The Role of the Gut Microbiota in Sanfilippo Syndrome’s Physiopathology: An Approach in Two Affected Siblings
Abstract
:1. Introduction
2. Results
2.1. Patient Description: Clinical Similarities and Differences between the Two MPS-IIIA Siblings
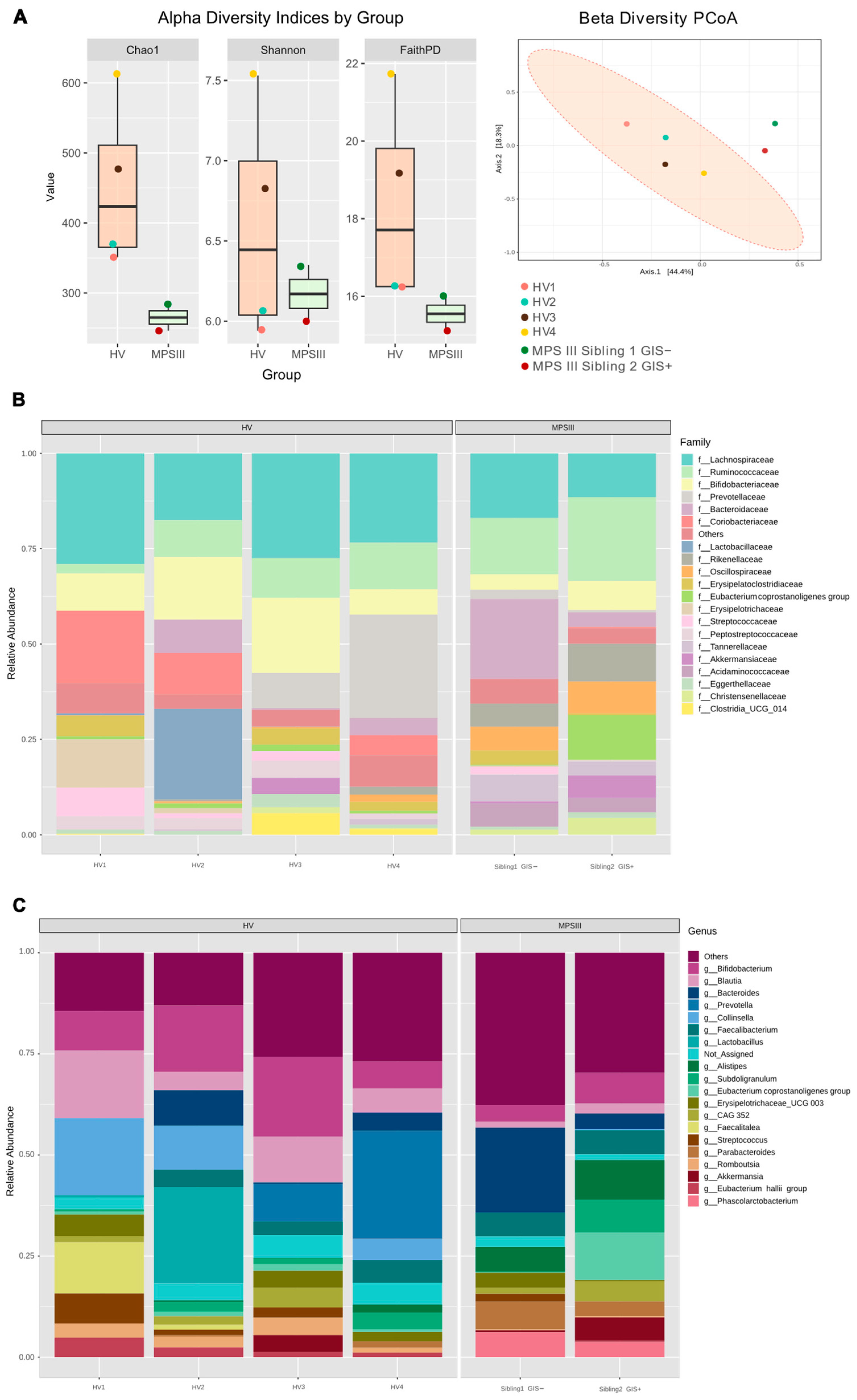
2.2. Microbial Composition, PUL Genes Abundance in MPS III Patients
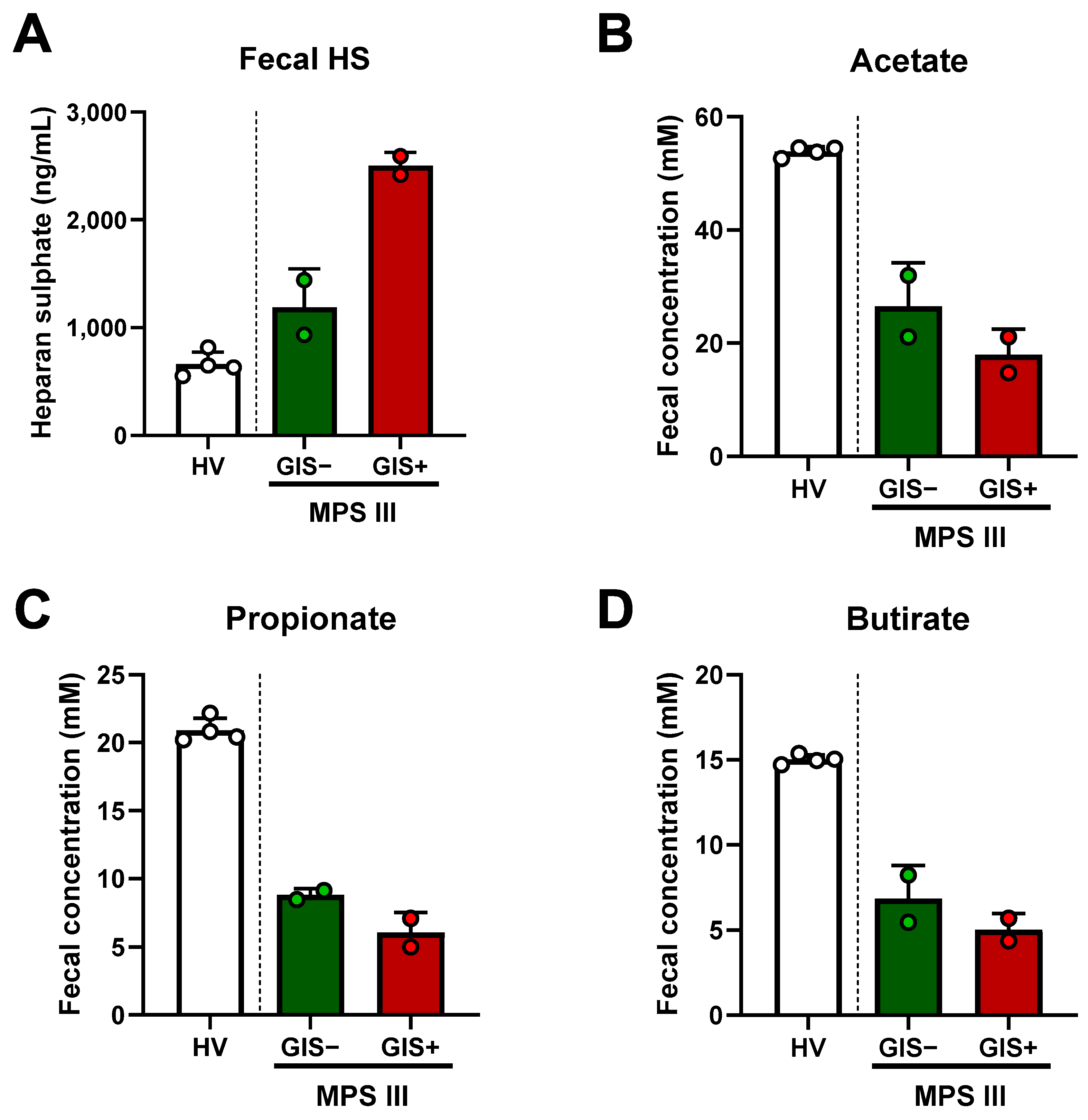
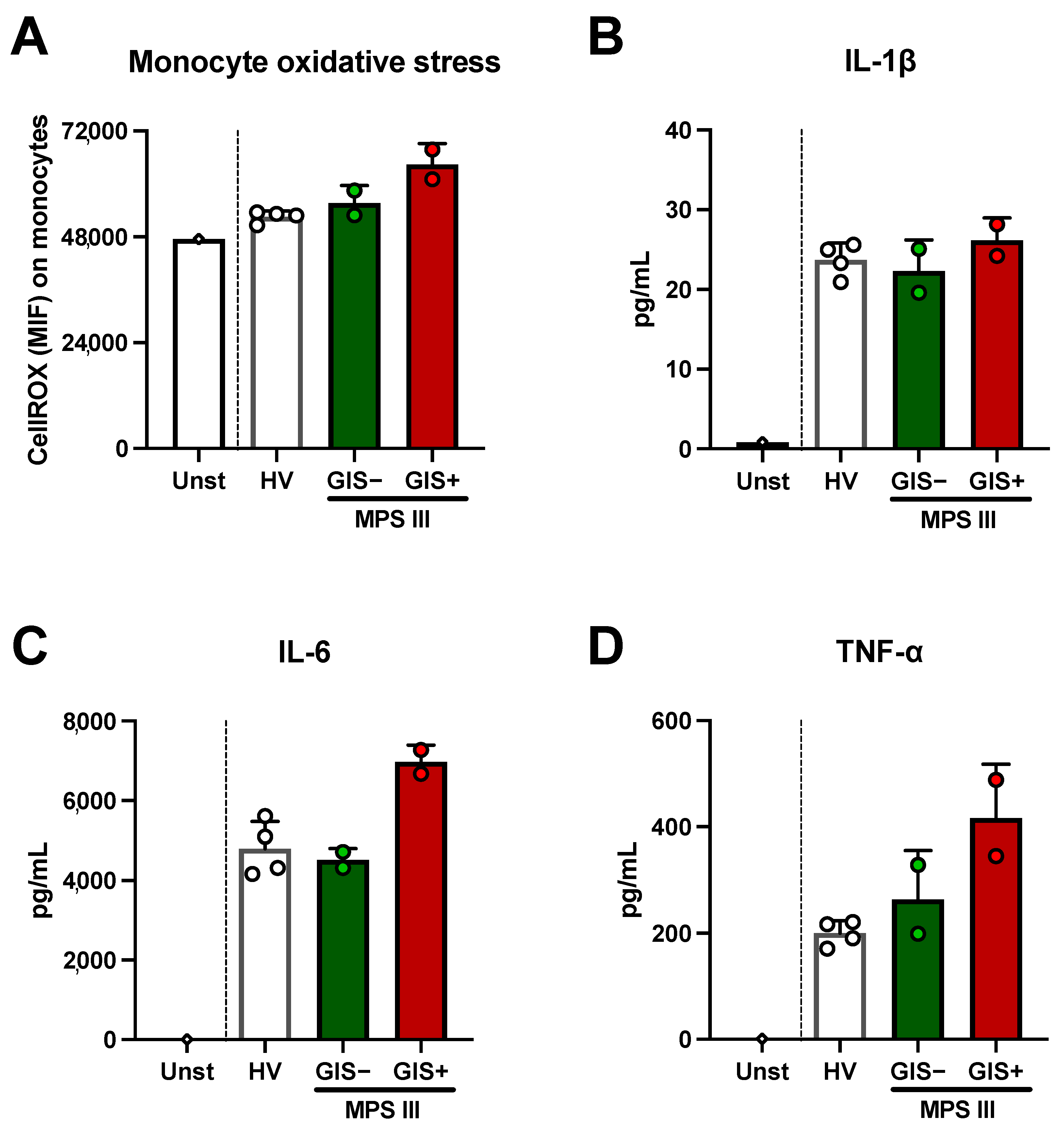
2.3. Microbial-Derived Metabolites and HS Levels in Stool: Role in Oxidative Stress
2.4. Bacteriodes thetaiotaomicron Isolate Exhibited In Vitro HS Degradation Ability
3. Discussion
4. Materials and Methods
4.1. Participants and Study Design
4.2. Microbiota Composition by 16S rDNA Sequencing
4.3. Relative Abundance of Polysaccharide Utilization Loci by qPCR
4.4. Levomepromazine MIC Determination
4.5. Fecal HS and SCFAs Determination
4.6. Monocyte Cell Culture and Fecal Metabolite Oxidative Stress and Inflammatory Assay
4.7. Bacteroides Spp. Isolation and In Vitro HS Degradation Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrade, F.; Aldámiz-Echevarría, L.; Llarena, M.; Couce, M.L. Sanfilippo Syndrome: Overall Review. Pediatr. Int. 2015, 57, 331–338. [Google Scholar] [CrossRef]
- Heon-Roberts, R.; Nguyen, A.L.A.; Pshezhetsky, A.V. Molecular Bases of Neurodegeneration and Cognitive Decline, the Major Burden of Sanfilippo Disease. J. Clin. Med. 2020, 9, 344. [Google Scholar] [CrossRef]
- Fecarotta, S.; Tarallo, A.; Damiano, C.; Minopoli, N.; Parenti, G. Pathogenesis of Mucopolysaccharidoses, an Update. Int. J. Mol. Sci. 2020, 21, 2515. [Google Scholar] [CrossRef]
- Benetó, N.; Vilageliu, L.; Grinberg, D.; Canals, I. Sanfilippo Syndrome: Molecular Basis, Disease Models and Therapeutic Approaches. Int. J. Mol. Sci. 2020, 21, 7819. [Google Scholar] [CrossRef] [PubMed]
- De Pasquale, V.; Pavone, L.M. Heparan Sulfate Proteoglycans: The Sweet Side of Development Turns Sour in Mucopolysaccharidoses. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165539. [Google Scholar] [CrossRef] [PubMed]
- Wijburg, F.A.; Heap, F.; Rust, S.; de Ruijter, J.; Tump, E.; Marchal, J.P.; Nestrasil, I.; Shapiro, E.; Jones, S.A.; Alexanderian, D. Long-Term Safety and Clinical Outcomes of Intrathecal Heparan-N-Sulfatase in Patients with Sanfilippo Syndrome Type A. Mol. Genet. Metab. 2021, 134, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Deiva, K.; Ausseil, J.; de Bournonville, S.; Zérah, M.; Husson, B.; Gougeon, M.-L.; Poirier-Beaudouin, B.; Zafeiriou, D.; Parenti, G.; Heard, J.-M.; et al. Intracerebral Gene Therapy in Four Children with Sanfilippo B Syndrome: 5.5-Year Follow-Up Results. Hum. Gene Ther. 2021, 32, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Rust, S.; Langford-Smith, K.; Weisberg, D.; Canal, M.; Breen, C.; Hepburn, M.; Tylee, K.; Vaz, F.M.; Vail, A.; et al. High Dose Genistein in Sanfilippo Syndrome: A Randomised Controlled Trial. J. Inherit. Metab. Dis. 2021, 44, 1248–1262. [Google Scholar] [CrossRef]
- Lavery, C.; Hendriksz, C.J.; Jones, S.A. Mortality in Patients with Sanfilippo Syndrome. Orphanet J. Rare Dis. 2017, 12, 168. [Google Scholar] [CrossRef]
- Thomas, S.; Ramaswami, U.; Cleary, M.; Yaqub, M.; Raebel, E.M. Gastrointestinal Manifestations in Mucopolysaccharidosis Type III: Review of Death Certificates and the Literature. J. Clin. Med. 2021, 10, 4445. [Google Scholar] [CrossRef]
- Roberts, A.L.K.D.; Howarth, G.S.; Liaw, W.C.; Moretta, S.; Kritas, S.; Lymn, K.A.; Yazbeck, R.; Tran, C.; Fletcher, J.M.; Butler, R.N.; et al. Gastrointestinal Pathology in a Mouse Model of Mucopolysaccharidosis Type IIIA. J. Cell Physiol. 2009, 219, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.O.; Lundgren, P.; Nath, K.; Thaiss, C.A. Metabolic Control by the Microbiome. Genome Med. 2022, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy Alters Gut Ecosystem and Improves Gastrointestinal and Autism Symptoms: An Open-Label Study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Vendrik, K.E.W.; Ooijevaar, R.E.; de Jong, P.R.C.; Laman, J.D.; van Oosten, B.W.; van Hilten, J.J.; Ducarmon, Q.R.; Keller, J.J.; Kuijper, E.J.; Contarino, M.F. Fecal Microbiota Transplantation in Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Cartmell, A.; Lowe, E.C.; Baslé, A.; Firbank, S.J.; Ndeh, D.A.; Murray, H.; Terrapon, N.; Lombard, V.; Henrissat, B.; Turnbull, J.E.; et al. How Members of the Human Gut Microbiota Overcome the Sulfation Problem Posed by Glycosaminoglycans. Proc. Natl. Acad. Sci. USA 2017, 114, 7037–7042. [Google Scholar] [CrossRef] [PubMed]
- Ndeh, D.; Baslé, A.; Strahl, H.; Yates, E.A.; McClurgg, U.L.; Henrissat, B.; Terrapon, N.; Cartmell, A. Metabolism of Multiple Glycosaminoglycans by Bacteroides thetaiotaomicron Is Orchestrated by a Versatile Core Genetic Locus. Nat. Commun. 2020, 11, 646. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.S.; Hameed, A.S.S.; Meng, X.; Liu, W. Utilization of Glycosaminoglycans by the Human Gut Microbiota: Participating Bacteria and Their Enzymatic Machineries. Gut Microbes 2022, 14, 2068367. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.S.; Jin, C.; Pereira, G.V.; Glowacki, R.W.P.; Gugel, S.R.; Singh, S.; Byrne, D.P.; Pudlo, N.A.; London, J.A.; Baslé, A.; et al. A Single Sulfatase Is Required to Access Colonic Mucin by a Gut Bacterium. Nature 2021, 598, 332–337. [Google Scholar] [CrossRef]
- Ruff, W.E.; Greiling, T.M.; Kriegel, M.A. Host-Microbiota Interactions in Immune-Mediated Diseases. Nat. Rev. Microbiol. 2020, 18, 521–538. [Google Scholar] [CrossRef]
- Ausseil, J.; Desmaris, N.; Bigou, S.; Attali, R.; Corbineau, S.; Vitry, S.; Parent, M.; Cheillan, D.; Fuller, M.; Maire, I.; et al. Early Neurodegeneration Progresses Independently of Microglial Activation by Heparan Sulfate in the Brain of Mucopolysaccharidosis IIIB Mice. PLoS ONE 2008, 3, e2296. [Google Scholar] [CrossRef] [PubMed]
- DiRosario, J.; Divers, E.; Wang, C.; Etter, J.; Charrier, A.; Jukkola, P.; Auer, H.; Best, V.; Newsom, D.L.; McCarty, D.M.; et al. Innate and Adaptive Immune Activation in the Brain of MPS IIIB Mouse Model. J. Neurosci. Res. 2009, 87, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.; Ellison, S.M.; Holley, R.J.; O’Leary, C.; Liao, A.; Asadi, J.; Glover, E.; Ghosh, A.; Jones, S.; Wilkinson, F.L.; et al. Haematopoietic Stem Cell Gene Therapy with IL-1Ra Rescues Cognitive Loss in Mucopolysaccharidosis IIIA. EMBO Mol. Med. 2020, 12, e11185. [Google Scholar] [CrossRef] [PubMed]
- Mandolfo, O.; Parker, H.; Bigger, B. Innate Immunity in Mucopolysaccharide Diseases. Int. J. Mol. Sci. 2022, 23, 1999. [Google Scholar] [CrossRef]
- Meyer, A.; Kossow, K.; Gal, A.; Mühlhausen, C.; Ullrich, K.; Braulke, T.; Muschol, N. Scoring Evaluation of the Natural Course of Mucopolysaccharidosis Type IIIA (Sanfilippo Syndrome Type A). Pediatrics 2007, 120, e1255–e1261. [Google Scholar] [CrossRef] [PubMed]
- Cyske, Z.; Anikiej-Wiczenbach, P.; Wisniewska, K.; Gaffke, L.; Pierzynowska, K.; Mański, A.; Wegrzyn, G. Sanfilippo Syndrome: Optimizing Care with a Multidisciplinary Approach. J. Multidiscip. Healthc. 2022, 15, 2097–2110. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-Term Benefit of Microbiota Transfer Therapy on Autism Symptoms and Gut Microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef]
- Chiang, H.-L.; Lin, C.-H. Altered Gut Microbiome and Intestinal Pathology in Parkinson’s Disease. J. Mov. Disord. 2019, 12, 67–83. [Google Scholar] [CrossRef]
- Bianchimano, P.; Britton, G.J.; Wallach, D.S.; Smith, E.M.; Cox, L.M.; Liu, S.; Iwanowski, K.; Weiner, H.L.; Faith, J.J.; Clemente, J.C.; et al. Mining the Microbiota to Identify Gut Commensals Modulating Neuroinflammation in a Mouse Model of Multiple Sclerosis. Microbiome 2022, 10, 174. [Google Scholar] [CrossRef]
- Cani, P.D. Gut Microbiota—At the Intersection of Everything? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 321–322. [Google Scholar] [CrossRef]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and Disease Markers Correlate with Gut Microbiome Composition across Thousands of People. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef] [PubMed]
- Saralegui, C.; García-Durán, C.; Romeu, E.; Hernáez-Sánchez, M.L.; Maruri, A.; Bastón-Paz, N.; Lamas, A.; Vicente, S.; Pérez-Ruiz, E.; Delgado, I.; et al. Statistical Evaluation of Metaproteomics and 16S rRNA Amplicon Sequencing Techniques for Study of Gut Microbiota Establishment in Infants with Cystic Fibrosis. Microbiol. Spectr. 2022, 10, e0146622. [Google Scholar] [CrossRef] [PubMed]
- Wampach, L.; Heintz-Buschart, A.; Fritz, J.V.; Ramiro-Garcia, J.; Habier, J.; Herold, M.; Narayanasamy, S.; Kaysen, A.; Hogan, A.H.; Bindl, L.; et al. Birth Mode Is Associated with Earliest Strain-Conferred Gut Microbiome Functions and Immunostimulatory Potential. Nat. Commun. 2018, 9, 5091. [Google Scholar] [CrossRef] [PubMed]
- Grondin, J.M.; Tamura, K.; Déjean, G.; Abbott, D.W.; Brumer, H. Polysaccharide Utilization Loci: Fueling Microbial Communities. J. Bacteriol. 2017, 199, e00860-16. [Google Scholar] [CrossRef] [PubMed]
- Foley, M.H.; Martens, E.C.; Koropatkin, N.M. SusE Facilitates Starch Uptake Independent of Starch Binding in B. thetaiotaomicron. Mol. Microbiol. 2018, 108, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.A.; Koropatkin, N.M. Host Glycan Utilization within the Bacteroidetes Sus-like Paradigm. Glycobiology 2021, 31, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Ratering, S.; Sadeghi, A.; Pokhrel, S.; Honermeier, B.; Schnell, S. The Response of the Soil Microbiota to Long-Term Mineral and Organic Nitrogen Fertilization Is Stronger in the Bulk Soil than in the Rhizosphere. Genes 2020, 11, 456. [Google Scholar] [CrossRef]
- D’Elia, J.N.; Salyers, A.A. Contribution of a Neopullulanase, a Pullulanase, and an Alpha-Glucosidase to Growth of Bacteroides thetaiotaomicron on Starch. J. Bacteriol. 1996, 178, 7173–7179. [Google Scholar] [CrossRef]
- Koropatkin, N.M.; Smith, T.J. SusG: A Unique Cell-Membrane-Associated Alpha-Amylase from a Prominent Human Gut Symbiont Targets Complex Starch Molecules. Structure 2010, 18, 200–215. [Google Scholar] [CrossRef]
- Reeves, A.R.; Wang, G.R.; Salyers, A.A. Characterization of Four Outer Membrane Proteins That Play a Role in Utilization of Starch by Bacteroides thetaiotaomicron. J. Bacteriol. 1997, 179, 643–649. [Google Scholar] [CrossRef]
- Cho, K.H.; Salyers, A.A. Biochemical Analysis of Interactions between Outer Membrane Proteins That Contribute to Starch Utilization by Bacteroides thetaiotaomicron. J. Bacteriol. 2001, 183, 7224–7230. [Google Scholar] [CrossRef]
- Zampini, L.; Padella, L.; Marchesiello, R.L.; Santoro, L.; Monachesi, C.; Giovagnoni, A.; Catassi, C.; Gabrielli, O.; Coppa, G.V.; Galeazzi, T. Importance of the Combined Urinary Procedure for the Diagnosis of Mucopolysaccharidoses. Clin. Chim. Acta 2017, 464, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Saville, J.T.; Flanigan, K.M.; Truxal, K.V.; McBride, K.L.; Fuller, M. Evaluation of Biomarkers for Sanfilippo Syndrome. Mol. Genet. Metab. 2019, 128, 68–74. [Google Scholar] [CrossRef]
- Benjdia, A.; Martens, E.C.; Gordon, J.I.; Berteau, O. Sulfatases and a Radical S-Adenosyl-L-Methionine (AdoMet) Enzyme Are Key for Mucosal Foraging and Fitness of the Prominent Human Gut Symbiont, Bacteroides thetaiotaomicron. J. Biol. Chem. 2011, 286, 25973–25982. [Google Scholar] [CrossRef]
- Kim, C.H. Complex Regulatory Effects of Gut Microbial Short-Chain Fatty Acids on Immune Tolerance and Autoimmunity. Cell Mol. Immunol. 2023, 20, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Ortiz, J.; Lorente-Ros, Á.; Briones-Figueroa, A.; Morán-Alvarez, P.; García-Fernández, A.; Garrote-Corral, S.; Amil-Casas, I.; Carrasco-Sayalero, Á.; Tejada-Velarde, A.; Camino-López, A.; et al. Serological Short-Chain Fatty Acid and Trimethylamine N-Oxide Microbial Metabolite Imbalances in Young Adults with Acute Myocardial Infarction. Heliyon 2023, 9, e20854. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Kuwabara, R.; de Haan, B.J.; Smink, A.M.; de Vos, P. Acetate and Butyrate Improve β-Cell Metabolism and Mitochondrial Respiration under Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 1542. [Google Scholar] [CrossRef]
- Barbed Ferrández, S.M.; García Romero, R.; Pérez Delgado, R.; Romagosa Sánchez-Monge, I.; Ros Arnal, I.; Torrecilla Idoipe, N. Intestinal lymphangiectasia in a patient with Sanfilippo B syndrome. Arch. Argent. Pediatr. 2021, 119, e138–e141. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Q.; Zheng, M.; Hao, S.; Lum, J.S.; Chen, X.; Huang, X.-F.; Yu, Y.; Zheng, K. Supplement of Microbiota-Accessible Carbohydrates Prevents Neuroinflammation and Cognitive Decline by Improving the Gut Microbiota-Brain Axis in Diet-Induced Obese Mice. J. Neuroinflamm. 2020, 17, 77. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface Components and Metabolites of Probiotics for Regulation of Intestinal Epithelial Barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Valstar, M.J.; Ruijter, G.J.G.; van Diggelen, O.P.; Poorthuis, B.J.; Wijburg, F.A. Sanfilippo Syndrome: A Mini-Review. J. Inherit. Metab. Dis. 2008, 31, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Lagadinou, M.; Onisor, M.O.; Rigas, A.; Musetescu, D.-V.; Gkentzi, D.; Assimakopoulos, S.F.; Panos, G.; Marangos, M. Antimicrobial Properties on Non-Antibiotic Drugs in the Era of Increased Bacterial Resistance. Antibiotics 2020, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Unno, T.; Kim, B.Y.; Park, M.S. Sex Differences in Gut Microbiota. World J. Men. S Health 2020, 38, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, P.; Liu, Y.; Zhong, X.; Wang, H.; Guo, Y. Sex Differences in Gut Microbiota in Patients with Major Depressive Disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, S.; Motooka, D.; Watanabe, S.; Kubo, R.; Jung, N.; Midorikawa, Y.; Shinozaki, N.O.; Sawai, Y.; Takeda, A.K.; Nakamura, S. Benchmark of 16S rRNA Gene Amplicon Sequencing Using Japanese Gut Microbiome Data from the V1–V2 and V3–V4 Primer Sets. BMC Genom. 2021, 22, 527. [Google Scholar] [CrossRef]
- Daskova, N.; Heczkova, M.; Modos, I.; Videnska, P.; Splichalova, P.; Pelantova, H.; Kuzma, M.; Gojda, J.; Cahova, M. Determination of Butyrate Synthesis Capacity in Gut Microbiota: Quantification of but Gene Abundance by qPCR in Fecal Samples. Biomolecules 2021, 11, 1303. [Google Scholar] [CrossRef]
- Teng, L.J.; Hsueh, P.R.; Tsai, J.C.; Chiang, F.L.; Chen, C.Y.; Ho, S.W.; Luh, K.T. PCR Assay for Species-Specific Identification of Bacteroides thetaiotaomicron. J. Clin. Microbiol. 2000, 38, 1672–1675. [Google Scholar] [CrossRef] [PubMed]
- Rohde, J.K.; Fuh, M.M.; Evangelakos, I.; Pauly, M.J.; Schaltenberg, N.; Siracusa, F.; Gagliani, N.; Tödter, K.; Heeren, J.; Worthmann, A. A Gas Chromatography Mass Spectrometry-Based Method for the Quantification of Short Chain Fatty Acids. Metabolites 2022, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- del Campo, R.; Martínez, E.; del Fresno, C.; Alenda, R.; Gómez-Piña, V.; Fernández-Ruíz, I.; Siliceo, M.; Jurado, T.; Toledano, V.; Arnalich, F.; et al. Translocated LPS Might Cause Endotoxin Tolerance in Circulating Monocytes of Cystic Fibrosis Patients. PLoS ONE 2011, 6, e29577. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Ortiz, J.; Llanos-González, E.; Toledano, V.; Del Campo, R.; Cubillos-Zapata, C.; Lozano-Rodríguez, R.; Ismail, A.; Prados, C.; Gómez-Campelo, P.; Aguirre, L.A.; et al. Pseudomonas aeruginosa Colonization Causes PD-L1 Overexpression on Monocytes, Impairing the Adaptive Immune Response in Patients with Cystic Fibrosis. J. Cyst. Fibros. 2019, 18, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Tierney, D.; Copsey, S.D.; Morris, T.; Perry, J.D. A New Chromogenic Medium for Isolation of Bacteroides fragilis Suitable for Screening for Strains with Antimicrobial Resistance. Anaerobe 2016, 39, 168–172. [Google Scholar] [CrossRef]
- Ladner, Y.D.; Alini, M.; Armiento, A.R. The Dimethylmethylene Blue Assay (DMMB) for the Quantification of Sulfated Glycosaminoglycans. Methods Mol. Biol. 2023, 2598, 115–121. [Google Scholar] [CrossRef]


| Sibling 1 | Sibling 2 | |
|---|---|---|
| Age (years) | 13 | 12 |
| Sex | Female | Male |
| Age at diagnosis (years) | 2 | 2 |
| Symptoms | Intellectual disability, behavioral disorder, epilepsy, sleep disorder, loss of walking and standing ability | Intellectual disability, behavioral disorder, epilepsy, sleep disorder, and recurrent diarrhea |
| Current treatment | Carbamazepine, tizanidine, trihexyphenidyl, vitamin D, clonazepam, melatonin | Carbamazepine, tizanidine, vitamin D, levomepromazine, clonazepam, melatonin |
| Urine total GAGs (mg/mmol creatinine) | 20.25 | 11.98 |
| Sulfamidase activity (nmol/mL/17 h) | 0.15 | 0.38 |
| ALT (U/L) | 42 | 50 |
| GGT (U/L) | 39 | 44 |
| Meyer scale | 0–1 | |
| - Motor | 0 | 1–2 |
| - Speech | 0 | 0 |
| - Cognitive | 0–1 | 1 |
| Daily diet | ||
| - Energy (Kcal) | 1080 | 1092 |
| - Fat (g per day) | 33 | 33 |
| - Proteins (g) | 43 | 43 |
| - Carbohydrates (g) | 147 | 149 |
| - Fiber (g) | 11.50 | 11.50 |
| Calcium (mg) | 632 | 632 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbero-Herranz, R.; Garriga-García, M.; Moreno-Blanco, A.; Palacios, E.; Ruiz-Sala, P.; Vicente-Santamaría, S.; Stanescu, S.; Belanger-Quintana, A.; Pintos-Morell, G.; Arconada, B.; et al. The Role of the Gut Microbiota in Sanfilippo Syndrome’s Physiopathology: An Approach in Two Affected Siblings. Int. J. Mol. Sci. 2024, 25, 8856. https://doi.org/10.3390/ijms25168856
Barbero-Herranz R, Garriga-García M, Moreno-Blanco A, Palacios E, Ruiz-Sala P, Vicente-Santamaría S, Stanescu S, Belanger-Quintana A, Pintos-Morell G, Arconada B, et al. The Role of the Gut Microbiota in Sanfilippo Syndrome’s Physiopathology: An Approach in Two Affected Siblings. International Journal of Molecular Sciences. 2024; 25(16):8856. https://doi.org/10.3390/ijms25168856
Chicago/Turabian StyleBarbero-Herranz, Raquel, María Garriga-García, Ana Moreno-Blanco, Esther Palacios, Pedro Ruiz-Sala, Saioa Vicente-Santamaría, Sinziana Stanescu, Amaya Belanger-Quintana, Guillem Pintos-Morell, Beatriz Arconada, and et al. 2024. "The Role of the Gut Microbiota in Sanfilippo Syndrome’s Physiopathology: An Approach in Two Affected Siblings" International Journal of Molecular Sciences 25, no. 16: 8856. https://doi.org/10.3390/ijms25168856
APA StyleBarbero-Herranz, R., Garriga-García, M., Moreno-Blanco, A., Palacios, E., Ruiz-Sala, P., Vicente-Santamaría, S., Stanescu, S., Belanger-Quintana, A., Pintos-Morell, G., Arconada, B., del Campo, R., & Avendaño-Ortiz, J. (2024). The Role of the Gut Microbiota in Sanfilippo Syndrome’s Physiopathology: An Approach in Two Affected Siblings. International Journal of Molecular Sciences, 25(16), 8856. https://doi.org/10.3390/ijms25168856






