Melanin as a Photothermal Agent in Antimicrobial Systems
Abstract
:1. Introduction
2. Melanin Photothermal Properties for Antibacterial Activity
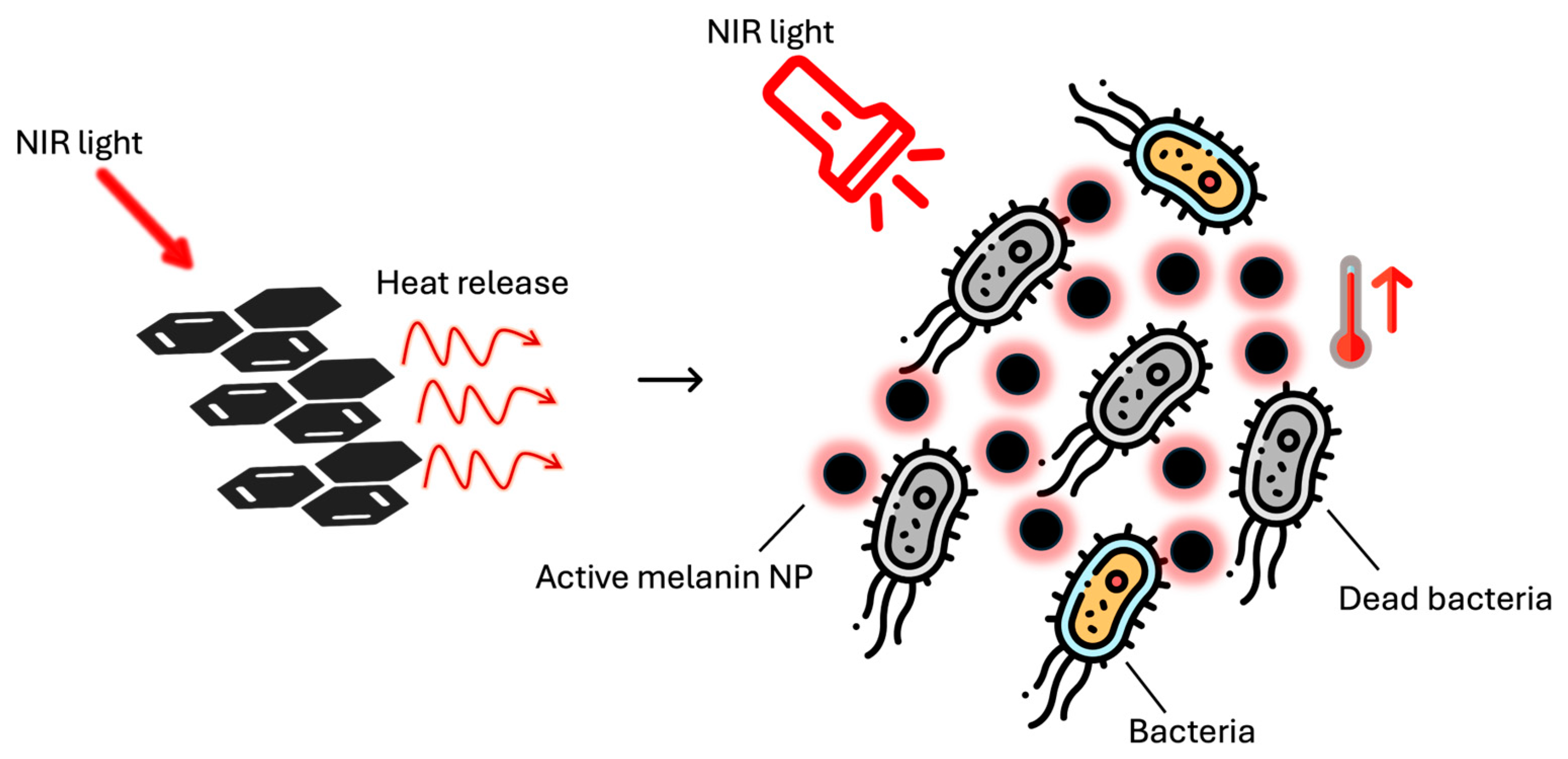
3. Antibacterial Systems Based on Melanin Action Only
Recent Advances in the Use of Melanin as an Antibacterial Agent
4. Antibacterial Systems Based on the Synergy of Melanin and Other Agents
Composite Nanomaterials for Improving Antimicrobial Activity of PDA
5. Future Challenges
5.1. Further Exploration of Melanin’s Properties
5.2. Surface Modification of Melanin for Better Interaction with Microorganisms
5.3. Biocompatibility of Composite Systems
5.4. Scale-Up: A Problem Related to Costs, Drug Administration Agencies’ Approval and Applications on Real-World Problems
5.5. Adverse Reaction to the Application of Photothermally Active Material for Human Health
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Jo, I.; Kim, Y.; Chung, W.; Kim, J.; Kim, S.; Lim, E.; Ahn, H.; Ryu, S. Microbiology and risk factors for gram-positive Cocci bacteremia in biliary infections. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Pasquarella, C.; Pitzurra, O.; Savino, A. The index of microbial air contamination. J. Hosp. Infect. 2000, 46, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Khan, A.U. Breaking the Spell: Combating Multidrug Resistant ‘Superbugs’. Front. Microbiol. 2016, 7, 174. [Google Scholar] [CrossRef]
- Alekshun, M.N.; Levy, S.B. Molecular Mechanisms of Antibacterial Multidrug Resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, S.; Khan, A.U. Nanoparticles vs. biofilms: A battle against another paradigm of antibiotic resistance. MedChemComm 2016, 7, 1479–1498. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef]
- Osaki, F.; Kanamori, T.; Sando, S.; Sera, T.; Aoyama, Y. A Quantum Dot Conjugated Sugar Ball and Its Cellular Uptake. On the Size Effects of Endocytosis in the Subviral Region. J. Am. Chem. Soc. 2004, 126, 6520–6521. [Google Scholar] [CrossRef]
- Sun, T.M.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.X.; Xia, Y.N. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chem.-Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.W.; Archer, L.A.; Yang, Z. Hollow Micro-/Nanostructures: Synthesis and Applications. Adv. Mater. 2008, 20, 3987–4019. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Polydopamine-Based Nanoprobes Application in Optical Biosensing. Biosensors 2023, 13, 956. [Google Scholar] [CrossRef]
- Menichetti, A.; Mordini, D.; Montalti, M. Polydopamine Nanosystems in Drug Delivery: Effect of Size, Morphology, and Surface Charge. Nanomaterials 2024, 14, 303. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zeng, X.; Chen, H.; Li, Z.; Zeng, W.; Mei, L.; Zhao, Y. Versatile Polydopamine Platforms: Synthesis and Promising Applications for Surface Modification and Advanced Nanomedicine. ACS Nano 2019, 13, 8537–8565. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Ai, K.L.; Lu, L.H. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, L.; Zhang, J.; Hu, J.; Duan, G.; Liu, X.; Li, Y.; Gu, Z. Polydopamine antibacterial materials. Mater. Horiz. 2021, 8, 1618–1633. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Obeng, E.; Sun, X.; Kwon, N.; Shen, J.; Yoon, J. Polydopamine, harness of the antibacterial potentials—A review. Mater. Today Bio 2022, 15, 100329. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-W.; Chiu, T.-H. Stable N-halamine on polydopamine coating for high antimicrobial efficiency. Eur. Polym. J. 2020, 130, 109654. [Google Scholar] [CrossRef]
- Liu, H.; Qu, X.; Tan, H.; Song, J.; Lei, M.; Kim, E.; Payne, G.F.; Liu, C. Role of polydopamine’s redox-activity on its pro-oxidant, radical-scavenging, and antimicrobial activities. Acta Biomater. 2019, 88, 181–196. [Google Scholar] [CrossRef]
- Ahmed, K.; Zaidi, S.F.; Mati-Ur, R.; Rehman, R.; Kondo, T. Hyperthermia and protein homeostasis: Cytoprotection and cell death. J. Therm. Biol. 2020, 91, 102615. [Google Scholar] [CrossRef]
- Song, W.; Yang, H.; Liu, S.; Yu, H.; Li, D.; Li, P.; Xing, R. Melanin: Insights into structure, analysis, and biological activities for future development. J. Mater. Chem. B 2023, 11, 7528–7543. [Google Scholar] [CrossRef] [PubMed]
- Mavridi-Printezi, A.; Guernelli, M.; Menichetti, A.; Montalti, M. Bio-Applications of Multifunctional Melanin Nanoparticles: From Nanomedicine to Nanocosmetics. Nanomaterials 2020, 10, 2276. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhou, X.H.; McCallum, N.C.; Hu, Z.Y.; Ni, Q.Z.; Kapoor, U.; Heil, C.M.; Cay, K.S.; Zand, T.; Mantanona, A.J.; et al. Unraveling the Structure and Function of Melanin through Synthesis. J. Am. Chem. Soc. 2021, 143, 2622–2637. [Google Scholar] [CrossRef] [PubMed]
- D’Alba, L.; Shawkey, M.D. Melanosomes: Biogenesis, properties, and evolution of an ancient organelle. Physiol. Rev. 2019, 99, 1–19. [Google Scholar] [CrossRef]
- Lino, V.; Manini, P. Dihydroxynaphthalene-Based Allomelanins: A Source of Inspiration for Innovative Technological Materials. ACS OMEGA 2022, 7, 15308–15314. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, W.; Gu, Z.; Wang, L.; Guo, L.; Ma, S.; Li, C.; Sun, J.; Han, B.; Chang, J. Recent advances and progress on melanin: From source to application. Int. J. Mol. Sci. 2023, 24, 4360. [Google Scholar] [CrossRef]
- Ju, K.Y.; Lee, Y.; Lee, S.; Park, S.B.; Lee, J.K. Bioinspired Polymerization of Dopamine to Generate Melanin-Like Nanoparticles Having an Excellent Free-Radical-Scavenging Property. Biomacromolecules 2011, 12, 625–632. [Google Scholar] [CrossRef]
- Xiao, M.; Li, Y.W.; Allen, M.C.; Deheyn, D.D.; Yue, X.J.; Zhao, J.Z.; Gianneschi, N.C.; Shawkey, M.D.; Dhinojwala, A. Bio-Inspired Structural Colors Produced via Self-Assembly of Synthetic Melanin Nanoparticles. Acs Nano 2015, 9, 5454–5460. [Google Scholar] [CrossRef]
- Jia, D.; Zou, Y.; Cheng, J.; Zhang, Y.; Zhang, H.; Lu, K.; Chen, H.; Zhang, Y.; Yu, Q. A multifunctional nanoplatform with “disruption and killing” function to improve the efficiency of conventional antibiotics for biofilm eradication. J. Mater. Sci. Technol. 2025, 205, 98–108. [Google Scholar] [CrossRef]
- Ajayi, A.; Akinjogunla, O.; Odeyemi, A.; Owolabi, A. Role of plasmids in antibiotic resistance in clinical infections and implications for epidemiological surveillance: A review. All Life 2024, 17, 2350414. [Google Scholar] [CrossRef]
- Rosso, L.; Lobry, J.R.; Bajard, S.; Flandrois, J.P. Convenient Model To Describe the Combined Effects of Temperature and pH on Microbial Growth. Appl. Environ. Microbiol. 1995, 61, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Peleg, M. On the Time Presentation in Differential Rate Equations of Dynamic Microbial Inactivation and Growth. Food Eng. Rev. 2024, 16, 179–191. [Google Scholar] [CrossRef]
- Gilbert, J.; Alverdy, J. Where do the pathogens that cause surgical site infections come from? Sci. Transl. Med. 2024, 16, eado1449. [Google Scholar] [CrossRef]
- Li, H.; Gänzle, M. Some Like It Hot: Heat Resistance of Escherichia coli in Food. Front. Microbiol. 2016, 7, 1763. [Google Scholar] [CrossRef] [PubMed]
- Atac, N.; Gunduz, H.; Koc, I.; Onbasli, K.; Khan, M.; Savani, S.; Sennaroglu, A.; Can, F.; Acar, H.; Kolemen, S. Selective antibacterial and antibiofilm activity of chlorinated hemicyanine against gram-positive bacteria. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2024, 316, 124324. [Google Scholar] [CrossRef] [PubMed]
- Doveri, L.; Fernandez, Y.; Dacarro, G. Nanomaterials for Photothermal Antimicrobial Surfaces. Acs Omega 2024, 9, 25575–25590. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int. Rev. Phys. Chem. 2000, 19, 409–453. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.; Qian, W.; El-Sayed, M. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef]
- Jaque, D.; Maestro, L.M.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Rodríguez, E.M.; Solé, J.G. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Mavridi-Printezi, A.; Menichetti, A.; Ferrazzano, L.; Montalti, M. Reversible Supramolecular Noncovalent Self-Assembly Determines the Optical Properties and the Formation of Melanin-like Nanoparticles. J. Phys. Chem. Lett. 2022, 13, 9829–9833. [Google Scholar] [CrossRef]
- Jablonski, N.; Chaplin, G. Human skin pigmentation as an adaptation to UV radiation. Proc. Natl. Acad. Sci. USA 2010, 107, 8962–8968. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chen, X.; Yang, P.; Liang, G.; Yang, Y.; Gu, Z.; Li, Y. Regulating the absorption spectrum of polydopamine. Science Advances 2020, 6, eabb4696. [Google Scholar] [CrossRef] [PubMed]
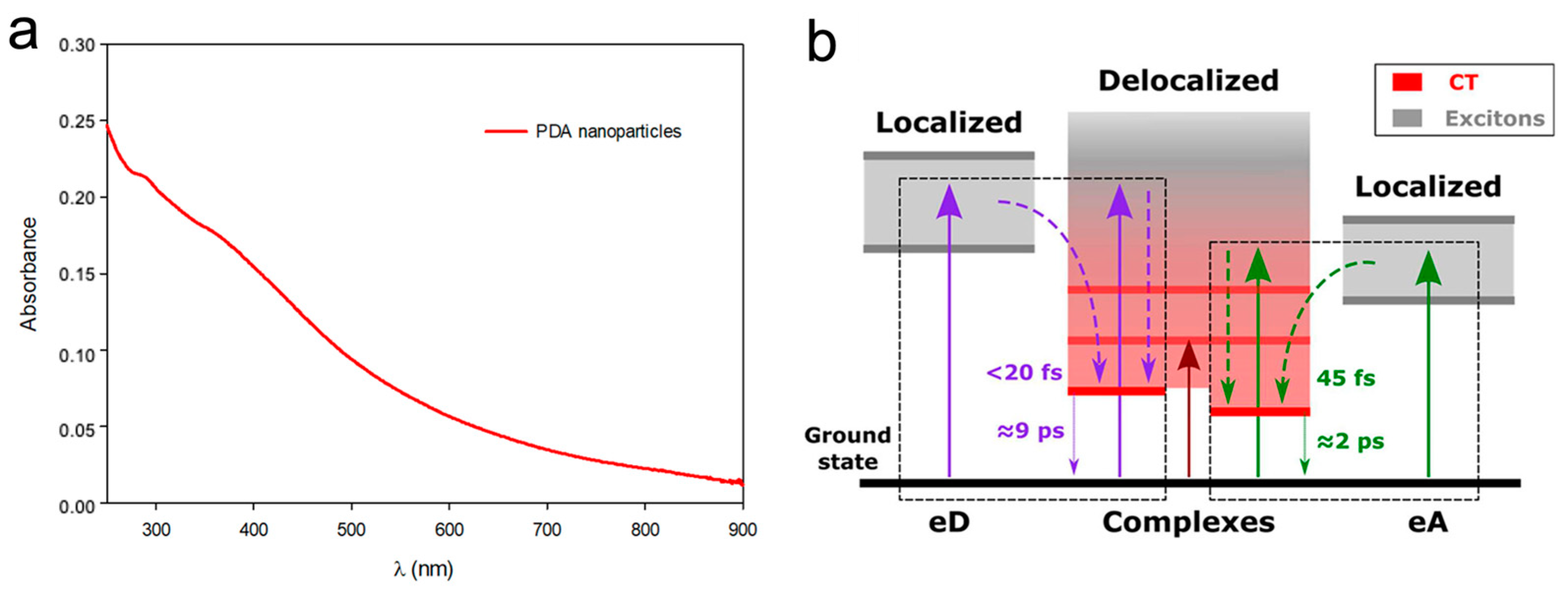
- Petropoulos, V.; Mavridi-Printezi, A.; Menichetti, A.; Mordini, D.; Kabacinski, P.; Gianneschi, N.; Montalti, M.; Maiuri, M.; Cerullo, G. Sub-50 fs Formation of Charge Transfer States Rules the Fate of Photoexcitations in Eumelanin-Like Materials. J. Phys. Chem. Lett. 2024, 15, 3639–3645. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration during Wound Healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, J.; Hu, J.; Cheng, Y.; Chen, X.; Gu, Z.; Li, Y. Bioinspired polydopamine hydrogels: Strategies and applications. Prog. Polym. Sci. 2023, 146, 101740. [Google Scholar] [CrossRef]
- Che, P.; Liu, W.; Chang, X.; Wang, A.; Han, Y. Multifunctional silver film with superhydrophobic and antibacterial properties. Nano Res. 2016, 9, 442–450. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Kholmanov, I.N.; Stoller, M.D.; Edgeworth, J.; Lee, W.H.; Li, H.; Lee, J.; Barnhart, C.; Potts, J.R.; Piner, R.; Akinwande, D.; et al. Nanostructured Hybrid Transparent Conductive Films with Antibacterial Properties. ACS Nano 2012, 6, 5157–5163. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Huang, S.; Xu, A.; Xue, W. Injectable Adhesive Self-Healing Multiple-Dynamic-Bond Crosslinked Hydrogel with Photothermal Antibacterial Activity for Infected Wound Healing. Chem. Mater. 2022, 34, 2655–2671. [Google Scholar] [CrossRef]
- Huang, Y.; Mu, L.; Zhao, X.; Han, Y.; Guo, B.L. Bacterial Growth-Induced Tobramycin Smart Release Self-Healing Hydrogel for Pseudomonas aeruginosa-Infected Burn Wound Healing. Acs Nano 2022, 16, 13022–13036. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Z.; Liang, Y.; He, J.; Guo, B. Multifunctional Tissue-Adhesive Cryogel Wound Dressing for Rapid Nonpressing Surface Hemorrhage and Wound Repair. ACS Appl. Mater. Interfaces 2020, 12, 35856–35872. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liang, Y.; Liang, Y.; Pan, G.; Guo, B. Injectable stretchable self-healing dual dynamic network hydrogel as adhesive anti-oxidant wound dressing for photothermal clearance of bacteria and promoting wound healing of MRSA infected motion wounds. Chem. Eng. J. 2022, 427, 132039. [Google Scholar] [CrossRef]
- Xiang, Y.; Qi, X.; Cai, E.; Zhang, C.; Wang, J.; Lan, Y.; Deng, H.; Shen, J.; Hu, R. Highly efficient bacteria-infected diabetic wound healing employing a melanin-reinforced biopolymer hydrogel. Chem. Eng. J. 2023, 460, 141852. [Google Scholar] [CrossRef]
- Zhou, X.H.; McCallum, N.C.; Hu, Z.Y.; Cao, W.; Gnanasekaran, K.; Feng, Y.N.; Stoddart, J.F.; Wang, Z.; Gianneschi, N.C. Artificial Allomelanin Nanoparticles. Acs Nano 2019, 13, 10980–10990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, D.; Tan, J.; Chang, Z.; Liu, X.; Ma, W.; Xu, Y. Near-Infrared Regulated Nanozymatic/Photothermal/Photodynamic Triple-Therapy for Combating Multidrug-Resistant Bacterial Infections via Oxygen-Vacancy Molybdenum Trioxide Nanodots. Small 2021, 17, 2005739. [Google Scholar] [CrossRef]
- Qi, R.; Cui, Y.; Liu, J.; Wang, X.; Yuan, H. Recent Advances of Composite Nanomaterials for Antibiofilm Application. Nanomaterials 2023, 13, 2725. [Google Scholar] [CrossRef]
- Wang, Z.; Zou, Y.; Li, Y.; Cheng, Y. Metal-Containing Polydopamine Nanomaterials: Catalysis, Energy, and Theranostics. Small 2020, 16, 1907042. [Google Scholar] [CrossRef]
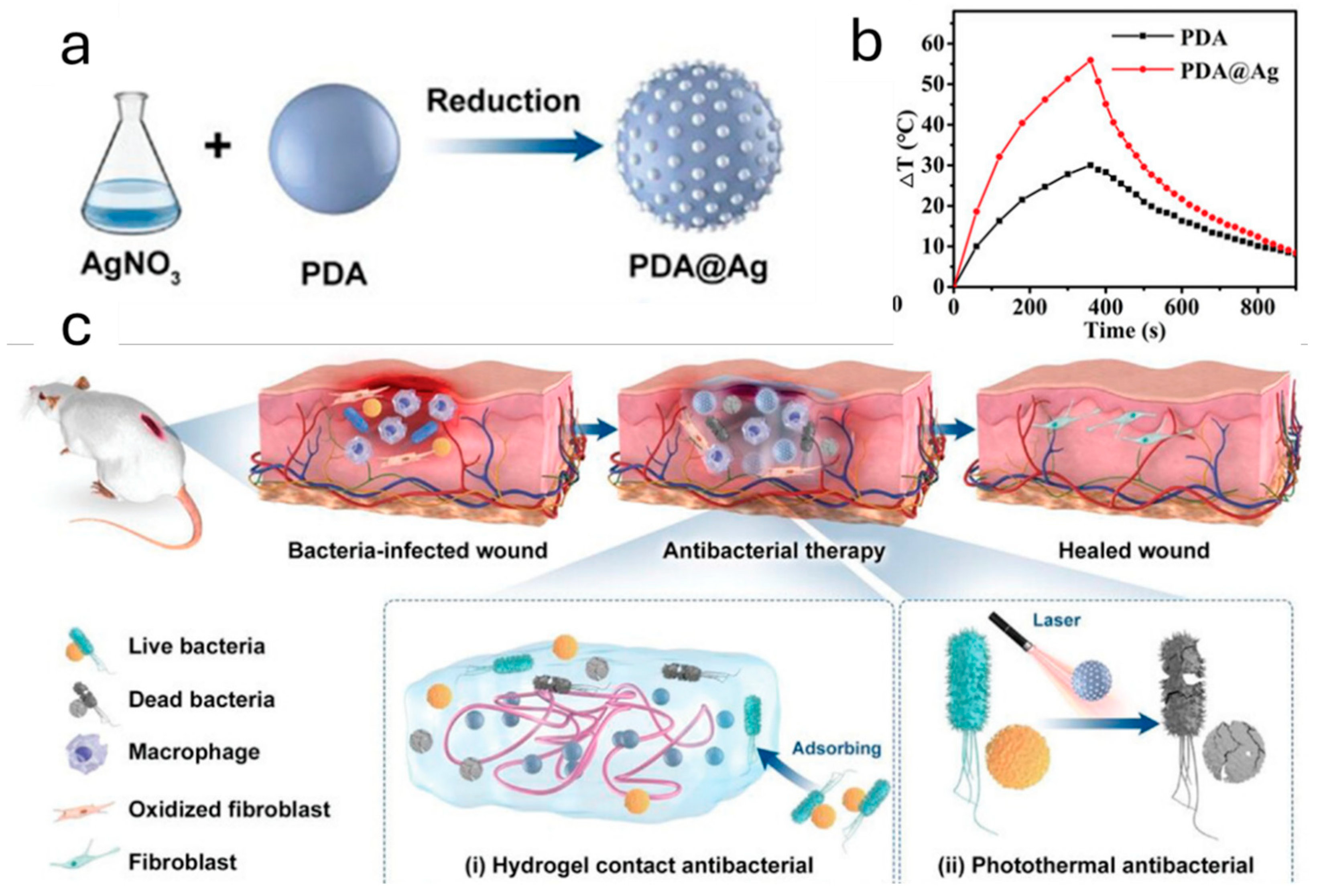
- Qi, X.; Huang, Y.; You, S.; Xiang, Y.; Cai, E.; Mao, R.; Pan, W.; Tong, X.; Dong, W.; Ye, F.; et al. Engineering Robust Ag-Decorated Polydopamine Nano-Photothermal Platforms to Combat Bacterial Infection and Prompt Wound Healing. Adv. Sci. 2022, 9, 2106015. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Xu, Q.; Chang, M.; Zhang, Y.; Wang, E.; Xing, M.; Gao, L.; Huan, Z.; Guo, F.; Chang, J. PDA/Cu Bioactive Hydrogel with “Hot Ions Effect” for Inhibition of Drug-Resistant Bacteria and Enhancement of Infectious Skin Wound Healing. Acs Appl. Mater. Interfaces 2020, 12, 31255–31269. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, Y.; Xin, H.; Yan, D.; Li, G.; Chen, R. Metal–phenolic network-based polydopamine@Cu within a polyvinyl alcohol hydrogel film for improved infected wound healing through antibacterial and pro-angiogenesis activity. Mater. Des. 2022, 221, 110904. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Z.; Zhang, N.; Gao, W.; Li, J.; Pu, Y.; He, B.; Xie, J. A Mg2+ polydopamine composite hydrogel for the acceleration of infected wound healing. Bioact. Mater. 2022, 15, 203–213. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, M.; Lv, N.; Cheng, C.; Huang, P.; Li, J.; Hu, Y.; Sun, M. Dual stimuli-responsive metal-organic framework-based nanosystem for synergistic photothermal/pharmacological antibacterial therapy. Acta Biomater. 2021, 122, 291–305. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Yang, T.; Qian, W.; Qi, C.; Mao, L.; Yu, X.; Zhu, H.; Luo, G.; Deng, J. Combined photothermal and antibiotic therapy for bacterial infection via acidity-sensitive nanocarriers with enhanced antimicrobial performance. Appl. Mater. Today 2018, 12, 415–429. [Google Scholar] [CrossRef]
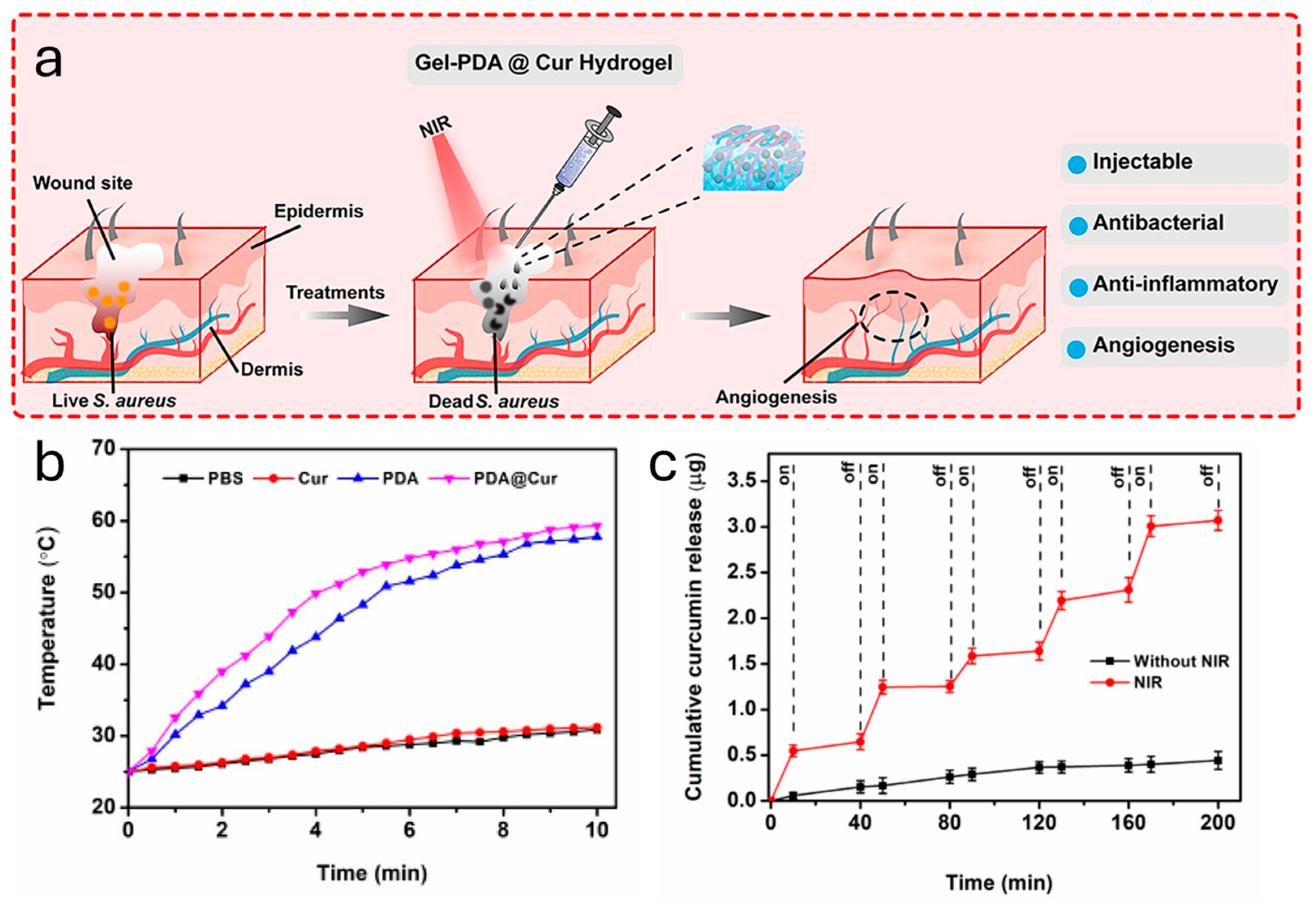
- Tao, B.L.; Lin, C.C.; Yuan, Z.; He, Y.; Chen, M.W.; Li, K.; Hu, J.W.; Yang, Y.L.; Xia, Z.Z.L.; Cai, K.Y. Near infrared light-triggered on-demand Cur release from Gel-PDA@Cur composite hydrogel for antibacterial wound healing. Chem. Eng. J. 2021, 403, 126182. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; An, J.; Fu, H.; Wu, X.; Pang, C.; Gao, H. Construction of an Antibacterial Membrane Based on Dopamine and Polyethylenimine Cross-Linked Graphene Oxide. ACS Biomater. Sci. Eng. 2019, 5, 2732–2739. [Google Scholar] [CrossRef]
- Huang, Y.R.; Li, Y.W.; Hu, Z.Y.; Yue, X.J.; Proetto, M.T.; Jones, Y.; Gianneschi, N.C. Mimicking Melanosomes: Polydopamine Nanoparticles as Artificial Microparasols. Acs Cent. Sci. 2017, 3, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Sunoqrot, S.; Mahmoud, N.; Ibrahim, L.; Al-Dabash, S.; Raschke, H.; Hergenröder, R. Tuning the Surface Chemistry of Melanin-Mimetic Polydopamine Nanoparticles Drastically Enhances Their Accumulation into Excised Human Skin. Acs Biomater. Sci. Eng. 2020, 6, 4424–4432. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, C.; Zhu, Y.; Zheng, L.; Zhang, L.; Hu, Y.; Yu, B.; Wang, Y.; Xu, F. Biofilm-Sensitive Photodynamic Nanoparticles for Enhanced Penetration and Antibacterial Efficiency. Adv. Funct. Mater. 2021, 31, 2103591. [Google Scholar] [CrossRef]
- Wu, H.; Wei, M.; Xu, Y.; Li, Y.; Zhai, X.; Su, P.; Ma, Q.; Zhang, H. PDA-Based Drug Delivery Nanosystems: A Potential Approach for Glioma Treatment. Int. J. Nanomed. 2022, 17, 3751–3775. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh-Narbat, M.; Memic, A.; McGowan, K.B.; Memic, A.; Tamayol, A. Advances in antimicrobial orthopaedic devices and FDA regulatory challenges. Prog. Biomed. Eng. 2024, 6, 032002. [Google Scholar] [CrossRef]
- Thomsen, S.; Pearce, J. Thermal Damage and Rate Processes in Biologic Tissues; Springer: Dordrecht, The Netherlands, 2011; pp. 487–549. [Google Scholar]
- Deng, X.; Shao, Z.; Zhao, Y. Solutions to the Drawbacks of Photothermal and Photodynamic Cancer Therapy. Adv. Sci. 2021, 8, 2002504. [Google Scholar] [CrossRef]
- Salimi, M.; Mosca, S.; Gardner, B.; Palombo, F.; Matousek, P.; Stone, N. Nanoparticle-Mediated Photothermal Therapy Limitation in Clinical Applications Regarding Pain Management. Nanomaterials 2022, 12, 922. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menichetti, A.; Mordini, D.; Montalti, M. Melanin as a Photothermal Agent in Antimicrobial Systems. Int. J. Mol. Sci. 2024, 25, 8975. https://doi.org/10.3390/ijms25168975
Menichetti A, Mordini D, Montalti M. Melanin as a Photothermal Agent in Antimicrobial Systems. International Journal of Molecular Sciences. 2024; 25(16):8975. https://doi.org/10.3390/ijms25168975
Chicago/Turabian StyleMenichetti, Arianna, Dario Mordini, and Marco Montalti. 2024. "Melanin as a Photothermal Agent in Antimicrobial Systems" International Journal of Molecular Sciences 25, no. 16: 8975. https://doi.org/10.3390/ijms25168975




