Atopic Dermatitis and Autism Spectrum Disorders: Common Role of Environmental and Clinical Co-Factors in the Onset and Severity of Their Clinical Course
Abstract
1. Introduction
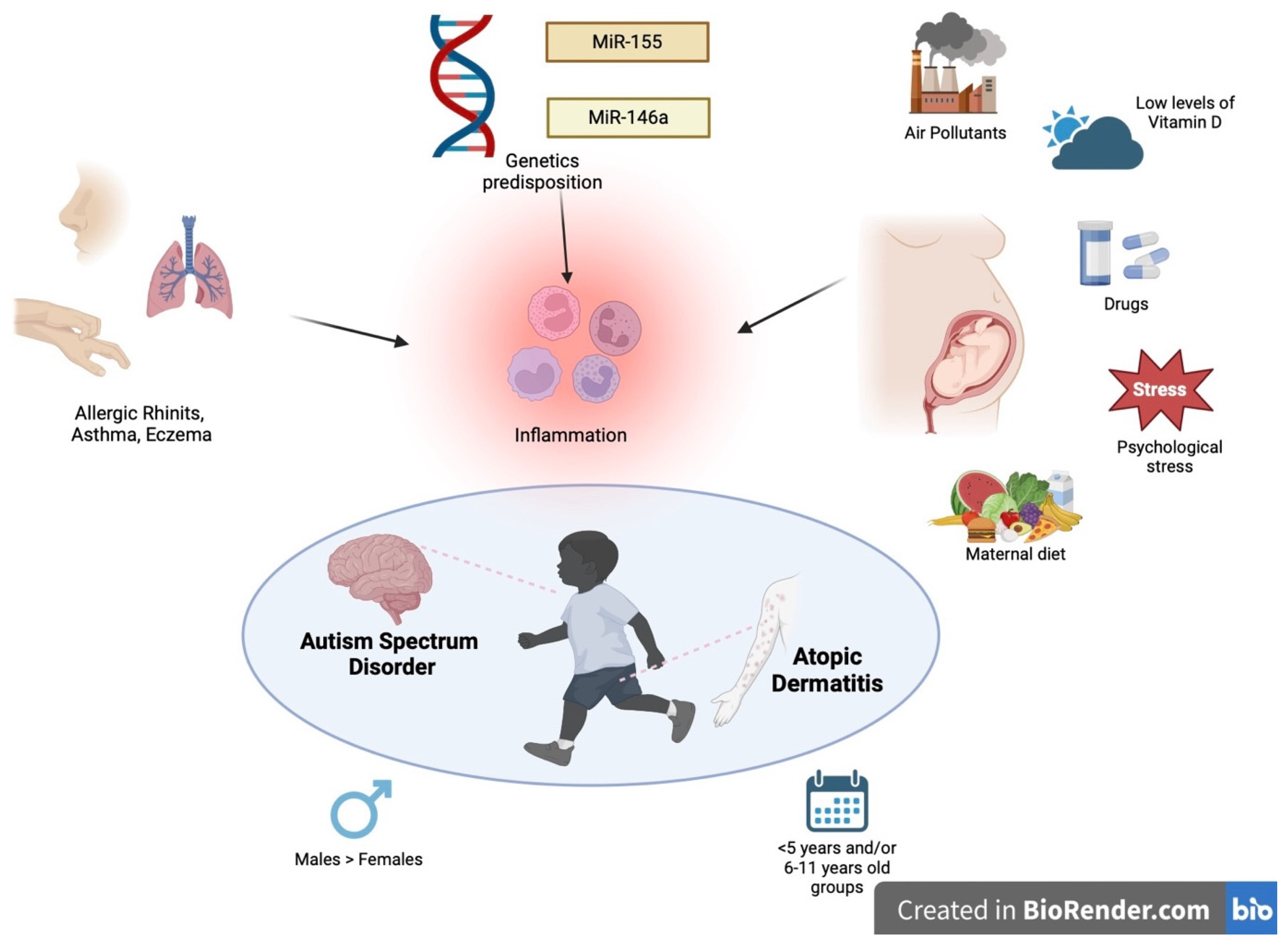
- -
- Genetic predisposition: Signal transducer and activator of transcription 6 (STAT6) is a possible common signaling pathway-y that supports the hypothesis of an etiological correlation between atopy and neurodevelopmental disorders. Genetic variants of STAT6 are associated with atopy due to its major role in the regulation of the Th2 immune response. On the other hand, STAT6 is also expressed in the central nervous system and plays a key role in the pathogenesis of some neuropsychiatric conditions, including ADHD, which in turn is generally comorbid with ASD [9]. Other gene mutations involved in AD pathogenesis have also been observed in ASD, such as GATA3 and ADBRD2. In addition, it has been hypothesized that microRNA (miRNA) also plays a fundamental role [12].
- -
- The production of pro-inflammatory cytokines (IL-6 and TNF alpha), which can cross the blood–brain barrier and activate neuroinflammatory mechanisms. The dysregulation of these cytokines is partially explained by alterations in the microbiota that may be found in both diseases.
- -
- Mast cell activation, responsible for the release of mediators involved in the disruption of the blood–brain barrier and subsequent brain damage.
- -
- The production of auto-antibodies against brain antigens (anti-MBP and anti-MAG) secondary to exposure to allergens. In fact, allergic responses are linked to an increased production of Th2 cells, which stimulate B lymphocytes to produce antibodies against allergens. In children with ASD, these could cross-react with sequence homologies with the brain, causing neuronal damage.
- -
- Maternal and neonatal vitamin D deficiency [10].
- Does the exposome favor the onset of both autism and atopic dermatitis before, during and after pregnancy?
- Does atopic eczema influence the severity of autism and vice versa?
- Is there a correlation between the age of occurrence of both AD and ASD?
- Can the presence of AD influence the onset of ASD/ADHD?
- Are there gender differences between AD and ASD patients?
- Are there comorbidities shared by both AD and ASD patients?
- Are there genetic or epigenetic factors shared by both diseases? (Figure 1).
2. The Common Role of the Exposome in AD and ASD
3. The Relationship between ASD and AD Severity
3.1. Studies Investigating Autism Severity in Patients with Eczema
3.2. Studies Investigating Eczema Severity in Patients with Autism
4. Age of Onset in Patients with Comorbid ASD and AD
5. The Role of AD in the Onset of ADHD/ASD
6. Gender Differences between Atopic Dermatitis and ASD
7. Common Comorbidities between AD and ASD
8. Link between the Genetics and Epigenetics of Autism and Atopic Dermatitis
9. Conclusions and Future Perspectives
| Key Points |
|
|
|
|
|
|
|
|
Funding
Conflicts of Interest
References
- Nutten, S. Atopic Dermatitis: Global Epidemiology and Risk Factors. Ann. Nutr. Metab. 2015, 66, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Patruno, C.; Balato, A.; Rongioletti, F.; Stingeni, L.; Balato, N. An Italian Multicentre Study on Adult Atopic Dermatitis: Persistent versus Adult-Onset Disease. Arch. Dermatol. Res. 2017, 309, 443–452. [Google Scholar] [CrossRef]
- Nettis, E.; Masciopinto, L.; Leo, E.; De Candia, N.; Albanesi, M.; Di Bona, D.; Quaranta, N.; Macchia, L. Dupilumab Elicits a Favorable Response in Type-2 Inflammatory Comorbidities of Severe Atopic Dermatitis. Clin. Mol. Allergy 2021, 19, 9. [Google Scholar] [CrossRef]
- Brancaccio, R.; Murdaca, G.; Casella, R.; Loverre, T.; Bonzano, L.; Nettis, E.; Gangemi, S. MIRNAs’ Cross-Involvement in Skin Allergies: A New Horizon for the Pathogenesis, Diagnosis and Therapy of Atopic Dermatitis, Allergic Contact Dermatitis and Chronic Spontaneous Urticaria. Biomedicines 2023, 11, 1266. [Google Scholar] [CrossRef]
- Quattrocki, E.A.; Friston, K.J. Autism, Oxytocin and Interoception. Neurosci. Biobehav. Rev. 2014, 47, 410–430. [Google Scholar] [CrossRef]
- Okoye, C.; Obialo-Ibeawuchi, C.M.; Obajeun, O.A.; Sarwar, S.; Tawfik, C.M.F.; Waleed, M.S.; Wasim, A.U.; Mohamoud, I.; Afolayan, A.Y.; Mbaezue, R.N. Early Diagnosis of Autism Spectrum Disorder: A Review and Analysis of the Risks and Benefits. Cureus 2023, 15, e43226. [Google Scholar] [CrossRef] [PubMed]
- Klin, A. Biomarkers in Autism Spectrum Disorder: Challenges, Advances, and the Need for Biomarkers of Relevance to Public Health. Focus 2018, 16, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Scandurra, V.; Gialloreti, L.E.; Barbanera, F.; Scordo, M.R.; Pierini, A.; Canitano, R. Neurodevelopmental Disorders and Adaptive Functions: A Study of Children with Autism Spectrum Disorders (ASD) and/or Attention Deficit and Hyperactivity Disorder (ADHD). Front. Psychiatry 2019, 10, 673. [Google Scholar] [CrossRef] [PubMed]
- Tonacci, A.; Pioggia, G.; Gangemi, S. Autism Spectrum Disorders and Atopic Dermatitis: A New Perspective from Country-Based Prevalence Data. Clin. Mol. Allergy 2021, 19, 27. [Google Scholar] [CrossRef]
- Billeci, L.; Tonacci, A.; Tartarisco, G.; Ruta, L.; Pioggia, G.; Gangemi, S. Association between Atopic Dermatitis and Autism Spectrum Disorders: A Systematic Review. Am. J. Clin. Dermatol. 2015, 16, 371–388. [Google Scholar] [CrossRef]
- Peterle, L.; Sanfilippo, S.; Tonacci, A.; Pomi, F.L.; Borgia, F.; Gangemi, S. Common Pathogenetic Traits of Atopic Dermatitis and Autism Spectrum Disorders, Potential Connections and Treatments: Trivial Th2 Inflammation or Much More? Front. Immunol. 2023, 14, 1201989. [Google Scholar] [CrossRef]
- Tonacci, A.; Bagnato, G.; Pandolfo, G.; Billeci, L.; Sansone, F.; Conte, R.; Gangemi, S. MicroRNA Cross-Involvement in Autism Spectrum Disorders and Atopic Dermatitis: A Literature Review. J. Clin. Med. 2019, 8, 88. [Google Scholar] [CrossRef]
- Papa, V.; Pomi, F.L.; Borgia, F.; Genovese, S.; Pioggia, G.; Gangemi, S. “Mens Sana in Cute Sana”—A State of the Art of Mutual Etiopathogenetic Influence and Relevant Pathophysiological Pathways between Skin and Mental Disorders: An Integrated Approach to Contemporary Psychopathological Scenarios. Cells 2023, 12, 1828. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Crumrine, D.; Kim, S.; Lee, Y.; Kim, B.; Abuabara, K.; Park, C.; Uchida, Y.; Wakefield, J.S.; Meyer, J.M.; et al. Phenotypic Overlap between Atopic Dermatitis and Autism. BMC Neurosci. 2021, 22, 43. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.R. Contributions of the Environment and Environmentally Vulnerable Physiology to Autism Spectrum Disorders. Curr. Opin. Neurol. 2010, 23, 103–110. [Google Scholar] [CrossRef]
- Kantor, R.; Silverberg, J.I. Environmental Risk Factors and Their Role in the Management of Atopic Dermatitis. Expert Rev. Clin. Immunol. 2016, 13, 15–26. [Google Scholar] [CrossRef]
- Lai, A.; Owens, K.; Patel, S.S.; Nicholas, M.W. The Impact of Air Pollution on Atopic Dermatitis. Curr. Allergy Asthma Rep. 2023, 23, 435–442. [Google Scholar] [CrossRef]
- Tsai, T.-N.; Wang, S.; Hsieh, C.; Wen, H.; Kuo, C.; Liu, H.; Sun, C.-W.; Chen, M.-L.; Wu, M.-T. Association between Prenatal Exposure to Metals and Atopic Dermatitis among Children Aged 4 Years in Taiwan. JAMA Netw. Open 2021, 4, e2131327. [Google Scholar] [CrossRef] [PubMed]
- Skogheim, T.S.; Weyde, K.V.F.; Engel, S.M.; Aase, H.; Surén, P.; Øie, M.G.; Biele, G.; Reichborn-Kjennerud, T.; Caspersen, I.H.; Hornig, M.; et al. Metal and Essential Element Concentrations during Pregnancy and Associations with Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder in Children. Environ. Int. 2021, 152, 106468. [Google Scholar] [CrossRef]
- Pham, C.; Symeonides, C.; O’Hely, M.; Sly, P.D.; Knibbs, L.D.; Thomson, S.; Vuillermin, P.; Saffery, R.; Ponsonby, A.; Author_Id, N. Early Life Environmental Factors Associated with Autism Spectrum Disorder Symptoms in Children at Age 2 Years: A Birth Cohort Study. Autism 2022, 26, 1864–1881. [Google Scholar] [CrossRef]
- Schettler, T. Human Exposure to Phthalates via Consumer Products. Andrology 2006, 29, 134–139. [Google Scholar] [CrossRef]
- Latini, G.; Verrotti, A.; De Felice, C. DI-2-Ethylhexyl Phthalate and Endocrine Disruption: A Review. Curr. Drug Targets—Immune Endocr. Metab. Disord. 2004, 4, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Whyatt, R.M.; Liu, X.; Rauh, V.; Calafat, A.M.; Just, A.C.; Hoepner, L.; Díaz, D.; Quinn, J.; Adibi, J.J.; Perera, F.P.; et al. Maternal Prenatal Urinary Phthalate Metabolite Concentrations and Child Mental, Psychomotor, and Behavioral Development at 3 Years of Age. Environ. Health Perspect. 2012, 120, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Sabir, S.; Rehman, K. Bisphenol A-Induced Metabolic Disorders: From Exposure to Mechanism of Action. Environ. Toxicol. Pharmacol. 2020, 77, 103373. [Google Scholar] [CrossRef]
- Testa, C.; Nuti, F.; Hayek, J.; De Felice, C.; Chelli, M.; Rovero, P.; Latini, G.; Papini, A.M. Di-(2-Ethylhexyl) Phthalate and Autism Spectrum Disorders. ASN Neuro 2012, 4, AN20120015. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, J.; Zheng, G.; Liang, J.; Hu, B.; Lou, Z.-Q.; Li, A.; Ding, Y. Prenatal Exposure to Di (2-Ethylhexyl) Phthalate Causes Autism-like Behavior through Inducing Nischarin Expression in the Mouse Offspring. Biochem. Biophys. Res. Commun. 2021, 585, 29–35. [Google Scholar] [CrossRef]
- Nadeem, A.; Al-Harbi, N.O.; Ahmad, S.F.; Alhazzani, K.; Attia, S.M.; Alsanea, S.; Alhoshani, A.; Mahmood, H.M.; Alfardan, A.S.; Bakheet, S.A. Exposure to the Plasticizer, Di-(2-Ethylhexyl) Phthalate during Juvenile Period Exacerbates Autism-like Behavior in Adult BTBR T + Tf/J Mice Due to DNA Hypomethylation and Enhanced Inflammation in Brain and Systemic Immune Cells. Prog Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110249. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Yanagisawa, R.; Inoue, K.; Ichinose, T.; Sadakane, K.; Yoshikawa, T. Di-(2-Ethylhexyl) Phthalate Enhances Atopic Dermatitis-Like Skin Lesionsin Mice. Environ. Health Perspect. 2006, 114, 1266–1269. [Google Scholar] [CrossRef]
- Yanagisawa, R.; Takano, H.; Inoue, K.; Koike, E.; Sadakane, K.; Ichinose, T. Effects of Maternal Exposure to Di-(2-Ethylhexyl) Phthalate during Fetal and/or Neonatal Periods on Atopic Dermatitis in Male Offspring. Environ. Health Perspect. 2008, 116, 1136–1141. [Google Scholar] [CrossRef]
- Choi, W.-J.; Kwon, H.-J.; Hong, S.; Lim, W.R.; Kim, H.-J.; Kim, J.-H.; Kim, C.-B.; Kim, K. Potential Nonmonotonous Association between Di(2-ethylhexyl) Phthalate Exposure and Atopic Dermatitis in K Orean Children. Br. J. Dermatol. 2014, 171, 854–860. [Google Scholar] [CrossRef]
- Sweeten, T.L.; Posey, D.J.; Shankar, S.; McDougle, C.J. High Nitric Oxide Production in Autistic Disorder: A Possible Role for Interferon-γ. Biol. Psychiatry 2004, 55, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Fluegge, K. Comment on: “Association between Atopic Dermatitis and Autism Spectrum Disorders: A Systematic Review. Am. J. Clin. Dermatol. 2016, 17, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Uchida, Y.; Moradian, S.; Crumrine, D.; Elias, P.M.; Bikle, D.D. Vitamin D Receptor and Coactivators SRC2 and 3 Regulate Epidermis-Specific Sphingolipid Production and Permeability Barrier Formation. J. Investig. Dermatol. 2009, 129, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Shan, L.; Du, L.; Feng, J.; Xu, Z.; Staal, W.; Jia, F. Serum Concentration of 25-Hydroxyvitamin D in Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Eur. Child Adolesc. Psychiatry 2015, 25, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Hattangdi-Haridas, S.R.; Lanham-New, S.A.; Wong, W.H.S.; Ho, M.H.K.; Darling, A.L. Vitamin D Deficiency and Effects of Vitamin D Supplementation on Disease Severity in Patients with Atopic Dermatitis: A Systematic Review and Meta-Analysis in Adults and Children. Nutrients 2019, 11, 1854. [Google Scholar] [CrossRef] [PubMed]
- Sidbury, R.; Sullivan, A.; Thadhani, R.; Camargo, C.A. Randomized Controlled Trial of Vitamin D Supplementation for Winter-Related Atopic Dermatitis in Boston: A Pilot Study. Br. J. Dermatol. 2008, 159, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A.; Ganmaa, D.; Sidbury, R.; Erdenedelger, K.; Radnaakhand, N.; Khandsuren, B. Randomized Trial of Vitamin D Supplementation for Winter-Related Atopic Dermatitis in Children. J. Allergy Clin. Immunol. 2014, 134, 831–835.e1. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Riley, A.W.; Volk, H.E.; Caruso, D.; Hironaka, L.K.; Sices, L.; Hong, X.; Wang, G.; Ji, Y.; Brucato, M.; et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr. Perinat. Epidemiol. 2017, 32, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Dou, L.; Zhang, Y.; Dou, Y.; Zhao, P.; Jiang, Y.; Gao, W.; Ji, M.; He, L.; Niu, D.; et al. Maternal Periconceptional Folate Status and Infant Atopic Dermatitis: A Prospective Cohort Study. Pediatr. Allergy Immunol. 2020, 32, 137–145. [Google Scholar] [CrossRef]
- Levine, S.Z.; Kodesh, A.; Viktorin, A.; Smith, L.A.; Uher, R.; Reichenberg, A.; Sandin, S. Association of Maternal Use of Folic Acid and Multivitamin Supplements in the Periods before and during Pregnancy with the Risk of Autism Spectrum Disorder in Offspring. JAMA Psychiatry 2018, 75, 176. [Google Scholar] [CrossRef]
- Kang, C.-M.; Chiang, B.; Wang, L. Maternal Nutritional Status and Development of Atopic Dermatitis in Their Offspring. Clin. Rev. Allergy Immunol. 2020, 61, 128–155. [Google Scholar] [CrossRef] [PubMed]
- Urbonaite, G.; Knyzeliene, A.; Bunn, F.S.; Smalskys, A.; Neniskyte, U. The Impact of Maternal High-Fat Diet on Offspring Neurodevelopment. Front. Neurosci. 2022, 16, 909762. [Google Scholar] [CrossRef]
- Roullet, F.; Lai, J.; Foster, J.A. In Utero Exposure to Valproic Acid and Autism—A Current Review of Clinical and Animal Studies. Neurotoxicol. Teratol. 2013, 36, 47–56. [Google Scholar] [CrossRef]
- Wu, X.; Hong, P.; Suolang, D.J.; Zhou, D.; Stefan, H. Drug-Induced Hypersensitivity Syndrome Caused by Valproic Acid as a Monotherapy for Epilepsy: First Case Report in Asian Population. Epilepsy Behav. Rep. 2017, 8, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Kashyap, A.; Undela, K. Valproic Acid and Stevens-Johnson Syndrome: A Systematic Review of Descriptive Studies. Int. J. Dermatol. 2019, 58, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- De Luca, F.; Losappio, L.; Mirone, C.; Schroeder, J.W.; Citterio, A.; Aversano, M.G.; Scibilia, J.; Pastorello, E.A. Tolerated Drugs in Subjects with Severe Cutaneous Adverse Reactions (SCARs) Induced by Anticonvulsants and Review of the Literature. Clin. Mol. Allergy 2017, 15, 16. [Google Scholar] [CrossRef]
- Moore, S.; Turnpenny, P.D.; Quinn, A.G.; Glover, S.J.; Lloyd, D.J.; Montgomery, T.; Dean, J. A Clinical Study of 57 Children with Fetal Anticonvulsant Syndromes. J. Med. Genet. 2000, 37, 489–497. [Google Scholar] [CrossRef]
- Dean, J.; Hailey, H.; Moore, S.; Lloyd, D.J.; Turnpenny, P.D.; Little, J. Long Term Health and Neurodevelopment in Children Exposed to Antiepileptic Drugs before Birth. J. Med. Genet. 2002, 39, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, G.A.; Al-Ayadhi, L. The Possible Relationship between Allergic Manifestations and Elevated Serum Levels of Brain Specific Auto-Antibodies in Autistic Children. J. Neuroimmunol. 2013, 261, 77–81. [Google Scholar] [CrossRef]
- Mostafa, G.A.; Hamza, R.T.; Elshahawi, H.H. Allergic Manifestations in Autistic Children: Relation to Disease Severity. J. Pediatr. Neurol. 2015, 6, 115–123. [Google Scholar] [CrossRef]
- Mostafa, G.A.; El-Sherif, D.F.; Hamza, R.T.; Shehab, A.A. Hyperserotonemia in Egyptian Autistic Children: Relation to Allergic Manifestations. J. Pediatr. Neurol. 2015, 6, 227–236. [Google Scholar] [CrossRef]
- Schopler, E.; Reichler, R.J.; DeVellis, R.F.; Daly, K. Toward Objective Classification of Childhood Autism: Childhood Autism Rating Scale (CARS). J. Autism Dev. Disord. 1980, 10, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Hitomi, Y.; Kambayashi, Y.; Hibino, Y.; Yamazaki, M.; Mitoma, J.; Asakura, H.; Hayashi, K.; Otaki, N.; Sagara, T.; et al. Epidemiological Study on the Involvements of Environmental Factors and Allergy in Child Mental Health Using the Autism Screening Questionnaire. Res. Autism Spectr. Disord. 2013, 7, 132–140. [Google Scholar] [CrossRef]
- Jameson, C.; Boulton, K.A.; Silove, N.; Guastella, A.J. Eczema and Related Atopic Diseases Are Associated with Increased Symptom Severity in Children with Autism Spectrum Disorder. Transl. Psychiatry 2022, 12, 415. [Google Scholar] [CrossRef] [PubMed]
- Esler, A.; Bal, V.H.; Guthrie, W.S.; Wetherby, A.M.; Weismer, S.E.; Lord, C. The Autism Diagnostic Observation Schedule, Toddler Module: Standardized Severity Scores. J. Autism Dev. Disord. 2015, 45, 2704–2720. [Google Scholar] [CrossRef] [PubMed]
- Tin, P.; Koudelka, C.; Simpson, E.L. Mental Health Comorbidity in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2013, 131, 428–433. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Esposito, M.; Gisondi, P.; Valenti, M.; Gori, N.; Giovanardi, G.; Bellinato, F.; De Simone, C.; Costanzo, A.; Fargnoli, M.C.; et al. Disease Severity Is Associated with Alexithymia in Patients with Atopic Dermatitis. Dermatology 2020, 236, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.C.; Lien, Y.T.; Wang, S.; Huang, S.L.; Chen, C.Y. Comorbidity of Atopic Disorders with Autism Spectrum Disorder and Attention Deficit/Hyperactivity Disorder. J. Pediatr. 2016, 171, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Shin, D.B.; Syed, M.; Abuabara, K.; Lemeshow, A.R.; Gelfand, J.M. Atopic Dermatitis and Risk of Major Neuropsychiatric Disorders in Children: A Population-based Cohort Study. J. Eur. Acad. Dermatol. Venereol. 2022, 37, 114–122. [Google Scholar] [CrossRef]
- Sumimoto, S.; Kawai, M.; Kasajima, Y.; Hamamoto, T. Increased Plasma Tumour Necrosis Factor-Alpha Concentration in Atopic Dermatitis. Arch. Dis. Child. 1992, 67, 277–279. [Google Scholar] [CrossRef]
- Xie, J.; Huang, L.; Li, X.; Li, H.; Zhou, Y.; Zhu, H.; Pan, T.; Kendrick, K.M.; Xu, W. Immunological Cytokine Profiling Identifies TNF-α as a Key Molecule Dysregulated in Autistic Children. Oncotarget 2017, 8, 82390–82398. [Google Scholar] [CrossRef] [PubMed]
- Danso, M.O.; Van Drongelen, V.; Mulder, A.A.; Van Esch, J.; Scott, H.C.; Van Smeden, J.; Ghalbzouri, A.E.; Bouwstra, J.A. TNF-A and TH2 Cytokines Induce Atopic Dermatitis–Like Features on Epidermal Differentiation Proteins and Stratum Corneum Lipids in Human Skin Equivalents. J. Investig. Dermatol. 2014, 134, 1941–1950. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Liu, S.; Luo, W.; Jiang, Y.; Gao, J. Association of Peripheral Blood Levels of Cytokines with Autism Spectrum Disorder: A Meta-Analysis. Front. Psychiatry 2021, 12, 670200. [Google Scholar] [CrossRef]
- Xu, G.; Strathearn, L.; Liu, B.; Bao, W. Prevalence of Autism Spectrum Disorder among US Children and Adolescents, 2014-2016. JAMA 2018, 319, 81. [Google Scholar] [CrossRef] [PubMed]
- Visser, S.N.; Danielson, M.L.; Bitsko, R.H.; Holbrook, J.; Kogan, M.D.; Ghandour, R.M.; Perou, R.; Blumberg, S.J. Trends in the Parent-Report of Health Care Provider-Diagnosed and Medicated Attention-Deficit/Hyperactivity Disorder: United States, 2003–2011. J. Am. Acad. Child. Adolesc. Psychiatry 2014, 53, 34–46.e2. [Google Scholar] [CrossRef]
- Bakkaloglu, B.; Anlar, B.; Anlar, F.Y.; Öktem, F.; Pehlivantürk, B.; Ünal, F.; Özbesler, C.; Gökler, B. Atopic Features in Early Childhood Autism. Eur. J. Paediatr. Neurol. 2008, 12, 476–479. [Google Scholar] [CrossRef]
- Schmitt, J.; Buske-Kirschbaum, A.; Roessner, V. Is Atopic Disease a Risk Factor for Attention-deficit/Hyperactivity Disorder? A Systematic Review. Allergy 2010, 65, 1506–1524. [Google Scholar] [CrossRef]
- Tsai, J.-D.; Chang, S.; Mou, C.-H.; Sung, F.; Lue, K. Association between Atopic Diseases and Attention-Deficit/Hyperactivity Disorder in Childhood: A Population-Based Case-Control Study. Ann. Epidemiol. 2013, 23, 185–188. [Google Scholar] [CrossRef]
- Buske-Kirschbaum, A.; Schmitt, J.; Plessow, F.; Romanos, M.; Weidinger, S.; Roessner, V. Psychoendocrine and Psychoneuroimmunological Mechanisms in the Comorbidity of Atopic Eczema and Attention Deficit/Hyperactivity Disorder. Psychoneuroendocrinology 2013, 38, 12–23. [Google Scholar] [CrossRef]
- Hu, C.-C.; Xu, X.; Xiong, G.; Xu, Q.; Zhou, B.; Li, C.; Qi, Q.; Liu, C.; Li, H.; Sun, Y.; et al. Alterations in Plasma Cytokine Levels in Chinese Children with Autism Spectrum Disorder. Autism Res. 2018, 11, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Angriman, M.; Comencini, E.; Vincenzi, B.; Maffeis, C. Association between Inflammatory Cytokines and ADHD Symptoms in Children and Adolescents with Obesity: A Pilot Study. Psychiatry Res. 2019, 278, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Van Der Schans, J.; Çiçek, R.; De Vries, T.W.; Hak, E.; Hoekstra, P.J. Association of Atopic Diseases and Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analyses. Neurosci. Biobehav. Rev. 2017, 74, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, C.; Koyama, M.; Ota, E.; Swa, T.; Mlunde, L.B.; Amiya, R.M.; Tachibana, Y.; Yamamoto-Hanada, K.; Mori, R. Allergic Diseases in Children with Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis. BMC Psychiatry 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Genuneit, J.; Braig, S.; Brandt, S.; Wabitsch, M.; Florath, I.; Brenner, H.; Rothenbacher, D. Infant Atopic Eczema and Subsequent Attention-deficit/Hyperactivity Disorder—A Prospective Birth Cohort Study. Pediatr. Allergy Immunol. 2013, 25, 51–56. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Chen, M.H.; Jeng, M.J.; Hsu, J.W.; Tsai, S.J.; Bai, Y.M.; Hung, G.Y.; Yen, H.J.; Chen, T.J.; Su, T.P. Longitudinal Association between Early Atopic Dermatitis and Subsequent Attention-Deficit or Autistic Disorder. Medicine 2016, 95, e5005. [Google Scholar] [CrossRef]
- Dirven-Meijer, P.C.; Glazenburg, E.J.; Mulder, P.; Oranje, A.P. Prevalence of Atopic Dermatitis in Children Younger than 4 Years in a Demarcated Area in Central Netherlands: The West Veluwe Study Group. Br. J. Dermatol. 2008, 158, 846–847. [Google Scholar] [CrossRef]
- Saeki, H.; Tsunemi, Y.; Fujita, H.; Kagami, S.; Sasaki, K.; Ohmatsu, H.; Watanabe, A.; Tamaki, K. Prevalence of Atopic Dermatitis Determined by Clinical Examination in Japanese Adults. J. Dermatol. 2006, 33, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.K.; Bergström, A.; Kull, I.; Melén, E.; Jonsson, M.; Lundin, S.; Wahlgren, C.-F.; Ballardini, N. Prevalence and Characteristics of Atopic Dermatitis among Young Adult Females and Males—Report from the Swedish Population-based Study BAMSE. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 698–704. [Google Scholar] [CrossRef]
- Kanda, N.; Hoashi, T.; Saeki, H. The Roles of Sex Hormones in the Course of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 4660. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Lee, J.M.; Moon, H.E.; Lee, D.H.; Kim, B.N.; Kim, J.; Kim, D.G.; Paek, S.H. A Short Review on the Current Understanding of Autism Spectrum Disorders. Exp. Neurobiol. 2016, 25, 1–13. [Google Scholar] [CrossRef]
- Bargiela, S.; Steward, R.; Mandy, W. The Experiences of Late-Diagnosed Women with Autism Spectrum Conditions: An Investigation of the Female Autism Phenotype. J. Autism Dev. Disord. 2016, 46, 3281–3294. [Google Scholar] [CrossRef] [PubMed]
- Giarelli, E.; Wiggins, L.D.; Rice, C.E.; Levy, S.E.; Kirby, R.S.; Pinto-Martin, J.; Mandell, D.S. Sex Differences in the Evaluation and Diagnosis of Autism Spectrum Disorders among Children. Disabil. Health J. 2010, 3, 107–116. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Lombardo, M.; Auyeung, B.; Ashwin, E.; Chakrabarti, B.; Knickmeyer, R. Why Are Autism Spectrum Conditions More Prevalent in Males? PLoS Biol. 2011, 9, e1001081. [Google Scholar] [CrossRef] [PubMed]
- Ferri, S.L.; Brodkin, E.S. Sex Differences in Autism Spectrum Disorder: A Review. Curr. Psychiatry Rep. 2018, 20, 9. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Auyeung, B.; Nørgaard-Pedersen, B.; Hougaard, D.M.; Abdallah, M.; Melgaard, L.; Cohen, A.; Chakrabarti, B.; Ruta, L.; Lombardo, M. Elevated Fetal Steroidogenic Activity in Autism. Mol. Psychiatry 2014, 20, 369–376. [Google Scholar] [CrossRef]
- Mueller, B.; Bale, T.L. Sex-Specific Programming of Offspring Emotionality after Stress Early in Pregnancy. J. Neurosci. 2008, 28, 9055–9065. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Knickmeyer, R.; Belmonte, M.K. Sex Differences in the Brain: Implications for Explaining Autism. Science 2005, 310, 819–823. [Google Scholar] [CrossRef]
- Pfaff, D.W.; Rapin, I.; Goldman, S. Male Predominance in Autism: Neuroendocrine Influences on Arousal and Social Anxiety. Autism Res. 2011, 4, 163–176. [Google Scholar] [CrossRef]
- Liu, N.; Yang, H.-P.; Han, L.; Ma, M. Oxytocin in Women’s Health and Disease. Front. Endocrinol. 2022, 13, 786271. [Google Scholar] [CrossRef]
- Chen, M.H.; Su, T.P.; Chen, Y.S.; Hsu, J.W.; Huang, K.; Chang, W.H.; Chen, T.J.; Pan, T.; Bai, Y.M. Is Atopy in Early Childhood a Risk Factor for ADHD and ASD? A Longitudinal Study. J. Psychosom. Res. 2014, 77, 316–321. [Google Scholar] [CrossRef]
- Lin, T.-C.; Lin, P.Y.; Su, T.P.; Chen, Y.S.; Hsu, J.W.; Huang, K.; Chang, W.H.; Chen, T.J.; Pan, T.; Chen, M.H.; et al. Autistic Spectrum Disorder, Attention Deficit Hyperactivity Disorder, and Allergy: Is There a Link? A Nationwide Study. Res. Autism Spectr. Disord. 2014, 8, 1333–1338. [Google Scholar] [CrossRef]
- Qu, X.; Lee, L.; Ladd-Acosta, C.; Hong, X.; Ji, Y.; Kalb, L.G.; Volk, H.E.; Wang, X. Association between Atopic Diseases and Neurodevelopmental Disabilities in a Longitudinal Birth Cohort. Autism Res. 2022, 15, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Nemet, S.; Asher, I.; Yoles, I.; Baevsky, T.; Sthoeger, Z. Early Childhood Allergy Linked with Development of Attention Deficit Hyperactivity Disorder and Autism Spectrum Disorder. Pediatr. Allergy Immunol. 2022, 33. [Google Scholar] [CrossRef]
- Rosenberg, R.E.; Law, J.K.; Yenokyan, G.; McGready, J.; Kaufmann, W.E.; Law, P.A. Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch. Pediatr. Adolesc. Med. 2009, 163, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.M.; Challman, T.D.; Bernier, R.; Bourgeron, T.; Chung, W.K.; Constantino, J.N.; Eichler, E.E.; Jacquemont, S.; Miller, D.T.; Mitchell, K.J.; et al. Insufficient Evidence for “Autism-Specific” Genes. Am. J. Hum. Genet. 2020, 106, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Sebat, J.; Lakshmi, B.; Malhotra, D.; Troge, J.; Lese-Martin, C.; Walsh, T.; Yamrom, B.; Yoon, S.; Krasnitz, A.; Kendall, J.; et al. Strong association of de novo copy number mutations with autism. Science 2007, 316, 445–449. [Google Scholar] [CrossRef]
- Levy, D.; Ronemus, M.; Yamrom, B.; Lee, Y.H.; Leotta, A.; Kendall, J.; Marks, S.; Lakshmi, B.; Pai, D.; Ye, K.; et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron 2011, 70, 886–897. [Google Scholar] [CrossRef]
- Sanders, S.J.; Ercan-Sencicek, A.G.; Hus, V.; Luo, R.; Murtha, M.T.; Moreno-De-Luca, D.; Chu, S.H.; Moreau, M.P.; Gupta, A.R.; Thomson, S.A.; et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 2011, 70, 863–885. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, H.; Ma, D.; Bucan, M.; Glessner, J.T.; Abrahams, B.S.; Salyakina, D.; Imielinski, M.; Bradfield, J.P.; Sleiman, P.M.; et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature 2009, 459, 528–533. [Google Scholar] [CrossRef]
- Butler, M.G.; Rafi, S.K.; Manzardo, A.M. High-resolution chromosome ideogram representation of currently recognized genes for autism spectrum disorders. Int. J. Mol. Sci. 2015, 16, 6464–6495. [Google Scholar] [CrossRef]
- Williams, L.A.; LaSalle, J.M. Future Prospects for Epigenetics in Autism Spectrum Disorder. Mol. Diagn. Ther. 2022, 26, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Bin, L.; Leung, D.Y.M. Genetic and epigenetic studies of atopic dermatitis. Allergy Asthma Clin. Immunol. 2016, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- De Pietri Tonelli, D.; Pulvers, J.N.; Haffner, C.; Murchison, E.P.; Hannon, G.J.; Huttner, W.B. miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development 2008, 135, 3911–3921. [Google Scholar] [CrossRef]
- Mannucci, C.; Casciaro, M.; Minciullo, P.L.; Calapai, G.; Navarra, M.; Gangemi, S. Involvement of microRNAs in skin disorders: A literature review. Allergy Asthma Proc. 2017, 38, 9–15. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Lepleux, M.; Makhlouf, M.; Martin, C.; Fregeac, J.; Siquier-Pernet, K.; Philippe, A.; Feron, F.; Gepner, B.; Rougeulle, C.; et al. Profiling olfactory stem cells from living patients identifies miRNAs relevant for autism pathophysiology. Mol. Autism 2016, 7, 1. [Google Scholar] [CrossRef]
- Rebane, A.; Runnel, T.; Aab, A.; Maslovskaja, J.; Rückert, B.; Zimmermann, M.; Plaas, M.; Kärner, J.; Treis, A.; Pihlap, M.; et al. MicroRNA-146a alleviates chronic skin inflammation in atopic dermatitis through suppression of innate immune responses in keratinocytes. J. Allergy Clin. Immunol. 2014, 134, 836–847.e11. [Google Scholar] [CrossRef]
- Sonkoly, E.; Janson, P.; Majuri, M.L.; Savinko, T.; Fyhrquist, N.; Eidsmo, L.; Xu, N.; Meisgen, F.; Wei, T.; Bradley, M.; et al. MiR-155 is overexpressed in patients with atopic dermatitis and modulates T-cell proliferative responses by targeting cytotoxic T lymphocyte-associated antigen 4. J. Allergy Clin. Immunol. 2010, 126, 581–589.e20. [Google Scholar] [CrossRef]
- Wei, H.; Zou, H.; Sheikh, A.M.; Malik, M.; Dobkin, C.; Brown, W.T.; Li, X. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J. Neuroinflamm. 2011, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.H.; Kreider, C.M.; Brasher, S.N.; Ansell, M. Clinical impact of early diagnosis of autism on the prognosis and parent-child relationships. Psychol. Res. Behav. Manag. 2017, 10, 283–292. [Google Scholar] [CrossRef]
- Briguglio, M.; Turriziani, L.; Currò, A.; Gagliano, A.; Di Rosa, G.; Caccamo, D.; Tonacci, A.; Gangemi, S. A Machine Learning Approach to the Diagnosis of Autism Spectrum Disorder and Multi-Systemic Developmental Disorder Based on Retrospective Data and ADOS-2 Score. Brain Sci. 2023, 13, 883. [Google Scholar] [CrossRef]

| Clinical Studies on ASD Severity in AD Patients | Method Used to Assess ASD Severity |
| Mostafa et al. (2008–2013) [49,50,51] | Japanese version of the Autism Screening Questionnaire (ASQ) |
| Shibata et al. (2013) [53] | Childhood Autism Rating Scale (CARS) |
| Jameson et al. (2022) [54] | ADOS-2 calibrated severity scores |
| Clinical studies on AD severity in ASD patients | Method used to assess AD severity |
| Yaghmaie et al. (2013) [56] | Assessed by the patient’s parent/guardian by posing the question: ‘‘Would you describe [his/her] eczema or skin allergy as mild, moderate, or severe?’’ |
| Liao et al. (2016) [58] | Number of clinical visits for AD the patients underwent under the age of two (1, 2–3, or 4 or more) |
| Wan et al. (2023) [59] | Based on treatment used, AD was classified as
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casella, R.; Miniello, A.; Buta, F.; Yacoub, M.-R.; Nettis, E.; Pioggia, G.; Gangemi, S. Atopic Dermatitis and Autism Spectrum Disorders: Common Role of Environmental and Clinical Co-Factors in the Onset and Severity of Their Clinical Course. Int. J. Mol. Sci. 2024, 25, 8936. https://doi.org/10.3390/ijms25168936
Casella R, Miniello A, Buta F, Yacoub M-R, Nettis E, Pioggia G, Gangemi S. Atopic Dermatitis and Autism Spectrum Disorders: Common Role of Environmental and Clinical Co-Factors in the Onset and Severity of Their Clinical Course. International Journal of Molecular Sciences. 2024; 25(16):8936. https://doi.org/10.3390/ijms25168936
Chicago/Turabian StyleCasella, Rossella, Andrea Miniello, Federica Buta, Mona-Rita Yacoub, Eustachio Nettis, Giovanni Pioggia, and Sebastiano Gangemi. 2024. "Atopic Dermatitis and Autism Spectrum Disorders: Common Role of Environmental and Clinical Co-Factors in the Onset and Severity of Their Clinical Course" International Journal of Molecular Sciences 25, no. 16: 8936. https://doi.org/10.3390/ijms25168936
APA StyleCasella, R., Miniello, A., Buta, F., Yacoub, M.-R., Nettis, E., Pioggia, G., & Gangemi, S. (2024). Atopic Dermatitis and Autism Spectrum Disorders: Common Role of Environmental and Clinical Co-Factors in the Onset and Severity of Their Clinical Course. International Journal of Molecular Sciences, 25(16), 8936. https://doi.org/10.3390/ijms25168936








