Enhanced CO2 Capture Potential of Chitosan-Based Composite Beads by Adding Activated Carbon from Coffee Grounds and Crosslinking with Epichlorohydrin
Abstract
1. Introduction
2. Results and Discussion
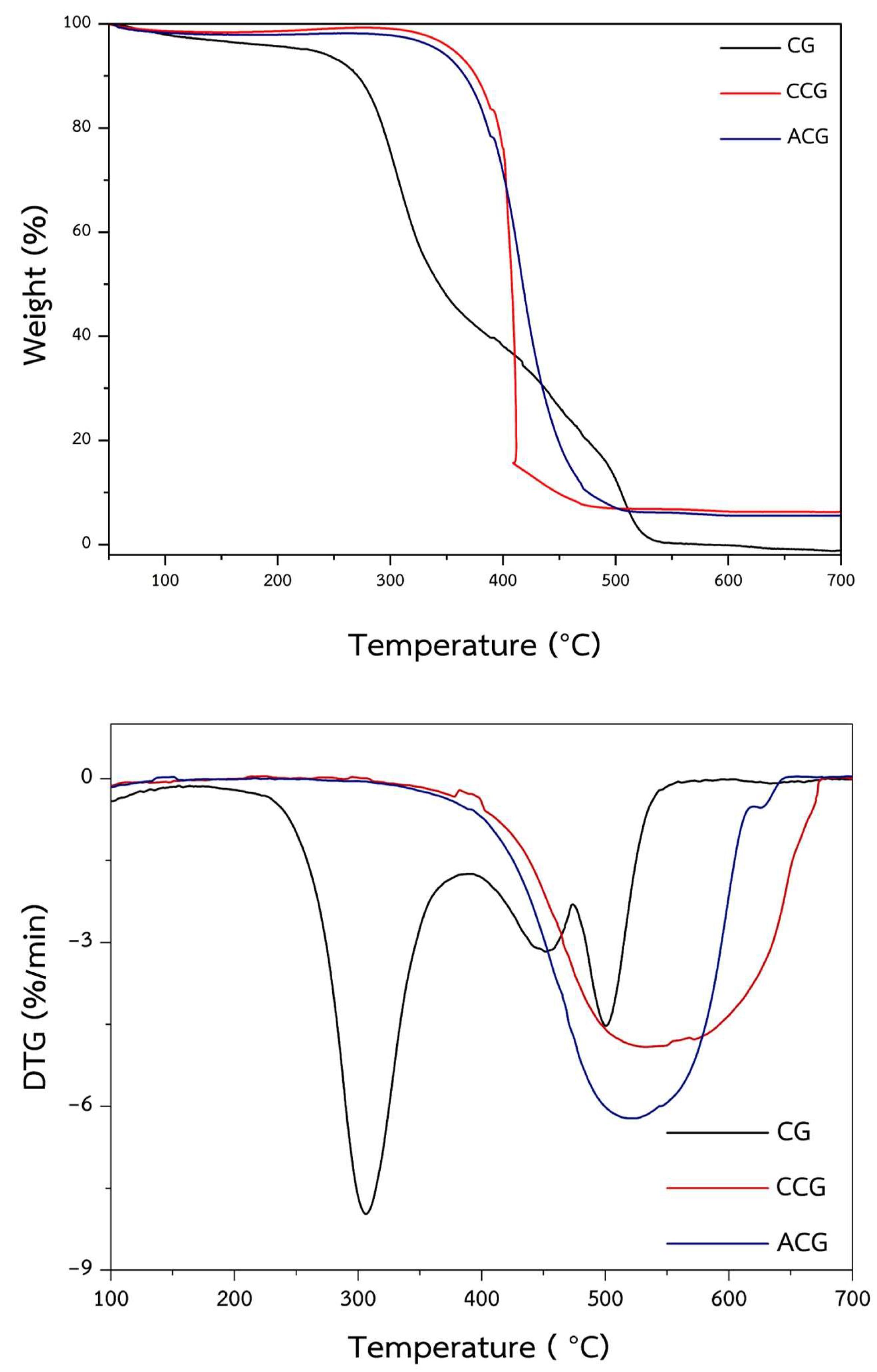
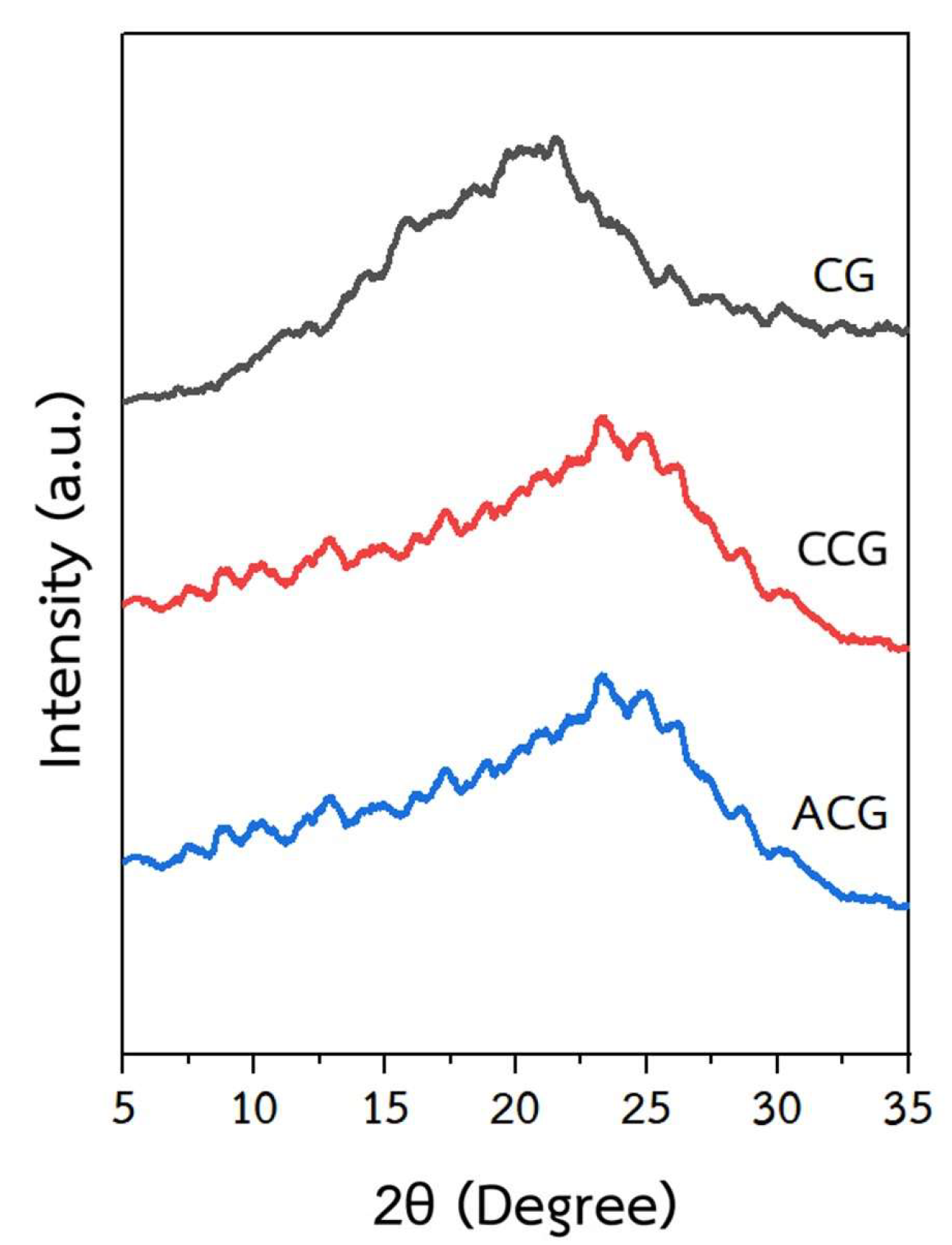
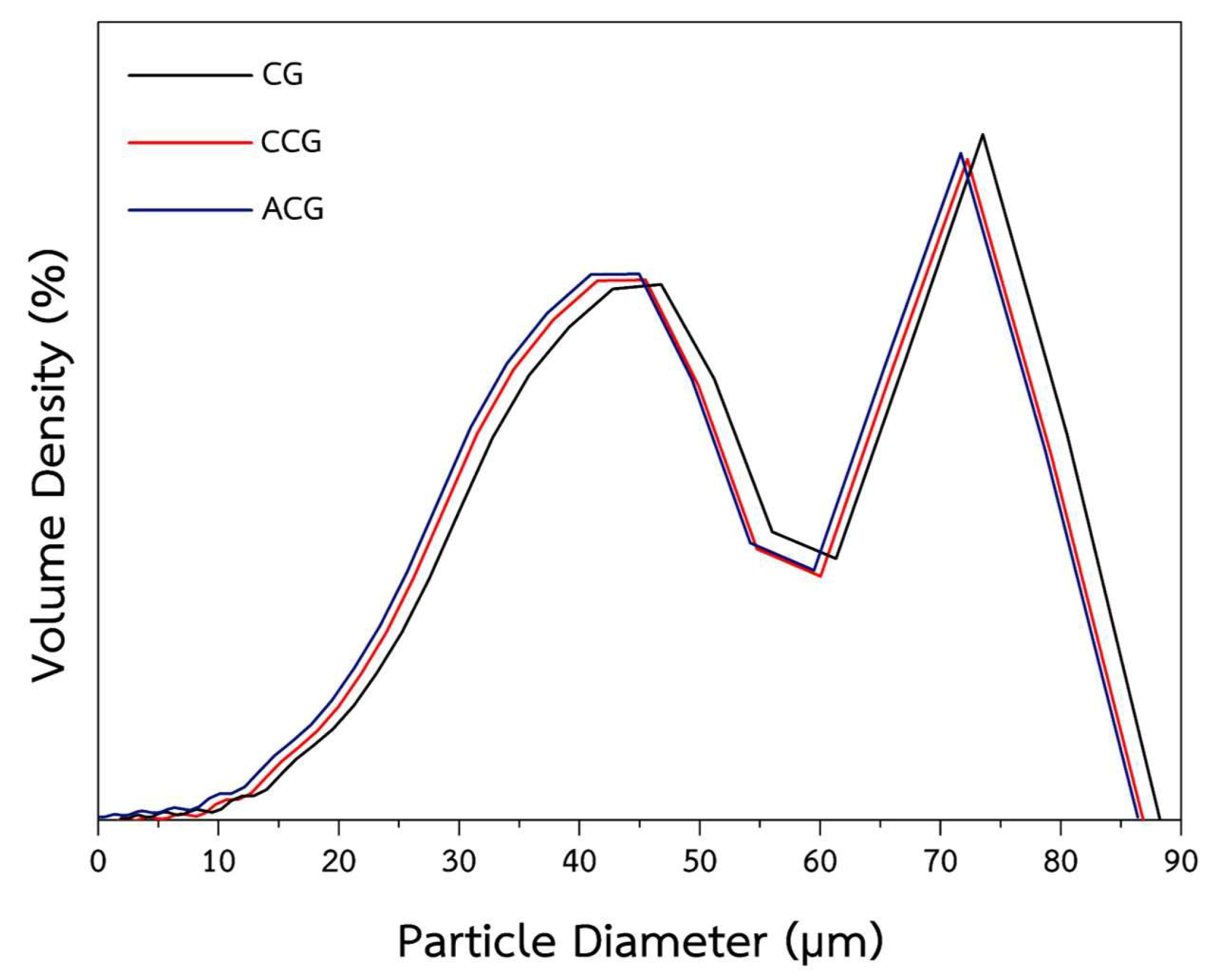
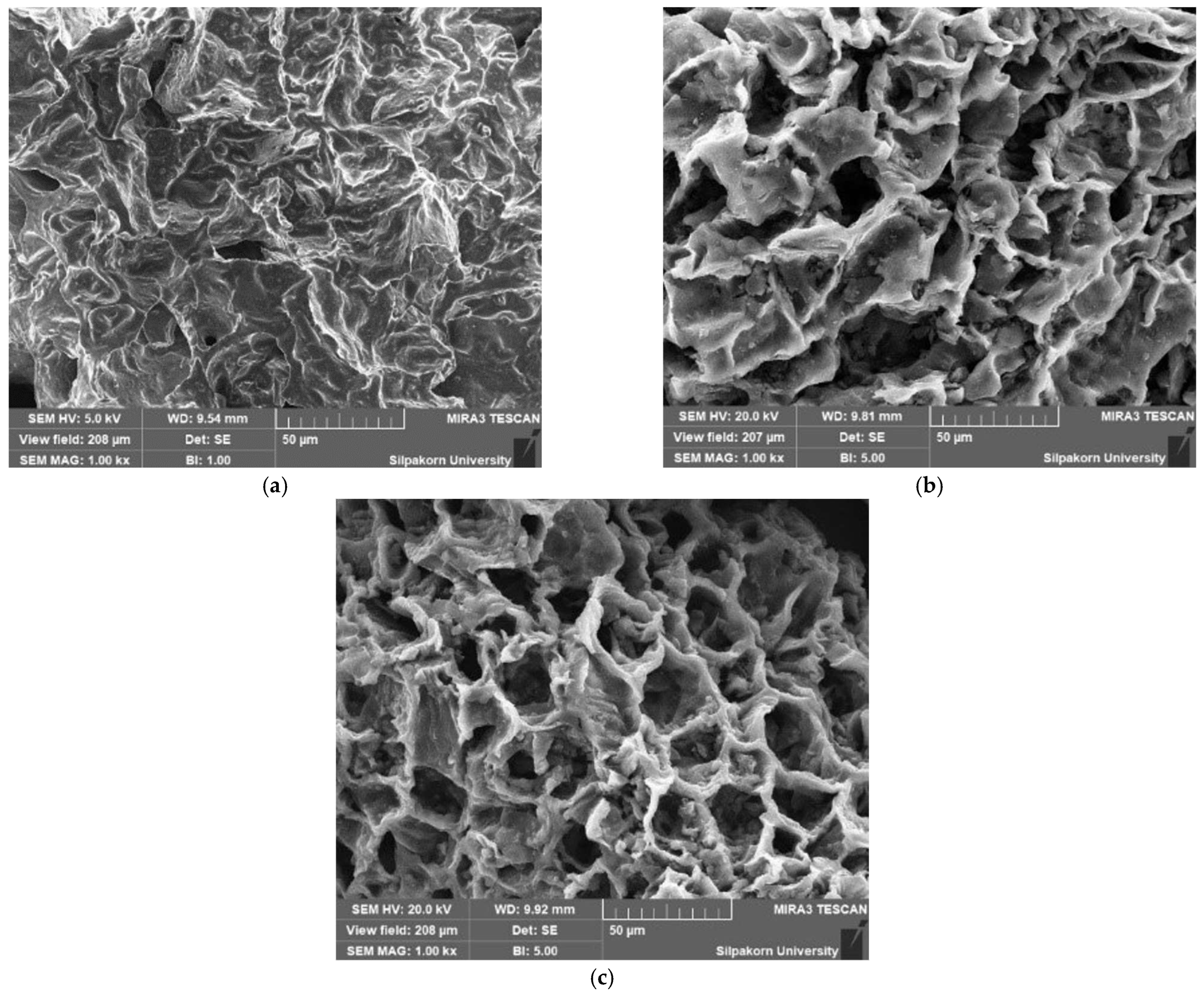
2.1. Characterization and Properties of CG, Carbonized Coffee Ground (CCG), and ACG
2.2. Characterization and Properties of CS/ACG Composite Materials
3. Experimental
3.1. Materials and Chemicals
3.2. Preparation of ACG
3.3. Synthesis of CS/ACG Composite Microsphere
3.4. Material Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Oliveira, M.R.; Cecilia, J.A.; Ballesteros-Plata, D.; Barroso-Martín, I.; Núñez, P.; Infantes-Molina, A.; Rodríguez-Castellón, E. Microwave-assisted synthesis of zeolite A from metakaolinite for CO2 adsorption. Int. J. Mol. Sci. 2023, 24, 14040. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Zhou, J.; Yang, F.; Lan, M.; Li, J.; Zhang, Z.; Chen, Z.; Xu, M.; Li, H.; Sanjayan, J.G. Analysis of theoretical carbon dioxide emissions from cement production: Methodology and application. J. Clean. Prod. 2022, 334, 130270. [Google Scholar] [CrossRef]
- Kopacka, G.; Wasiluk, K.; Majewski, P.W.; Kopyt, M.; Kwiatkowski, P.; Megiel, E. Aluminium-based metal–organic framework nano cuboids and nanoflakes with embedded gold nanoparticles for carbon dioxide fixation with epoxides into cyclic esters. Int. J. Mol. Sci. 2024, 25, 1020. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Deng, Z.; Davis, S.; Ciais, P. Monitoring global carbon emissions in 2022. Nat. Rev. Earth Environ. 2023, 4, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Liu, H.; Zeng, Z.; Pan, W. Recent progress of catalysts for synthesis of cyclic carbonates from CO2 and epoxides. J. CO2 Util. 2023, 68, 102355. [Google Scholar] [CrossRef]
- Mousa, A.O.; Chuang, C.-H.; Kuo, S.-W.; Mohamed, M.G. Strategic design and synthesis of ferrocene linked porous organic frameworks toward tunable CO2 capture and energy storage. Int. J. Mol. Sci. 2023, 24, 12371. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Li, H.; Chen, S.; Teng, F. Investment decision on carbon capture and utilization (CCU) technologies—A real option model based on technology learning effect. Appl. Energy 2022, 322, 119514. [Google Scholar] [CrossRef]
- Peres, C.B.; Resende, P.M.R.; Nunes, L.J.R.; Morais, L.C. Advances in carbon capture and use (CCU) technologies: A comprehensive review and CO2 mitigation potential analysis. Clean Technol. 2022, 4, 1193–1207. [Google Scholar] [CrossRef]
- Dziejarski, B.; Serafin, J.; Andersson, K.; Krzyżyńska, R. CO2 capture materials: A review of current trends and future challenges. Mater. Today Sustain. 2023, 24, 100483. [Google Scholar] [CrossRef]
- Chagas, J.A.O.; Crispim, G.O.; Pinto, B.P.; Gil, R.A.S.S.; Mota, C.J.A. Synthesis, characterization, and CO2 uptake of adsorbents prepared by hydrothermal carbonization of chitosan. ACS Omega 2020, 5, 29520–29529. [Google Scholar] [CrossRef]
- Huang, H.; Wang, L.; Zhang, X.; Zhao, H.; Gu, Y. CO2-selective capture from light hydrocarbon mixtures by metal-organic frameworks: A review. Clean Technol. 2023, 5, 1–24. [Google Scholar] [CrossRef]
- Nandanwar, P.; Jugade, R.; Gomase, V.; Shekhawat, A.; Bambal, A.; Saravanan, D.; Pandey, S. Chitosan-biopolymer-entrapped activated charcoal for adsorption of reactive orange dye from aqueous phase and CO2 from gaseous phase. J. Compos. Sci. 2023, 7, 103. [Google Scholar] [CrossRef]
- Foungchuen, J.; Pairin, N.; Phalakornkule, C. Impregnation of chitosan onto activated carbon for adsorption selectivity towards CO2: Biohydrogen purification. KMUTNB Int. J. Appl. Sci. Technol. 2016, 9, 197–209. [Google Scholar] [CrossRef]
- Keramati, M.; Ghoreyshi, A.A. Improving CO2 adsorption onto activated carbon through functionalization by chitosan and triethylenetetramine. Phys. E Low Dimens. Syst. Nanostruct. 2014, 57, 161–168. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Boominathan, T.; Sivaramakrishna, A. Recent advances in the synthesis, properties, and applications of modified chitosan derivatives: Challenges and opportunities. Top. Curr. Chem. 2021, 379, 19. [Google Scholar] [CrossRef] [PubMed]
- Nikoshvili, L.Z.; Tikhonov, B.B.; Ivanov, P.E.; Stadolnikova, P.Y.; Sulman, M.G.; Matveeva, V.G. Recent progress in chitosan-containing composite materials for sustainable approaches to adsorption and catalysis. Catalysts 2023, 13, 367. [Google Scholar] [CrossRef]
- Sneddon, G.; Ganin, A.Y.; Yiu, H.H.P. Sustainable CO2 adsorbents prepared by coating chitosan onto mesoporous silicas for large-scale carbon capture technology. Energy Technol. 2015, 3, 249–258. [Google Scholar] [CrossRef]
- Danon, A.; Stair, P.C.; Weitz, E. FTIR study of CO2 adsorption on amine-grafted SBA-15: Elucidation of adsorbed species. J. Phys. Chem. C 2011, 115, 11540–11549. [Google Scholar] [CrossRef]
- Shao, L.; Wan, H.; Wang, L.; Wang, J.; Liu, Z.; Wu, Z.; Zhan, P.; Zhang, L.; Ma, X.; Huang, J. N-doped highly microporous carbon derived from the self-assembled lignin/chitosan composites beads for selective CO2 capture and efficient p-nitrophenol adsorption. Sep. Purif. Technol. 2023, 313, 123440. [Google Scholar] [CrossRef]
- Phalakornkule, C.; Foungchuen, J.; Pitakchon, T. Impregnation of chitosan onto activated carbon for high adsorption selectivity towards CO2: CO2 capture from biohydrogen, biogas and flue gas. J. Sustain. Energy Environ. 2012, 3, 153–157. [Google Scholar]
- Marin, L.; Dragoi, B.; Olaru, N.; Perju, E.; Coroaba, A.; Doroftei, F.; Scavia, G.; Destri, S.; Zappia, S.; Porzio, W. Nanoporous furfuryl-imine-chitosan fibers as a new pathway towards eco-materials for CO2 adsorption. Eur. Polym. J. 2019, 120, 109214. [Google Scholar] [CrossRef]
- Hassan, H.H.; Abeer, S.; Nadim, H. Removal of malachite green from water using hydrothermally carbonized pine needles. RSC Adv. 2015, 5, 7909–7920. [Google Scholar] [CrossRef]
- Özhan, A.; Şahin, Ö.; Küçük, M.M.; Saka, C. Preparation and characterization of activated carbon from pine cone by microwave-induced ZnCl2 activation and its effects on the adsorption of methylene blue. Cellulose 2014, 21, 2457–2467. [Google Scholar] [CrossRef]
- Buasri, A.; Chaiyut, N.; Loryuenyong, V.; Rodklum, C.; Chaikwan, T.; Kumphan, N.; Jadee, K.; Klinklom, P.; Wittayarounayut, W. Transesterification of waste frying oil for synthesizing biodiesel by KOH supported on coconut shell activated carbon in packed bed reactor. Sci. Asia 2012, 38, 283–288. [Google Scholar] [CrossRef]
- Buasri, A.; Ananganjanakit, T.; Peangkom, N.; Khantasema, P.; Pleeram, K.; Lakaeo, A.; Arthnukarn, J.; Loryuenyong, V. A facile route for the synthesis of reduced graphene oxide (RGO) by DVD laser scribing and its applications in the environment-friendly electrochromic devices (ECD). J. Optoelectron. Adv. M 2017, 19, 492–500. [Google Scholar]
- Buasri, A.; Ojchariyakul, S.; Kaewmanechai, P.; Eakviriyapichat, W.; Loryuenyong, V. The fabrication of multicolor electrochromic device based on graphene conductive ink/poly(Lactic acid) thin films by voltage-step method. Optoelectron. Adv. Mater.–Rapid Commun. 2018, 12, 388–393. [Google Scholar]
- Ozdemir, I.; Şahin, M.; Orhan, R.; Erdem, M. Preparation and characterization of activated carbon from grape stalk by zinc chloride activation. Fuel Process. Technol. 2014, 125, 200–206. [Google Scholar] [CrossRef]
- Shang, H.; Lu, Y.; Zhao, F.; Chao, C.; Zhang, B.; Zhang, H. Preparing high surface area porous carbon from biomass by carbonization in a molten salt medium. RSC Adv. 2015, 5, 75728. [Google Scholar] [CrossRef]
- Buasri, A.; Chaiyut, N.; Nakweang, C. Preparing activated carbon from palm shell for biodiesel fuel production. Chiang Mai J. Sci. 2011, 38, 572–578. [Google Scholar]
- Buasri, A.; Chaiyut, N.; Loryuenyong, V.; Rodklum, C.; Chaikwan, T.; Kumphan, N. Continuous process for biodiesel production in packed bed reactor from waste frying oil using potassium hydroxide supported on Jatropha curcas fruit shell as solid catalyst. Appl. Sci. 2012, 2, 641–653. [Google Scholar] [CrossRef]
- Mukherjee, A.; Saha, B.; Niu, C.; Dalai, A.K. Preparation of activated carbon from spent coffee grounds and functionalization by deep eutectic solvent: Effect of textural properties and surface chemistry on CO2 capture performance. J. Environ. Chem. Eng. 2022, 10, 108815. [Google Scholar] [CrossRef]
- Malini, K.; Selvakumar, D.; Kumar, N.S. Activated carbon from biomass: Preparation, factors improving basicity and surface properties for enhanced CO2 capture capacity—A review. J. CO2 Util. 2023, 67, 102318. [Google Scholar] [CrossRef]
- Rivera, X.C.S.; Gallego-Schmid, A.; Najdanovic-Visak, V.; Azapagic, A. Life cycle environmental sustainability of valorization routes for spent coffee grounds: From waste to resources. Resour. Conserv. Recy. 2020, 157, 104751. [Google Scholar] [CrossRef]
- Campos, G.A.F.; Perez, J.P.H.; Block, I.; Sagu, S.T.; Celis, P.S.; Taubert, A.; Rawel, H.M. Preparation of activated carbons from spent coffee grounds and coffee parchment and assessment of their adsorbent efficiency. Processes 2021, 9, 1396. [Google Scholar] [CrossRef]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; de Rezende, T.R.; Schwarz, S.; Lachenmeier, D.W. A review of coffee by-products including leaf, flower, cherry, husk, silver skin, and spent grounds as novel foods within the european union. Foods 2020, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef]
- Saratale, G.D.; Bhosale, R.; Shobana, S.; Banu, J.R.; Pugazhendhi, A.; Mahmoud, E.; Sirohi, R.; Bhatia, S.K.; Atabani, A.E.; Mulone, V.; et al. A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Bioresour. Technol. 2020, 314, 123800. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, Z.; Yu, T.; Yan, F. Spent coffee grounds: Present and future of environmentally friendly applications on industries-A review. Trends Food Sci. Technol. 2024, 143, 104312. [Google Scholar] [CrossRef]
- Mensah, R.Q.; Tantayotai, P.; Rattanaporn, K.; Chuetor, S.; Kirdponpattara, S.; Kchaou, M.; Show, P.-L.; Mussatto, S.I.; Sriariyanun, M. Properties and applications of green-derived products from spent coffee grounds—Steps towards sustainability. Bioresour. Technol. Rep. 2024, 26, 101859. [Google Scholar] [CrossRef]
- Dutta, S.D.; Patel, D.K.; Ganguly, K.; Lim, K.-T. Isolation and characterization of cellulose nanocrystals from coffee grounds for tissue engineering. Mater. Lett. 2021, 287, 129311. [Google Scholar] [CrossRef]
- Baby, R.; Saifullah, B.; Hussein, M.Z. Carbon nanomaterials for the treatment of heavy metal-contaminated water and environmental remediation. Nanoscale Res. Lett. 2019, 14, 341. [Google Scholar] [CrossRef]
- Chen, X.; He, L. Microwave irradiation assisted preparation of chitosan composite microsphere for dye adsorption. Int. J. Polym. Sci. 2017, 2017, 2672597. [Google Scholar] [CrossRef]
- Chiou, M.-S.; Ho, P.-Y.; Li, H.-Y. Adsorption of anionic dyes in acid solutions using chemically cross-linked chitosan beads. Dyes Pigments 2004, 60, 69–84. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Enhanced abilities of highly swollen chitosan beads for color removal and tyrosinase immobilization. J. Hazard. Mater. 2001, 81, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Khandaker, T.; Hossain, M.S.; Dhar, P.K.; Rahman, M.S.; Hossain, M.A.; Ahmed, M.B. Efficacies of carbon-based adsorbents for carbon dioxide capture. Processes 2020, 8, 654. [Google Scholar] [CrossRef]
- Yuliusman; Nasruddin; Afdhol, M.K.; Haris, F.; Amiliana, R.A.; Hanafi, A.; Ramadhan, I.T. Production of activated carbon from coffee grounds using chemical and physical activation method. Adv. Sci. Lett. 2017, 23, 5751–5755. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; José ATeixeira Mussatto, S.I. Extraction of polysaccharides by autohydrolysis of spent coffee grounds and evaluation of their antioxidant activity. Carbohydr. Polym. 2017, 157, 258–266. [Google Scholar] [CrossRef]
- Yang, L.; Nazari, L.; Yuan, Z.; Corscadden, K.; Xu, C.; He, Q. Hydrothermal liquefaction of spent coffee grounds in water medium for bio-oil production. Biomass Bioenergy 2016, 86, 191–198. [Google Scholar] [CrossRef]
- Ren, L.; Hemar, Y.; Perera, C.O.; Lewis, G.; Krissansen, G.W.; Buchanan, P.K. Antibacterial and antioxidant activities of aqueous extracts of eight edible mushrooms. Bioact. Carbohydr. Diet. Fibre 2014, 3, 41–51. [Google Scholar] [CrossRef]
- Arauzo, P.J.; Maziarka, P.; Olszewski, M.; Isemin, R.; Muratova, N.; Ronsse, F.; Kruse, A. Valorization of the poultry litter through wet torrefaction and different activation treatments. Sci. Total Environ. 2020, 732, 139288. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Chen, N.; Wan, L.; Li, G.; Chen, T.; Yang, F.; Sun, S. Preparation of high-performance activated carbon from coffee grounds after extraction of bio-oil. Molecules 2021, 26, 257. [Google Scholar] [CrossRef] [PubMed]
- Reffas, A.; Bernardet, V.; David, B.; Reinert, L.; Bencheikh Lehocine, M.; Dubois, M.; Batisse, N.; Duclaux, L. Carbons prepared from coffee grounds by H3PO4 activation: Characterization and adsorption of methylene blue and Nylosan Red N-2RBL. J. Hazard. Mater. 2010, 175, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Dziejarski, B.; Hernández-Barreto, D.F.; Moreno-Piraján, J.C.; Giraldo, L.; Serafin, J.; Knutsson, P.; Andersson, K.; Krzyżyńska, R. Upgrading recovered carbon black (rCB) from industrial-scale end-of-life tires (ELTs) pyrolysis to activated carbons: Material characterization and CO2 capture abilities. Environ. Res. 2024, 247, 118169. [Google Scholar] [CrossRef] [PubMed]
- Aouay, F.; Attia, A.; Dammak, L.; Amar, R.B.; Deratani, A. Activated carbon prepared from waste coffee grounds: Characterization and adsorption properties of dyes. Materials 2024, 17, 3078. [Google Scholar] [CrossRef] [PubMed]
- Romdhani, M.; Attia, A.; Charcosset, C.; Mahouche-Chergui, S.; Ates, A.; Duplay, J.; Ben Amar, R. Optimization of paracetamol and chloramphenicol removal by novel activated carbon derived from sawdust using response surface methodology. Sustainability 2023, 15, 2516. [Google Scholar] [CrossRef]
- Bernal, V.; Giraldo, L.; Moreno-Piraján, J.C. Physicochemical properties of activated carbon: Their effect on the adsorption of pharmaceutical compounds and adsorbate–adsorbent interactions. C 2018, 4, 62. [Google Scholar] [CrossRef]
- Li, Y.; Zou, B.; Hu, C.; Cao, M. Nitrogen-doped porous carbon nanofiber webs for efficient CO2 capture and conversion. Carbon 2016, 99, 79–89. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Cui, Z.; Fu, Z.; Yang, L.; Liu, G.; Li, M. Coffee grounds derived N enriched microporous activated carbons: Efficient adsorbent for post-combustion CO2 capture and conversion. J. Colloid Interface Sci. 2020, 578, 491–499. [Google Scholar] [CrossRef]
- Dhelipan, M.; Arunchander, A.; Sahu, A.K.; Kalpana, D. Activated carbon from orange peels as supercapacitor electrode and catalyst support for oxygen reduction reaction in proton exchange membrane fuel cell. J. Saudi Chem. Soc. 2017, 21, 487–494. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Cwikla-Bundyra, W.; Bogusz, A.; Skwarek, E.; Ok, Y.S. Characterization of nanoparticles of biochars from different biomass. J. Anal. Appl. Pyrol. 2016, 121, 165–172. [Google Scholar] [CrossRef]
- Ma, S.; Jing, F.; Sohi, S.P.; Chen, J. New insights into contrasting mechanisms for PAE adsorption on millimeter, micron- and nano-scale biochar. Environ. Sci. Pollut. Res. 2019, 26, 18636–18650. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Redzwan, G.; Sahu, J.N.; Mukherjee, S.; Gupta, B.S.; Hashim, M.A. Hexavalent chromium adsorption by a novel activated carbon prepared by microwave activation. BioRes 2014, 9, 1498–1518. [Google Scholar] [CrossRef]
- Laksaci, H.; Khelifi, A.; Trari, M.; Addoun, A. Synthesis and characterization of microporous activated carbon from coffee grounds using potassium hydroxides. J. Clean. Prod. 2017, 147, 254–262. [Google Scholar] [CrossRef]
- Rosson, E.; Garbo, F.; Marangoni, G.; Bertani, R.; Lavagnolo, M.C.; Moretti, E.; Talon, A.; Mozzon, M.; Sgarbossa, P. Activated carbon from spent coffee grounds: A good competitor of commercial carbons for water decontamination. Appl. Sci. 2020, 10, 5598. [Google Scholar] [CrossRef]
- Omidi-Khaniabadi, Y.; Jafari, A.; Nourmoradi, H.; Taheri, F.; Saeedi, S. Adsorption of 4-chlorophenol from aqueous solution using activated carbon synthesized from aloe vera green wastes. J. Adv. Environ. Health Res. 2015, 3, 120–129. [Google Scholar]
- Wang, Y.; Lu, C.; Cao, X.; Wang, Q.; Yang, G.; Chen, J. Porous carbon spheres derived from hemicelluloses for supercapacitor application. Int. J. Mol. Sci. 2022, 23, 7101. [Google Scholar] [CrossRef]
- Xia, H.; Peng, J.; Zhang, L. Preparation of high surface area activated carbon from Eupatorium adenophorum using K2CO3 activation by microwave heating. Green Process. Synth. 2015, 4, 299–305. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Coromina, H.M.; Walsh, D.A.; Mokaya, R. Biomass-derived activated carbon with simultaneously enhanced CO2 uptake for both pre and post combustion capture applications. J. Mater. Chem. A 2016, 4, 280–289. [Google Scholar] [CrossRef]
- Munim, S.A.; Saddique, M.T.; Raza, Z.A.; Majeed, M.I. Fabrication of cellulose-mediated chitosan adsorbent beads and their surface chemical characterization. Polym. Bull. 2020, 77, 183–196. [Google Scholar] [CrossRef]
- Wang, T.; Wusigale; Kuttappan, D.; Amalaradjou, M.A.; Luo, Y.; Luo, Y. Polydopamine-coated chitosan hydrogel beads for synthesis and immobilization of silver nanoparticles to simultaneously enhance antimicrobial activity and adsorption kinetics. Adv. Compos. Hybrid Mater. 2021, 4, 696–706. [Google Scholar] [CrossRef]
- Luo, X.; Liu, L.; Wang, L.; Liu, X.; Cai, Y. Facile synthesis and low concentration tylosin adsorption performance of chitosan/cellulose nanocomposite microspheres. Carbohydr. Polym. 2019, 206, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Nitsae, M.; Madjid, A.; Hakim, L.; Sabarudin, A. Preparation of chitosan beads using tripolyphosphate and ethylene glycol diglycidyl ether as crosslinker for Cr(VI) adsorption. Chem. Chem. Technol. 2016, 10, 105–113. [Google Scholar] [CrossRef]
- Phuoc, N.M.; Thien, L.T.; Phuong, N.T.T.; Duong, N.T.H.; Dung, N.V.; Long, N.Q. Novel chitosan-zeolite X composite beads prepared by phase-inversion method for CO2 adsorptive capture. Chemosphere 2024, 352, 141327. [Google Scholar] [CrossRef]
- Wood, B.C.; Bhide, S.Y.; Dutta, D.; Kandagal, V.S.; Pathak, A.D.; Punnathanam, S.N.; Ayappa, K.G.; Narasimhan, S. Methane and carbon dioxide adsorption on edge-functionalized graphene: A comparative DFT study. J. Chem. Phys. 2012, 137, 054702. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Agag, T.; Ishida, H.; Qutubuddin, S. Biobased chitosan hybrid aerogels with superior adsorption: Role of graphene oxide in CO2 capture. RSC Adv. 2013, 3, 16011–16020. [Google Scholar] [CrossRef]
- Fathalian, F.; Moghadamzadeh, H.; Hemmati, A.; Ghaemi, A. Efficient CO2 adsorption using chitosan, graphene oxide, and zinc oxide composite. Sci. Rep. 2024, 14, 3186. [Google Scholar] [CrossRef] [PubMed]
- Benhouria, A.; Zaghouane-Boudiaf, H.; Bourzami, R.; Djerboua, F.; Hameed, B.H.; Boutahala, M. Cross-linked chitosan-epichlorohydrin/bentonite composite for reactive orange 16 dye removal: Experimental study and molecular dynamic simulation. Int. J. Biol. Macromol. 2023, 242, 124786. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. A review of several reported procedures to determine the degree of N-acetylation for chitin and chitosan using infrared spectroscopy. Carbohydr. Polym. 2008, 71, 497–508. [Google Scholar] [CrossRef]
- Rafigh, S.M.; Heydarinasab, A. Mesoporous chitosan−SiO2 nanoparticles: Synthesis, characterization, and CO2 adsorption capacity. ACS Sustain. Chem. Eng. 2017, 5, 10379–10386. [Google Scholar] [CrossRef]
- Valechha, A.; Thote, J.; Labhsetwar, N.; Rayalu, S. Biopolymer based adsorbents for the post combustion CO2 capture. Int. J. Knowl. Eng. 2012, 3, 103–106. [Google Scholar]
- Kumar, S.; de A. e Silva, J.; Wani, M.Y.; Dias, C.M.F.; Sobral, A.J.F.N. Studies of carbon dioxide capture on porous chitosan derivative. J. Dispers. Sci. Technol. 2015, 37, 155–158. [Google Scholar] [CrossRef]



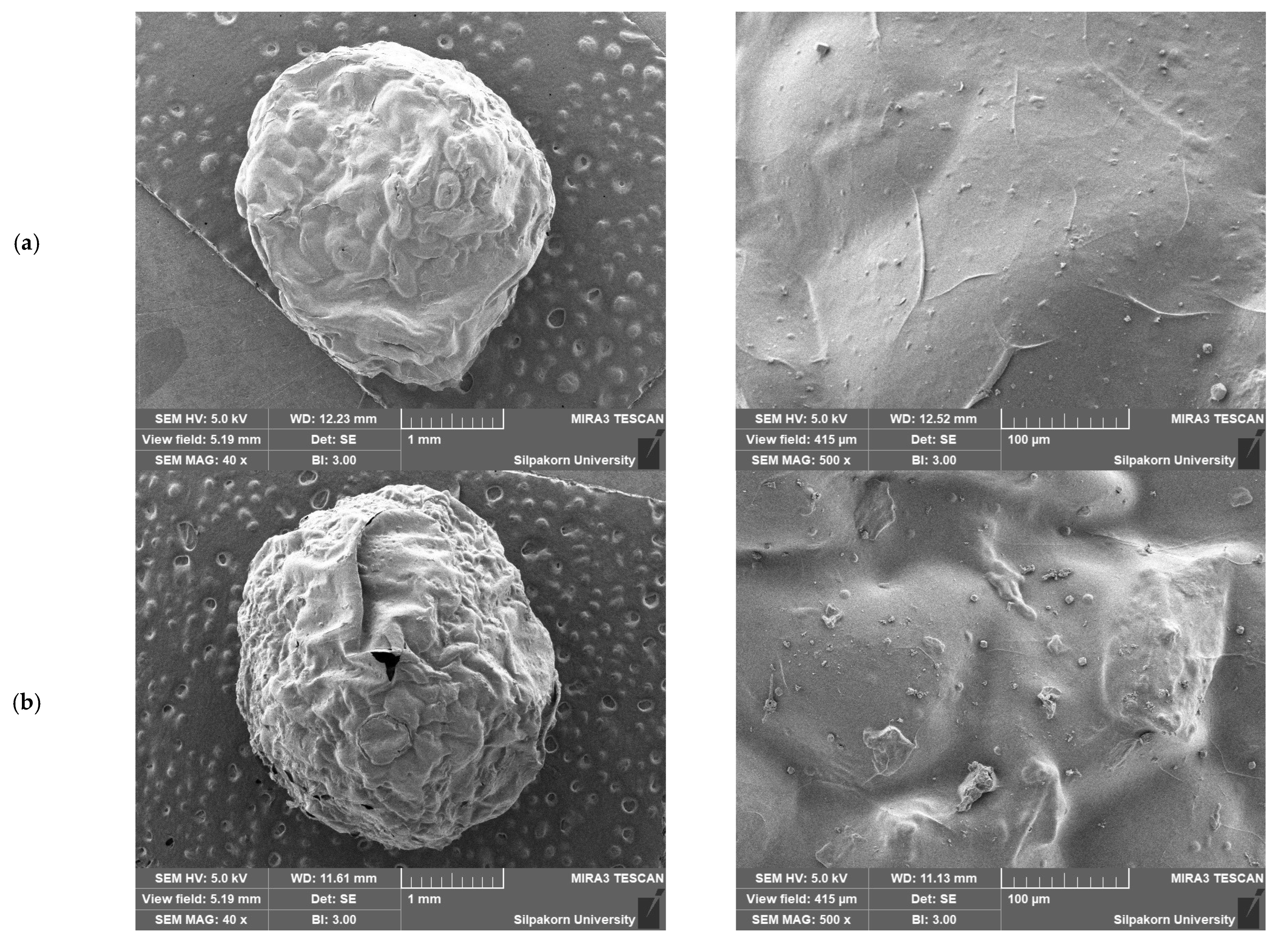

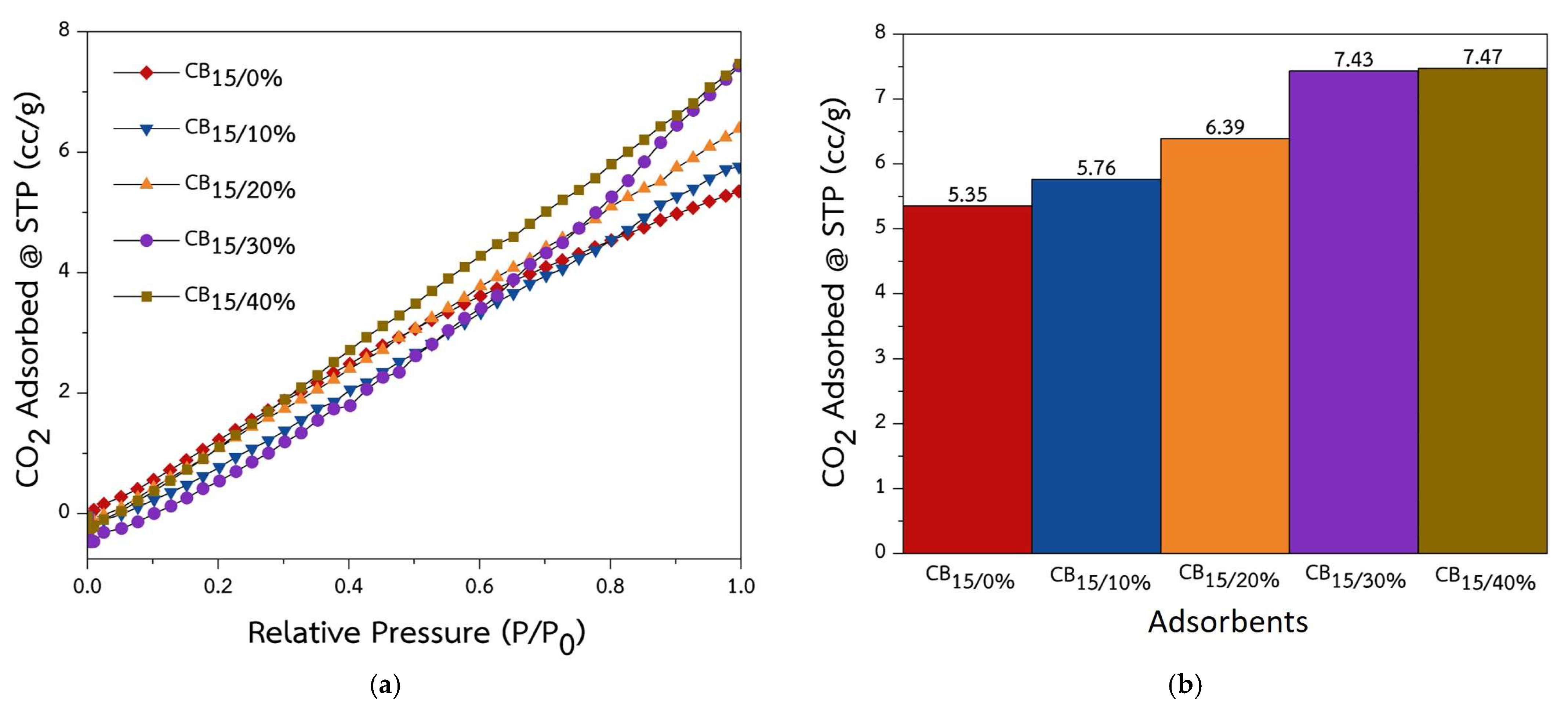




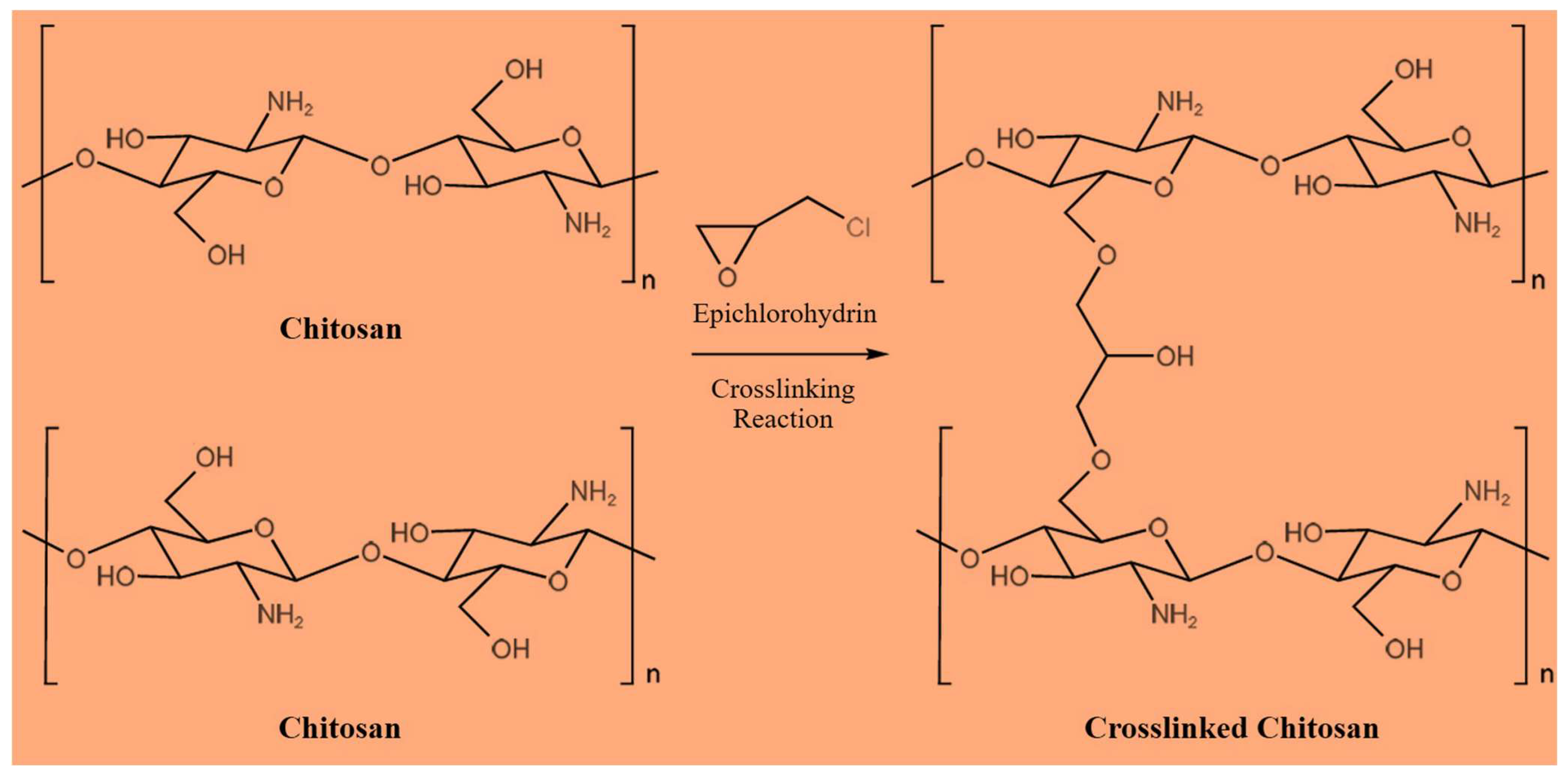


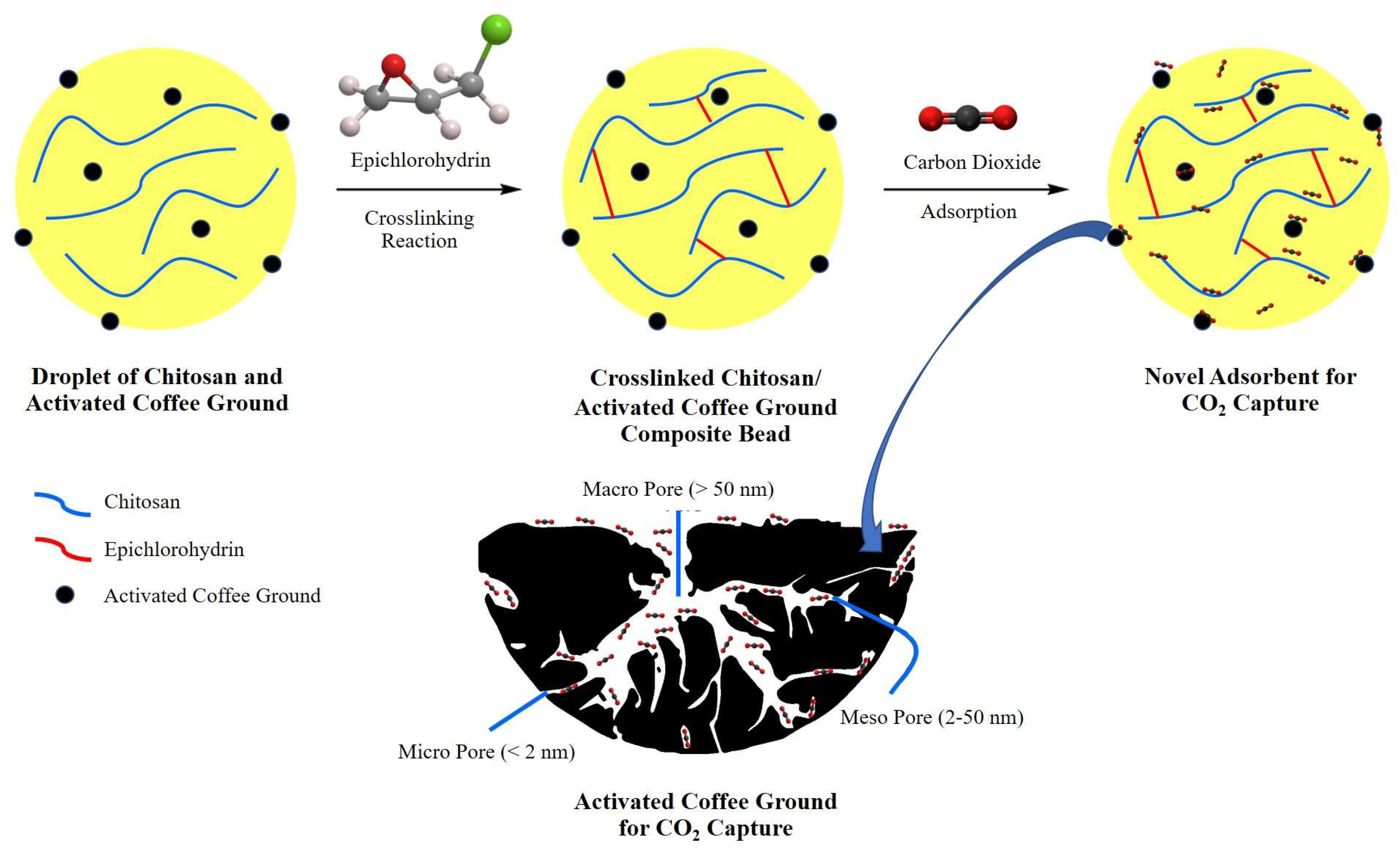
| Sample | Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| CG | 2.90 | 3.31 | 0.57 |
| CCG | 4.39 | 3.94 | 0.62 |
| ACG | 5.60 | 3.71 | 0.87 |
| Sample | CO2 Adsorption (mmol/g) | Reference |
|---|---|---|
| Chitosan/Activated Coffee Ground | 0.34 | This Work |
| Imine-Chitosan | 0.11 | [85] |
| Triphenyl Amine Porous Chitosan Derivative | 0.85 | [86] |
| Chitosan-Hydrothermal Carbonization | 0.30 | [10] |
| Chitosan/Fumed Silica | 0.29 | [19] |
| Sample | CS (g) | ACG (g) | ACG (%w/w) | EP (g) |
|---|---|---|---|---|
| CB15/0% | 15 | 0 | 0 | 5 |
| CB15/10% | 15 | 1.5 | 10 | 5 |
| CB15/15% | 15 | 3.0 | 20 | 5 |
| CB15/20% | 15 | 4.5 | 30 | 5 |
| CB15/25% | 15 | 6.0 | 40 | 5 |
| CB20/0% | 20 | 0 | 0 | 5 |
| CB20/10% | 20 | 2.0 | 10 | 5 |
| CB20/20% | 20 | 4.0 | 20 | 5 |
| CB20/30% | 20 | 6.0 | 30 | 5 |
| CB20/40% | 20 | 8.0 | 40 | 5 |
| CB25/0% | 25 | 0 | 0 | 5 |
| CB25/10% | 25 | 2.5 | 10 | 5 |
| CB25/20% | 25 | 5.0 | 20 | 5 |
| CB25/30% | 25 | 7.5 | 30 | 5 |
| CB25/40% | 25 | 10.0 | 40 | 5 |
| Sample | CS (g) | ACG (g) | ACG (%w/w) | EP (g) |
|---|---|---|---|---|
| CB20/40%EP2 | 20 | 8.0 | 40 | 2 |
| CB20/40%EP3 | 20 | 8.0 | 40 | 3 |
| CB20/40%EP4 | 20 | 8.0 | 40 | 4 |
| CB20/40%EP5 | 20 | 8.0 | 40 | 5 |
| CB20/40%EP6 | 20 | 8.0 | 40 | 6 |
| CB20/40%EP7 | 20 | 8.0 | 40 | 7 |
| CB20/40%EP8 | 20 | 8.0 | 40 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loryuenyong, V.; Nakhlo, W.; Srikaenkaew, P.; Yaidee, P.; Buasri, A.; Eiad-Ua, A. Enhanced CO2 Capture Potential of Chitosan-Based Composite Beads by Adding Activated Carbon from Coffee Grounds and Crosslinking with Epichlorohydrin. Int. J. Mol. Sci. 2024, 25, 8916. https://doi.org/10.3390/ijms25168916
Loryuenyong V, Nakhlo W, Srikaenkaew P, Yaidee P, Buasri A, Eiad-Ua A. Enhanced CO2 Capture Potential of Chitosan-Based Composite Beads by Adding Activated Carbon from Coffee Grounds and Crosslinking with Epichlorohydrin. International Journal of Molecular Sciences. 2024; 25(16):8916. https://doi.org/10.3390/ijms25168916
Chicago/Turabian StyleLoryuenyong, Vorrada, Worranuch Nakhlo, Praifha Srikaenkaew, Panpassa Yaidee, Achanai Buasri, and Apiluck Eiad-Ua. 2024. "Enhanced CO2 Capture Potential of Chitosan-Based Composite Beads by Adding Activated Carbon from Coffee Grounds and Crosslinking with Epichlorohydrin" International Journal of Molecular Sciences 25, no. 16: 8916. https://doi.org/10.3390/ijms25168916
APA StyleLoryuenyong, V., Nakhlo, W., Srikaenkaew, P., Yaidee, P., Buasri, A., & Eiad-Ua, A. (2024). Enhanced CO2 Capture Potential of Chitosan-Based Composite Beads by Adding Activated Carbon from Coffee Grounds and Crosslinking with Epichlorohydrin. International Journal of Molecular Sciences, 25(16), 8916. https://doi.org/10.3390/ijms25168916










