Ethyl Acetate Fractions of Salvia miltiorrhiza Bunge (Danshen) Crude Extract Modulate Fibrotic Signals to Ameliorate Diabetic Kidney Injury
Abstract
1. Introduction
2. Results
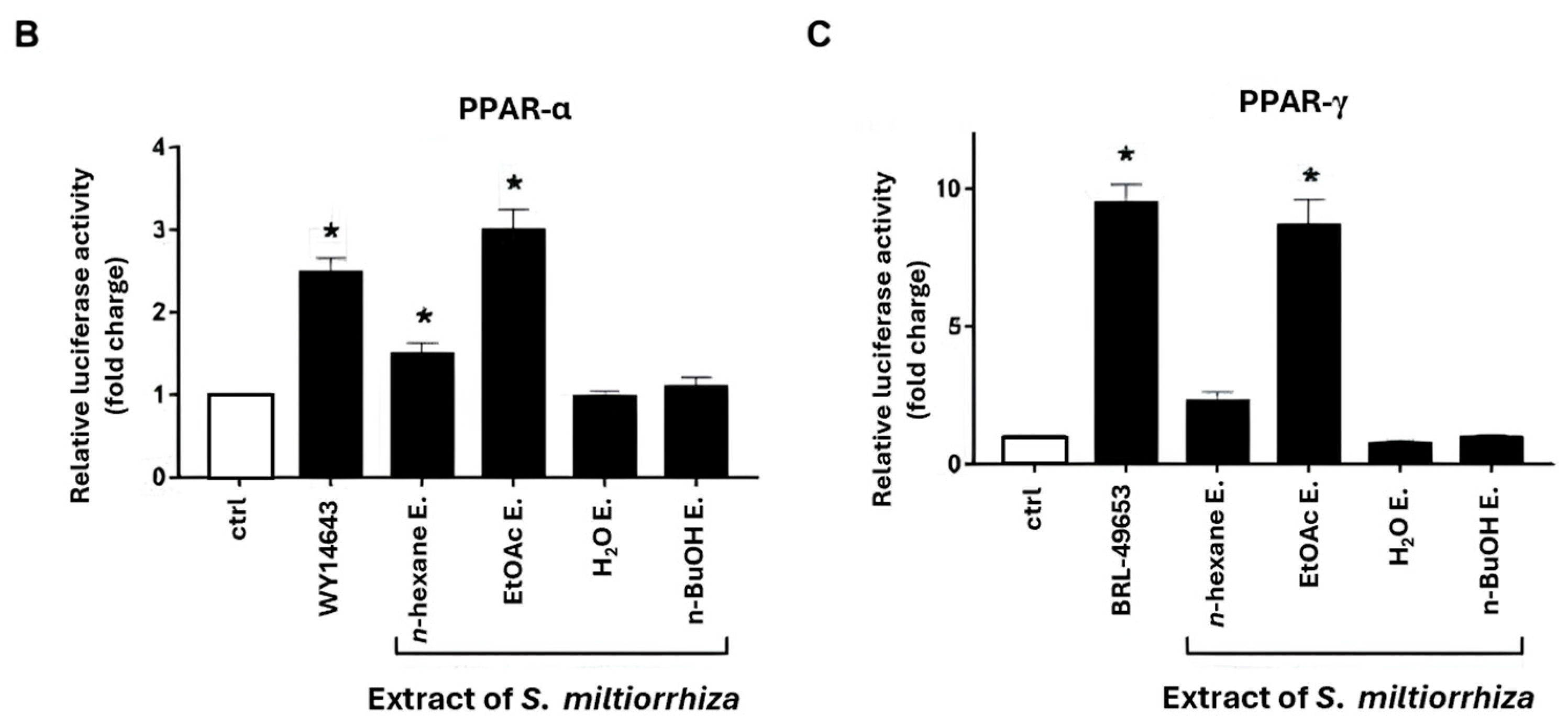
2.1. Effects of S. miltiorrhiza Extracts on PPAR-α and PPAR-γ Transcriptional Activities in Renal Mesangial Cells Using Dual-Luciferase Reporter Assay
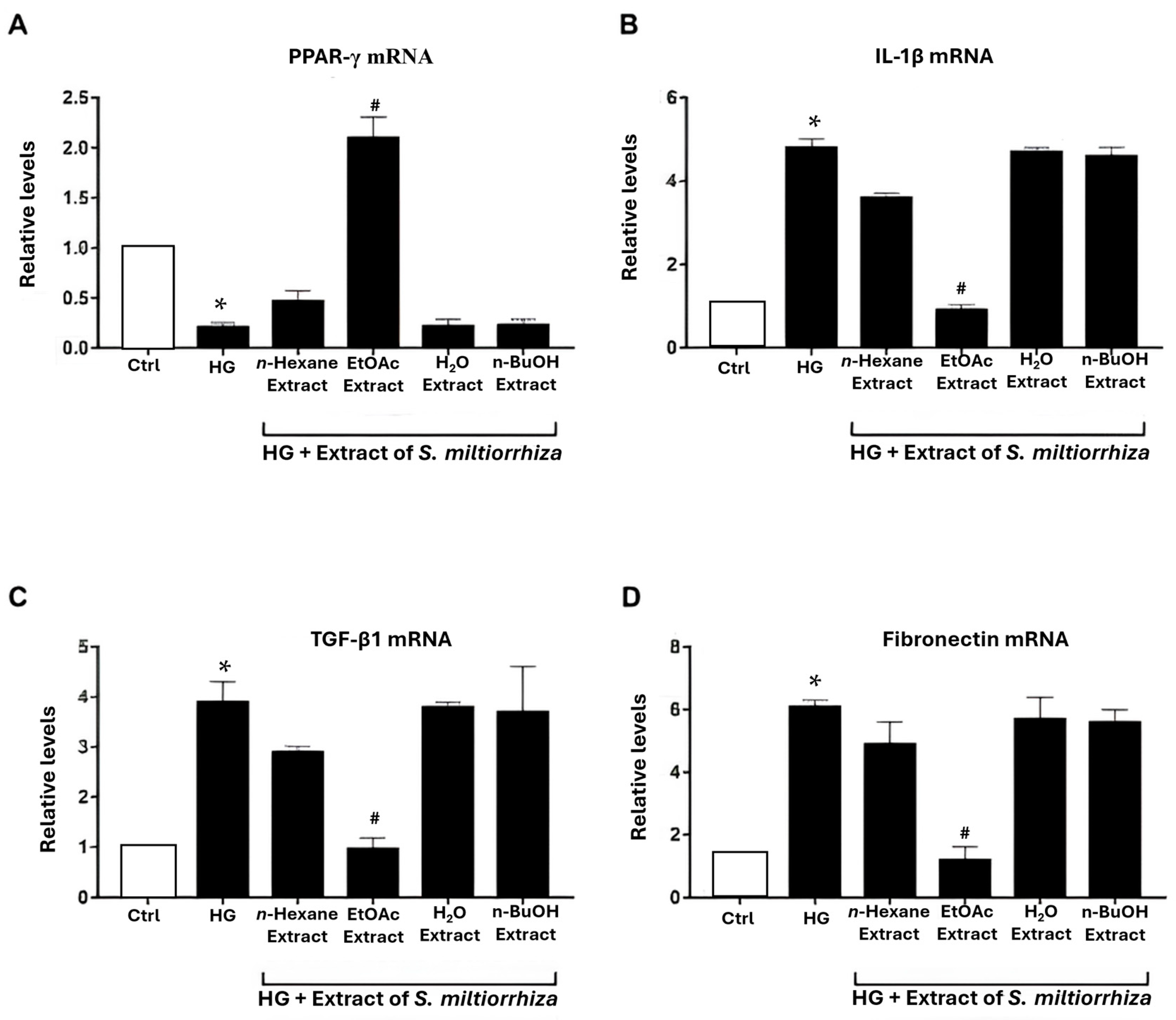
2.2. Effects of S. miltiorrhiza EtOAc Extract on Renal Inflammation/Fibrosis-Related Genes in HG-Stressed Mesangial Cells
2.3. Inhibitory Effects of S. miltiorrhiza Extracts on TGF-β1-Induced Myofibroblast Activation
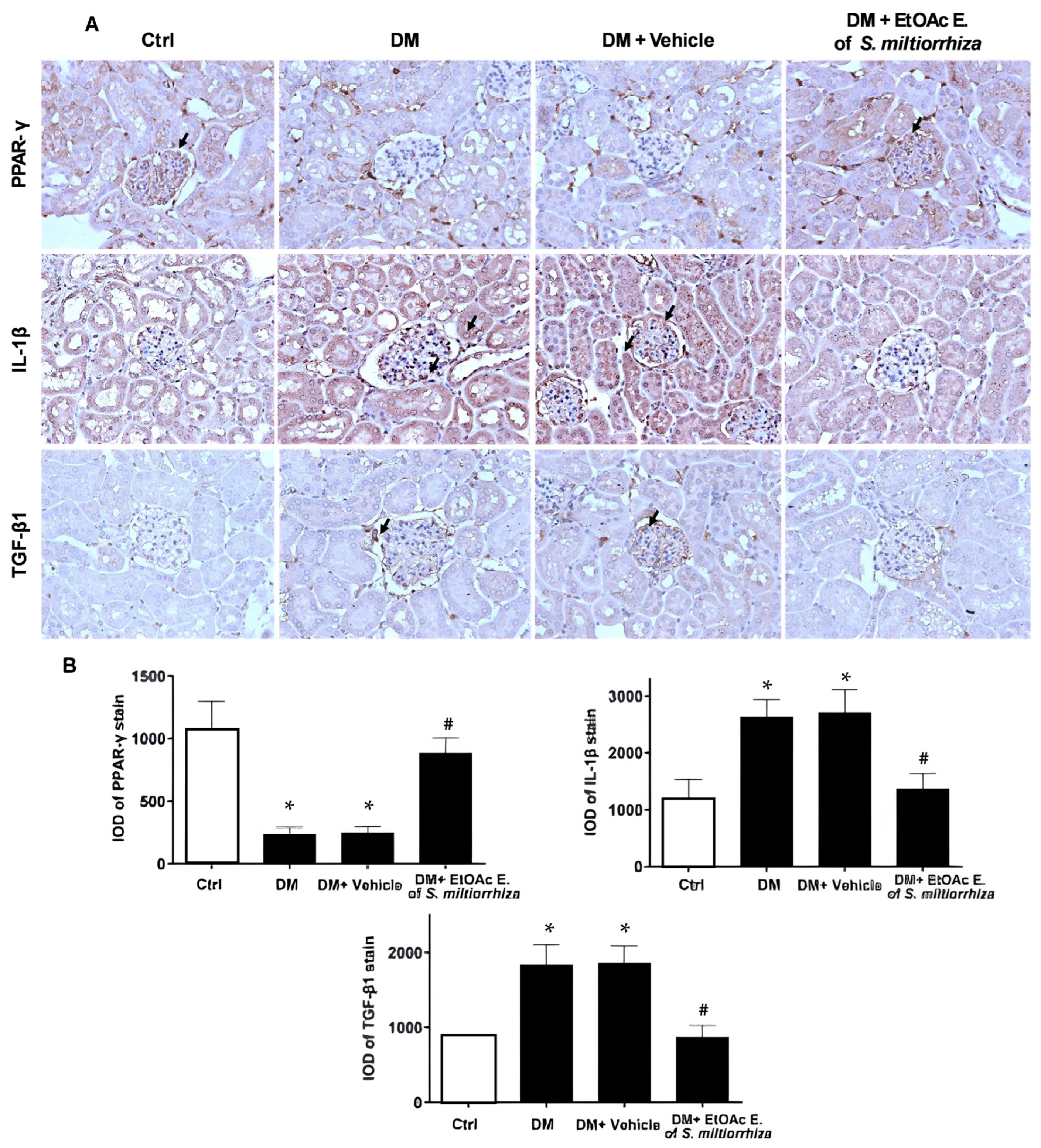
2.4. In Vivo Effects of EtOAc Extract of S. miltiorrhiza on Renal Gene Expression in STZ-Induced Diabetic Mice
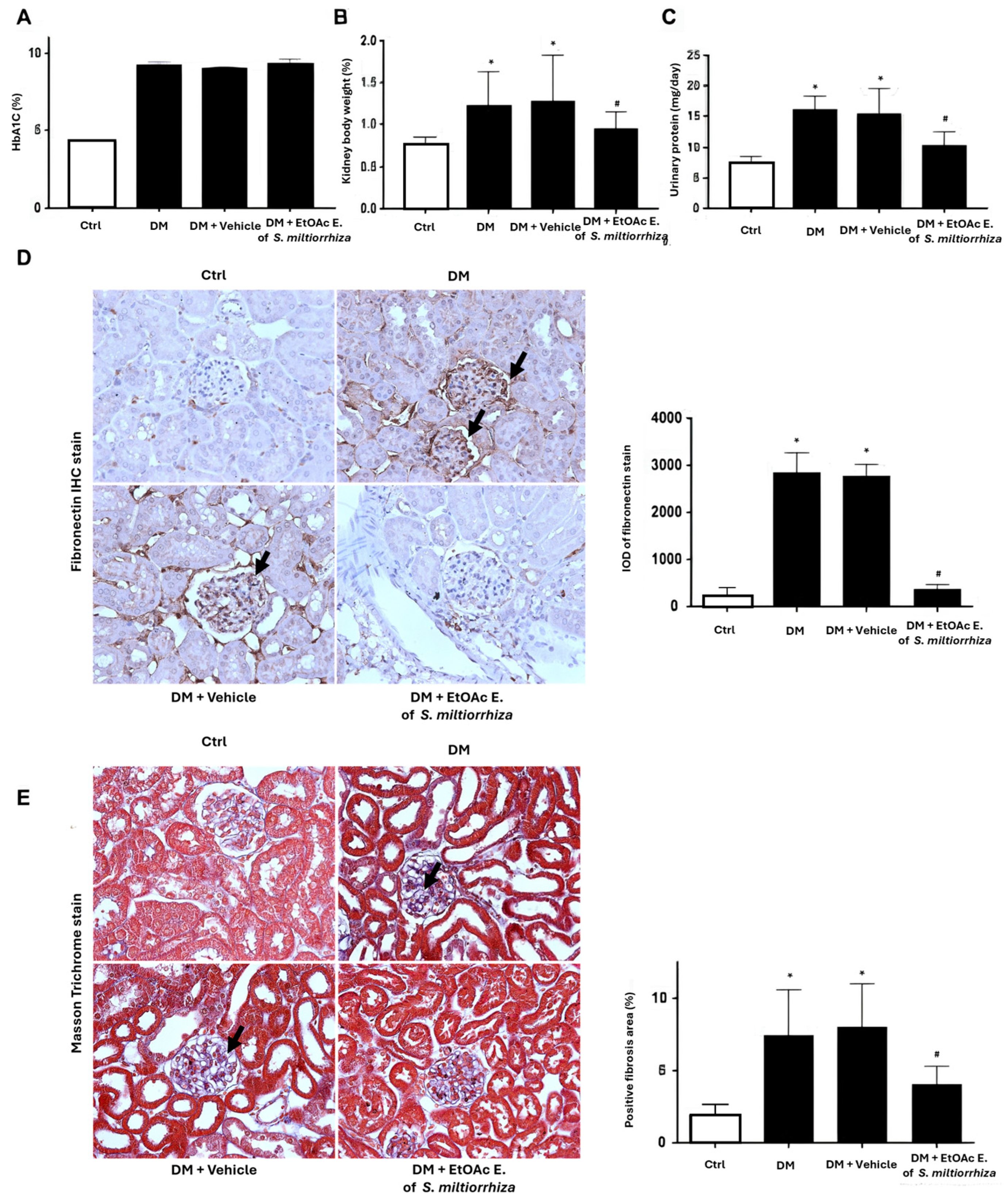
2.5. Renoprotective Effects of S. miltiorrhiza EtOAc Extract STZ-Induced Diabetic Mice
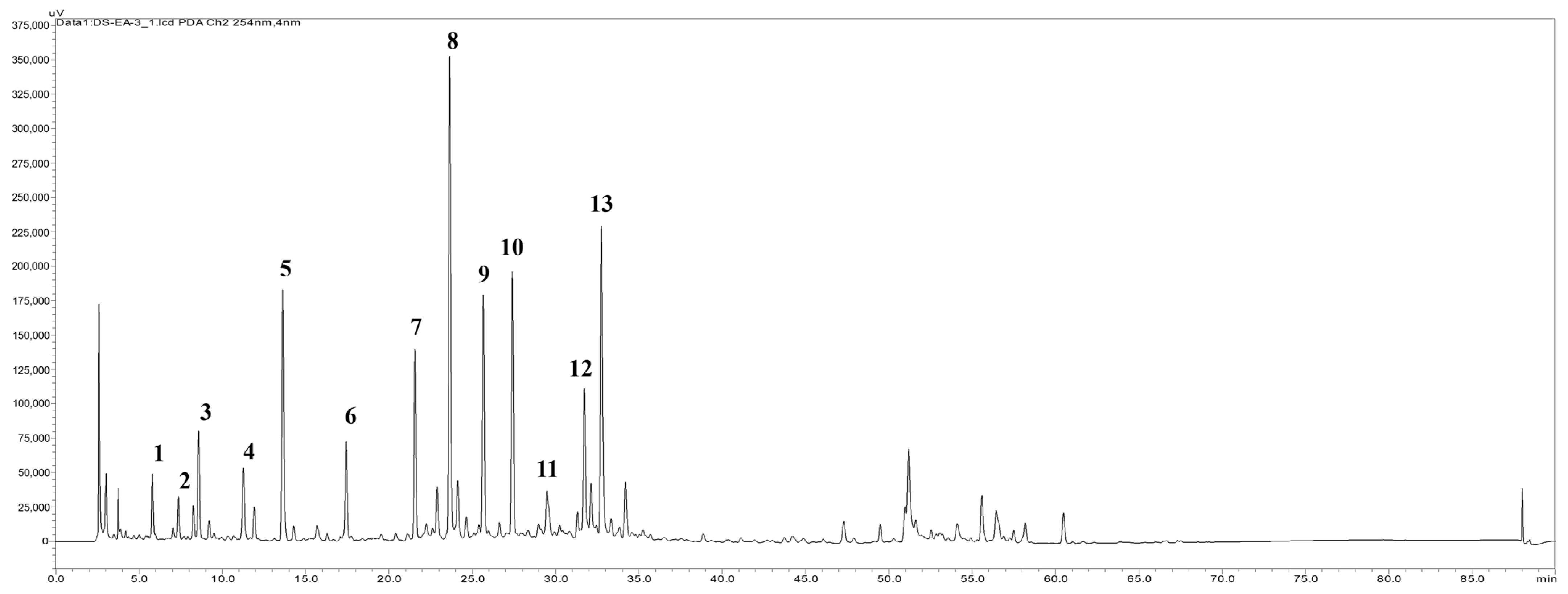
2.6. HPLC Fingerprint Profile of the EtOAc Extract and the Isolation Components
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.1.1. In Vitro Model of High Glucose Stress
4.1.2. In Vitro Model of Myofibroblast Activation
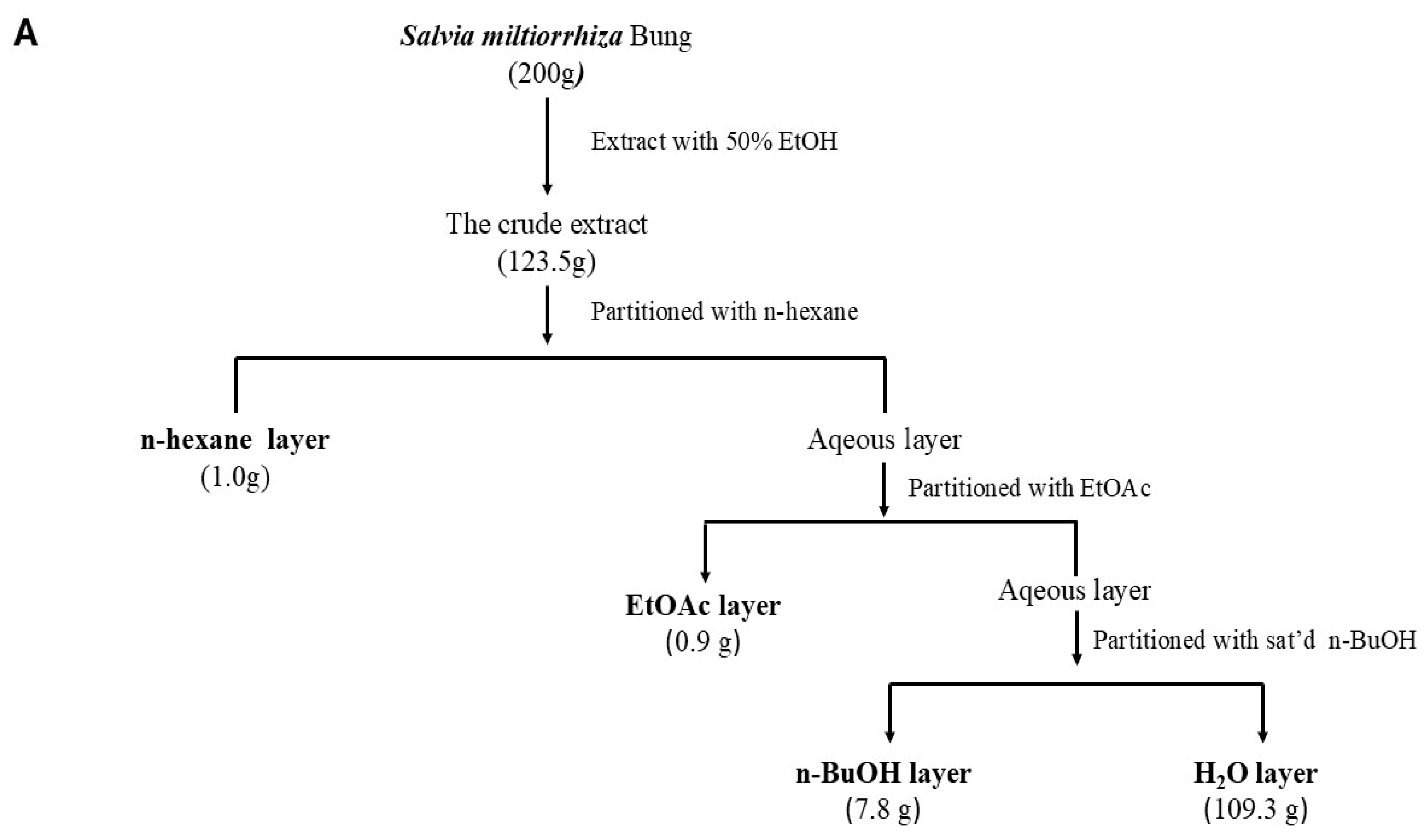
4.2. Preparation of the S. miltiorrhiza (Danshen) Extract
4.3. Main Compounds Isolation and HPLC Profile of the EtOAc Extract
4.4. Measurement of PPAR-α and PPAR-γ Activities by Reporter Assay
4.5. RNA Extraction and Quantitative Reverse Transcription-PCR (qRT-PCR)
4.6. Protein Extraction and Western Blot Analysis
4.7. Streptozotocin (STZ)-Induced Diabetic Animal Model and Treatment
4.8. Urine and Blood Biochemistry
4.9. Histological and Immunohistochemical Examinations
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Sabanayagam, C.; Chee, M.L.; Banu, R.; Cheng, C.Y.; Lim, S.C.; Tai, E.S.; Coffman, T.; Wong, T.Y. Association of Diabetic Retinopathy and Diabetic Kidney Disease with All-Cause and Cardiovascular Mortality in a Multiethnic Asian Population. JAMA Netw. Open 2019, 2, e191540. [Google Scholar] [CrossRef]
- Ritz, E.; Zeng, X.X.; Rychlik, I. Clinical manifestation and natural history of diabetic nephropathy. Contrib. Nephrol. 2011, 170, 19–27. [Google Scholar] [CrossRef]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.; Zoungas, S.; Rossing, P.; Groop, P.H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Primers 2015, 1, 15018. [Google Scholar] [CrossRef] [PubMed]
- Wada, J.; Makino, H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. 2013, 124, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Fukami, K.; Ueda, S.; Okuda, S. Molecular mechanisms of diabetic nephropathy and its therapeutic intervention. Curr. Drug Targets 2007, 8, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.S.; Hathaway, C.K.; Smithies, O.; Kakoki, M. Transforming growth factor-beta1 and diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2016, 310, F689–F696. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gu, Z. The role of peroxisome proliferator-activated receptors in kidney diseases. Front. Pharmacol. 2022, 13, 832732. [Google Scholar] [CrossRef] [PubMed]
- Kökény, G.; Calvier, L.; Hansmann, G. PPARγ and TGFβ-major regulators of metabolism, inflammation, and fibrosis in the lungs and kidneys. Int. J. Mol. Sci. 2021, 22, 10431. [Google Scholar] [CrossRef]
- Chung, S.; Park, C.W. Role of peroxisome proliferator-activated receptor α in diabetic nephropathy. Diabetes Metab. J. 2011, 35, 327–336. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, Y.; Guan, Y. PPARγ as a therapeutic target in diabetic nephropathy and other renal diseases. Curr. Opin. Nephrol. Hypertens. 2012, 21, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Gao, X.; Wang, S.; Huang, M.; Sun, Z.; Dong, H.; Yu, H.; Wang, G. PPAR-α agonist fenofibrate prevented diabetic nephropathy by inhibiting M1 macrophages via improving endothelial cell function in db/db Mice. Front. Med. 2021, 8, 652558. [Google Scholar] [CrossRef]
- Jia, Z.; Sun, Y.; Yang, G.; Zhang, A.; Huang, S.; Heiney, K.M.; Zhang, Y. New insights into the PPAR γ agonists for the treatment of diabetic nephropathy. PPAR Res. 2014, 2014, 818530. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Fu, L.; Nile, S.H.; Zhang, J.; Kai, G. Salvia miltiorrhiza in treating cardiovascular diseases: A review on its pharmacological and clinical applications. Front. Pharmacol. 2019, 10, 753. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Y.; Ren, G.; Hu, X.; Wang, C.; Yang, Z.; Li, Z.; Mao, W.; Lu, D. Tanshinone IIA sodium sulfonate regulates antioxidant system, inflammation, and endothelial dysfunction in atherosclerosis by downregulation of CLIC1. Eur. J. Pharmacol. 2017, 815, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Wang, F.J.; Lyu, M.; Yang, J.; Zhang, P.; Zhu, Y. Ability to suppress TGF-beta-activated myofibroblast differentiation distinguishes the anti-pulmonary fibrosis efficacy of two Danshen-containing Chinese herbal medicine prescriptions. Front. Pharmacol. 2019, 10, 412. [Google Scholar] [CrossRef]
- Peng, L.Y.; An, L.; Sun, N.Y.; Ma, Y.; Zhang, X.W.; Liu, W.H.; Liu, B.L.; Li, P.; Chen, J. Salvia miltiorrhiza restrains reactive oxygen species-associated pulmonary fibrosis via targeting Nrf2-Nox4 redox balance. Am. J. Chin. Med. 2019, 47, 1113–1131. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.F.; Chang, H.C.; Huang, S.L.; Chen, C.L.; Chen, W.T.; Yang, C.C. Prescribed renoprotective Chinese herbal medicines were associated with a lower risk of all-cause and disease-specific mortality among patients with chronic kidney disease: A population-based follow-up study in Taiwan. Evid. Based Complement. Alternat Med. 2017, 2017, 5632195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Tao, Y.Y.; Yuan, J.L.; Shen, L.; Liu, C.H. Salvianolic acid B prevents epithelial-to-mesenchymal transition through the TGF-beta1 signal transduction pathway in vivo and in vitro. BMC Cell Biol. 2010, 11, 31. [Google Scholar] [CrossRef]
- Tang, Q.; Zhong, H.; Xie, F.; Xie, J.; Chen, H.; Yao, G. Expression of miR-106b-25 induced by salvianolic acid B inhibits epithelial-to-mesenchymal transition in HK-2 cells. Eur. J. Pharmacol. 2014, 741, 97–103. [Google Scholar] [CrossRef]
- Chen, G.; Fu, Y.; Wu, X. Protective effect of Salvia miltiorrhiza extract against renal ischemia-reperfusion-induced injury in rats. Molecules 2012, 17, 1191–1202. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wang, Y.L.; Gao, C.; Gu, Y.T.; Huang, J.; Wang, J.H.; Wang, J.H.; Zhang, Z. Salvianolic acid A attenuates kidney injury and inflammation by inhibiting NF-kappaB and p38 MAPK signaling pathways in 5/6 nephrectomized rats. Acta Pharmacol. Sin. 2018, 39, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Zhu, R.; Tian, Y.; Chen, B.; Li, R.; Li, L.; Wang, L.; Che, Y.; Zhao, D.; Mo, F.; et al. Salvia miltiorrhiza in diabetes: A review of its pharmacology, phytochemistry, and safety. Phytomedicine 2019, 58, 152871. [Google Scholar] [CrossRef] [PubMed]
- Meim, X.D.; Cao, Y.F.; Che, Y.Y.; Li, J.; Shang, Z.P.; Zhao, W.J.; Qiao, Y.J.; Zhang, J.Y. Danshen: A phytochemical and pharmacological overview. Chin. J. Nat. Med. 2019, 17, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Yin, J.; Yang, Y.; Chen, S.; Gao, X. Renoprotection of danshen injection on streptozotocin-induced diabetic rats, associated with tubular function and structure. J. Ethnopharmacol. 2014, 151, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Huang, B.; Fu, X.; Yang, J.; Lin, Y.; Lin, F. Effects of tanshinone IIA on the regulation of renal proximal tubular fibrosis. Mol. Med. Rep. 2017, 15, 4247–4252. [Google Scholar] [CrossRef]
- Xu, L.; Shen, P.; Bi, Y.; Chen, J.; Xiao, Z.; Zhang, X.; Wang, Z. Danshen injection ameliorates STZ-induced diabetic nephropathy in association with suppression of oxidative stress, pro-inflammatory factors and fibrosis. Int. Immunopharmacol. 2016, 38, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Maeda, Y.; Sonoda, N.; Makimura, H.; Kimura, S.; Maeno, S.; Takayanagi, R.; Inoguchi, T. Renoprotective effect of a novel selective PPARalpha modulator K-877 in db/db mice: A role of diacylglycerol-protein kinase C-NAD(P)H oxidase pathway. Metabolism 2017, 71, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Q.; Wang, C.; Lou, Z.; Li, Q. Trigonelline reduced diabetic nephropathy and insulin resistance in type 2 diabetic rats through peroxisome proliferator-activated receptor-gamma. Exp. Ther. Med. 2019, 18, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Ding, L.; He, X.; Takahashi, Y.; Ma, J.X. Interaction of PPARγ with the canonic Wnt pathway in the regulation of renal fibrosis. Diabetes 2016, 65, 3730–3743. [Google Scholar] [CrossRef]
- Lin, C.L.; Hsu, Y.C.; Lee, P.H.; Lei, C.C.; Wang, J.Y.; Huang, Y.T.; Wang, S.Y.; Wang, F.S. Cannabinoid receptor 1 disturbance of PPARγ2 augments hyperglycemia induction of mesangial inflammation and fibrosis in renal glomeruli. J. Mol. Med. 2014, 92, 779–792. [Google Scholar] [CrossRef]
- Yin, Z.; Zhao, Y.; He, M.; Li, H.; Fan, J.; Nie, X.; Yan, M.; Chen, C.; Wang, D.W. MiR-30c/PGC-1beta protects against diabetic cardiomyopathy via PPARγ. Cardiovasc. Diabetol. 2019, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Patial, V. Peroxisome proliferator-activated receptor gamma and its natural agonists in the treatment of kidney diseases. Front. Pharmacol. 2022, 13, 991059. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Jiang, Y.R. Tangeretin inhibition of high-glucose-Induced IL-1β, IL-6, TGF-β1, and VEGF expression in human RPE Cells. J. Diabetes Res. 2020, 2020, 9490642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pang, S.; Deng, B.; Qian, L.; Chen, J.; Zou, J.; Zheng, J.; Yang, L.; Zhang, C.; Chen, X.; et al. High glucose induces renal mesangial cell proliferation and fibronectin expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is inhibited by resveratrol. Int. J. Biochem. Cell Biol. 2012, 44, 629–638. [Google Scholar] [CrossRef]
- Malmström, J.; Lindberg, H.; Lindberg, C.; Bratt, C.; Wieslander, E.; Delander, E.L.; Särnstrand, B.; Burns, J.S.; Mose-Larsen, P.; Fey, S.; et al. Transforming growth factor-beta 1 specifically induce proteins involved in the myofibroblast contractile apparatus. Mol. Cell Proteom. 2004, 3, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Lauritano, C.; Longobardi, C.; Andretta, E.; Elagoz, A.M.; Rapisarda, P.; Di Iorio, M.; Florio, S.; Ciarcia, R. Effects of a red orange and lemon extract in obese diabetic zucker rats: Role of nicotinamide adenine dinucleotide phosphate oxidase. J. Clin. Med. 2020, 9, 1600. [Google Scholar] [CrossRef]
- Ebaid, H.; Bashandy, S.A.; Abdel-Mageed, A.M.; Al-Tamimi, J.; Hassan, I.; Alhazza, I.M. Folic acid and melatonin mitigate diabetic nephropathy in rats via inhibition of oxidative stress. Nutr. Metab. 2020, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- AL-Qabbaa, S.M.; Qaboli, S.I.; Alshammari, T.K.; Alamin, M.A.; Alrajeh, H.M.; Almuthnabi, L.A.; Alotaibi, R.R.; Alonazi, A.S.; Bin Dayel, A.F.; Alrasheed, N.M.; et al. Sitagliptin mitigates diabetic nephropathy in a rat model of streptozotocin-induced type 2 diabetes: Possible role of PTP1B/JAK-STAT pathway. Int. J. Mol. Sci. 2023, 24, 6532. [Google Scholar] [CrossRef]
- Balakumar, P.; Kadian, S.; Mahadevan, N. Are PPAR alpha agonists a rational therapeutic strategy for preventing abnormalities of the diabetic kidney? Pharmacol. Res. 2012, 65, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Kim, H.W.; Ko, S.H.; Chung, H.W.; Lim, S.W.; Yang, C.W.; Chang, Y.S.; Sugawara, A.; Guan, Y.; Breyer, M.D. Accelerated diabetic nephropathy in mice lacking the peroxisome proliferator-activated receptor alpha. Diabetes 2006, 55, 885–893. [Google Scholar] [CrossRef]
- Ding, L.; Wo, L.; Du, Z.; Tang, L.; Song, Z.; Dou, X. Danshen protects against early-stage alcoholic liver disease in mice via inducing PPARα activation and subsequent 4-HNE degradation. PLoS ONE 2017, 12, e0186357. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Q.; Zhou, C.J.; Zhang, Y.P.; Zhang, X.Q.; Xu, W.; Lin, J.; Wang, P.J. Salvianolic acid B ameliorates hyperglycemia and dyslipidemia in db/db mice through the AMPK Pathway. Cell Physiol. Biochem. 2016, 40, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, J. Salvianolic acid B prevents steroid-induced osteonecrosis of the femoral head via PPARγ expression in rats. Exp. Ther. Med. 2017, 13, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Gonzalez, J.F.; Mora-Fernandez, C. The role of inflammatory cytokines in diabetic nephropathy. J. Am. Soc. Nephrol. 2008, 19, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Q.; Liu, G.; Zhang, N. Therapeutic potentials and mechanisms of the Chinese traditional medicine Danshensu. Eur. J. Pharmacol. 2019, 864, 172710. [Google Scholar] [CrossRef] [PubMed]
- Kolset, S.O.; Reinholt, F.P.; Jenssen, T. Diabetic nephropathy and extracellular matrix. J. Histochem. Cytochem. 2012, 60, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, X.; Xiao, W.; Fu, J.; Harris, R.C.; Lindenmeyer, M.; Cohen, C.D.; Guillot, N.; Baron, M.H.; Wang, N.; et al. BAMBI elimination enhances alternative TGF-β signaling and glomerular dysfunction in diabetic mice. Diabetes 2015, 64, 2220–2233. [Google Scholar] [CrossRef]
- Fujimoto, M.; Maezawa, Y.; Yokote, K.; Joh, K.; Kobayashi, K.; Kawamura, H.; Nishimura, M.; Roberts, A.B.; Saito, Y.; Mori, S. Mice lacking Smad3 are protected against streptozotocin-induced diabetic glomerulopathy. Biochem. Biophys. Res. Commun. 2003, 305, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR. Experiments 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.S.; Wang, C.J.; Chen, Y.J.; Chang, P.R.; Huang, Y.T.; Sun, Y.C.; Huang, H.C.; Yang, Y.J.; Yang, K.D. Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1alpha and VEGF-A expression in shock wave-stimulated osteoblasts. J. Biol. Chem. 2004, 279, 10331–10337. [Google Scholar] [CrossRef]
- Lin, C.L.; Hsu, Y.C.; Huang, Y.T.; Shih, Y.H.; Wang, C.J.; Chiang, W.C.; Chang, P.J. A KDM6A-KLF10 reinforcing feedback mechanism aggravates diabetic podocyte dysfunction. EMBO Mol. Med. 2019, 11, e9828. [Google Scholar] [CrossRef] [PubMed]

| Genes | Sense (Forward) | Antisense (Reverse) |
|---|---|---|
| PPAR-γ | 5′-CCAAAGCCTGAGCCCAGA-3′ | 5′-GCACCACTCCCATGGCAT-3′ |
| IL-1β | 5′-CCAGGATGAGGACCCAAGCA-3′ | 5′-TCCCGACCATTGCTGTTTCC-3′ |
| TGF-β1 | 5′-TGAGTGGCTGTCTTTTGACG-3′ | 5′-TGGGACTGATCCCATTGATT-3′ |
| Fibronectin | 5′-CAGCCCCTGATTGGAGTC-3′ | 5′-TGGGTGACACCTGAGTGAAC-3′ |
| β-actin | 5′-CGCCAACCGCGAGAAGAT-3′ | 5′-CGTCACCGGAGTCCATCA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-C.; Shih, Y.-H.; Ho, C.; Liu, C.-C.; Liaw, C.-C.; Lin, H.-Y.; Lin, C.-L. Ethyl Acetate Fractions of Salvia miltiorrhiza Bunge (Danshen) Crude Extract Modulate Fibrotic Signals to Ameliorate Diabetic Kidney Injury. Int. J. Mol. Sci. 2024, 25, 8986. https://doi.org/10.3390/ijms25168986
Hsu Y-C, Shih Y-H, Ho C, Liu C-C, Liaw C-C, Lin H-Y, Lin C-L. Ethyl Acetate Fractions of Salvia miltiorrhiza Bunge (Danshen) Crude Extract Modulate Fibrotic Signals to Ameliorate Diabetic Kidney Injury. International Journal of Molecular Sciences. 2024; 25(16):8986. https://doi.org/10.3390/ijms25168986
Chicago/Turabian StyleHsu, Yung-Chien, Ya-Hsueh Shih, Cheng Ho, Cheng-Chi Liu, Chia-Ching Liaw, Hui-Yi Lin, and Chun-Liang Lin. 2024. "Ethyl Acetate Fractions of Salvia miltiorrhiza Bunge (Danshen) Crude Extract Modulate Fibrotic Signals to Ameliorate Diabetic Kidney Injury" International Journal of Molecular Sciences 25, no. 16: 8986. https://doi.org/10.3390/ijms25168986
APA StyleHsu, Y.-C., Shih, Y.-H., Ho, C., Liu, C.-C., Liaw, C.-C., Lin, H.-Y., & Lin, C.-L. (2024). Ethyl Acetate Fractions of Salvia miltiorrhiza Bunge (Danshen) Crude Extract Modulate Fibrotic Signals to Ameliorate Diabetic Kidney Injury. International Journal of Molecular Sciences, 25(16), 8986. https://doi.org/10.3390/ijms25168986






