The Regulation of MicroRNA-21 by Interleukin-6 and Its Role in the Development of Fibrosis in Endometriotic Lesions
Abstract
1. Introduction
2. Results
2.1. Progression of Endometriosis in the Mouse Model
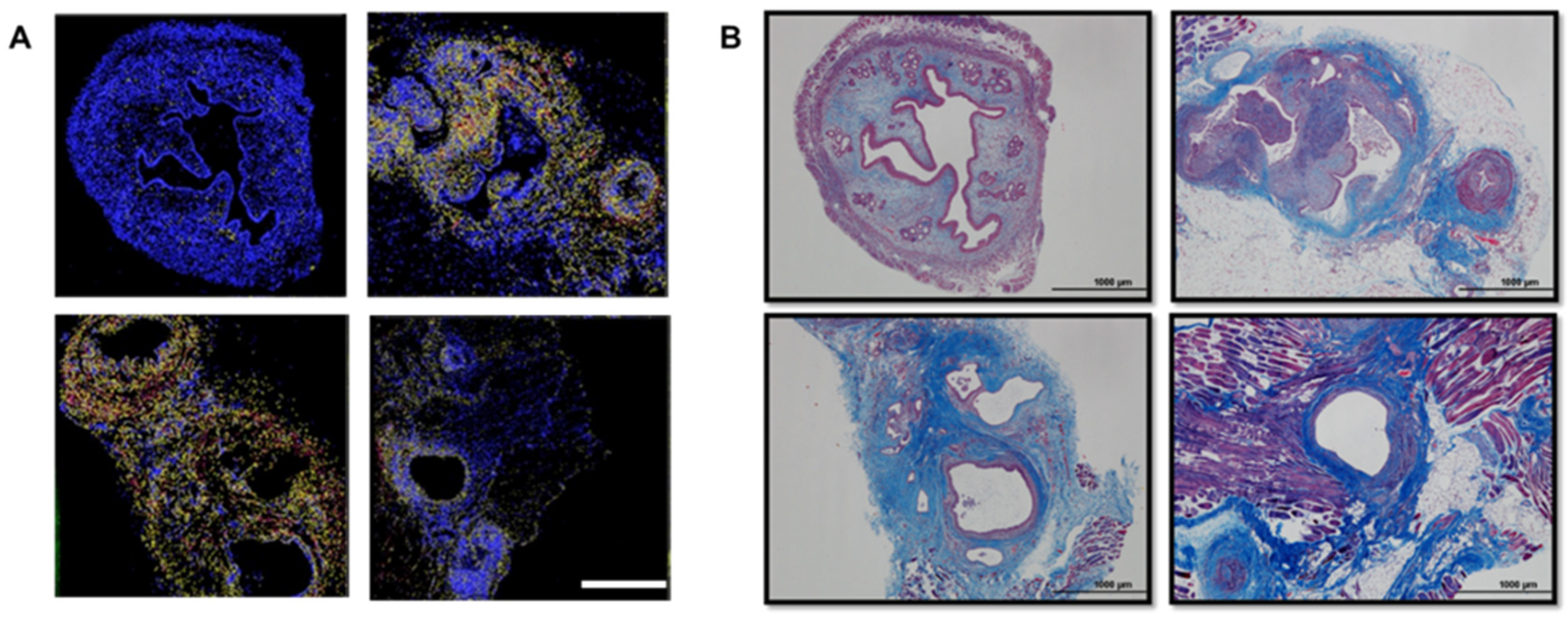
2.2. Development of Fibrosis in the Mouse and Baboon Models
2.3. Small-RNA Library and Ingenuity Pathway Analysis (IPA)
2.4. miR-21 Is Increased in Endometriotic Lesions
2.5. miR-21 Is Predominantly Expressed in the Stroma of Endometriotic Lesions
2.6. miR-21 Expression and Fibrosis Development in the Mouse Model
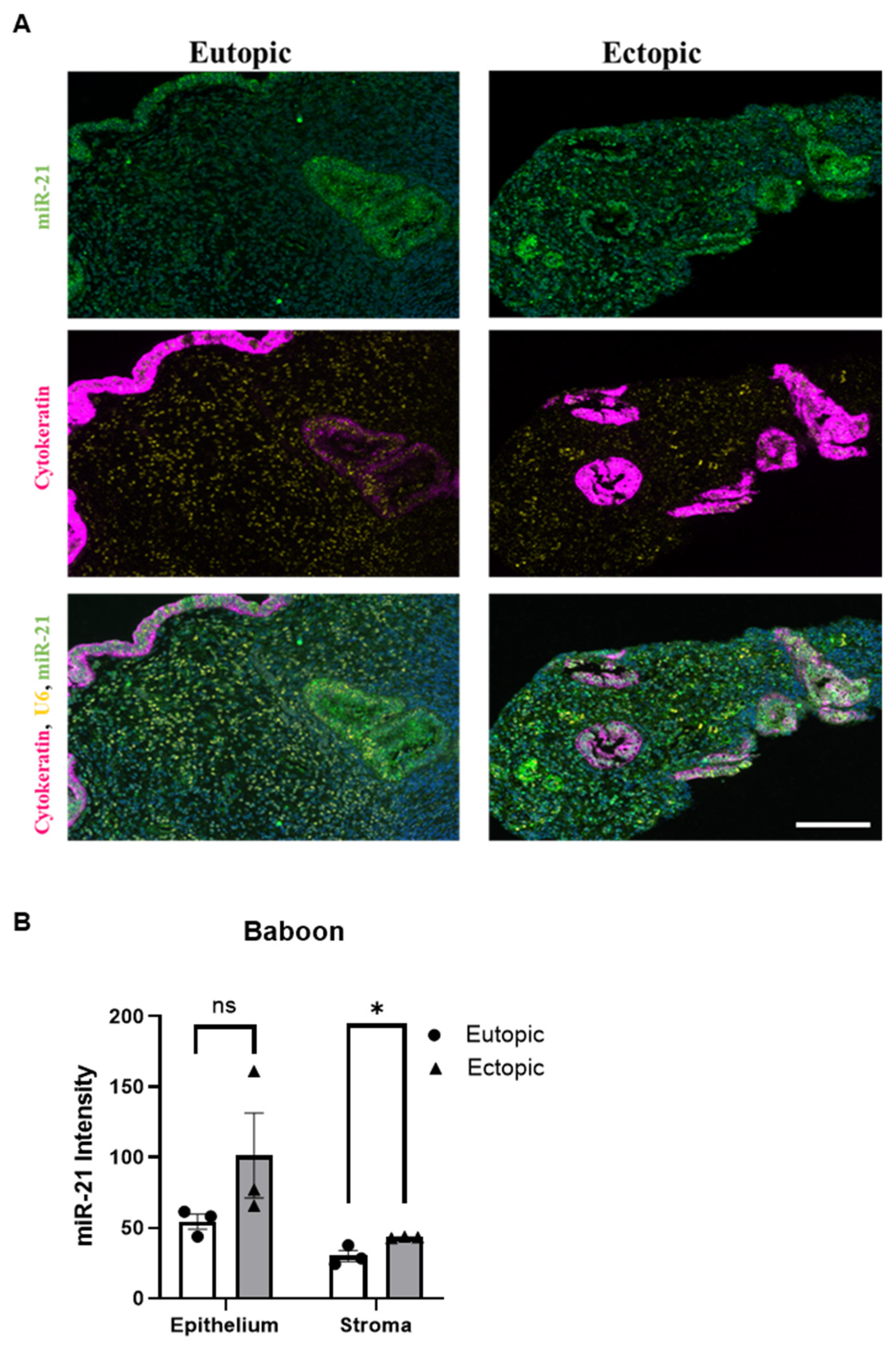
2.7. miR-21 Expression in the Baboon Model
2.8. CTGF Expression Is Correlated with the Presence of Fibrosis in the Mouse Model, Baboon Model and Women with Endometriosis
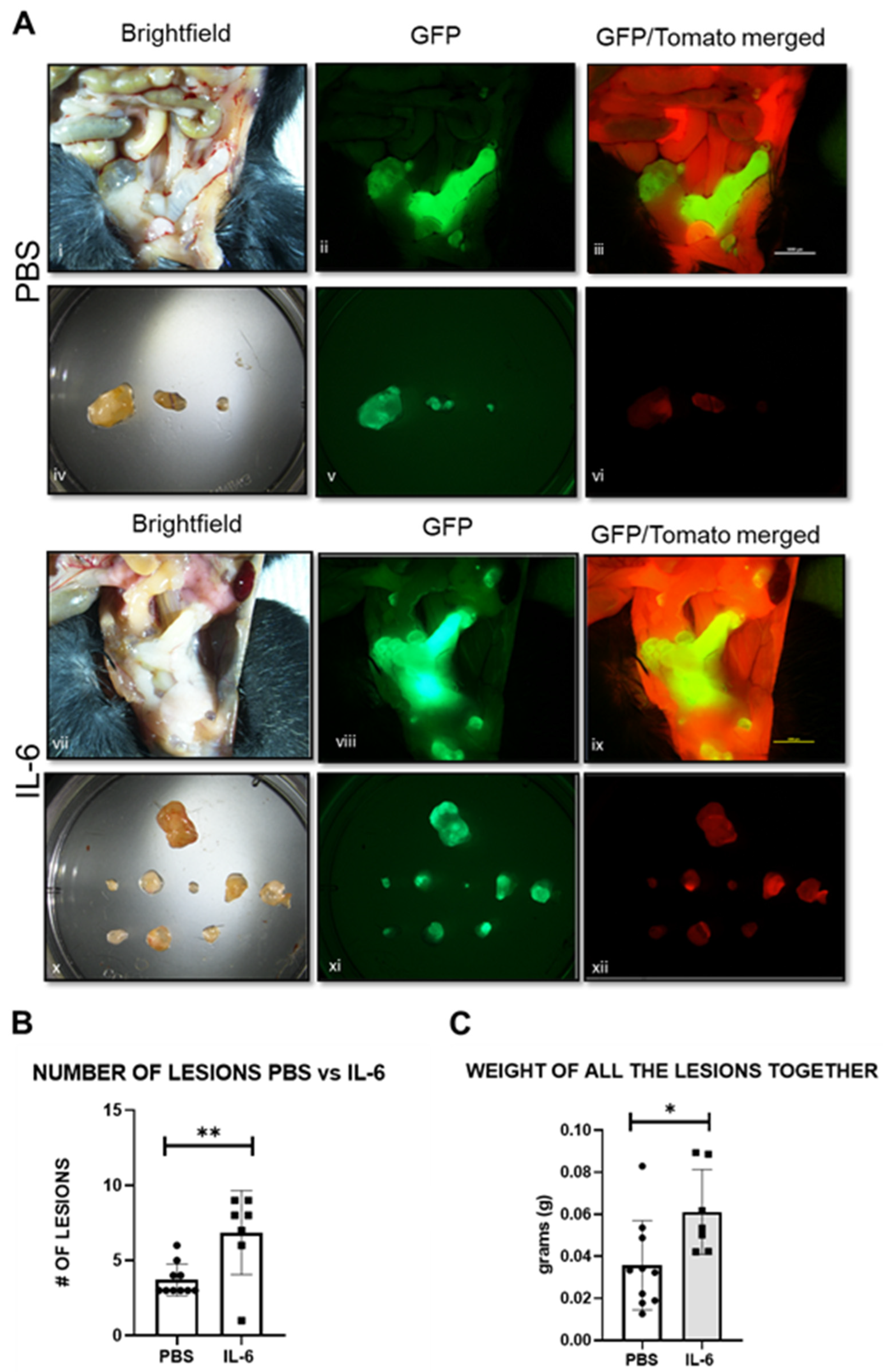
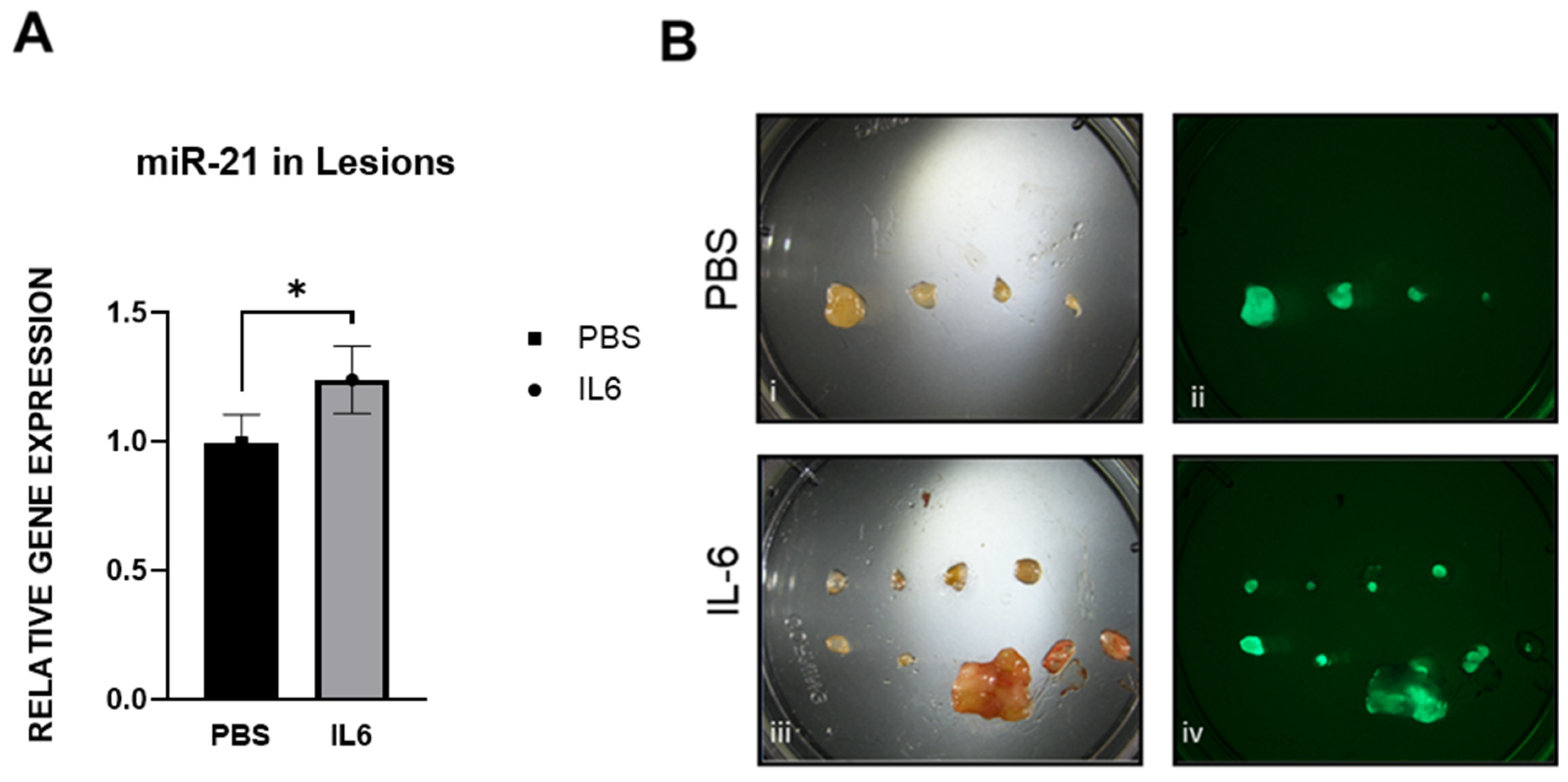
2.9. IL-6 Upregulates miR-21 in the Mouse Model
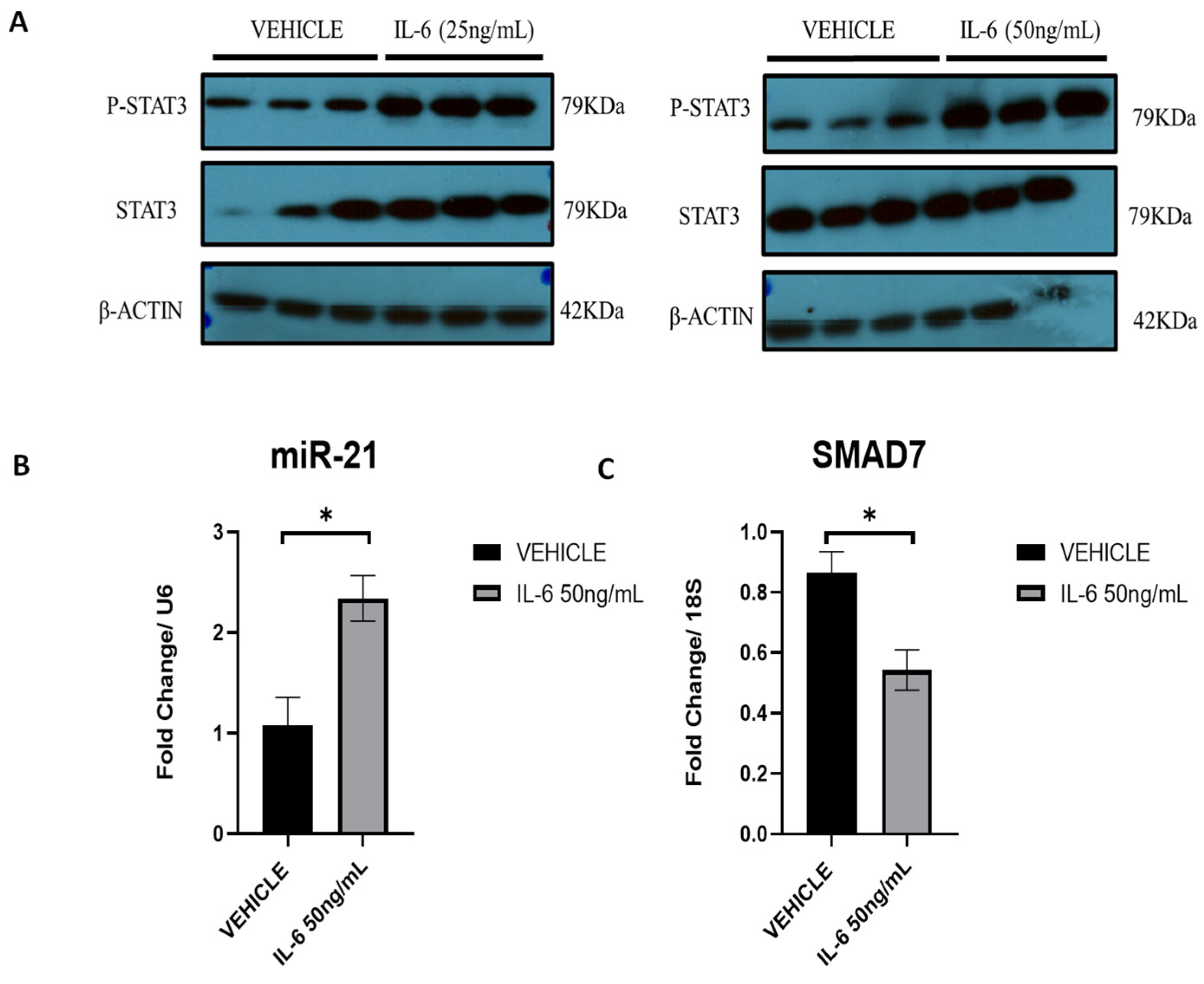
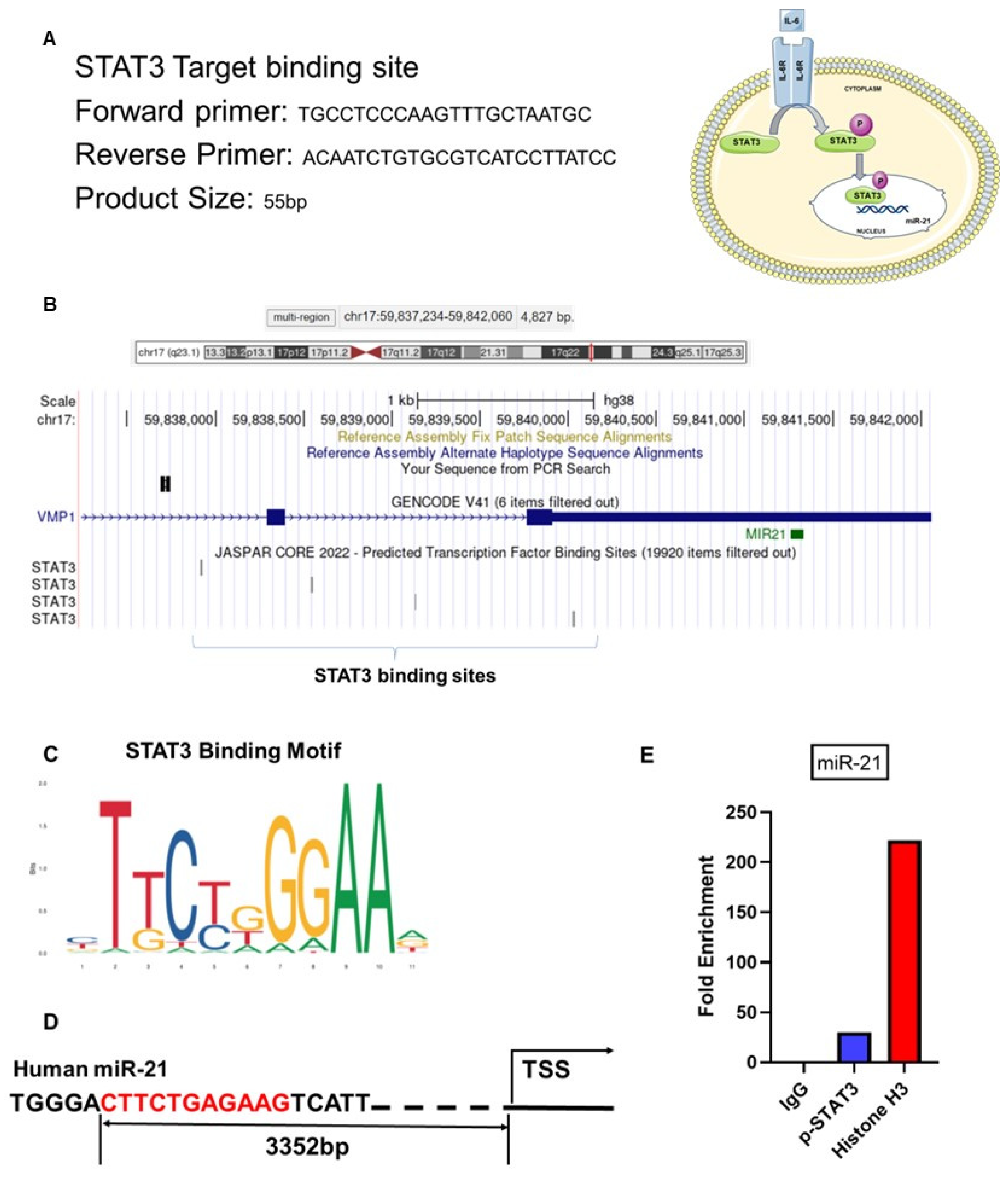
2.10. IL-6 Upregulates miR-21 via p-STAT3 in Ectopic Stromal Cells
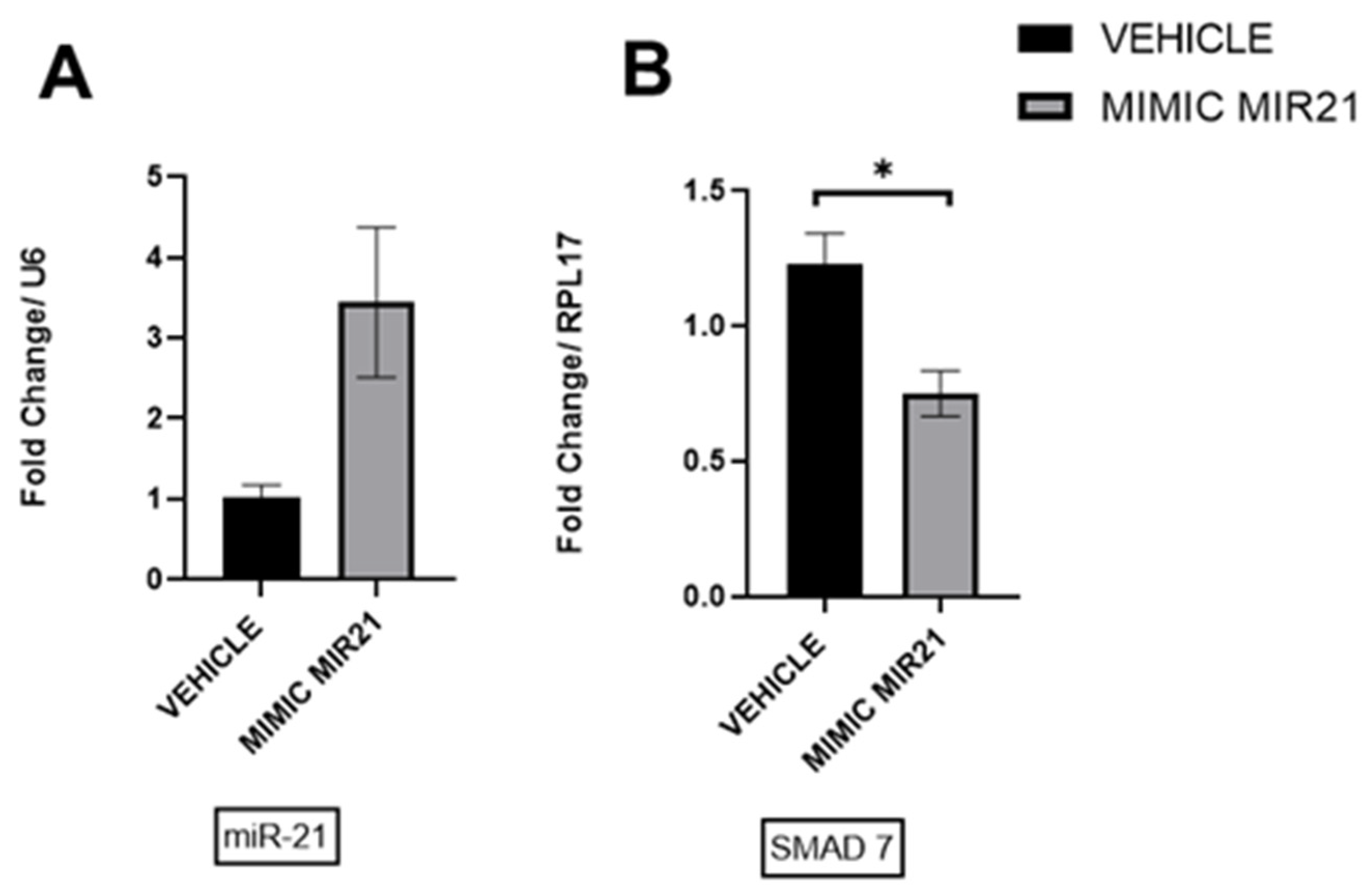
2.11. Upregulation of miR-21 in Ectopic Stromal Cells
3. Discussion
4. Materials and Methods
4.1. Induction of Endometriosis and IL-6 Treatment in the Mouse Model
4.2. Baboon Endometriosis Model
4.3. Human Endometrial and Endometriotic Samples
4.4. RNA Isolation and RT-qPCR
4.5. In Situ Hybridization (ISH)
4.6. Masson’s Trichrome Staining
4.7. Small RNA-Sequencing
4.8. Ingenuity Pathway Analysis (IPA)
4.9. Cell Culture and Transfection
4.10. CUT&RUN Assay
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef]
- Vigano, P.; Candiani, M.; Monno, A.; Giacomini, E.; Vercellini, P.; Somigliana, E. Time to redefine endometriosis including its pro-fibrotic nature. Hum. Reprod. 2018, 33, 347–352. [Google Scholar] [CrossRef]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Vigano, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Falcone, T.; Lebovic, D.I. Clinical Management of Endometriosis. Obstet. Gynecol. 2011, 118, 691–705. [Google Scholar] [CrossRef]
- Martire, F.G.; Giorgi, M.; D’Abate, C.; Colombi, I.; Ginetti, A.; Cannoni, A.; Fedele, F.; Exacoustos, C.; Centini, G.; Zupi, E.; et al. Deep Infiltrating Endometriosis in Adolescence: Early Diagnosis and Possible Prevention of Disease Progression. J. Clin. Med. 2024, 13, 550. [Google Scholar] [CrossRef]
- Treloar, S.A.; Bell, T.A.; Nagle, C.M.; Purdie, D.M.; Green, A.C. Early menstrual characteristics associated with subsequent diagnosis of endometriosis. Am. J. Obstet. Gynecol. 2010, 202, 534.e1–534.e6. [Google Scholar] [CrossRef]
- Vercellini, P.; Vigano, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef]
- Wu, G.; Bersinger, N.A.; Mueller, M.D.; von Wolff, M. Intrafollicular inflammatory cytokines but not steroid hormone concentrations are increased in naturally matured follicles of women with proven endometriosis. J. Assist. Reprod. Genet. 2017, 34, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ogata, A.; Narazaki, M. Tocilizumab: An Updated Review of Its Use in the Treatment of Rheumatoid Arthritis and Its Application for Other Immune-Mediated Diseases. Clin. Med. Insights Ther. 2013, 5, CMT-S9282. [Google Scholar] [CrossRef]
- Murakami, M.; Kamimura, D.; Hirano, T. Pleiotropy and Specificity: Insights from the Interleukin 6 Family of Cytokines. Immunity 2019, 50, 812–831. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Yoo, J.Y.; Kim, T.H.; Shin, J.H.; Langenheim, J.F.; Ferguson, S.D.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Jeong, J.W. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum. Reprod. 2015, 30, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Niu, G.; Kortylewski, M.; Burdelya, L.; Shain, K.; Zhang, S.; Bhattacharya, R.; Gabrilovich, D.; Heller, R.; Coppola, D.; et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat. Med. 2004, 10, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Loffler, D.; Brocke-Heidrich, K.; Pfeifer, G.; Stocsits, C.; Hackermuller, J.; Kretzschmar, A.K.; Burger, R.; Gramatzki, M.; Blumert, C.; Bauer, K.; et al. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood 2007, 110, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Jeong, J.W.; Fazleabas, A.T.; Tayade, C.; Young, S.L.; Lessey, B.A. Protein Inhibitor of Activated STAT3 (PIAS3) Is Down-Regulated in Eutopic Endometrium of Women with Endometriosis. Biol. Reprod. 2016, 95, 11. [Google Scholar] [CrossRef] [PubMed]
- Tscherner, A.; Brown, A.C.; Stalker, L.; Kao, J.; Dufort, I.; Sirard, M.A.; LaMarre, J. STAT3 signaling stimulates miR-21 expression in bovine cumulus cells during in vitro oocyte maturation. Sci. Rep. 2018, 8, 11527. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.M.; Creighton, C.J.; Han, D.Y.; Zariff, A.; Anderson, M.L.; Gunaratne, P.H.; Matzuk, M.M. Functional microRNA involved in endometriosis. Mol. Endocrinol. 2011, 25, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O.; Hamilton, A.E.; Aghajanova, L.; Vo, K.C.; Nezhat, C.N.; Lessey, B.A.; Giudice, L.C. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol. Hum. Reprod. 2009, 15, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Sandoghsaz, R.S.; Montazeri, F.; Shafienia, H.; Mehdi Kalantar, S.; Javaheri, A.; Samadi, M. Expression of miR-21 &IL-4 in endometriosis. Hum. Immunol. 2024, 85, 110746. [Google Scholar] [CrossRef]
- Giudice, L.C.; Evers, J.L. , Healy, D.L. Endometriosis: Science and Practice; John Wiley & Sons: New York, NY, USA, 2012; p. 600. [Google Scholar]
- Kumarswamy, R.; Volkmann, I.; Thum, T. Regulation and function of miRNA-21 in health and disease. Ribonucleic Acid Biol. 2011, 8, 706–713. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S. MicroRNAs in fibrosis: Opportunities and challenges. Arthritis Res. Ther. 2016, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Charrier, A.; Chen, R.; Chen, L.; Kemper, S.; Hattori, T.; Takigawa, M.; Brigstock, D.R. Connective tissue growth factor (CCN2) and microRNA-21 are components of a positive feedback loop in pancreatic stellate cells (PSC) during chronic pancreatitis and are exported in PSC-derived exosomes. J. Cell Commun. Signal. 2014, 8, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Itagaki, R.; Zheng, X.; Batkai, S.; Thum, S.; Ahmad, F.; Van Aelst, L.N.; Sharma, A.; Piccoli, M.T.; Weinberger, F.; et al. miR-21 promotes fibrosis in an acute cardiac allograft transplantation model. Cardiovasc. Res. 2016, 110, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chen, H.; Ge, D.; Xu, Y.; Xu, H.; Yang, Y.; Gu, M.; Zhou, Y.; Zhu, J.; Ge, T.; et al. Mir-21 Promotes Cardiac Fibrosis After Myocardial Infarction Via Targeting Smad7. Cell Physiol. Biochem. 2017, 42, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Luo, H.; Li, Y.; Zhou, Y.; Jiang, Y.; Chai, J.; Xiao, X.; You, Y.; Zuo, X. MicroRNA-21 in scleroderma fibrosis and its function in TGF-beta-regulated fibrosis-related genes expression. J. Clin. Immunol. 2013, 33, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Friggeri, A.; Yang, Y.; Milosevic, J.; Ding, Q.; Thannickal, V.J.; Kaminski, N.; Abraham, E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 2010, 207, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O. Fibrosis as a molecular hallmark of endometriosis pathophysiology. Fertil. Steril. 2022, 118, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Vissers, G.; Giacomozzi, M.; Verdurmen, W.; Peek, R.; Nap, A. The role of fibrosis in endometriosis: A systematic review. Hum. Reprod. Update 2024. [Google Scholar] [CrossRef] [PubMed]
- Vigano, P.; Ottolina, J.; Bartiromo, L.; Bonavina, G.; Schimberni, M.; Villanacci, R.; Candiani, M. Cellular Components Contributing to Fibrosis in Endometriosis: A Literature Review. J. Minim. Invasive Gynecol. 2020, 27, 287–295. [Google Scholar] [CrossRef]
- Kang, H. Role of MicroRNAs in TGF-beta Signaling Pathway-Mediated Pulmonary Fibrosis. Int. J. Mol. Sci. 2017, 18, 2527. [Google Scholar] [CrossRef]
- Huang, Y.; He, Y.; Li, J. MicroRNA-21: A central regulator of fibrotic diseases via various targets. Curr. Pharm. Des. 2015, 21, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Marí-Alexandre, J.; García-Oms, J.; Barceló-Molina, M.; Gilabert-Aguilar, J.; Estellés, A.; Braza-Boíls, A.; Gilabert-Estellés, J. MicroRNAs and angiogenesis in endometriosis. Thromb. Res. 2015, 135, S38–S40. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-Y.; Kim, T.H.; Shin, J.-H.; Marquardt, R.M.; Müller, U.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Yoon, H.-G.; Jeong, J.-W. Loss of MIG-6 results in endometrial progesterone resistance via ERBB2. Nat. Commun. 2022, 13, 1101. [Google Scholar] [CrossRef]
- Haikalis, M.E.; Wessels, J.M.; Leyland, N.A.; Agarwal, S.K.; Foster, W.G. MicroRNA expression pattern differs depending on endometriosis lesion type. Biol. Reprod. 2018, 98, 623–633. [Google Scholar] [CrossRef]
- Talebloo, N.; Bernal, M.A.O.; Kenyon, E.; Mallett, C.L.; Fazleabas, A.; Moore, A. Detection of Endometriosis Lesions Using Gd-Based Collagen I Targeting Probe in Murine Models of Endometriosis. Mol. Imaging Biol. 2023, 25, 833–843. [Google Scholar] [CrossRef]
- Nielsen, B.S.; Jorgensen, S.; Fog, J.U.; Sokilde, R.; Christensen, I.J.; Hansen, U.; Brunner, N.; Baker, A.; Moller, S.; Nielsen, H.J. High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients. Clin. Exp. Metastasis 2011, 28, 27–38. [Google Scholar] [CrossRef]
- Sempere, L.F.; Preis, M.; Yezefski, T.; Ouyang, H.; Suriawinata, A.A.; Silahtaroglu, A.; Conejo-Garcia, J.R.; Kauppinen, S.; Wells, W.; Korc, M. Fluorescence-based codetection with protein markers reveals distinct cellular compartments for altered MicroRNA expression in solid tumors. Clin. Cancer Res. 2010, 16, 4246–4255. [Google Scholar] [CrossRef] [PubMed]
- Kadera, B.E.; Li, L.; Toste, P.A.; Wu, N.; Adams, C.; Dawson, D.W.; Donahue, T.R. MicroRNA-21 in Pancreatic Ductal Adenocarcinoma Tumor-Associated Fibroblasts Promotes Metastasis. PLoS ONE 2013, 8, e71978. [Google Scholar] [CrossRef]
- Schipper, J.; Westerhuis, J.J.; Beddows, I.; Madaj, Z.; Monsma, D.; Hostetter, G.; Kiupel, M.; Conejo-Garcia, J.R.; Sempere, L.F. Loss of microRNA-21 leads to profound stromal remodeling and short survival in K-Ras-driven mouse models of pancreatic cancer. Int. J. Cancer 2020, 147, 2265–2278. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M.D.; Pickard, K.M.; Nielsen, B.S.; Sayan, A.E.; Jenei, V.; Mellone, M.; Mitter, R.; Primrose, J.N.; Thomas, G.J.; Packham, G.K.; et al. Pleiotropic actions of miR-21 highlight the critical role of deregulated stromal microRNAs during colorectal cancer progression. Cell Death Dis. 2013, 4, e684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Chen, Y.; Suo, L.; Chen, H.; Zhu, L.; Wan, G.; Han, X. Activin a promotes myofibroblast differentiation of endometrial mesenchymal stem cells via STAT3-dependent Smad/CTGF pathway. Cell Commun. Signal. 2019, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.; Williams, S.; Kothapalli, D.; Klapper, H.; Grotendorst, G.R. Stimulation of Fibroblast Cell Growth, Matrix Production, and Granulation Tissue Formation by Connective Tissue Growth Factor. J. Investig. Dermatol. 1996, 107, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Tall, E.G.; Bernstein, A.M.; Oliver, N.; Gray, J.L.; Masur, S.K. TGF-beta-stimulated CTGF production enhanced by collagen and associated with biogenesis of a novel 31-kDa CTGF form in human corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5002–5011. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ortega, M.; Rodriguez-Vita, J.; Sanchez-Lopez, E.; Carvajal, G.; Egido, J. TGF-beta signaling in vascular fibrosis. Cardiovasc. Res. 2007, 74, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Jeung, I.C.; Park, A.; Park, Y.J.; Jung, H.; Kim, T.D.; Lee, H.G.; Choi, I.; Yoon, S.R. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum. Reprod. 2014, 29, 2176–2189. [Google Scholar] [CrossRef] [PubMed]
- Kashanian, M.; Sariri, E.; Vahdat, M.; Ahmari, M.; Moradi, Y.; Sheikhansari, N. A comparison between serum levels of interleukin-6 and CA125 in patients with endometriosis and normal women. Med. J. Islam. Repub. Iran 2015, 29, 280. [Google Scholar] [PubMed]
- Keenan, J.A.; Chen, T.T.; Chadwell, N.L.; Torry, D.S.; Caudle, M.R. IL-1 beta, TNF-alpha, and IL-2 in peritoneal fluid and macrophage-conditioned media of women with endometriosis. Am. J. Reprod. Immunol. 1995, 34, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, A.; Bruse, C.; Carlberg, M.; Carlström, K. Interleukin 1β, interleukin-6, and tumor necrosis factor-α in endometriotic tissue and in endometrium. Fertil. Steril. 2001, 75, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fu, X.; Wu, T.; Yang, L.; Hu, C.; Wu, R. Role of Interleukin-6 and Its Receptor in Endometriosis. Med. Sci. Monit. 2017, 23, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Iwabe, T.; Terakawa, N. Role of cytokines in endometriosis. Fertil. Steril. 2001, 76, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. ; Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Fujishita, A.; Sekine, I.; Ishimaru, T.; Masuzaki, H. Immunopathogenesis of pelvic endometriosis: Role of hepatocyte growth factor, macrophages and ovarian steroids. Am. J. Reprod. Immunol. 2008, 60, 383–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; van Boxel-Dezaire, A.H.; Cheon, H.; Yang, J.; Stark, G.R. STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 16975–16980. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E.; Darnell, J.E., Jr. Stats: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Afshar, Y.; Hastings, J.; Roqueiro, D.; Jeong, J.W.; Giudice, L.C.; Fazleabas, A.T. Changes in eutopic endometrial gene expression during the progression of experimental endometriosis in the baboon, Papio anubis. Biol. Reprod. 2013, 88, 44. [Google Scholar] [CrossRef] [PubMed]
- Hastings, J.M.; Fazleabas, A.T. A baboon model for endometriosis: Implications for fertility. Reprod. Biol. Endocrinol. 2006, 4 (Suppl. S1), S7. [Google Scholar] [CrossRef] [PubMed]
- Fazleabas, A.T. A baboon model for inducing endometriosis. Methods Mol. Med. 2006, 121, 95–99. [Google Scholar] [CrossRef]
- Joshi, N.R.; Miyadahira, E.H.; Afshar, Y.; Jeong, J.W.; Young, S.L.; Lessey, B.A.; Serafini, P.C.; Fazleabas, A.T. Progesterone Resistance in Endometriosis Is Modulated by the Altered Expression of MicroRNA-29c and FKBP4. J. Clin. Endocrinol. Metab. 2017, 102, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Joshi, N.R.; Burns, G.W.; Hrbek, S.M.; Vegter, E.L.; Ochoa-Bernal, M.A.; Song, Y.; Moldovan, G.E.; Sempere, L.F.; Miyadahira, E.H.; et al. MicroRNA-210-3p Regulates Endometriotic Lesion Development by Targeting IGFBP3 in Baboons and Women with Endometriosis. Reprod. Sci. 2023, 30, 2932–2944. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sempere, L.F.; Zaluzec, E.; Kenyon, E.; Kiupel, M.; Moore, A. Automated Five-Color Multiplex Co-detection of MicroRNA and Protein Expression in Fixed Tissue Specimens. Methods Mol. Biol. 2020, 2148, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Q.; Xu, C.-B. A convenient method for quantifying collagen fibers in atherosclerotic lesions by ImageJ software. Int. J. Clin. Exp. Med. 2017, 10, 14927–14935. [Google Scholar]
- Song, Y.; Joshi, N.R.; Vegter, E.; Hrbek, S.; Lessey, B.A.; Fazleabas, A.T. Establishment of an Immortalized Endometriotic Stromal Cell Line from Human Ovarian Endometrioma. Reprod. Sci. 2020, 27, 2082–2091. [Google Scholar] [CrossRef] [PubMed]
- Skene, P.J.; Henikoff, J.G.; Henikoff, S. Targeted in situ genome-wide profiling with high efficiency for low cell numbers. Nat. Protoc. 2018, 13, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Pouly, J.L.; Canis, M. Persistent activation of signal transducer and activator of transcription 3 via interleukin-6 trans-signaling is involved in fibrosis of endometriosis. Hum. Reprod. 2022, 37, 1489–1504. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochoa Bernal, M.A.; Song, Y.; Joshi, N.; Burns, G.W.; Paul, E.N.; Vegter, E.; Hrbek, S.; Sempere, L.F.; Fazleabas, A.T. The Regulation of MicroRNA-21 by Interleukin-6 and Its Role in the Development of Fibrosis in Endometriotic Lesions. Int. J. Mol. Sci. 2024, 25, 8994. https://doi.org/10.3390/ijms25168994
Ochoa Bernal MA, Song Y, Joshi N, Burns GW, Paul EN, Vegter E, Hrbek S, Sempere LF, Fazleabas AT. The Regulation of MicroRNA-21 by Interleukin-6 and Its Role in the Development of Fibrosis in Endometriotic Lesions. International Journal of Molecular Sciences. 2024; 25(16):8994. https://doi.org/10.3390/ijms25168994
Chicago/Turabian StyleOchoa Bernal, Maria Ariadna, Yong Song, Niraj Joshi, Gregory W. Burns, Emmanuel N. Paul, Erin Vegter, Samantha Hrbek, Lorenzo F. Sempere, and Asgerally T. Fazleabas. 2024. "The Regulation of MicroRNA-21 by Interleukin-6 and Its Role in the Development of Fibrosis in Endometriotic Lesions" International Journal of Molecular Sciences 25, no. 16: 8994. https://doi.org/10.3390/ijms25168994
APA StyleOchoa Bernal, M. A., Song, Y., Joshi, N., Burns, G. W., Paul, E. N., Vegter, E., Hrbek, S., Sempere, L. F., & Fazleabas, A. T. (2024). The Regulation of MicroRNA-21 by Interleukin-6 and Its Role in the Development of Fibrosis in Endometriotic Lesions. International Journal of Molecular Sciences, 25(16), 8994. https://doi.org/10.3390/ijms25168994






