Construct, Face, and Predictive Validity of Parkinson’s Disease Rodent Models
Abstract
1. Introduction
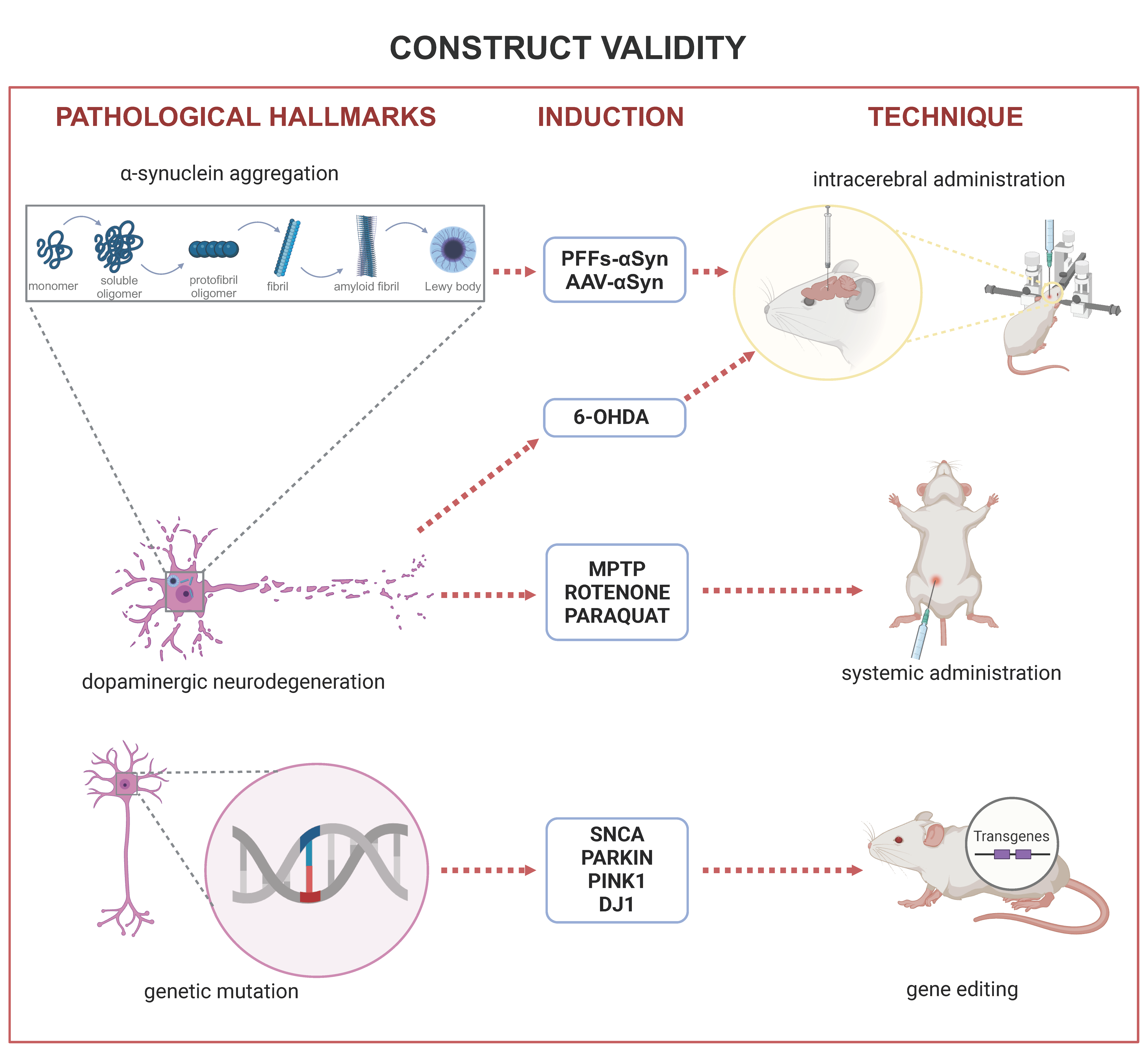
2. Construct Validity
2.1. Degeneration of Dopaminergic Neurons
2.2. Lewy Bodies
2.3. Gene Mutations
2.3.1. SNCA
2.3.2. PARKIN
2.3.3. PINK1
2.3.4. DJ-1
2.3.5. Genetic Variability and Clinical Trial
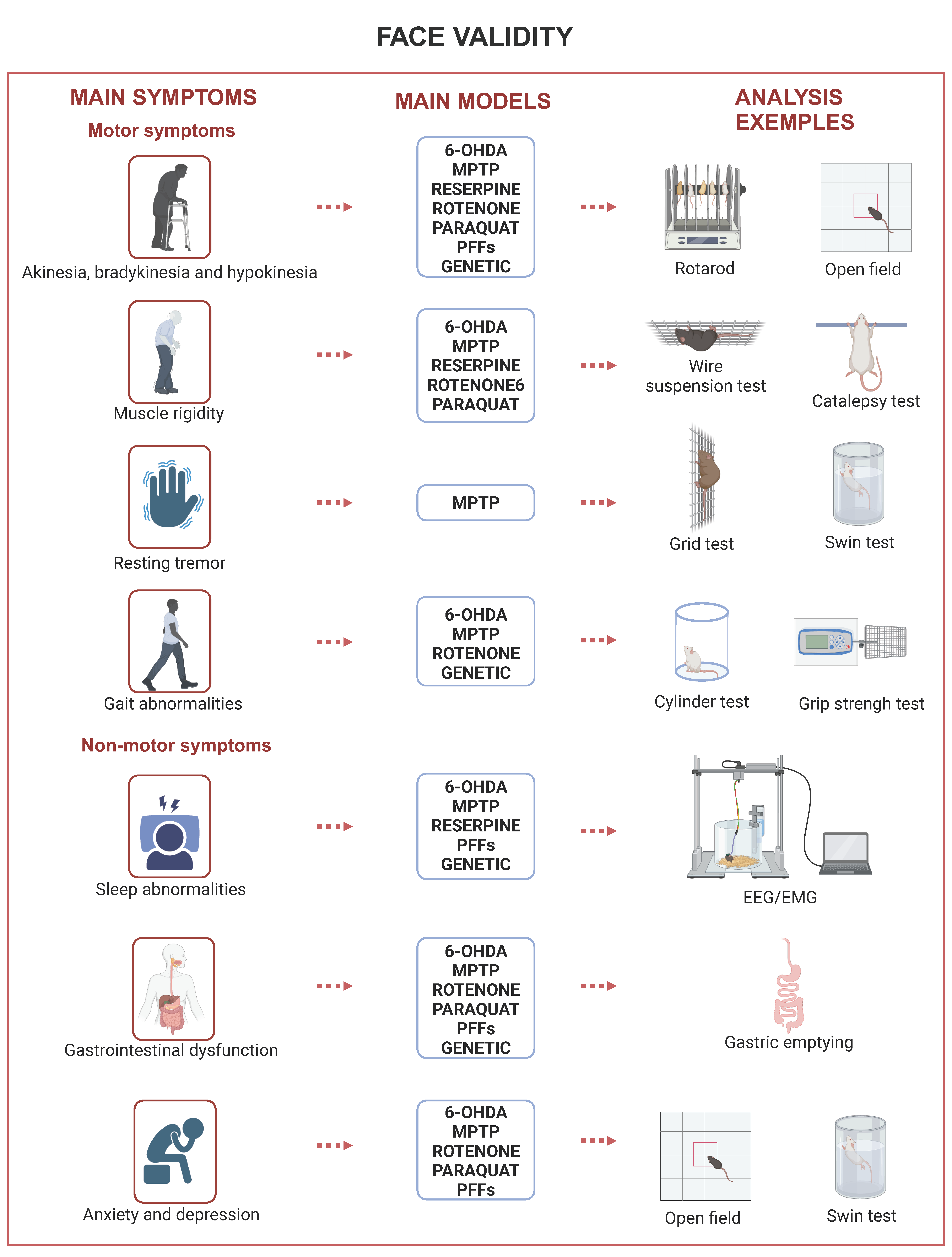
3. Face Validity
3.1. Motor Symptoms
3.1.1. Akinesia, Bradykinesia, and Hypokinesia
3.1.2. Muscle Rigidity
3.1.3. Resting Tremors
3.1.4. Gait Abnormalities
3.2. Non-Motor Symptoms
3.2.1. Sleep Abnormalities
3.2.2. Gastrointestinal Dysfunction
3.2.3. Anxiety and Depression
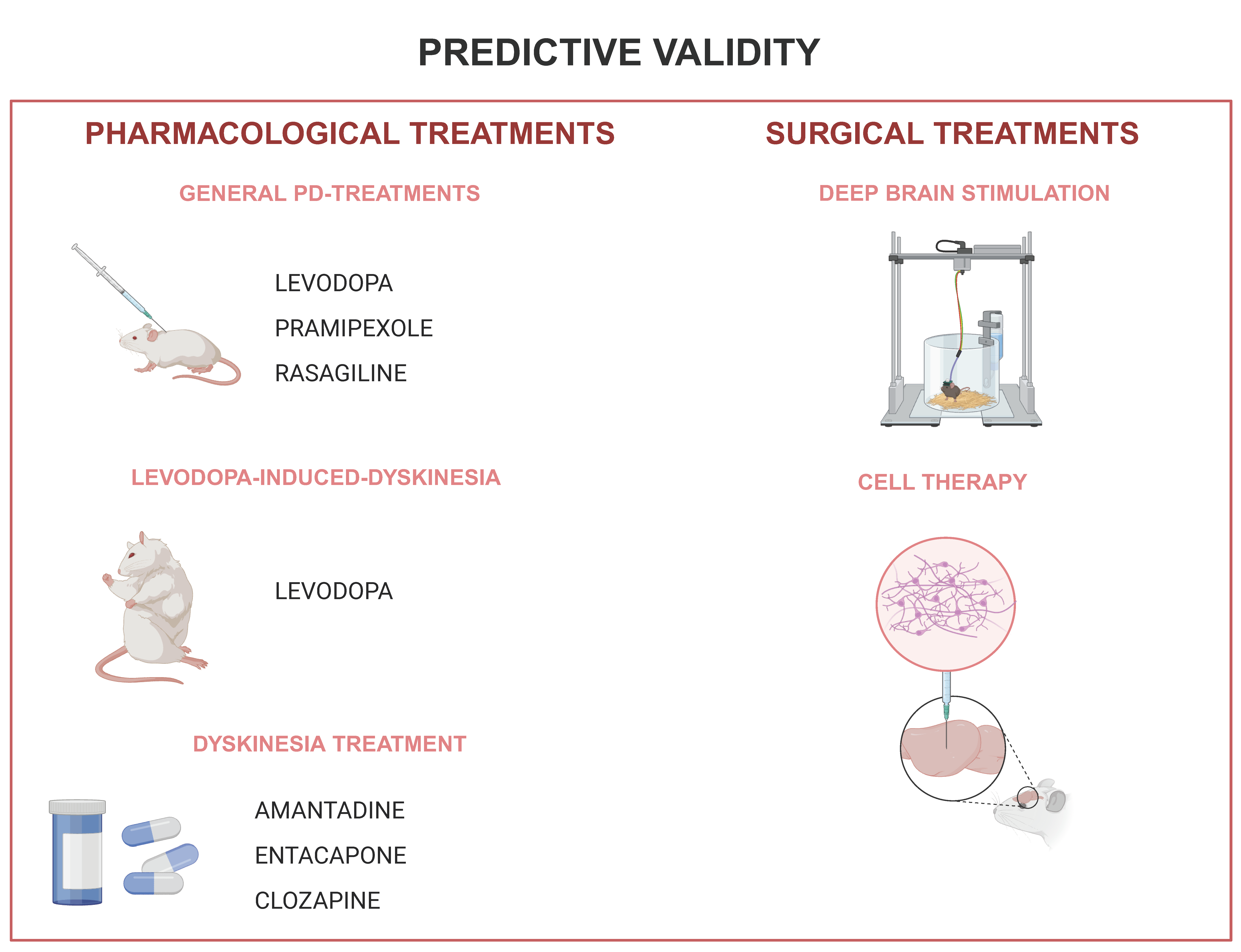
4. Predictive Validity
4.1. Pharmacological Treatments
4.2. Treatment Adverse Effect
4.3. Surgical Treatments
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Gibb, W.R.; Lees, A.J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 745–752. [Google Scholar] [CrossRef]
- Ramesh, S.; Arachchige, A.S.P.M. Depletion of dopamine in Parkinson’s disease and relevant therapeutic options: A review of the literature. AIMS Neurosci. 2023, 10, 200–231. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Raymick, J.; Imam, S. Neuroprotective and Therapeutic Strategies against Parkinson’s Disease: Recent Perspectives. Int. J. Mol. Sci. 2016, 17, 904. [Google Scholar] [CrossRef] [PubMed]
- Kouli, A.; Torsney, K.M.; Kuan, W.-L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Codon Publications: Brisbane, Australia, 2018; pp. 3–26. [Google Scholar]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.P.; Ribeiro, D.L.; Dos Santos, K.B.; Padovan-Neto, F.E. The 6-hydroxydopamine rat model of Parkinson’s disease. J. Vis. Exp. 2021, 176, e62923. [Google Scholar] [CrossRef]
- Kin, K.; Yasuhara, T.; Kameda, M.; Date, I. Animal Models for Parkinson’s Disease Research: Trends in the 2000s. Int. J. Mol. Sci. 2019, 20, 5402. [Google Scholar] [CrossRef]
- Ke, M.; Chong, C.-M.; Zhu, Q.; Zhang, K.; Cai, C.Z.; Lu, J.H.; Qin, D.; Su, H. Comprehensive Perspectives on Experimental Models for Parkinson’s Disease. Aging Dis. 2021, 12, 223–246. [Google Scholar] [CrossRef]
- Manning-BoĞ, A.B.; Langston, J.W. Model fusion, the next phase in developing animal models for Parkinson’s disease. Neurotox. Res. 2007, 11, 219–240. [Google Scholar] [CrossRef]
- Willner, P. Validation criteria for animal models of human mental disorders: Learned helplessness as a paradigm case. Prog. Neuropsychopharmacol. Biol. Psychiatry 1986, 10, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Thiele, S.L.; Warre, R.; Nash, J.E. Development of a Unilaterally-lesioned 6-OHDA Mouse Model of Parkinson’s Disease. J. Vis. Exp. 2012, 60, e3234. [Google Scholar] [CrossRef]
- Decressac, M.; Mattsson, B.; Björklund, A. Comparison of the behavioural and histological characteristics of the 6-OHDA and α-synuclein rat models of Parkinson’s disease. Exp. Neurol. 2012, 235, 306–315. [Google Scholar] [CrossRef]
- Slézia, A.; Hegedüs, P.; Rusina, E.; Lengyel, K.; Solari, N.; Kaszas, A.; Balázsfi, D.; Botzanowski, B.; Acerbo, E.; Missey, F.; et al. Behavioral, neural and ultrastructural alterations in a graded-dose 6-OHDA mouse model of early-stage Parkinson’s disease. Sci. Rep. 2023, 13, 19478. [Google Scholar] [CrossRef]
- Gubinelli, F.; Sarauskyte, L.; Venuti, C.; Kulacz, I.; Cazzolla, G.; Negrini, M.; Anwer, D.; Vecchio, I.; Jakobs, F.; Manfredsson, F.P.; et al. Characterisation of functional deficits induced by AAV overexpression of alpha-synuclein in rats. Curr. Res. Neurobiol. 2023, 4, 100065. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.S.; Geng, W.S.; Jia, J.J. Neurotoxin-Induced Animal Models of Parkinson Disease: Pathogenic Mechanism and Assessment. ASN Neuro 2018, 10, 1759091418777438. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Q.; Wang, B.; Shen, T.; Luo, W.; Liu, T. Preclinical reserpine models recapitulating motor and non-motor features of Parkinson’s disease: Roles of epigenetic upregulation of alpha-synuclein and autophagy impairment. Front. Pharmacol. 2022, 13, 944376. [Google Scholar] [CrossRef]
- Leão, A.H.F.F.; Sarmento-Silva, A.J.; Santos, J.R.; Ribeiro, A.M.; Silva, R.H. Molecular, Neurochemical, and Behavioral Hallmarks of Reserpine as a Model for Parkinson’s Disease: New Perspectives to a Long-Standing Model. Brain Pathol. 2015, 25, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Lama, J.; Buhidma, Y.; Fletcher, E.J.R.; Duty, S. Animal models of Parkinson’s disease: A guide to selecting the optimal model for your research. Neuronal Signal. 2021, 5, NS20210026. [Google Scholar] [CrossRef]
- Cannon, J.R.; Tapias, V.; Na, H.M.; Honick, A.S.; Drolet, R.E.; Greenamyre, J.T. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol. Dis. 2009, 34, 279–290. [Google Scholar] [CrossRef]
- Sherer, T.B.; Kim, J.H.; Betarbet, R.; Greenamyre, J.T. Subcutaneous Rotenone Exposure Causes Highly Selective Dopaminergic Degeneration and α-Synuclein Aggregation. Exp. Neurol. 2003, 179, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Van Laar, A.D.; Webb, K.R.; Keeney, M.T.; Van Laar, V.S.; Zharikov, A.; Burton, E.A.; Hastings, T.G.; Glajch, K.E.; Hirst, W.D.; Greenamyre, J.T.; et al. Transient exposure to rotenone causes degeneration and progressive parkinsonian motor deficits, neuroinflammation, and synucleinopathy. NPJ Parkinson’s Dis. 2023, 9, 121. [Google Scholar] [CrossRef]
- McCormack, A.L.; Thiruchelvam, M.; Manning-Bog, A.B.; Thiffault, C.; Langston, J.W.; Cory-Slechta, D.A.; Di Monte, D.A. Environmental Risk Factors and Parkinson’s Disease: Selective Degeneration of Nigral Dopaminergic Neurons Caused by the Herbicide Paraquat. Neurobiol Dis 2002, 10, 119–127. [Google Scholar] [CrossRef]
- McCormack, A.L.; Atienza, J.G.; Johnston, L.C.; Andersen, J.K.; Vu, S.; Di Monte, D.A. Role of Oxidative Stress in Paraquat-Induced Dopaminergic Cell Degeneration. J. Neurochem. 2005, 93, 1030–1037. [Google Scholar] [CrossRef]
- Peng, J.; Mao, X.O.; Stevenson, F.F.; Hsu, M.; Andersen, J.K. The Herbicide Paraquat Induces Dopaminergic Nigral Apoptosis through Sustained Activation of the JNK Pathway. J. Biol. Chem. 2004, 279, 32626–32632. [Google Scholar] [CrossRef]
- Manning-Boǧ, A.B.; McCormack, A.L.; Purisai, M.G.; Bolin, L.M.; Di Monte, D.A. α-Synuclein Overexpression Protects against Paraquat-Induced Neurodegeneration. J. Neurosci. 2003, 23, 3095. [Google Scholar] [CrossRef]
- Manning-Bog, A.B.; McCormack, A.L.; Li, J.; Uversky, V.N.; Fink, A.L.; Di Monte, D.A. The Herbicide Paraquat Causes Up-Regulation and Aggregation of α-Synuclein in Mice: PARAQUAT AND α-SYNUCLEIN. J. Biol. Chem. 2002, 277, 1641–1644. [Google Scholar] [CrossRef]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef]
- Luk, K.C.; Covell, D.J.; Kehm, V.M.; Zhang, B.; Song, I.Y.; Byrne, M.D.; Pitkin, R.M.; Decker, S.C.; Trojanowski, J.Q.; Lee, V.M.Y. Molecular and Biological Compatibility with Host Alpha-Synuclein Influences Fibril Pathogenicity. Cell Rep. 2016, 16, 3373. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.X.; Cornblath, E.J.; Darwich, A.; Zhang, B.; Brown, H.; Gathagan, R.J.; Sandler, R.M.; Bassett, D.S.; Trojanowski, J.Q.; Lee, V.M.Y. Spread of α-Synuclein Pathology through the Brain Connectome Is Modulated by Selective Vulnerability and Predicted by Network Analysis. Nat. Neurosci. 2019, 22, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Van der Perren, A.; Casteels, C.; Van Laere, K.; Gijsbers, R.; Van den Haute, C.; Baekelandt, V. Development of an Alpha-Synuclein Based Rat Model for Parkinson’s Disease via Stereotactic Injection of a Recombinant Adeno-Associated Viral Vector. J. Vis. Exp. 2016, 2016, 53670. [Google Scholar] [CrossRef]
- Karikari, A.A.; McFleder, R.L.; Ribechini, E.; Blum, R.; Bruttel, V.; Knorr, S.; Gehmeyr, M.; Volkmann, J.; Brotchie, J.M.; Ahsan, F.; et al. Neurodegeneration by α-Synuclein-Specific T Cells in AAV-A53T-α-Synuclein Parkinson’s Disease Mice. Brain Behav. Immun. 2022, 101, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Wegrzynowicz, M.; Bar-On, D.; Calo’, L.; Anichtchik, O.; Iovino, M.; Xia, J.; Ryazanov, S.; Leonov, A.; Giese, A.; Dalley, J.W.; et al. Depopulation of Dense α-Synuclein Aggregates Is Associated with Rescue of Dopamine Neuron Dysfunction and Death in a New Parkinson’s Disease Model. Acta Neuropathol. 2019, 138, 575–595. [Google Scholar] [CrossRef]
- Masliah, E.; Rockenstein, E.; Veinbergs, I.; Mallory, M.; Hashimoto, M.; Takeda, A.; Sagara, Y.; Sisk, A.; Mucke, L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: Implications for neurodegenerative disorders. Science 2000, 287, 1265–1269. [Google Scholar] [CrossRef]
- Chesselet, M.F.; Richter, F.; Zhu, C.; Magen, I.; Watson, M.B.; Subramaniam, S.R. A Progressive Mouse Model of Parkinson’s Disease: The Thy1-ASyn (“Line 61”) Mice. Neurotherapeutics 2012, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Rockenstein, E.; Mallory, M.; Hashimoto, M.; Song, D.; Shults, C.W.; Lang, I.; Masliah, E. Differential Neuropathological Alterations in Transgenic Mice Expressing Alpha-Synuclein from the Platelet-Derived Growth Factor and Thy-1 Promoters. J. Neurosci. Res. 2002, 68, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Ungerstedt, U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur. J. Pharmacol. 1968, 5, 107–110. [Google Scholar] [CrossRef]
- Cenci, M.A.; Björklund, A. Animal models for preclinical Parkinson’s research: An update and critical appraisal. In Progress in Brain Research; Elsevier B.V.: Amsterdam, The Netherlands, 2020; pp. 27–59. [Google Scholar]
- Hattori, N.; Mizuno, Y. Mitochondrial Dysfunction in Parkinson’s Disease. Exp. Neurobiol. 2015, 24, 103–116. [Google Scholar]
- Schapira, A.H.V.; Cooper, J.M.; Dexter, D.; Clark, J.B.; Jenner, P.; Marsden, C.D. Mitochondrial Complex I Deficiency in Parkinson’s Disease. J. Neurochem. 1990, 54, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Sauer, H.; Oertel, W.H. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: A combined retrograde tracing and immunocytochemical study in the rat. Neuroscience 1994, 59, 401–415. [Google Scholar] [CrossRef]
- Przedbroski, S.; Leviver, M.; Jiang, H.; Ferreira, M.; Jackson-Lewis, V.; Donaldson, D.; Togasaki, D.M. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by instrastriatal injection of 6-hydroxydopamine. Neuroscience 1995, 67, 631–647. [Google Scholar] [CrossRef]
- Bové, J.; Perier, C. Neurotoxin-based models of Parkinson’s disease. Neuroscience 2012, 211, 51–76. [Google Scholar] [CrossRef]
- Przedborski, S.; Jackson-Lewis, V.; Naini, A.B.; Jakowec, M.; Petzinger, G.; Miller, R.; Akram, M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): A technical review of its utility and safety. J. Neurochem. 2001, 76, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.S.; Novikova, L.; Roels, C. MPTP treatment in mice does not transmit and cause Parkinsonian neurotoxicity in non-treated cagemates through close contact. Neurosci. Res. 2005, 52, 371–378. [Google Scholar] [CrossRef]
- Giovanni, A.; Sieber, B.A.; Heikkila, R.E.; Sonsalla, P.K. Studies on species sensitivity to the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Part 1: Systemic administration. J. Pharmacol. Exp. Ther. 1994, 270, 1000–1007. [Google Scholar] [PubMed]
- Staal, R.G.W.; Sonsalla, P.K. Inhibition of Brain Vesicular Monoamine Transporter (VMAT2) Enhances 1-Methyl-4-phenylpyridinium Neurotoxicity In Vivo in Rat Striata. J. Pharmacol. Exp. Ther. 2000, 293, 336–342. [Google Scholar] [PubMed]
- Prediger, R.D.S.; Batista, L.C.; Medeiros, R.; Pandolfo, P.; Florio, J.C.; Takahashi, R.N. The risk is in the air: Intranasal administration of MPTP to rats reproducing clinical features of Parkinson’s disease. Exp. Neurol. 2006, 202, 391–403. [Google Scholar] [CrossRef]
- Prediger, R.D.S.; Aguiar, A.S.; Rojas-Mayorquin, A.E.; Figueiredo, C.P.; Matheus, F.C.; Ginestet, L.; Chevarin, C.; Bel, E.D.; Mongeau, R.; Hamon, M.; et al. Single intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL/6 mice models early preclinical phase of Parkinson’s disease. Neurotoxic. Res. 2010, 17, 114–129. [Google Scholar] [CrossRef]
- Carta, A.R.; Carboni, E.; Spiga, S. The MPTP/Probenecid Model of Progressive Parkinson’s Disease. In Dopamine: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2013; pp. 295–308. [Google Scholar]
- Kim, A.; Nigmatullina, R.; Zalyalova, Z.; Soshnikova, N.; Krasnov, A.; Vorobyeva, N.; Georgieva, S.; Kudrin, V.; Narkevich, V.; Ugrumov, M. Upgraded Methodology for the Development of Early Diagnosis of Parkinson’s Disease Based on Searching Blood Markers in Patients and Experimental Models. Mol. Neurobiol. 2019, 56, 3437–3450. [Google Scholar] [CrossRef]
- Berry, C.; La Vecchia, C.; Nicotera, P. Paraquat and Parkinson’s disease. Cell Death Differ. 2010, 17, 1115–1125. [Google Scholar] [CrossRef]
- Cannon, J.R.; Greenamyre, J.T. Gene-environment interactions in Parkinson’s disease: Specific evidence in humans and mammalian models. Neurobiol. Dis. 2013, 57, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Terron, A.; Bal-Price, A.; Paini, A.; Monnet-Tschudi, F.; Bennekou, S.H.; EFSA WG EPI1 Members; Leist, M.; Schildknecht, S. An adverse outcome pathway for parkinsonian motor deficits associated with mitochondrial complex I inhibition. Arch. Toxicol. 2018, 92, 41–82. [Google Scholar] [CrossRef]
- Ren, J.P.; Zhao, Y.W.; Sun, X.J. Toxic influence of chronic oral administration of paraquat on nigrostriatal dopaminergic neurons in C57BL/6 mice. Chin. Med. J. 2009, 122, 2366–2371. [Google Scholar] [PubMed]
- Niso-Santano, M.; González-Polo, R.A.; Bravo-San Pedro, J.M.; Gómez-Sánchez, R.; Lastres-Becker, I.; Ortiz-Ortiz, M.A.; Soler, G.; Morán, J.M.; Cuadrado, A.; Fuentes, J.M.; et al. Activation of apoptosis signal-regulating kinase 1 is a key factor in paraquat-induced cell death: Modulation by the Nrf2/Trx axis. Free Radic. Biol. Med. 2010, 48, 1370–1381. [Google Scholar] [CrossRef]
- Potashkin, J.A.; Blume, S.R.; Runkle, N.K. Limitations of Animal Models of Parkinson’s Disease. Parkinson’s Dis. 2011, 2011, 658083. [Google Scholar] [CrossRef] [PubMed]
- Jagmag, S.A.; Tripathi, N.; Shukla, S.D.; Maiti, S.; Khurana, S. Evaluation of Models of Parkinson’s Disease. Front. Neurosci. 2016, 9, 503. [Google Scholar] [CrossRef] [PubMed]
- Freyaldenhoven, T.E.; Cadet, J.L.; Ali, S.F. The dopamine-depleting effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in CD-1 mice are gender-dependent. Brain Res. 1996, 735, 232–238. [Google Scholar] [CrossRef]
- Gillies, G.E.; Murray, H.E.; Dexter, D.; McArthur, S. Sex dimorphisms in the neuroprotective effects of estrogen in an animal model of Parkinson’s disease. Pharmacol. Biochem. Behav. 2004, 78, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Melcangi, R.C.; Giatti, S.; Garcia-Segura, L.M. Levels and actions of neuroactive steroids in the nervous system under physiological and pathological conditions: Sex-specific features. Neurosci. Biobehav. Rev. 2016, 67, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.; Boyer, R.; Auburger, G.; Leube, B.; Ulm, G.; Mezey, E.; Harta, G.; Brownstein, M.J.; Jonnalagada, S.; Chernova, T.; et al. The ubiquitin pathway in Parkinson’s disease. Nature 1998, 395, 451–452. [Google Scholar] [CrossRef]
- Setsuie, R.; Wang, Y.L.; Mochizuki, H.; Osaka, H.; Hayakawa, H.; Ichihara, N.; Li, H.; Furuta, A.; Sano, Y.; Sun, Y.J.; et al. Dopaminergic neuronal loss in transgenic mice expressing the Parkinson’s disease-associated UCH-L1 I93M mutant. Neurochem. Int. 2007, 50, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Konnova, E.A.; Swanberg, M. Animal Models of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Codon Publications: Brisbane, Australia, 2018; pp. 83–106. [Google Scholar]
- Oliveras-Salvá, M.; Van Der Perren, A.; Casadei, N.; Stroobants, S.; Nuber, S.; D’Hooge, R.; Van den Haute, C.; Baekelandt, V. RAAV2/7 vector-mediated overexpression of alpha-synuclein in mouse substantia nigra induces protein aggregation and progressive dose-dependent neurodegeneration. Mol. Neurodegener. 2013, 8, 44. [Google Scholar] [CrossRef]
- Cookson, M.R. α-Synuclein and neuronal cell death. Mol. Neurodegener. 2009, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Maroteaux, L.; Campanelli, J.T.; Scheller, R.H. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988, 8, 2804–2815. [Google Scholar] [CrossRef]
- Nemani, V.M.; Lu, W.; Berge, V.; Nakamura, K.; Onoa, B.; Lee, M.K.; Chaudhry, F.A.; Nicoll, R.A.; Edwards, R.H. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 2010, 65, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Chen, X.; Rizo, J.; Jahn, R.; Südhof, T.C. A Broken α-Helix in Folded α-Synuclein. J. Biol. Chem. 2003, 278, 15313–15318. [Google Scholar] [CrossRef] [PubMed]
- Hijaz, B.A.; Volpicelli-Daley, L.A. Initiation and propagation of α-synuclein aggregation in the nervous system. Mol. Neurodegener. 2020, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Le, W. Biomarkers for Parkinson’s Disease: How Good Are They? Neurosci. Bull. 2020, 36, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Visanji, N.P.; Brotchie, J.M.; Kalia, L.V.; Koprich, J.B.; Tandon, A.; Watts, J.C.; Lang, A.E. α-Synuclein-Based Animal Models of Parkinson’s Disease: Challenges and Opportunities in a New Era. Trends Neurosci. 2016, 39, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Benito, M.; Granado, N.; García-Sanz, P.; Michel, A.; Dumoulin, M.; Moratalla, R. Modeling Parkinson’s Disease with the Alpha-Synuclein Protein. Front. Pharmacol. 2020, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Gubinelli, F.; Cazzolla, G.; Negrini, M.; Kulacz, I.; Mehrdadian, A.; Tomasello, G.; Venuti, C.; Sarauskyte, L.; Jacobs, F.; Manfredsson, F.P.; et al. Lateralized deficits after unilateral AAV-vector based overexpression of alpha-synuclein in the midbrain of rats on drug-free behavioral tests. Behav. Brain Res. 2022, 429, 113887. [Google Scholar] [CrossRef]
- Polinski, N.K.; Volpicelli-Daley, L.A.; Sortwell, C.E.; Luk, K.C.; Cremades, N.; Gottler, L.M.; Froula, J.; Duffy, M.F.; Lee, V.M.Y.; Martinez, T.N.; et al. Best Practices for Generating and Using Alpha-Synuclein Pre-Formed Fibrils to Model Parkinson’s Disease in Rodents. J. Parkinson’s Dis. 2018, 8, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.C.; Kehm, V.M.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 2012, 209, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Polinski, N.K. A Summary of Phenotypes Observed in the In Vivo Rodent Alpha-Synuclein Preformed Fibril Model. J. Parkinson’s Dis. 2021, 11, 1555–1567. [Google Scholar] [CrossRef]
- Lamontagne-Proulx, J.; Coulombe, K.; Morissette, M.; Rieux, M.; Calon, F.; Di Paolo, T.; Soulet, D. Sex and Age Differences in a Progressive Synucleinopathy Mouse Model. Biomolecules 2023, 13, 977. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Heng, Y.; Mou, Z.; Huang, J.Y.; Yuan, Y.H.; Chen, N.H. Reassessment of subacute MPTP-treated mice as animal model of Parkinson’s disease. Acta Pharmacol. Sin. 2017, 38, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef]
- Kumar, A.; Leinisch, F.; Kadiiska, M.B.; Corbett, J.; Mason, R.P. Formation and Implications of Alpha-Synuclein Radical in Maneb- and Paraquat-Induced Models of Parkinson’s Disease. Mol. Neurobiol. 2016, 53, 2983–2994. [Google Scholar] [CrossRef]
- Wills, J.; Credle, J.; Oaks, A.W.; Duka, V.; Lee, J.H.; Jones, J.; Sidhu, A. Paraquat, but Not Maneb, Induces Synucleinopathy and Tauopathy in Striata of Mice through Inhibition of Proteasomal and Autophagic Pathways. PLoS ONE 2012, 7, e30745. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the α-Synuclein Gene Identified in Families with Parkinson’s Disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef]
- Bonifati, V.; Rizzu, P.; Van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.J.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 Gene Associated with Autosomal Recessive Early-Onset Parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef]
- Meade, R.M.; Fairlie, D.P.; Mason, J.M. Alpha-Synuclein Structure and Parkinson’s Disease—Lessons and Emerging Principles. Mol. Neurodegener. 2019, 14, 29. [Google Scholar] [CrossRef]
- Valente, E.M.; Abou-Sleiman, P.M.; Caputo, V.; Muqit, M.M.K.; Harvey, K.; Gispert, S.; Ali, Z.; Del Turco, D.; Bentivoglio, A.R.; Healy, D.G.; et al. Hereditary Early-Onset Parkinson’s Disease Caused by Mutations in PINK1. Science 2004, 304, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Lill, C.M.; Hansen, J.; Olsen, J.H.; Binder, H.; Ritz, B.; Bertram, L. Impact of Parkinson’s Disease Risk Loci on Age at Onset. Mov. Disord. 2015, 30, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.; Vila, M.; Lincoln, S.; McCormack, A.; Picciano, M.; LaFrancois, J.; Yu, X.; Dickson, D.; Langston, W.J.; McGowan, E.; et al. Lack of Nigral Pathology in Transgenic Mice Expressing Human α-Synuclein Driven by the Tyrosine Hydroxylase Promoter. Neurobiol. Dis. 2001, 8, 535–539. [Google Scholar] [CrossRef]
- Prasad, K.; Tarasewicz, E.; Ohman Strickland, P.A.; O’Neill, M.; Mitchell, S.N.; Merchant, K.; Tep, S.; Hilton, K.; Datwani, A.; Buttini, M.; et al. Biochemical and Morphological Consequences of Human α-Synuclein Expression in a Mouse α-Synuclein Null Background. Eur. J. Neurosci. 2011, 33, 642–656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lautenschläger, J.; Stephens, A.D.; Fusco, G.; Ströhl, F.; Curry, N.; Zacharopoulou, M.; Michel, C.H.; Laine, R.; Nespovitaya, N.; Fantham, M.; et al. C-terminal calcium binding of α-synuclein modulates synaptic vesicle interaction. Nat. Commun. 2018, 9, 712. [Google Scholar] [CrossRef] [PubMed]
- Brucale, M.; Sandal, M.; Di Maio, S.; Rampioni, A.; Tessari, I.; Tosatto, L.; Bisaglia, M.; Bubacco, L.; Samorì, B. Pathogenic mutations shift the equilibria of α-synuclein single molecules towards structured conformers. ChemBioChem 2009, 10, 176–183. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Südhof, T.C. Systematic mutagenesis of α-synuclein reveals distinct sequence requirements for physiological and pathological activities. J. Neurosci. 2012, 32, 15227–15242. [Google Scholar] [CrossRef]
- Petrucci, S.; Ginevrino, M.; Valente, E.M. Phenotypic spectrum of alpha-synuclein mutations: New insights from patients and cellular models. Park. Relat. Disord. 2016, 22 (Suppl. S1), S16–S20. [Google Scholar] [CrossRef] [PubMed]
- Kasten, M.; Klein, C. The many faces of alpha-synuclein mutations. Mov. Disord. 2013, 28, 697–701. [Google Scholar] [CrossRef]
- Khalaf, O.; Fauvet, B.; Oueslati, A.; Dikiy, I.; Mahul-Mellier, A.L.; Ruggeri, F.S.; Mbefo, M.K.; Vercruysse, F.; Dietler, G.; Lee, S.J.; et al. The H50Q mutation enhances αα-synuclein aggregation, secretion, and toxicity. J. Biol. Chem. 2014, 289, 21856–21876. [Google Scholar] [CrossRef]
- Liddle, R.A. Parkinson’s disease from the gut. Brain Res. 2018, 1693, 201–206. [Google Scholar] [CrossRef]
- Plaas, M.; Karis, A.; Innos, J.; Rebane, E.; Baekelandt, V.; Vaarmann, A.; Luuk, H.; Vasar, E.; Kõks, S. Alpha-synuclein A30P point-mutation generates age-dependent nigrostriatal deficiency in mice. J. Physiol. Pharmacol. 2008, 59, 205–216. [Google Scholar]
- Kuo, Y.M.; Li, Z.; Jiao, Y.; Gaborit, N.; Pani, A.K.; Orrison, B.M.; Bruneau, B.G.; Giasson, B.I.; Smeyne, R.J.; Gershon, M.D.; et al. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated α-synuclein gene mutations precede central nervous system changes. Hum. Mol. Genet. 2010, 19, 1633–1650. [Google Scholar] [CrossRef] [PubMed]
- Zarranz, J.J.; Alegre, J.; Gómez-Esteban, J.C.; Lezcano, E.; Ros, R.; Ampuero, I.; Vidal, L.; Hoenicka, J.; Ro-driguez, O.; Atarés, B.; et al. The New Mutation, E46K, of α-Synuclein Causes Parkinson and Lewy Body Dementia. Ann. Neurol. 2004, 55, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Ornelas, L.; Viennet, T.; Rovere, M.; Jiang, H.; Liu, L.; Nuber, S.; Ericsson, M.; Arthanari, H.; Selkoe, D.J. Altered conformation of α-synuclein drives dysfunction of synaptic vesicles in a synaptosomal model of Parkinson’s disease. Cell Rep. 2021, 36, 109333. [Google Scholar] [CrossRef] [PubMed]
- Seirafi, M.; Kozlov, G.; Gehring, K. Parkin structure and function. FEBS J. 2015, 282, 2076–2088. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.A.; Palmiter, R.D. Parkin-deficient mice are not a robust model of parkinsonism. Proc. Natl. Acad. Sci. USA 2005, 102, 2174–2179. [Google Scholar] [CrossRef]
- Kitada, T.; Asakawa, S.; Hattori, N.; Matsumine, H.; Yamamura, Y.; Minoshima, S.; Yokochi, M.; Mizuno, Y.; Shimizu, N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 1998, 392, 605–608. [Google Scholar] [CrossRef]
- Narendra, D.; Tanaka, A.; Suen, D.-F.; Youle, R.J.; Narendra, D.; Youle’, R.J. Parkin Is Recruited Selectively to Impaired Mitochondria and Promotes Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008, 183, 795–803. [Google Scholar] [CrossRef]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef]
- Morais, V.A.; Haddad, D.; Craessaerts, K.; De Bock, P.J.; Swerts, J.; Vilain, S.; Aerts, L.; Overbergh, L.; Grünwald, A.; Seibler, P.; et al. PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling. Science 2014, 344, 203–207. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Olzmann, J.A.; Chin, L.S.; Li, L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007, 5, e172. [Google Scholar] [CrossRef] [PubMed]
- Oliveras-Salvá, M.; Van Rompuy, A.S.; Heeman, B.; Van Den Haute, C.; Baekelandt, V. Loss-of-function rodent models for parkin and PINK1. J. Park. Dis. 2011, 1, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Exner, N.; Lutz, A.K.; Haass, C.; Winklhofer, K.F. Mitochondrial dysfunction in Parkinson’s disease: Molecular mechanisms and pathophysiological consequences. EMBO J. 2012, 31, 3038–3062. [Google Scholar] [CrossRef] [PubMed]
- Soto, I.; McManus, R.; Navarrete, W.; Kasanga, E.A.; Doshier, K.; Nejtek, V.A.; Salvatore, M.F. Aging accelerates locomotor decline in PINK1 knockout rats in association with decreased nigral, but not striatal, dopamine and tyrosine hydroxylase expression. Exp. Neurol. 2024, 376, 114771. [Google Scholar] [CrossRef]
- Doty, R.L.; Stern, M.B.; Pfeiffer, C.; Gollomp, S.M.; Hurtig, H.I. Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1992, 55, 138–142. [Google Scholar] [CrossRef]
- Glasl, L.; Kloos, K.; Giesert, F.; Roethig, A.; Di Benedetto, B.; Kühn, R.; Zhang, J.; Hafen, U.; Zerle, J.; Hofmann, A.; et al. Pink1-deficiency in mice impairs gait, olfaction and serotonergic innervation of the olfactory bulb. Exp. Neurol. 2012, 235, 214–227. [Google Scholar] [CrossRef]
- Chen, L.; Cagniard, B.; Mathews, T.; Jones, S.; Koh, H.C.; Ding, Y.; Carvey, P.M.; Ling, Z.; Kang, U.J.; Zhuang, X. Age-dependent motor deficits and dopaminergic dysfunction in DJ-1 null mice. J. Biol. Chem. 2005, 280, 21418–21426. [Google Scholar] [CrossRef] [PubMed]
- Kordower, J.H.; Olanow, C.W.; Dodiya, H.B.; Chu, Y.; Beach, T.G.; Adler, C.H.; Halliday, G.M.; Bartus, R.T. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 2013, 136, 2419–2431. [Google Scholar] [CrossRef]
- Kim, R.H.; Smith, P.D.; Aleyasin, H.; Hayley, S.; Mount, M.P.; Pownall, S.; Wakeham, A.; You-Ten, A.J.; Kalia, S.K.; Horne, P.; et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc. Natl. Acad. Sci. USA 2005, 102, 5215–5220. [Google Scholar] [CrossRef]
- Andres-Mateos, E.; Perier, C.; Zhang, L.; Blanchard-Fillion, B.; Greco, T.M.; Thomas, B.; Seok Ko, H.; Sasaki, M.; Ischiropoulos, H.; Przedborski, S.; et al. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc. Natl. Acad. Sci. USA 2007, 104, 14807–14812. [Google Scholar] [CrossRef] [PubMed]
- Dolgacheva, L.P.; Berezhnov, A.V.; Fedotova, E.I.; Zinchenko, V.P.; Abramov, A.Y. Role of DJ-1 in the mechanism of pathogenesis of Parkinson’s disease. J. Bioenerg. Biomembr. 2019, 51, 175–188. [Google Scholar] [CrossRef]
- Leonard, H.; Blauwendraat, C.; Krohn, L.; Faghri, F.; Iwaki, H.; Ferguson, G.; Day-Williams, A.G.; Stone, D.J.; Singleton, A.B.; Nalls, M.A.; et al. Genetic variability and potential effects on clinical trial outcomes: Perspectives in Parkinson’s disease. J. Med. Genet. 2020, 57, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Prasuhn, J.; Brüggemann, N.; Hessler, N.; Berg, D.; Gasser, T.; Brockmann, K.; Olbrich, D.; Ziegler, A.; König, I.R.; Klein, C.; et al. An omics-based strategy using coenzyme Q10 in patients with Parkinson’s disease: Concept evaluation in a double-blind randomized placebo-controlled parallel group trial. Neurol. Res. Pract. 2019, 1, 31. [Google Scholar] [CrossRef]
- McKinney, W.T.; Bunney, W.E. Animal Model of Depression: I. Review of Evidence: Implications for Research. Arch. Gen. Psychiatry 1969, 21, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Abramson, L.Y.; Seligman, M.E.; Teasdale, J.D. Learned helplessness in humans: Critique and reformulation. J. Abnorm. Psychol. 1978, 87, 49–74. [Google Scholar] [CrossRef]
- Jolicoeur, F.B.; Rivest, R.; Drumheller, A. Hypokinesia, rigidity, and tremor induced by hypothalamic 6-OHDA lesions in the rat. Brain Res. Bull. 1991, 26, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Wolfarth, S.; Konieczny, J.; Śmiałowska, M.; Schulze, G.; Ossowska, K. Influence of 6-hydroxydopamine lesion of the dopaminergic nigrostriatal pathway on the muscle tone and electromyographic activity measured during passive movements. Neuroscience 1996, 74, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Kabuto, H.; Makino, H.; Ogawa, N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J. Neurosci. Methods 1997, 73, 45–48. [Google Scholar] [CrossRef]
- Rosa, I.; Di Censo, D.; Ranieri, B.; Di Giovanni, G.; Scarnati, E.; Alecci, M.; Galante, A.; Florio, T.M. Comparison between tail suspension swing test and standard rotation test in revealing early motor behavioral changes and neurodegeneration in 6-OHDA hemiparkinsonian rats. Int. J. Mol. Sci. 2020, 21, 2874. [Google Scholar] [CrossRef]
- Chuang, C.-S.; Su, H.-L.; Cheng, F.-C.; Hsu, S.-H.; Chuang, C.-F.; Liu, C.-S. Quantitative evaluation of motor function before and after engraftment of dopaminergic neurons in a rat model of Parkinson’s disease. J. Biomed. Sci. 2010, 17, 9. [Google Scholar] [CrossRef]
- Wang, X.H.; Lu, G.; Hu, X.; Tsang, K.S.; Kwong, W.H.; Wu, F.X.; Meng, H.W.; Jiang, S.; Liu, S.W.; Ng, H.K.; et al. Quantitative assessment of gait and neurochemical correlation in a classical murine model of Parkinson’s disease. BMC Neurosci. 2012, 13, 142. [Google Scholar] [CrossRef]
- Henry, B.; Crossman, A.R.; Brotchie, J.M. Characterization of Enhanced Behavioral Responses to L-DOPA Following Repeated Administration in the 6-Hydroxydopamine-Lesioned Rat Model of Parkinson’s Disease. Exp. Neurol. 1998, 151, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Cenci, M.A.; Lee, C.S.; Bjö Rklund, A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin-and glutamic acid decarboxylase mRNA. Eur. J. Neurosci. 1998, 10, 2694–2706. [Google Scholar]
- Steece-Collier, K.; Collier, T.J.; Danielson, P.D.; Kurlan, R.; Yurek, D.M.; Sladek, J.R. Embryonic mesencephalic grafts increase levodopa-induced forelimb hyperkinesia in Parkinsonian rats. Mov. Disord. 2003, 18, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Real, C.C.; Ferreira, A.F.B.; Chaves-Kirsten, G.P.; Torrão, A.S.; Pires, R.S.; Britto, L.R.G. BDNF receptor blockade hinders the beneficial effects of exercise in a rat model of Parkinson’s disease. Neuroscience 2013, 237, 118–129. [Google Scholar] [CrossRef]
- Real, C.C.; Doorduin, J.; Kopschina Feltes, P.; Vállez García, D.; de Paula Faria, D.; Britto, L.R.; de Vries, E.F.J. Evaluation of exercise-induced modulation of glial activation and dopaminergic damage in a rat model of Parkinson’s disease using [11C]PBR28 and [18F]FDOPA PET. J. Cereb. Blood Flow Metab. 2019, 39, 989–1004. [Google Scholar] [CrossRef]
- De Castro Medeiros, D.; Plewnia, C.; Mendes, R.V.; Pisanò, C.A.; Boi, L.; Moraes, M.F.D.; Aguiar, C.L.; Fisone, G.A. A mouse model of sleep disorders in Parkinson’s disease showing distinct effects of dopamine D2-like receptor activation. Prog. Neurobiol. 2023, 231, 102536. [Google Scholar] [CrossRef]
- Feng, X.Y.; Yan, J.T.; Zhang, X.L.; Zhu, J.X. Gastrointestinal non-motor dysfunction in Parkinson’s disease model rats with 6-hydroxydopamine. Physiol. Res. 2019, 68, 295–303. [Google Scholar] [CrossRef]
- Santiago, R.M.; Barbieiro, J.; Lima, M.M.S.; Dombrowski, P.A.; Andreatini, R.; Vital, M.A.B.F. Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson’s disease are predominantly associated with serotonin and dopamine. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Nikkhah, G.; Bentlage, C.; Bjarklund, A. Forelimb Akinesia in the Rat Parkinson Model: Differential Effects of Dopamine Agonists and Nigral Transplants as Assessed by a New Stepping Test. J. Neurosci. 1995, 15, 3863–3875. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Rong, Q. Effect of Different MPTP Administration Intervals on Mouse Models of Parkinson’s Disease. Contrast Media Mol. Imaging 2022, 2022, 2112146. [Google Scholar] [CrossRef]
- Potts, L.F.; Wu, H.; Singh, A.; Marcilla, I.; Luquin, M.R.; Papa, S.M. Modeling Parkinson’s disease in monkeys for translational studies, a critical analysis. Exp. Neurol. 2014, 256, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Fadda, P.; Klephan, K.J.; Hull, C.; Teismann, P.; Platt, B.; Riedel, G. Neurochemical, histological, and behavioral profiling of the acute, sub-acute, and chronic MPTP mouse model of Parkinson’s disease. J. Neurochem. 2023, 164, 121–142. [Google Scholar] [CrossRef] [PubMed]
- Blume, S.R.; Cass, D.K.; Tseng, K.Y. Stepping test in mice: A reliable approach in determining forelimb akinesia in MPTP-induced Parkinsonism. Exp. Neurol. 2009, 219, 208–211. [Google Scholar] [CrossRef]
- Ogawa, N.; Hirose, Y.; Ohara, S.; Ono, T.; Watanabe, Y. A simple quantitative bradykinesia test in MPTP-treated mice. Res. Commun. Chem. Pathol. Pharmacol. 1985, 50, 435–441. [Google Scholar] [PubMed]
- Kim, S.T.; Son, H.J.; Choi, J.H.; Ji, I.J.; Hwang, O. Vertical grid test and modified horizontal grid test are sensitive methods for evaluating motor dysfunctions in the MPTP mouse model of Parkinson’s disease. Brain Res. 2010, 1306, 176–183. [Google Scholar] [CrossRef]
- Haobam, R.; Sindhu, K.M.; Chandra, G.; Mohanakumar, K.P. Swim-test as a function of motor impairment in MPTP model of Parkinson’s disease: A comparative study in two mouse strains. Behav. Brain Res. 2005, 163, 159–167. [Google Scholar] [CrossRef]
- Lima, M.M.S.; Andersen, M.L.; Reksidler, A.B.; Vital, M.A.B.F.; Tufik, S. The role of the substantia nigra pars compacta in regulating sleep patterns in rats. PLoS ONE 2007, 2, e513. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Jiang, R.; Xie, W.; Liu, X.; Tang, Y.; Xiao, H.; Gao, J.; Jia, Y.; Bai, Q. Intestinal Pathology and Gut Microbiota Alterations in a Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Mouse Model of Parkinson’s Disease. Neurochem. Res. 2018, 43, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Dovonou, A.; Bolduc, C.; Soto Linan, V.; Gora, C.; Peralta, M.R., III; Lévesque, M. Animal models of Parkinson’s disease: Bridging the gap between disease hallmarks and research questions. Transl. Neurodegener. 2023, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, V.S.; Santos, J.R.; Leão, A.H.F.F.; Medeiros, A.M.; Melo, T.G.; Izídio, G.S.; Cabral, A.; Ribeiro, R.A.; Abílio, V.C.; Ribeiro, A.M.; et al. Repeated treatment with a low dose of reserpine as a progressive model of Parkinson’s disease. Behav. Brain Res. 2012, 231, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Leal, P.C.; Lins, L.C.R.F.; de Gois, A.M.; Marchioro, M.; Santos, J.R. Commentary: Evaluation of models of Parkinson’s disease. Front. Neurosci. 2016, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Bispo, J.M.M.; Melo, J.E.C.; Gois, A.M.; Leal, P.C.; Lins, L.C.R.F.; Souza, M.F.; Medeiros, K.A.A.L.; Ribeiro, A.M.; Silva, R.H.; Marchioro, M.; et al. Sex differences in the progressive model of parkinsonism induced by reserpine in rats. Behav. Brain Res. 2019, 363, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.R.; Cunha, J.A.S.; Dierschnabel, A.L.; Campêlo, C.L.C.; Leão, A.H.F.F.; Silva, A.F.; Engelberth, R.C.G.J.; Izídio, G.S.; Cavalcante, J.S.; Abílio, V.C.; et al. Cognitive, motor and tyrosine hydroxylase temporal impairment in a model of parkinsonism induced by reserpine. Behav. Brain Res. 2013, 253, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.L.; Tsai, C.H.; Lu, M.K.; Liu, H.J.; Chen, Y.C.; Chang, F.C. Interleukin-1beta mediates sleep alteration in rats with rotenone-induced parkinsonism. Sleep 2007, 30, 413–425. [Google Scholar] [CrossRef]
- Antkiewicz-Michaluk, L.; Wąsik, A.; Możdżeń, E.; Romańska, I.; Michaluk, J. Antidepressant-like effect of tetrahydroisoquinoline amines in the animal model of depressive disorder induced by repeated administration of a low dose of reserpine: Behavioral and neurochemical studies in the rat. Neurotox. Res. 2014, 26, 85–98. [Google Scholar] [CrossRef]
- Silva-Martins, S.; Beserra-Filho, J.I.A.; Maria-Macêdo, A.; Custódio-Silva, A.C.; Soares-Silva, B.; Silva, S.P.; Lambertucci, R.H.; Silva, R.H.; dos Santos, J.R.; Gandhi, S.R.; et al. Myrtenol complexed with β-cyclodextrin ameliorates behavioural deficits and reduces oxidative stress in the reserpine-induced animal model of Parkinsonism. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1488–1499. [Google Scholar] [CrossRef]
- Casanova, Y.; Negro, S.; Barcia, E. Application of neurotoxin- And pesticide-induced animal models of Parkinson’s disease in the evaluation of new drug delivery systems. Acta Pharm. 2022, 72, 35–58. [Google Scholar] [CrossRef]
- Fleming, S.M.; Zhu, C.; Fernagut, P.O.; Mehta, A.; DiCarlo, C.D.; Seaman, R.L.; Chesselet, M.F. Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Exp. Neurol. 2004, 187, 418–429. [Google Scholar] [CrossRef]
- Real, C.C.; Binda, K.H.; Thomsen, M.B.; Lillethorup, T.P.; Brooks, D.J.; Landau, A.M. Selecting the Best Animal Model of Parkinson’s Disease for Your Research Purpose: Insight from in vivo PET Imaging Studies. Curr. Neuropharmacol. 2023, 21, 1241–1272. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.E.; Stringer, A.; Bobrovskaya, L. Rotenone induces gastrointestinal pathology and microbiota alterations in a rat model of Parkinson’s disease. Neurotoxicology 2018, 65, 174–185. [Google Scholar] [CrossRef]
- Dodiya, H.B.; Forsyth, C.B.; Voigt, R.M.; Engen, P.A.; Patel, J.; Shaikh, M.; Green, S.J.; Naqib, A.; Roy, A.; Kordower, J.H.; et al. Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol. Dis. 2020, 135, 104352. [Google Scholar] [CrossRef]
- Tasselli, M.; Chaumette, T.; Paillusson, S.; Monnet, Y.; Lafoux, A.; Huchet-Cadiou, C.; Aubert, P.; Hunot, S.; Derkinderen, P.; Neunlist, M. Effects of oral administration of rotenone on gastrointestinal functions in mice. Neurogastroenterol. Motil. 2013, 25, e183–e193. [Google Scholar] [CrossRef]
- Duty, S.; Jenner, P. Themed Issue: Translational Neuropharmacology-Using Appropriate Animal Models to Guide Clinical Drug Development Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011, 164, 1357. [Google Scholar] [CrossRef]
- Maulana, S.; Kamilah, S.N.; Muslim, C.; Ruyani, A.; Astuti, R.R.S. Assessing the Neurotoxicological Effect of the Acute Paraquat Aerosols Exposure in Causing Parkinsonism on Mouse through Behavioral Assays. J. Farm. Dan Ilmu Kefarmasian Indones. 2022, 9, 298–304. [Google Scholar] [CrossRef]
- Bove, C.; Anselmi, L.; Travagli, X.R.A. Altered gastric tone and motility response to brain-stem dopamine in a rat model of parkinsonism. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G1–G7. [Google Scholar] [CrossRef]
- Tinakoua, A.; Bouabid, S.; Faggiani, E.; De Deurwaerdère, P.; Lakhdar-Ghazal, N.; Benazzouz, A. The impact of combined administration of paraquat and maneb on motor and non-motor functions in the rat. Neuroscience 2015, 311, 118–129. [Google Scholar] [CrossRef]
- Okuda, S.; Uemura, N.; Sawamura, M.; Taguchi, T.; Ikuno, M.; Uemura, M.T.; Yamakado, H.; Takahashi, R. Rapid Induction of Dopaminergic Neuron Loss Accompanied by Lewy Body-Like Inclusions in A53T BAC-SNCA Transgenic Mice. Neurotherapeutics 2022, 19, 289–304. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, W.B.; Shen, B.; Dong, H.; Zhao, J.; Tang, Y.L.; Fan, Y.; Yang, Y.F.; Sun, Y.M.; Luo, S.S.; et al. Propagated a-synucleinopathy recapitulates REM sleep behaviour disorder followed by parkinsonian phenotypes in mice. Brain 2020, 143, 3374–3392. [Google Scholar] [CrossRef] [PubMed]
- Manfredsson, F.P.; Luk, K.C.; Benskey, M.J.; Gezer, A.; Garcia, J.; Kuhn, N.C.; Sandoval, I.M.; Patterson, J.R.; O’Mara, A.; Yonkers, R.; et al. Induction of alpha-synuclein pathology in the enteric nervous system of the rat and non-human primate results in gastrointestinal dysmotility and transient CNS pathology. Neurobiol. Dis. 2018, 112, 106–118. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, S.H.; Kam, T.I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.e7. [Google Scholar] [CrossRef]
- Goldberg, M.S.; Pisani, A.; Haburcak, M.; Vortherms, T.A.; Kitada, T.; Costa, C.; Tong, Y.; Martella, G.; Tscherter, A.; Martins, A.; et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial parkinsonism-linked gene DJ-1. Neuron 2005, 45, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhong, S.; Tan, Y.; Zeng, W.Q.; Wang, J.; Cheng, C.; Yang, X.; Wu, Y.; Cao, X.; Xu, Y. The Rodent Models of Dyskinesia and Their Behavioral Assessment. Front. Neurol. 2019, 10, 1016. [Google Scholar] [CrossRef]
- Yang, K.M.; Blue, K.V.; Mulholland, H.M.; Kurup, M.P.; Kelm-Nelson, C.A.; Ciucci, M.R. Characterization of oromotor and limb motor dysfunction in the DJ1-/- model of Parkinson disease. Behav. Brain Res. 2018, 339, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Barkow, J.C.; Freed, C.R. Running wheel exercise reduces α-synuclein aggregation and improves motor and cognitive function in a transgenic mouse model of Parkinson’s disease. PLoS ONE 2017, 12, e0190160. [Google Scholar] [CrossRef] [PubMed]
- Dave, K.D.; De Silva, S.; Sheth, N.P.; Ramboz, S.; Beck, M.J.; Quang, C.; Switzer, R.C.; Ahmad, S.O.; Sunkin, S.M.; Walker, D.; et al. Phenotypic characterization of recessive gene knockout rat models of Parkinson’s disease. Neurobiol. Dis. 2014, 70, 190–203. [Google Scholar] [CrossRef]
- Bastioli, G.; Regoni, M.; Cazzaniga, F.; De Luca, C.M.G.; Bistaffa, E.; Zanetti, L.; Moda, F.; Valtorta, F.; Sassone, J. Animal models of autosomal recessive parkinsonism. Biomedicines 2021, 9, 812. [Google Scholar] [CrossRef]
- Shinzawa, K.; Sumi, H.; Ikawa, M.; Matsuoka, Y.; Okabe, M.; Sakoda, S.; Tsujimoto, Y. Neuroaxonal dystrophy caused by group VIA phospholipase A2 deficiency in mice: A model of human neurodegenerative disease. J. Neurosci. 2008, 28, 2212–2220. [Google Scholar] [CrossRef]
- McDowell, K.A.; Shin, D.; Roos, K.P.; Chesselet, M.F. Sleep dysfunction and EEG alterations in mice overexpressing alpha-synuclein. J. Parkinson’s Dis. 2014, 4, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haq, R.; Schlachetzki, J.C.M.; Boktor, J.C.; Cantu-Jungles, T.M.; Thron, T.; Zhang, M.; Bostick, J.W.; Khazaei, T.; Chilakala, S.; Morais, L.H.; et al. A prebiotic diet modulates microglial states and motor deficits in α-synuclein overexpressing mice. eLife 2022, 11, e81453. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wang, V.; Huang, E.Y.K.; Chou, Y.C.; Kuo, T.T.; Olson, L.; Hoffer, B.J. Delayed Dopamine Dysfunction and Motor Deficits in Female Parkinson Model Mice. Int. J. Mol. Sci. 2019, 20, 6251. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, W.J.; Guseman, T.L.; Pienaar, I.S.; Dluzen, D.E.; Young, J.W. A novel biomechanical analysis of gait changes in the MPTP mouse model of Parkinson’s disease. PeerJ 2015, 2015, e1175. [Google Scholar] [CrossRef] [PubMed]
- Tamás, A.; Lubics, A.; Szalontay, L.; Lengvári, I.; Reglodi, D. Age and gender differences in behavioral and morphological outcome after 6-hydroxydopamine-induced lesion of the substantia nigra in rats. Behav. Brain Res. 2005, 158, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.C.; Meurer, Y.S.R.; Bioni, V.S.; Cunha, D.M.G.; Gonçalves, N.; Lopes-Silva, L.B.; Becegato, M.; Soares, M.B.L.; Marinho, G.F.; Santos, J.R.; et al. Female Rats Are Resistant to Cognitive, Motor and Dopaminergic Deficits in the Reserpine-Induced Progressive Model of Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 757714. [Google Scholar] [CrossRef]
- Patil, P.; Chaware, V.; Redasani, V. An Overview of Animal Models and Symptomatic Treatment of Parkinson’s disease. Asian J. Pharm. Res. Dev. 2023, 11, 132–135. [Google Scholar] [CrossRef]
- Magno, L.A.V.; Collodetti, M.; Tenza-Ferrer, H.; Romano-Silva, M.A. Cylinder test to assess sensory-motor function in a mouse model of parkinson s disease. Bio-Protocol 2019, 9, e3337. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Wessolleck, J.; Papazoglou, A.; Metz, G.A.; Nikkhah, G. Walking pattern analysis after unilateral 6-OHDA lesion and transplantation of foetal dopaminergic progenitor cells in rats. Behav. Brain Res. 2009, 199, 317–325. [Google Scholar] [CrossRef]
- Dauer, W.; Kholodilov, N.; Vila, M.; Trillat, A.C.; Goodchild, R.; Larsen, K.E.; Staal, R.; Tieu, K.; Schmitz, Y.; Yuan, C.A.; et al. Resistance of alpha-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc. Natl. Acad. Sci. USA 2002, 99, 14524–14529. [Google Scholar] [CrossRef]
- Johnson, M.E.; Stecher, B.; Labrie, V.; Brundin, L.; Brundin, P. Triggers, Facilitators, and Aggravators: Redefining Parkinson’s Disease Pathogenesis. Trends Neurosci. 2019, 42, 4–13. [Google Scholar] [CrossRef]
- Riedel, O.; Klotsche, J.; Spottke, A.; Deuschl, G.; Förstl, H.; Henn, F.; Heuser, I.; Oertel, W.; Reichmann, H.; Riederer, P.; et al. Frequency of dementia, depression, and other neuropsychiatric symptoms in 1,449 outpatients with Parkinson’s disease. J. Neurol. 2010, 257, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Umbach, D.M.; Peddada, S.D.; Xu, Z.; Tröster, A.I.; Huang, X.; Chen, H. Potential sex differences in nonmotor symptoms in early drug-naive Parkinson disease. Neurology 2015, 84, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Fifel, K.; Piggins, H.; Deboer, T. Modeling sleep alterations in Parkinson’s disease: How close are we to valid translational animal models? Sleep Med. Rev. 2016, 25, 95–111. [Google Scholar] [CrossRef]
- Mitchell, H.A.; Bogenpohl, J.W.; Liles, L.C.; Epstein, M.P.; Bozyczko-Coyne, D.; Williams, M.; Weinshenker, D. Behavioral responses of dopamine β-hydroxylase knockout mice to modafinil suggest a dual noradrenergic-dopaminergic mechanism of action. Pharmacol. Biochem. Behav. 2008, 91, 217–222. [Google Scholar] [CrossRef]
- Hunsley, M.S.; Palmiter, R.D. Altered sleep latency and arousal regulation in mice lacking norepinephrine. Pharmacol. Biochem. Behav. 2004, 78, 765–773. [Google Scholar] [CrossRef]
- Sakata, M.; Sei, H.; Toida, K.; Fujihara, H.; Urushihara, R.; Morita, Y. Mesolimbic dopaminergic system is involved in diurnal blood pressure regulation. Brain Res. 2002, 928, 194–201. [Google Scholar] [CrossRef] [PubMed]
- De Castro Medeiros, D.; Aguiar, C.L.; Moraes, M.F.D.; Fisone, G. Sleep Disorders in Rodent Models of Parkinson’s Disease. Front. Pharmacol. 2019, 10, 1414. [Google Scholar] [CrossRef]
- Taylor, T.N.; Caudle, W.M.; Shepherd, K.R.; Noorian, A.R.; Jackson, C.R.; Iuvone, P.M.; Weinshenker, D.; Greene, J.G.; Miller, G.W. Nonmotor symptoms of Parkinson’s disease revealed in an animal model with reduced monoamine storage capacity. J. Neurosci. 2009, 29, 8103–8113. [Google Scholar] [CrossRef]
- Sun, M.F.; Zhu, Y.L.; Zhou, Z.L.; Jia, X.B.; Xu, Y.D.; Yang, Q.; Cui, C.; Shen, Y.Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Toti, L.; Travagli, R.A. Gastric dysregulation induced by microinjection of 6-OHDA in the substantia nigra pars compacta of rats is determined by alterations in the brain-gut axis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 307, G1013–G1023. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef] [PubMed]
- Caudal, D.; Alvarsson, A.; Björklund, A.; Svenningsson, P. Depressive-like phenotype induced by AAV-mediated overexpression of human α-synuclein in midbrain dopaminergic neurons. Exp. Neurol. 2015, 273, 243–252. [Google Scholar] [CrossRef]
- Chia, S.J.; Tan, E.K.; Chao, Y.X. Historical perspective: Models of Parkinson’s disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.N.; Greene, J.G.; Miller, G.W. Behavioral phenotyping of mouse models of Parkinson’s disease. Behav. Brain Res. 2010, 211, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meredith, G.E.; Kang, U.J. Behavioral models of Parkinson’s disease in rodents: A new look at an old problem. Mov. Disord. 2006, 21, 1595–1606. [Google Scholar] [CrossRef]
- McDowell, K.; Chesselet, M.F. Animal models of the non-motor features of Parkinson’s disease. Neurobiol. Dis. 2012, 46, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Tan, E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease: A review. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Paillé, V.; Henry, V.; Lescaudron, L.; Brachet, P.; Damier, P. Rat model of Parkinson’s disease with bilateral motor abnormalities, reversible with levodopa, and dyskinesias. Mov. Disord. 2007, 22, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.A.; Lee, W.Y.; Kim, Y.S.; Kang, U.J. The effects of chronic L-DOPA therapy on pharmacodynamic parameters in a rat model of motor response fluctuations. Exp. Neurol. 2003, 184, 304–312. [Google Scholar] [CrossRef]
- Mierau, J.; Schingnitz, G. Biochemical and pharmacological studies on pramipexole, a potent and selective dopamine D2 receptor agonist. Eur. J. Pharmacol. 1992, 215, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Maj, J.; Rogóz, Z.; Skuza, G.; Kołodziejczyk, K. The behavioural effects of pramipexole, a novel dopamine receptor agonist. Eur. J. Pharmacol. 1997, 324, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Negro, S.; Slowing, K.; Fernández-Carballido, A.; Barcia, E. An effective novel delivery strategy of rasagiline for Parkinson’s disease. Int. J. Pharm. 2011, 419, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.A.; Miller, D.R.; Annarumma, S.; Rusch, C.T.; Ramirez-Zamora, A.; Khoshbouei, H. Levodopa-induced dyskinesia: A historical review of Parkinson’s disease, dopamine, and modern advancements in research and treatment. J. Neurol. 2022, 269, 2892–2909. [Google Scholar] [CrossRef] [PubMed]
- Paci, C.; Thomas, A.; Onofrj, M. Amantadine for dyskinesia in patients affected by severe Parkinson’s disease. Neurol. Sci. 2001, 22, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lloret, S.; Rascol, O. Efficacy and safety of amantadine for the treatment of L-DOPA-induced dyskinesia. J. Neural Transm. 2018, 125, 1237–1250. [Google Scholar] [CrossRef]
- Marin, C.; Aguilar, E.; Bonastre, M.; Tolosa, E.; Obeso, J.A. Early administration of entacapone prevents levodopa-induced motor fluctuations in hemiparkinsonian rats. Exp. Neurol. 2005, 192, 184–193. [Google Scholar] [CrossRef]
- Durif, F.; Debilly, B.; Galitzky, M.; Morand, D.; Viallet, F.; Borg, M.; Thobois, S.; Broussolle, E.; Rascol, O. Clozapine improves dyskinesias in Parkinson disease: A double-blind, placebo-controlled study. Neurology 2004, 62, 381–388. [Google Scholar] [CrossRef]
- Lundblad, M.; Andersson, M.; Winkler, C.; Kirik, D.; Wierup, N.; Cenci Nilsson, M.A. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur. J. Neurosci. 2002, 15, 120–132. [Google Scholar] [CrossRef]
- Moro, E.; Esselink, R.J.A.; Xie, J.; Hommel, M.; Benabid, A.L.; Pollak, P. The impact on Parkinson’s disease of electrical parameter settings in STN stimulation. Neurology 2002, 59, 706–713. [Google Scholar] [CrossRef]
- Lozano, A.M.; Dostrovsky, J.; Chen, R.; Ashby, P. Deep brain stimulation for Parkinson’s disease: Disrupting the disruption. Lancet Neurol. 2002, 1, 225–231. [Google Scholar] [CrossRef]
- Temel, Y. Deep Brain Stimulation in Animal Models, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Carlsson, A.; Lindqvist, M.; Magnusson, T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature 1957, 180, 1200. [Google Scholar] [CrossRef]
- Cotzias, G.C.; Van Woert, M.H.; Schiffer, L.M. Aromatic amino acids and modification of parkinsonism. N. Engl. J. Med. 1967, 276, 374–379. [Google Scholar] [CrossRef]
- Rascol, O.; Fabbri, M.; Poewe, W. Amantadine in the treatment of Parkinson’s disease and other movement disorders. Lancet Neurol. 2021, 20, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Geerts, H. Of mice and men: Bridging the translational disconnect in CNS drug discovery. CNS Drugs 2009, 23, 915–926. [Google Scholar] [CrossRef]
- Zeiss, C.J.; Allore, H.G.; Beck, A.P. Established patterns of animal study design undermine translation of disease-modifying therapies for Parkinson’s disease. PLoS ONE 2017, 12, e0171790. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- Arrowsmith, J.; Miller, P. Trial Watch: Phase II and Phase III attrition rates 2011–2012. Nat. Rev. Drug Discov. 2013, 12, 569–570. [Google Scholar] [CrossRef]
- Hartung, T. The (misleading) role of animal models in drug development. Front. Drug Discov. 2024, 4, 1355044. [Google Scholar] [CrossRef]
- Kimmelman, J.; London, A.J.; Ravina, B.; Ramsay, T.; Bernstein, M.; Fine, A.; Stahnisch, F.W.; Emborg, M.E. Launching invasive, first-in-human trials against Parkinson’s disease: Ethical considerations. Mov. Disord. 2009, 24, 1893–1901. [Google Scholar] [CrossRef]
- Pusztai, L.; Hatzis, C.; Andre, F. Reproducibility of research and preclinical validation: Problems and solutions. Nat. Rev. Clin. Oncol. 2013, 10, 720–724. [Google Scholar] [CrossRef]
- Quinn, N.P. Classification of fluctuations in patients with Parkinson’s disease. Neurology 1998, 51, S25–S29. [Google Scholar] [CrossRef] [PubMed]
- Widnell, K. Pathophysiology of motor fluctuations in Parkinson’s disease. Mov. Disord. 2005, 20 (Suppl. S11), S17–S22. [Google Scholar] [CrossRef]
- Papa, S.M.; Engber, T.M.; Kask, A.M.; Chase, T.N. Motor fluctuations in levodopa treated parkinsonian rats: Relation to lesion extent and treatment duration. Brain Res. 1994, 662, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.K.; Kwatra, M.; Wang, J.; Ko, H.S. Levodopa-Induced Dyskinesia in Parkinson’s Disease: Pathogenesis and Emerging Treatment Strategies. Cells 2022, 11, 3736. [Google Scholar] [CrossRef]
- Calabresi, P.; Di Filippo, M.; Ghiglieri, V.; Tambasco, N.; Picconi, B. Levodopa-induced dyskinesias in patients with Parkinson’s disease: Filling the bench-to-bedside gap. Lancet Neurol. 2010, 9, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Iderberg, H.; Francardo, V.; Pioli, E.Y. Animal models of l-DOPA-induced dyskinesia: An update on the current options. Neuroscience 2012, 211, 13–27. [Google Scholar] [CrossRef]
- Wadolowski, R.; Solomon, I. Comparison of basal and hypoxic respiratory behaviors in 6-OHDA and rotenone induced SN lesion Parkinson’s disease rat models. Physiol. Am. Physiol. Soc. 2023, 38, 5735082. [Google Scholar] [CrossRef]
- Konitsiotis, S.; Tsironis, C. Levodopa-induced dyskinesia and rotational behavior in hemiparkinsonian rats: Independent features or components of the same phenomenon? Behav. Brain Res. 2006, 170, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Cenci, M.A.; Ohlin, K.E.; Rylander, D. Plastic effects of L-DOPA treatment in the basal ganglia and their relevance to the development of dyskinesia. Park. Relat. Disord. 2009, 15 (Suppl. S3), S59–S63. [Google Scholar] [CrossRef] [PubMed]
- Björklund, A.; Dunnett, S.B. The Amphetamine Induced Rotation Test: A Re-Assessment of Its Use as a Tool to Monitor Motor Impairment and Functional Recovery in Rodent Models of Parkinson’s Disease. J. Parkinson’s Dis. 2019, 9, 17–29. [Google Scholar] [CrossRef]
- Cenci, M.A.; Crossman, A.R. Animal models of l-dopa-induced dyskinesia in Parkinson’s disease. Mov. Disord. 2018, 33, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Restrepo, J.; Won, L.; Hwang, D.Y.; Kim, K.S.; Kang, U.J. Chronic 3,4-dihydroxyphenylalanine treatment induces dyskinesia in aphakia mice, a novel genetic model of Parkinson’s disease. Neurobiol. Dis. 2007, 27, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Duty, S. Targeting glutamate receptors to tackle the pathogenesis, clinical symptoms and levodopa-induced dyskinesia associated with Parkinson’s disease. CNS Drugs 2012, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.; Macias, R.; Pavón, N.; López, G.; Rodríguez-Oroz, M.C.; Rodríguez, R.; Alvarez, M.; Pedroso, I.; Teijeiro, J.; Fernández, R.; et al. Therapeutic efficacy of unilateral subthalamotomy in Parkinson’s disease: Results in 89 patients followed for up to 36 months. J. Neurol. Neurosurg. Psychiatry 2009, 80, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef]
- Merino Ruiz, M.C.; Guimarães, R.P.; Mortari, M.R. Parkinson’s disease rodent models: Are they suitable for DBS research? J. Neurosci. Methods 2022, 380, 109687. [Google Scholar] [CrossRef]
- Máñez-Miró, J.U.; Rodríguez-Rojas, R.; Del Álamo, M.; Martínez-Fernández, R.; Obeso, J.A. Present and future of subthalamotomy in the management of Parkinson’s disease: A systematic review. Expert. Rev. Neurother. 2021, 21, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Rizelio, V.; Szawka, R.E.; Xavier, L.L.; Achaval, M.; Rigon, P.; Saur, L.; Matheussi, F.; Delattre, A.M.; Anselmo-Franci, J.A.; Meneses, M.; et al. Lesion of the subthalamic nucleus reverses motor deficits but not death of nigrostriatal dopaminergic neurons in a rat 6-hydroxydopamine-lesion model of Parkinson’s disease. Braz. J. Med. Biol. Res. 2010, 43, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Knorr, S.; Musacchio, T.; Paulat, R.; Matthies, C.; Endres, H.; Wenger, N.; Harms, C.; Ip, C.W. Experimental deep brain stimulation in rodent models of movement disorders. Exp. Neurol. 2022, 348, 113926. [Google Scholar] [CrossRef]
- Benazzouz, A.; Gao, D.M.; Ni, Z.G.; Piallat, B.; Bouali-Benazzouz, R.; Benabid, A.L. Effect of high-frequency stimulation of the subthalamic nucleus on the neuronal activities of the substantia nigra pars reticulata and ventrolateral nucleus of the thalamus in the rat. Neuroscience 2000, 99, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Filali, M.; Hutchison, W.D.; Palter, V.N.; Lozano, A.M.; Dostrovsky, J.O. Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp. Brain Res. 2004, 156, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Lane, E.L.; Lelos, M.J. Defining the unknowns for cell therapies in Parkinson’s disease. Dis. Model. Mech. 2022, 15, dmm049543. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Björklund, A. Cell Therapy in Parkinson’s Disease. NeuroRx 2004, 1, 382–393. [Google Scholar] [CrossRef]
- Nieto, M.; Gil-Bea, F.J.; Dalfó, E.; Cuadrado, M.; Cabodevilla, F.; Sánchez, B.; Catena, S.; Sesma, T.; Ribé, E.; Ferrer, I.; et al. Increased sensitivity to MPTP in human α-synuclein A30P transgenic mice. Neurobiol. Aging 2006, 27, 848–856. [Google Scholar] [CrossRef]
- Song, D.D.; Shults, C.W.; Sisk, A.; Rockenstein, E.; Masliah, E. Enhanced substantia nigra mitochondrial pathology in human α-synuclein transgenic mice after treatment with MPTP. Exp. Neurol. 2004, 186, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Drolet, R.E.; Behrouz, B.; Lookingland, K.J.; Goudreau, J.L. Mice Lacking α-Synuclein have an Attenuated Loss of Striatal Dopamine Following Prolonged Chronic MPTP Administration. Neurotoxicology 2004, 25, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, O.M.; Fornai, F.; Alessandrí, M.G.; Takamori, S.; Geppert, M.; Jahn, R.; Südhof, T.C. Role of α-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice. Neuroscience 2003, 118, 985–1002. [Google Scholar] [CrossRef] [PubMed]
- Klivenyi, P.; Siwek, D.; Gardian, G.; Yang, L.; Starkov, A.; Cleren, C.; Ferrante, R.J.; Kowall, N.W.; Abeliovich, A.; Beal, M.F. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol. Dis. 2006, 21, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Peelaerts, W.; Bousset, L.; Van Der Perren, A.; Moskalyuk, A.; Pulizzi, R.; Giugliano, M.; Van Den Haute, C.; Melki, R.; Baekelandt, V. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 2015, 522, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Hoban, D.B.; Shrigley, S.; Mattsson, B.; Breger, L.S.; Jarl, U.; Cardoso, T.; Wahlestedt, J.N.; Luk, K.C.; Björklund, A.; Parmar, M. Impact of α-synuclein pathology on transplanted hESC-derived dopaminergic neurons in a humanized α-synuclein rat model of PD. Proc. Natl. Acad. Sci. USA 2020, 117, 15209–15220. [Google Scholar] [CrossRef]
- Negrini, M.; Tomasello, G.; Davidsson, M.; Fenyi, A.; Adant, C.; Hauser, S.; Espa, E.; Gubinelli, F.; Manfredsson, F.P.; Melki, R.; et al. Sequential or Simultaneous Injection of Preformed Fibrils and AAV Overexpression of Alpha-Synuclein Are Equipotent in Producing Relevant Pathology and Behavioral Deficits. J. Parkinson’s Dis. 2022, 12, 1133–1153. [Google Scholar] [CrossRef] [PubMed]
| Model | Da Neurons Loss | Asyn-Aggregation |
|---|---|---|
| 6-OHDA | Yes [7,12,13,14] | No [15] |
| MPTP | Yes [16] | No |
| RESERPINE | Yes [17,18] | Yes [19] |
| ROTENONE | Yes [20,21,22] | Yes [20,21,22] |
| PARAQUAT | Yes [23,24,25] | Yes [26,27] |
| PFFs | Yes [28,29,30] | Yes [28,29,30] |
| AAV-aSYN | Yes [31,32] | Yes [31,32] |
| TRANSGENIC | Yes [33] | Yes [33,34,35,36] |
| Model | Motor Symptoms | Tests for Motor Symptoms | Non-Motor Symptoms | Tests for Non-Motor Symptoms |
|---|---|---|---|---|
| 6-OHDA | Dysfunctions in locomotor activities (akinesia, bradykinesia, hypokinesia), muscle rigidity [123,124], dyskinesia, and gait abnormalities [90]. | Open field, rotarod, pole test [125], tail suspension test (TST), tail suspension swing test (TSST) [126], Catwalk [127,128], rotational sensitization [129], grasping test or movement-induced reflex electromyographic activity [123,124], AIMs and other rating scales for dyskinesia [130,131], rotational motor behavior (apomorphine or amphetamine tests) [132], and cylinder test [133]. | Sleep abnormalities [134] Gastrointestinal dysfunction [135] Anxiety and depression [136] | EEG Gastric emptying [135] Sucrose preference test Forced swimming test Open field test [136] |
| MPTP | Dysfunctions in locomotor activities (akinesia, bradykinesia, hypokinesia), dyskinesia, resting tremors, muscle rigidity, and gait abnormalities [137,138,139]. | Open field, rotarod, balance beam test [140] stepping test [141], pole test [142], horizontal and vertical grid tests [143], swim-test [144], catalepsy test, cylinder test, Catwalk [128]. | Sleep abnormalities [145] Gastrointestinal dysfunction [146] Anxiety and depression [136] | EEG [145] Stool Collection [146] Sucrose preference test Forced swimming test Open field test [136] |
| RESERPINE | Akinesia [147], oral dyskinesia [148,149], and muscle rigidity. | Open field, rotarod, catalepsy test, and oral dyskinesia assessment [148,150,151]. | Sleep abnormalities [152] Anxiety [153] depression [154] | EEG [152] Open field Elevated plus maze [154], |
| ROTENONE | Dysfunctions in locomotor activities (akinesia, bradykinesia, hypokinesia), muscle rigidity [155], gait abnormalities [156]. | Open field, rotarod, pole test, forced swimming test [157], catalepsy test, cylinder test. | Gastrointestinal dysfunction [158] Anxiety and depression [136] | Stool collection [159] Urine collection [159] Gastric emptying [158] Bead latency [160] Sucrose preference test Forced swimming test Open field test [136] |
| PARAQUAT | Dysfunctions in locomotor activities (akinesia, bradykinesia, hypokinesia) [161], and forelimb rigidity [162]. | Open field, rotarod, inclined plane test, swimming test, catalepsy test, wire suspension test [161]. | Gastrointestinal dysfunction [163] Anxiety and depression [164] | Gastric motility [163] Sucrose preference test Forced swimming test Open field test Elevated plus maze [164] |
| PFFs | Dysfunctions in locomotor activities (akinesia, bradykinesia, hypokinesia) [28,147], dyskinesia. | Open field, rotarod, wire hang test, tail suspension test (TST) [28], muscular strength test, elevated plus maze test, forced swim test, Y-maze test, apomorphine-induced rotational behavior test [165]. | Sleep abnormalities [166] Gastrointestinal dysfunction [167] Anxiety and depression [168] | EEG [166] Stool collection [167] Open field Elevated plus maze Tail suspension [168] |
| GENETIC | Dysfunctions in locomotor and sensorimotor activities (akinesia, bradykinesia, hypokinesia) [169], dyskinesia [170], and gait abnormalities [171]. | Open field, rotarod, tapered balance beam test [171], pole test, adhesive removal test [172], grip strength [173], tail suspension test [174], AIM scores [170], cylinder test [175], ink test [98]. | Sleep Abnormalities [176] Gastrointestinal dysfunction [177] | EEG [176] Stool collection [177] |
| Treatment | Humans | Rodents |
|---|---|---|
| Pharmacological Treatment | ||
| Levodopa | 300–1500 mg/day [203,204] | 12.5–100 mg/kg [205,206] |
| Pramipexole | 0.125–4.5 mg/day [203,204] | 0.05–1 mg/kg [207,208] |
| Rasagiline | 1 mg/day [203] | 1 mg/kg [209] |
| Dyskinesia | ||
| Levodopa | - | 1–300 mg/kg [210] |
| Dyskinesia Treatment | ||
| Amantadine | 300 mg/day [211] | 10–60 mg/kg [212] |
| Entacapone | 600–1600 mg/day [203] | 30 mg/kg/day [213] |
| Clozapine | 39.4 + −4.5 mg/day [214] | 8 mg/kg dose [215] |
| Surgical TreatmENT | ||
| DBS | 130–185 Hz; 60–90 μs pulses [216], eletrodes size: 6 mm2 [217] | 130–185 Hz; 60–90 μs pulses [218], eletrodes size: 1–100 μm2 [217]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, R.P.; Resende, M.C.S.d.; Tavares, M.M.; Belardinelli de Azevedo, C.; Ruiz, M.C.M.; Mortari, M.R. Construct, Face, and Predictive Validity of Parkinson’s Disease Rodent Models. Int. J. Mol. Sci. 2024, 25, 8971. https://doi.org/10.3390/ijms25168971
Guimarães RP, Resende MCSd, Tavares MM, Belardinelli de Azevedo C, Ruiz MCM, Mortari MR. Construct, Face, and Predictive Validity of Parkinson’s Disease Rodent Models. International Journal of Molecular Sciences. 2024; 25(16):8971. https://doi.org/10.3390/ijms25168971
Chicago/Turabian StyleGuimarães, Rayanne Poletti, Maria Clara Souza de Resende, Miguel Mesquita Tavares, Caio Belardinelli de Azevedo, Miguel Cesar Merino Ruiz, and Márcia Renata Mortari. 2024. "Construct, Face, and Predictive Validity of Parkinson’s Disease Rodent Models" International Journal of Molecular Sciences 25, no. 16: 8971. https://doi.org/10.3390/ijms25168971
APA StyleGuimarães, R. P., Resende, M. C. S. d., Tavares, M. M., Belardinelli de Azevedo, C., Ruiz, M. C. M., & Mortari, M. R. (2024). Construct, Face, and Predictive Validity of Parkinson’s Disease Rodent Models. International Journal of Molecular Sciences, 25(16), 8971. https://doi.org/10.3390/ijms25168971







