Biosynthesis of Polyhydroalkanoates Doped with Silver Nanoparticles Using Pseudomonas putida and Pseudomonas aeruginosa for Antibacterial Polymer Applications
Abstract
1. Introduction
2. Results
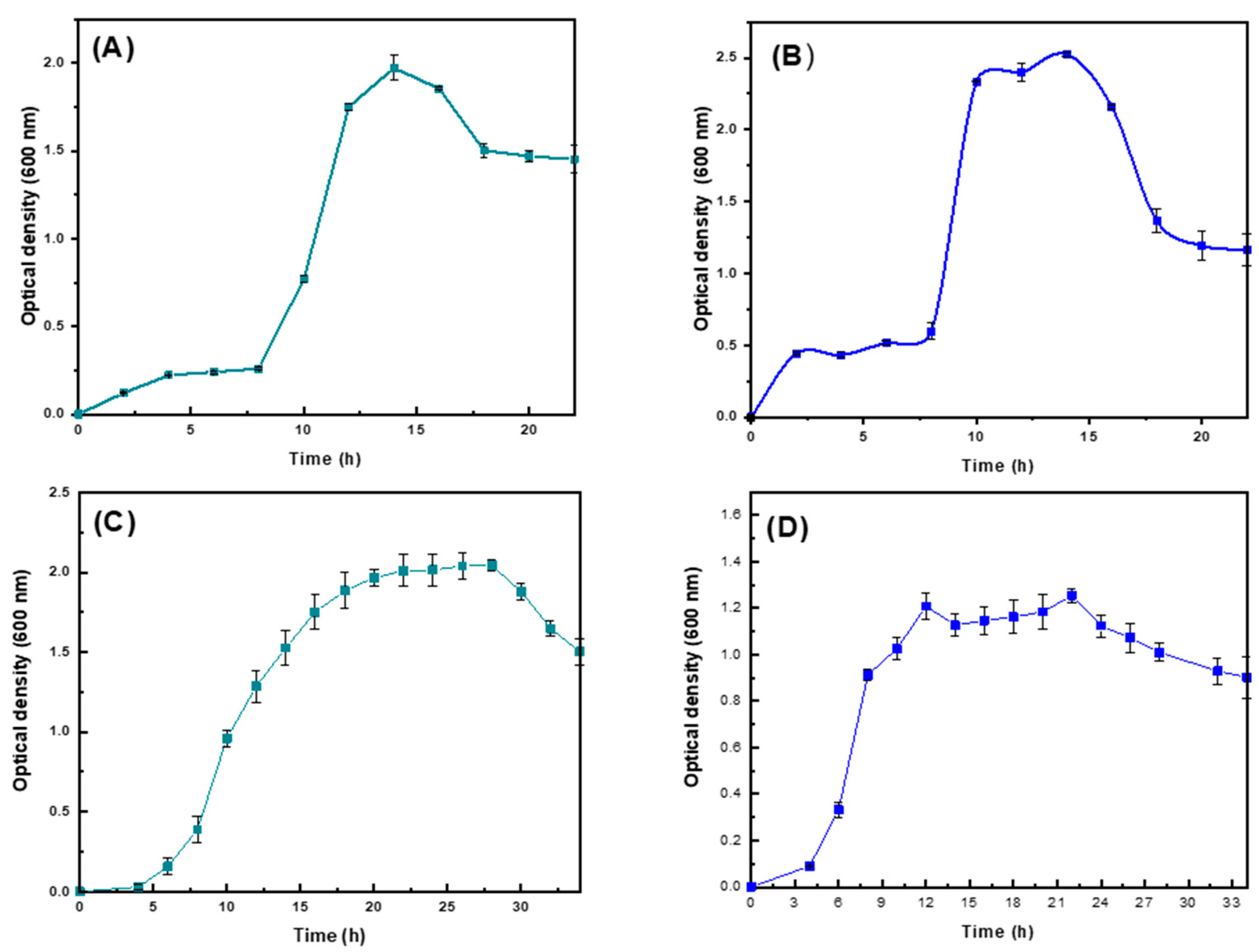
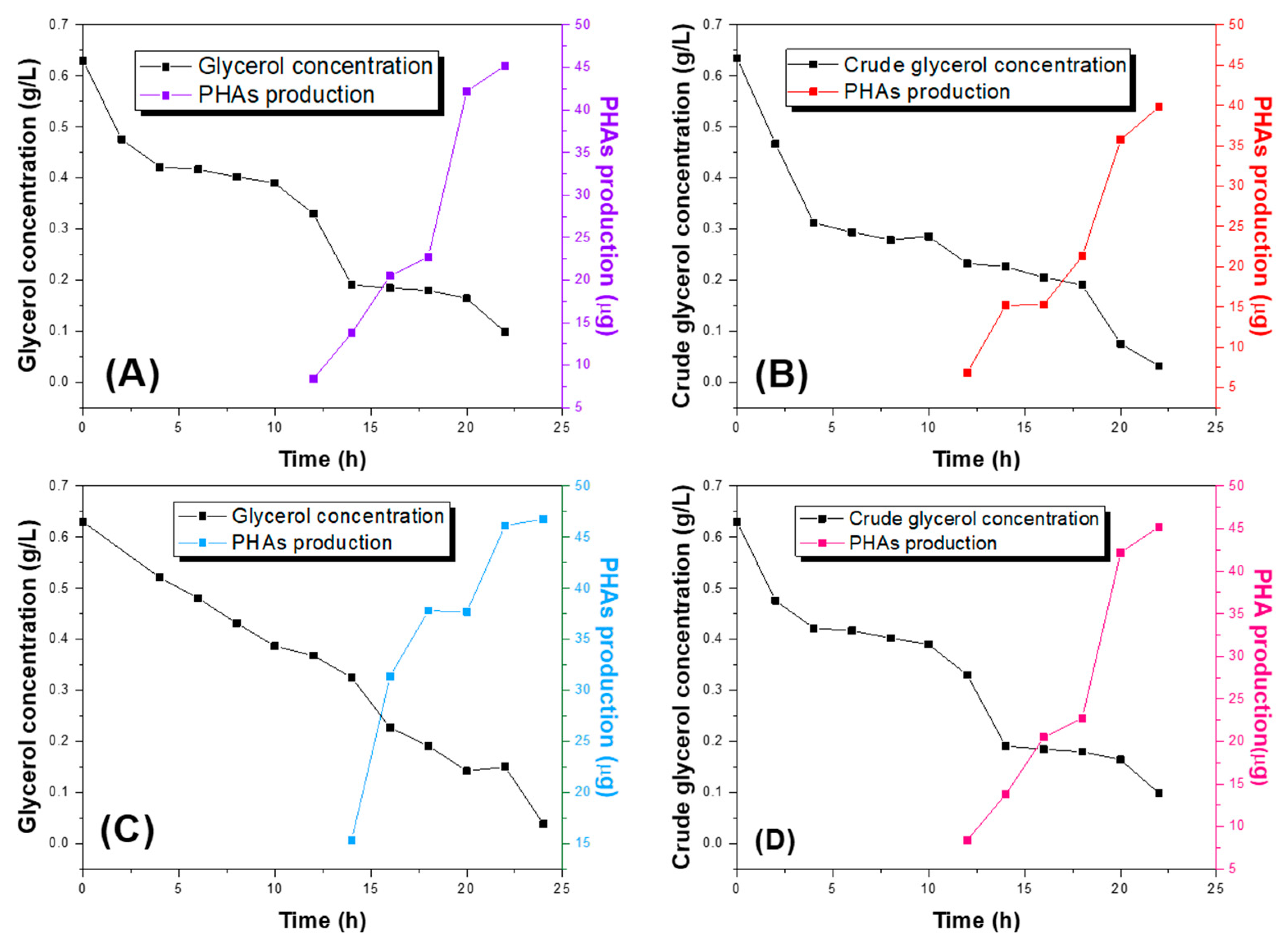
2.1. Evaluation of PHAs Synthesis in the Different Strains of Pseudomonas putida and Pseudomonas aeruginosa
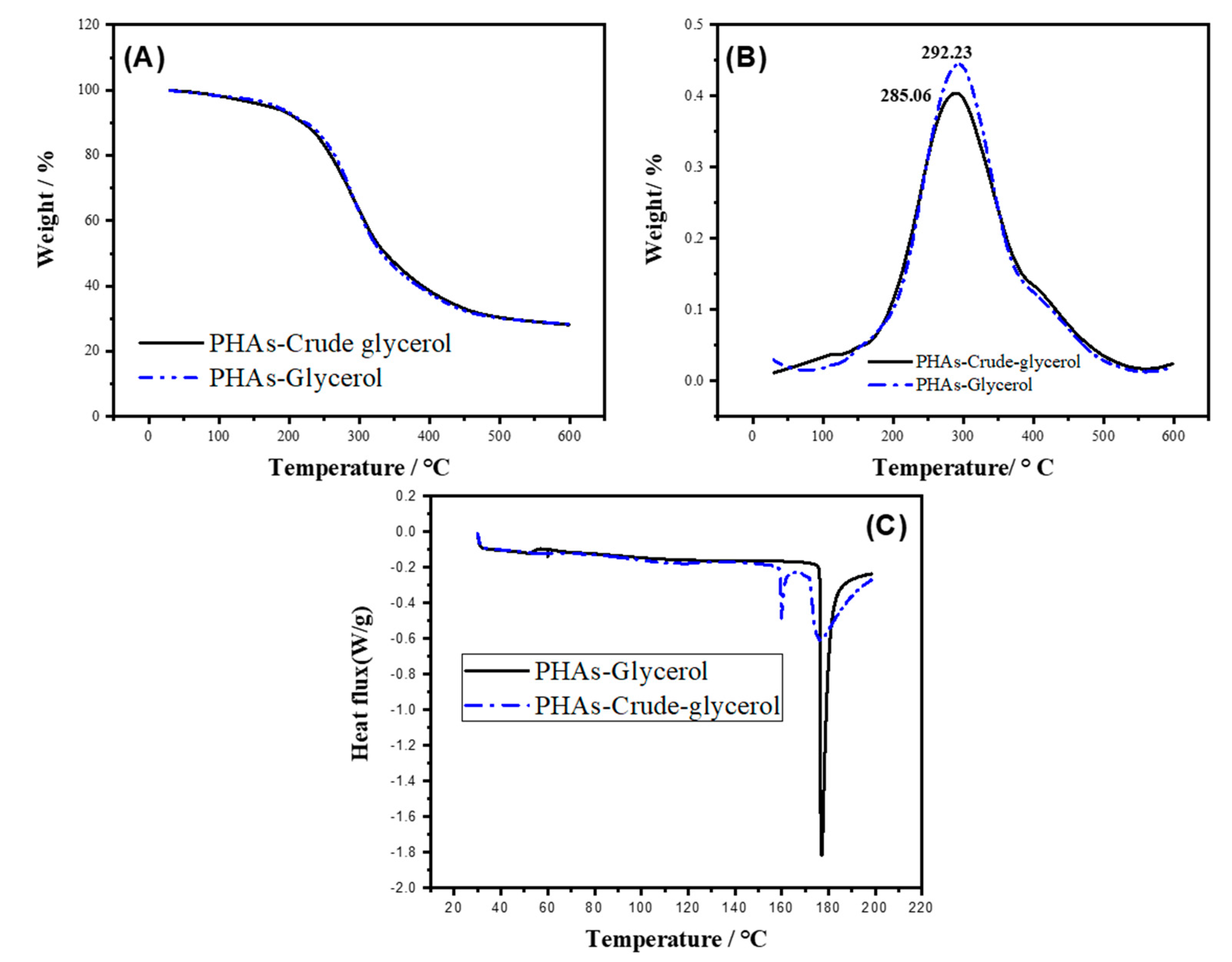
2.2. TGA of the PHAs Produced
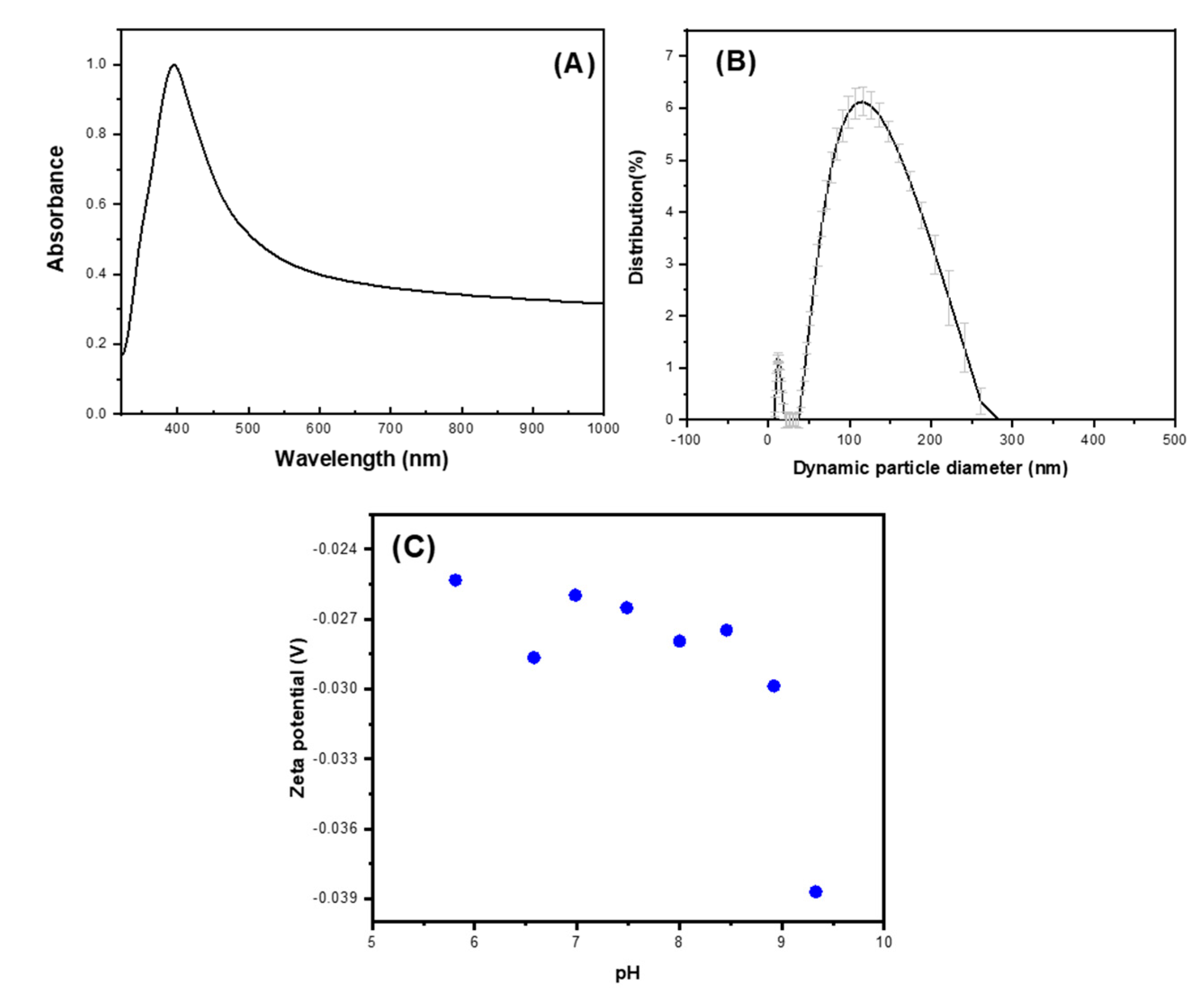
2.3. Silver Nanoparticles Characterization
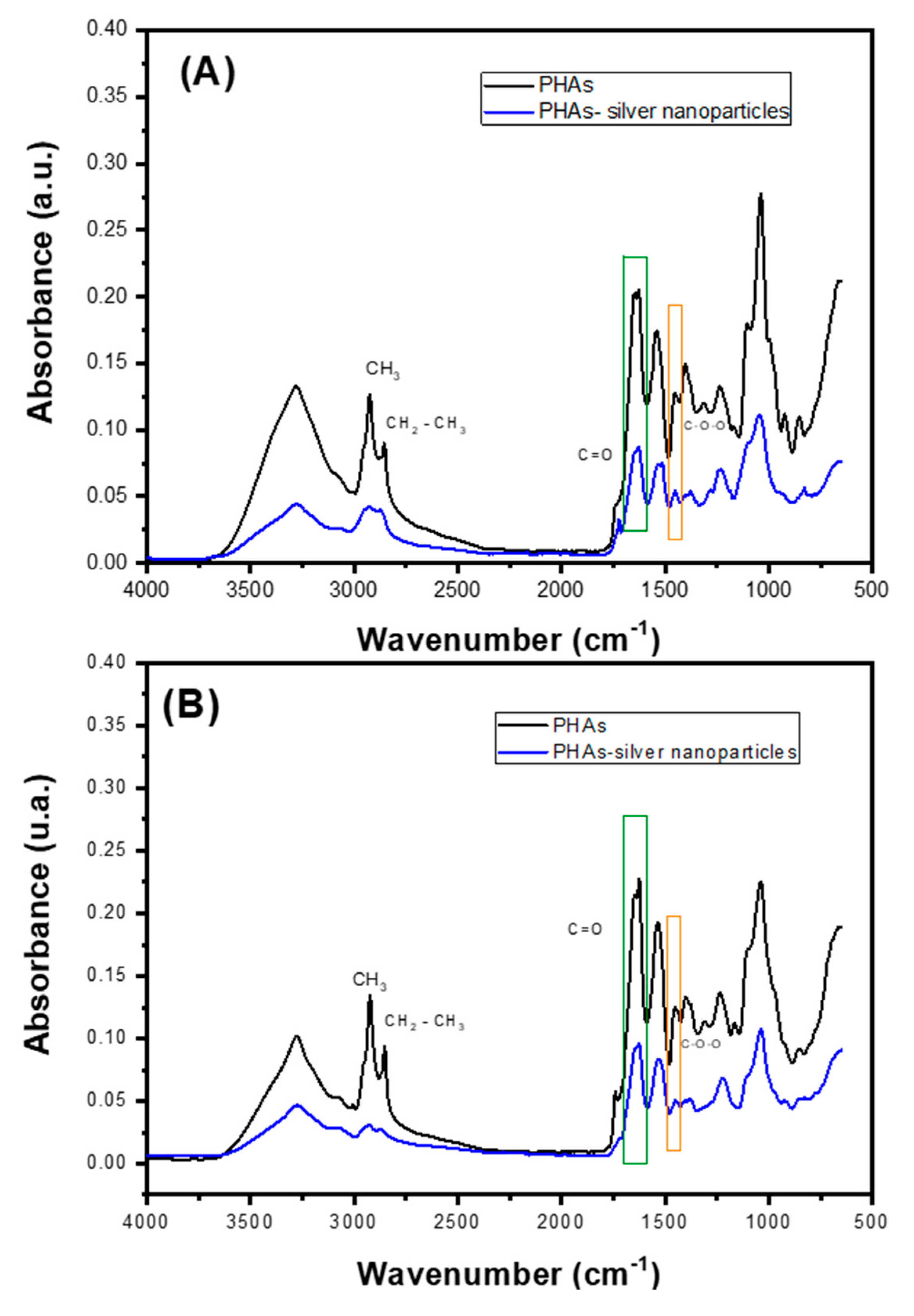
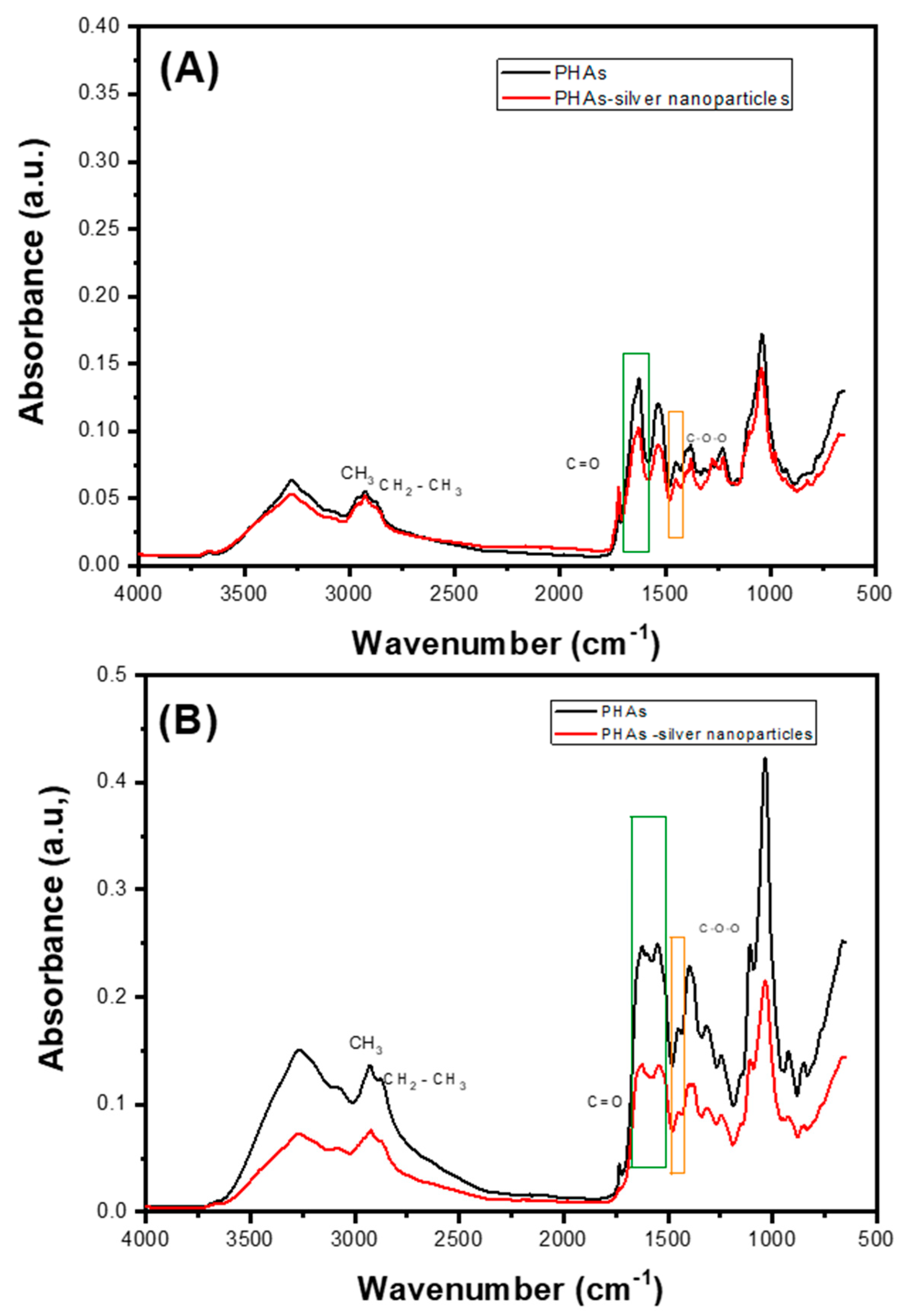
2.4. Characterizations of PHAs with Silver Nanoparticles
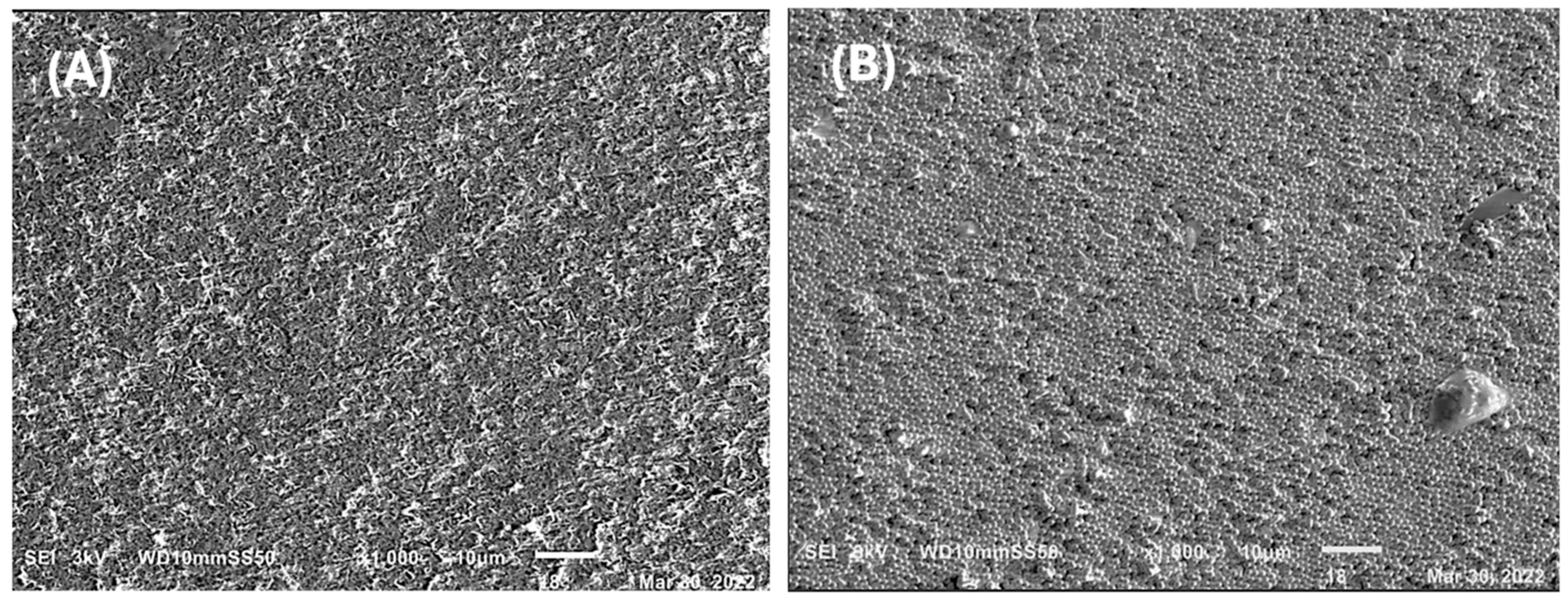
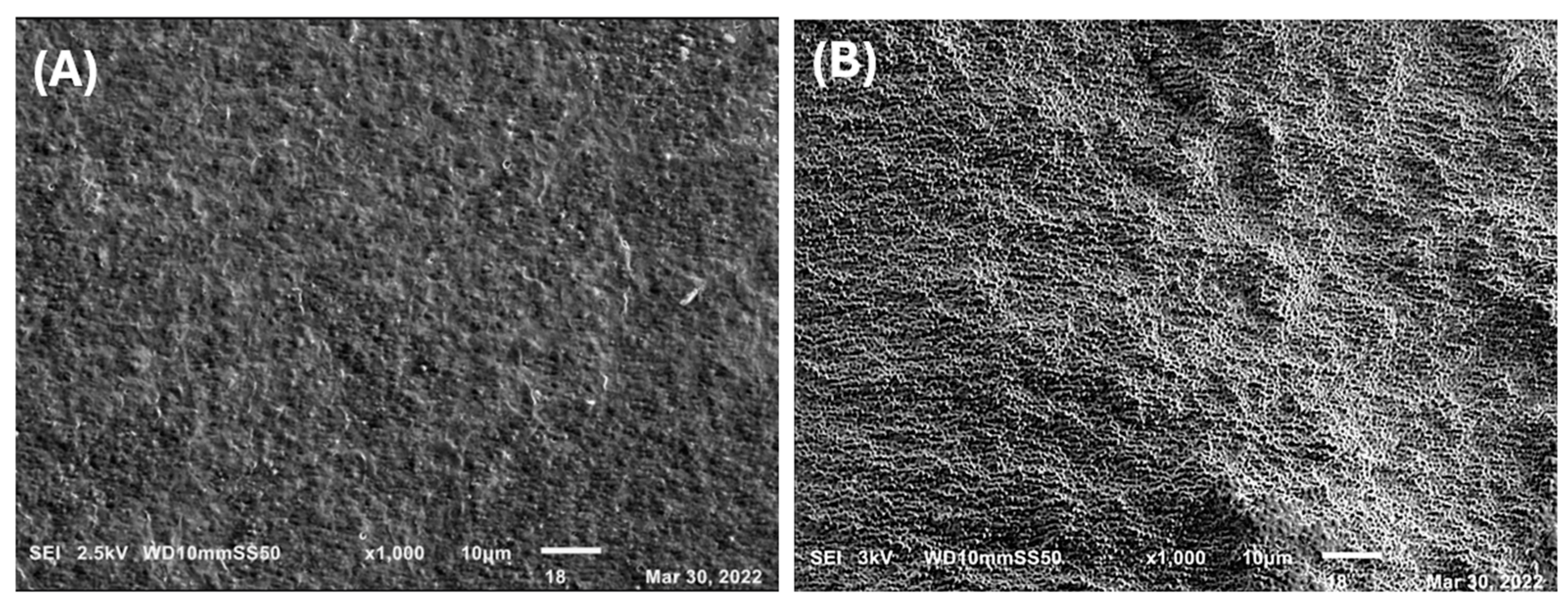
2.5. Micrographs of the PHAs-Silver Nanoparticles Nanocomposite
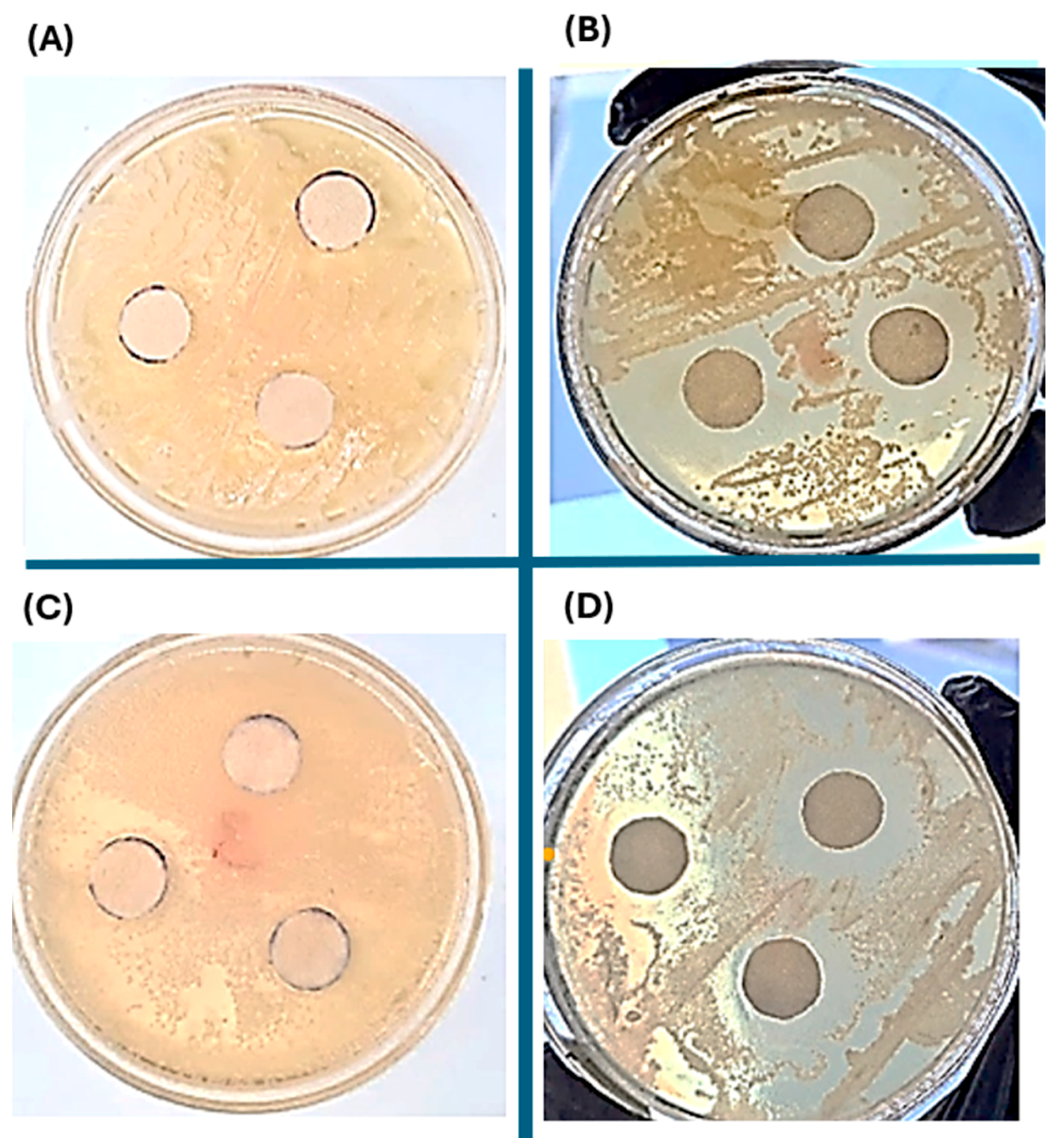
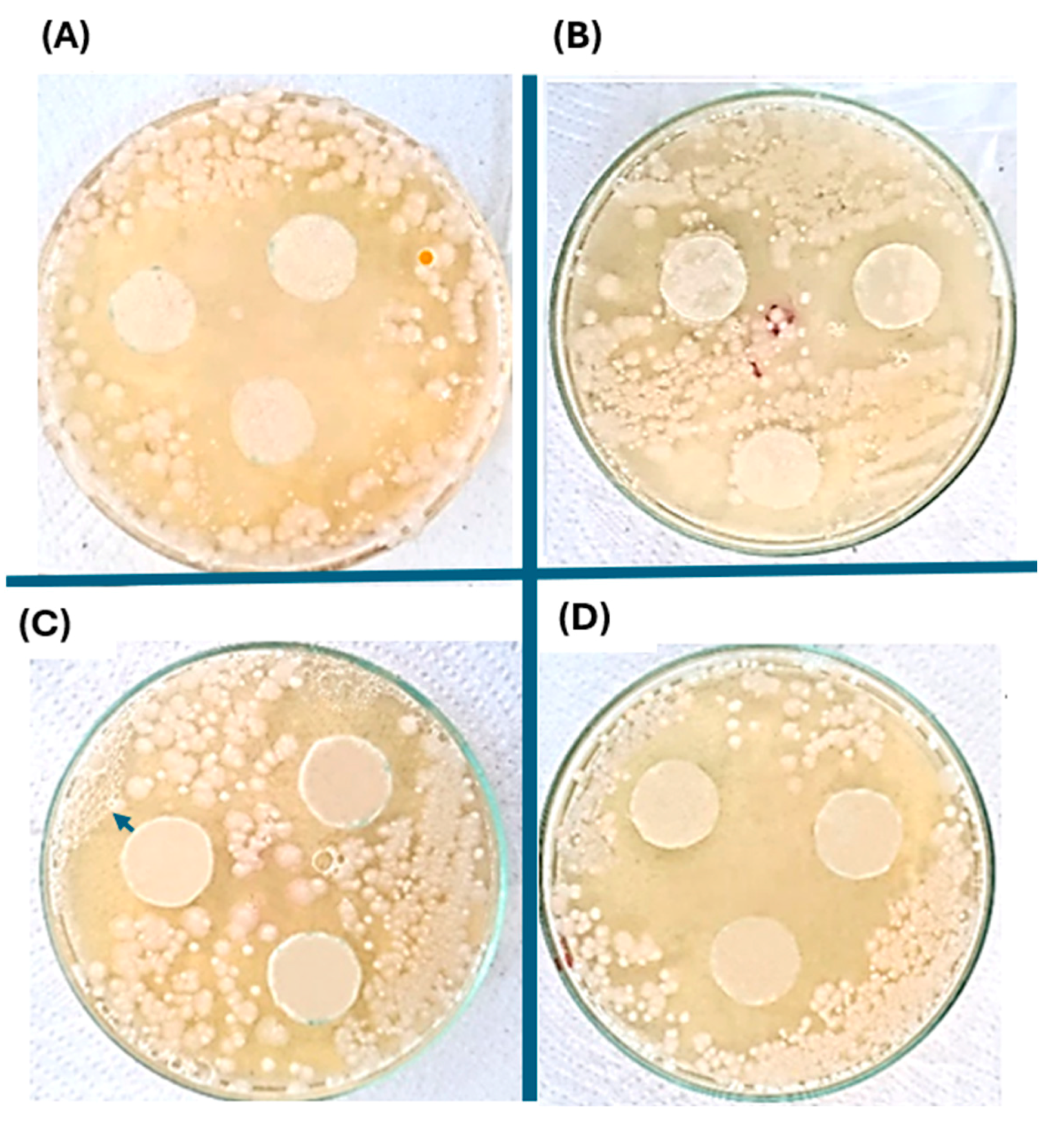
2.6. Antibacterial Evaluation of the Silver Nanoparticles-PHAs Composite
3. Materials and Methods
3.1. Inoculation and Incubation of Bacteria for PHAs Production
3.2. Extraction of PHAs from Microbial Cultures of Pseudomonas aeruginosa and Pseudomonas putida
3.3. Quantification of PHAs through the Spectrophotometric Assay for Poly-β-Hydroxybutyric Acid
3.4. Physicochemical Characterization of PHAs
3.5. Synthesis and Characterization of Silver Nanoparticles
3.6. Synthesis of the Nanocomposite (PHAs-Silver Nanoparticles)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PHAs | Polyhydroxyalkanoates |
References
- Rond’ošová, S.; Legerská, B.; Chmelová, D.; Ondrejovič, M.; Miertuš, S. Optimization of Growth Conditions to Enhance PHA Production by Cupriavidus necátor. Fermentations 2022, 8, 451. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.; Lee, J. Exploiting Polyhydroxyalkanoates for Biomedical Applications. Polymers 2023, 15, 1937. [Google Scholar] [CrossRef] [PubMed]
- Rai, R. Biosynthesis of Polyhydroxyalkanoates and Its Medical Applications. Ph.D. Thesis, University of Westminster School of Life Sciences, London, UK, 2010. [Google Scholar]
- Teeka, J.; Imai, T.; Cheng, X.; Reungsang, A. Screening of PHA-Producing Bacteria Using Bio- diesel-Derived Waste Glycerol as a Sole Carbon Source. J. Water Environ. 2010, 8, 373–381. [Google Scholar] [CrossRef][Green Version]
- Rocha-Meneses, L.; Hari, A.; Inayat, A.; Yousef, L.A.; Alarab, S.; Abdallah, M.; Shanableh, A.; Ghenai, C.; Shanmugam, S.; Kikas, T. Recent advances on biodiesel production from waste cooking oil (WCO): A review of reactors, catalysts, and optimization techniques impacting the production. Fuel 2023, 348, 128514. [Google Scholar] [CrossRef]
- Nisa, F. U Chapter 12—Biofuel: A unique solution for the future energy crisis. In Environmental Sustainability of Biofuels; Elsevier: Amsterdam, The Netherlands, 2023; pp. 219–236. [Google Scholar]
- Henriques, R. Über partielle Verseifung von Ölen und Fetten II. Angew. Chem. 1898, 11, 697–702. [Google Scholar] [CrossRef]
- Garlapati, V.K.; Shankar, U.; Budhiraja, A. Bioconversion technologies of crude glycerol to value added industrial products. Biotechnol. Rep. 2016, 5, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Poblete-Castro, I.; Wittmann, C.; Nikel, P.I. Biochemistry, genetics and biotechnology of glycerol utilization in Pseudomonas species. Microb. Biotechnol. 2020, 13, 32–53. [Google Scholar] [CrossRef]
- Singh, R.; Langyan, S.; Rohtagi, B.; Darjee, S.; Khandelwal, A.; Shrivastava, M.; Kothari, R.; Mohan, H.; Raina, S.; Kaur, J.; et al. Production of biofuels options by contribution of effective and suitable enzymes: Technological developments and challenges. Mater. Sci. Energy Technol. 2022, 5, 294–310. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Lee, C.; Mat Nanyan, N.S.; Tay, G.S. A systematic review on utilization of biodiesel-derived crude glycerol in sustainable polymers preparation. Int. J. Biol. Macromol. 2024, 261, 129536. [Google Scholar] [CrossRef]
- Rossini Simões, C.; Pereira da Silva, M.W.; Magalhães de Souza, R.F.; Roja Hacha, R.; Gutierrez Merma, A.; Torem, M.L.; Cianga Silva, F.P. Biosurfactants: An Overview of Their Properties, Production, and Application in Mineral Flotation. Resources 2024, 13, 81. [Google Scholar] [CrossRef]
- Tsuge, T.; Yano, K.; Imazu, S.; Numata, K.; Kikkawa, Y.; Abe, H.; Taguchi, S. Biosynthesis of Polyhydroxyalkanoate (PHA) Copolymer from Fructose Using Wild-Type and Laboratory-Evolved PHA Synthases. Macromol. Biosci. 2005, 5, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Tsuge, T.; Matsumoto, K.; Nakae, S.; Taguchi, S.; Doi, Y. Investigation of metabolic pathways for biopolyester production. RIKEN Rev. 2001, 42, 71–74. [Google Scholar]
- Silby, M.W.; Winstanley, C.; Godfrey, S.A.C.; Levy, S.B.; Jackson, R.W. Pseudomonas genomes: Diverse and adaptable. FEMS Microbiol. Rev. 2011, 35, 652–680. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, R.A.J.; Hill, D.J.; Kenward, M.A.; Williams, C.D.; Radecka, I. Bacterial synthesis of biodegradable polyhydroxyalka-noates. J. Appl. Microbiol. 2007, 102, 1437–1449. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonavo-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Pulignam, I.; Appaturi, J.N.; Parumasivam, T.; Ahmad, A.; Sudesh, K. Biomedical Applications of Polyhydroxyalkanoate in Tissue Engineering. Polymers 2022, 14, 2141. [Google Scholar] [CrossRef]
- Mamata; Agarwal, A.; Awasthi, A.; Awasthi, K.; Dutta, A. Antibacterial activities of GO–Ag nanocomposites with various loading concentrations of Ag nanoparticles. Appl. Phys. A 2023, 129, 838. [Google Scholar] [CrossRef]
- Naganthran, A.; Verasoundarapandian, G.; Khakid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Che Abdullah, C.A.; Ahmad, S.A. Synthesis characterization and biomedical application of silver nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef]
- Javid, H.; Nawaz, A.; Riaz, N.; Mukhtar, H.; UI-Haq, I.; Ahmed Shah, K.; Khan, H.; Naqvi, S.M.; Shakoor, S.; Rasool, A.; et al. Biosynthesis of Polyhydroxyalkanoates (PHAs) by the Valorization of Biomass and 494 Synthetic Waste. Molecules 2020, 25, 5539. [Google Scholar] [CrossRef]
- Sillema, W.D.; Cal, A.J.; Hathwaik, U.I.; Orts, W.J.; Lee, C.C. Polyhydroxyalkanoate production in Pseudomonas putida from alkanoic acids of varying lengths. PLoS ONE 2023, 18, e0284377. [Google Scholar]
- Liu, H.; Chen, Y.; Zhang, Y.; Zhao, W.; Guo, H.; Wang, S.; Xia, W.; Wang, S.; Liu, R.; Yang, C. Enhanced production of polyhydroxyalkanoates in Pseudomonas putida KT2440 by a combination of genome streamlining and promoter engineering. Int. J. Biol. Macromol. 2022, 209, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Spiers, A.J.; Buckling, A. The causes of Pseudomonas diversity. Microbiology 2000, 146, 2345–2350. [Google Scholar] [CrossRef] [PubMed]
- Getachew, A.; Berhanu, A.; Birhane, A. Production of Sterilized Medium Chain Length Polyhydroxyalkanoates (Smcl- Pha) as a Biofilm to Tissue Engineering Application. J. Tissue Sci. Eng. 2016, 7, 167. [Google Scholar] [CrossRef]
- Guo, W.; Duan, J.; Geng, W.; Feng, J.; Wang, S.; Song, C. Comparison of medium-chain-length polyhydroxyalkanoates synthases from Pseudomonas mendocina NK-01 with the same substrate specificity. Microbiol. Res. 2000, 168, 231–237. [Google Scholar] [CrossRef]
- Shamala, T.R.; Divyashree, M.S.; Davis, R.; Kumari, K.S.L.; Vijayendra, S.V.N.; Raj, B. Production and characterization of bacterial polyhydroxyalkanoate copolymers and evaluation of their blends by fourier transform infrared spectroscopy and scanning electron microscopy. Indian J. Microbiol. 2009, 49, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Hsu, J.; Shih, M.; Hsieh, C.; Ju, W.; Chen, Y.; Lee, B.; Hou, C. Process Optimization of Silver Nanoparticle Synthesis and Its Application in Mercury Detection. Micromachines 2021, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Talabani, R.F.; Hamad, S.M.; Barzinjy, A.A.; Demir, U. Biosynthesis of Silver Nanoparticles and Their Applications in Harvesting Sunlight for Solar Thermal Generation. Nanomaterials 2021, 11, 2421. [Google Scholar] [CrossRef] [PubMed]
- Elzey, S.; Grassian, V.H. Agglomeration isolation dissolution of commercially manufactured silver nanoparticles in aqueous environments. J. Nanopart. Res. 2010, 12, 1945–1958. [Google Scholar] [CrossRef]
- Schuster, D. Encyclopedia of Emulsion Technology, Vol. 3: Basic Theory, Measurement, Applications; CRC Press: Boca Raton, FL, USA, 1987; Volume 3. [Google Scholar]
- Hansen, P.E.; Vakili, M.; Kamounah, F.S.; Spanget-Larsen, J. NH Stretching Frequencies of Intramolecularly Hydrogen-Bonded Systems: An Experimental and Theoretical Study. Molecules 2021, 26, 7651. [Google Scholar] [CrossRef]
- Gumel, A.M.; Annuar, M.S.M.; Heidelberg, T. Biosynthesis and Characterization of Polyhydroxyalkanoates Copolymers Produced by Pseudomonas putida Bet001 Isolated from Palm Oil Mill Effluent. PLoS ONE 2012, 7, 0045214. [Google Scholar] [CrossRef]
- Kalaoglu-Altan, O.I.; Baskan, H.; Meireman, T.; Basnett, P.; Azimi, B.; Fusco, A.; Funel, N.; Donnarumma, G.; Lazzeri, A.; Roy, I.; et al. Silver nanoparticle-coated polyhydroxyalkanoate based electrospun fibers for wound dressing applications. Materials 2021, 14, 4907. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mat. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microb. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.; Ko, K.K.; Oh, L.H.; Lee, B.T. Fabrication of silver nanoparticles and their antimicrobial mechanisms. Eur. Cells Mater. 2006, 11, 58. [Google Scholar]
- Kaviya, S.; Santhanalakshmi, J.; Viswanathan, B. Green synthesis of silver nanoparticles using Polyalthia longifolia leaf extract along with D-sorbitol: Study of antibacterial activity. J. Nanotech. 2011, 40, 1–13. [Google Scholar]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Guardão Franca, R.D.; Dionísio, M.; Sevrin, C.; Grandfils, C.; Reis, M.A.M.; Laorenço, N.D. Polyhydrox-yalkanoates from a Mixed Microbial Culture: Extraction Optimization and Polymer Characterization. Polymers 2022, 14, 2155. [Google Scholar] [CrossRef] [PubMed]
- Mahato, R.P.; Kumar, S.; Singh, P. Optimization of Growth Conditions to Produce Sustainable Polyhydroxyalkanoate Bioplastic by Pseudomonas aeruginosa EO1. Front. Microbiol. 2021, 12, 711588. [Google Scholar] [CrossRef] [PubMed]
- Rampado, R.; Giordano, F.; Moracci, L.; Crotti, S.; Caliceti, P.; Agosti, M.; Taraballi, F. Optimization of a detergent-based protocol for membrane proteins purification from mammalian cells. J. Pharm. Biomed. Anal. 2022, 219, 114926. [Google Scholar] [CrossRef] [PubMed]
- Lemoigne, M. Produits de deshydration et de polymerisation de L’acide = Oxybutyrique. Bull. Soc. Chim. Biol. 1926, 8, 770–782. [Google Scholar]
- Law, J.H.; Slepecky, R.A. Assay of poly-beta-hydroxybutyric acid. J. Bacteriol. 1961, 82, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, U.T.; Velidandi, A.; Nageswara Rao, G.V.S. Sodium borohydride mediated synthesis of nano-sized silver particles: Their characterization, anti-microbial and cytotoxicity studies. Mater. Chem. Phys. 2023, 15, 126997. [Google Scholar] [CrossRef]
- Balcucho, J.; Narváez, D.M.; Tarazona, N.A.; Castro-Mayorga, J.L. Microbially Synthesized Polymer-Metal Nanoparticles Composites as Promising Wound Dressings to Overcome Methicillin-Resistance Staphylococcus aureus Infections. Polymers 2023, 15, 920. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.; Steinbüchel, A. Poly (3-hydroxybutyrate) Granule-Associated Proteins: Impacts on Poly (3-hydroxybutyrate) Synthesis and Degradatio. Biomacromolecules 2005, 6, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Frone, A.N.; Nicolae, C.A.; Eremia, M.C.; Tofan, V.; Ghiurea, M.; Chiulan, I.; Radu, E.; Damian, C.M.; Panaitescu, D.M. Low molecular weight and polymeric modifiers as toughening agents in poly (3-hydroxybutyrate) films. Polymers 2020, 12, 2446. [Google Scholar] [CrossRef]
- Sedlacek, P.; Pernicova, I.; Novackova, I.; Kourilova, X.; Kalina, M.; Kovalcik, A.; Koller, M.; Nebesarova, J.; Krzyzanek, V.; Hrubanova, K.; et al. Introducing the Newly Isolated Bacterium Aneurinibacillus sp. H1 as an Auspicious Thermophilic Producer of Various Polyhydroxyalkanoates (PHA) Copolymers–2. Material Study on the Produced Copolymers. Polymers 2020, 12, 1298. [Google Scholar] [CrossRef]
| Pseudomonas aureginosa | Pseudonomas putida | |
|---|---|---|
| Glycerol | 0.084757708 (g/L) | 0.099539043 (g/L) |
| Crude glycerol | 0.08499393 (g/L) | 0.097921247 (g/L) |
| Pseudomonas aureginosa Inhibitory Zone Distances (mm) | Pseudonomas putida Inhibitory Zone Distances (mm) | |
|---|---|---|
| Glycerol | 11.36 | 5.49 |
| Crude glycerol | 10.53 | 12.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Romero, C.L.; Chávez-Ramírez, A.U.; Flores-Juárez, C.R.; Arjona, N.; Álvarez-López, A.; del Bosque Plata, L.; Vallejo-Becerra, V.; Galindo-de-la-Rosa, J.d.D. Biosynthesis of Polyhydroalkanoates Doped with Silver Nanoparticles Using Pseudomonas putida and Pseudomonas aeruginosa for Antibacterial Polymer Applications. Int. J. Mol. Sci. 2024, 25, 8996. https://doi.org/10.3390/ijms25168996
Cruz-Romero CL, Chávez-Ramírez AU, Flores-Juárez CR, Arjona N, Álvarez-López A, del Bosque Plata L, Vallejo-Becerra V, Galindo-de-la-Rosa JdD. Biosynthesis of Polyhydroalkanoates Doped with Silver Nanoparticles Using Pseudomonas putida and Pseudomonas aeruginosa for Antibacterial Polymer Applications. International Journal of Molecular Sciences. 2024; 25(16):8996. https://doi.org/10.3390/ijms25168996
Chicago/Turabian StyleCruz-Romero, Carmen Liliana, Abraham Ulises Chávez-Ramírez, Cyntia R. Flores-Juárez, Noé Arjona, Alejandra Álvarez-López, Laura del Bosque Plata, Vanessa Vallejo-Becerra, and Juan de Dios Galindo-de-la-Rosa. 2024. "Biosynthesis of Polyhydroalkanoates Doped with Silver Nanoparticles Using Pseudomonas putida and Pseudomonas aeruginosa for Antibacterial Polymer Applications" International Journal of Molecular Sciences 25, no. 16: 8996. https://doi.org/10.3390/ijms25168996
APA StyleCruz-Romero, C. L., Chávez-Ramírez, A. U., Flores-Juárez, C. R., Arjona, N., Álvarez-López, A., del Bosque Plata, L., Vallejo-Becerra, V., & Galindo-de-la-Rosa, J. d. D. (2024). Biosynthesis of Polyhydroalkanoates Doped with Silver Nanoparticles Using Pseudomonas putida and Pseudomonas aeruginosa for Antibacterial Polymer Applications. International Journal of Molecular Sciences, 25(16), 8996. https://doi.org/10.3390/ijms25168996






