Synthesis, Structure, Electrochemical Properties, and Antioxidant Activity of Organogermanium(IV) Catecholate Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. X-ray Structure
2.2.1. The Molecular Structures in the Crystal State
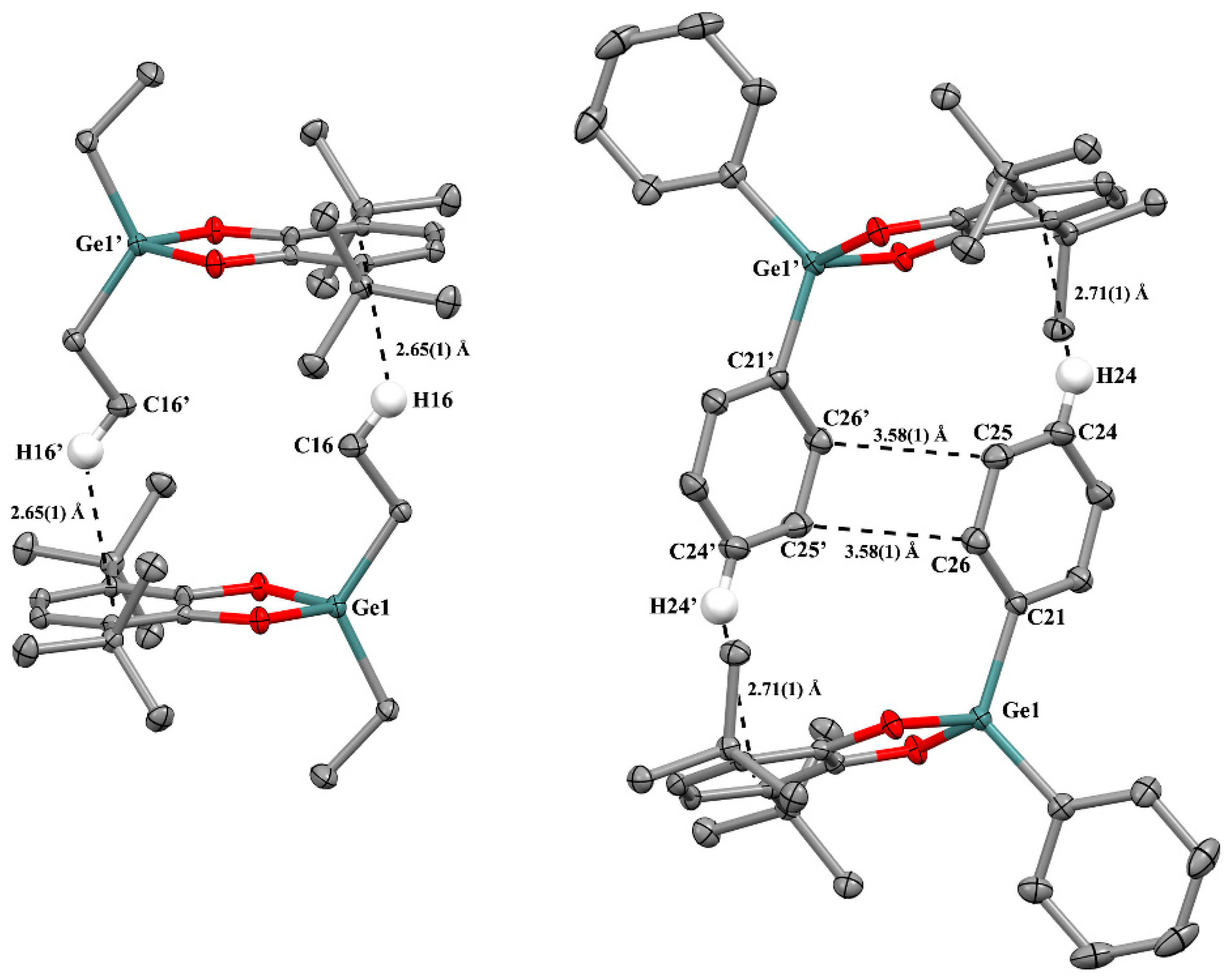
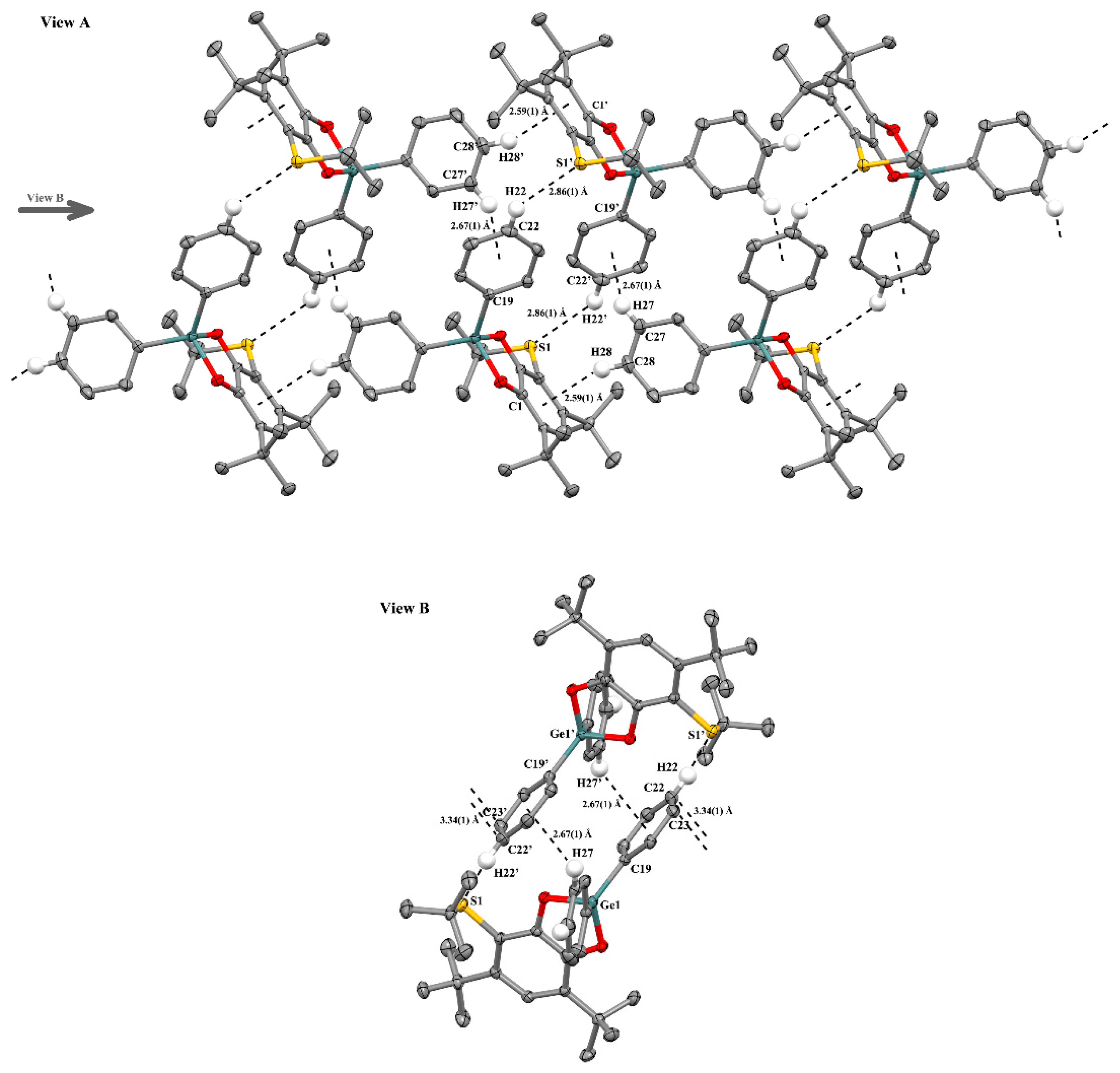
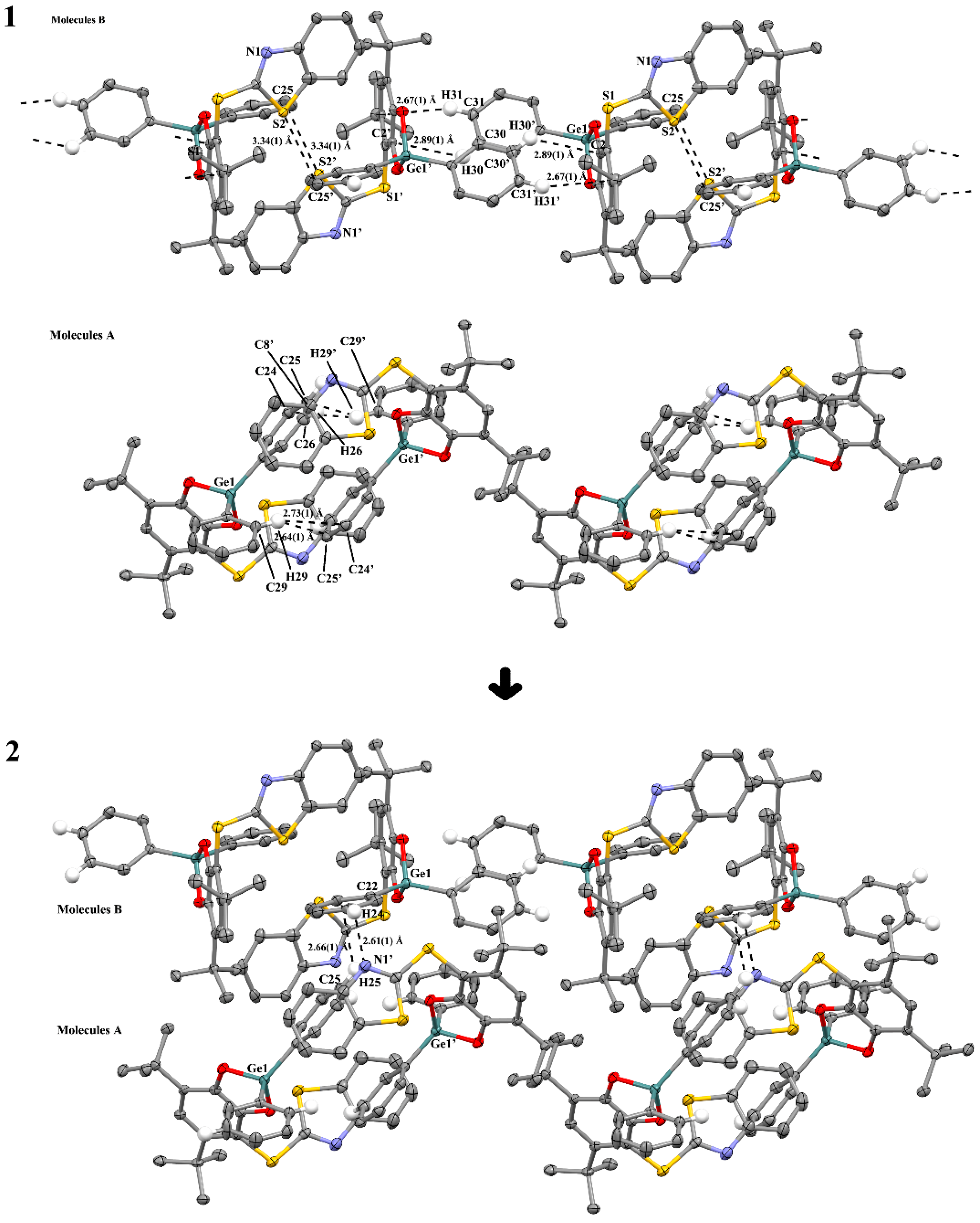
2.2.2. The Features of Crystal Packing
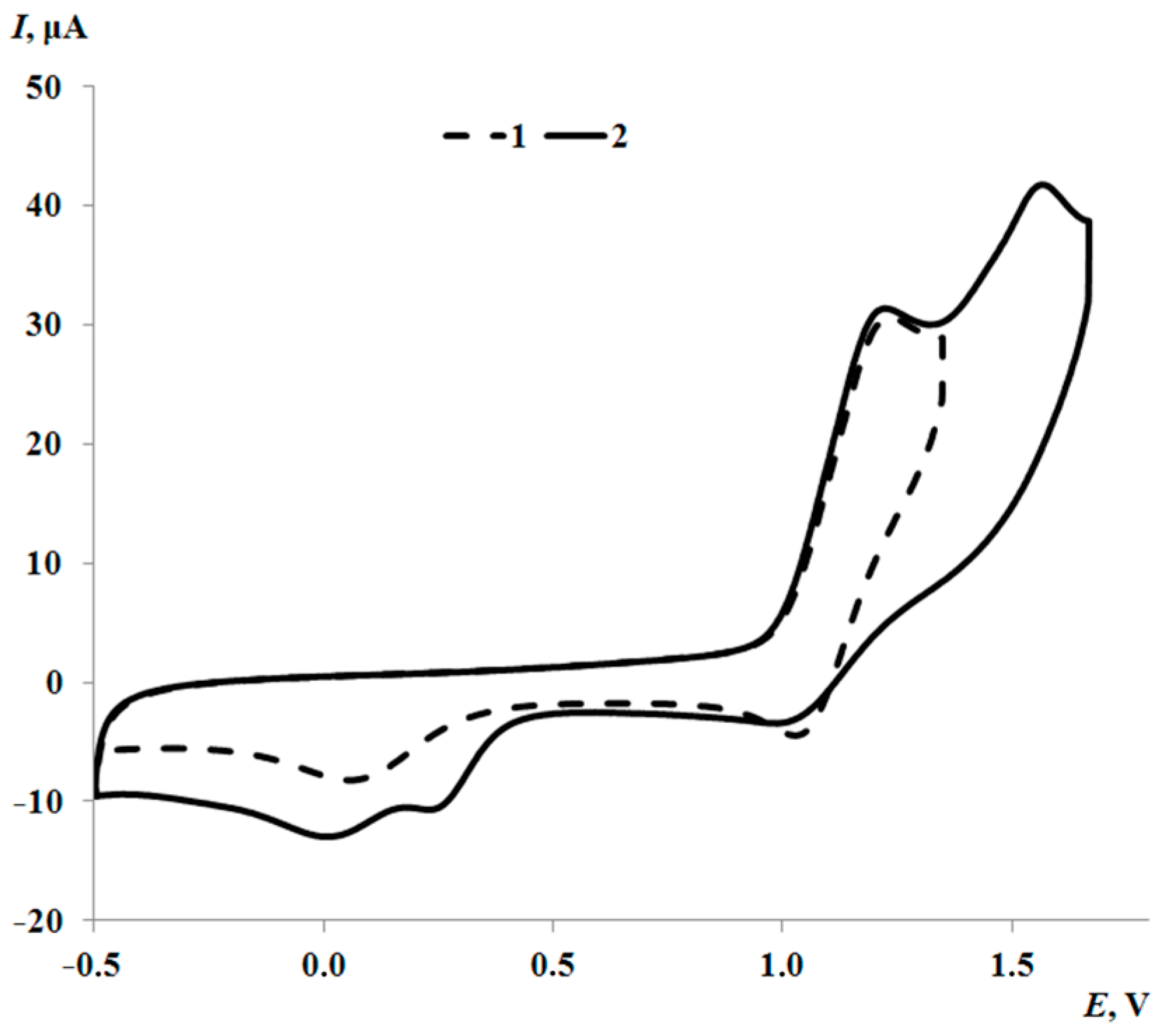
2.3. Electrochemistry
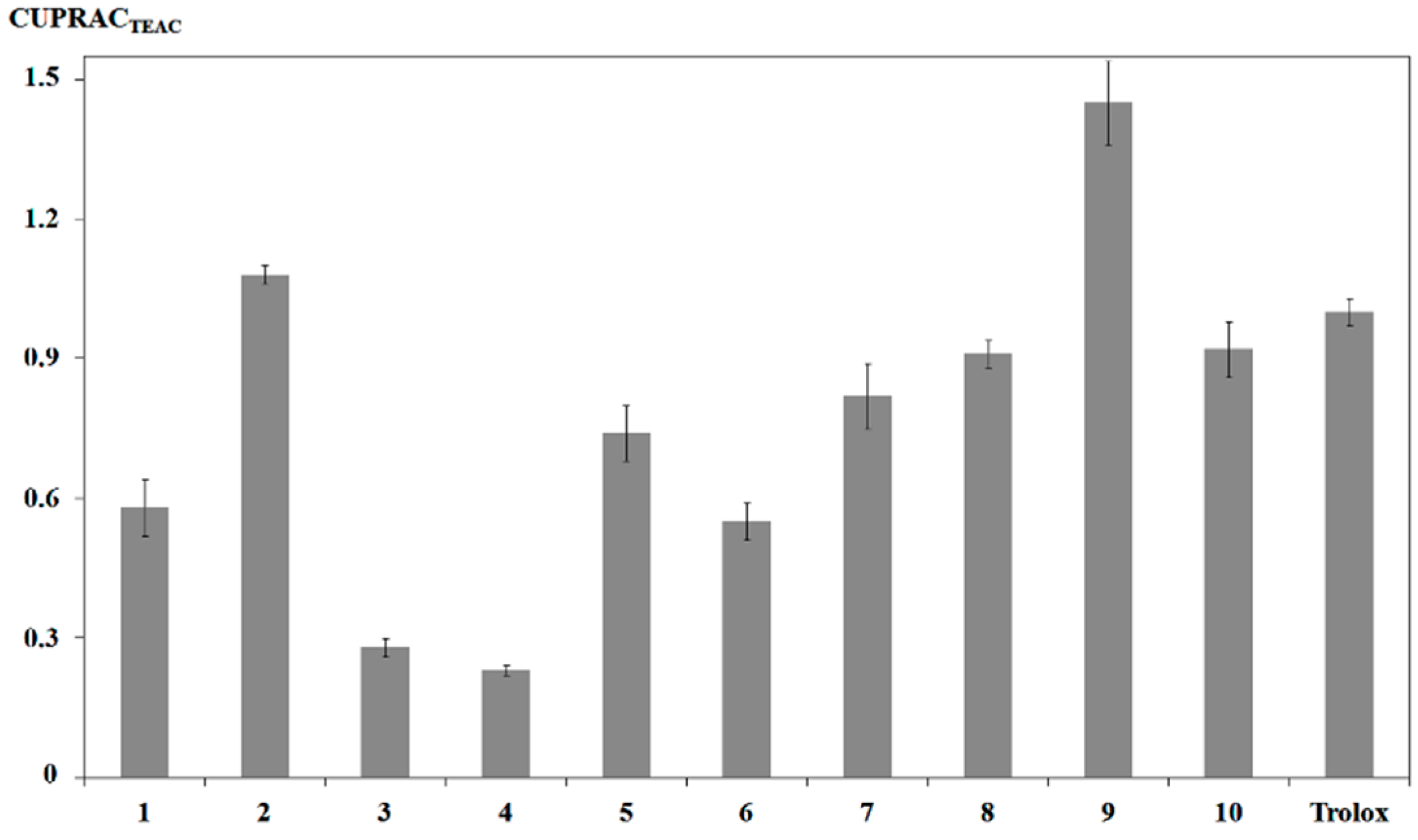
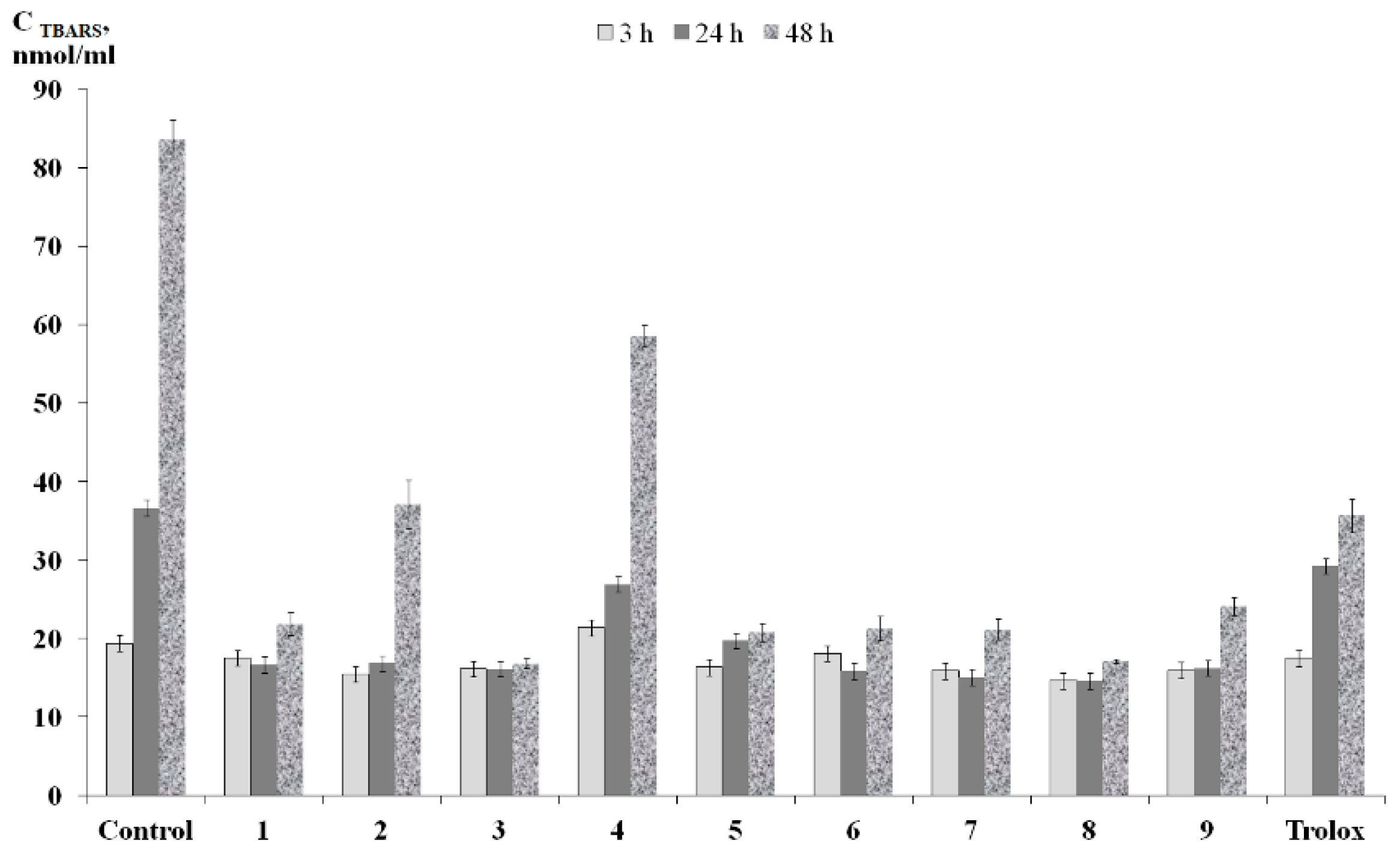
2.4. Radical Scavenging and Antioxidant Activity
3. Materials and Methods
3.1. General
3.2. Spectroscopic Studies
3.3. Single-Crystal X-ray Analysis
3.4. Synthesis and Characterization
3.5. Electrochemistry
3.6. DPPH Assay
3.7. ABTS Assay
3.8. CUPRAC Assay
3.9. Lipid Peroxidation Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Organogermanium Compounds: Theory, Experiment, and Applications; Lee, V.Y. (Ed.) Wiley: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Nikolaevskaya, E.N.; Syroeshkin, M.A.; Egorov, M.P. Organic derivatives of group 14 elements: General aspects of synthesis, modern trends, and application prospects. Mendeleev Commun. 2023, 33, 733–745. [Google Scholar] [CrossRef]
- Dasgupta, R.; Khan, S. N-heterocyclic germylenes and stannylenes: Synthesis, reactivity and catalytic application in a nutshell. Adv. Organomet. Chem. 2020, 74, 105–152. [Google Scholar] [CrossRef]
- Tschernuth, F.S.; Kostenko, A.; Stigler, S.; Gradenegger, A.; Inoue, S. A neutral germanium-centred hard and soft Lewis superacid and its unique reactivity towards hydrosilanes. Dalton Trans. 2024, 53, 74–81. [Google Scholar] [CrossRef]
- Basu, D.; Ghosh, B.; Srivastava, D.; Patra, N.; Nayek, H.P. Mononuclear organogermanium(iv) catalysts for a [3 + 2] cycloaddition reaction. Dalton Trans. 2024, 53, 5648–5657. [Google Scholar] [CrossRef] [PubMed]
- Peddi, P.; Khan, S.; Gonnade, R.G.; Yildiz, C.B.; Majumdar, M. Intramolecular donor-stabilized tetra-coordinated germanium(iv) di-cations and their Lewis acidic properties. Chem. Sci. 2023, 14, 13755–13764. [Google Scholar] [CrossRef] [PubMed]
- Greb, L.; Ebner, F.; Ginzburg, Y.; Sigmund, L.M. Element-Ligand Cooperativity with p-Block Elements. Eur. J. Inorg. Chem. 2020, 2020, 3030–3047. [Google Scholar] [CrossRef]
- Druzhkov, N.O.; Kazakov, G.G.; Shavyrin, A.S.; Baranov, E.V.; Egorova, E.N.; Piskunov, A.V.; Abakumov, G.A. Stabilization of low valent 14 group metal complexes by 9, 10-diamidophenanthrene ligand. Inorg. Chem. Comm. 2018, 90, 92–96. [Google Scholar] [CrossRef]
- Kristinsdottir, L.; Oldroyd, N.L.; Grabiner, R.; Knights, A.W.; Heilmann, A.; Protchenko, A.V.; Niu, H.; Kolychev, E.L.; Campos, J.; Hicks, J.; et al. Synthetic, structural and reaction chemistry of N-heterocyclic germylene and stannylene compounds featuring N-boryl substituents. Dalton Trans. 2019, 48, 11951–11960. [Google Scholar] [CrossRef] [PubMed]
- Karlov, S.S.; Zaitseva, G.S.; Egorova, M.P. Tetrylenes based on tri- and tetradentate ONO-, NNO-, NNN-, and ONNO-type ligands: Synthesis, structure, and reactivity. Russ. Chem. Bull. 2019, 68, 1129–1142. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Cherepakhin, V.S.; Churakov, A.V.; Peregudov, A.S.; Tarasevich, B.N.; Egorov, M.P.; Zaitseva, G.S.; Karlov, S.S. Extending the family of stable heavier carbenes: New tetrylenes based on N,N,O-ligands. Inorg. Chim. Acta 2016, 443, 91–100. [Google Scholar] [CrossRef]
- Arsenyeva, K.V.; Klimashevskaya, A.V.; Zherebtsov, M.A.; Chegerev, M.G.; Cherkasov, A.V.; Yakushev, I.A.; Piskunov, A.V. Redox-Active Germylene Based on 2,4,6,8-Tetra-tert-butylphenoxazin-1-one: Synthesis, Structure, and Chemical Properties. Russ. J. Coord. Chem. 2022, 48, 464–477. [Google Scholar] [CrossRef]
- Kazakov, G.G.; Druzhkov, N.O.; Baranov, E.V.; Piskunov, A.V.; Cherkasov, V.K. The reactivity of N-heterocyclic germylenes and stannylenes based on 9,10-phenanthrendiimines towards metal carbonyls and sulfur. J. Organomet. Chem. 2021, 946–947, 121887. [Google Scholar] [CrossRef]
- Weyer, N.; Guthardt, R.; Correia Bicho, B.A.; Oetzel, J.; Bruhn, C.; Siemeling, U. Stable N-Heterocyclic Germylenes of the Type [Fe{(η5-C5H4)NR}2Ge] and Their Oxidation Reactions with Sulfur, Selenium, and Diphenyl Diselenide. Z. Anorg. Allg. Chem. 2019, 645, 188–197. [Google Scholar] [CrossRef]
- Arsenyeva, K.V.; Ershova, I.V.; Chegerev, M.G.; Cherkasov, A.V.; Aysin, R.R.; Lalov, A.V.; Fukin, G.K.; Piskunov, A.V. Reactivity of O,N-heterocyclic germylene and stannylene towards μ-dithiobis(tricarbonyliron). J. Organomet. Chem. 2020, 927, 121524. [Google Scholar] [CrossRef]
- Arsenyeva, K.V.; Chegerev, M.G.; Cherkasov, A.V.; Pashanova, K.I.; Ershova, I.V.; Trofimova, O.Y.; Piskunov, A.V. Facile synthesis of digermylene oxide and its reactivity towards vanadocene: The first example of Cp2V—germylene coordination. Mendeleev Commun. 2021, 31, 330–333. [Google Scholar] [CrossRef]
- Dasgupta, R.; Das, S.; Hiwase, S.; Pati, S.K.; Khan, S. N-Heterocyclic Germylene and Stannylene Catalyzed Cyanosilylation and Hydroboration of Aldehydes. Organometallics 2019, 38, 1429–1435. [Google Scholar] [CrossRef]
- Arsenyeva, K.V.; Pashanova, K.I.; Trofimova, O.Y.; Ershova, I.V.; Chegerev, M.G.; Starikova, A.A.; Cherkasov, A.V.; Syroeshkin, M.A.; Kozmenkova, A.Y.; Piskunov, A.V. O,N-Heterocyclic germylenes as efficient catalysts for hydroboration and cyanosilylation of benzaldehyde. New J. Chem. 2021, 45, 11758–11767. [Google Scholar] [CrossRef]
- Piskunov, A.V.; Aivaz’yan, I.A.; Poddel’sky, A.I.; Fukin, G.K.; Baranov, E.V.; Cherkasov, V.K.; Abakumov, G.A. New Germanium Complexes Containing Ligands Based on 4,6-Di-tert -butyl-N-(2,6-diisopropylphenyl)-o-iminobenzoquinone in Different Redox States. Eur. J. Inorg. Chem. 2008, 2008, 1435–1444. [Google Scholar] [CrossRef]
- Piskunov, A.V.; Arsenyeva, K.V.; Klimashevskaya, A.V.; Cherkasov, A.V. Heterometallic Germanium(IV) Complexes Based on the N-Phenyl-Substituted o-Amidophenolate Ligand. Russ. J. Coord. Chem. 2022, 48, 278–286. [Google Scholar] [CrossRef]
- Lado, A.V.; Piskunov, A.V.; Zhdanovich, I.V.; Fukin, G.K.; Baranov, E.V. Novel Germanium(IV) Catecholate Complexes. Russ. J. Coord. Chem. 2008, 34, 251–255. [Google Scholar] [CrossRef]
- Chegerev, M.G.; Starikova, A.A.; Piskunov, A.V.; Cherkasov, V.K. Valence Tautomerism in Main-Group Complexes? Computational Modeling of Si, Ge, Sn, and Pb Bischelates with o-Iminoquinone Ligands. Eur. J. Inorg. Chem. 2016, 2016, 252–258. [Google Scholar] [CrossRef]
- Nikolaevskaya, E.N.; Shangin, P.G.; Starikova, A.A.; Jouikov, V.V.; Egorov, M.P.; Syroeshkin, M.A. Easily electroreducible halogen-free germanium complexes with biologically active pyridines. Inorg. Chim. Acta 2019, 495, 119007. [Google Scholar] [CrossRef]
- Shangin, P.G.; Krylova, I.V.; Lalov, A.V.; Kozmenkova, A.Y.; Saverina, E.A.; Buikin, P.A.; Korlyukov, A.A.; Starikova, A.A.; Nikolaevskaya, E.N.; Egorov, M.P.; et al. Supramolecular D⋯A-layered structures based on germanium complexes with 2,3-dihydroxynaphthalene and N,N′-bidentate ligands. RSC Adv. 2021, 11, 21527–21536. [Google Scholar] [CrossRef] [PubMed]
- Gon, M.; Yaegashi, M.; Tanaka, K.; Chujo, Y. Near-Infrared Emissive Hypervalent Compounds with Germanium(IV)-Fused Azobenzene π-Conjugated Systems. Chem. Eur. J. 2023, 29, e202203423. [Google Scholar] [CrossRef] [PubMed]
- Kansuzyan, A.V.; Farafonova, S.D.; Saverina, E.A.; Krylova, I.V.; Balycheva, V.A.; Akyeva, A.Y.; Medvedev, A.G.; Nikolaevskaya, E.N.; Egorov, M.P.; Prikhodchenko, P.V.; et al. Highly soluble germanium dioxide as a new source of germanium for derivatization with organic compounds. Mendeleev Commun. 2022, 32, 25–27. [Google Scholar] [CrossRef]
- Roth, D.; Wadepohl, H.; Greb, L. Bis(perchlorocatecholato)germane: Hard and Soft Lewis Superacid with Unlimited Water Stability. Angew. Chem. Int. Ed. 2020, 59, 20930–20934. [Google Scholar] [CrossRef]
- Glavinović, M.; Krause, M.; Yang, L.; McLeod, J.A.; Liu, L.; Baines, K.M.; Friščić, T.; Lumb, J.P. A chlorine-free protocol for processing germanium. Sci. Adv. 2017, 3, e1700149. [Google Scholar] [CrossRef] [PubMed]
- Akbulatov, A.F.; Akyeva, A.Y.; Shangin, P.G.; Emelianov, N.A.; Krylova, I.V.; Markova, M.O.; Labutskaya, L.D.; Mumyatov, A.V.; Tuzharov, E.I.; Bunin, D.A.; et al. Sn and Ge Complexes with Redox-Active Ligands as Efficient Interfacial Membrane-like Buffer Layers for p-i-n Perovskite Solar Cells. Membranes 2023, 13, 439. [Google Scholar] [CrossRef] [PubMed]
- Saverina, E.A.; Kapaev, R.R.; Stishenko, P.V.; Galushko, A.S.; Balycheva, V.A.; Ananikov, V.P.; Egorov, M.P.; Jouikov, V.V.; Troshin, P.A.; Syroeshkin, M.A. 2-Carboxyethylgermanium Sesquioxide as A Promising Anode Material for Li-Ion Batteries. ChemSusChem. 2020, 13, 3137–3146. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.T.; Cosby, T.P.L.; Boyle, P.D.; Baines, K.M. Selective dimerization of α-methylstyrene by tunable bis(catecholato)germane Lewis acid catalysts. Dalton Trans. 2021, 50, 15906–15913. [Google Scholar] [CrossRef] [PubMed]
- Basu, D.; Pada, H. Nayek Bis(catecholato)germane: An effective catalyst for Friedel–Crafts alkylation reaction. Dalton Trans. 2022, 51, 10587–10594. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.T.; Nanan, D.A.R.; Baines, K.M. Expanding the scope of bis(catecholato)germane catalysis: Hydrosilylation, hydroboration, Friedel–Crafts alkylation and oligomerization. Dalton Trans. 2023, 52, 10363–10371. [Google Scholar] [CrossRef] [PubMed]
- Lukevics, E.; Ignatovich, L. Chapter 15. Biological Activity of Organogermanium Compounds. In Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: The Use of Metals in Medicine; Gielen, M., Tieknik, E.R.T., Eds.; J. Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 279–295. [Google Scholar] [CrossRef]
- Menchikov, L.G.; Ignatenko, M.A. Molecular-biological problems of drug design and mechanism of drug action biological activity of organogermanium compounds (a review). Pharm. Chem. J. 2013, 46, 835–838. [Google Scholar] [CrossRef]
- Kadomtseva, A.V.; Mochalov, G.M.; Kuzina, O.V. Biologically Active Coordination Compounds of Germanium. Synthesis and Physicochemical Properties. Russ. J. Org. Chem. 2021, 57, 879–888. [Google Scholar] [CrossRef]
- Menchikov, L.G.; Popov, A.V. Physiological Activity of Trace Element Germanium including Anticancer Properties. Biomedicines 2023, 11, 1535. [Google Scholar] [CrossRef] [PubMed]
- Narokha, V.; Nizhenkovska, I.; Kuznetsova, O. Potential of germanium-based compounds in coronavirus infection. Acta Pharm. 2022, 72, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Milaeva, E.R.; Shpakovsky, D.B.; Gracheva, Y.A.; Antonenko, T.A.; Ksenofontova, T.D.; Nikitin, E.A.; Berseneva, D.A. Novel selective anticancer agents based on Sn and Au complexes. Mini-review. Pure Appl. Chem. 2020, 92, 1201–1216. [Google Scholar] [CrossRef]
- Barbanente, A.; Ditaranto, N.; Laghezza, A.; Tortorella, P.; Intini, F.P.; Pacifico, C.; Natilea, G.; Margiotta, N. Cisplatin and zoledronic acid: Two drugs combined in a Pt(II) complex with potential antitumor activity towards bone tumors and metastases. Dalton Trans. 2023, 52, 6117–6128. [Google Scholar] [CrossRef]
- Lim, D.H.; Li, M.; Seo, J.-A.; Lim, K.-M.; Ham, S.W. A novel organogermanium protected atopic dermatitis induced by oxazolone. Bioorg. Med. Chem. Lett. 2010, 20, 4032–4034. [Google Scholar] [CrossRef]
- Lim, D.H.; Li, M.; Kim, E.-h.; Ham, S.W. Synthesis of Novel Organogermanium Derivative Conjugated with Vitamin C and Study of its Antioxidant Effects. Bull. Korean Chem. Soc. 2010, 31, 1839–1840. [Google Scholar] [CrossRef]
- Jiang, J.; Yao, S.; Cai, H.-H.; Yang, P.-H.; Cai, J. Synthesis and synergetic effects of chrysin–organogermanium(IV) complex as potential anti-oxidant. Bioorg. Med. Chem. Lett. 2013, 23, 5727–5732. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-P.; Xie, W.-L.; Cai, H.-H.; Cai, J.-Y.; Yang, P.-H. Hydroxyl radical scavenging mechanism of human erythrocytes by quercetin–germanium(IV) complex. Eur. J. Pharm. Sci. 2012, 47, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Yao, S.; Cai, J.; Yang, P.-h. Synthesis and synergetic anti-tumor activity evaluation of dihydroartemisininorganogermanium(IV) compound. Bioorg. Med. Chem. Lett. 2014, 24, 5294–5297. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Zeng, J.; Luo, J.-J.; Yang, P.-H.; Cai, J.-Y. Synthesis and biological evaluation of Germanium(IV)–polyphenol complexes as potential anti-cancer agents. Bioorg. Med. Chem. Lett. 2013, 23, 2902–2908. [Google Scholar] [CrossRef]
- Nakamura, T.; Shimada, Y.; Takeda, T.; Sato, K.; Akiba, M.; Fukaya, H. Organogermanium compound, Ge-132, forms complexes with adrenaline, ATP and other physiological cis-diol compounds. Future Med. Chem. 2015, 7, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Karpenko, R.G.; Krylova, I.V.; Kamernitskii, A.V. Germylated steroids. 3. Synthesis of trialkylgermylated steroids. Russ. Chem. Bull. 2011, 60, 2100–2102. [Google Scholar] [CrossRef]
- Vishtorskaya, A.A.; Saverina, E.A.; Pechennikov, V.M.; Krylova, I.V.; Lalov, A.V.; Syroeshkin, M.A.; Egorov, M.P.; Jouikov, V.V. Assessing Ge-132 as an antioxidant in organic and water-containing media. J. Organomet. Chem. 2018, 858, 8–13. [Google Scholar] [CrossRef]
- Riviere, P.; Castel, A.; Satge, J.; Guyot, D. Cycloaddition of germylenes to 3,5-di-tert-butyl ortoquinone. J. Organomet. Chem. 1986, 315, 157–164. [Google Scholar] [CrossRef]
- Riviere, P.; Castel, A.; Ko, Y.H.; Desor, D. Etude de la reaction de germyllithiums sur la di-t-butyl-3,5-orthoquinone: Mise en evidence d’un mecanisme par transfert monoélectronique. J. Organomet. Chem. 1990, 386, 147–156. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef]
- Del Rio, N.; Baceiredo, A.; Saffon-Merceron, N.; Hashizume, D.; Lutters, D.; Müller, T.; Kato, T. A Stable Heterocyclic Amino(phosphanylidene-σ4-phosphorane)Germylene. Angew. Chem. Int. Ed. 2016, 55, 4753–4758. [Google Scholar] [CrossRef]
- Schäfer, H.; Saak, W.; Weidenbruch, M. Azadigermiridines by Addition of Diazomethane or Trimethylsilyldiazomethane to a Digermene. Organometallics 1999, 18, 3159–3163. [Google Scholar] [CrossRef]
- Mohapatra, C.; Scharf, L.T.; Scherpf, T.; Mallick, B.; Feichtner, K.-S.; Schwarz, C.; Gessner, V.H. Isolation of a Diylide-Stabilized Stannylene and Germylene: Enhanced Donor Strength through Coplanar Lone Pair Alignment. Angew. Chem. Int. Ed. 2019, 58, 7459–7463. [Google Scholar] [CrossRef]
- Wolff, B.; Weiss, A. Novel Octahedral Si and Ge Complexes with a Hexadentate Diphenol Ligand. Angew. Chem. Int. Ed. 1986, 25, 162–163. [Google Scholar] [CrossRef]
- Tacke, R.; Stewart, A.; Becht, J.; Burschka, C.; Richter, I. Di[(hydroxyalkyl)dimethylammonium] tris[benzene-1,2-diolato(2–)]silicates and their germanium analogs: Syntheses, crystal structure analyses, and NMR studies. Can. J. Chem. 2000, 78, 1380–1387. [Google Scholar] [CrossRef]
- Mugridge, J.S.; Fiedler, D.; Raymond, K.N. A ferrocene-based catecholamide ligand: The consequences of ligand swivel for directed supramolecular self-assembly. J. Coord. Chem. 2010, 63, 2779–2789. [Google Scholar] [CrossRef]
- Davis, A.V.; Firman, T.K.; Hay, B.P.; Raymond, K.N. d-Orbital Effects on Stereochemical Non-Rigidity: Twisted TiIV Intramolecular Dynamics. J. Am. Chem Soc. 2006, 128, 9484–9496. [Google Scholar] [CrossRef] [PubMed]
- Baramov, T.; Keijzer, K.; Irran, E.; Mçsker, E.; Baik, M.-H.; Süssmuth, R. Synthesis and Structural Characterization of Hexacoordinate Silicon, Germanium, and Titanium Complexes of the E. coli Siderophore Enterobactin. Chem. Eur. J. 2013, 19, 10536–10542. [Google Scholar] [CrossRef] [PubMed]
- Nikolaevskaya, E.N.; Saveriva, E.A.; Starikova, A.A.; Farhati, A.; Kiskin, M.A.; Syroeshkin, M.A.; Egorov, M.P.; Jouikov, V.V. Halogen-free GeO2 conversion: Electrochemical reduction vs. complexation in (DTBC)2Ge[Py(CN)n] (n = 0…2) complexes. Dalton Trans. 2018, 17127–17133. [Google Scholar] [CrossRef]
- Holmes, R.R.; Day, R.O.; Sau, A.C.; Poutasse, C.A.; Holmes, J.M. Pentacoordinated molecules. 64. Synthesis and molecular structure of five-coordinated phenyl-substituted anionic germanium(IV) complexes. Influence of the central atom on geometry. Inorg. Chem. 1986, 25, 607–611. [Google Scholar] [CrossRef]
- Chen, K.-H.; Liu, Y.-H.; Chiu, C.-W. A Non-innocent Ligand Supported Germylene and Its Diverse Reactions. Organometallics 2020, 39, 4645–4650. [Google Scholar] [CrossRef]
- Nanjo, M.; Goto, M.; Nakashima, Y. Synthesis and structure of hypercoordinated germanate complexes with naphthalene-2,3-dialkoxide ligands. Inorg. Chim. Acta 2021, 528, 120608. [Google Scholar] [CrossRef]
- Nanjo, M.; Yoneda, T.; Iwamatsu, K. Hypercoordinate germanium complexes with phenanthrene-9,10-diolate ligands: Synthesis, structure, and electronic properties. Mendeleev Commun. 2022, 32, 12–15. [Google Scholar] [CrossRef]
- Christ, J.; Epps, C.; Pritchard, V.; Schmeh, D.; Pierpont, C.; Nordlander, E. Synthesis and Reactivity of Catecholate Complexes Containing Quadruply Bonded Metal Ions. Inorg. Chem. 2010, 49, 2029–2031. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Nordlander, E.; Schmeh, D.; Shoemaker, R.; Pierpont, C.G. Coordination Complexes of Molybdenum with 3,6-Di-tert-butylcatechol. Addition Products of DMSO, Pyridine N-oxide, and Triphenylarsine Oxide to the Putative [MoVIO(3,6-DBCat)2] Monomer and Self-Assembly of the Chiral [{MoVIO(3,6-DBCat)2}4] Square. Inorg. Chem. 2004, 43, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Sarkar, B.; Kumbhakar, D.; Mondal, T.K.; Mobin, S.M.; Fiedler, J.; Urbanos, F.A.; Jimenez-Aparicio, R.; Kaim, W.; Lahiri, G.K. Bis(acetylacetonato)ruthenium Complexes of Noninnocent 1,2-Dioxolene Ligands: Qualitatively Different Bonding in Relation to Monoimino and Diimino Analogues. Chem. Eur. J. 2011, 17, 11030. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Restorp, P.; Nordlander, E.; Pierpont, C.G. Oxo-deficient dioxylene complexes of Mo(VI) containing 3,6-di-tert-butylcatechol. Chem. Commun. 2001, 2686–2687. [Google Scholar] [CrossRef]
- Scherer, T.M.; Hartenbach, I.; Lissner, F.; Schwederski, B.; Hübner, R.; Fiedler, J.; Záliš, S.; Sarkar, B.; Kaim, W. Analysis of Multiple Redox Sites in Complexes [M(C5Me5)(Q)(NO)]n, M=Ru or Os, Q = o-Quinones. Z. Anorg. Allg. Chem. 2021, 647, 867–875. [Google Scholar] [CrossRef]
- Klementyeva, S.V.; Smolentsev, A.I.; Abramov, P.A.; Konchenko, S.N. Yttrium 3,5-di-tert-butyl-catecholates supported by 2,6-diisopropylphenyl substituted β-diketiminate. Inorg. Chem. Commun. 2017, 86, 154–158. [Google Scholar] [CrossRef]
- Astaf’eva, T.V.; Rumyantcev, R.V.; Arsenyev, M.V.; Fukin, G.K.; Cherkasov, V.K.; Poddel’sky, A.I. 1D Coordination polymers based on triphenylantimony(V) 3-formyl-substituted catecholates. J. Organometal. Chem. 2022, 958, 122190. [Google Scholar] [CrossRef]
- Okhlopkova, L.S.; Poddel’sky, A.I.; Smolyaninov, I.V.; Fukin, G.K.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Triphenylantimony(V) Catecholato Complexes with 4-(2,6-Dimethylphenyliminomethyl)pyridine. Structure, Redox Properties: The Influence of Pyridine Ligand. J. Organometal. Chem. 2019, 897, 32–41. [Google Scholar] [CrossRef]
- Nishio, M. CH/π hydrogen bonds in crystals. Cryst. Eng. Commun. 2004, 6, 130–158. [Google Scholar] [CrossRef]
- Mantina, M.; Chamberlin, A.C.; Valero, R.; Cramer, C.J.; Truhlar, D.G. Consistent van der Waals radii for the whole main group. J. Phys. Chem. A 2009, 113, 5806–5812. [Google Scholar] [CrossRef] [PubMed]
- Piskunov, A.V.; Pashanova, K.I.; Fukin, G.K.; Bogomyakov, A.S.; Smolyaninov, I.V.; Berberova, N.T. Copper(II) complexes bearing o-iminosemiquinonate ligands with augmented aromatic substituents. Polyhedron 2016, 119, 286–292. [Google Scholar] [CrossRef]
- Piskunov, A.V.; Trofimova, O.Y.; Fukin, G.K.; Ketkov, S.Y.; Smolyaninov, I.V.; Cherkasov, V.K. Tin(IV) and lead(IV) complexes with a tetradentate redox-active ligand. Dalton Trans. 2012, 41, 10970–10979. [Google Scholar] [CrossRef] [PubMed]
- Baryshnikova, S.V.; Bellan, E.V.; Poddel’sky, A.I.; Arsenyev, M.V.; Fukin, G.K.; Piskunov, A.V.; Cherkasov, V.K.; Abakumov, G.A.; Smolyaninov, I.V.; Berberova, N.T. Tin(IV) and antimony(V) complexes bearing catecholate ligands connected to ferrocene—Syntheses, molecular structures, and electrochemical properties. Eur. J. Inorg. Chem. 2016, 2016, 5230–5241. [Google Scholar] [CrossRef]
- Ershova, I.V.; Meshcheryakova, I.N.; Trofimova, O.Y.; Pashanova, K.I.; Arsenyeva, K.V.; Khamaletdinova, N.M.; Smolyaninov, I.V.; Arsenyev, M.V.; Cherkasov, A.V.; Piskunov, A.V. Complexes of Metal Halides with Unreduced o-(Imino)quinones. Inorg. Chem. 2021, 60, 12309–12322. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Fukin, G.K.; Cherkasov, V.K.; Berberova, N.; Abakumov, G.A. Triaryl/trialkylantimony(V) catecholates with electron-acceptor groups. J. Organomet. Chem. 2015, 789–790, 8–13. [Google Scholar] [CrossRef]
- Baryshnikova, S.V.; Poddel’sky, A.I.; Bellan, E.V.; Smolyaninov, I.V.; Cherkasov, A.V.; Fukin, G.K.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Ferrocene-Containing Tin(IV) Complexes Based on o-Benzoquinone and o-Iminobenzoquinone Ligands. Synthesis, Molecular Structure, and Electrochemical Properties. Inorg. Chem. 2020, 59, 6774–6784. [Google Scholar] [CrossRef] [PubMed]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Druzhkov, N.O.; Fukin, G.K. Heterometallic antimony(V)-zinc and antimony(V)-copper complexes comprising catecholate and diazadiene as redox active centers. J. Organomet. Chem. 2021, 952, 121994. [Google Scholar] [CrossRef]
- Okhlopkova, L.S.; Poddel’sky, A.I.; Smolyaninov, I.V. Heterometallic Complexes Based on Triphenylantimony(V) Quinone-Catecholate. Russ. J. Coord. Chem. 2020, 46, 762–771. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Poddel’sky, A.I.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Electrochemical transformations of catecholate and o-amidophenolate complexes with triphenylantimony(V). Russ. J. Coord. Chem. 2010, 36, 644–650. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Fukin, G.K.; Berberova, N.T.; Poddel’sky, A.I. Triphenylantimony(V) Catecholates of the type (3-RS-4,6-DBCat)SbPh3—Catechol Thioether Derivatives: Structure, Electrochemical Properties and Antiradical Activity. Molecules 2021, 26, 2171. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Poddel’sky, A.I.; Smolyaninova, S.A.; Arsenyev, M.V.; Fukin, G.K.; Berberova, N.T. Polyfunctional sterically hindered catechols with additional phenolic group and their triphenylantimony(V) catecholates: Synthesis, structure, and redox properties. Molecules 2020, 25, 1770. [Google Scholar] [CrossRef] [PubMed]
- Filonova, G.E.; Nikolaevskaya, E.N.; Kansuzyan, A.V.; Krylova, I.V.; Egorov, M.P.; Jouikov, V.V.; Syroeshkin, M.A. Antioxidant Properties of Adrenaline in the Presence of Ge-132. Eur. J. Org. Chem. 2019, 2019, 4128–4132. [Google Scholar] [CrossRef]
- Nikolaevskaya, E.N.; Kansuzyan, A.V.; Filonova, G.E.; Zelenova, V.A.; Pechennikov, V.M.; Krylova, I.V.; Egorov, M.P.; Jouikov, V.V.; Syroeshkin, M.A. Germanium Dioxide and the Antioxidant Properties of Catechols. Eur. J. Inorg. Chem. 2019, 2019, 676–681. [Google Scholar] [CrossRef]
- Okhlopkova, L.S.; Poddel’sky, A.I.; Smolyaninov, I.V.; Baranov, E.V. Mononuclear Antimony(V) Catecholate Complexes with Additional Pyridine Ligands. Russ. J. Coord. Chem. 2020, 46, 466–476. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Shataeva, A.I.; Baranov, E.V.; Fukin, G.K. Binuclear Triphenylantimony(V) Catecholates through N-Donor Linkers: Structural Features and Redox Properties. Molecules 2022, 27, 6484. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Pitikova, O.V.; Poddel’sky, A.I.; Berberova, N.T. Electrochemical transformations and antiradical activity of asymmetrical RS-substituted pyrocatechols. Russ. Chem. Bull. 2018, 67, 1857–1867. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Antonova, N.A.; Poddel’sky, A.I.; Smolyaninova, S.A.; Osipova, V.P.; Berberova, N.T. Radical scavenging activity of sterically hindered catecholate and o-amidophenolate complexes of LSbVPh3 type. J. Organomet. Chem. 2011, 696, 2611–2620. [Google Scholar] [CrossRef]
- Li, L.; Ruan, T.; Lyu, Y.; Wu, B. Advances in Effect of Germanium or Germanium Compounds on Animals—A Review. J. Biosci. Med. 2017, 5, 56–73. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Antonova, N.A.; Poddel’sky, A.I.; Osipova, V.P.; Kolyada, M.N.; Berberova, N.T. The Influence of Ph3Sb(V)L Complexes with Redox Active Ligands on Lipid Peroxidation in Vivo. Dokl. Chem. 2012, 443, 72–76. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Antonova, N.A.; Poddel’sky, A.I.; Osipova, V.P.; Berberova, N.T.; Pimenov, Y.T. The influence of some triphenylantimony(V) catecholates and o-amidophenolates on lipid peroxidation in vitro. Appl. Organomet. Chem. 2012, 26, 277–283. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Poddel’skii, A.I.; Antonova, N.A.; Smolyaninova, S.A.; Berberova, N.T. Antiradical Activity of Morpholine and Piperazine Functionalized Triphenylantimony(V) Catecholates. Russ. J. Coord. Chem. 2013, 39, 165–174. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Poddel’sky, A.I.; Smolyaninova, S.A.; Luzhnova, S.A.; Berberova, N.T. Anti and prooxidant activity of triphenylantimony(V) catecholates derived from alkyl gallates. Russ. Chem. Bull. 2015, 64, 2223–2231. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Pitikova, O.V.; Korchagina, E.O.; Poddel’sky, A.I.; Fukin, G.K.; Luzhnova, S.A.; Tichkomirov, A.M.; Ponomareva, E.N.; Berberova, N.T. Catechol thioethers with physiologically active fragments: Electrochemistry, antioxidant and cryoprotective activities. Bioorg. Chem. 2019, 89, 103003. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Burmistrova, D.A.; Pomortseva, N.P.; Poddel’sky, A.I.; Berberova, N.T. Antioxidant Activity of Catechol Thioethers with Heterocyclic Moieties in Reactions with Radical Promoters. Dokl. Chem. 2022, 504, 100–105. [Google Scholar] [CrossRef]
- Perrin, D.D.; Armarego, W.L.F.; Perrin, D.R. Purification of Laboratory Chemicals; Pergamon: Oxford, UK, 1980. [Google Scholar]
- Sheldrick, G.M. SADABS; Bruker AXS Inc.: Madison, WI, USA, 1997. [Google Scholar]
- Sheldrick, M. SHELXT 2014/4 (Sheldrick, 2014).
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanism of antioxidant activity using the DPPH free radical method. Food. Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Re, R.; Pellergrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Başkan, K.S.; Erçağ, E.; Çelik, S.E.; Baki, S.; Yıldız, L.; Karaman, S.; Apak, R. A comprehensive review of CUPRAC methodology. Anal. Methods 2011, 3, 2439–2453. [Google Scholar] [CrossRef]
- Stroev, E.N.; Makarova, V.G. Praktikum Po Biologiheskoy Khimii [Practical Work in Biological Chemistry]; Vushaya Shkola: Moscow, Russia, 1986. [Google Scholar]
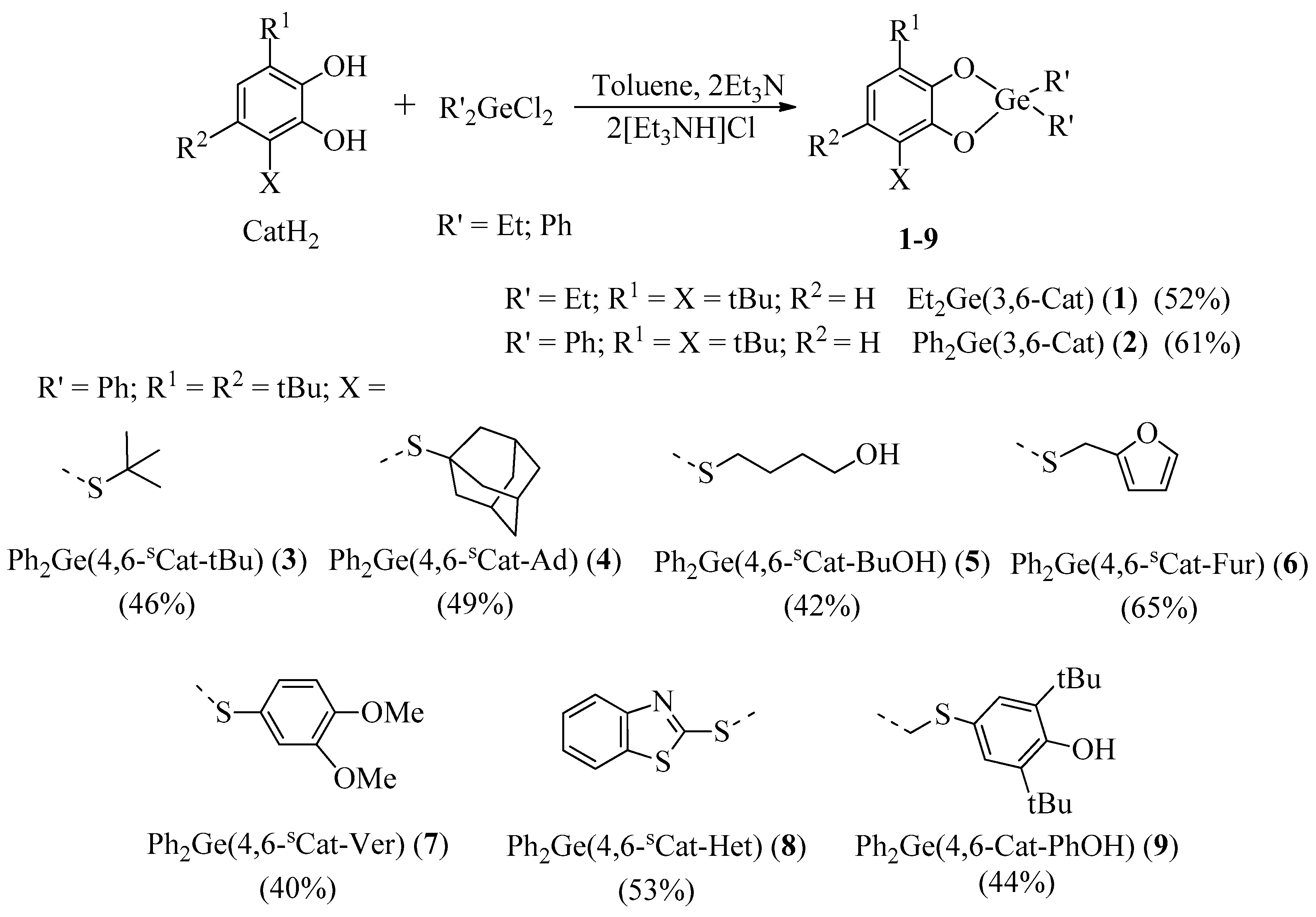
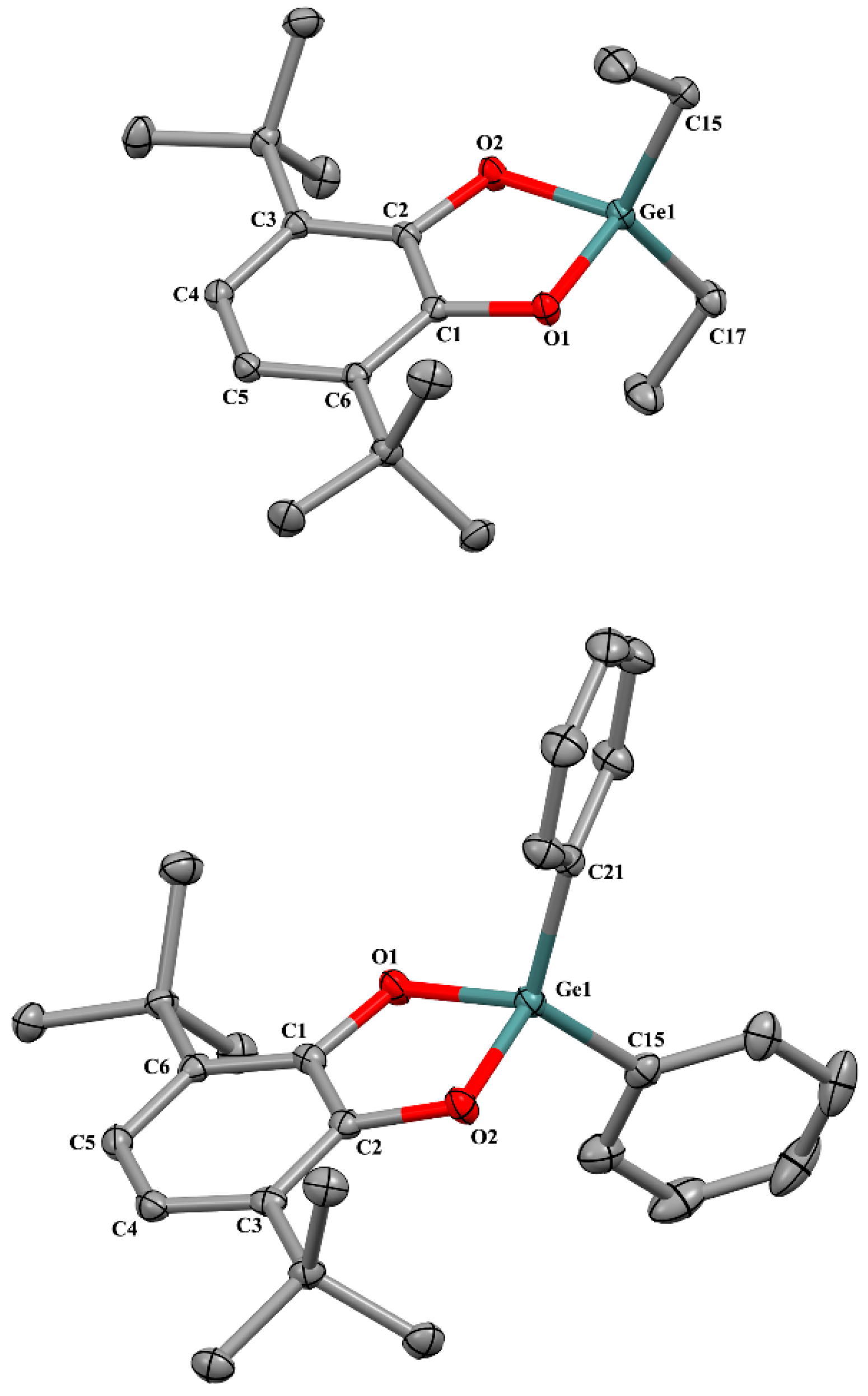
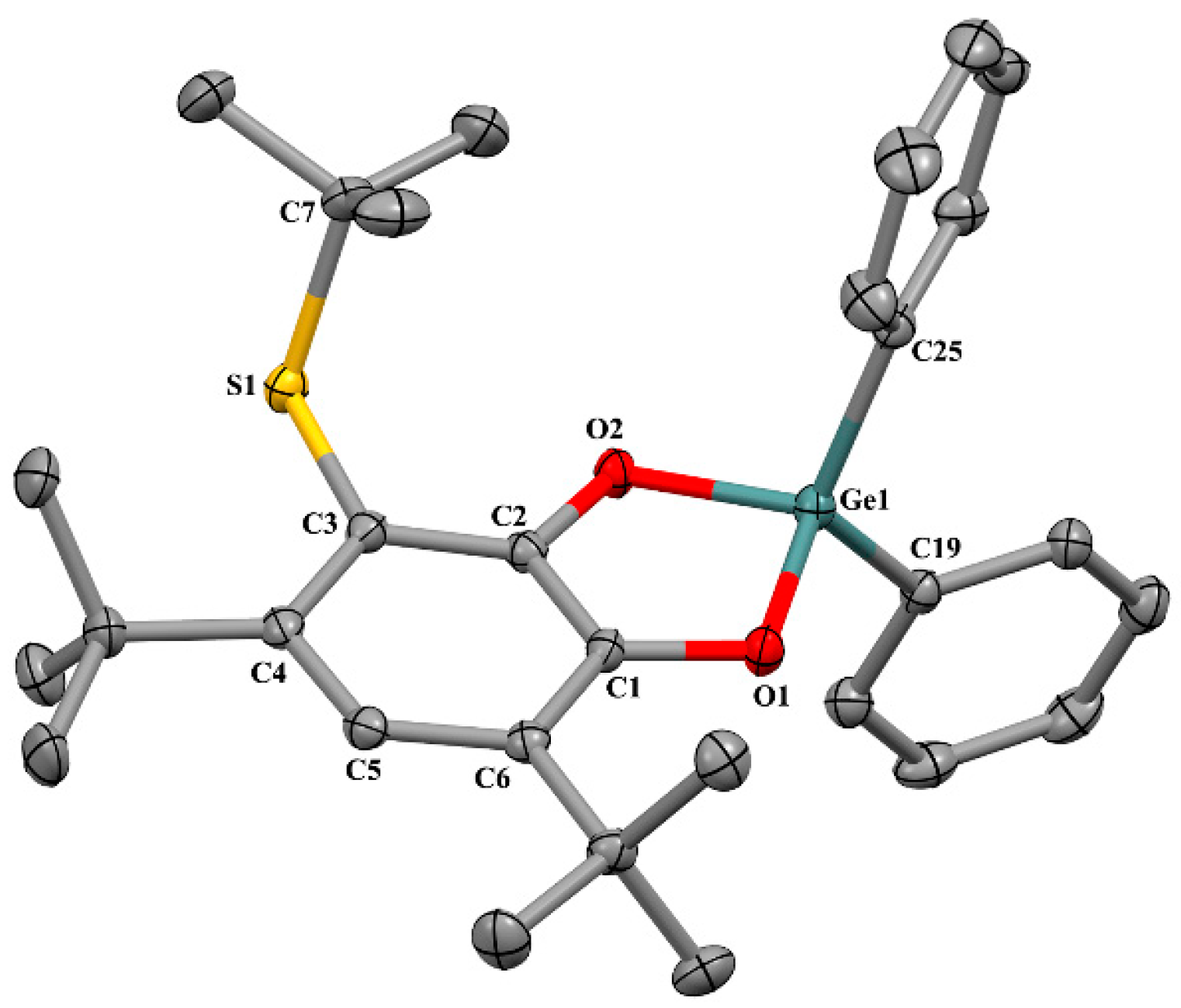

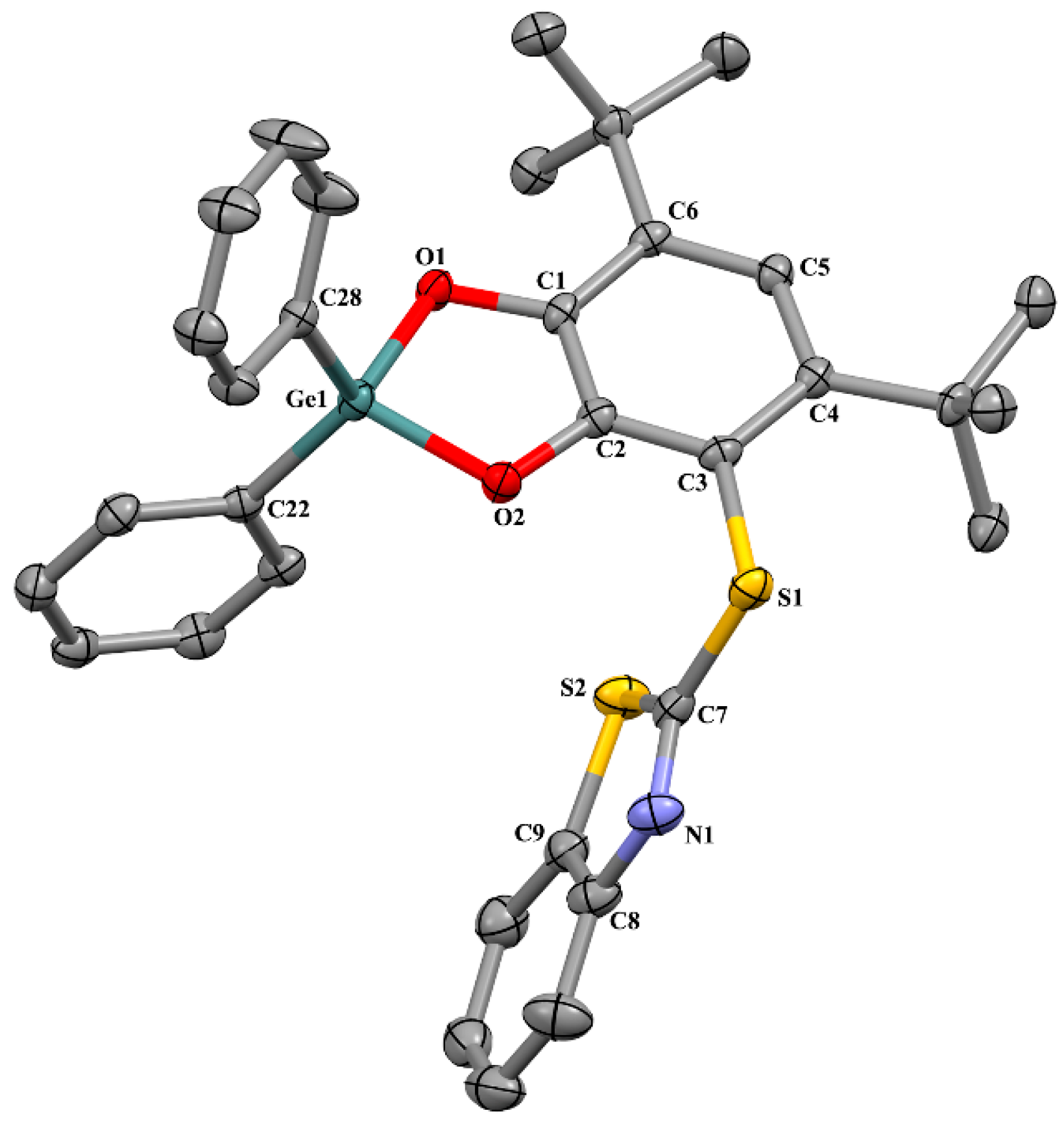



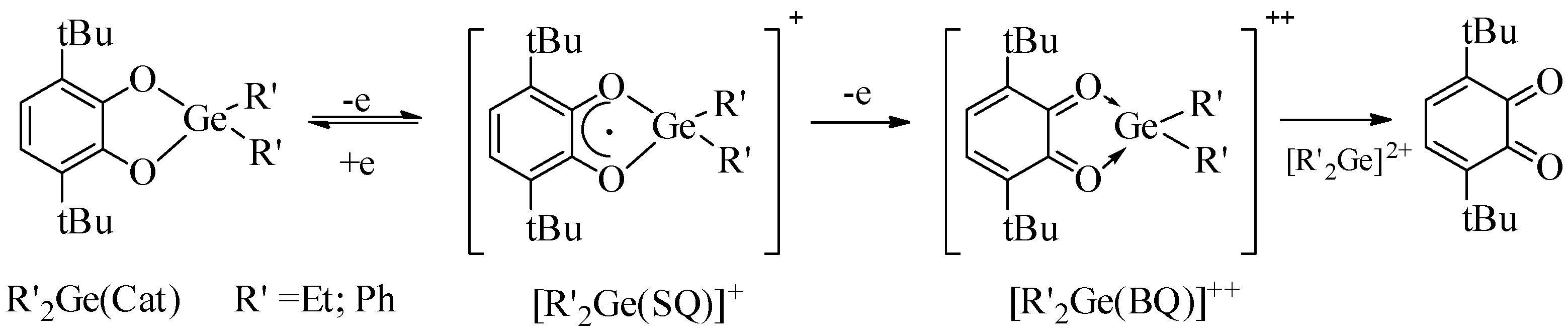



| Bond | 1 | 2 * | 3 | 6 ** | 8 *, *** |
|---|---|---|---|---|---|
| Ge1–O1 | 1.8130(8) | 1.801(1)/1.800(1) | 1.805(1) | 1.800(1) | 1.816(2)/1.812(2) |
| Ge1–O2 | 1.8099(9) | 1.802(1)/1.804(1) | 1.813(2) | 1.812(1) | 1.817(2)/1.815(2) |
| Ge1–C | Ge1–C15 1.928(1) | Ge1–C15 1.913(2)/1.922(2) | Ge1–C19 1.913(2) | Ge1–C20 1.912(2) | Ge1–C22 1.910(2)/1.914(3) |
| Ge1–C17 1.930(1) | Ge1–C21 1.909(2)/1.924(2) | Ge1–C25 1.916(2) | Ge1–C26 1.918(2) | Ge1–C28 1.916(3)/1.915(2) | |
| O1–C1 | 1.378(1) | 1.376(2)/1.387(2) | 1.387(2) | 1.385(2) | 1.382(2)/1.380(3) |
| O2–C2 | 1.379(1) | 1.384(2)/1.388(2) | 1.371(2) | 1.374(2) | 1.372(3)/1.365(3) |
| C1–C2 | 1.406(2) | 1.407(3)/1.401(3) | 1.406(2) | 1.406(2) | 1.401(4)/1.401(3) |
| C2–C3 | 1.399(2) | 1.395(2)/1.397(2) | 1.402(3) | 1.394(3) | 1.390(3)/1.397(3) |
| C3–C4 | 1.400(2) | 1.397(2)/1.396(3) | 1.424(2) | 1.413(3) | 1.424(3)/1.418(4) |
| C4–C5 | 1.392(2) | 1.388(3)/1.389(3) | 1.403(2) | 1.400(2) | 1.390(4)/1.399(3) |
| C5–C6 | 1.399(2) | 1.396(2)/1.397(2) | 1.410(3) | 1.404(3) | 1.405(3)/1.401(3) |
| C1–C6 | 1.401(2) | 1.396(2)/1.394(2) | 1.390(2) | 1.384(3) | 1.393(3)/1.389(3) |
| S1–C3 | - | - | 1.786(2) | 1.778(2) | 1.781(3)/1.784(2) |
| S1–C7 | - | - | 1.875(2) | 1.827(2) | 1.747(2)/1.748(2) |
| № | Compound | Eox11/2, V | Ic/Ia | Eox2p, V | Eox3p, V |
|---|---|---|---|---|---|
| 1 | Et2Ge(3,6-Cat) | 1.03 | 1.0 | 1.46 | - |
| 2 | Ph2Ge(3,6-Cat) | 1.13 | 0.6 | 1.45 | - |
| 3 | Ph2Ge(4,6-sCat-tBu) | 1.15 | 0.4 | 1.47 | 1.78 |
| 4 | Ph2Ge(4,6-sCat-Ad) | 1.17 | 0.6 | 1.45 | 1.86 |
| 5 | Ph2Ge(4,6-sCat-BuOH) | 1.08 | 0.8 | 1.38 | 1.70 |
| 6 | Ph2Ge(4,6-sCat-Fur) | 1.23 * | - | 1.43 | 1.82 |
| 7 | Ph2Ge(4,6-sCat-Ver) | 1.18 | 0.5 | 1.42 ** | 1.72 |
| 8 | Ph2Ge(4,6-sCat-Het) | 1.22 * | - | 1.48 | - |
| 9 | Ph2Ge(4,6-Cat-PhOH) | 1.12 * | - | 1.51 | - |
| 10 | Ph2Ge(3,5-Cat) | 1.14 | 0.6 | 1.36 | - |
| a Ph3Sb(4,6-Cat-PhOH) | 0.89 | 0.8 | 1.39 | - | |
| a Ph3Sb(4,6-sCat-Bu) | 0.94 | 0.7 | 1.40 | 1.69 | |
| a Ph3Sb(3,6-Cat) | 0.89 | 0.8 | 1.40 | - |
| N | Compound | IC50 (DPPH), µM | TEC50, Min | IC50 (ABTS+), µM | ABTSTEAC |
|---|---|---|---|---|---|
| 1 | Et2Ge(3,6-Cat) | 80.2 ± 2.7 | 55 | 115.5 ± 3.2 | 0.20 ± 0.02 |
| 2 | Ph2Ge(3,6-Cat) | 59.0 ± 0.5 | 60 | 37.3 ± 0.8 | 0.39 ± 0.09 |
| 3 | Ph2Ge(4,6-sCat-tBu) | 60.1 ± 0.9 | 37 | 113.0 ± 4.5 | 0.21 ± 0.01 |
| 4 | Ph2Ge(4,6-sCat-Ad) | 178.0 ± 3.6 | 120 | 76.6 ± 1.8 | 0.33 ± 0.01 |
| 5 | Ph2Ge(4,6-sCat-BuOH) | 76.4 ± 1.0 | 80 | 32.0 ± 1.5 | 0.54 ± 0.04 |
| 6 | Ph2Ge(4,6-sCat-Fur) | 85.6 ± 3.2 | 110 | 60.3 ± 1.9 | 0.29 ± 0.03 |
| 7 | Ph2Ge(4,6-sCat-Ver) | 156.0 ± 4.2 | 180 | 34.1 ± 0.7 | 0.52 ± 0.03 |
| 8 | Ph2Ge(4,6-sCat-Het) | 12.0 ± 0.5 | 57 | 29.2 ± 0.9 | 0.58 ± 0.02 |
| 9 | Ph2Ge(4,6-Cat-PhOH) | 7.5 ± 0.3 | 50 | 35.2 ± 0.5 | 0.42 ± 0.08 |
| 10 | Ph2Ge(3,5-Cat) | 130.4 ± 2.0 | 150 | 45.2 ± 0.9 | 0.35 ± 0.03 |
| Trolox | 12.0 ± 0.50 | 10 | 16.0 ± 1.0 | 1.00 ± 0.03 | |
| a Ph3Sb(4,6-sCat-Bu) | 29.5 ± 1.20 | 170 | - | - | |
| a Ph3Sb(3,6-Cat) | 17.3 ± 0.50 | 60 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burmistrova, D.A.; Pomortseva, N.P.; Voronina, Y.K.; Kiskin, M.A.; Dolgushin, F.M.; Berberova, N.T.; Eremenko, I.L.; Poddel’sky, A.I.; Smolyaninov, I.V. Synthesis, Structure, Electrochemical Properties, and Antioxidant Activity of Organogermanium(IV) Catecholate Complexes. Int. J. Mol. Sci. 2024, 25, 9011. https://doi.org/10.3390/ijms25169011
Burmistrova DA, Pomortseva NP, Voronina YK, Kiskin MA, Dolgushin FM, Berberova NT, Eremenko IL, Poddel’sky AI, Smolyaninov IV. Synthesis, Structure, Electrochemical Properties, and Antioxidant Activity of Organogermanium(IV) Catecholate Complexes. International Journal of Molecular Sciences. 2024; 25(16):9011. https://doi.org/10.3390/ijms25169011
Chicago/Turabian StyleBurmistrova, Daria A., Nadezhda P. Pomortseva, Yulia K. Voronina, Mikhail A. Kiskin, Fedor M. Dolgushin, Nadezhda T. Berberova, Igor L. Eremenko, Andrey I. Poddel’sky, and Ivan V. Smolyaninov. 2024. "Synthesis, Structure, Electrochemical Properties, and Antioxidant Activity of Organogermanium(IV) Catecholate Complexes" International Journal of Molecular Sciences 25, no. 16: 9011. https://doi.org/10.3390/ijms25169011
APA StyleBurmistrova, D. A., Pomortseva, N. P., Voronina, Y. K., Kiskin, M. A., Dolgushin, F. M., Berberova, N. T., Eremenko, I. L., Poddel’sky, A. I., & Smolyaninov, I. V. (2024). Synthesis, Structure, Electrochemical Properties, and Antioxidant Activity of Organogermanium(IV) Catecholate Complexes. International Journal of Molecular Sciences, 25(16), 9011. https://doi.org/10.3390/ijms25169011








