Region-Specific Gene Expression Changes Associated with Oleoylethanolamide-Induced Attenuation of Alcohol Self-Administration
Abstract
:1. Introduction
2. Results
2.1. Bodyweight
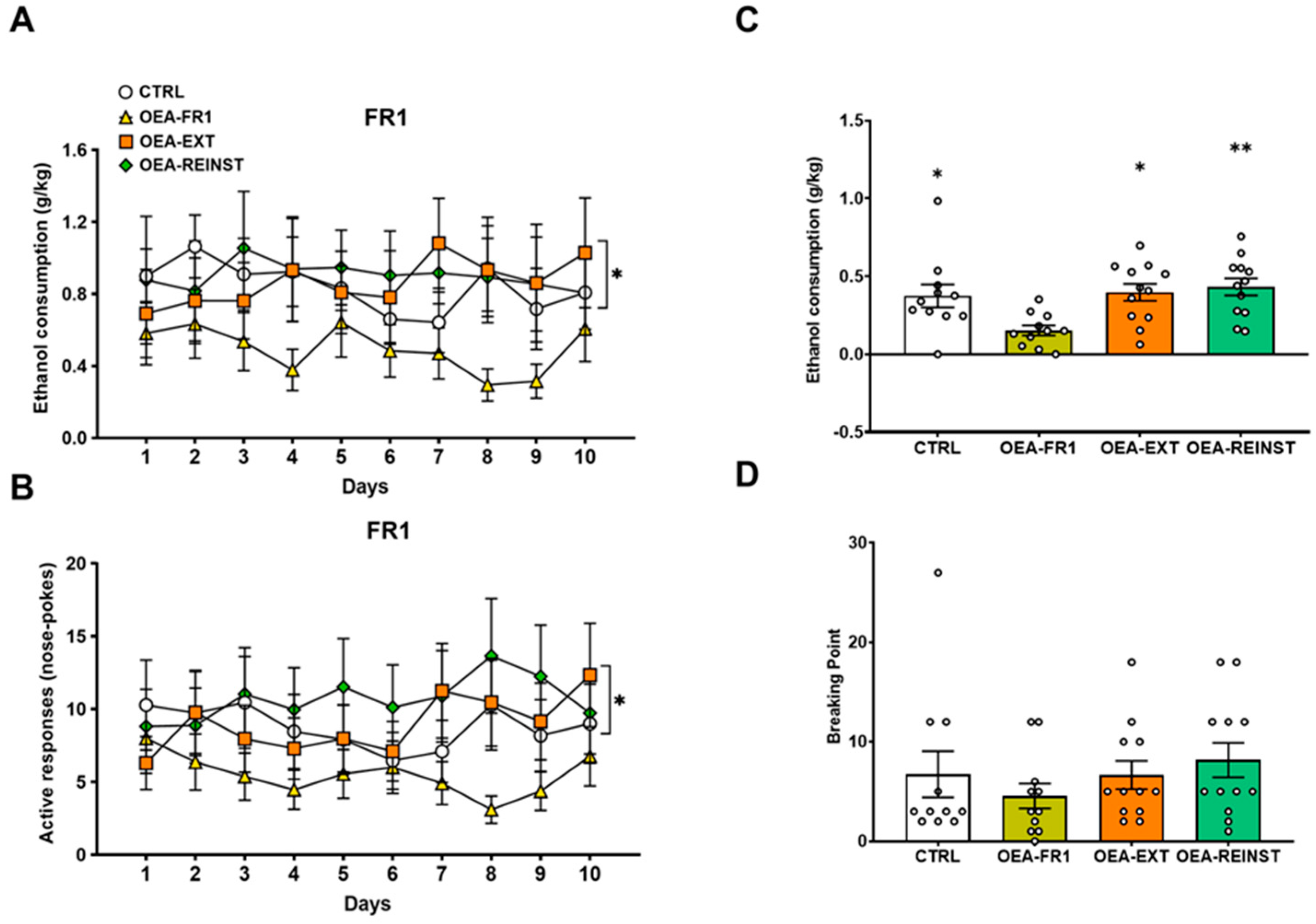
2.2. DID and Oral Alcohol SA
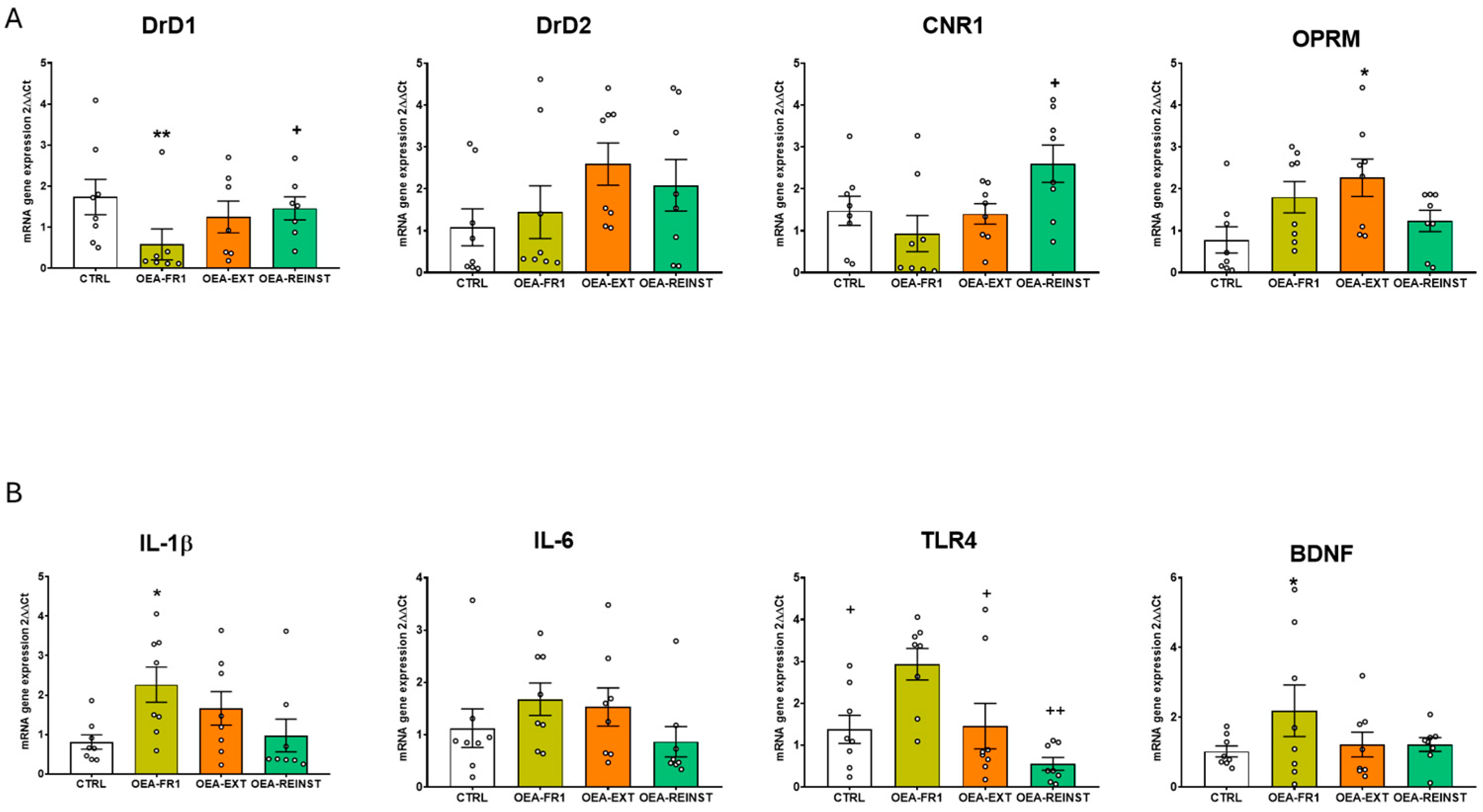
2.3. OEA Affects Gene Expression in the Striatum and Hippocampus
3. Discussion
4. Methods and Materials
4.1. Experimental Design
4.2. Drugs
4.3. Habituation to Alcohol
4.4. Oral Alcohol Self-Administration (SA)
4.4.1. Training (15 Days)
4.4.2. FR1 (10 Days)
4.4.3. PR (1 Day)
4.4.4. Extinction Sessions
4.4.5. Cue-Induced Reinstatement of Alcohol Seeking (1 Day)
4.5. Tissue Sampling and Biochemical Analyses
RNA Isolation, Reverse Transcription, and Quantitative RT-PCR
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, A.F.; Heilig, M.; Perez, A.; Probst, C.; Rehm, J. Alcohol use disorders. Lancet 2019, 394, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Singla, R.; Maheshwari, O.; Fontaine, C.J.; Gil-Mohapel, J. Alcohol use disorder: Neurobiology and therapeutics. Biomedicines 2022, 10, 1192. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Bhaseen, S.; Krishnamurthy, S.; Sahu, A.N. Neurochemical evidence of preclinical and clinical reports on target-based therapy in alcohol used disorder. Neurochem. Res. 2020, 45, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Olsen, R.W. Alcohol use disorders and current pharmacological therapies: The role of GABAA receptors. Acta Pharmacol. Sin. 2014, 35, 981–993. [Google Scholar] [CrossRef]
- Czerwińska-Błaszczyk, A.; Pawlak, E.; Pawłowski, T. The significance of toll-like receptors in the neuroimmunologic background of alcohol dependence. Front. Psychiatry 2022, 12, 797123. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Schwarz, M.; Delzenne, N.M.; Stärkel, P.; de Timary, P. Alterations of kynurenine pathway in alcohol use disorder and abstinence: A link with gut microbiota, peripheral inflammation and psychological symptoms. Transl. Psychiatry 2021, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, N.; Wallen, G.R.; Yang, L.; Alkhatib, J.; Schwandt, M.L.; Feng, D.; Gao, B.; Diazgranados, N.; Ramchandani, V.A.; Barb, J.J. An exploratory study of pro-inflammatory cytokines in individuals with alcohol use disorder: MCP-1 and IL-8 associated with alcohol consumption, sleep quality, anxiety, depression, and liver biomarkers. Front. Psychiatry 2022, 13, 931280. [Google Scholar] [CrossRef] [PubMed]
- Ray, L.A.; Bujarski, S.; Grodin, E.; Hartwell, E.; Green, R.J.; Venegas, A.; Lim, A.C.; Gillis, A.; Miotto, K. State-of-the-art behavioral and pharmacological treatments for alcohol use disorder. Am. J. Drug Alcohol Abus. 2018, 45, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Sagheddu, C.; Torres, L.H.; Marcourakis, T.; Pistis, M. Endocannabinoid-Like Lipid Neuromodulators in the Regulation of Dopamine Signaling: Relevance for Drug Addiction. Front. Synaptic Neurosci. 2020, 12, 588660. [Google Scholar] [CrossRef]
- Rodríguez De Fonseca, F.; Navarro, M.; Gómez, R.; Escuredo, L.; Nava, F.; Fu, J.; Murillo-Rodríguez, E.; Giuffrida, A.; Loverme, J.; Gaetani, S.; et al. An anorexic lipid mediator regulated by feeding. Nature 2001, 414, 209–212. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Fu, J.; Astarita, G.; Li, X.; Gaetani, S.; Campolongo, P.; Cuomo, V.; Piomelli, D. The Lipid Messenger OEA Links Dietary Fat Intake to Satiety. Cell Metab. 2008, 8, 281–288. [Google Scholar] [CrossRef]
- Pagotto, U.; Marsicano, G.; Cota, D.; Lutz, B.; Pasquali, R. The Emerging Role of the Endocannabinoid System in Endocrine Regulation and Energy Balance. Endocr. Rev. 2006, 27, 73–100. [Google Scholar] [CrossRef] [PubMed]
- Bilbao, A.; Serrano, A.; Cippitelli, A.; Pavón, F.J.; Giuffrida, A.; Suárez, J.; García-Marchena, N.; Baixeras, E.; Gómez de Heras, R.; Orio, L.; et al. Role of the satiety factor oleoylethanolamide in alcoholism. Addict. Biol. 2016, 21, 859–872. [Google Scholar] [CrossRef]
- Best, L.M.; Williams, B.; Le Foll, B.; Mansouri, E.; Bazinet, R.P.; Lin, L.; De Luca, V.; Lagzdins, D.; Rusjan, P.; Tyndale, R.F.; et al. Lower brain fatty acid amide hydrolase in treatment-seeking patients with alcohol use disorder: A positron emission tomography study with [C-11]CURB. Neuropsychopharmacology 2020, 45, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marchena, N.; Pavon, F.J.; Pastor, A.; Araos, P.; Pedraz, M.; Romero-Sanchiz, P.; Calado, M.; Suarez, J.; Castilla-Ortega, E.; Orio, L.; et al. Plasma concentrations of oleoylethanolamide and other acylethanolamides are altered in alcohol-dependent patients: Effect of length of abstinence. Addict. Biol. 2017, 22, 1366–1377. [Google Scholar] [CrossRef]
- Sánchez-Marín, L.; Pavón-Morón, F.J.; Rodríguez de Fonseca, F.; Serrano, A. Attenuation of oleoylethanolamide-induced reduction of alcohol consumption in adult rats exposed intermittently to alcohol during adolescence. Neurosci. Lett. 2022, 781, 136670. [Google Scholar] [CrossRef]
- Guzmán, M.; Verme, J.L.; Fu, J.; Oveisi, F.; Blázquez, C.; Piomelli, D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor α (PPAR-α). J. Biol. Chem. 2004, 279, 27849–27854. [Google Scholar] [CrossRef]
- Orio, L.; Alen, F.; Pavón, F.J.; Serrano, A.; García-Bueno, B. Oleoylethanolamide, neuroinflammation, and alcohol abuse. Front. Mol. Neurosci. 2019, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- González-Portilla, M.; Mellado, S.; Montagud-Romero, S.; Rodríguez de Fonseca, F.; Pascual, M.; Rodríguez-Arias, M. Oleoylethanolamide attenuates cocaine-primed reinstatement and alters dopaminergic gene expression in the striatum. Behav. Brain Funct. 2023, 19, 8. [Google Scholar] [CrossRef]
- Antón, M.; Alén, F.; Gomez de Heras, R.; Serrano, A.; Pavón, F.J.; Leza, J.C.; García-Bueno, B.; Rodríguez de Fonseca, F.; Orio, L. Oleoylethanolamide prevents neuroimmune HMGB1/TLR4/NF-kB danger signaling in rat frontal cortex and depressive-like behavior induced by ethanol binge administration. Addict. Biol. 2017, 22, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Campolongo, P.; Roozendaal, B.; Trezza, V.; Cuomo, V.; Astarita, G.; Fu, J.; McGaugh, J.L.; Piomelli, D. Fat-induced satiety factor oleoylethanolamide enhances memory consolidation. Proc. Natl. Acad. Sci. USA 2009, 106, 8027–8031. [Google Scholar] [CrossRef] [PubMed]
- Moya, M.; San Felipe, D.; Ballesta, A.; Alén, F.; Rodríguez de Fonseca, F.; García-Bueno, B.; Marco, E.M.; Orio, L. Cerebellar and cortical TLR4 activation and behavioral impairments in Wernicke-Korsakoff Syndrome: Pharmacological effects of oleoylethanolamide. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 108, 110190. [Google Scholar] [CrossRef] [PubMed]
- Hirth, N.; Meinhardt, M.W.; Noori, H.R.; Salgado, H.; Torres-Ramirez, O.; Uhrig, S.; Broccoli, L.; Vengeliene, V.; Roßmanith, M.; Perreau-Lenz, S.; et al. Convergent evidence from alcohol-dependent humans and rats for a hyperdopaminergic state in protracted abstinence. Proc. Natl. Acad. Sci. USA 2016, 113, 3024–3029. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.Z.; Cheer, J.F.; Tonini, R. Modulating the Neuromodulators: Dopamine, Serotonin, and the Endocannabinoid System. Trends Neurosci. 2021, 44, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.S.V.; Barrett, D.A.; Randall, M.D. ‘Entourage’ effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br. J. Pharmacol. 2008, 155, 837–846. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, N.M.W.J.; Lange, J.H.M.; Kruse, C.G.; Herremans, A.H.; Schoffelmeer, A.N.M.; van Drimmelen, M.; De Vries, T.J. SLV330, a cannabinoid CB1 receptor antagonist, attenuates ethanol and nicotine seeking and improves inhibitory response control in rats. Behav. Brain Res. 2011, 217, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Economidou, D.; Mattioli, L.; Cifani, C.; Perfumi, M.; Massi, M.; Cuomo, V.; Trabace, L.; Ciccocioppo, R. Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology 2006, 183, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Hungud, B.L.; Szakall, I.; Adam, A.; Basavarajappa, B.S.; Vadasz, C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J. Neurochem. 2003, 84, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Lazenka, M.F.; Selley, D.E.; Sim-Selley, L.J. Brain regional differences in CB1 receptor adaptation and regulation of transcription. Life Sci. 2013, 92, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Soria-Gomez, E.; Pagano Zottola, A.C.; Mariani, Y.; Desprez, T.; Barresi, M.; Bonilla-del Río, I.; Muguruza, C.; Le Bon-Jego, M.; Julio-Kalajzić, F.; Flynn, R.; et al. Subcellular specificity of cannabinoid effects in striatonigral circuits. Neuron 2021, 109, 1513–1526.e11. [Google Scholar] [CrossRef] [PubMed]
- Shahen-Zoabi, S.; Smoum, R.; Bingor, A.; Grad, E.; Nemirovski, A.; Shekh-Ahmad, T.; Mechoulam, R.; Yaka, R. N-oleoyl glycine and N-oleoyl alanine attenuate alcohol self-administration and preference in mice. Transl. Psychiatry 2023, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hansson, A.C.; Gründer, G.; Hirth, N.; Noori, H.R.; Spanagel, R.; Sommer, W.H. Dopamine and opioid systems adaptation in alcoholism revisited: Convergent evidence from positron emission tomography and postmortem studies. Neurosci. Biobehav. Rev. 2019, 106, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Hall, F.S.; Goeb, M.; Li, X.F.; Sora, I.; Uhl, G.R. μ-Opioid receptor knockout mice display reduced cocaine conditioned place preference but enhanced sensitization of cocaine-induced locomotion. Mol. Brain Res. 2004, 121, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Comerota, M.M.; Gedam, M.; Xiong, W.; Jin, F.; Deng, L.; Wang, M.C.; Wang, J.; Zheng, H. Oleoylethanolamide facilitates PPARα and TFEB signaling and attenuates Aβ pathology in a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2023, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Sayd, A.; Antón, M.; Alén, F.; Caso, J.R.; Pavón, J.; Leza, J.C.; de Fonseca, F.R.; García-Bueno, B.; Orio, L. Systemic Administration of Oleoylethanolamide Protects from Neuroinflammation and Anhedonia Induced by LPS in Rats. Int. J. Neuropsychopharmacol. 2015, 18, pyu111. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Lawrimore, C.J.; Walter, T.J.; Coleman, L.G., Jr. The role of neuroimmune signaling in alcoholism. Neuropharmacology 2017, 122, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Lu, B. BDNF and activity-dependent synaptic modulation. Learn. Mem. 2003, 10, 86–98. [Google Scholar] [CrossRef]
- Masana, Y.; Wanaka, A.; Kato, H.; Asai, T.; Tohyama, M. Localization of trkB mRNA in postnatal brain development. J. Neurosci. Res. 1993, 35, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.C.; Guo, H.; Zhou, H.; Suo, D.Q.; Li, W.J.; Zhou, Y.; Zhao, Y.; Yang, W.S.; Jin, X. Chronic oleoylethanolamide treatment improves spatial cognitive deficits through enhancing hippocampal neurogenesis after transient focal cerebral ischemia. Biochem. Pharmacol. 2015, 94, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Yu, H.L.; Tian-Lan Zhang, F.; Quan, Z.S. Antidepressant-like effects of oleoylethanolamide in a mouse model of chronic unpredictable mild stress. Pharmacol. Biochem. Behav. 2015, 133, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Silva-Peña, D.; Rivera, P.; Alén, F.; Vargas, A.; Rubio, L.; García-Marchena, N.; Pavón, F.J.; Serrano, A.; De Fonseca, F.R.; Suárez, J. Oleoylethanolamide modulates BDNF-ERK signaling and neurogenesis in the hippocampi of rats exposed to δ9-THC and ethanol binge drinking during adolescence. Front. Mol. Neurosci. 2019, 12, 442287. [Google Scholar] [CrossRef] [PubMed]
- Reguilón, M.D.; Ferrer-Pérez, C.; Manzanedo, C.; Miñarro, J.; Rodríguez-Arias, M. Voluntary wheel running during adolescence prevents the increase in ethanol intake induced by social defeat in male mice. Psychopharmacology 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ródenas-González, F.; Arenas, M.C.; Blanco-Gandía, M.C.; Manzanedo, C.; Rodríguez-Arias, M. Vicarious social defeat increases conditioned rewarding effects of cocaine and ethanol intake in female mice. Biomedicines 2023, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.S.; Best, K.; Belknap, J.K.; Finn, D.A.; Crabbe, J.C. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav. 2005, 84, 53–63. [Google Scholar] [CrossRef]
- Navarrete, F.; Rubio, G.; Manzanares, J. Effects of naltrexone plus topiramate on ethanol self-administration and tyrosine hydroxylase gene expression changes. Addict. Biol. 2014, 19, 862–873. [Google Scholar] [CrossRef]
- Maddern, X.J.; Ursich, L.T.; Bailey, G.; Pearl, A.; Anversa, R.G.; Lawrencem, A.J.; Walkerm, L.C. Sex Differences in Alcohol Use: Is It All About Hormones? Endocrinology 2024, 165, bqae088. [Google Scholar] [CrossRef] [PubMed]
- McHugh, R.K.; Weiss, R.D. Alcohol Use Disorder and Depressive Disorders. Alcohol Res. 2019, 40, arcr.v40.1.01. [Google Scholar] [CrossRef] [PubMed]
- Delk, J.; Bensley, K.; Ye, Y.; Subbaraman, M.S.; Phillips, A.Z.; Karriker-Jaffe, K.J.; Mulia, N. Intersectional disparities in outpatient alcohol treatment completion by gender and race and ethnicity. Alcohol Clin. Exp. Res. 2024, 48, 389–399. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Portilla, M.; Montagud-Romero, S.; Mellado, S.; de Fonseca, F.R.; Pascual, M.; Rodríguez-Arias, M. Region-Specific Gene Expression Changes Associated with Oleoylethanolamide-Induced Attenuation of Alcohol Self-Administration. Int. J. Mol. Sci. 2024, 25, 9002. https://doi.org/10.3390/ijms25169002
González-Portilla M, Montagud-Romero S, Mellado S, de Fonseca FR, Pascual M, Rodríguez-Arias M. Region-Specific Gene Expression Changes Associated with Oleoylethanolamide-Induced Attenuation of Alcohol Self-Administration. International Journal of Molecular Sciences. 2024; 25(16):9002. https://doi.org/10.3390/ijms25169002
Chicago/Turabian StyleGonzález-Portilla, Macarena, Sandra Montagud-Romero, Susana Mellado, Fernando Rodríguez de Fonseca, María Pascual, and Marta Rodríguez-Arias. 2024. "Region-Specific Gene Expression Changes Associated with Oleoylethanolamide-Induced Attenuation of Alcohol Self-Administration" International Journal of Molecular Sciences 25, no. 16: 9002. https://doi.org/10.3390/ijms25169002






