ShSPI Inhibits Thrombosis Formation and Ischemic Stroke In Vivo
Abstract
1. Introduction
2. Results
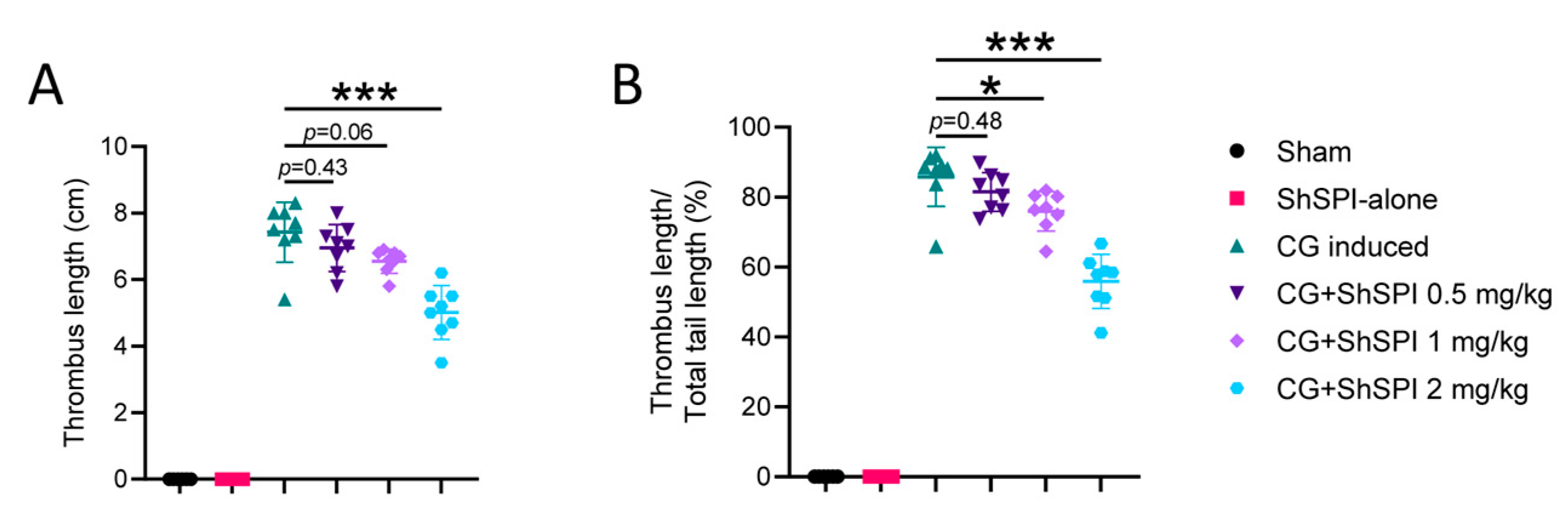
2.1. ShSPI Pretreatment Inhibits CG-Induced Thrombus Formation in an In Vivo Mouse Model
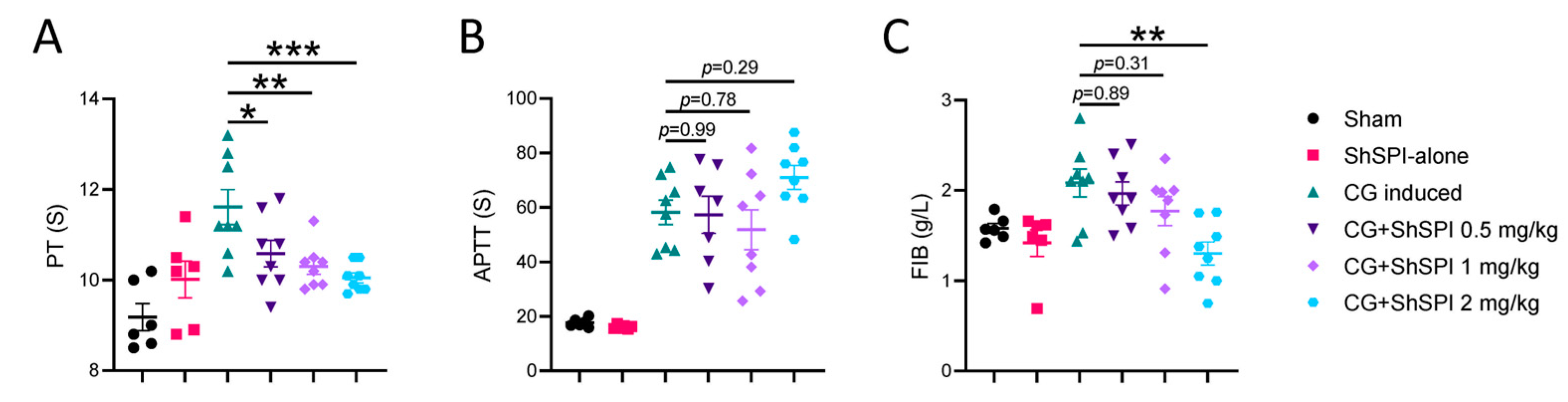
2.2. ShSPI Inhibits CG-Induced Coagulation Dysfunction
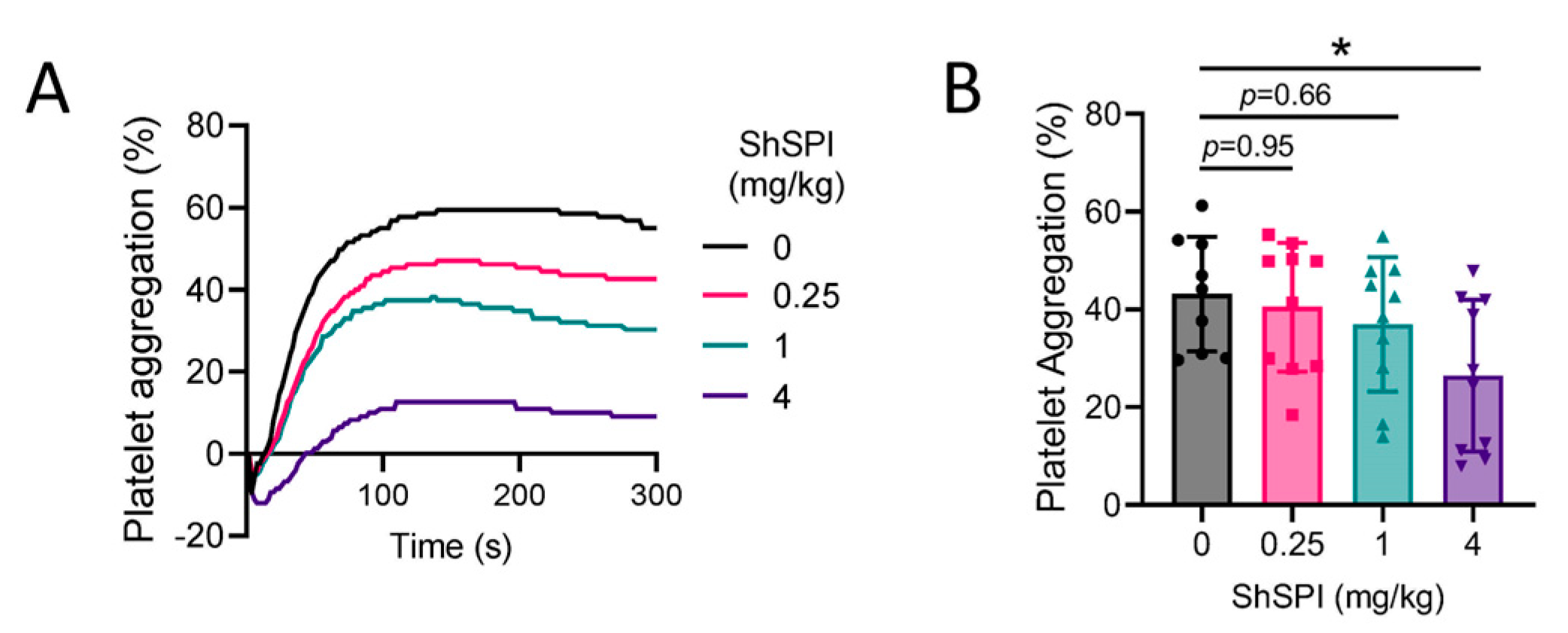
2.3. ShSPI Inhibits Platelet Activation
2.4. ShSPI Modulates Oxidative Stress
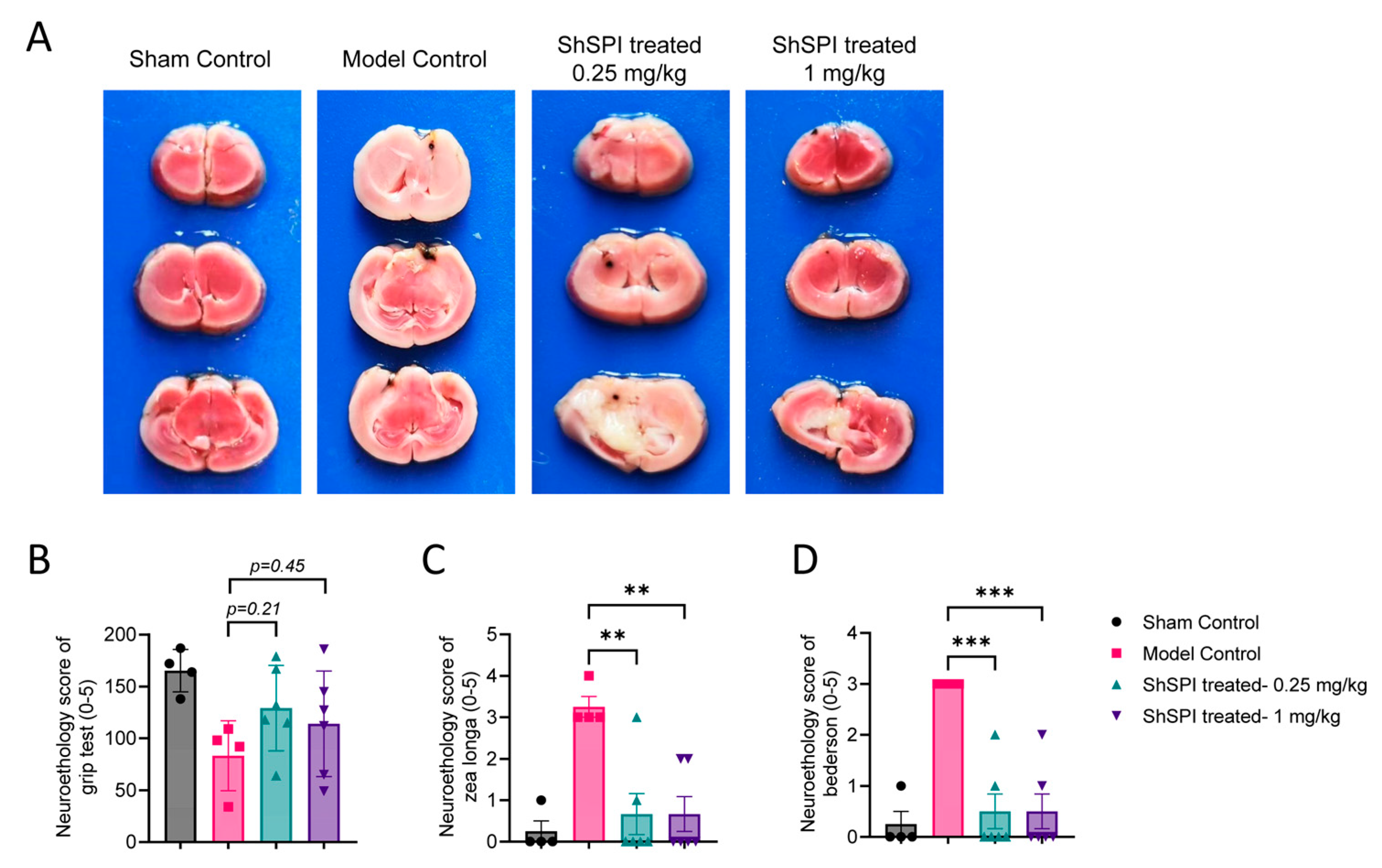
2.5. ShSPI Attenuates IS Severity In Vivo
3. Discussion
4. Materials and Methods
4.1. Synthesis and Characterization of ShSPI
4.2. Cell Lines and Cytotoxicity Assay
4.3. Mice
4.4. Carrageenan (CG)-Induced Tail Thrombosis Model
4.5. Coagulation Function Analysis
4.6. Preparation and Activation of Platelets
4.7. Transient MCAO (tMCAO)
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis: Epidemiologic Aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Massberg, S.; Grahl, L.; von Bruehl, M.-L.; Manukyan, D.; Pfeiler, S.; Goosmann, C.; Brinkmann, V.; Lorenz, M.; Bidzhekov, K.; Khandagale, A.B.; et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010, 16, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Szapary, L.; Tornyos, D.; Kupo, P.; Lukacs, R.; El Alaoui El Abdallaoui, O.; Komocsi, A. Combination of antiplatelet and anticoagulant therapy, component network meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2022, 9, 1036609. [Google Scholar] [CrossRef] [PubMed]
- Balint, A.; Tornyos, D.; El Alaoui El Abdallaoui, O.; Kupo, P.; Komocsi, A. Network Meta-Analysis of Ticagrelor for Stroke Prevention in Patients at High Risk for Cardiovascular or Cerebrovascular Events. Stroke 2021, 52, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Prisco, D.; Eikelboom, J.W. Factor XI inhibitors: Cardiovascular perspectives. Eur. Heart J. 2023, 44, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Elnour, A.A.; Komocsi, A.; Kupo, P.; El Khidir, I.Y.; Zachariah, S.; Sam, K.G.; Asim, S.; Sadeq, A. The Role of Direct Oral Anticoagulant in Patients with Acute Coronary Syndrome on Single or Dual Antiplatelet Regime: Review of Opportunities and Challenges. Curr Rev. Clin Exp. Pharmacol. 2021, 16, 52–63. [Google Scholar] [CrossRef]
- Demoulin, S.; Godfroid, E.; Hermans, C. Dual inhibition of factor XIIa and factor XIa as a therapeutic approach for safe thromboprotection. J. Thromb. Haemost. 2021, 19, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Zhang, J.; Chen, X.; Liu, M.; Zhao, H.; Jin, L.; Zhang, Z.; Luan, N.; Meng, P.; Wang, J.; et al. Role of LL-37 in thrombotic complications in patients with COVID-19. Cell. Mol. Life Sci. 2022, 79, 309. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, Z.; Fang, M.; Han, Y.; Wang, G.; Wang, S.; Xue, M.; Li, Y.; Zhang, L.; Wu, J.; et al. Transferrin plays a central role in coagulation balance by interacting with clotting factors. Cell Res. 2020, 30, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Marinaccio, L.; Stefanucci, A.; Scioli, G.; Della Valle, A.; Zengin, G.; Cichelli, A.; Mollica, A. Peptide Human Neutrophil Elastase Inhibitors from Natural Sources: An Overview. Int. J. Mol. Sci. 2022, 23, 2924. [Google Scholar] [CrossRef]
- Zeng, W.; Song, Y.; Wang, R.; He, R.; Wang, T. Neutrophil elastase: From mechanisms to therapeutic potential. J. Pharm. Anal. 2023, 13, 355–366. [Google Scholar] [CrossRef]
- Renesto, P.; Chignard, M. Enhancement of cathepsin G-induced platelet activation by leukocyte elastase: Consequence for the neutrophil-mediated platelet activation. Blood 1993, 82, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Leinweber, J.; Mizurini, D.M.; Francischetti, I.M.B.; Fleischer, M.; Hermann, D.M.; Kleinschnitz, C.; Langhauser, F. Elastase inhibitor agaphelin protects from acute ischemic stroke in mice by reducing thrombosis, blood–brain barrier damage, and inflammation. Brain Behav. Immun. 2021, 93, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Renesto, P.; Balloy, V.; Kamimura, T.; Masuda, K.; Imaizumi, A.; Chignard, M. Inhibition by recombinant SLPI and half–SLPI (Asn55–Ala107) of elastase and cathepsin G activities: Consequence for neutrophil–platelet cooperation. Br. J. Pharmacol. 1993, 108, 1100–1106. [Google Scholar] [CrossRef]
- Wang, C.; Chen, M.; Lu, X.; Yang, S.; Yang, M.; Fang, Y.; Lai, R.; Duan, Z. Isolation and Characterization of Poeciguamerin, a Peptide with Dual Analgesic and Anti-Thrombotic Activity from the Poecilobdella manillensis Leech. Int. J. Mol. Sci. 2023, 24, 11097. [Google Scholar] [CrossRef] [PubMed]
- Luan, N.; Zhao, Q.; Duan, Z.; Ji, M.; Xing, M.; Zhu, T.; Mwangi, J.; Rong, M.; Liu, J.; Lai, R. Identification and Characterization of ShSPI, a Kazal-Type Elastase Inhibitor from the Venom of Scolopendra Hainanum. Toxins 2019, 11, 708. [Google Scholar] [CrossRef] [PubMed]
- Portier, I.; Manne, B.K.; Kosaka, Y.; Tolley, N.D.; Denorme, F.; Babur, O.; Reddy, A.P.; Wilmarth, P.A.; Aslan, J.E.; Weyrich, A.S.; et al. Aging-related alterations in mechanistic target of rapamycin signaling promote platelet hyperreactivity and thrombosis. J. Thromb. Haemost. 2024, 6, S1538-7836(24)00317-9, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Occhipinti, G.; Giacoppo, D.; Agnello, F.; Laudani, C.; Spagnolo, M.; Mauro, M.S.; Rochira, C.; Finocchiaro, S.; Mazzone, P.M.; et al. Antithrombotic Therapy for Primary and Secondary Prevention of Ischemic Stroke: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 82, 1538–1557. [Google Scholar] [CrossRef]
- Talasaz, A.H.; Sadeghipour, P.; Ortega-Paz, L.; Kakavand, H.; Aghakouchakzadeh, M.; Beavers, C.; Fanikos, J.; Eikelboom, J.W.; Siegal, D.M.; Monreal, M.; et al. Optimizing antithrombotic therapy in patients with coexisting cardiovascular and gastrointestinal disease. Nat. Rev. Cardiol. 2024, 21, 574–592. [Google Scholar] [CrossRef]
- Tang, X.; Fang, M.; Cheng, R.; Zhang, Z.; Wang, Y.; Shen, C.; Han, Y.; Lu, Q.; Du, Y.; Liu, Y.; et al. Iron-Deficiency and Estrogen Are Associated With Ischemic Stroke by Up-Regulating Transferrin to Induce Hypercoagulability. Circ. Res. 2020, 127, 651–663. [Google Scholar] [CrossRef]
- Pircher, J.; Czermak, T.; Ehrlich, A.; Eberle, C.; Gaitzsch, E.; Margraf, A.; Grommes, J.; Saha, P.; Titova, A.; Ishikawa-Ankerhold, H.; et al. Cathelicidins prime platelets to mediate arterial thrombosis and tissue inflammation. Nat. Commun. 2018, 9, 1523. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, X.; Liao, Z.; Shen, C.; Cheng, R.; Fang, M.; Wang, G.; Li, Y.; Tang, S.; Xie, L.; et al. Hypoxia and low temperature upregulate transferrin to induce hypercoagulability at high altitude. Blood 2022, 140, 2063–2075. [Google Scholar] [CrossRef] [PubMed]
- Si-Tahar, M.; Pidard, D.; Balloy, V.; Moniatte, M.; Kieffer, N.; Van Dorsselaer, A.; Chignard, M. Human neutrophil elastase proteolytically activates the platelet integrin alphaIIbbeta3 through cleavage of the carboxyl terminus of the alphaIIb subunit heavy chain. Involvement in the potentiation of platelet aggregation. J. Biol. Chem. 1997, 272, 11636–11647. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Valois, M.V.; Ottaiano, T.F.; Miranda, A.; Hansen, D.; Sampaio, M.U.; Oliva, M.L.V.; de Abreu Maffei, F.H. The recombinant plant Bauhinia bauhinioides elastase inhibitor reduces rat thrombus without alterations in hemostatic parameters. Sci. Rep. 2021, 11, 13475. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Kuroda, S.; Shichinohe, H.; Kahata, K.; Shiratori, S.; Ikeda, S.; Harada, T.; Hirata, K.; Tha, K.K.; Aragaki, M.; et al. Intracerebral transplantation of MRI-trackable autologous bone marrow stromal cells for patients with subacute ischemic stroke. Med 2024, 5, 432–444.E4. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shi, K.; Wang, Y.; Shi, F.-D. Neurovascular Inflammation and Complications of Thrombolysis Therapy in Stroke. Stroke 2023, 54, 2688–2697. [Google Scholar] [CrossRef]
- Yang, J.; Cha, L.; Wang, Y.; Zhang, Q.; Tang, X.; Shao, J.; Duan, Z. L-Palmitoylcarnitine potentiates plasmin and tPA to inhibit thrombosis. Nat. Prod. Bioprospect. 2023, 13, 48. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, C.; Fang, M.; Han, Y.; Long, C.; Liu, W.; Yang, M.; Liu, M.; Zhang, D.; Cao, Q.; et al. Novel contact-kinin inhibitor sylvestin targets thromboinflammation and ameliorates ischemic stroke. Cell. Mol. Life Sci. 2022, 79, 240. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, N.; Cao, H.; Wang, Y.; Zhang, H.; Lin, K.; Hu, J.; Rong, M.; Liu, C. ShSPI Inhibits Thrombosis Formation and Ischemic Stroke In Vivo. Int. J. Mol. Sci. 2024, 25, 9003. https://doi.org/10.3390/ijms25169003
Luan N, Cao H, Wang Y, Zhang H, Lin K, Hu J, Rong M, Liu C. ShSPI Inhibits Thrombosis Formation and Ischemic Stroke In Vivo. International Journal of Molecular Sciences. 2024; 25(16):9003. https://doi.org/10.3390/ijms25169003
Chicago/Turabian StyleLuan, Ning, Han Cao, Yunfei Wang, Haihao Zhang, Kangyang Lin, Jingping Hu, Mingqiang Rong, and Cunbao Liu. 2024. "ShSPI Inhibits Thrombosis Formation and Ischemic Stroke In Vivo" International Journal of Molecular Sciences 25, no. 16: 9003. https://doi.org/10.3390/ijms25169003
APA StyleLuan, N., Cao, H., Wang, Y., Zhang, H., Lin, K., Hu, J., Rong, M., & Liu, C. (2024). ShSPI Inhibits Thrombosis Formation and Ischemic Stroke In Vivo. International Journal of Molecular Sciences, 25(16), 9003. https://doi.org/10.3390/ijms25169003






