Toxic Features and Metabolomic Intervention of Glabrene, an Impurity Found in the Pharmaceutical Product of Glabridin
Abstract
1. Introduction
2. Results
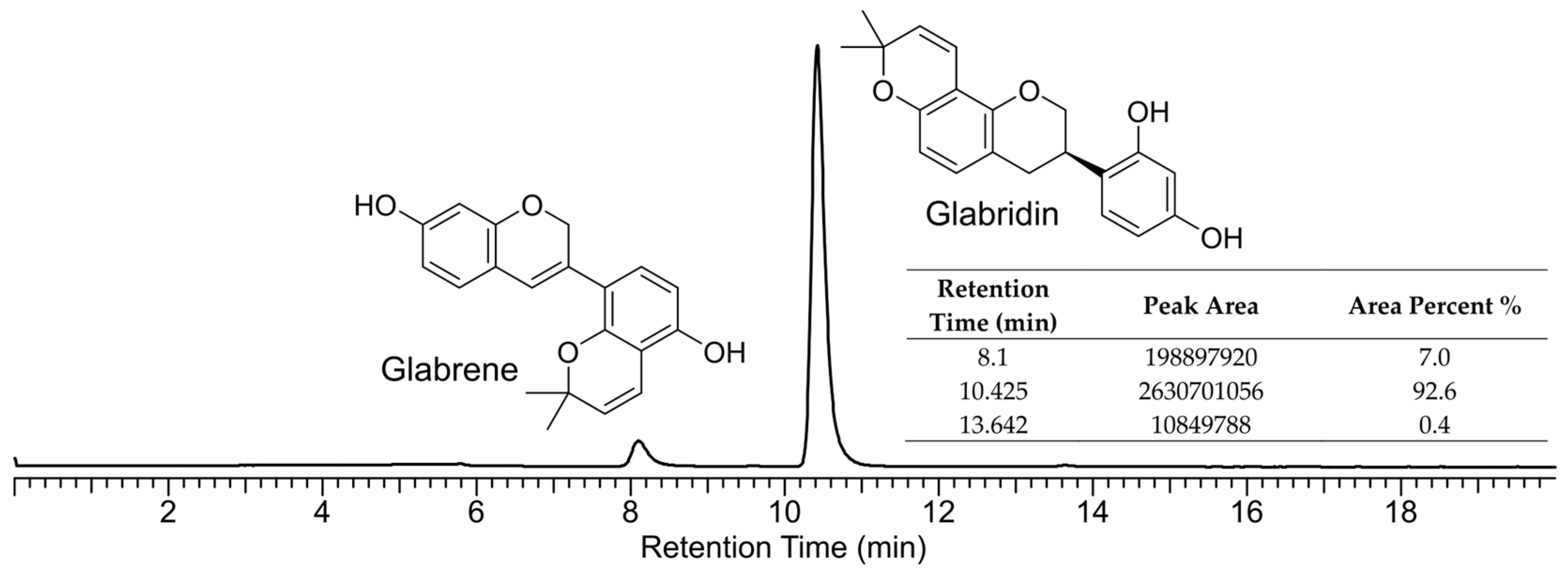
2.1. Chemical Verification of Glabrene
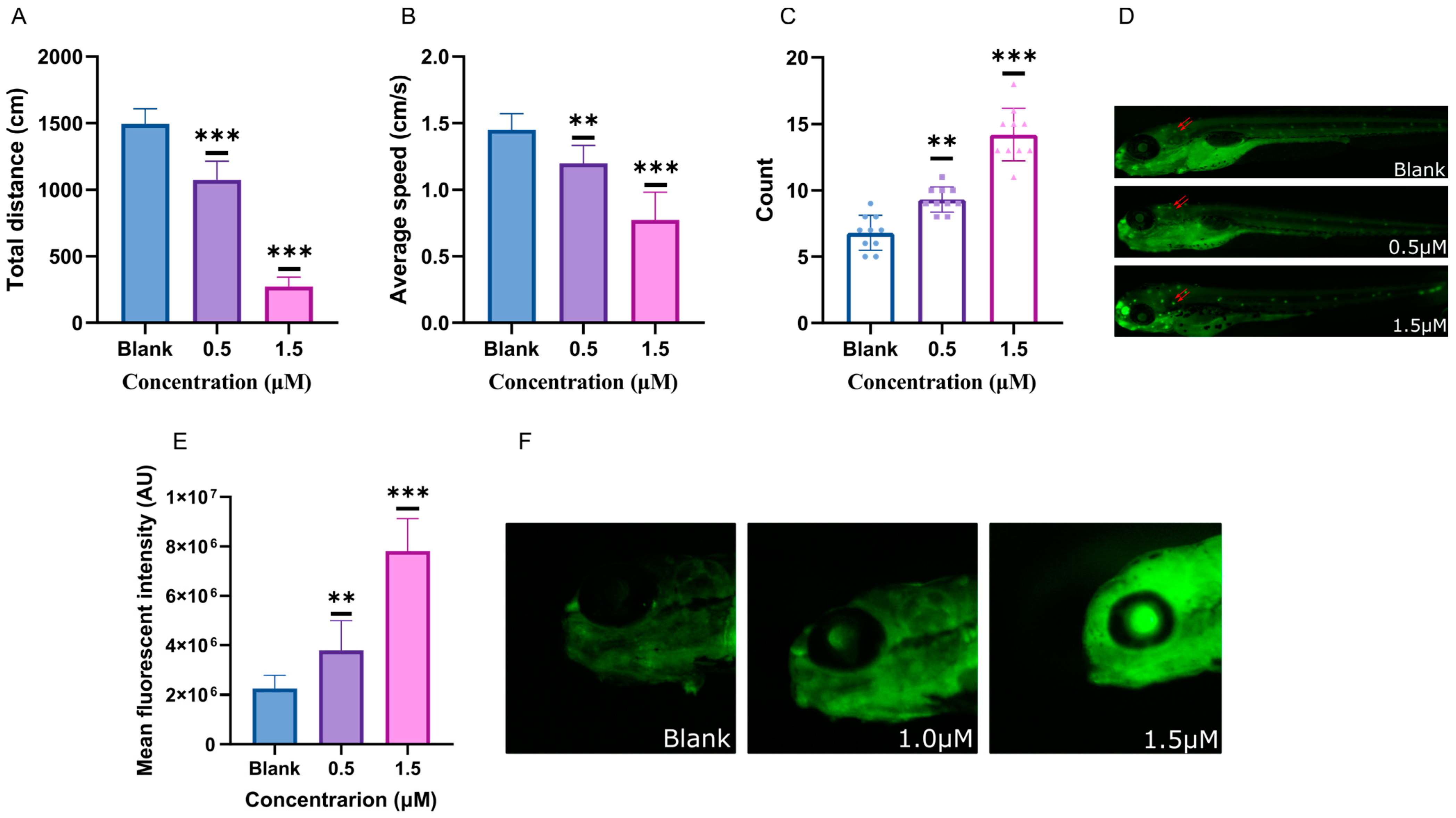
2.2. Developmental Toxicity Assessment of Glabrene
2.3. Behavioral Characteristics Intervented by Glabrene
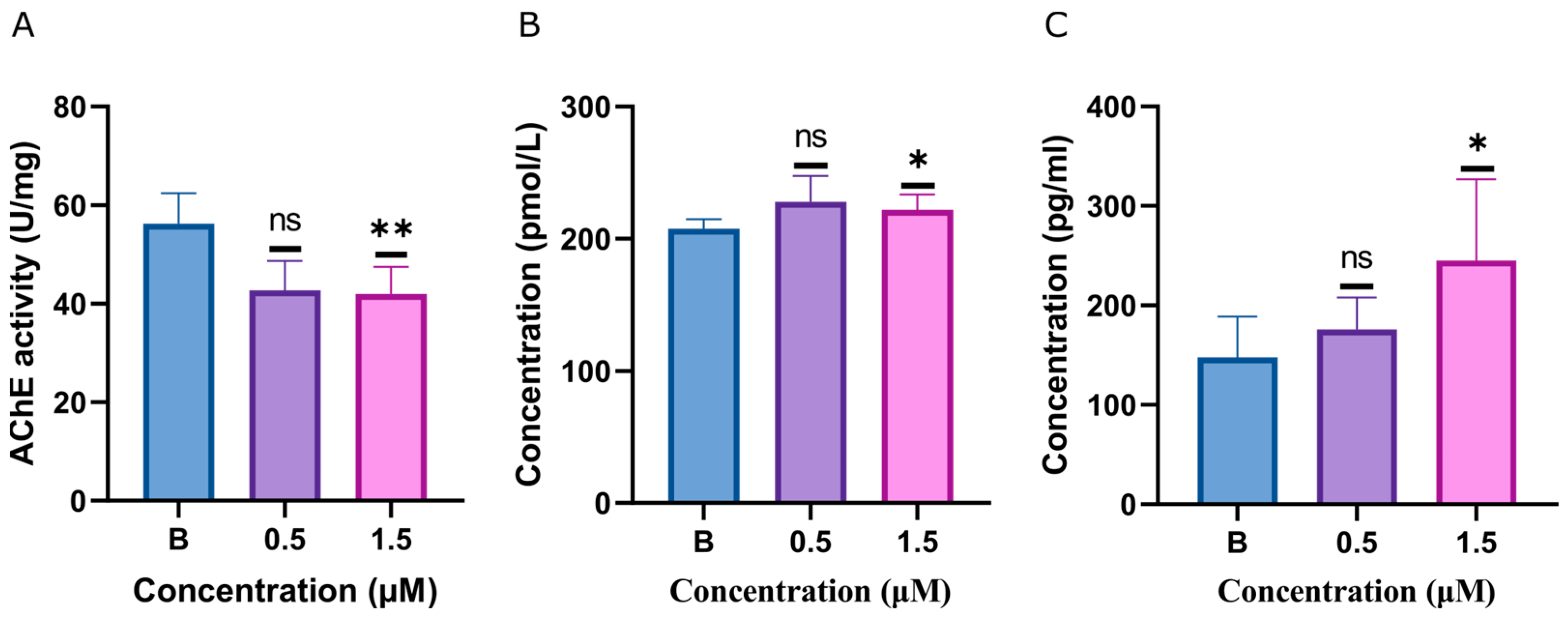
2.4. ACh, AChE, and Dopamine Levels Disturbed by Glabrene
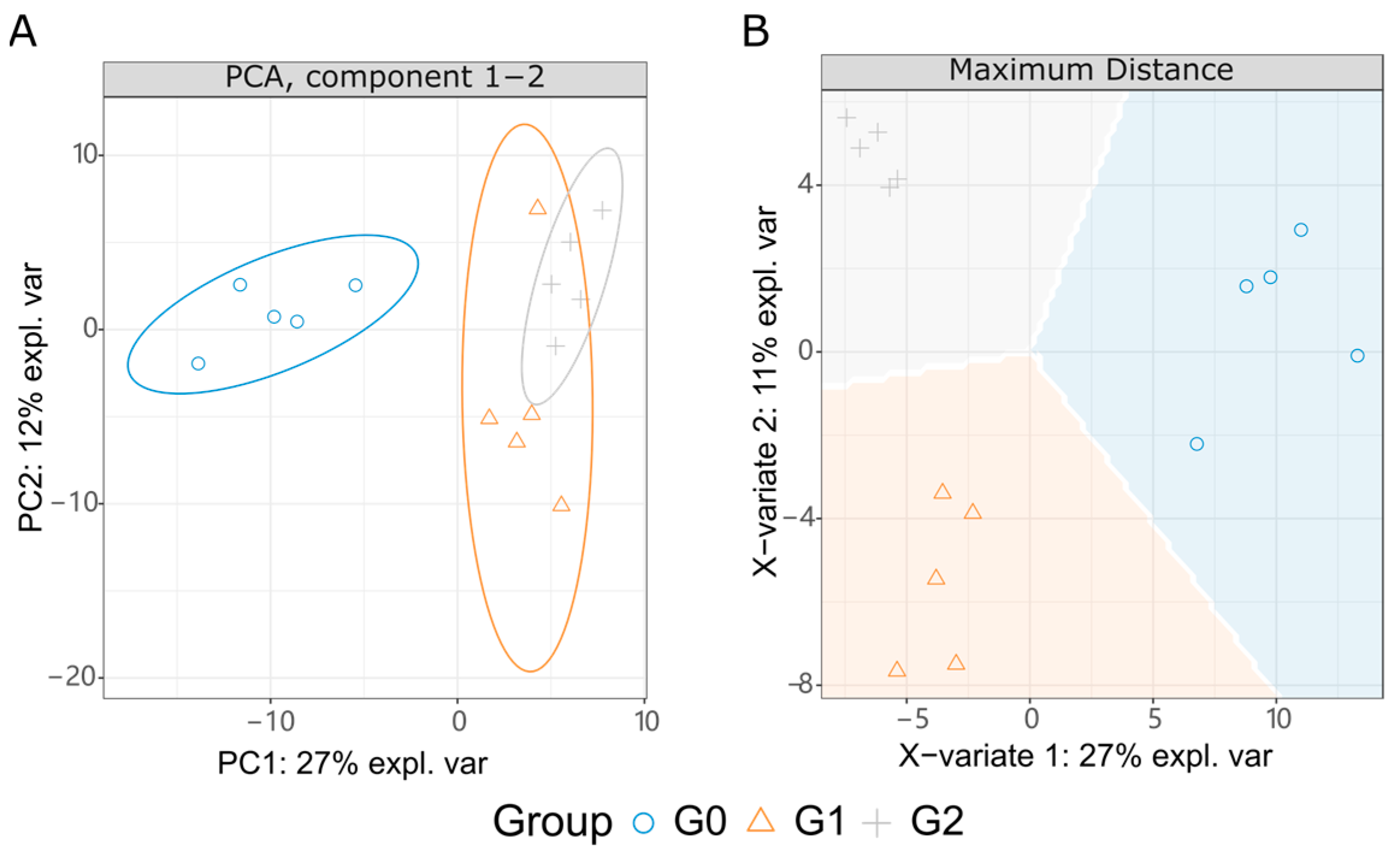
2.5. Metabolome Variation Interfered by Glabrene
3. Discussion
3.1. Glabrene Exhibited Conspicuous Zebrafish Lethal Toxicity and Affected Body Development
3.2. Glabrene Caused Nerve Damage, Disrupting the Balance of Neurotransmitters
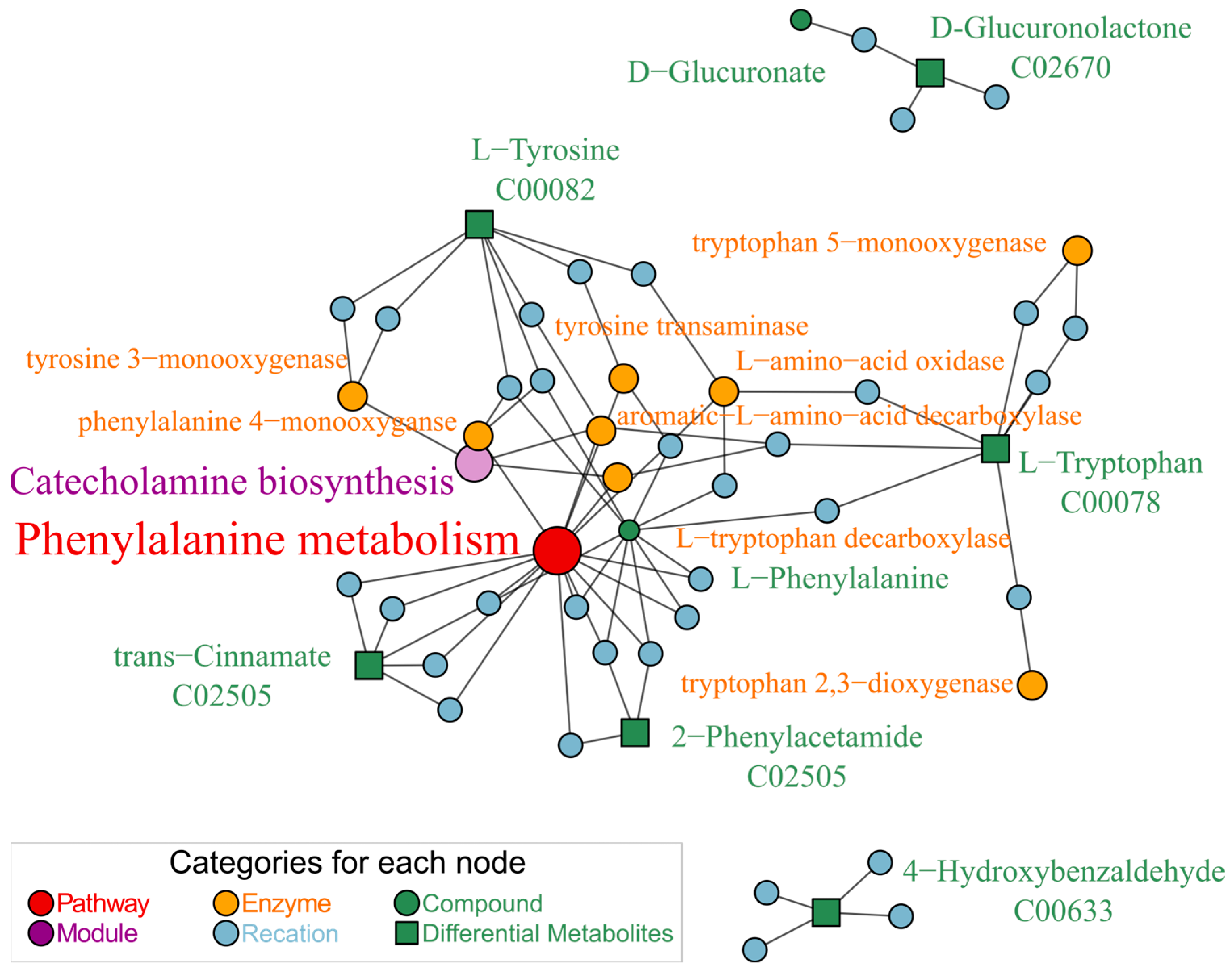
3.3. Glabrene Affected the Metabolism of Phenylalanine In Vivo
3.4. Glabrene Interfered with Neurotransmitters
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Zebrafish Maintenance
4.3. Glabrene Separation and Purification
4.4. Acute Toxicity Assay on Zebrafish
4.5. Development Toxicity Evaluation
4.6. Cartilage Tissue Observation
4.7. Neurometric Characterization Bioassay
4.8. AChE Level Analysis and Enzyme-Linked Immunoreaction Assay
4.9. Metabonomic Sample Preparation and Data Acquisition
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Q.; Wu, S.; Liang, W.; Tan, J.; Liu, X.; Yuan, Y.; Li, X.; Zhao, H. Glabrol Impurity Exacerbates Glabridin Toxicity in Zebrafish Embryos by Increasing Myofibril Disorganization. J. Ethnopharmacol. 2022, 287, 114963. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, X.; Huang, Y. Inhibitory Mechanisms of Glabridin on Tyrosinase. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2016, 168, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, X.; Zhong, B.; Liao, Q.; Wang, X.; Xie, Y.; He, X. Review on the Diverse Biological Effects of Glabridin. Drug Des. Devel. Ther. 2023, 17, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, A.; Chand, J.; Dash, A.; Bhatt, S.; Dhiman, S.; Wazir, P.; Singh, B.; Goswami, A.; Nandi, U. Glabridin Plays Dual Action to Intensify Anti-Metastatic Potential of Paclitaxel via Impeding CYP2C8 in Liver and CYP2J2/EETs in Tumor of an Orthotopic Mouse Model of Breast Cancer. Chem. Biol. Interact. 2023, 382, 110605. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Agha, M.V.; Ahmad, F.; Anver, R.; Sheikhan, K.S.A.M.; Mateo, J.; Alam, M.; Buddenkotte, J.; Uddin, S.; Steinhoff, M. Metabolomics Analyses Reveal the Crucial Role of ERK in Regulating Metabolic Pathways Associated with the Proliferation of Human Cutaneous T-Cell Lymphoma Cells Treated with Glabridin. Cell Prolif. 2024, e13701. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Mu, W.; Li, H.; Yan, Y.; Zhan, X.; Luo, W.; Wang, Z.; Kan, W.; Zhao, J.; Hui, S.; et al. Glabridin Improves Autoimmune Disease in Trex1-Deficient Mice by Reducing Type I Interferon Production. Mol. Med. 2023, 29, 167. [Google Scholar] [CrossRef]
- Weng, J.; Wang, Y.; Tan, Z.; Yuan, Y.; Huang, S.; Li, Z.; Li, Y.; Zhang, L.; Du, Z. Glabridin Reduces Neuroinflammation by Modulating Inflammatory Signals in LPS-Induced in Vitro and in Vivo Models. Inflammopharmacology 2024, 32, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhou, C.; Yu, K.-H.; Lin, Y.-S.; Wang, L.-B.; Zhang, Y.; Liu, S.-X.; Xu, W.-Y.; Sun, Y.; Zhou, T.-L.; et al. Glabridin Functions as a Quorum Sensing Inhibitor to Inhibit Biofilm Formation and Swarming Motility of Multidrug-Resistant Acinetobacter Baumannii. Infect. Drug Resist. 2023, 16, 5697–5705. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Liu, H.; Qiu, Z.; Xiao, Z.; Lu, Z. Glabridin Therapy Reduces Chronic Allodynia, Spinal Microgliosis, and Dendritic Spine Generation by Inhibiting Fractalkine-CX3CR1 Signaling in a Mouse Model of Tibial Fractures. Brain Sci. 2023, 13, 739. [Google Scholar] [CrossRef]
- Khalesi, M. Ochratoxin A in Liquorice Products—A Review. Food Addit. Contam. Part A 2015, 32, 2086–2092. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, M.-M.; Sun, Y.; Wang, L.-F.; Wang, H.; Zhang, Y.-J.; Li, H.-Y.; Zhuang, P.-W.; Yang, Z. Synergistic Promotion on Tyrosinase Inhibition by Antioxidants. Molecules 2018, 23, 106. [Google Scholar] [CrossRef]
- Ao, M.; Shi, Y.; Cui, Y.; Guo, W.; Wang, J.; Yu, L. Factors Influencing Glabridin Stability. Nat. Prod. Commun. 2010, 5, 1934578X1000501214. [Google Scholar] [CrossRef]
- Bodor, Z.; Kovacs, Z.; Rashed, M.S.; Kókai, Z.; Dalmadi, I.; Benedek, C. Sensory and Physicochemical Evaluation of Acacia and Linden Honey Adulterated with Sugar Syrup. Sensors 2020, 20, 4845. [Google Scholar] [CrossRef]
- Nerya, O.; Vaya, J.; Musa, R.; Izrael, S.; Ben-Arie, R.; Tamir, S. Glabrene and Isoliquiritigenin as Tyrosinase Inhibitors from Licorice Roots. J. Agric. Food Chem. 2003, 51, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Tamir, S.; Eizenberg, M.; Somjen, D.; Izrael, S.; Vaya, J. Estrogen-like Activity of Glabrene and Other Constituents Isolated from Licorice Root. J. Steroid Biochem. Mol. Biol. 2001, 78, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Celano, R.; Docimo, T.; Piccinelli, A.L.; Rizzo, S.; Campone, L.; Di Sanzo, R.; Carabetta, S.; Rastrelli, L.; Russo, M. Specialized Metabolite Profiling of Different Glycyrrhiza glabra Organs by Untargeted UHPLC-HRMS. Ind. Crops Prod. 2021, 170, 113688. [Google Scholar] [CrossRef]
- Çevik, D.; Yılmazgöz, B.; Kan, Y.; Güzelcan, E.A.; Durmaz, I.; Çetin-Atalay, R.; Kırmızıbekmez, H. Bioactivity-Guided Isolation of Cytotoxic Secondary Metabolites from the Roots of Glycyrrhiza glabra and Elucidation of Their Mechanisms of Action—ScienceDirect. Ind. Crops Prod. 2018, 124, 389–396. [Google Scholar] [CrossRef]
- Kim, M.J.; Cho, S.H.; Seo, Y.; Kim, S.-D.; Park, H.-C.; Kim, B.-J. Neuro-Restorative Effect of Nimodipine and Calcitriol in 1-Methyl 4-Phenyl 1,2,3,6 Tetrahydropyridine-Induced Zebrafish Parkinson’s Disease Model. J. Korean Neurosurg. Soc. 2023. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Tamura, Y.; Mizutani, K. Isolation and Synthesis of Two New 3-Arylcoumarin Derivatives from the Root of Glycyrrhiza glabra (Licorice), and Structure Revision of an Antioxidant Isoflavonoid Glabrene. Nat. Prod. Lett. 1997, 9, 289–296. [Google Scholar] [CrossRef]
- Yang, L.; Ho, N.Y.; Alshut, R.; Legradi, J.; Weiss, C.; Reischl, M.; Mikut, R.; Liebel, U.; Müller, F.; Strähle, U. Zebrafish Embryos as Models for Embryotoxic and Teratological Effects of Chemicals. Reprod. Toxicol. 2009, 28, 245–253. [Google Scholar] [CrossRef]
- Sieber, S.; Grossen, P.; Bussmann, J.; Campbell, F.; Kros, A.; Witzigmann, D.; Huwyler, J. Zebrafish as a Preclinical in Vivo Screening Model for Nanomedicines. Adv. Drug Deliv. Rev. 2019, 151–152, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Husain, I.; Bala, K.; Khan, I.A.; Khan, S.I. A Review on Phytochemicals, Pharmacological Activities, Drug Interactions, and Associated Toxicities of Licorice (Glycyrrhiza sp.). Food Front. 2021, 2, 449–485. [Google Scholar] [CrossRef]
- Jiang, Z.; Feng, L. Study on the Improvement of Immune Microenvironment in Lung Cancer by the Combination of Three-Step Analgesics and Glabrene Based on Network Pharmacology and Molecular Docking Analysis. J. Biol. Reg. Homeos. Ag. 2023, 37, 5589–5598. [Google Scholar] [CrossRef]
- Yang, C.; Alam, A.; Alhumaydhi, F.A.; Khan, M.S.; Alsagaby, S.A.; Al Abdulmonem, W.; Hassan, M.I.; Shamsi, A.; Bano, B.; Yadav, D.K. Bioactive Phytoconstituents as Potent Inhibitors of Tyrosine-Protein Kinase Yes (YES1): Implications in Anticancer Therapeutics. Molecules 2022, 27, 3060. [Google Scholar] [CrossRef]
- Postlethwait, J.H.; Woods, I.G.; Ngo-Hazelett, P.; Yan, Y.L.; Kelly, P.D.; Chu, F.; Huang, H.; Hill-Force, A.; Talbot, W.S. Zebrafish Comparative Genomics and the Origins of Vertebrate Chromosomes. Genome Res. 2000, 10, 1890–1902. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Cubbage, C.C.; Mabee, P.M. Development of the Cranium and Paired Fins in the Zebrafish Danio rerio (Ostariophysi, Cyprinidae). J. Morphol. 1996, 229, 121–160. [Google Scholar] [CrossRef]
- Wullimann, M.F. Secondary Neurogenesis and Telencephalic Organization in Zebrafish and Mice: A Brief Review. Integr. Zool. 2009, 4, 123–133. [Google Scholar] [CrossRef]
- Simmler, C.; Pauli, G.F.; Chen, S.-N. Phytochemistry and Biological Properties of Glabridin. Fitoterapia 2013, 90, 160–184. [Google Scholar] [CrossRef]
- Cerulli, A.; Masullo, M.; Montoro, P.; Piacente, S. Licorice (Glycyrrhiza glabra, G. Uralensis, and G. Inflata) and Their Constituents as Active Cosmeceutical Ingredients. Cosmetics 2022, 9, 7. [Google Scholar] [CrossRef]
- Agahi, F.; Juan, C.; Font, G.; Juan-García, A. Neurotoxicity of Zearalenone’s Metabolites and Beauvericin Mycotoxins via Apoptosis and Cell Cycle Disruption. Toxicology 2021, 456, 152784. [Google Scholar] [CrossRef] [PubMed]
- Bownik, A.; Wlodkowic, D. Applications of Advanced Neuro-Behavioral Analysis Strategies in Aquatic Ecotoxicology. Sci. Total Environ. 2021, 772, 145577. [Google Scholar] [CrossRef] [PubMed]
- Meijide, F.J.; Da Cuña, R.H.; Prieto, J.P.; Dorelle, L.S.; Babay, P.A.; Lo Nostro, F.L. Effects of Waterborne Exposure to the Antidepressant Fluoxetine on Swimming, Shoaling and Anxiety Behaviours of the Mosquitofish Gambusia Holbrooki. Ecotoxicol. Environ. Saf. 2018, 163, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Plemel, J.R.; Caprariello, A.V.; Keough, M.B.; Henry, T.J.; Tsutsui, S.; Chu, T.H.; Schenk, G.J.; Klaver, R.; Yong, V.W.; Stys, P.K. Unique Spectral Signatures of the Nucleic Acid Dye Acridine Orange Can Distinguish Cell Death by Apoptosis and Necroptosis. J. Cell Biol. 2017, 216, 1163–1181. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Patriarchi, T.; Cho, J.R.; Merten, K.; Howe, M.W.; Marley, A.; Xiong, W.-H.; Folk, R.W.; Broussard, G.J.; Liang, R.; Jang, M.J.; et al. Ultrafast Neuronal Imaging of Dopamine Dynamics with Designed Genetically Encoded Sensors. Science 2018, 360, eaat4422. [Google Scholar] [CrossRef] [PubMed]
- Graves, S.M.; Xie, Z.; Stout, K.A.; Zampese, E.; Burbulla, L.F.; Shih, J.C.; Kondapalli, J.; Patriarchi, T.; Tian, L.; Brichta, L.; et al. Dopamine Metabolism by a Monoamine Oxidase Mitochondrial Shuttle Activates the Electron Transport Chain. Nat. Neurosci. 2020, 23, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Tufi, S.; Lamoree, M.; de Boer, J.; Leonards, P. Simultaneous Analysis of Multiple Neurotransmitters by Hydrophilic Interaction Liquid Chromatography Coupled to Tandem Mass Spectrometry. J. Chromatogr. A 2015, 1395, 79–87. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, X.; Chen, L.; Xu, L.; Cai, Y.; Hou, S.; Zheng, M.; Liu, F. Design, Synthesis, and Evaluation of 8-Aminoquinoline-Melatonin Derivatives as Effective Multifunctional Agents for Alzheimer’s Disease. Ann. Transl. Med. 2022, 10, 303. [Google Scholar] [CrossRef]
- Nedogreeva, O.A.; Evtushenko, N.A.; Manolova, A.O.; Peregud, D.I.; Yakovlev, A.A.; Lazareva, N.A.; Gulyaeva, N.V.; Stepanichev, M.Y. Oxidative Damage of Proteins Precedes Loss of Cholinergic Phenotype in the Septal Neurons of Olfactory Bulbectomized Mice. Curr. Alzheimer Res. 2021, 18, 1140–1151. [Google Scholar] [CrossRef]
- Cragg, S.J. Meaningful Silences: How Dopamine Listens to the ACh Pause. Trends Neurosci. 2006, 29, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Bymaster, F.P.; Felder, C.; Ahmed, S.; McKinzie, D. Muscarinic Receptors as a Target for Drugs Treating Schizophrenia. Curr. Drug Targets—CNS Neurol. Disord. 2002, 1, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Issac, P.K.; Guru, A.; Velayutham, M.; Pachaiappan, R.; Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Harikrishnan, R.; Arockiaraj, J. Oxidative Stress Induced Antioxidant and Neurotoxicity Demonstrated in Vivo Zebrafish Embryo or Larval Model and Their Normalization Due to Morin Showing Therapeutic Implications. Life Sci. 2021, 283, 119864. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, Y.J.; Lim, Y.; Oh, B.; Kim, J.Y.; Bouwman, J.; Kwon, O. Combination of Diet Quality Score, Plasma Carotenoids, and Lipid Peroxidation to Monitor Oxidative Stress. Oxidative Med. Cell. Longev. 2018, 2018, 8601028. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.H.-L.; Cheng, S.S.-Y.; Cheung, Y.-T.; Wuwongse, S.; Zhang, N.Q.; Ho, Y.-S.; Lee, S.M.-Y.; Chang, R.C.-C. A Reciprocal Relationship between Reactive Oxygen Species and Mitochondrial Dynamics in Neurodegeneration. Redox Biol. 2018, 14, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Liu, Y.-H.; Liu, D.-Z.; Xu, J.-Y.; Zhang, Q. Insulin-Mimic Components in Acer Truncatum Leaves: Bio-Guided Isolation, Annual Variance Profiling and Regulating Pathway Investigated by Omics. Pharm. Basel Switz. 2021, 14, 662. [Google Scholar] [CrossRef] [PubMed]
- Teh, L.E.; Kue, C.S.; Ng, C.H.; Lau, B.F. Toxicity Effect of Bougainvillea Glabra (Paper Flower) Water Extracts on Zebrafish Embryo. INNOSC Theranostics Pharmacol. Sci. 2019, 2, 780. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, Z.; Song, Q.; Ren, Z.; Xiao, J.; Li, L.; Zhang, Q. Metabolic Exploration of the Developmental Abnormalities and Neurotoxicity of Esculentoside B, the Main Toxic Factor in Phytolaccae Radix. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2023, 176, 113777. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Samanta, P.; Lee, S.; Lee, J.; Cho, J.-W.; Chun, H.-S.; Yoon, S.; Kim, W.-K. Developmental and Neurotoxicity of Acrylamide to Zebrafish. Int. J. Mol. Sci. 2021, 22, 3518. [Google Scholar] [CrossRef]
- Alafiatayo, A.A.; Lai, K.-S.; Syahida, A.; Mahmood, M.; Shaharuddin, N.A. Phytochemical Evaluation, Embryotoxicity, and Teratogenic Effects of Curcuma Longa Extract on Zebrafish (Danio rerio). Evid.-Based Complement. Altern. Med. ECAM 2019, 2019, 3807207. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Y.; Zhang, H.; Rajendran, R.S.; Wang, R.; Hsiao, C.-D.; Li, J.; Xia, Q.; Liu, K. Isoliquiritigenin Triggers Developmental Toxicity and Oxidative Stress-Mediated Apoptosis in Zebrafish Embryos/Larvae via Nrf2-HO1/JNK-ERK/Mitochondrion Pathway. Chemosphere 2020, 246, 125727. [Google Scholar] [CrossRef] [PubMed]
- Beler, M.; Ünal, İ.; Cansız, D.; Emekli-Alturfan, E. Alcian Blue Staining for Chondrocranium Development in Zebrafish. Methods Mol. Biol. Clifton NJ 2024, 2753, 447–457. [Google Scholar] [CrossRef]
- Hoyberghs, J.; Ball, J.; Trznadel, M.; Beekhuijzen, M.; Burbank, M.; Wilhelmi, P.; Muriana, A.; Powles-Glover, N.; Letamendia, A.; Van Cruchten, S. Biological Variability Hampers the Use of Skeletal Staining Methods in Zebrafish Embryo Developmental Toxicity Assays. Reprod. Toxicol. 2024, 127, 108615. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Chen, C.; Liu, F.; Hu, H.; Dong, S.; Shen, Q.; Zeng, J.; Huang, L.; Liao, X.; Cao, Z.; et al. Pinoresinol Diglucoside Mitigates Dexamethasone-Induced Osteoporosis and Chondrodysplasia in Zebrafish. Toxicol. Appl. Pharmacol. 2024, 484, 116884. [Google Scholar] [CrossRef] [PubMed]
- Schn?Rr, S.J.; Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Measuring Thigmotaxis in Larval Zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.-D.; Shi, W.-J.; Li, S.-Y.; Zhang, J.-G.; Lu, Z.-J.; Long, X.-B.; Liu, X.; Huang, C.-S.; Ying, G.-G. Ephedrine and Cocaine Cause Developmental Neurotoxicity and Abnormal Behavior in Zebrafish. Aquat. Toxicol. 2023, 265, 106765. [Google Scholar] [CrossRef]
- Wu, L.; Dang, Y.; Liang, L.-X.; Gong, Y.-C.; Zeeshan, M.; Qian, Z.; Geiger, S.D.; Vaughn, M.G.; Zhou, Y.; Li, Q.-Q.; et al. Perfluorooctane Sulfonates Induces Neurobehavioral Changes and Increases Dopamine Neurotransmitter Levels in Zebrafish Larvae. Chemosphere 2022, 297, 134234. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Zhu, Z.-J.; Ren, S.-P.; Deng, Y.-C.; Xu, J.-Y.; Zhang, S.-M.; Gao, J.-M.; Zhang, Q. Metabolomic Navigated Citrus Waste Repurposing to Restore Amino Acids Disorder in Neural Lesion. Food Chem. 2022, 387, 132933. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Jiang, H.; Guo, D.; Huang, W.; Ren, H.; Zhang, Q. Toxic Features and Metabolomic Intervention of Glabrene, an Impurity Found in the Pharmaceutical Product of Glabridin. Int. J. Mol. Sci. 2024, 25, 8985. https://doi.org/10.3390/ijms25168985
Li X, Jiang H, Guo D, Huang W, Ren H, Zhang Q. Toxic Features and Metabolomic Intervention of Glabrene, an Impurity Found in the Pharmaceutical Product of Glabridin. International Journal of Molecular Sciences. 2024; 25(16):8985. https://doi.org/10.3390/ijms25168985
Chicago/Turabian StyleLi, Xue, Haixin Jiang, Dongxue Guo, Wen Huang, Houpu Ren, and Qiang Zhang. 2024. "Toxic Features and Metabolomic Intervention of Glabrene, an Impurity Found in the Pharmaceutical Product of Glabridin" International Journal of Molecular Sciences 25, no. 16: 8985. https://doi.org/10.3390/ijms25168985
APA StyleLi, X., Jiang, H., Guo, D., Huang, W., Ren, H., & Zhang, Q. (2024). Toxic Features and Metabolomic Intervention of Glabrene, an Impurity Found in the Pharmaceutical Product of Glabridin. International Journal of Molecular Sciences, 25(16), 8985. https://doi.org/10.3390/ijms25168985







