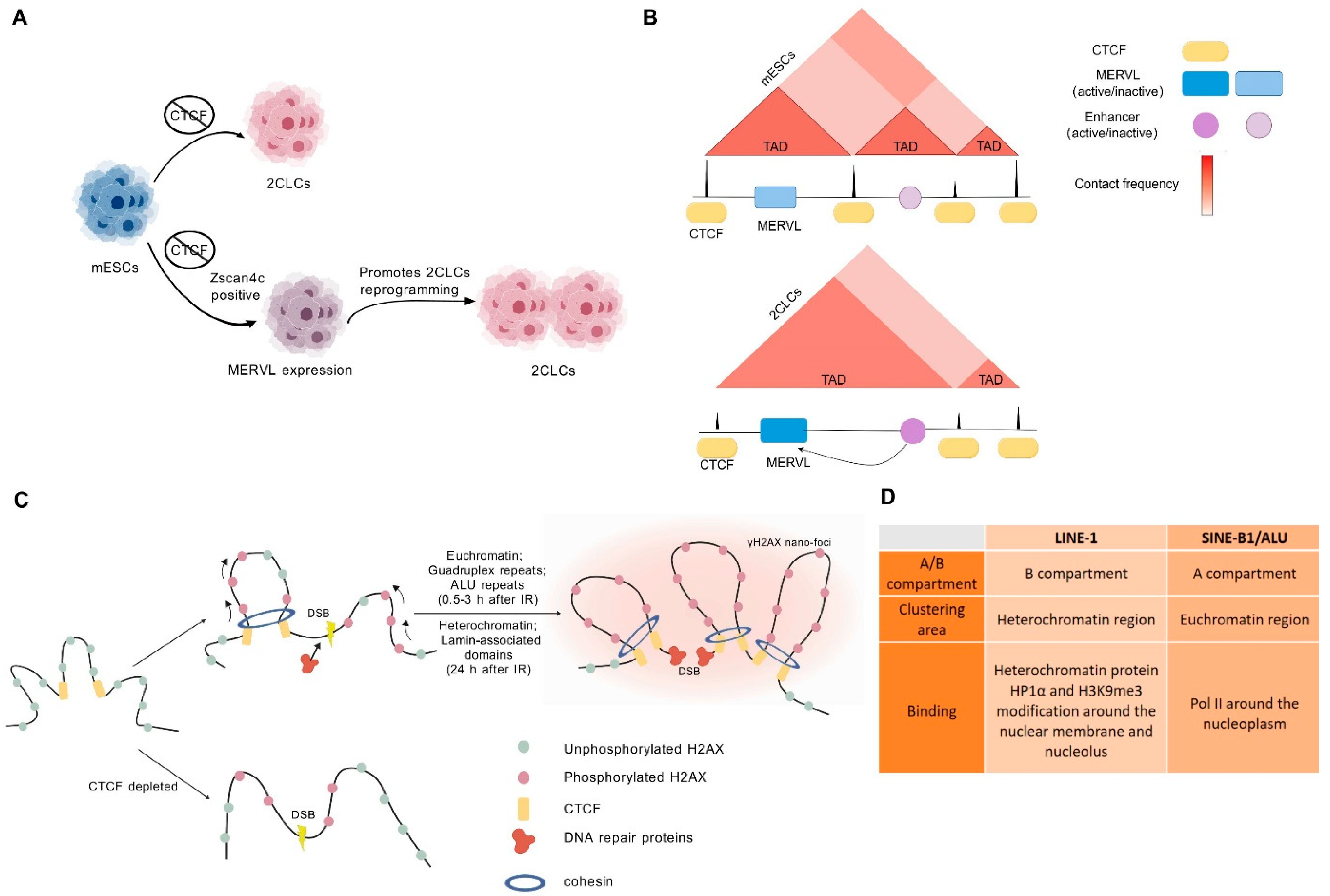
7. Dynamic Chromatin Structure in Response to DNA Damage
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McClintock, B. The significance of responses of the genome to challenge. Science 1984, 226, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, S.J.; Storer, J.M.; Hartley, G.A.; Grady, P.G.S.; Gershman, A.; de Lima, L.G.; Limouse, C.; Halabian, R.; Wojenski, L.; Rodriguez, M.; et al. From telomere to telomere: The transcriptional and epigenetic state of human repeat elements. Science 2022, 376, eabk3112. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E.; Freudenreich, C.H. Structure-forming repeats and their impact on genome stability. Curr. Opin. Genet. Dev. 2021, 67, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.H.; Boeke, J.D. Human transposon tectonics. Cell 2012, 149, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Cordaux, R.; Batzer, M.A. The impact of retrotransposons on human genome evolution. Nat. Rev. Genet. 2009, 10, 691–703. [Google Scholar] [CrossRef]
- Liao, X.; Zhu, W.; Zhou, J.; Li, H.; Xu, X.; Zhang, B.; Gao, X. Repetitive DNA sequence detection and its role in the human genome. Commun. Biol. 2023, 6, 954. [Google Scholar] [CrossRef] [PubMed]
- Musson, R.; Gasior, L.; Bisogno, S.; Ptak, G.E. DNA damage in preimplantation embryos and gametes: Specification, clinical relevance and repair strategies. Hum. Reprod. Update 2022, 28, 376–399. [Google Scholar] [CrossRef]
- Casas-Delucchi, C.S.; Daza-Martin, M.; Williams, S.L.; Coster, G. The mechanism of replication stalling and recovery within repetitive DNA. Nat. Commun. 2022, 13, 3953. [Google Scholar] [CrossRef]
- Bzymek, M.; Lovett, S.T. Instability of repetitive DNA sequences: The role of replication in multiple mechanisms. Proc. Natl. Acad. Sci. USA 2001, 98, 8319–8325. [Google Scholar] [CrossRef]
- Zheng, T.; Zhou, H.; Li, X.; Peng, D.; Yang, Y.; Zeng, Y.; Liu, H.; Ren, J.; Zhao, Y. RBMX is required for activation of ATR on repetitive DNAs to maintain genome stability. Cell Death Differ. 2020, 27, 3162–3176. [Google Scholar] [CrossRef]
- Song, X.; Aw, J.T.M.; Ma, F.; Cheung, M.F.; Leung, D.; Herrup, K. DNA Repair Inhibition Leads to Active Export of Repetitive Sequences to the Cytoplasm Triggering an Inflammatory Response. J. Neurosci. 2021, 41, 9286–9307. [Google Scholar] [CrossRef] [PubMed]
- Frankish, A.; Carbonell-Sala, S.; Diekhans, M.; Jungreis, I.; Loveland, J.E.; Mudge, J.M.; Sisu, C.; Wright, J.C.; Arnan, C.; Barnes, I.; et al. GENCODE: Reference annotation for the human and mouse genomes in 2023. Nucleic Acids Res. 2023, 51, D942–D949. [Google Scholar] [CrossRef] [PubMed]
- Balzano, E.; Pelliccia, F.; Giunta, S. Genome (in)stability at tandem repeats. Semin. Cell Dev. Biol. 2021, 113, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Gemmell, N.J. Repetitive DNA: Genomic dark matter matters. Nat. Rev. Genet. 2021, 22, 342. [Google Scholar] [CrossRef]
- Jurka, J.; Kapitonov, V.V.; Kohany, O.; Jurka, M.V. Repetitive sequences in complex genomes: Structure and evolution. Annu. Rev. Genom. Hum. Genet. 2007, 8, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Kazazian, H.H., Jr. Mobile elements: Drivers of genome evolution. Science 2004, 303, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.R.; Doucet, A.J.; Kopera, H.C.; Moldovan, J.B.; Garcia-Perez, J.L.; Moran, J.V. The Influence of LINE-1 and SINE Retrotransposons on Mammalian Genomes. Microbiol. Spectr. 2015, 3, MDNA3-0061-2014. [Google Scholar] [CrossRef]
- Ostertag, E.M.; Goodier, J.L.; Zhang, Y.; Kazazian, H.H., Jr. SVA elements are nonautonomous retrotransposons that cause disease in humans. Am. J. Hum. Genet. 2003, 73, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Hancks, D.C.; Goodier, J.L.; Mandal, P.K.; Cheung, L.E.; Kazazian, H.H., Jr. Retrotransposition of marked SVA elements by human L1s in cultured cells. Hum. Mol. Genet. 2011, 20, 3386–3400. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Shi, G. Retrotransposons in pluripotent stem cells. Cell Regen. 2020, 9, 4. [Google Scholar] [CrossRef]
- Hutchins, A.P.; Pei, D. Transposable elements at the center of the crossroads between embryogenesis, embryonic stem cells, reprogramming, and long non-coding RNAs. Sci. Bull. 2015, 60, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Oomen, M.E.; Torres-Padilla, M.E. Jump-starting life: Balancing transposable element co-option and genome integrity in the developing mammalian embryo. EMBO Rep. 2024, 25, 1721–1733. [Google Scholar] [CrossRef]
- Ma, G.; Babarinde, I.A.; Zhou, X.; Hutchins, A.P. Transposable Elements in Pluripotent Stem Cells and Human Disease. Front. Genet. 2022, 13, 902541. [Google Scholar] [CrossRef]
- Wang, J.; Xie, G.; Singh, M.; Ghanbarian, A.T.; Rasko, T.; Szvetnik, A.; Cai, H.; Besser, D.; Prigione, A.; Fuchs, N.V.; et al. Primate-specific endogenous retrovirus-driven transcription defines naive-like stem cells. Nature 2014, 516, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Mouse embryonic development requires transposable element expression. Nat. Genet. 2023, 55, 367–368. [CrossRef]
- Macfarlan, T.S.; Gifford, W.D.; Driscoll, S.; Lettieri, K.; Rowe, H.M.; Bonanomi, D.; Firth, A.; Singer, O.; Trono, D.; Pfaff, S.L. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 2012, 487, 57–63. [Google Scholar] [CrossRef]
- Pontis, J.; Pulver, C.; Playfoot, C.J.; Planet, E.; Grun, D.; Offner, S.; Duc, J.; Manfrin, A.; Lutolf, M.P.; Trono, D. Primate-specific transposable elements shape transcriptional networks during human development. Nat. Commun. 2022, 13, 7178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zheng, R.; Li, M.; Yan, C.; Lan, X.; Tong, B.; Lu, P.; Jiang, W. Active endogenous retroviral elements in human pluripotent stem cells play a role in regulating host gene expression. Nucleic Acids Res. 2022, 50, 4959–4973. [Google Scholar] [CrossRef]
- Guo, Y.; Li, T.D.; Modzelewski, A.J.; Siomi, H. Retrotransposon renaissance in early embryos. Trends Genet. 2024, 40, 39–51. [Google Scholar] [CrossRef]
- Friedli, M.; Turelli, P.; Kapopoulou, A.; Rauwel, B.; Castro-Diaz, N.; Rowe, H.M.; Ecco, G.; Unzu, C.; Planet, E.; Lombardo, A.; et al. Loss of transcriptional control over endogenous retroelements during reprogramming to pluripotency. Genome Res. 2014, 24, 1251–1259. [Google Scholar] [CrossRef]
- Lopez-Flores, I.; Garrido-Ramos, M.A. The repetitive DNA content of eukaryotic genomes. Genome Dyn. 2012, 7, 1–28. [Google Scholar]
- Hancks, D.C.; Kazazian, H.H., Jr. Active human retrotransposons: Variation and disease. Curr. Opin. Genet. Dev. 2012, 22, 191–203. [Google Scholar] [CrossRef]
- Tam, P.L.F.; Leung, D. The Molecular Impacts of Retrotransposons in Development and Diseases. Int. J. Mol. Sci. 2023, 24, 16418. [Google Scholar] [CrossRef]
- Li, T.D.; Murano, K.; Kitano, T.; Guo, Y.; Negishi, L.; Siomi, H. TDP-43 safeguards the embryo genome from L1 retrotransposition. Sci. Adv. 2022, 8, eabq3806. [Google Scholar] [CrossRef]
- Rowe, H.M.; Jakobsson, J.; Mesnard, D.; Rougemont, J.; Reynard, S.; Aktas, T.; Maillard, P.V.; Layard-Liesching, H.; Verp, S.; Marquis, J.; et al. KAP1 controls endogenous retroviruses in embryonic stem cells. Nature 2010, 463, 237–240. [Google Scholar] [CrossRef]
- Wolf, D.; Goff, S.P. TRIM28 mediates primer binding site-targeted silencing of murine leukemia virus in embryonic cells. Cell 2007, 131, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Goff, S.P. Embryonic stem cells use ZFP809 to silence retroviral DNAs. Nature 2009, 458, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.M.; Goyal, P.; Maksakova, I.A.; Bilenky, M.; Leung, D.; Tang, J.X.; Shinkai, Y.; Mager, D.L.; Jones, S.; Hirst, M.; et al. DNA methylation and SETDB1/H3K9me3 regulate predominantly distinct sets of genes, retroelements, and chimeric transcripts in mESCs. Cell Stem Cell 2011, 8, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dai, H.; Martos, S.N.; Xu, B.; Gao, Y.; Li, T.; Zhu, G.; Schones, D.E.; Wang, Z. Distinct roles of DNMT1-dependent and DNMT1-independent methylation patterns in the genome of mouse embryonic stem cells. Genome Biol. 2015, 16, 115. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Y.; Macfarlan, T.S. The Role of KRAB-ZFPs in Transposable Element Repression and Mammalian Evolution. Trends Genet. 2017, 33, 871–881. [Google Scholar] [CrossRef]
- Tan, X.; Xu, X.; Elkenani, M.; Smorag, L.; Zechner, U.; Nolte, J.; Engel, W.; Pantakani, D.V. Zfp819, a novel KRAB-zinc finger protein, interacts with KAP1 and functions in genomic integrity maintenance of mouse embryonic stem cells. Stem Cell Res. 2013, 11, 1045–1059. [Google Scholar] [CrossRef]
- Shi, H.; Strogantsev, R.; Takahashi, N.; Kazachenka, A.; Lorincz, M.C.; Hemberger, M.; Ferguson-Smith, A.C. ZFP57 regulation of transposable elements and gene expression within and beyond imprinted domains. Epigenet. Chromatin 2019, 12, 49. [Google Scholar] [CrossRef]
- He, Q.; Kim, H.; Huang, R.; Lu, W.; Tang, M.; Shi, F.; Yang, D.; Zhang, X.; Huang, J.; Liu, D.; et al. The Daxx/Atrx Complex Protects Tandem Repetitive Elements during DNA Hypomethylation by Promoting H3K9 Trimethylation. Cell Stem Cell 2015, 17, 273–286. [Google Scholar] [PubMed]
- Srinivasan, R.; Nady, N.; Arora, N.; Hsieh, L.J.; Swigut, T.; Narlikar, G.J.; Wossidlo, M.; Wysocka, J. Zscan4 binds nucleosomal microsatellite DNA and protects mouse two-cell embryos from DNA damage. Sci. Adv. 2020, 6, eaaz9115. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.; Rousseau, P.; Hardikar, S.; Veland, N.; Wong, J.; Autexier, C.; Chen, T. Zscan4 Inhibits Maintenance DNA Methylation to Facilitate Telomere Elongation in Mouse Embryonic Stem Cells. Cell Rep. 2017, 20, 1936–1949. [Google Scholar]
- Wang, G.; Vasquez, K.M. Dynamic alternative DNA structures in biology and disease. Nat. Rev. Genet. 2023, 24, 211–234. [Google Scholar] [PubMed]
- Gold, M.A.; Whalen, J.M.; Freon, K.; Hong, Z.; Iraqui, I.; Lambert, S.A.E.; Freudenreich, C.H. Restarted replication forks are error-prone and cause CAG repeat expansions and contractions. PLoS Genet. 2021, 17, e1009863. [Google Scholar]
- Natale, F.; Scholl, A.; Rapp, A.; Yu, W.; Rausch, C.; Cardoso, M.C. DNA replication and repair kinetics of Alu, LINE-1 and satellite III genomic repetitive elements. Epigenet. Chromatin 2018, 11, 61. [Google Scholar]
- Vallier, L. Cell Cycle Rules Pluripotency. Cell Stem Cell 2015, 17, 131–132. [Google Scholar] [CrossRef]
- Gonzales, K.A.; Liang, H.; Lim, Y.S.; Chan, Y.S.; Yeo, J.C.; Tan, C.P.; Gao, B.; Le, B.; Tan, Z.Y.; Low, K.Y.; et al. Deterministic Restriction on Pluripotent State Dissolution by Cell-Cycle Pathways. Cell 2015, 162, 564–579. [Google Scholar]
- Pauklin, S.; Vallier, L. The cell-cycle state of stem cells determines cell fate propensity. Cell 2013, 155, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, C.; Tripathi, V.; Manolika, E.M.; Heijink, A.M.; Ricci, G.; Merzouk, S.; de Boer, H.R.; Demmers, J.; van Vugt, M.; Ray Chaudhuri, A. RIF1 promotes replication fork protection and efficient restart to maintain genome stability. Nat. Commun. 2019, 10, 3287. [Google Scholar] [CrossRef] [PubMed]
- Li, P.S.; Wang, L.; Bennett, B.D.; Wang, J.J.; Li, J.L.; Qin, Y.F.; Takaku, M.; Wade, P.A.; Wong, J.M.; Hu, G. Rif1 promotes a repressive chromatin state to safeguard against endogenous retrovirus activation. Nucleic Acids Res. 2017, 45, 12723–12738. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.R.; Barral, P.; Vannier, J.B.; Borel, V.; Steger, M.; Tomas-Loba, A.; Sartori, A.A.; Adams, I.R.; Batista, F.D.; Boulton, S.J. RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resection. Mol. Cell 2013, 49, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Mattarocci, S.; Reinert, J.K.; Bunker, R.D.; Fontana, G.A.; Shi, T.; Klein, D.; Cavadini, S.; Faty, M.; Shyian, M.; Hafner, L.; et al. Rif1 maintains telomeres and mediates DNA repair by encasing DNA ends. Nat. Struct. Mol. Biol. 2017, 24, 588–595. [Google Scholar] [PubMed]
- Dan, J.; Liu, Y.; Liu, N.; Chiourea, M.; Okuka, M.; Wu, T.; Ye, X.; Mou, C.; Wang, L.; Wang, L.; et al. Rif1 maintains telomere length homeostasis of ESCs by mediating heterochromatin silencing. Dev. Cell 2014, 29, 7–19. [Google Scholar] [PubMed]
- Ochs, F.; Karemore, G.; Miron, E.; Brown, J.; Sedlackova, H.; Rask, M.B.; Lampe, M.; Buckle, V.; Schermelleh, L.; Lukas, J.; et al. Stabilization of chromatin topology safeguards genome integrity. Nature 2019, 574, 571–574. [Google Scholar] [CrossRef]
- Liu, C.; Yu, P.; Ren, Z.; Yao, F.; Wang, L.; Hu, G.; Li, P.; Zhao, Q. Rif1 Regulates Self-Renewal and Impedes Mesendodermal Differentiation of Mouse Embryonic Stem Cells. Stem Cell Rev. Rep. 2023, 19, 1540–1553. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa-Sugata, N.; Yamazaki, S.; Mita-Yoshida, K.; Ono, T.; Nishito, Y.; Masai, H. Loss of full-length DNA replication regulator Rif1 in two-cell embryos is associated with zygotic transcriptional activation. J. Biol. Chem. 2021, 297, 101367. [Google Scholar]
- Pommier, Y.; Nussenzweig, A.; Takeda, S.; Austin, C. Human topoisomerases and their roles in genome stability and organization. Nat. Rev. Mol. Cell Biol. 2022, 23, 407–427. [Google Scholar]
- Pommier, Y.; Sun, Y.; Huang, S.N.; Nitiss, J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 2016, 17, 703–721. [Google Scholar] [PubMed]
- Antony, S.; Arimondo, P.B.; Sun, J.S.; Pommier, Y. Position- and orientation-specific enhancement of topoisomerase I cleavage complexes by triplex DNA structures. Nucleic Acids Res. 2004, 32, 5163–5173. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.E.; Kim, J.H.; Chung, I.K. Topoisomerase II-mediated DNA cleavage on the cruciform structure formed within the 5′ upstream region of the human beta-globin gene. Mol. Cells 1998, 8, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Punchihewa, C.; Carver, M.; Yang, D. DNA sequence selectivity of human topoisomerase I-mediated DNA cleavage induced by camptothecin. Protein Sci. 2009, 18, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Choi, I.Y.; Kang, M.R.; Kim, S.S.; Muller, M.T.; Spitzner, J.R.; Chung, I.K. DNA topoisomerase II cleavage of telomeres in vitro and in vivo. Biochim. Biophys. Acta 1998, 1395, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Govoni, M.; Farabegoli, F.; Pession, A.; Novello, F. Inhibition of topoisomerase II activity and its effect on nucleolar structure and function. Exp. Cell Res. 1994, 211, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Tichy, E.D.; Stephan, Z.A.; Osterburg, A.; Noel, G.; Stambrook, P.J. Mouse embryonic stem cells undergo charontosis, a novel programmed cell death pathway dependent upon cathepsins, p53, and EndoG, in response to etoposide treatment. Stem Cell Res. 2013, 10, 428–441. [Google Scholar] [PubMed]
- Udvardy, A.; Schedl, P. Chromatin structure, not DNA sequence specificity, is the primary determinant of topoisomerase II sites of action in vivo. Mol. Cell Biol. 1991, 11, 4973–4984. [Google Scholar] [PubMed]
- Kas, E.; Laemmli, U.K. In vivo topoisomerase II cleavage of the Drosophila histone and satellite III repeats: DNA sequence and structural characteristics. EMBO J. 1992, 11, 705–716. [Google Scholar] [CrossRef]
- Meuleman, W.; Muratov, A.; Rynes, E.; Halow, J.; Lee, K.; Bates, D.; Diegel, M.; Dunn, D.; Neri, F.; Teodosiadis, A.; et al. Index and biological spectrum of human DNase I hypersensitive sites. Nature 2020, 584, 244–251. [Google Scholar] [CrossRef]
- Yiangou, L.; Grandy, R.A.; Osnato, A.; Ortmann, D.; Sinha, S.; Vallier, L. Cell cycle regulators control mesoderm specification in human pluripotent stem cells. J. Biol. Chem. 2019, 294, 17903–17914. [Google Scholar] [CrossRef] [PubMed]
- Stambrook, P.J.; Tichy, E.D. Preservation of genomic integrity in mouse embryonic stem cells. Adv. Exp. Med. Biol. 2010, 695, 59–75. [Google Scholar] [PubMed]
- Hong, Y.; Stambrook, P.J. Restoration of an absent G1 arrest and protection from apoptosis in embryonic stem cells after ionizing radiation. Proc. Natl. Acad. Sci. USA 2004, 101, 14443–14448. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.H.; Yoon, S.; Kim, K.P. Combined Ectopic Expression of Homologous Recombination Factors Promotes Embryonic Stem Cell Differentiation. Mol. Ther. 2018, 26, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.W.; Kim, D.K.; Kim, K.P.; Park, K.S. Rad51 regulates cell cycle progression by preserving G2/M transition in mouse embryonic stem cells. Stem Cells Dev. 2014, 23, 2700–2711. [Google Scholar] [CrossRef] [PubMed]
- Mujoo, K.; Pandita, R.K.; Tiwari, A.; Charaka, V.; Chakraborty, S.; Singh, D.K.; Hambarde, S.; Hittelman, W.N.; Horikoshi, N.; Hunt, C.R.; et al. Differentiation of Human Induced Pluripotent or Embryonic Stem Cells Decreases the DNA Damage Repair by Homologous Recombination. Stem Cell Rep. 2017, 9, 1660–1674. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.M.; Chan, Y.L.; Weichselbaum, R.W.; Bishop, D.K. Non-enzymatic roles of human RAD51 at stalled replication forks. Nat. Commun. 2019, 10, 4410. [Google Scholar] [CrossRef]
- Malacaria, E.; Pugliese, G.M.; Honda, M.; Marabitti, V.; Aiello, F.A.; Spies, M.; Franchitto, A.; Pichierri, P. Rad52 prevents excessive replication fork reversal and protects from nascent strand degradation. Nat. Commun. 2019, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Daza-Martin, M.; Starowicz, K.; Jamshad, M.; Tye, S.; Ronson, G.E.; MacKay, H.L.; Chauhan, A.S.; Walker, A.K.; Stone, H.R.; Beesley, J.F.J.; et al. Isomerization of BRCA1-BARD1 promotes replication fork protection. Nature 2019, 571, 521–527. [Google Scholar]
- Choi, E.H.; Yoon, S.; Koh, Y.E.; Seo, Y.J.; Kim, K.P. Maintenance of genome integrity and active homologous recombination in embryonic stem cells. Exp. Mol. Med. 2020, 52, 1220–1229. [Google Scholar]
- Kalmbach, K.; Robinson, L.G., Jr.; Wang, F.; Liu, L.; Keefe, D. Telomere length reprogramming in embryos and stem cells. Biomed. Res. Int. 2014, 2014, 925121. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bailey, S.M.; Okuka, M.; Munoz, P.; Li, C.; Zhou, L.; Wu, C.; Czerwiec, E.; Sandler, L.; Seyfang, A.; et al. Telomere lengthening early in development. Nat. Cell Biol. 2007, 9, 1436–1441. [Google Scholar] [CrossRef]
- Le, R.; Huang, Y.; Zhang, Y.; Wang, H.; Lin, J.; Dong, Y.; Li, Z.; Guo, M.; Kou, X.; Zhao, Y.; et al. Dcaf11 activates Zscan4-mediated alternative telomere lengthening in early embryos and embryonic stem cells. Cell Stem Cell 2021, 28, 732–747.e9. [Google Scholar]
- Sapir, E.; Gozaly-Chianea, Y.; Al-Wahiby, S.; Ravindran, S.; Yasaei, H.; Slijepcevic, P. Effects of BRCA2 deficiency on telomere recombination in non-ALT and ALT cells. Genome Integr. 2011, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Kaul, Z.; Gocha, A.S.; Martinez, A.R.; Harris, J.; Parvin, J.D.; Groden, J. Association of BLM and BRCA1 during Telomere Maintenance in ALT Cells. PLoS ONE 2014, 9, e103819. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahiby, S.; Slijepcevic, P. Chromosomal aberrations involving telomeres in BRCA1 deficient human and mouse cell lines. Cytogenet. Genome Res. 2005, 109, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Giscard d’Estaing, S.; Perrin, D.; Lenoir, G.M.; Guerin, J.F.; Dante, R. Upregulation of the BRCA1 gene in human germ cells and in preimplantation embryos. Fertil. Steril. 2005, 84, 785–788. [Google Scholar] [CrossRef]
- Gowen, L.C.; Johnson, B.L.; Latour, A.M.; Sulik, K.K.; Koller, B.H. Brca1 deficiency results in early embryonic lethality characterized by neuroepithelial abnormalities. Nat. Genet. 1996, 12, 191–194. [Google Scholar] [CrossRef]
- Chen, J.; Li, P.; Song, L.; Bai, L.; Huen, M.S.Y.; Liu, Y.; Lu, L.Y. 53BP1 loss rescues embryonic lethality but not genomic instability of BRCA1 total knockout mice. Cell Death Differ. 2020, 27, 2552–2567. [Google Scholar]
- Snouwaert, J.N.; Gowen, L.C.; Latour, A.M.; Mohn, A.R.; Xiao, A.; DiBiase, L.; Koller, B.H. BRCA1 deficient embryonic stem cells display a decreased homologous recombination frequency and an increased frequency of non-homologous recombination that is corrected by expression of a brca1 transgene. Oncogene 1999, 18, 7900–7907. [Google Scholar] [CrossRef]
- Pulvers, J.N.; Huttner, W.B. Brca1 is required for embryonic development of the mouse cerebral cortex to normal size by preventing apoptosis of early neural progenitors. Development 2009, 136, 1859–1868. [Google Scholar] [CrossRef]
- Ludwig, T.; Chapman, D.L.; Papaioannou, V.E.; Efstratiadis, A. Targeted mutations of breast cancer susceptibility gene homologs in mice: Lethal phenotypes of Brca1, Brca2, Brca1/Brca2, Brca1/p53, and Brca2/p53 nullizygous embryos. Genes. Dev. 1997, 11, 1226–1241. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Tan, W.; Song, Q.; Pei, H.; Li, J. BRCA1 and Breast Cancer: Molecular Mechanisms and Therapeutic Strategies. Front. Cell Dev. Biol. 2022, 10, 813457. [Google Scholar] [CrossRef] [PubMed]
- Gorodetska, I.; Kozeretska, I.; Dubrovska, A. BRCA Genes: The Role in Genome Stability, Cancer Stemness and Therapy Resistance. J. Cancer 2019, 10, 2109–2127. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Guo, M.; Cui, J.; Cai, H.; Wang, S.M. Heterozygotic Brca1 mutation initiates mouse genome instability at embryonic stage. Oncogenesis 2022, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Pao, G.M.; Huynh, A.M.; Suh, H.; Tonnu, N.; Nederlof, P.M.; Gage, F.H.; Verma, I.M. BRCA1 tumour suppression occurs via heterochromatin-mediated silencing. Nature 2011, 477, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Racca, C.; Britton, S.; Hedouin, S.; Francastel, C.; Calsou, P.; Larminat, F. BRCA1 prevents R-loop-associated centromeric instability. Cell Death Dis. 2021, 12, 896. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Osterman, M.D.; Li, M. Tissue specificity of DNA damage response and tumorigenesis. Cancer Biol. Med. 2019, 16, 396–414. [Google Scholar] [CrossRef] [PubMed]
- Li, G.M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008, 18, 85–98. [Google Scholar] [CrossRef]
- Chao, E.C.; Lipkin, S.M. Molecular models for the tissue specificity of DNA mismatch repair-deficient carcinogenesis. Nucleic Acids Res. 2006, 34, 840–852. [Google Scholar] [CrossRef]
- Kim, M.; Trinh, B.N.; Long, T.I.; Oghamian, S.; Laird, P.W. Dnmt1 deficiency leads to enhanced microsatellite instability in mouse embryonic stem cells. Nucleic Acids Res. 2004, 32, 5742–5749. [Google Scholar] [CrossRef]
- Elliott, B.; Jasin, M. Repair of double-strand breaks by homologous recombination in mismatch repair-defective mammalian cells. Mol. Cell Biol. 2001, 21, 2671–2682. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Markouli, C.; Geens, M.; Barbe, L.; Sermon, K.; Spits, C. Human embryonic stem cells show low-grade microsatellite instability. Mol. Hum. Reprod. 2014, 20, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Tichy, E.D.; Liang, L.; Deng, L.; Tischfield, J.; Schwemberger, S.; Babcock, G.; Stambrook, P.J. Mismatch and base excision repair proficiency in murine embryonic stem cells. DNA Repair 2011, 10, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Madden-Hennessey, K.; Gupta, D.; Radecki, A.A.; Guild, C.; Rath, A.; Heinen, C.D. Loss of mismatch repair promotes a direct selective advantage in human stem cells. Stem Cell Rep. 2022, 17, 2661–2673. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S.; Wang, J.; Myeroff, L.; Parsons, R.; Sun, L.; Lutterbaugh, J.; Fan, R.S.; Zborowska, E.; Kinzler, K.W.; Vogelstein, B.; et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science 1995, 268, 1336–1338. [Google Scholar] [CrossRef] [PubMed]
- Deininger, P.L.; Batzer, M.A. Alu repeats and human disease. Mol. Genet. Metab. 1999, 67, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Do, A.T.; LaRocque, J.R. The role of Drosophila mismatch repair in suppressing recombination between diverged sequences. Sci. Rep. 2015, 5, 17601. [Google Scholar] [CrossRef]
- Larocque, J.R.; Jasin, M. Mechanisms of recombination between diverged sequences in wild-type and BLM-deficient mouse and human cells. Mol. Cell Biol. 2010, 30, 1887–1897. [Google Scholar] [CrossRef]
- Chen, C.C.; Avdievich, E.; Zhang, Y.; Zhang, Y.; Wei, K.; Lee, K.; Edelmann, W.; Jasin, M.; LaRocque, J.R. EXO1 suppresses double-strand break induced homologous recombination between diverged sequences in mammalian cells. DNA Repair 2017, 57, 98–106. [Google Scholar] [CrossRef]
- Baldwin, E.T.; van Eeuwen, T.; Hoyos, D.; Zalevsky, A.; Tchesnokov, E.P.; Sanchez, R.; Miller, B.D.; Di Stefano, L.H.; Ruiz, F.X.; Hancock, M.; et al. Structures, functions and adaptations of the human LINE-1 ORF2 protein. Nature 2024, 626, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.L. The ORF1 protein encoded by LINE-1: Structure and function during L1 retrotransposition. J. Biomed. Biotechnol. 2006, 2006, 45621. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.L.; Cruceanu, M.; Branciforte, D.; Wai-Lun Li, P.; Kwok, S.C.; Hodges, R.S.; Williams, M.C. LINE-1 retrotransposition requires the nucleic acid chaperone activity of the ORF1 protein. J. Mol. Biol. 2005, 348, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Xiao-Jie, L.; Hui-Ying, X.; Qi, X.; Jiang, X.; Shi-Jie, M. LINE-1 in cancer: Multifaceted functions and potential clinical implications. Genet. Med. 2016, 18, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Sahakyan, A.B.; Murat, P.; Mayer, C.; Balasubramanian, S. G-quadruplex structures within the 3′ UTR of LINE-1 elements stimulate retrotransposition. Nat. Struct. Mol. Biol. 2017, 24, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Suarez, N.A.; Macia, A.; Muotri, A.R. LINE-1 retrotransposons in healthy and diseased human brain. Dev. Neurobiol. 2018, 78, 434–455. [Google Scholar] [CrossRef] [PubMed]
- Garza, R.; Atacho, D.A.M.; Adami, A.; Gerdes, P.; Vinod, M.; Hsieh, P.; Karlsson, O.; Horvath, V.; Johansson, P.A.; Pandiloski, N.; et al. LINE-1 retrotransposons drive human neuronal transcriptome complexity and functional diversification. Sci. Adv. 2023, 9, eadh9543. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.H. Transposable elements in cancer. Nat. Rev. Cancer 2017, 17, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Kazazian, H.H., Jr.; Moran, J.V. Mobile DNA in Health and Disease. N. Engl. J. Med. 2017, 377, 361–370. [Google Scholar] [CrossRef]
- Yang, L.; Metzger, G.A.; Padilla Del Valle, R.; Delgadillo Rubalcaba, D.; McLaughlin, R.N., Jr. Evolutionary insights from profiling LINE-1 activity at allelic resolution in a single human genome. EMBO J. 2024, 43, 112–131. [Google Scholar] [CrossRef]
- Percharde, M.; Lin, C.J.; Yin, Y.; Guan, J.; Peixoto, G.A.; Bulut-Karslioglu, A.; Biechele, S.; Huang, B.; Shen, X.; Ramalho-Santos, M. A LINE1-Nucleolin Partnership Regulates Early Development and ESC Identity. Cell 2018, 174, 391–405.e19. [Google Scholar] [CrossRef]
- Belgnaoui, S.M.; Gosden, R.G.; Semmes, O.J.; Haoudi, A. Human LINE-1 retrotransposon induces DNA damage and apoptosis in cancer cells. Cancer Cell Int. 2006, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Stoiljkovic, M.; Song, E.; Gao, X.B.; Yasumoto, Y.; Kudo, E.; Carvalho, F.; Kong, Y.; Park, A.; Shanabrough, M.; et al. LINE-1 activation in the cerebellum drives ataxia. Neuron 2022, 110, 3278–3287.e8. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, C.P.; Sagalovskiy, I.; Guo, Q.; Nezos, A.; Kapsogeorgou, E.K.; Lu, P.; Liang Zhou, J.; Kirou, K.A.; Seshan, S.V.; Moutsopoulos, H.M.; et al. Expression of Long Interspersed Nuclear Element 1 Retroelements and Induction of Type I Interferon in Patients With Systemic Autoimmune Disease. Arthritis Rheumatol. 2016, 68, 2686–2696. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Yu, J. New Understanding of the Relevant Role of LINE-1 Retrotransposition in Human Disease and Immune Modulation. Front. Cell Dev. Biol. 2020, 8, 657. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, M.; Ito, T.; Petrashen, A.P.; Elias, A.E.; Skvir, N.J.; Criscione, S.W.; Caligiana, A.; Brocculi, G.; Adney, E.M.; Boeke, J.D.; et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 2019, 566, 73–78. [Google Scholar] [CrossRef]
- St Laurent, G., III; Hammell, N.; McCaffrey, T.A. A LINE-1 component to human aging: Do LINE elements exact a longevity cost for evolutionary advantage? Mech. Ageing Dev. 2010, 131, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Peze-Heidsieck, E.; Bonnifet, T.; Znaidi, R.; Ravel-Godreuil, C.; Massiani-Beaudoin, O.; Joshi, R.L.; Fuchs, J. Retrotransposons as a Source of DNA Damage in Neurodegeneration. Front. Aging Neurosci. 2021, 13, 786897. [Google Scholar] [CrossRef] [PubMed]
- Erwin, J.A.; Paquola, A.C.; Singer, T.; Gallina, I.; Novotny, M.; Quayle, C.; Bedrosian, T.A.; Alves, F.I.; Butcher, C.R.; Herdy, J.R.; et al. L1-associated genomic regions are deleted in somatic cells of the healthy human brain. Nat. Neurosci. 2016, 19, 1583–1591. [Google Scholar] [CrossRef]
- Morrish, T.A.; Garcia-Perez, J.L.; Stamato, T.D.; Taccioli, G.E.; Sekiguchi, J.; Moran, J.V. Endonuclease-independent LINE-1 retrotransposition at mammalian telomeres. Nature 2007, 446, 208–212. [Google Scholar] [CrossRef]
- Morrish, T.A.; Gilbert, N.; Myers, J.S.; Vincent, B.J.; Stamato, T.D.; Taccioli, G.E.; Batzer, M.A.; Moran, J.V. DNA repair mediated by endonuclease-independent LINE-1 retrotransposition. Nat. Genet. 2002, 31, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Villasante, A.; de Pablos, B.; Mendez-Lago, M.; Abad, J.P. Telomere maintenance in Drosophila: Rapid transposon evolution at chromosome ends. Cell Cycle 2008, 7, 2134–2138. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.O.; Slicko, A.; Yamashita, Y.M. The retrotransposon R2 maintains Drosophila ribosomal DNA repeats. Proc. Natl. Acad. Sci. USA 2023, 120, e2221613120. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Wang, Q.; Mendez-Dorantes, C.; Burns, K.H.; Chiarle, R. Frequency and mechanisms of LINE-1 retrotransposon insertions at CRISPR/Cas9 sites. Nat. Commun. 2022, 13, 3685. [Google Scholar] [CrossRef] [PubMed]
- Pascarella, G.; Hon, C.C.; Hashimoto, K.; Busch, A.; Luginbuhl, J.; Parr, C.; Hin Yip, W.; Abe, K.; Kratz, A.; Bonetti, A.; et al. Recombination of repeat elements generates somatic complexity in human genomes. Cell 2022, 185, 3025–3040.e6. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Zhang, X.; Shi, J.; Yao, M.; Lin, J.; Li, J.; Liu, H.; Li, H.; Shi, G.; Wang, Z.; et al. Dynamically reorganized chromatin is the key for the reprogramming of somatic cells to pluripotent cells. Sci. Rep. 2015, 5, 17691. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, J.; Yang, X.; Zhou, C.; Guo, J.; Wu, C.; Qin, Y.; Guo, L.; He, J.; Yu, S.; et al. Chromatin Accessibility Dynamics during iPSC Reprogramming. Cell Stem Cell 2017, 21, 819–833.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, F.; Tian, Y.; Xu, W.; Zhu, Q.; Li, Z.; Qiu, L.; Lu, X.; Peng, B.; Liu, X.; et al. PARylated PDHE1alpha generates acetyl-CoA for local chromatin acetylation and DNA damage repair. Nat. Struct. Mol. Biol. 2023, 30, 1719–1734. [Google Scholar] [CrossRef]
- Dixon, J.R.; Jung, I.; Selvaraj, S.; Shen, Y.; Antosiewicz-Bourget, J.E.; Lee, A.Y.; Ye, Z.; Kim, A.; Rajagopal, N.; Xie, W.; et al. Chromatin architecture reorganization during stem cell differentiation. Nature 2015, 518, 331–336. [Google Scholar] [CrossRef]
- Davidson, I.F.; Barth, R.; Zaczek, M.; van der Torre, J.; Tang, W.; Nagasaka, K.; Janissen, R.; Kerssemakers, J.; Wutz, G.; Dekker, C.; et al. CTCF is a DNA-tension-dependent barrier to cohesin-mediated loop extrusion. Nature 2023, 616, 822–827. [Google Scholar] [CrossRef]
- Chen, X.; Ke, Y.; Wu, K.; Zhao, H.; Sun, Y.; Gao, L.; Liu, Z.; Zhang, J.; Tao, W.; Hou, Z.; et al. Key role for CTCF in establishing chromatin structure in human embryos. Nature 2019, 576, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, T.; Vega-Sendino, M.; Tillo, D.; Wu, W.; Zolnerowich, N.; Pavani, R.; Tran, A.D.; Domingo, C.N.; Franco, M.; Markiewicz-Potoczny, M.; et al. CTCF is a barrier for 2C-like reprogramming. Nat. Commun. 2021, 12, 4856. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Sanchez, A.; Noordermeer, D.; Greenberg, M.V.C. The impact of DNA methylation on CTCF-mediated 3D genome organization. Nat. Struct. Mol. Biol. 2024, 31, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Saldana-Meyer, R.; Rodriguez-Hernaez, J.; Escobar, T.; Nishana, M.; Jacome-Lopez, K.; Nora, E.P.; Bruneau, B.G.; Tsirigos, A.; Furlan-Magaril, M.; Skok, J.; et al. RNA Interactions Are Essential for CTCF-Mediated Genome Organization. Mol. Cell 2019, 76, 412–422.e5. [Google Scholar] [CrossRef]
- Hansen, A.S.; Hsieh, T.S.; Cattoglio, C.; Pustova, I.; Saldana-Meyer, R.; Reinberg, D.; Darzacq, X.; Tjian, R. Distinct Classes of Chromatin Loops Revealed by Deletion of an RNA-Binding Region in CTCF. Mol. Cell 2019, 76, 395–411.e13. [Google Scholar] [CrossRef]
- Wang, W.; Gao, R.; Yang, D.; Ma, M.; Zang, R.; Wang, X.; Chen, C.; Kou, X.; Zhao, Y.; Chen, J.; et al. ADNP modulates SINE B2-derived CTCF-binding sites during blastocyst formation in mice. Genes. Dev. 2024, 38, 168–188. [Google Scholar] [CrossRef]
- van de Nobelen, S.; Rosa-Garrido, M.; Leers, J.; Heath, H.; Soochit, W.; Joosen, L.; Jonkers, I.; Demmers, J.; van der Reijden, M.; Torrano, V.; et al. CTCF regulates the local epigenetic state of ribosomal DNA repeats. Epigenet. Chromatin 2010, 3, 19. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, Z.; Stong, N.; Plasschaert, R.; Moczan, A.; Chen, H.S.; Hu, S.; Wikramasinghe, P.; Davuluri, R.V.; Bartolomei, M.S.; et al. A role for CTCF and cohesin in subtelomere chromatin organization, TERRA transcription, and telomere end protection. EMBO J. 2012, 31, 4165–4178. [Google Scholar] [CrossRef]
- Lawson, H.A.; Liang, Y.; Wang, T. Transposable elements in mammalian chromatin organization. Nat. Rev. Genet. 2023, 24, 712–723. [Google Scholar] [CrossRef]
- Huang, K.; Jia, J.; Wu, C.; Yao, M.; Li, M.; Jin, J.; Jiang, C.; Cai, Y.; Pei, D.; Pan, G.; et al. Ribosomal RNA gene transcription mediated by the master genome regulator protein CCCTC-binding factor (CTCF) is negatively regulated by the condensin complex. J. Biol. Chem. 2013, 288, 26067–26077. [Google Scholar] [CrossRef]
- Arnould, C.; Rocher, V.; Saur, F.; Bader, A.S.; Muzzopappa, F.; Collins, S.; Lesage, E.; Le Bozec, B.; Puget, N.; Clouaire, T.; et al. Chromatin compartmentalization regulates the response to DNA damage. Nature 2023, 623, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Du, Z.; Wei, L.; Fang, H.; Dong, Q.; Niu, J.; Li, Y.; Gao, J.; Zhang, M.Q.; et al. The loss of heterochromatin is associated with multiscale three-dimensional genome reorganization and aberrant transcription during cellular senescence. Genome Res. 2021, 31, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Iacovoni, J.S.; Caron, P.; Lassadi, I.; Nicolas, E.; Massip, L.; Trouche, D.; Legube, G. High-resolution profiling of gammaH2AX around DNA double strand breaks in the mammalian genome. EMBO J. 2010, 29, 1446–1457. [Google Scholar] [CrossRef]
- Tanwar, V.S.; Jose, C.C.; Cuddapah, S. Role of CTCF in DNA damage response. Mutat. Res. Rev. Mutat. Res. 2019, 780, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Purman, C.; Porter, S.I.; Nganga, V.; Saini, A.; Hayer, K.E.; Gurewitz, G.L.; Sleckman, B.P.; Bednarski, J.J.; Bassing, C.H.; et al. DNA double-strand breaks induce H2Ax phosphorylation domains in a contact-dependent manner. Nat. Commun. 2020, 11, 3158. [Google Scholar] [CrossRef]
- Arnould, C.; Rocher, V.; Finoux, A.L.; Clouaire, T.; Li, K.; Zhou, F.; Caron, P.; Mangeot, P.E.; Ricci, E.P.; Mourad, R.; et al. Loop extrusion as a mechanism for formation of DNA damage repair foci. Nature 2021, 590, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Natale, F.; Rapp, A.; Yu, W.; Maiser, A.; Harz, H.; Scholl, A.; Grulich, S.; Anton, T.; Horl, D.; Chen, W.; et al. Identification of the elementary structural units of the DNA damage response. Nat. Commun. 2017, 8, 15760. [Google Scholar] [CrossRef]
- Kang, M.A.; Lee, J.S. A Newly Assigned Role of CTCF in Cellular Response to Broken DNAs. Biomolecules 2021, 11, 363. [Google Scholar] [CrossRef]
- Velazquez Camacho, O.; Galan, C.; Swist-Rosowska, K.; Ching, R.; Gamalinda, M.; Karabiber, F.; De La Rosa-Velazquez, I.; Engist, B.; Koschorz, B.; Shukeir, N.; et al. Major satellite repeat RNA stabilize heterochromatin retention of Suv39h enzymes by RNA-nucleosome association and RNA:DNA hybrid formation. Elife 2017, 6, e25293. [Google Scholar] [CrossRef]
- Novo, C.L.; Wong, E.V.; Hockings, C.; Poudel, C.; Sheekey, E.; Wiese, M.; Okkenhaug, H.; Boulton, S.J.; Basu, S.; Walker, S.; et al. Satellite repeat transcripts modulate heterochromatin condensates and safeguard chromosome stability in mouse embryonic stem cells. Nat. Commun. 2022, 13, 3525. [Google Scholar] [CrossRef]
- Lu, J.Y.; Chang, L.; Li, T.; Wang, T.; Yin, Y.; Zhan, G.; Han, X.; Zhang, K.; Tao, Y.; Percharde, M.; et al. Homotypic clustering of L1 and B1/Alu repeats compartmentalizes the 3D genome. Cell Res. 2021, 31, 613–630. [Google Scholar] [CrossRef]
- Burton, A.; Brochard, V.; Galan, C.; Ruiz-Morales, E.R.; Rovira, Q.; Rodriguez-Terrones, D.; Kruse, K.; Le Gras, S.; Udayakumar, V.S.; Chin, H.G.; et al. Heterochromatin establishment during early mammalian development is regulated by pericentromeric RNA and characterized by non-repressive H3K9me3. Nat. Cell Biol. 2020, 22, 767–778. [Google Scholar] [CrossRef]
- Sakashita, A.; Kitano, T.; Ishizu, H.; Guo, Y.; Masuda, H.; Ariura, M.; Murano, K.; Siomi, H. Transcription of MERVL retrotransposons is required for preimplantation embryo development. Nat. Genet. 2023, 55, 484–495. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Preissl, S.; Amaral, M.L.; Grinstein, J.D.; Farah, E.N.; Destici, E.; Qiu, Y.; Hu, R.; Lee, A.Y.; et al. Transcriptionally active HERV-H retrotransposons demarcate topologically associating domains in human pluripotent stem cells. Nat. Genet. 2019, 51, 1380–1388. [Google Scholar] [CrossRef]
- Buttler, C.A.; Chuong, E.B. Emerging roles for endogenous retroviruses in immune epigenetic regulation. Immunol. Rev. 2022, 305, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Kigami, D.; Minami, N.; Takayama, H.; Imai, H. MuERV-L is one of the earliest transcribed genes in mouse one-cell embryos. Biol. Reprod. 2003, 68, 651–654. [Google Scholar] [CrossRef]
- Jachowicz, J.W.; Bing, X.; Pontabry, J.; Boskovic, A.; Rando, O.J.; Torres-Padilla, M.E. LINE-1 activation after fertilization regulates global chromatin accessibility in the early mouse embryo. Nat. Genet. 2017, 49, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, X.; Zang, R.; Chen, J.; Fidalgo, M.; Sanchez-Priego, C.; Yang, J.; Caichen, A.; Ma, F.; Macfarlan, T.; et al. DUX-miR-344-ZMYM2-Mediated Activation of MERVL LTRs Induces a Totipotent 2C-like State. Cell Stem Cell 2020, 26, 234–250.e7. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, F.; Chen, R.; Xie, D.; Yang, J.; Zhao, X.; Guo, R.; Zhang, Y.; Shen, Y.; Goke, J.; et al. Zscan4c activates endogenous retrovirus MERVL and cleavage embryo genes. Nucleic Acids Res. 2019, 47, 8485–8501. [Google Scholar] [CrossRef]
- Bourque, G.; Burns, K.H.; Gehring, M.; Gorbunova, V.; Seluanov, A.; Hammell, M.; Imbeault, M.; Izsvak, Z.; Levin, H.L.; Macfarlan, T.S.; et al. Ten things you should know about transposable elements. Genome Biol. 2018, 19, 199. [Google Scholar] [CrossRef]
- Deloger, M.; Cavalli, F.M.; Lerat, E.; Biemont, C.; Sagot, M.F.; Vieira, C. Identification of expressed transposable element insertions in the sequenced genome of Drosophila melanogaster. Gene 2009, 439, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sela, N.; Kim, E.; Ast, G. The role of transposable elements in the evolution of non-mammalian vertebrates and invertebrates. Genome Biol. 2010, 11, R59. [Google Scholar] [CrossRef] [PubMed]
- Russ, E.; Iordanskiy, S. Endogenous Retroviruses as Modulators of Innate Immunity. Pathogens 2023, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Leavy, O. Antiviral immunity: Immune control of endogenous retroviruses. Nat. Rev. Immunol. 2012, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Young, G.R.; Eksmond, U.; Salcedo, R.; Alexopoulou, L.; Stoye, J.P.; Kassiotis, G. Resurrection of endogenous retroviruses in antibody-deficient mice. Nature 2012, 491, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Z.; Wu, Z.; Ren, J.; Fan, Y.; Sun, L.; Cao, G.; Niu, Y.; Zhang, B.; Ji, Q.; et al. Resurrection of endogenous retroviruses during aging reinforces senescence. Cell 2023, 186, 287–304.e26. [Google Scholar] [CrossRef]
- Fablet, M. Host control of insect endogenous retroviruses: Small RNA silencing and immune response. Viruses 2014, 6, 4447–4464. [Google Scholar] [CrossRef]
- Smalheiser, N.R.; Torvik, V.I. Mammalian microRNAs derived from genomic repeats. Trends Genet. 2005, 21, 322–326. [Google Scholar] [CrossRef]
- Piriyapongsa, J.; Marino-Ramirez, L.; Jordan, I.K. Origin and evolution of human microRNAs from transposable elements. Genetics 2007, 176, 1323–1337. [Google Scholar] [CrossRef]
- Gim, J.A.; Ha, H.S.; Ahn, K.; Kim, D.S.; Kim, H.S. Genome-Wide Identification and Classification of MicroRNAs Derived from Repetitive Elements. Genom. Inform. 2014, 12, 261–267. [Google Scholar] [CrossRef]
- Haas, S.; Trumpp, A. An Intrinsic Interferon Program Protects Stem Cells from Viral Infection. Dev. Cell 2018, 44, 279–280. [Google Scholar] [CrossRef]
- Wu, X.; Dao Thi, V.L.; Huang, Y.; Billerbeck, E.; Saha, D.; Hoffmann, H.H.; Wang, Y.; Silva, L.A.V.; Sarbanes, S.; Sun, T.; et al. Intrinsic Immunity Shapes Viral Resistance of Stem Cells. Cell 2018, 172, 423–438.e25. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Frontini, F.; Qi, W.; Hariharan, A.; Ronner, M.; Wipplinger, M.; Blanquart, C.; Rehrauer, H.; Fonteneau, J.F.; Felley-Bosco, E. Endogenous retrovirus expression activates type-I interferon signaling in an experimental mouse model of mesothelioma development. Cancer Lett. 2021, 507, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.; Chen, V.C.; Rozario, P.; Ng, W.L.; Kong, P.S.; Sia, W.R.; Kang, A.E.Z.; Su, Q.; Nguyen, L.H.; Zhu, F.; et al. Bat ASC2 suppresses inflammasomes and ameliorates inflammatory diseases. Cell 2023, 186, 2144–2159.e22. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. The human placenta: New perspectives on its formation and function during early pregnancy. Proc. Biol. Sci. 2023, 290, 20230191. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Li, R.; Chen, M.; Zheng, R.; Wang, J.; Sun, C.; Qu, Y. Endogenous retrovirus HERVH-derived lncRNA UCA1 controls human trophoblast development. Proc. Natl. Acad. Sci. USA 2024, 121, e2318176121. [Google Scholar] [CrossRef] [PubMed]
- Ichiyanagi, T.; Katoh, H.; Mori, Y.; Hirafuku, K.; Boyboy, B.A.; Kawase, M.; Ichiyanagi, K. B2 SINE Copies Serve as a Transposable Boundary of DNA Methylation and Histone Modifications in the Mouse. Mol. Biol. Evol. 2021, 38, 2380–2395. [Google Scholar] [CrossRef]
- Arnaud, P.; Goubely, C.; Pelissier, T.; Deragon, J.M. SINE retroposons can be used in vivo as nucleation centers for de novo methylation. Mol. Cell Biol. 2000, 20, 3434–3441. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, G.; Pang, Q.; Lin, Z.; Zhang, X.; Huang, K. Repetitive Sequence Stability in Embryonic Stem Cells. Int. J. Mol. Sci. 2024, 25, 8819. https://doi.org/10.3390/ijms25168819
Shi G, Pang Q, Lin Z, Zhang X, Huang K. Repetitive Sequence Stability in Embryonic Stem Cells. International Journal of Molecular Sciences. 2024; 25(16):8819. https://doi.org/10.3390/ijms25168819
Chicago/Turabian StyleShi, Guang, Qianwen Pang, Zhancheng Lin, Xinyi Zhang, and Kaimeng Huang. 2024. "Repetitive Sequence Stability in Embryonic Stem Cells" International Journal of Molecular Sciences 25, no. 16: 8819. https://doi.org/10.3390/ijms25168819
APA StyleShi, G., Pang, Q., Lin, Z., Zhang, X., & Huang, K. (2024). Repetitive Sequence Stability in Embryonic Stem Cells. International Journal of Molecular Sciences, 25(16), 8819. https://doi.org/10.3390/ijms25168819





