Mechanobiology and Primary Cilium in the Pathophysiology of Bone Marrow Myeloproliferative Diseases
Abstract
1. Introduction
2. Methods
Search Strategy and Selection
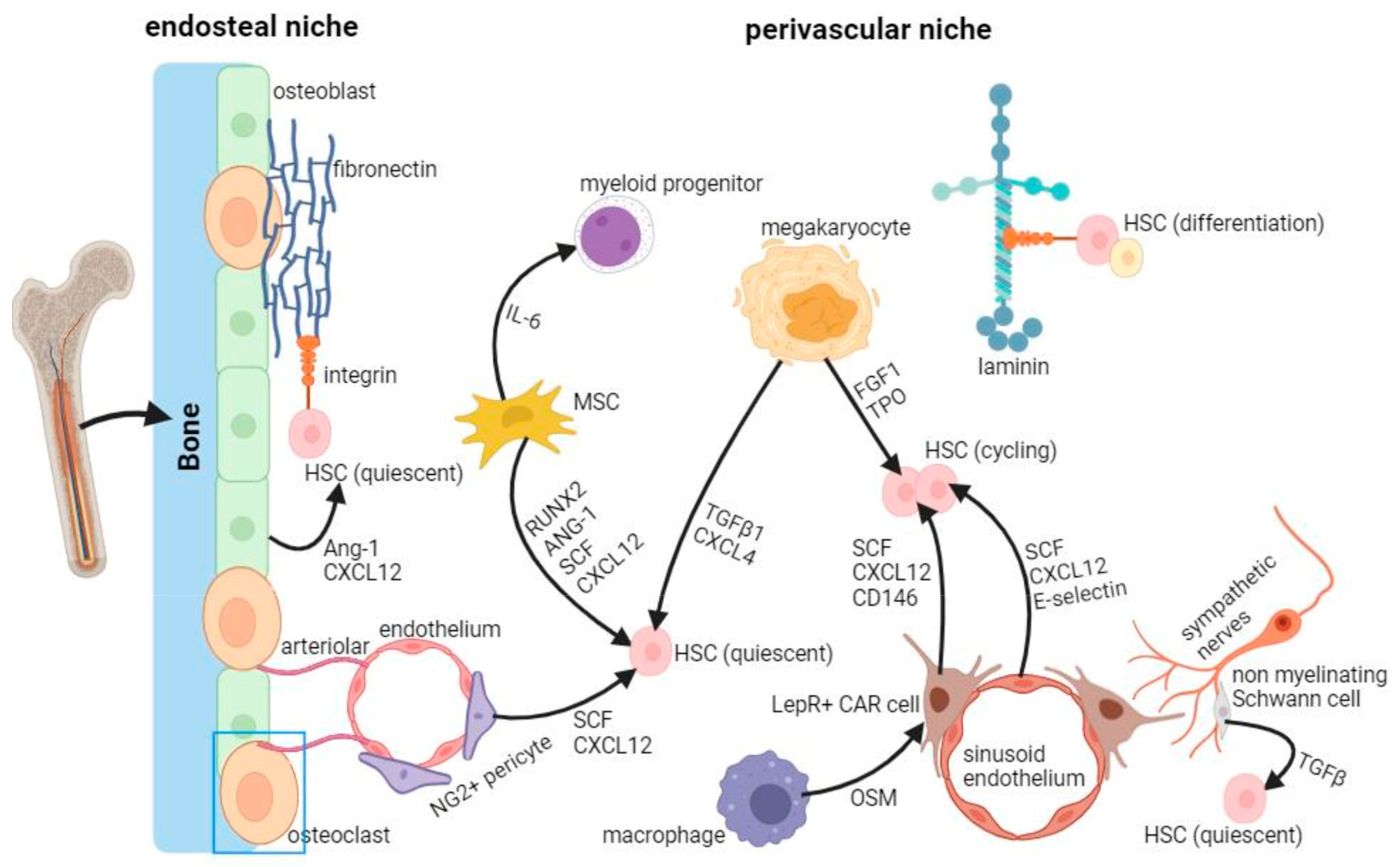
3. Mechanobiology of HSCs in the Bone Marrow Niche
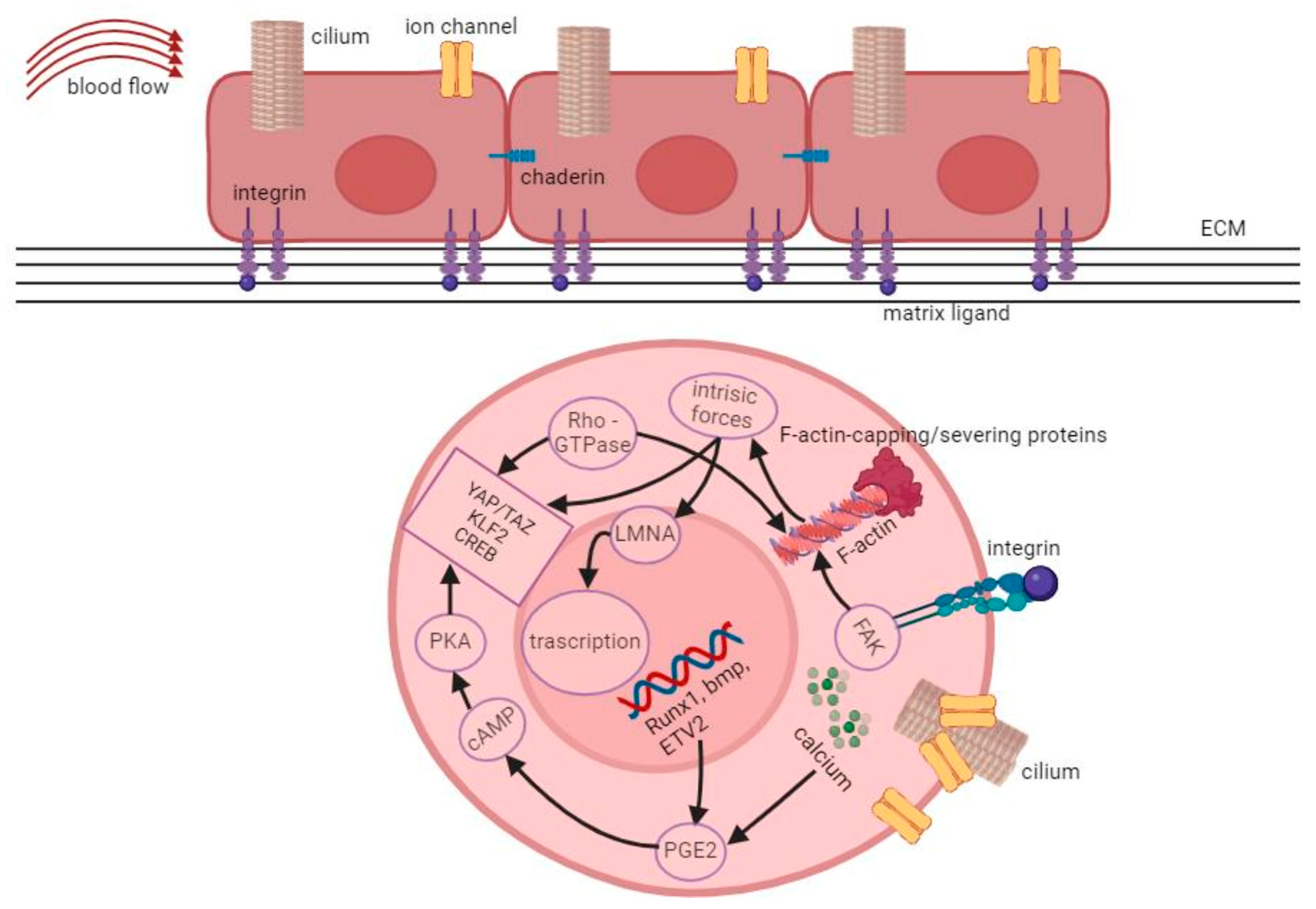
3.1. Intracellular Forces and the Role of the Cytoskeleton
3.2. Cell-Cell and Cell-Substrate Interactions
3.3. Vascular Cells as Transducers of Mechanical Signals to HSC in the Niche
4. The Role of Primary Cilium
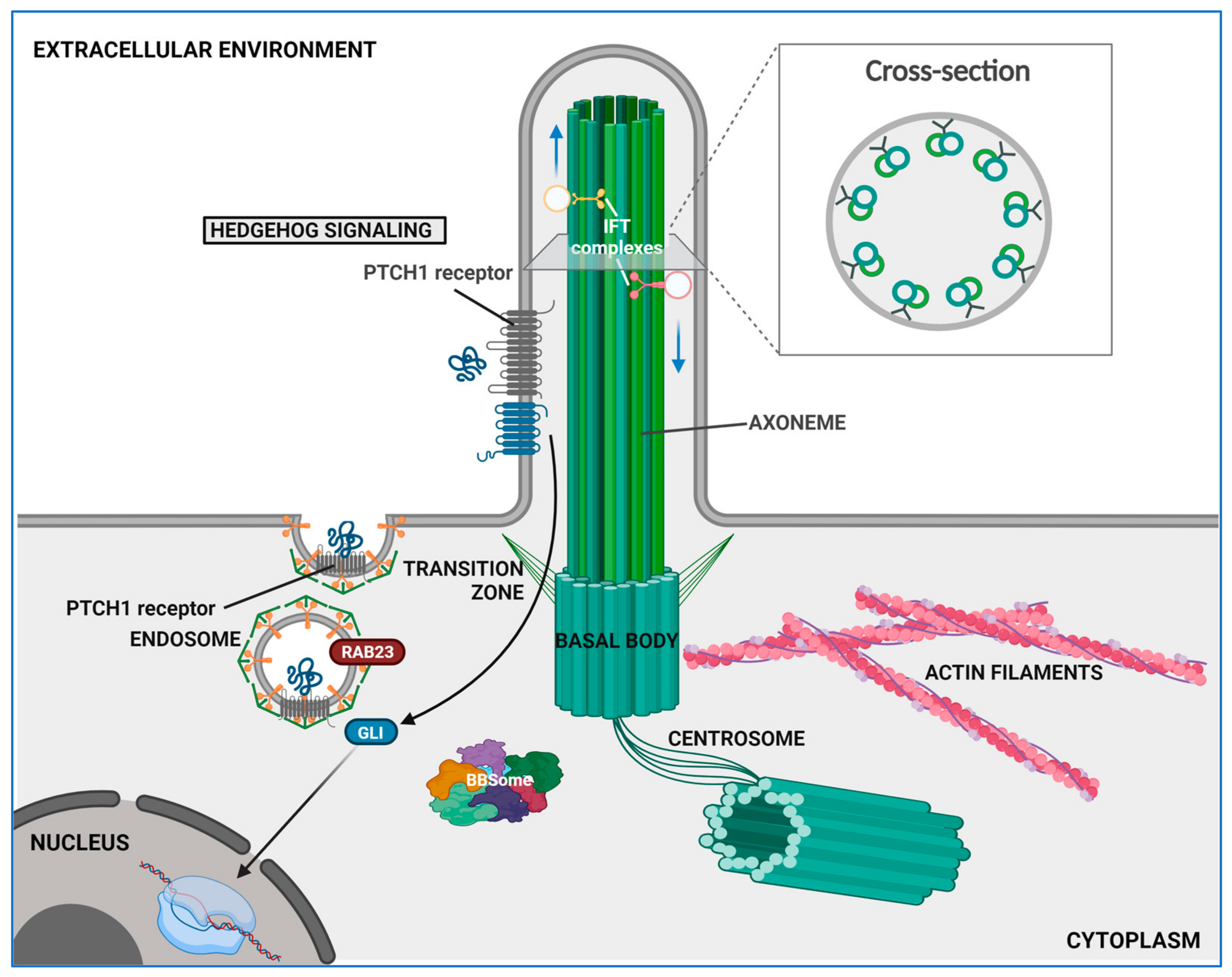
4.1. Structural and Functional Overview
4.2. Ciliary Signaling and Their Relevance in HSC Biology
4.3. The Functional Significance of the Primary Cilium in the Bone Marrow Niche
5. Altered Mechanobiology in MPN
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ng, Z.Y.; Fuller, K.A.; Mazza-Parton, A.; Erber, W.N. Morphology of Myeloproliferative Neoplasms. Int. J. Lab. Hematol. 2023, 45 (Suppl. S2), 59–70. [Google Scholar] [CrossRef] [PubMed]
- Cree, I.A. The WHO Classification of Haematolymphoid Tumours. Leukemia 2022, 36, 1701–1702. [Google Scholar] [CrossRef] [PubMed]
- Dameshek, W. Some Speculations on the Myeloproliferative Syndromes [Editorial]. Blood 1951, 6, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Loscocco, G.G.; Guglielmelli, P.; Vannucchi, A.M. Impact of Mutational Profile on the Management of Myeloproliferative Neoplasms: A Short Review of the Emerging Data. Onco Targets Ther. 2020, 13, 12367–12382. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Kralovics, R. Progress in Elucidation of Molecular Pathophysiology of Myeloproliferative Neoplasms and Its Application to Therapeutic Decisions. Int. J. Hematol. 2020, 111, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Gianelli, U.; Thiele, J.; Orazi, A.; Gangat, N.; Vannucchi, A.M.; Tefferi, A.; Kvasnicka, H.M. International Consensus Classification of Myeloid and Lymphoid Neoplasms: Myeloproliferative Neoplasms. Virchows Arch. 2023, 482, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Loghavi, S.; Kanagal-Shamanna, R.; Khoury, J.D.; Medeiros, L.J.; Naresh, K.N.; Nejati, R.; Patnaik, M.M. Fifth Edition of the World Health Classification of Tumors of the Hematopoietic and Lymphoid Tissue: Myeloid Neoplasms. Mod. Pathol. 2024, 37, 100397. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.K.; Piontek, K.; Boletta, A.; Liu, L.; Qian, F.; Xu, P.-N.; Germino, F.J.; Germino, G.G. PKD1 Induces P21waf1 and Regulation of the Cell Cycle via Direct Activation of the JAK-STAT Signaling Pathway in a Process Requiring PKD2. Cell 2002, 109, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Low, S.H.; Vasanth, S.; Larson, C.H.; Mukherjee, S.; Sharma, N.; Kinter, M.T.; Kane, M.E.; Obara, T.; Weimbs, T. Polycystin-1, STAT6, and P100 Function in a Pathway That Transduces Ciliary Mechanosensation and Is Activated in Polycystic Kidney Disease. Dev. Cell 2006, 10, 57–69. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT Signaling Pathway: From Bench to Clinic. Sig Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Mekahli, D.; Parys, J.B.; Bultynck, G.; Missiaen, L.; De Smedt, H. Polycystins and Cellular Ca2+ Signaling. Cell. Mol. Life Sci. 2013, 70, 2697–2712. [Google Scholar] [CrossRef] [PubMed]
- Rolles, B.; Mullally, A. Molecular Pathogenesis of Myeloproliferative Neoplasms. Curr. Hematol. Malig. Rep. 2022, 17, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Curto-Garcia, N.; Harrison, C.; McLornan, D.P. Bone Marrow Niche Dysregulation in Myeloproliferative Neoplasms. Haematologica 2020, 105, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Bernardo, M.E. Mesenchymal Stromal Cells: Role in the BM Niche and in the Support of Hematopoietic Stem Cell Transplantation. Hemasphere 2018, 2, e151. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.D.; Dumbali, S.; Wenzel, P.L. Mechanoregulation in Hematopoiesis and Hematologic Disorders. Curr. Stem Cell Rep. 2020, 6, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Chaudhry, P.; Merchant, A.A. Primary Cilia Are Present on Human Blood and Bone Marrow Cells and Mediate Hedgehog Signaling. Exp. Hematol. 2016, 44, 1181–1187.e2. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Vining, K.H.; Mooney, D.J. Mechanical Forces Direct Stem Cell Behaviour in Development and Regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef]
- García-Cardeña, G.; Slegtenhorst, B.R. Hemodynamic Control of Endothelial Cell Fates in Development. Annu. Rev. Cell Dev. Biol. 2016, 32, 633–648. [Google Scholar] [CrossRef]
- Shi, G.; Zhang, P.; Zhang, X.; Li, J.; Zheng, X.; Yan, J.; Zhang, N.; Yang, H. The Spatiotemporal Heterogeneity of the Biophysical Microenvironment during Hematopoietic Stem Cell Development: From Embryo to Adult. Stem Cell Res. Ther. 2023, 14, 251. [Google Scholar] [CrossRef]
- Shin, J.-W.; Spinler, K.R.; Swift, J.; Chasis, J.A.; Mohandas, N.; Discher, D.E. Lamins Regulate Cell Trafficking and Lineage Maturation of Adult Human Hematopoietic Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 18892–18897. [Google Scholar] [CrossRef] [PubMed]
- Röselová, P.; Obr, A.; Holoubek, A.; Grebeňová, D.; Kuželová, K. Adhesion Structures in Leukemia Cells and Their Regulation by Src Family Kinases. Cell Adhes. Migr. 2018, 12, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-Dependent Matrix Remodelling Is Required for the Generation and Maintenance of Cancer-Associated Fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Lancino, M.; Majello, S.; Herbert, S.; De Chaumont, F.; Tinevez, J.-Y.; Olivo-Marin, J.-C.; Herbomel, P.; Schmidt, A. Anisotropic Organization of Circumferential Actomyosin Characterizes Hematopoietic Stem Cells Emergence in the Zebrafish. eLife 2018, 7, e37355. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef]
- Shin, J.-W.; Swift, J.; Ivanovska, I.; Spinler, K.R.; Buxboim, A.; Discher, D.E. Mechanobiology of Bone Marrow Stem Cells: From Myosin-II Forces to Compliance of Matrix and Nucleus in Cell Forms and Fates. Differentiation 2013, 86, 77–86. [Google Scholar] [CrossRef]
- Lee-Thedieck, C.; Schertl, P.; Klein, G. The Extracellular Matrix of Hematopoietic Stem Cell Niches. Adv. Drug Deliv. Rev. 2022, 181, 114069. [Google Scholar] [CrossRef]
- Sugiyama, D.; Kulkeaw, K.; Mizuochi, C. TGF-Beta-1 up-Regulates Extra-Cellular Matrix Production in Mouse Hepatoblasts. Mech. Dev. 2013, 130, 195–206. [Google Scholar] [CrossRef]
- Hoggatt, J.; Singh, P.; Tate, T.A.; Chou, B.-K.; Datari, S.R.; Fukuda, S.; Liu, L.; Kharchenko, P.V.; Schajnovitz, A.; Baryawno, N.; et al. Rapid Mobilization Reveals a Highly Engraftable Hematopoietic Stem Cell. Cell 2018, 172, 191–204.e10. [Google Scholar] [CrossRef]
- Privratsky, J.R.; Newman, D.K.; Newman, P.J. PECAM-1: Conflicts of Interest in Inflammation. Life Sci. 2010, 87, 69–82. [Google Scholar] [CrossRef]
- Priest, A.V.; Shafraz, O.; Sivasankar, S. Biophysical Basis of Cadherin Mediated Cell-Cell Adhesion. Exp. Cell Res. 2017, 358, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.; Watson, S.; Lord, M.S.; Eamegdool, S.S.; Bax, D.V.; Nivison-Smith, L.B.; Kondyurin, A.; Ma, L.; Oberhauser, A.F.; Weiss, A.S.; et al. Substrate Elasticity Provides Mechanical Signals for the Expansion of Hemopoietic Stem and Progenitor Cells. Nat. Biotechnol. 2010, 28, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.Y.; Giroux, S.; Golub, R.; Klaine, M.; Jalil, A.; Boucontet, L.; Godin, I.; Cumano, A. Characterization of Purified Intraembryonic Hematopoietic Stem Cells as a Tool to Define Their Site of Origin. Proc. Natl. Acad. Sci. USA 2005, 102, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Lachowski, D.; Cortes, E.; Robinson, B.; Rice, A.; Rombouts, K.; Del Río Hernández, A.E. FAK Controls the Mechanical Activation of YAP, a Transcriptional Regulator Required for Durotaxis. FASEB J. 2018, 32, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Lundin, V.; Sugden, W.W.; Theodore, L.N.; Sousa, P.M.; Han, A.; Chou, S.; Wrighton, P.J.; Cox, A.G.; Ingber, D.E.; Goessling, W.; et al. YAP Regulates Hematopoietic Stem Cell Formation in Response to the Biomechanical Forces of Blood Flow. Dev. Cell 2020, 52, 446–460.e5. [Google Scholar] [CrossRef] [PubMed]
- Hines, M.; Nielsen, L.; Cooper-White, J. The Hematopoietic Stem Cell Niche: What Are We Trying to Replicate? J. Chem. Tech. Amp. Biotech. 2008, 83, 421–443. [Google Scholar] [CrossRef]
- Kopp, H.-G.; Avecilla, S.T.; Hooper, A.T.; Rafii, S. The Bone Marrow Vascular Niche: Home of HSC Differentiation and Mobilization. Physiology 2005, 20, 349–356. [Google Scholar] [CrossRef]
- Choi, J.S.; Harley, B.A.C. Marrow-Inspired Matrix Cues Rapidly Affect Early Fate Decisions of Hematopoietic Stem and Progenitor Cells. Sci. Adv. 2017, 3, e1600455. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Li, N.; Evans, S.M.; Diaz, M.F.; Wenzel, P.L. Biomechanical Force in Blood Development: Extrinsic Physical Cues Drive pro-Hematopoietic Signaling. Differentiation 2013, 86, 92–103. [Google Scholar] [CrossRef]
- Ji, R.P.; Phoon, C.K.L.; Aristizábal, O.; McGrath, K.E.; Palis, J.; Turnbull, D.H. Onset of Cardiac Function During Early Mouse Embryogenesis Coincides with Entry of Primitive Erythroblasts into the Embryo Proper. Circ. Res. 2003, 92, 133–135. [Google Scholar] [CrossRef]
- Suo, J.; Ferrara, D.E.; Sorescu, D.; Guldberg, R.E.; Taylor, W.R.; Giddens, D.P. Hemodynamic Shear Stresses in Mouse Aortas: Implications for Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, Y.; Bruns, I.; Scheiermann, C.; Ahmed, J.; Pinho, S.; Zhang, D.; Mizoguchi, T.; Wei, Q.; Lucas, D.; Ito, K.; et al. Arteriolar Niches Maintain Haematopoietic Stem Cell Quiescence. Nature 2013, 502, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.P.C.; Li, Y.-S.; Zhao, Y.; Chen, K.-D.; Li, S.; Lao, J.; Yuan, S.; Shyy, J.Y.-J.; Chien, S. DNA Microarray Analysis of Gene Expression in Endothelial Cells in Response to 24-h Shear Stress. Physiol. Genom. 2001, 7, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Shay-Salit, A.; Shushy, M.; Wolfovitz, E.; Yahav, H.; Breviario, F.; Dejana, E.; Resnick, N. VEGF Receptor 2 and the Adherens Junction as a Mechanical Transducer in Vascular Endothelial Cells. Proc. Natl. Acad. Sci. USA 2002, 99, 9462–9467. [Google Scholar] [CrossRef] [PubMed]
- Nauli, S.M.; Kawanabe, Y.; Kaminski, J.J.; Pearce, W.J.; Ingber, D.E.; Zhou, J. Endothelial Cilia Are Fluid Shear Sensors That Regulate Calcium Signaling and Nitric Oxide Production Through Polycystin-1. Circulation 2008, 117, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yang, M.; Capitano, M.; Guo, B.; Liu, S.; Wan, J.; Broxmeyer, H.E.; Huang, X. Pharmacological Activation of Nitric Oxide Signaling Promotes Human Hematopoietic Stem Cell Homing and Engraftment. Leukemia 2021, 35, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, K.W. Beiträge Zur Kenntniss Einiger Drüsen Und Epithelien. Arch. Für Mikrosk. Anat. 1898, 52, 552–706. [Google Scholar] [CrossRef]
- Singla, V.; Reiter, J.F. The Primary Cilium as the Cell’s Antenna: Signaling at a Sensory Organelle. Science 2006, 313, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Tiberio, F.; Parolini, O.; Lattanzi, W. Ciliary Signalling and Mechanotransduction in the Pathophysiology of Craniosynostosis. Genes 2021, 12, 1073. [Google Scholar] [CrossRef]
- Mill, P.; Christensen, S.T.; Pedersen, L.B. Primary Cilia as Dynamic and Diverse Signalling Hubs in Development and Disease. Nat. Rev. Genet. 2023, 24, 421–441. [Google Scholar] [CrossRef]
- Kee, H.L.; Dishinger, J.F.; Lynne Blasius, T.; Liu, C.-J.; Margolis, B.; Verhey, K.J. A Size-Exclusion Permeability Barrier and Nucleoporins Characterize a Ciliary Pore Complex That Regulates Transport into Cilia. Nat. Cell Biol. 2012, 14, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalo, F.R.; Reiter, J.F. Open Sesame: How Transition Fibers and the Transition Zone Control Ciliary Composition. Cold Spring Harb. Perspect. Biol. 2017, 9, a028134. [Google Scholar] [CrossRef] [PubMed]
- Nechipurenko, I.V. The Enigmatic Role of Lipids in Cilia Signaling. Front. Cell Dev. Biol. 2020, 8, 777. [Google Scholar] [CrossRef] [PubMed]
- Hoey, D.A.; Downs, M.E.; Jacobs, C.R. The Mechanics of the Primary Cilium: An Intricate Structure with Complex Function. J. Biomech. 2012, 45, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Insinna, C.; Ott, C.; Stauffer, J.; Pintado, P.A.; Rahajeng, J.; Baxa, U.; Walia, V.; Cuenca, A.; Hwang, Y.-S.; et al. Early Steps in Primary Cilium Assembly Require EHD1/EHD3-Dependent Ciliary Vesicle Formation. Nat. Cell Biol. 2015, 17, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Molla-Herman, A.; Ghossoub, R.; Blisnick, T.; Meunier, A.; Serres, C.; Silbermann, F.; Emmerson, C.; Romeo, K.; Bourdoncle, P.; Schmitt, A.; et al. The Ciliary Pocket: An Endocytic Membrane Domain at the Base of Primary and Motile Cilia. J. Cell Sci. 2010, 123, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Spasic, M.; Jacobs, C.R. Primary Cilia: Cell and Molecular Mechanosensors Directing Whole Tissue Function. Semin. Cell Dev. Biol. 2017, 71, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ju, L.; Rushdi, M.; Ge, C.; Zhu, C. Receptor-Mediated Cell Mechanosensing. MBoC 2017, 28, 3134–3155. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.L.; Guevarra, M.D.; Nguyen, A.M.; Chua, M.C.; Wang, Y.; Jacobs, C.R. The Primary Cilium Functions as a Mechanical and Calcium Signaling Nexus. Cilia 2015, 4, 7. [Google Scholar] [CrossRef]
- Praetorius, H.A.; Spring, K.R. Bending the MDCK Cell Primary Cilium Increases Intracellular Calcium. J. Membr. Biol. 2001, 184, 71–79. [Google Scholar] [CrossRef]
- Yoder, B.K.; Hou, X.; Guay-Woodford, L.M. The Polycystic Kidney Disease Proteins, Polycystin-1, Polycystin-2, Polaris, and Cystin, Are Co-Localized in Renal Cilia. J. Am. Soc. Nephrol. 2002, 13, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Saternos, H.; Ley, S.; AbouAlaiwi, W. Primary Cilia and Calcium Signaling Interactions. Int. J. Mol. Sci. 2020, 21, 7109. [Google Scholar] [CrossRef] [PubMed]
- Sachs, F. Stretch-Activated Ion Channels: What Are They? Physiology 2010, 25, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Espinha, L.C.; Hoey, D.A.; Fernandes, P.R.; Rodrigues, H.C.; Jacobs, C.R. Oscillatory Fluid Flow Influences Primary Cilia and Microtubule Mechanics. Cytoskeleton 2014, 71, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Price, C.; Zhou, X.; Li, W.; Wang, L. Real-Time Measurement of Solute Transport within the Lacunar-Canalicular System of Mechanically Loaded Bone: Direct Evidence for Load-Induced Fluid Flow. J. Bone Miner. Res. 2011, 26, 277–285. [Google Scholar] [CrossRef]
- Hung, C.T.; Allen, F.D.; Pollack, S.R.; Brighton, C.T. Intracellular Ca2+ Stores and Extracellular Ca2+ Are Required in the Real-Time Ca2+ Response of Bone Cells Experiencing Fluid Flow. J. Biomech. 1996, 29, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.J.; Frikha-Benayed, D.; Louie, J.; Stephen, S.; Spray, D.C.; Thi, M.M.; Seref-Ferlengez, Z.; Majeska, R.J.; Weinbaum, S.; Schaffler, M.B. Osteocyte Calcium Signals Encode Strain Magnitude and Loading Frequency in Vivo. Proc. Natl. Acad. Sci. USA 2017, 114, 11775–11780. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.S.; Joca, H.C.; Law, R.A.; Williams, K.M.; Kerr, J.P.; Shi, G.; Khairallah, R.J.; Martin, S.S.; Konstantopoulos, K.; Ward, C.W.; et al. Microtubules Tune Mechanotransduction through NOX2 and TRPV4 to Decrease Sclerostin Abundance in Osteocytes. Sci. Signal. 2017, 10, eaan5748. [Google Scholar] [CrossRef]
- Johnson, G.P.; Fair, S.; Hoey, D.A. Primary Cilium-Mediated MSC Mechanotransduction Is Dependent on Gpr161 Regulation of Hedgehog Signalling. Bone 2021, 145, 115846. [Google Scholar] [CrossRef]
- Riffault, M.; Johnson, G.P.; Owen, M.M.; Javaheri, B.; Pitsillides, A.A.; Hoey, D.A. Loss of Adenylyl Cyclase 6 in Leptin Receptor-Expressing Stromal Cells Attenuates Loading-Induced Endosteal Bone Formation. JBMR Plus 2020, 4, e10408. [Google Scholar] [CrossRef]
- Wheway, G.; Nazlamova, L.; Hancock, J.T. Signaling through the Primary Cilium. Front. Cell Dev. Biol. 2018, 6, 8. [Google Scholar] [CrossRef]
- Haycraft, C.J.; Banizs, B.; Aydin-Son, Y.; Zhang, Q.; Michaud, E.J.; Yoder, B.K. Gli2 and Gli3 Localize to Cilia and Require the Intraflagellar Transport Protein Polaris for Processing and Function. PLoS Genet. 2005, 1, e53. [Google Scholar] [CrossRef] [PubMed]
- Caron, A.; Xu, X.; Lin, X. Wnt/β-Catenin Signaling Directly Regulates Foxj1 Expression and Ciliogenesis in Zebrafish Kupffer’s Vesicle. Development 2012, 139, 514–524. [Google Scholar] [CrossRef]
- Cano, D.A.; Murcia, N.S.; Pazour, G.J.; Hebrok, M. Orpk Mouse Model of Polycystic Kidney Disease Reveals Essential Role of Primary Cilia in Pancreatic Tissue Organization. Development 2004, 131, 3457–3467. [Google Scholar] [CrossRef] [PubMed]
- Lienkamp, S.; Ganner, A.; Walz, G. Inversin, Wnt Signaling and Primary Cilia. Differentiation 2012, 83, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H. Involvement of Wnt Signaling in Primary Cilia Assembly and Disassembly. FEBS J. 2020, 287, 5027–5038. [Google Scholar] [CrossRef]
- Gerdes, J.M.; Liu, Y.; Zaghloul, N.A.; Leitch, C.C.; Lawson, S.S.; Kato, M.; Beachy, P.A.; Beales, P.L.; DeMartino, G.N.; Fisher, S.; et al. Disruption of the Basal Body Compromises Proteasomal Function and Perturbs Intracellular Wnt Response. Nat. Genet. 2007, 39, 1350–1360. [Google Scholar] [CrossRef]
- Corbit, K.C.; Shyer, A.E.; Dowdle, W.E.; Gaulden, J.; Singla, V.; Reiter, J.F. Kif3a Constrains β-Catenin-Dependent Wnt Signalling through Dual Ciliary and Non-Ciliary Mechanisms. Nat. Cell Biol. 2008, 10, 70–76. [Google Scholar] [CrossRef]
- Christian Wigley, W.; Fabunmi, R.P.; Lee, M.G.; Marino, C.R.; Muallem, S.; DeMartino, G.N.; Thomas, P.J. Dynamic Association of Proteasomal Machinery with the Centrosome. J. Cell Biol. 1999, 145, 481–490. [Google Scholar] [CrossRef]
- Porazinski, S.; Wang, H.; Asaoka, Y.; Behrndt, M.; Miyamoto, T.; Morita, H.; Hata, S.; Sasaki, T.; Krens, S.F.G.; Osada, Y.; et al. YAP Is Essential for Tissue Tension to Ensure Vertebrate 3D Body Shape. Nature 2015, 521, 217–221. [Google Scholar] [CrossRef]
- Goode, D.K.; Obier, N.; Vijayabaskar, M.S.; Lie-A-Ling, M.; Lilly, A.J.; Hannah, R.; Lichtinger, M.; Batta, K.; Florkowska, M.; Patel, R.; et al. Dynamic Gene Regulatory Networks Drive Hematopoietic Specification and Differentiation. Dev. Cell 2016, 36, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Pala, R.; Alomari, N.; Nauli, S. Primary Cilium-Dependent Signaling Mechanisms. Int. J. Mol. Sci. 2017, 18, 2272. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, P.; Wei, Y.; Gao, Y.; Patient, R.; Liu, F. A Blood Flow-Dependent Klf2a-NO Signaling Cascade Is Required for Stabilization of Hematopoietic Stem Cell Programming in Zebrafish Embryos. Blood 2011, 118, 4102–4110. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, Q.; Shan, W.; Cai, S.; Tie, R.; Xu, Y.; Lin, Y.; Qian, P.; Huang, H. Biomechanical Cues as Master Regulators of Hematopoietic Stem Cell Fate. Cell. Mol. Life Sci. 2021, 78, 5881–5902. [Google Scholar] [CrossRef] [PubMed]
- AbouAlaiwi, W.A.; Takahashi, M.; Mell, B.R.; Jones, T.J.; Ratnam, S.; Kolb, R.J.; Nauli, S.M. Ciliary Polycystin-2 Is a Mechanosensitive Calcium Channel Involved in Nitric Oxide Signaling Cascades. Circ. Res. 2009, 104, 860–869. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, L.; Wang, S.; Huang, B.; Jing, Y.; Su, J. Bone Marrow Mesenchymal Stromal Cells: Identification, Classification, and Differentiation. Front. Cell Dev. Biol. 2022, 9, 787118. [Google Scholar] [CrossRef]
- Salem, H.K.; Thiemermann, C. Mesenchymal Stromal Cells: Current Understanding and Clinical Status. Stem Cells 2010, 28, 585–596. [Google Scholar] [CrossRef]
- Tummala, P.; Arnsdorf, E.J.; Jacobs, C.R. The Role of Primary Cilia in Mesenchymal Stem Cell Differentiation: A Pivotal Switch in Guiding Lineage Commitment. Cel. Mol. Bioeng. 2010, 3, 207–212. [Google Scholar] [CrossRef]
- Raman, N.; Imran, S.A.M.; Ahmad Amin Noordin, K.B.; Zaman, W.S.W.K.; Nordin, F. Mechanotransduction in Mesenchymal Stem Cells (MSCs) Differentiation: A Review. Int. J. Mol. Sci. 2022, 23, 4580. [Google Scholar] [CrossRef]
- Ma, Z.; Qin, M.; Liang, H.; Chen, R.; Cai, S.; Huang, Z.; Tai, G. Primary Cilia-Dependent Signaling Is Involved in Regulating Mesenchymal Stem Cell Proliferation and Pluripotency Maintenance. J. Mol. Hist. 2020, 51, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Hoey, D.A.; Chua, M.; Bellon, R.; Jacobs, C.R. Mechanical Signals Promote Osteogenic Fate through a Primary Cilia-mediated Mechanism. FASEB J. 2016, 30, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xu, J. Immune Modulation by Mesenchymal Stem Cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.; Drohat, P.; Crawford, B.; Hornicek, F.J.; Best, T.M.; Kouroupis, D. Mesenchymal Stem/Stromal Cells: Immunomodulatory and Bone Regeneration Potential after Tumor Excision in Osteosarcoma Patients. Bioengineering 2023, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Mbalaviele, G.; Jaiswal, N.; Meng, A.; Cheng, L.; Van Den Bos, C.; Thiede, M. Human Mesenchymal Stem Cells Promote Human Osteoclast Differentiation from CD34+ Bone Marrow Hematopoietic Progenitors. Endocrinology 1999, 140, 3736–3743. [Google Scholar] [CrossRef]
- Lattanzi, W.; Parolisi, R.; Barba, M.; Bonfanti, L. Osteogenic and Neurogenic Stem Cells in Their Own Place: Unraveling Differences and Similarities Between Niches. Front. Cell. Neurosci. 2015, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; MacArthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and Haematopoietic Stem Cells Form a Unique Bone Marrow Niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Sagaradze, G.D.; Basalova, N.A.; Efimenko, A.Y.; Tkachuk, V.A. Mesenchymal Stromal Cells as Critical Contributors to Tissue Regeneration. Front. Cell Dev. Biol. 2020, 8, 576176. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.; Frenette, P.S. Haematopoietic Stem Cell Activity and Interactions with the Niche. Nat. Rev. Mol. Cell Biol. 2019, 20, 303–320. [Google Scholar] [CrossRef]
- Basten, S.G.; Giles, R.H. Functional Aspects of Primary Cilia in Signaling, Cell Cycle and Tumorigenesis. Cilia 2013, 2, 6. [Google Scholar] [CrossRef]
- Teves, M.E.; Strauss, J.F.; Sapao, P.; Shi, B.; Varga, J. The Primary Cilium: Emerging Role as a Key Player in Fibrosis. Curr. Rheumatol. Rep. 2019, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- La Spina, E.; Giallongo, S.; Giallongo, C.; Vicario, N.; Duminuco, A.; Parenti, R.; Giuffrida, R.; Longhitano, L.; Li Volti, G.; Cambria, D.; et al. Mesenchymal Stromal Cells in Tumor Microenvironment Remodeling of BCR-ABL Negative Myeloproliferative Diseases. Front. Oncol. 2023, 13, 1141610. [Google Scholar] [CrossRef] [PubMed]
- Decker, M.; Martinez-Morentin, L.; Wang, G.; Lee, Y.; Liu, Q.; Leslie, J.; Ding, L. Leptin-Receptor-Expressing Bone Marrow Stromal Cells Are Myofibroblasts in Primary Myelofibrosis. Nat. Cell Biol. 2017, 19, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.L.; Santra, T.; Owens, P.; Morrison, A.M.; Barry, F. Primary Cilium-Associated Genes Mediate Bone Marrow Stromal Cell Response to Hypoxia. Stem Cell Res. 2014, 13, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Leng, F.; Gao, Y.; He, W.; Wang, J.; Xian, C.J.; Ma, H.; Chen, K. Protection of Primary Cilia Is an Effective Countermeasure against the Impairment of Osteoblast Function Induced by Simulated Microgravity. J. Cell. Mol. Medi 2023, 27, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.K.; Kim, J.S.; Lee, G.B.; Lim, J.H.; Park, K.M. Oxidative Stress Following Acute Kidney Injury Causes Disruption of Lung Cell Cilia and Their Release into the Bronchoaveolar Lavage Fluid and Lung Injury, Which Are Exacerbated by Idh2 Deletion. Redox Biol. 2021, 46, 102077. [Google Scholar] [CrossRef]
- Sreekumar, V.; Aspera-Werz, R.; Ehnert, S.; Strobel, J.; Tendulkar, G.; Heid, D.; Schreiner, A.; Arnscheidt, C.; Nussler, A.K. Resveratrol Protects Primary Cilia Integrity of Human Mesenchymal Stem Cells from Cigarette Smoke to Improve Osteogenic Differentiation in Vitro. Arch. Toxicol. 2018, 92, 1525–1538. [Google Scholar] [CrossRef]
- Bae, J.-E.; Kang, G.M.; Min, S.H.; Jo, D.S.; Jung, Y.-K.; Kim, K.; Kim, M.-S.; Cho, D.-H. Primary Cilia Mediate Mitochondrial Stress Responses to Promote Dopamine Neuron Survival in a Parkinson’s Disease Model. Cell Death Dis. 2019, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Liu, T.; Sun, Y.; Cai, N.; Xuan, Y.; Wei, Z.; Cui, B.; Jing, L.; Ma, H.; Xian, C.J.; et al. Simulated Microgravity-induced Oxidative Stress and Loss of Osteogenic Potential of Osteoblasts Can Be Prevented by Protection of Primary Cilia. J. Cell. Physiol. 2023, 238, 2692–2709. [Google Scholar] [CrossRef]
- Clement, C.A.; Ajbro, K.D.; Koefoed, K.; Vestergaard, M.L.; Veland, I.R.; Henriques de Jesus, M.P.R.; Pedersen, L.B.; Benmerah, A.; Andersen, C.Y.; Larsen, L.A.; et al. TGF-β Signaling Is Associated with Endocytosis at the Pocket Region of the Primary Cilium. Cell Rep. 2013, 3, 1806–1814. [Google Scholar] [CrossRef]
- Mead, A.J.; Mullally, A. Myeloproliferative Neoplasm Stem Cells. Blood 2017, 129, 1607–1616. [Google Scholar] [CrossRef]
- Lee, J.Y.; Stearns, T. FOP Is a Centriolar Satellite Protein Involved in Ciliogenesis. PLoS ONE 2013, 8, e58589. [Google Scholar] [CrossRef]
- Longhitano, L.; Tibullo, D.; Vicario, N.; Giallongo, C.; La Spina, E.; Romano, A.; Lombardo, S.; Moretti, M.; Masia, F.; Coda, A.R.D.; et al. IGFBP-6/Sonic Hedgehog/TLR4 Signalling Axis Drives Bone Marrow Fibrotic Transformation in Primary Myelofibrosis. Aging 2021, 13, 25055–25071. [Google Scholar] [CrossRef] [PubMed]
- Green, D.E.; Rubin, C.T. Consequences of Irradiation on Bone and Marrow Phenotypes, and Its Relation to Disruption of Hematopoietic Precursors. Bone 2014, 63, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Luo, Y.; Zhou, D. Hematopoietic Stem Cell Injury Induced by Ionizing Radiation. Antioxid. Redox Signal. 2014, 20, 1447–1462. [Google Scholar] [CrossRef] [PubMed]
- Gault, N.; Verbiest, T.; Badie, C.; Romeo, P.-H.; Bouffler, S. Hematopoietic Stem and Progenitor Cell Responses to Low Radiation Doses—Implications for Leukemia Risk. Int. J. Radiat. Biol. 2019, 95, 892–899. [Google Scholar] [CrossRef]
- Tang, C.; Li, M.-H.; Chen, Y.-L.; Sun, H.-Y.; Liu, S.-L.; Zheng, W.-W.; Zhang, M.-Y.; Li, H.; Fu, W.; Zhang, W.-J.; et al. Chemotherapy-Induced Niche Perturbs Hematopoietic Reconstitution in B-Cell Acute Lymphoblastic Leukemia. J. Exp. Clin. Cancer Res. 2018, 37, 204. [Google Scholar] [CrossRef]
- Tikhonova, A.N.; Dolgalev, I.; Hu, H.; Sivaraj, K.K.; Hoxha, E.; Cuesta-Domínguez, Á.; Pinho, S.; Akhmetzyanova, I.; Gao, J.; Witkowski, M.; et al. The Bone Marrow Microenvironment at Single-Cell Resolution. Nature 2019, 569, 222–228. [Google Scholar] [CrossRef]
- Chen, L.; Hu, H.; Qiu, W.; Shi, K.; Kassem, M. Actin Depolymerization Enhances Adipogenic Differentiation in Human Stromal Stem Cells. Stem Cell Res. 2018, 29, 76–83. [Google Scholar] [CrossRef]

| Polycythemia Vera | Essential Thrombocythemia | Primary Myelofibrosis |
|---|---|---|
| Major criteria | ||
|
|
|
| Minor criteria | ||
|
|
|
| Criteria for diagnosis | ||
| For the diagnosis of PV, three major criteria are required or, alternatively, two major criteria and the minor criterion. | For the diagnosis of ET, all four major criteria are required or the first three major criteria and the minor criterion. | For the diagnosis of PMF, patients must present all three major criteria, and at least one minor criterion. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiberio, F.; Coda, A.R.D.; Tosi, D.D.; Luzi, D.; Polito, L.; Liso, A.; Lattanzi, W. Mechanobiology and Primary Cilium in the Pathophysiology of Bone Marrow Myeloproliferative Diseases. Int. J. Mol. Sci. 2024, 25, 8860. https://doi.org/10.3390/ijms25168860
Tiberio F, Coda ARD, Tosi DD, Luzi D, Polito L, Liso A, Lattanzi W. Mechanobiology and Primary Cilium in the Pathophysiology of Bone Marrow Myeloproliferative Diseases. International Journal of Molecular Sciences. 2024; 25(16):8860. https://doi.org/10.3390/ijms25168860
Chicago/Turabian StyleTiberio, Federica, Anna Rita Daniela Coda, Domiziano Dario Tosi, Debora Luzi, Luca Polito, Arcangelo Liso, and Wanda Lattanzi. 2024. "Mechanobiology and Primary Cilium in the Pathophysiology of Bone Marrow Myeloproliferative Diseases" International Journal of Molecular Sciences 25, no. 16: 8860. https://doi.org/10.3390/ijms25168860
APA StyleTiberio, F., Coda, A. R. D., Tosi, D. D., Luzi, D., Polito, L., Liso, A., & Lattanzi, W. (2024). Mechanobiology and Primary Cilium in the Pathophysiology of Bone Marrow Myeloproliferative Diseases. International Journal of Molecular Sciences, 25(16), 8860. https://doi.org/10.3390/ijms25168860







