Emerging Therapeutic Approaches and Genetic Insights in Stargardt Disease: A Comprehensive Review
Abstract
:1. Introduction
2. Disease Overview
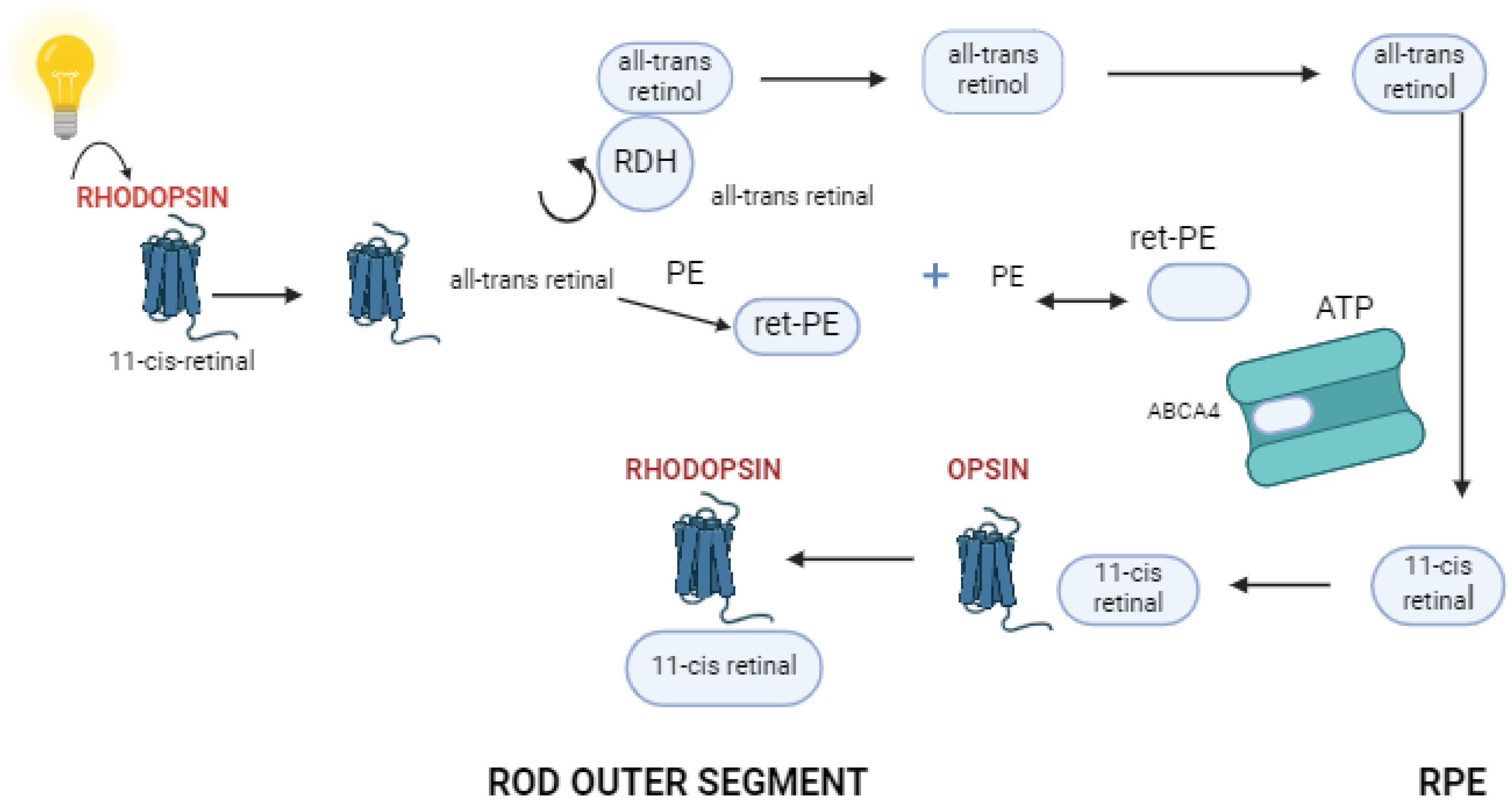
3. Function of Retinal Pigment Epithelium and Photoreceptor Cells
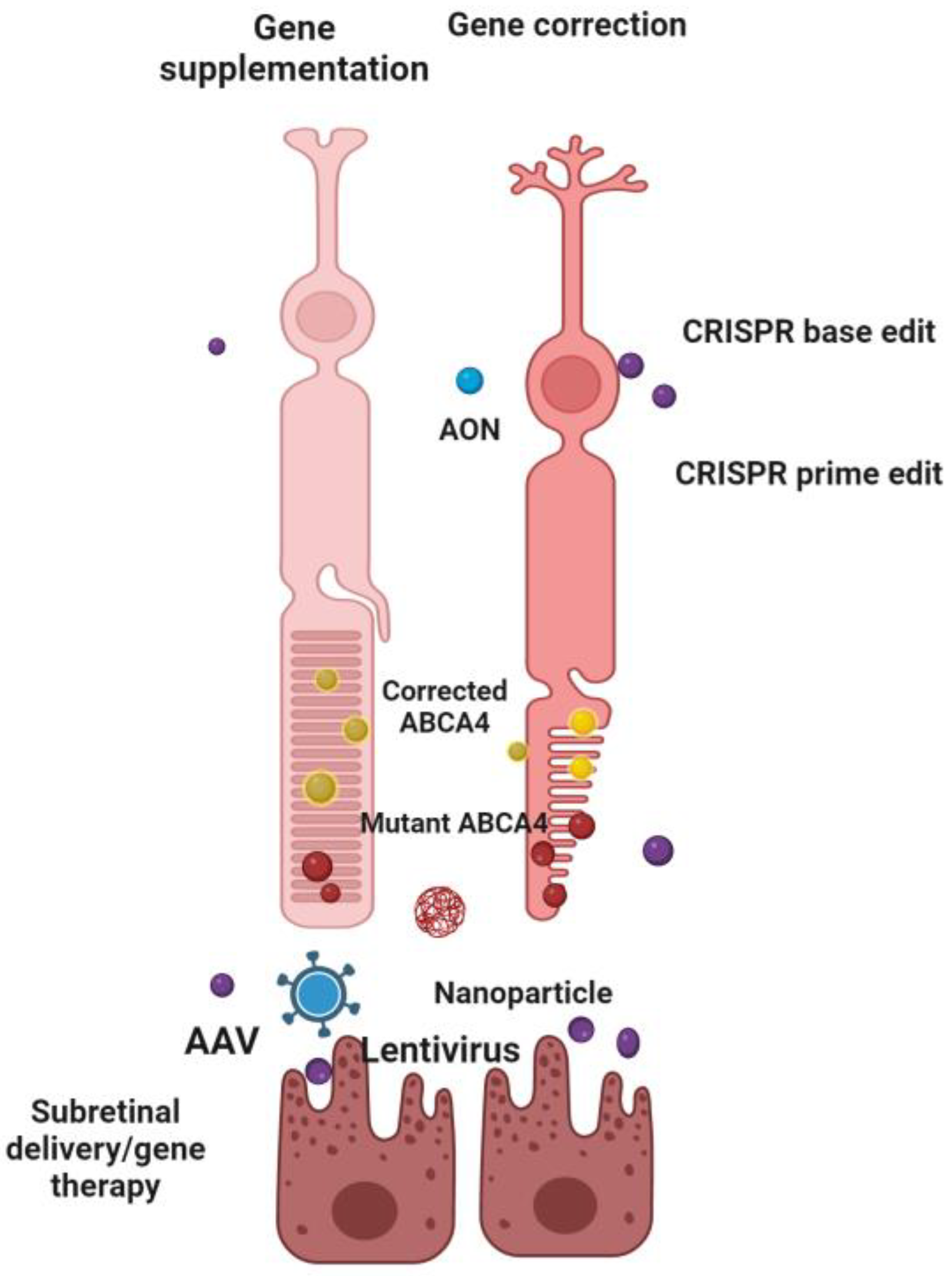
4. Novel Therapies in Stargardt Disease
4.1. Gene Therapy
4.1.1. Lentiviral Vectors
4.1.2. Adeno-Associated Viral Vectors
4.1.3. Other Genetic Approaches
| Strategy/ Intervention | Sponsor/Name of Study | Therapy | Clinical Trial/Website | Timeline | Phase |
|---|---|---|---|---|---|
| Gene therapy | |||||
| Gene supplementation | Ocugen/ GARDian West China Hospital | AAV (OCU410ST) AAV (JWK006) | NCT05956626 NCT06300476 | 2023/2025 2023/2026 | Phase 1/2 Phase 1/2 |
| Gene supplementation. | Sanofi | Lentivirus (SAR422459) | NCT01367444 NCT01736592 | 2011/2019 2012/2033 | Phase 1/2 Phase 1/2 |
| Gene supplementation | Splice Bio AAVantgarde Bio | Dual AAV | https://splice.bio/pipeline/; accessed on 26 June 2024 https://www.aavantgardebio.com/technology-pipeline/; accessed on 15 June 2024 | expected 2024/2025 | Phase 1/2 |
| Gene modulation | - | AON | N/A | N/A | N/A |
| Gene editing | Ascidian Therapeutics/ STELLAR | ACDN-01 | NCT06467344 | 2024/2030 | Phase 1/2 |
| Gene editing | - | CRISPR | - | N/A | N/A |
| Gene coding | Saliogen | SGT-1001 | https://www.saliogen.com/pipeline/; accessed on 20 May 2024 | expected 2024 | N/A |
| Optogenetic therapy | Nanoscope Therapeutics/ STARLIGHT | vMCO-010 | NCT05417126 | 2022/2023 | Phase 2 |
| Raytherapeutics | RTx-021 | https://raytherapeutics.com/pipeline/; accessed on 2 June 2024 | N/A | N/A | |
4.2. Cell Replacement Therapy
4.3. Pharmacological Therapy
4.4. Dietary Supplements
4.5. Future Perspectives in Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanna, P.; Strauss, R.W.; Fujinami, K.; Michaelides, M. Stargardt disease: Clinical features, molecular genetics, animal models and therapeutic options. Br. J. Ophthalmol. 2017, 101, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shah, S.M.; Mangwani-Mordani, S.; Gregori, N.Z. Updates on Emerging Interventions for Autosomal Recessive ABCA4-Associated Stargardt Disease. J. Clin. Med. 2023, 12, 6229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lenis, T.L.; Hu, J.; Ng, S.Y.; Jiang, Z.; Sarfare, S.; Lloyd, M.B.; Esposito, N.J.; Samuel, W.; Jaworski, C.; Bok, D.; et al. Expression of ABCA4 in the retinal pigment epithelium and its implications for Stargardt macular degeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E11120–E11127. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, T.; Baumann, B.; Allikmets, R. Stargardt Disease: Genetics and Molecular Mechanisms. In Inherited Retinal Disease: Diagnosis and Clinical Management; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–125. ISBN 9780128146358. [Google Scholar]
- Cicinelli, M.V.; Battista, M.; Starace, V.; Battaglia Parodi, M.; Bandello, F. Monitoring and Management of the Patient with Stargardt Disease. Clin. Optom. 2019, 11, 151–165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galvin, O.; Chi, G.; Brady, L.; Hippert, C.; Rubido, M.D.V.; Daly, A.; Michaelides, M. The impact of inherited retinal diseases in the Republic of Ireland (ROI) and the United Kingdom (UK) from a cost-of-illness perspective. Clin. Ophthalmol. 2020, 14, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Wittenborn, J.S.; Zhang, X.; Feagan, C.W.; Crouse, W.L.; Shrestha, S.; Kemper, A.R.; Hoerger, T.J.; Saaddine, J.B. The economic burden of vision loss and eye disorders among the United States population younger than 40 years. Ophthalmology 2013, 120, 1728–1735. [Google Scholar] [CrossRef]
- Sabel, B.A.; Wang, J.; Cárdenas-Morales, L.; Faiq, M.; Heim, C. Mental stress as consequence and cause of vision loss: The dawn of psychosomatic ophthalmology for preventive and personalized medicine. EPMA J. 2018, 9, 133–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heath Jeffery, R.C.; Mukhtar, S.A.; McAllister, I.L.; Morgan, W.H.; Mackey, D.A.; Chen, F.K. Inherited retinal diseases are the most common cause of blindness in the working-age population in Australia. Ophthalmic Genet. 2021, 42, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Arsenijevic, Y.; Berger, A.; Udry, F.; Kostic, C. Lentiviral Vectors for Ocular Gene Therapy. Pharmaceutics 2022, 14, 1605. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piotter, E.; McClements, M.E.; MacLaren, R.E. Therapy Approaches for Stargardt Disease. Biomolecules 2021, 11, 1179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, X.; Ruan, G.; Zhang, J.; Luo, F.; Zhao, K. Genetic Diagnosis of Familial Exudative Vitreoretinopathy by Targeted Next-Generation Sequencing. Ophthalmic Genet. 2021, 42, 196–202. [Google Scholar] [CrossRef]
- Cremers, F.P.M.; Lee, W.; Collin, R.W.J.; Allikmets, R. Clinical spectrum, genetic complexity and therapeutic approaches for retinal disease caused by ABCA4 mutations. Prog. Retin. Eye Res. 2020, 79, 100861. [Google Scholar] [CrossRef] [PubMed]
- Spiteri Cornish, K.; Ho, J.; Downes, S.; Scott, N.W.; Bainbridge, J.; Lois, N. The Epidemiology of Stargardt Disease in the United Kingdom. Ophthalmol. Retina 2017, 1, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Runhart, E.H.; Dhooge, P.; Meester-Smoor, M.; Pas, J.; Pott, J.W.R.; van Leeuwen, R.; Kroes, H.Y.; Bergen, A.A.; de Jong-Hesse, Y.; Thiadens, A.A.; et al. Stargardt disease: Monitoring incidence and diagnostic trends in the Netherlands using a nationwide disease registry. Acta Ophthalmol. 2022, 100, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, C.; Wu, D.; Tang, C.; Ren, C.; Yu, M.; He, H.; Lu, Y.; Xie, H.; Xu, H.; et al. Genetic Diagnosis of Hereditary Retinal Degeneration in China Using Next-Generation Sequencing. Front. Genet. 2021, 12, 646058. [Google Scholar] [CrossRef]
- Huckfeldt, R.M.; East, J.S.; Stone, E.M.; Sohn, E.H. Phenotypic Variation in a Family with Pseudodominant Stargardt Disease. JAMA Ophthalmol. 2016, 134, 580–583. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Rabiolo, A.; Brambati, M.; Viganò, C.; Bandello, F.; Battaglia Parodi, M. Factors Influencing Retinal Pigment Epithelium-Atrophy Progression Rate in Stargardt Disease. Transl. Vis. Sci. Technol. 2020, 9, 33. [Google Scholar] [CrossRef]
- Burke, T.R.; Duncker, T.; Woods, R.L.; Greenberg, J.P.; Zernant, J.; Tsang, S.H.; Smith, R.T.; Allikmets, R.; Sparrow, J.R.; Delori, F.C. Quantitative fundus autofluorescence in recessive Stargardt disease. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2841–2852. [Google Scholar] [CrossRef]
- Charng, J.; Xiao, D.; Mehdizadeh, M.; Attia, M.S.; Arunachalam, S.; Lamey, T.M.; Thompson, J.A.; McLaren, T.L.; De Roach, J.N.; Mackey, D.A.; et al. Deep learning segmentation of hyperautofluorescent fleck lesions in Stargardt disease. Sci. Rep. 2020, 10, 16491. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, J.; Li, D. Functions and Diseases of the Retinal Pigment Epithelium. Front. Pharmacol. 2021, 12, 727870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Léveillard, T.; Sahel, J.A. Metabolic and redox signaling in the retina. Free Radic. Biol. Med. 2023, 74, 3649–3665. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Li, S.; Moghrabi, W.N.; Sun, H.; Travis, G.H. RPE65 is the retinoid isomerase in bovine retinal pigment epithelium. Biochemistry 2005, 44, 14963–14973. [Google Scholar] [CrossRef]
- Mannino, G.; Cristaldi, M.; Giurdanella, G.; Perrotta, R.E.; Lo Furno, D.; Giuffrida, R.; Rusciano, D. ARPE-19 conditioned medium promotes neural differentiation of adipose-derived mesenchymal stem cells. World J. Stem Cells 2021, 13, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Mack, D.L.; Lin, B.; Wei, X.; Kosa, E.; Chuang, Y.A.; Chew, J.; Wang, K.; Byrnes, C.; Snyder, E.Y.; O’Rourke, J.R.; et al. Dicer reduction induces progressive gene-independent loss of functionality in Drosophila GABAergic neurons. J. Neurosci. Res. 2011, 89, 972–979. [Google Scholar] [CrossRef]
- Han, Z.; Conley, S.M.; Naash, M.I. Gene therapy for Stargardt disease associated with ABCA4 mutations. Proc. Natl. Acad. Sci. USA 2019, 116, 7951–7956. [Google Scholar] [CrossRef]
- Reingruber, J.; Holcman, D. The dynamics of phosphodiesterase activation in rods and cones. Biophys. J. 2008, 94, 1954–1970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fishman, G.A.; Gilbert, L.D.; Anderson, R.J.; Marmor, M.F.; Weleber, R.G.; Tratner, I.; Moradi, R.; Stone, E.M. Rapid-onset widespread chorioretinal atrophy in patients with pseudodominant Stargardt disease and ABCA4 mutations. JAMA Ophthalmol. 2015, 133, 1198–1203. [Google Scholar] [CrossRef]
- Radu, R.A.; Han, Y.; Bui, T.V.; Nusinowitz, S.; Bok, D.; Lichter, J.B.; Travis, G.H.; Mata, N.L. Reductions in Serum Vitamin A in Stargardt’s Patients Treated with Oral Fenretinide. Investig. Ophthalmol. Vis. Sci. 2011, 53, 4409–4415. [Google Scholar] [CrossRef]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and Safety of Voretigene Neparvovec (AAV2-HRPE65v2) in Patients with RPE65-Mediated Inherited Retinal Dystrophy: A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; Xue, K.; de la Camara, C.M.-F.; Nanda, A.; Davies, A.; Wood, L.J.; Salvetti, A.P.; Fischer, M.D.; Aylward, J.W.; Barnard, A.R.; et al. Initial Results from a First-in-Human Gene Therapy Trial on X-Linked Retinitis Pigmentosa Caused by Mutations in RPGR. Nat. Med. 2020, 26, 354–359. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; Birtel, J.; McClements, M.E.; Shanks, M.E.; Clouston, P.; Downes, S.M.; Issa, P.C.; MacLaren, R.E. Clinical and Molecular Characterization of PROM1-Related Retinal Degeneration. JAMA Netw. Open 2019, 2, e195752. [Google Scholar] [CrossRef] [PubMed]
- Binley, K.; Widdowson, P.; Loader, J.; Kelleher, M.; Iqball, S.; Ferrige, G.; de Belin, J.; Carlucci, M.; Angell-Manning, D.; Hurst, F.; et al. Transduction of photoreceptors with equine infectious anemia virus lentiviral vectors: Safety and biodistribution of StarGen for Stargardt disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4061–4071. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.A.; Erker, L.R.; Audo, I.; Choi, D.; Mohand-Said, S.; Sestakauskas, K.; Benoit, P.; Appelqvist, T.; Krahmer, M.; Segaut-Prevost, C.; et al. Three-year safety results of SAR422459 (EIAV-ABCA4) gene therapy in patients with ABCA4-associated Stargardt disease: An open-label dose-escalation phase I/IIa clinical trial, cohorts 1–5. Am. J. Ophthalmol. 2022, 240, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.A.; Choi, D.; Erker, L.R.; Pennesi, M.E.; Yang, P.; Chegarnov, E.N.; Steinkamp, P.N.; Schlechter, C.L.; Dhaenens, C.M.; Mohand-Said, S.; et al. Test-retest variability of functional and structural parameters in patients with Stargardt disease participating in the SAR422459 gene therapy trial. Transl. Vis. Sci. Technol. 2016, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- McClements, M.E.; Barnard, A.R.; Singh, M.S.; Charbel Issa, P.; Jiang, Z.; Radu, R.A.; MacLaren, R.E. An AAV Dual Vector Strategy Ameliorates the Stargardt Phenotype in Adult Abca4-/-Mice. Hum. Gene Ther. 2019, 30, 590–600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trapani, I. Methods in Molecular Biology. In Efficient Non-Viral Gene Therapy for Stargardt’s Disease with pH-Sensitive Multifunctional Lipid ECO Plasmid DNA Nanoparticles; Association for Research in Vision and Ophthalmology: Rockville, MD, USA, 2018. [Google Scholar] [CrossRef]
- Notario, A.; Trapani, I.; Tiberi, P.; Minopoli, R.; Iodice, C.; D’Avino, C.; Marrocco, E.; Manfredi, A.; Giacca, M.; Auricchio, A. Efficient Non-viral Gene Therapy for Stargardt’s Disease with pH-Sensitive Multifunctional Lipid ECO Plasmid DNA Nanoparticles. Mol. Ther. 2021, 29, 2248–2267. [Google Scholar] [CrossRef]
- Han, Z.; Conley, S.M.; Makkia, R.S.; Cooper, M.J.; Naash, M.I. DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J. Clin. Investig. 2012, 122, 3221–3226. [Google Scholar] [CrossRef]
- Sun, D.; Schur, R.M.; Sears, A.E.; Gao, S.Q.; Vaidya, A.; Sun, W.; Maeda, A.; Kern, T.; Palczewski, K.; Lu, Z.R. Non-viral Gene Therapy for Stargardt Disease with ECO/pRHO-ABCA4 Self-Assembled Nanoparticles. Mol. Ther. 2020, 28, 293–303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sangermano, R.; Garanto, A.; Khan, M.; Runhart, E.H.; Bauwens, M.; Bax, N.M.; van den Born, L.I.; Khan, M.I.; Cornelis, S.S.; Verheij, J.B.G.M.; et al. Deep-intronic ABCA4 variants explain missing heritability in Stargardt disease and allow correction of splice defects by antisense oligonucleotides. Genet. Med. 2019, 21, 1751–1760. [Google Scholar] [CrossRef]
- Albert, S.; Garanto, A.; Sangermano, R.; Khan, M.; Bax, N.M.; Hoyng, C.B.; Zernant, J.; Lee, W.; Allikmets, R.; Collin, R.W.J.; et al. Identification and Rescue of Splice Defects Caused by Two Neighboring Deep-Intronic ABCA4 Mutations Underlying Stargardt Disease. Am. J. Hum. Genet. 2018, 102, 517–527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garanto, A.; Duijkers, L.; Tomkiewicz, T.Z.; Collin, R.W.J. Antisense Oligonucleotide Screening to Optimize the Rescue of the Splicing Defect Caused by the Recurrent Deep-Intronic ABCA4 Variant c.4539+2001G>A in Stargardt Disease. Investig. Ophthalmol. Vis. Sci. 2018, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Bauwens, M.; Garanto, A.; Sangermano, R.; Naessens, S.; Weisschuh, N.; De Zaeytijd, J.; Khan, M.; Sadler, F.; Balikova, I.; Van Cauwenbergh, C.; et al. ABCA4-associated disease as a model for missing heritability in autosomal recessive disorders: Novel noncoding splice, cis-regulatory, structural, and recurrent hypomorphic variants. Genet. Med. 2019, 21, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Parmar, V.M.; Parmar, T.; Arai, E.; Perusek, L.; Maeda, A. A2E-associated cell death and inflammation in retinal pigmented epithelial cells from human induced pluripotent stem cells. Stem Cell Res. 2018, 27, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Malcuit, C.; Wang, S.; Girman, S.; Francis, P.; Lemieux, L.; Lanza, R.; Lund, R. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells 2009, 27, 2126–2135. [Google Scholar] [CrossRef] [PubMed]
- Song, W.K.; Park, K.-M.; Kim, H.-J.; Lee, J.H.; Choi, J.; Chong, S.Y.; Shim, S.H.; Del Priore, L.V.; Lanza, R. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: Preliminary results in Asian patients. Stem Cell Rep. 2015, 4, 860–872. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Lanza, R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Stargardt Disease. Medicines 2021, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Radu, R.A.; Yuan, Q.; Hu, J.; Peng, J.H.; Lloyd, M.; Nusinowitz, S.; Bok, D.; Travis, G.H. Accelerated Accumulation of Lipofuscin Pigments in the RPE of a Mouse Model for ABCA4-Mediated Retinal Dystrophies Following Vitamin A Supplementation. Invest. Ophthalmol. Vis. Sci. 2008, 49, 3821–3829. [Google Scholar] [CrossRef]
- Scholl, H.P.; Shah, S.M.; Kay, C.N.; Tsang, S.H.; Stepien, K.E.; Bernstein, P.S.; Lam, B.L.; Gorin, M.B.; Washington, I.; Saad, L. TEASE: A phase 2 clinical trial assessing the tolerability and effects of oral once-a-day ALK-001 on Stargardt disease. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2685. [Google Scholar]
- Steinhoff, J.S.; Lass, A.; Schupp, M. Biological Functions of RBP4 and Its Relevance for Human Diseases. Front. Physiol. 2021, 12, 659977. [Google Scholar] [CrossRef] [PubMed]
- Flores-Cortez, Y.A.; Barragán-Bonilla, M.I.; Mendoza-Bello, J.M.; González-Calixto, C.; Flores-Alfaro, E.; Espinoza-Rojo, M. Interplay of retinol binding protein 4 with obesity and associated chronic alterations (Review). Mol. Med. Rep. 2022, 26, 244. [Google Scholar] [CrossRef]
- Grigg, J.R.; Chen, F.K.; Chen, T.-C.; Jamieson, R.V.; Mata, N.L.; Liao, W. A Phase 1b/2 Study of the Safety and Tolerability of Tinlarebant in Adolescent STGD1 Subjects. Investig. Ophthalmol. Vis. Sci. 2022, 63, 3518. [Google Scholar]
- Grigg, J.R.; Chen, F.; Chen, T.-C.; Jamieson, R.V.; Scholl, H.P.; Michaelides, M.; Zernant, J.; Allikmets, R.; Nguyen, Q.D.; Mata, N.L. Safety, Tolerability, and Efficacy of Tinlarebant from the 24-Month Phase 2 study in Adolescent Patients Affected by Stargardt Disease. Investig. Ophthalmol. Vis. Sci. 2024, 65, 1026. [Google Scholar]
- Dhooge, P.P.A.; Möller, P.T.; Meland, N.; Stingl, K.; Boon, C.J.F.; Lotery, A.J.; Parodi, M.B.; Herrmann, P.; Klein, W.; Fsadni, M.G.; et al. Repeatability of Quantitative Autofluorescence Imaging in a Multicenter Study Involving Patients with Recessive Stargardt Disease 1. Transl. Vis. Sci. Technol. 2023, 12, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, Y.; Taubitz, T.; Tschulakow, A.V.; Heiduschka, P.; Szewczyk, G.; Burnet, M.; Peters, T.; Biesemeier, A.; Sarna, T.; Schraermeyer, U.; et al. Removal of RPE lipofuscin results in rescue from retinal degeneration in a mouse model of advanced Stargardt disease: Role of reactive oxygen species. Free Radic. Biol. Med. 2022, 182, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Julien-Schraermeyer, S.; Illing, B.; Tschulakow, A.; Taubitz, T.; Guezguez, J.; Burnet, M.; Schraermeyer, U. Penetration, distribution, and elimination of remofuscin/soraprazan in Stargardt mouse eyes following a single intravitreal injection using pharmacokinetics and transmission electron microscopic autoradiography: Implication for the local treatment of Stargardt’s disease and dry age-related macular degeneration. Pharmacol. Res. Perspect. 2020, 8, e00683. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghenciu, L.A.; Hațegan, O.A.; Bolintineanu, S.L.; Dănilă, A.-I.; Faur, A.C.; Prodan-Bărbulescu, C.; Stoicescu, E.R.; Iacob, R.; Șișu, A.M. Immune-Mediated Ocular Surface Disease in Diabetes Mellitus—Clinical Perspectives and Treatment: A Narrative Review. Biomedicines 2024, 12, 1303. [Google Scholar] [CrossRef]
- Ghenciu, L.A.; Hațegan, O.A.; Bolintineanu, S.L.; Dănilă, A.-I.; Iacob, R.; Stoicescu, E.R.; Lupu, M.A.; Olariu, T.R. Human Ocular Toxoplasmosis in Romania: History, Epidemiology, and Public Health: A Narrative Review. Microorganisms 2024, 12, 1541. [Google Scholar] [CrossRef]
- Pinilla, I.; Maneu, V.; Campello, L.; Fernández-Sánchez, L.; Martínez-Gil, N.; Kutsyr, O.; Sánchez-Sáez, X.; Sánchez-Castillo, C.; Lax, P.; Cuenca, N. Inherited Retinal Dystrophies: Role of Oxidative Stress and Inflammation in Their Physiopathology and Therapeutic Implications. Antioxidants 2022, 11, 1086. [Google Scholar] [CrossRef]
- Drolet, D.W.; Green, L.S.; Gold, L.; Janjic, N. Reviews Fit for the Eye: Aptamers in Ocular Disorders. Nucleic Acid Ther. 2016, 26, 127–146. [Google Scholar] [CrossRef]
- Park, Y.G.; Park, Y.S.; Kim, I.B. Complement System and Potential Therapeutics in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 6851. [Google Scholar] [CrossRef] [PubMed]
- Csaky, K.G.; Bok, D.; Radu, R.A.; Sadda, S.R. Complement C5 Inhibition as a Potential Treatment for Autosomal Recessive Stargardt Disease (STGD1): Design of a Clinical Trial Assessing a Novel Treatment and Primary Outcome Measure. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1569. [Google Scholar]
- Bavik, C.; Henry, S.H.; Zhang, Y.; Mitts, K.; McGinn, T.; Budzynski, E.; Pashko, A.; Lieu, K.L.; Zhong, S.; Blumberg, B.; et al. Visual Cycle Modulation as an Approach toward Preservation of Retinal Integrity. PLoS ONE 2015, 10, e0124940. [Google Scholar] [CrossRef]
- Kubota, R.; Birch, D.G.; Gregory, J.K.; Koester, J.M. Randomised study evaluating the pharmacodynamics of emixustat hydrochloride in subjects with macular atrophy secondary to Stargardt disease. Br. J. Ophthalmol. 2022, 106, 403–408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mata, N.L.; Lichter, J.B.; Vogel, R.; Han, Y.; Bui, T.V.; Singerman, L.J. Investigation of oral fenretinide for treatment of geographic atrophy in age-related macular degeneration. Retina 2013, 33, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Farnoodian, M.; Barone, F.; Boyle, M.; Gupta, R.; Nelson, L.M.; Bose, D.A.; Jun, B.; Gordon, W.C.; Do, K.V.; Kautzmann Guerin, M.-A.; et al. Metformin Attenuates the Hallmarks of Stargardt Disease. Investig. Ophthalmol. Vis. Sci. 2023, 64, 476. [Google Scholar]
- Radu, R.A.; Mata, N.L.; Nusinowitz, S.; Liu, X.; Travis, G.H. Treatment with isotretinoin inhibits lipofuscin accumulation in a mouse model of recessive Stargardt’s macular degeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 4742–4747. [Google Scholar] [CrossRef]
- Jurgensmeier, C.; Bhosale, P.; Bernstein, P.S. Evaluation of 4-methylpyrazole as a potential therapeutic dark adaptation inhibitor. Curr. Eye Res. 2007, 32, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.M.; Ciulla, T.A.; Berrocal, A.M.; Gregori, N.Z.; Flynn, H.W., Jr.; Lam, B.L. Stargardt macular dystrophy and evolving therapies. Expert Opin. Biol. Ther. 2018, 18, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Piccardi, M.; Fadda, A.; Martelli, F.; Marangoni, D.; Magli, A.; Minnella, A.M.; Bertelli, M.; Di Marco, S.; Bisti, S.; Falsini, B. Antioxidant Saffron and Central Retinal Function in ABCA4-Related Stargardt Macular Dystrophy. Nutrients 2019, 11, 2461. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.; Mitrofanis, J.; Johnstone, D.M.; Falsini, B.; Bisti, S.; Adam, P.; Nuevo, A.B.; George-Weinstein, M.; Mason, R.; Eells, J. Acquired Resilience: An Evolved System of Tissue Protection in Mammals. Dose-Response 2018, 16, 1559325818803428. [Google Scholar] [CrossRef] [PubMed]
- Hodge, W.; Barnes, D.; Schachter, H.M.; Pan, Y.; Lowcock, E.C.; Zhang, L.; Sampson, M.; Morrison, A.; Tran, K.; Miguelez, M.; et al. Effects of Omega-3 Fatty Acids on Eye Health: Summary. 2005 Jul. In AHRQ Evidence Report Summaries; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2005; p. 117. [Google Scholar]
- Prokopiou, K.; Kolovos, P.; Tsangari, H.; Bandello, F.; Rossetti, L.M.; Mastropasqua, L.; Mohand-Said, S.; Georgiou, T. A prospective, multicentre, randomised, double-blind study designed to assess the potential effects of omega-3 fatty acids supplementation in dry age-related macular degeneration or Stargardt disease. Investig. Ophthalmol. Vis. Sci. 2022, 63, 377. [Google Scholar]
- Liu, B.; Deng, X.; Jiang, Q.; Jin, H.; Wang, H.; Wang, X. Metformin Targets Abca4 to Reduce Lipofuscin Accumulation in Stargardt Disease Models. Proc. Natl. Acad. Sci. USA 2020, 117, 29089–29098. [Google Scholar]
- Prokopiou, E.; Kolovos, P.; Georgiou, C.; Kalogerou, M.; Potamiti, L.; Sokratous, K.; Kyriacou, K.; Georgiou, T. Omega-3 fatty acids supplementation protects the retina from age-associated degeneration in aged C57BL/6J mice. BMJ Open Ophthalmol. 2019, 4, e000326. [Google Scholar] [CrossRef]
- Siles, L.; Ruiz-Nogales, S.; Navinés-Ferrer, A.; Méndez-Vendrell, P.; Pomares, E. Efficient correction of Abca4 variants by CRISPR-Cas9 in hiPSCs derived from stargardt disease patients. Mol. Ther. Nucleic Acids 2023, 32, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, M.L.; Papathanasiou, S.; Doerfler, P.A.; Blaine, L.J.; Sun, L.; Yao, Y.; Zhang, C.-Z.; Weiss, M.J.; Pellman, D. Chromothripsis as an on-target consequence of CRISPR-Cas9 genome editing. Nat. Genet. 2021, 53, 895–905. [Google Scholar] [CrossRef]
- Kuntam, S.; Cingaram, P.R. CRISPR-Cas9 in hiPSCs: A new era in personalized treatment for Stargardt disease. Mol. Ther. Nucleic Acids 2023, 32, 896–897. [Google Scholar] [CrossRef] [PubMed]

| Strategy/ Intervention | Sponsor/Name of Study | Therapy | Clinical Trial/Website | Timeline | Phase |
|---|---|---|---|---|---|
| Stem cell therapy | Federal University of São Paulo Astellas | hESC-RPE | NCT02903576 NCT02941991 | 2015/2019 2013/2019 | Phase I/II - |
| MD Stem Cells/ SCOTS | BMSC | NCT01920867 | 2012/2020 | - | |
| CHABiotech Astellas | MA09-hRPE | NCT01625559 NCT01345006 NCT01469832 NCT02445612 | 2012/2015 2011/2015 2011/2015 2012/2019 | Phase I Phase I/II Phase I/II N/A | |
| BlueRock Therapeutics/FUJIFILM Cellular Dynamics/Opsis Therapeutics | iPSC (OpCT-001) | - | N/A | N/A |
| Strategy/ Intervention | Sponsor/Name of Study | Therapy | Clinical Trial/Website | Timeline | Phase |
|---|---|---|---|---|---|
| Drug therapy | |||||
| Deuterated vitamin A | Alkeus Pharmaceuticals TEASE | ALK-001 | NCT02402660 NCT02230228 NCT04239625 | 2015/2025 2014/2015 2019/2026 | Phase 2 Phase 1 Phase 2 |
| RBP4 inhibition | Belitebio/ DRAGON DRAGON II PHOENIX | Tinlarebant | NCT05244304 NCT06388083 NCT05949593 | 2022/2025 2024/2027 2023/2027 | Phase 3 Phase 2/3 Phase 3 |
| Stargazer Pharmaceuticals | STG-001 | NCT04489511 | 2020/2021 | Phase 2 | |
| Sirion Therapeutics | Fenretinide | NCT00429936 | 2006/2010 | Phase 2 | |
| Stargazer Pharmaceuticals | A1120 | - | N/A | N/A | |
| Removal of lipofuscin | Katairo/ STARTT | Soraprazan (Remofuscin) | EudraCT No. 2018-001496-20 | 2019/2023 | Phase 2 |
| C5 inhibition | Astellas Pharma | Avacincaptad pegol (Zimura) | NCT03364153 | 2018/2025 | Phase 2 |
| Visual cycle modulator | Kubota Vision/seaSTAR | Emixustat | NCT03033108 NCT03772665 | 2017/2017 2019/2022 | Phase 2 Phase 3 |
| Increase macroautophagy | National Eye Institute | Metformin | NCT04545736 | 2020/2027 | Phase 1/2 |
| Strategy/ Intervention | Sponsor/Name of Study | Therapy | Clinical Trial/Website | Timeline | Phase |
|---|---|---|---|---|---|
| Diet | |||||
| Ophthalmos Research and Education Institute/ MADEOS | Omega-3 fatty acids | NCT03297515 | 2019/2020 | Not applicable | |
| Catholic University of the Sacred Heart/ STARSAF02 | Saffron | NCT01278277 | 2011/2017 | Phase 1/2 | |
| National Eye Institute | DHA | NCT00060749 | 2003/2007 | Phase I | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghenciu, L.A.; Hațegan, O.A.; Stoicescu, E.R.; Iacob, R.; Șișu, A.M. Emerging Therapeutic Approaches and Genetic Insights in Stargardt Disease: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 8859. https://doi.org/10.3390/ijms25168859
Ghenciu LA, Hațegan OA, Stoicescu ER, Iacob R, Șișu AM. Emerging Therapeutic Approaches and Genetic Insights in Stargardt Disease: A Comprehensive Review. International Journal of Molecular Sciences. 2024; 25(16):8859. https://doi.org/10.3390/ijms25168859
Chicago/Turabian StyleGhenciu, Laura Andreea, Ovidiu Alin Hațegan, Emil Robert Stoicescu, Roxana Iacob, and Alina Maria Șișu. 2024. "Emerging Therapeutic Approaches and Genetic Insights in Stargardt Disease: A Comprehensive Review" International Journal of Molecular Sciences 25, no. 16: 8859. https://doi.org/10.3390/ijms25168859






