In Vitro Investigation of the Anti-Fibrotic Effects of 1-Phenyl-2-Pentanol, Identified from Moringa oleifera Lam., on Hepatic Stellate Cells
Abstract
:1. Introduction
2. Results
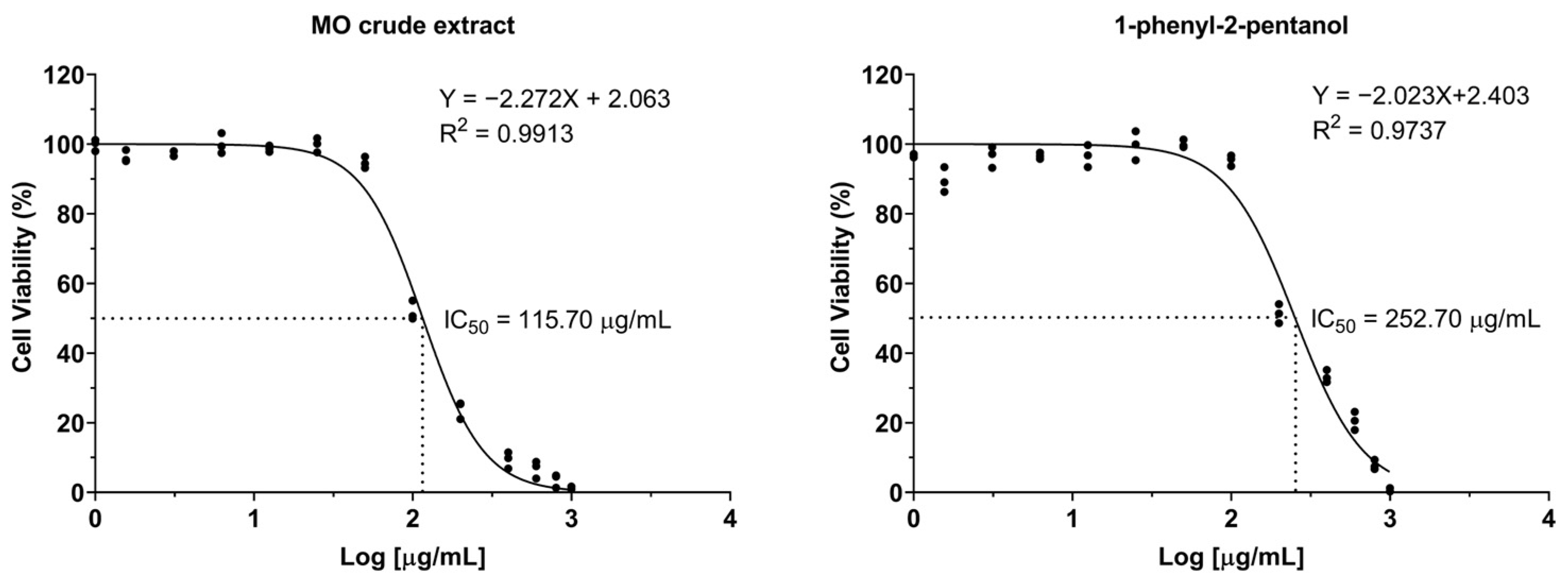
2.1. The Effects of Crude MO Extract and 1-PHE on Cell Viability
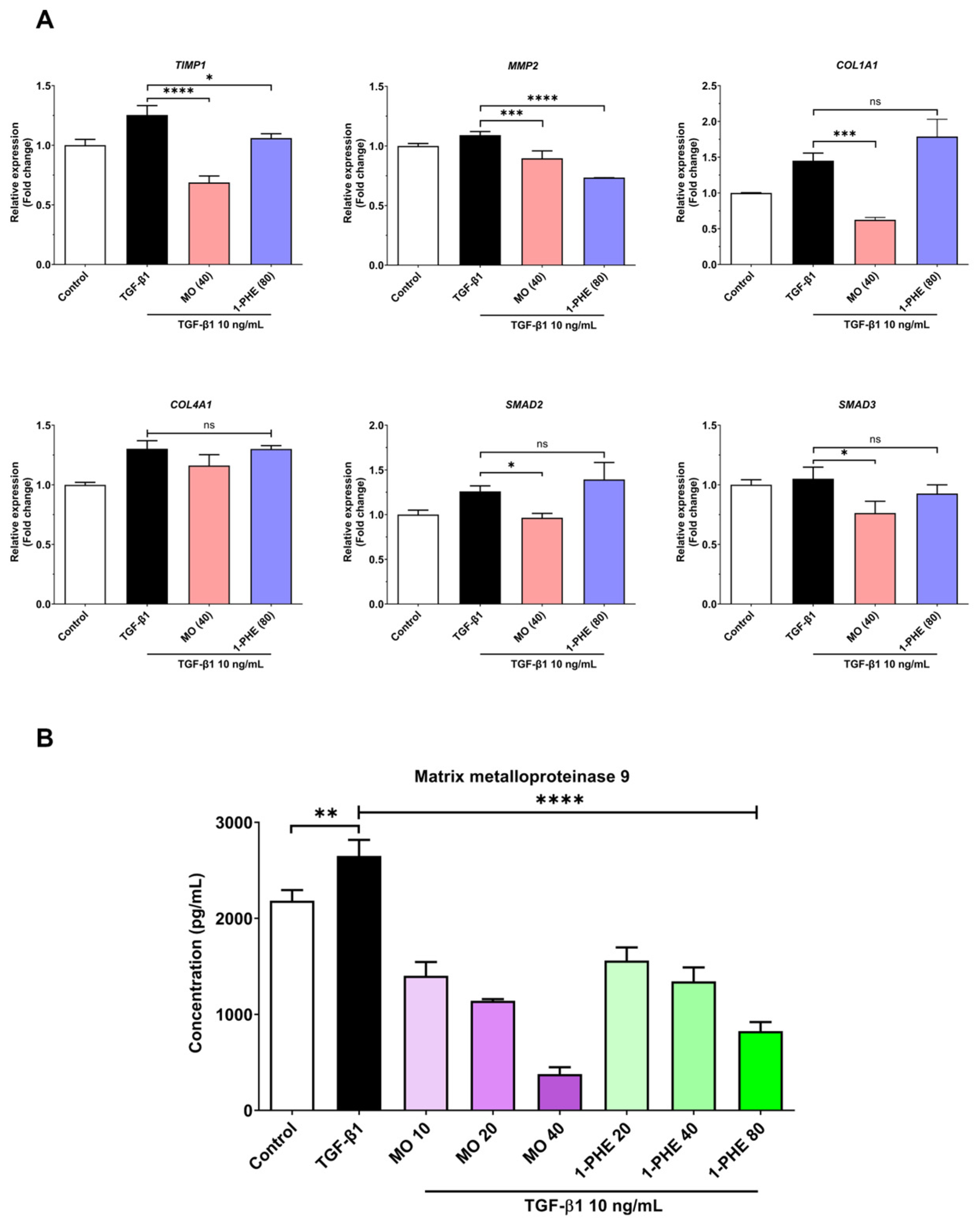
2.2. The Effects of Crude MO Extract and 1-PHE on Expression of Liver Fibrotic Markers
2.3. Proteomic Analysis of 1-PHE Treatment in LX-2 Cells
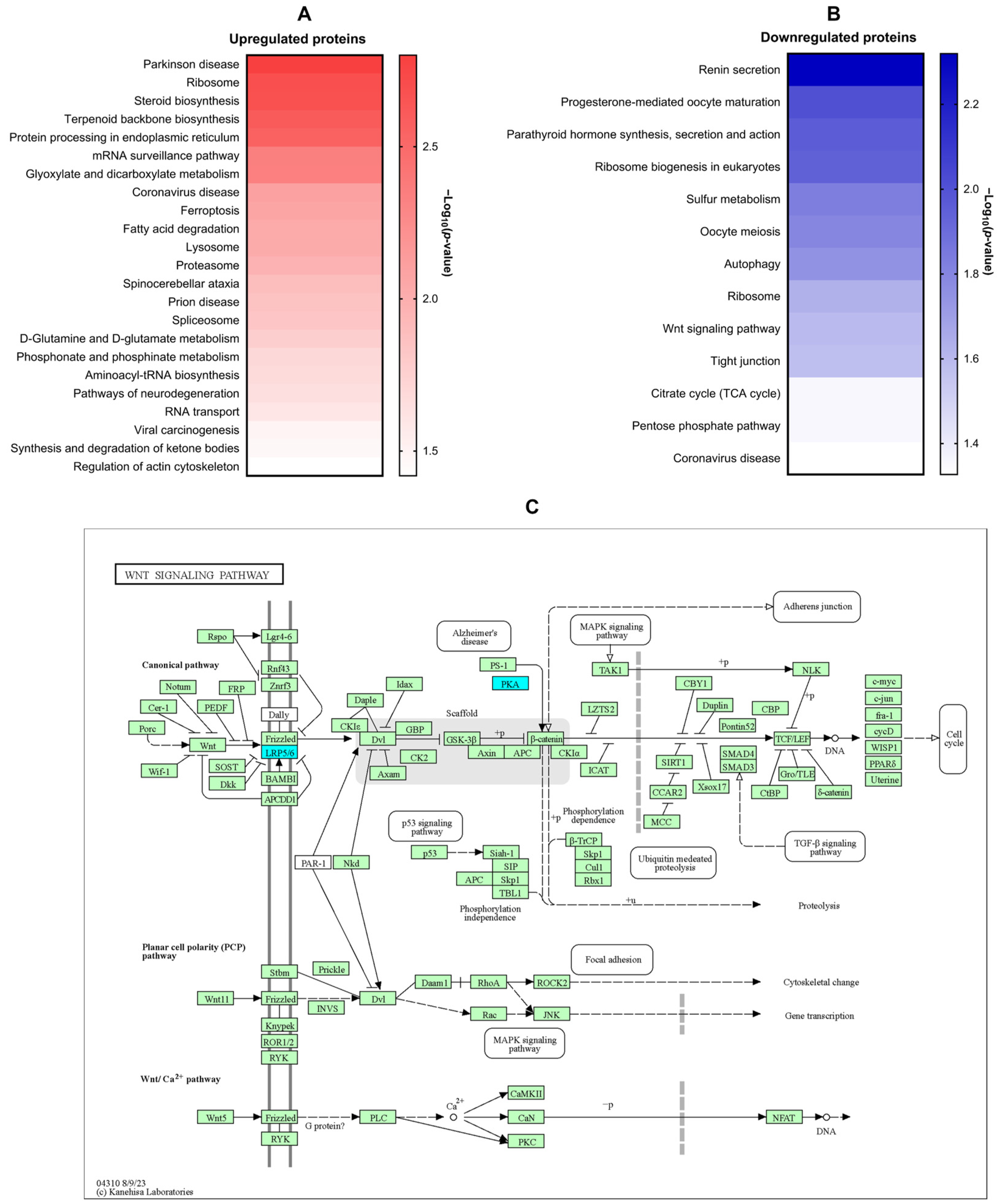
2.4. Molecular Docking Analysis of Candidate Target Proteins
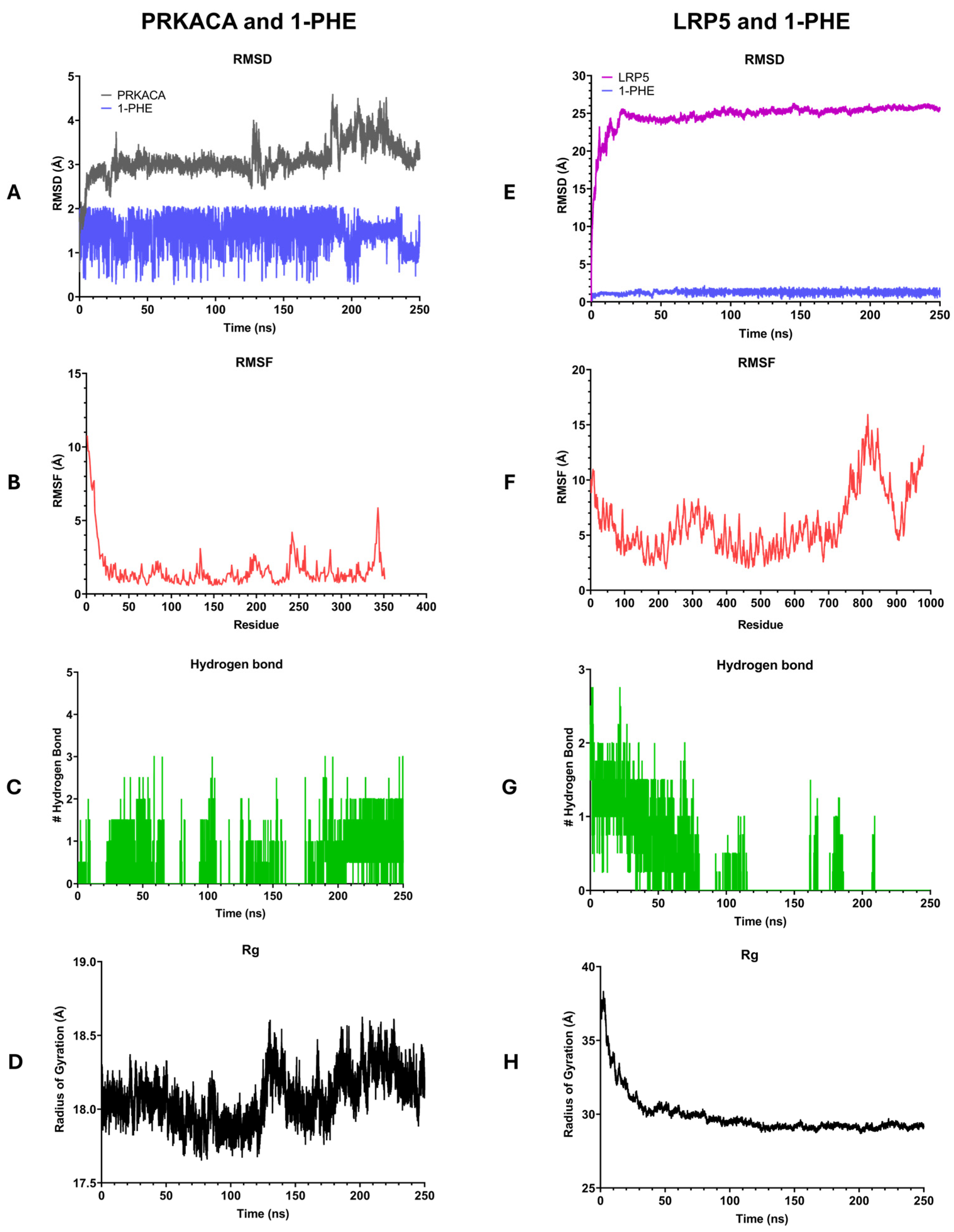
2.5. Molecular Dynamics Simulation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Line and Culture
4.3. Preparation of MO Leaf Crude Extract and Active Compound
4.4. Cytotoxicity Assessment by Resazurin Reduction Assay
4.5. Real-Time Quantitative Reverse Transcription PCR
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Sample Preparation for LC-MS/MS Analysis
4.8. LC-MS/MS Setting and Data Processing for Proteomic Analysis
4.9. Bioinformatic Analysis of Proteomic Data
4.10. Molecular Docking Analysis
4.11. Molecular Dynamics (MD) Simulation
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parola, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Asp. Med. 2019, 65, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Suzuki, S.; Senoo, H. Hepatic Stellate Cells: Unique Characteristics in Cell Biology and Phenotype. Cell Struct. Funct. 2003, 28, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Sun, H.; Xue, T.; Gan, C.; Liu, H.; Xie, Y.; Yao, Y.; Ye, T. Liver Fibrosis: Therapeutic Targets and Advances in Drug Therapy. Front. Cell Dev. Biol. 2021, 9, 730176. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Chouhan, K.; Weiskirchen, S.; Weiskirchen, R. Cellular Mechanisms of Liver Fibrosis. Front. Pharmacol. 2021, 12, 671640. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Nevzorova, Y.A.; Luedde, T.; Zimmermann, H.; Kroy, D.; Strnad, P.; Berres, M.-L.; Bernhagen, J.; Tacke, F.; Nattermann, J.; et al. Liver Fibrosis—From Mechanisms of Injury to Modulation of Disease. Front. Med. 2022, 8, 814496. [Google Scholar] [CrossRef]
- Pellicoro, A.; Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Liver fibrosis and repair: Immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014, 14, 181–194. [Google Scholar] [CrossRef]
- Pan, X.; Ma, X.; Jiang, Y.; Wen, J.; Yang, L.; Chen, D.; Cao, X.; Peng, C. A Comprehensive Review of Natural Products against Liver Fibrosis: Flavonoids, Quinones, Lignans, Phenols, and Acids. Evid. Based Complement. Alternat. Med. 2020, 2020, 7171498. [Google Scholar] [CrossRef]
- El-Tantawy, W.H.; Temraz, A. Anti-fibrotic activity of natural products, herbal extracts and nutritional components for prevention of liver fibrosis: Review. Arch. Physiol. Biochem. 2022, 128, 382–393. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, Y.; Wen, J.; Zhao, Y.; Zeng, J.; Guo, Y. A comprehensive review of natural products to fight liver fibrosis: Alkaloids, terpenoids, glycosides, coumarins and other compounds. Eur. J. Pharmacol. 2020, 888, 173578. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Liu, Z.; Ci, L.; Shuai, C.; Lv, X.; Li, J. Research progress on the anti-hepatic fibrosis action and mechanism of natural products. Int. Immunopharmacol. 2019, 75, 105765. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-R.; Chen, X.-P.; Lu, J.-J.; Wang, Y.; Wang, Y.-T. Potent natural products and herbal medicines for treating liver fibrosis. Chin. Med. 2015, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Lu, G.; Ye, L.; Shi, R.; Zhu, M.; Yu, X.; Li, Z.; Jia, X.; Feng, L. Moringa oleifera Lam.: A comprehensive review on active components, health benefits and application. RSC Adv. 2023, 13, 24353–24384. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-B.; Chen, G.-L.; Guo, M.-Q. Antioxidant and Anti-Inflammatory Activities of the Crude Extracts of Moringa oleifera from Kenya and Their Correlations with Flavonoids. Antioxidants 2019, 8, 296. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Y.; Wu, J.; Li, N.; Guo, M. Anti-Inflammatory Properties and Potential Bioactive Components from Moringa oleifera Leaves Revealed by Affinity Ultrafiltration LC–MS and Molecular Docking. ACS Food Sci. Technol. 2021, 1, 1953–1962. [Google Scholar] [CrossRef]
- Luetragoon, T.; Pankla Sranujit, R.; Noysang, C.; Thongsri, Y.; Potup, P.; Suphrom, N.; Nuengchamnong, N.; Usuwanthim, K. Bioactive Compounds in Moringa oleifera Lam. Leaves Inhibit the Pro-Inflammatory Mediators in Lipopolysaccharide-Induced Human Monocyte-Derived Macrophages. Molecules 2020, 25, 191. [Google Scholar] [CrossRef]
- Bhadresha, K.; Thakore, V.; Brahmbhatt, J.; Upadhyay, V.; Jain, N.; Rawal, R. Anticancer effect of Moringa oleifera leaves extract against lung cancer cell line via induction of apoptosis. Adv. Cancer Biol. Metastasis 2022, 6, 100072. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Albalawi, S.M.; Athar, M.T.; Khan, A.Q.; Al-Shahrani, H.; Islam, M. Moringa oleifera as an Anti-Cancer Agent against Breast and Colorectal Cancer Cell Lines. PLoS ONE 2015, 10, 135814. [Google Scholar] [CrossRef]
- Luetragoon, T.; Pankla Sranujit, R.; Noysang, C.; Thongsri, Y.; Potup, P.; Suphrom, N.; Nuengchamnong, N.; Usuwanthim, K. Anti-Cancer Effect of 3-Hydroxy-β-Ionone Identified from Moringa oleifera Lam. Leaf on Human Squamous Cell Carcinoma 15 Cell Line. Molecules 2020, 25, 3563. [Google Scholar] [CrossRef]
- van den Berg, J.; Kuipers, S. The antibacterial action of Moringa oleifera: A systematic review. S. Afr. J. Bot. 2022, 151, 224–233. [Google Scholar] [CrossRef]
- Segwatibe, M.K.; Cosa, S.; Bassey, K. Antioxidant and Antimicrobial Evaluations of Moringa oleifera Lam Leaves Extract and Isolated Compounds. Molecules 2023, 28, 899. [Google Scholar] [CrossRef] [PubMed]
- Enerijiofi, K.E.; Akapo, F.H.; Erhabor, J.O. GC–MS analysis and antibacterial activities of Moringa oleifera leaf extracts on selected clinical bacterial isolates. Bull. Natl. Res. Cent. 2021, 45, 179. [Google Scholar] [CrossRef]
- Wilujeng, L.K.; Safitri, F.N.; Supriono, S.; Kalim, H.; Poeranto, S. The effect of Moringa oleifera (Lam) leaves ethanol extracts as anti-inflammatory and anti-fibrotic through TNF-α and p38-MAPK expression: In vivo model of liver fibrosis approach. AIP Conf. Proc. 2021, 2353, 30046. [Google Scholar]
- Hamza, A.A. Ameliorative effects of Moringa oleifera Lam seed extract on liver fibrosis in rats. Food Chem. Toxicol. 2010, 48, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Wisitpongpun, P.; Suphrom, N.; Potup, P.; Nuengchamnong, N.; Calder, P.C.; Usuwanthim, K. In Vitro Bioassay-Guided Identification of Anticancer Properties from Moringa oleifera Lam. Leaf against the MDA-MB-231 Cell Line. Pharmaceuticals 2020, 13, 464. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- QuickCalcs, G. EC Anything Calculator. Available online: https://www.graphpad.com/quickcalcs/Ecanything1/ (accessed on 17 February 2023).
- Duspara, K.; Bojanic, K.; Pejic, J.I.; Kuna, L.; Kolaric, T.O.; Nincevic, V.; Smolic, R.; Vcev, A.; Glasnovic, M.; Curcic, I.B.; et al. Targeting the Wnt Signaling Pathway in Liver Fibrosis for Drug Options: An Update. J. Clin. Transl. Hepatol. 2021, 9, 960–971. [Google Scholar] [CrossRef]
- Nishikawa, K.; Osawa, Y.; Kimura, K. Wnt/β-Catenin Signaling as a Potential Target for the Treatment of Liver Cirrhosis Using Antifibrotic Drugs. Int. J. Mol. Sci. 2018, 19, 3103. [Google Scholar] [CrossRef]
- Miao, C.-g.; Yang, Y.-y.; He, X.; Huang, C.; Huang, Y.; Zhang, L.; Lv, X.-W.; Jin, Y.; Li, J. Wnt signaling in liver fibrosis: Progress, challenges and potential directions. Biochimie 2013, 95, 2326–2335. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Al-Ashmawy, G.M.; Fakher, H.E.; Khedr, N.F. The role of WNT/β-catenin signaling pathway and glutamine metabolism in the pathogenesis of CCl4-induced liver fibrosis: Repositioning of niclosamide and concerns about lithium. Cytokine 2020, 136, 155250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Q.; Deng, X.-W.; Xu, G.-Q.; Lin, J.; Lu, H.-Z.; Chen, J. Mechanical homeostasis imbalance in hepatic stellate cells activation and hepatic fibrosis. Front. Mol. Biosci. 2023, 10, 1183808. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Caballero-Díaz, D. Transforming Growth Factor-β-Induced Cell Plasticity in Liver Fibrosis and Hepatocarcinogenesis. Front. Oncol. 2018, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Naim, A.; Pan, Q.; MS, B. Matrix Metalloproteinases (MMPs) in Liver Diseases. J. Clin. Exp. Hepatol. 2017, 7, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Roeb, E. Matrix metalloproteinases and liver fibrosis (translational aspects). Matrix Biol. 2018, 68, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Pan, S.; Shang, S.; Li, C. Interaction between the Wnt/β-catenin signaling pathway and the EMMPRIN/MMP-2, 9 route in periodontitis. J. Periodontal Res. 2018, 53, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Crampton, S.P.; Hughes, C.C.W. Wnt Signaling Induces Matrix Metalloproteinase Expression and Regulates T Cell Transmigration. Immunity 2007, 26, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Ingraham, C.A.; Park, G.C.; Makarenkova, H.P.; Crossin, K.L. Matrix Metalloproteinase (MMP)-9 Induced by Wnt Signaling Increases the Proliferation and Migration of Embryonic Neural Stem Cells at Low O2 Levels. J. Biol. Chem. 2011, 286, 17649–17657. [Google Scholar] [CrossRef]
- Geervliet, E.; Bansal, R. Matrix Metalloproteinases as Potential Biomarkers and Therapeutic Targets in Liver Diseases. Cells 2020, 9, 1212. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. Available online: https://www.ncbi.nlm.nih.gov/books/NBK144065/ (accessed on 11 February 2023).
- Robert, S.; Gicquel, T.; Bodin, A.; Lagente, V.; Boichot, E. Characterization of the MMP/TIMP Imbalance and Collagen Production Induced by IL-1β or TNF-α Release from Human Hepatic Stellate Cells. PLoS ONE 2016, 11, 153118. [Google Scholar] [CrossRef] [PubMed]
- Krobthong, S.; Yingchutrakul, Y.; Samutrtai, P.; Hitakarun, A.; Siripattanapipong, S.; Leelayoova, S.; Mungthin, M.; Choowongkomon, K. Utilizing Quantitative Proteomics to Identify Species-Specific Protein Therapeutic Targets for the Treatment of Leishmaniasis. ACS Omega 2022, 7, 12580–12588. [Google Scholar] [CrossRef] [PubMed]
- Griss, J.; Viteri, G.; Sidiropoulos, K.; Nguyen, V.; Fabregat, A.; Hermjakob, H. ReactomeGSA—Efficient Multi-Omics Comparative Pathway Analysis. Mol. Cell. Proteom. 2020, 19, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Goedhart, J.; Luijsterburg, M.S. VolcaNoseR is a web app for creating, exploring, labeling and sharing volcano plots. Sci. Rep. 2020, 10, 20560. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, 90–97. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, 90. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold Protein Structure Database in 2024: Providing structure coverage for over 214 million protein sequences. Nucleic Acids Res. 2024, 52, 368–375. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Shapovalov, M.V.; Dunbrack, R.L., Jr. A smoothed backbone-dependent rotamer library for proteins derived from adaptive kernel density estimates and regressions. Structure 2011, 19, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Khamto, N.; Utama, K.; Boontawee, P.; Janthong, A.; Tatieng, S.; Arthan, S.; Choommongkol, V.; Sangthong, P.; Yenjai, C.; Suree, N.; et al. Inhibitory Activity of Flavonoid Scaffolds on SARS-CoV-2 3CLpro: Insights from the Computational and Experimental Investigations. J. Chem. Inf. Model. 2024, 64, 874–891. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
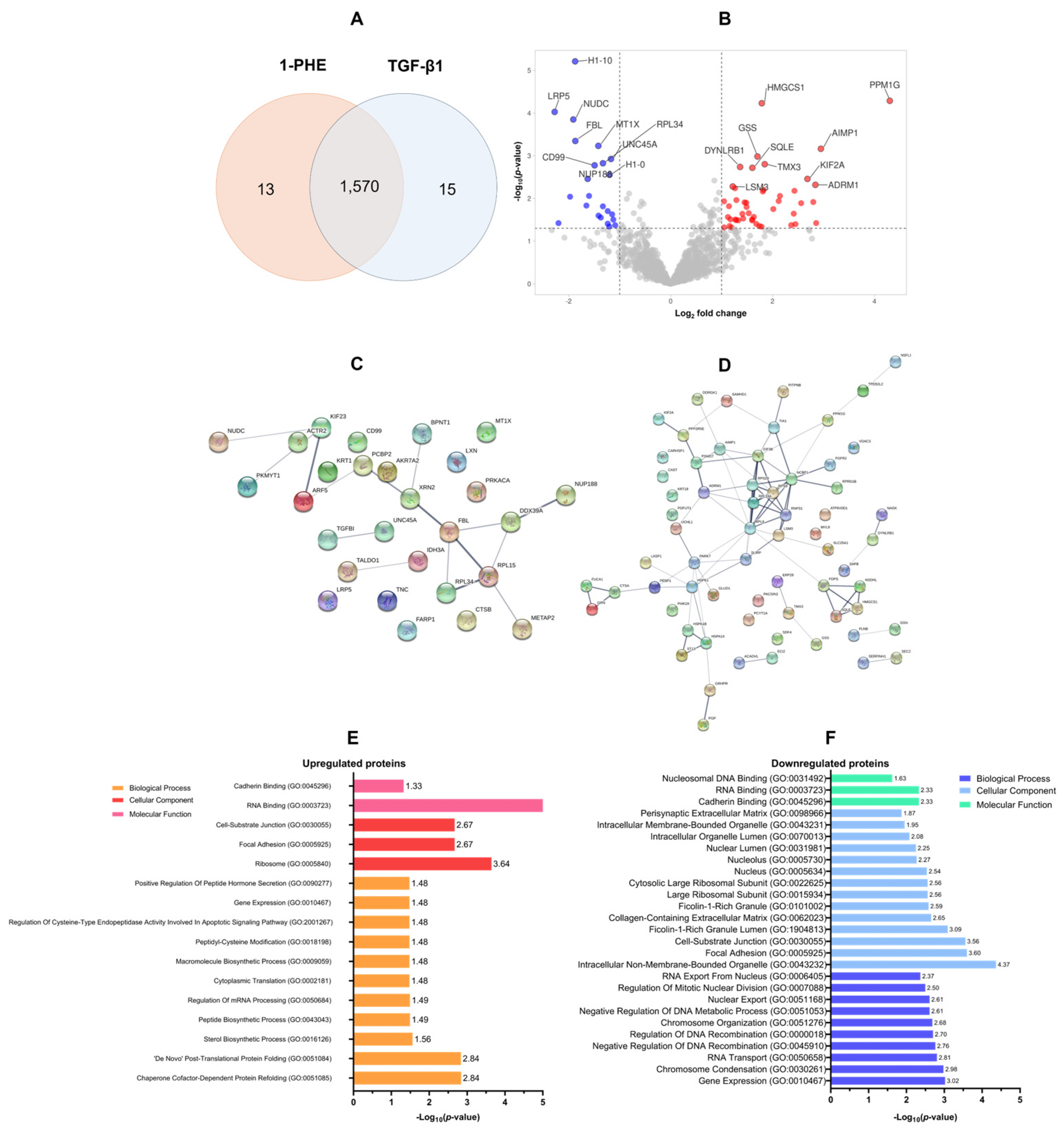

| Uniprot ID | Gene ID | Description | Log2 Fold Change | −log10 p-Value |
|---|---|---|---|---|
| Top 10 upregulated proteins | ||||
| O15355 | PPM1G | Protein phosphatase | 4.30 | 4.29 |
| Q12904 | AIMP1 | Aminoacyl tRNA synthase complex-interacting multifunctional protein 1 | 2.95 | 3.16 |
| Q16537 | PPP2R5E | Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit epsilon isoform | 2.86 | 1.42 |
| Q16186 | ADRM1 | Proteasomal ubiquitin receptor ADRM1 | 2.84 | 2.32 |
| P04066 | FUCA1 | Tissue alpha-L-fucosidase | 2.80 | 1.92 |
| O00139 | KIF2A | Kinesin-like protein KIF2A | 2.68 | 2.46 |
| Q9UNF0 | PACSIN2 | Protein kinase C and casein kinase substrate in neurons protein 2 | 2.56 | 1.89 |
| Q9BRK5 | SDF4 | 45 kDa calcium-binding protein | 2.45 | 1.40 |
| Q0VDF9 | HSPA14 | Heat shock 70 kDa protein 14 | 2.43 | 2.18 |
| Q09161 | NCBP1 | Nuclear cap-binding protein subunit 1 | 2.42 | 1.64 |
| Top-10 downregulated proteins | ||||
| O75197 | LRP5 | Low-density-lipoprotein-receptor-related protein 5 | −2.28 | 4.03 |
| Q15582 | TGFBI | Transforming growth factor-beta-induced protein ig-h3 | −2.20 | 1.42 |
| P04264 | KRT1 | Keratin, type II cytoskeletal 1 | −1.97 | 2.04 |
| Q9Y266 | NUDC | Nuclear migration protein nudC | −1.91 | 3.85 |
| Q92522 | H1FX | Histone H1x | −1.87 | 5.21 |
| P22087 | FBL | rRNA 2′-O-methyltransferase fibrillarin | −1.87 | 3.35 |
| Q9BS40 | LXN | Latexin | −1.65 | 1.83 |
| Q5SRE5 | NUP188 | Nucleoporin NUP188 homolog | −1.63 | 2.46 |
| P07858 | CTSB | Cathepsin B | −1.60 | 2.06 |
| Protein Name | Ligand | AutoDock Vina Score | Interacting Amino Acid |
|---|---|---|---|
| Protein kinase cAMP-activated catalytic subunit alpha (PRKACA) | 1-PHE | −6.033 | Val57, Ala70, Glu127, Leu173 |
| 3SB | −6.306 | Leu49, Val57, Ala70, Lys72, Asp166, Asn171, Leu173, Thr183, Asp184 | |
| Low-density-lipoprotein-receptor-related protein 5 (LRP5) | 1-PHE | −6.055 | Ala454, Ile455, Ala553, Val555, Ala598, Val600 |
| Gene ID | Description | Forward (5′ → 3′) | Reverse (5′ → 3′) |
|---|---|---|---|
| TIMP1 | TIMP metallopeptidase inhibitor 1 | CAAGATGTATAAAGGGTTCCAAGC | TCCATCCTGCAGTTTTCCAG |
| MMP2 | Matrix metallopeptidase 2 | AAGTATGGCTTCTGCCCTGA | ATTTGTTGCCCAGGAAAGTG |
| COL1A1 | Collagen type I alpha 1 chain | CCGGCTCCTGCTCCTCTTAGCG | CGTTCTGTACGCAGGTGATTGGTGG |
| COL4A1 | Collagen type IV alpha 1 chain | CCTGGCTTGAAAAACAGCTC | CCCTGCTGAGGTCTGTGAAC |
| SMAD2 | SMAD family member 2 | TGCTCTGAAATTTGGGGACTGA | GACGACCATCAAGAGACCTGG |
| SMAD3 | SMAD family member 3 | ATCGTGAAGCGCCTGCTG | CATCCAGGGACCTGGGGA |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | ATGACATCAAGAAGGTGGTG | CATACCAGGAAATGAGCTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buakaew, W.; Krobthong, S.; Yingchutrakul, Y.; Khamto, N.; Sutana, P.; Potup, P.; Thongsri, Y.; Daowtak, K.; Ferrante, A.; Léon, C.; et al. In Vitro Investigation of the Anti-Fibrotic Effects of 1-Phenyl-2-Pentanol, Identified from Moringa oleifera Lam., on Hepatic Stellate Cells. Int. J. Mol. Sci. 2024, 25, 8995. https://doi.org/10.3390/ijms25168995
Buakaew W, Krobthong S, Yingchutrakul Y, Khamto N, Sutana P, Potup P, Thongsri Y, Daowtak K, Ferrante A, Léon C, et al. In Vitro Investigation of the Anti-Fibrotic Effects of 1-Phenyl-2-Pentanol, Identified from Moringa oleifera Lam., on Hepatic Stellate Cells. International Journal of Molecular Sciences. 2024; 25(16):8995. https://doi.org/10.3390/ijms25168995
Chicago/Turabian StyleBuakaew, Watunyoo, Sucheewin Krobthong, Yodying Yingchutrakul, Nopawit Khamto, Pornsuda Sutana, Pachuen Potup, Yordhathai Thongsri, Krai Daowtak, Antonio Ferrante, Catherine Léon, and et al. 2024. "In Vitro Investigation of the Anti-Fibrotic Effects of 1-Phenyl-2-Pentanol, Identified from Moringa oleifera Lam., on Hepatic Stellate Cells" International Journal of Molecular Sciences 25, no. 16: 8995. https://doi.org/10.3390/ijms25168995
APA StyleBuakaew, W., Krobthong, S., Yingchutrakul, Y., Khamto, N., Sutana, P., Potup, P., Thongsri, Y., Daowtak, K., Ferrante, A., Léon, C., & Usuwanthim, K. (2024). In Vitro Investigation of the Anti-Fibrotic Effects of 1-Phenyl-2-Pentanol, Identified from Moringa oleifera Lam., on Hepatic Stellate Cells. International Journal of Molecular Sciences, 25(16), 8995. https://doi.org/10.3390/ijms25168995








