Sepsis Biomarkers: Advancements and Clinical Applications—A Narrative Review
Abstract
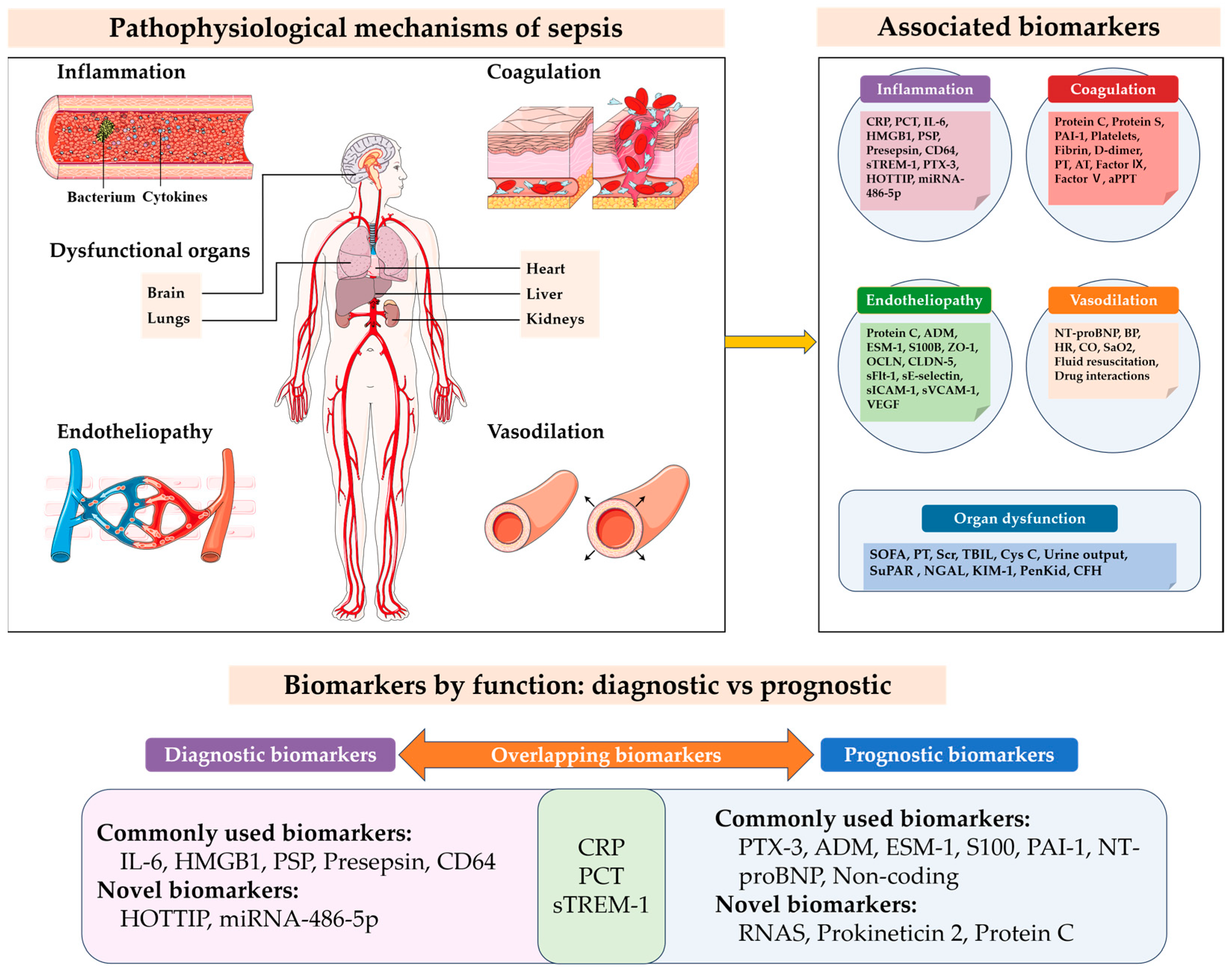
1. Introduction
2. What Is an Ideal Sepsis Biomarker?
3. Methods
3.1. Inclusion and Exclusion Criteria
3.2. Search Strategies
3.3. Study Selection
3.4. Data Collection
4. Biomarkers for Sepsis Diagnosis
4.1. Commonly Used Diagnostic Biomarkers
4.1.1. C-Reactive Protein (CRP)
4.1.2. Procalcitonin (PCT)
4.1.3. Interleukin-6 (IL-6)
4.1.4. High-Mobility Group Box 1 (HMGB1)
4.1.5. Pancreatic Stone Protein (PSP)
4.1.6. Presepsin
4.1.7. Cluster of Differentiation 64 (CD64)
4.1.8. Soluble Triggering Receptor Expressed on Myeloid Cells-1 (sTREM-1)
4.2. Novel Diagnostic Biomarkers
4.2.1. Circular RNAs (circRNAs)
4.2.2. HOXA Distal Transcript Antisense RNA (HOTTIP)
4.2.3. microRNA-486-5p
5. Biomarkers for Sepsis Prognosis
5.1. Commonly Used Prognostic Biomarkers
5.1.1. Pentraxin-3 (PTX-3)
5.1.2. Adrenomedullin (ADM)
5.1.3. Endothelial Cell-Specific Molecule-1 (ESM-1)
5.1.4. Plasminogen Activator Inhibitor-1 (PAI-1)
5.1.5. S100 Calcium-Binding Protein B (S100B)
5.1.6. N-Terminal-Pro Hormone BNP (NT-proBNP)
5.1.7. Non-Coding RNAs
5.1.8. Others
5.2. Novel Prognostic Biomarkers
5.2.1. Prokineticin 2
5.2.2. Protein C (PC)
| Biomarker | Source | Biological Function | Clinical Applications | Testing Methods | Strengths | Limitations | Refs. |
|---|---|---|---|---|---|---|---|
| Commonly used prognostic biomarkers | |||||||
| PTX-3 | Various cells (macrophages, dendritic cells) | Acute inflammatory response |
|
|
|
| [47,91,92,93,94,95] |
| ADM | Vascular smooth muscle and endothelial cells | Vasodilation, reduced endothelial permeability |
|
|
|
| [19,96,97,98,99,100] |
| ESM-1 | Endothelial cells | Regulation of angiogenesis and inflammation |
|
|
|
| [102,103,104,105] |
| PAI-1 | Various cells (endothelial cells, platelets, and adipocytes, etc.) | Inhibition of fibrinolysis |
|
|
|
| [106,107,108,109,110] |
| S100B | Glial cells | Reflects blood–brain barrier disruption and brain injury |
|
|
|
| [111,112,113,114,115] |
| NT-proBNP | Cardiac ventricular myocytes | Response to cardiac pressure changes |
|
|
|
| [116,117,118,119] |
| lncRNAs CASC2 | Various tissues | Regulation of gene expression, cell proliferation, differentiation, apoptosis |
|
|
|
| [120,121,122] |
| miRNAs | Various cells | Post-transcriptional regulation of gene expression |
|
|
|
| [84,90] |
| sPD-L1 | Immune cells and tumor cells | Immune suppression |
|
|
|
| [124,125] |
| Novel prognostic biomarkers | |||||||
| Prokineticin 2 | Various tissues (CNS, gastrointestinal tract, and immune cells). | Regulation of multiple biological processes |
|
|
|
| [131] |
| PC | Liver | Anticoagulant, regulates coagulation cascade |
|
|
|
| [20,132,133,134,135] |
6. Biomarkers for Sepsis-Associated Acute Kidney Injury (Sepsis-AKI)
6.1. Inflammatory Biomarkers
6.2. Microcirculatory Disturbance Biomarkers
7. Multi-Biomarker Approach
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Executive Summary: Surviving Sepsis Campaign: International Guidelines for the Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, 1974–1982. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann-Struzek, C.; Mellhammar, L.; Rose, N.; Cassini, A.; Rudd, K.E.; Schlattmann, P.; Allegranzi, B.; Reinhart, K. Incidence and Mortality of Hospital- and ICU-Treated Sepsis: Results from an Updated and Expanded Systematic Review and Meta-Analysis. Intensive Care Med. 2020, 46, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Gul, F.; Arslantas, M.K.; Cinel, I.; Kumar, A. Changing Definitions of Sepsis. Turk. J. Anaesthesiol. Reanim. 2017, 45, 129–138. [Google Scholar] [CrossRef]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.-L.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003, 29, 530–538. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Raith, E.P.; Udy, A.A.; Bailey, M.; McGloughlin, S.; MacIsaac, C.; Bellomo, R.; Pilcher, D.V. Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults With Suspected Infection Admitted to the Intensive Care Unit. JAMA 2017, 317, 290–300. [Google Scholar] [CrossRef]
- Polat, G.; Ugan, R.A.; Cadirci, E.; Halici, Z. Sepsis and Septic Shock: Current Treatment Strategies and New Approaches. Eurasian J. Med. 2017, 49, 53–58. [Google Scholar] [CrossRef]
- Von Groote, T.; Meersch-Dini, M. Biomarkers for the Prediction and Judgement of Sepsis and Sepsis Complications: A Step towards Precision Medicine? J. Clin. Med. 2022, 11, 5782. [Google Scholar] [CrossRef]
- Phua, J.; Ngerng, W.; See, K.; Tay, C.; Kiong, T.; Lim, H.; Chew, M.; Yip, H.; Tan, A.; Khalizah, H.; et al. Characteristics and Outcomes of Culture-Negative versus Culture-Positive Severe Sepsis. Crit. Care 2013, 17, R202. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef]
- Baldirà, J.; Ruiz-Rodríguez, J.C.; Ruiz-Sanmartin, A.; Chiscano, L.; Cortes, A.; Sistac, D.Á.; Ferrer-Costa, R.; Comas, I.; Villena, Y.; Larrosa, M.N.; et al. Use of Biomarkers to Improve 28-Day Mortality Stratification in Patients with Sepsis and SOFA ≤ 6. Biomedicines 2023, 11, 2149. [Google Scholar] [CrossRef] [PubMed]
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.-L. Biomarkers of Sepsis: Time for a Reappraisal. Crit. Care 2020, 24, 287. [Google Scholar] [CrossRef] [PubMed]
- Sanz Codina, M.; Zeitlinger, M. Biomarkers Predicting Tissue Pharmacokinetics of Antimicrobials in Sepsis: A Review. Clin. Pharmacokinet. 2022, 61, 593–617. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, P.; Coelho, L.; Dal-Pizzol, F.; Ferrer, R.; Huttner, A.; Conway Morris, A.; Nobre, V.; Ramirez, P.; Rouze, A.; Salluh, J.; et al. How to Use Biomarkers of Infection or Sepsis at the Bedside: Guide to Clinicians. Intensive Care Med. 2023, 49, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-K.; Lan, H.-M.; Han, S.-T.; Wu, C.-C.; Chen, K.-F. Current Evidence and Limitation of Biomarkers for Detecting Sepsis and Systemic Infection. Biomedicines 2020, 8, 494. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Choi, J.-H. An Update on Sepsis Biomarkers. Infect. Chemother. 2020, 52, 1. [Google Scholar] [CrossRef]
- Barichello, T.; Generoso, J.S.; Singer, M.; Dal-Pizzol, F. Biomarkers for Sepsis: More than Just Fever and Leukocytosis—A Narrative Review. Crit. Care 2022, 26, 14. [Google Scholar] [CrossRef]
- Méndez Hernández, R.; Ramasco Rueda, F. Biomarkers as Prognostic Predictors and Therapeutic Guide in Critically Ill Patients: Clinical Evidence. J. Pers. Med. 2023, 13, 333. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, P.; Salluh, J.I.F. Biomarker-Guided Antibiotic Therapy in Adult Critically Ill Patients: A Critical Review. Ann. Intensive Care 2012, 2, 32. [Google Scholar] [CrossRef]
- Larsen, F.F.; Petersen, J.A. Novel Biomarkers for Sepsis: A Narrative Review. Eur. J. Intern. Med. 2017, 45, 46–50. [Google Scholar] [CrossRef]
- Kraus, V.B. Biomarkers as Drug Development Tools: Discovery, Validation, Qualification and Use. Nat. Rev. Rheumatol. 2018, 14, 354–362. [Google Scholar] [CrossRef] [PubMed]
- McFadyen, J.D.; Zeller, J.; Potempa, L.A.; Pietersz, G.A.; Eisenhardt, S.U.; Peter, K. C-Reactive Protein and Its Structural Isoforms: An Evolutionary Conserved Marker and Central Player in Inflammatory Diseases and Beyond. Subcell. Biochem. 2020, 94, 499–520. [Google Scholar] [CrossRef] [PubMed]
- The Captain Study Group; Parlato, M.; Philippart, F.; Rouquette, A.; Moucadel, V.; Puchois, V.; Blein, S.; Bedos, J.-P.; Diehl, J.-L.; Hamzaoui, O.; et al. Circulating Biomarkers May Be Unable to Detect Infection at the Early Phase of Sepsis in ICU Patients: The CAPTAIN Prospective Multicenter Cohort Study. Intensive Care Med. 2018, 44, 1061–1070. [Google Scholar] [CrossRef]
- Li, S.; Rong, H.; Guo, Q.; Chen, Y.; Zhang, G.; Yang, J. Serum Procalcitonin Levels Distinguish Gram-Negative Bacterial Sepsis from Gram-Positive Bacterial and Fungal Sepsis. J. Res. Med. Sci. 2016, 21, 39. [Google Scholar] [CrossRef]
- Quirant-Sánchez, B.; Plans-Galván, O.; Lucas, E.; Argudo, E.; Martinez-Cáceres, E.M.; Arméstar, F. HLA-DR Expression on Monocytes and Sepsis Index Are Useful in Predicting Sepsis. Biomedicines 2023, 11, 1836. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, J.; Brooks, K.; Samms-Vaughan, M.; Paneth, N. C-Reactive Protein as a Predictor of Neonatal Sepsis. Psychol. Health Med. 2022, 29, 1134–1141. [Google Scholar] [CrossRef]
- Mierzchała-Pasierb, M.; Lipińska-Gediga, M. Sepsis Diagnosis and Monitoring—Procalcitonin as Standard, but What Next? Anaesthesiol. Intensive Ther. 2019, 51, 299–305. [Google Scholar] [CrossRef]
- Li, Z.; He, L.; Li, S.; He, W.; Zha, C.; Ou, W.; Hou, Q.; Wang, W.; Sun, X.; Liang, H. Combination of Procalcitonin and C-reactive Protein Levels in the Early Diagnosis of Bacterial Co-infections in Children with H1N1 Influenza. Influenza Other Respir. Viruses 2019, 13, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Faix, J.D. Biomarkers of Sepsis. Crit. Rev. Clin. Lab. Sci. 2013, 50, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Escadafal, C.; Incardona, S.; Fernandez-Carballo, B.L.; Dittrich, S. The Good and the Bad: Using C Reactive Protein to Distinguish Bacterial from Non-Bacterial Infection among Febrile Patients in Low-Resource Settings. BMJ Glob. Health 2020, 5, e002396. [Google Scholar] [CrossRef] [PubMed]
- Largman-Chalamish, M.; Wasserman, A.; Silberman, A.; Levinson, T.; Ritter, O.; Berliner, S.; Zeltser, D.; Shapira, I.; Rogowski, O.; Shenhar-Tsarfaty, S. Differentiating between Bacterial and Viral Infections by Estimated CRP Velocity. PLoS ONE 2022, 17, e0277401. [Google Scholar] [CrossRef]
- Xu, H.-G.; Tian, M.; Pan, S.-Y. Clinical Utility of Procalcitonin and Its Association with Pathogenic Microorganisms. Crit. Rev. Clin. Lab. Sci. 2022, 59, 93–111. [Google Scholar] [CrossRef]
- Zaki, H.A.; Bensliman, S.; Bashir, K.; Iftikhar, H.; Fayed, M.H.; Salem, W.; Elmoheen, A.; Yigit, Y. Accuracy of Procalcitonin for Diagnosing Sepsis in Adult Patients Admitted to the Emergency Department: A Systematic Review and Meta-Analysis. Syst. Rev. 2024, 13, 37. [Google Scholar] [CrossRef]
- Nakajima, A.; Yazawa, J.; Sugiki, D.; Mizuguchi, M.; Sagara, H.; Fujisiro, M.; Shibazaki, M.; Hitani, A.; To, M.; Haruki, K. Clinical Utility of Procalcitonin as a Marker of Sepsis: A Potential Predictor of Causative Pathogens. Intern. Med. 2014, 53, 1497–1503. [Google Scholar] [CrossRef][Green Version]
- Ulla, M.; Pizzolato, E.; Lucchiari, M.; Loiacono, M.; Soardo, F.; Forno, D.; Morello, F.; Lupia, E.; Moiraghi, C.; Mengozzi, G.; et al. Diagnostic and Prognostic Value of Presepsin in the Management of Sepsis in the Emergency Department: A Multicenter Prospective Study. Crit. Care 2013, 17, R168. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, W.; Hou, W.; Jiang, C.; Hu, J.; Sun, L.; Hu, L.; Wu, J.; Shang, A. The Diagnostic Utility of IL-10, IL-17, and PCT in Patients with Sepsis Infection. Front. Public Health 2022, 10, 923457. [Google Scholar] [CrossRef] [PubMed]
- Keçe, E.; Yaka, E.; Yılmaz, S.; Doğan, N.Ö.; Alyeşil, C.; Pekdemir, M. Comparison of Diagnostic and Prognostic Utility of Lactate and Procalcitonin for Sepsis in Adult Cancer Patients Presenting to Emergency Department with Systemic Inflammatory Response Syndrome. Turk. J. Emerg. Med. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Lin, C.-T.; Lu, J.-J.; Chen, Y.-C.; Kok, V.C.; Horng, J.-T. Diagnostic Value of Serum Procalcitonin, Lactate, and High-Sensitivity C-Reactive Protein for Predicting Bacteremia in Adult Patients in the Emergency Department. PeerJ 2017, 5, e4094. [Google Scholar] [CrossRef] [PubMed]
- Tsalik, E.L.; Jaggers, L.B.; Glickman, S.W.; Langley, R.J.; Van Velkinburgh, J.C.; Park, L.P.; Fowler, V.G.; Cairns, C.B.; Kingsmore, S.F.; Woods, C.W. Discriminative Value of Inflammatory Biomarkers for Suspected Sepsis. J. Emerg. Med. 2012, 43, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Miglietta, F.; Faneschi, M.L.; Lobreglio, G.; Palumbo, C.; Rizzo, A.; Cucurachi, M.; Portaccio, G.; Guerra, F.; Pizzolante, M. Procalcitonin, C-Reactive Protein and Serum Lactate Dehydrogenase in the Diagnosis of Bacterial Sepsis, SIRS and Systemic Candidiasis. Infez. Med. 2015, 23, 230–237. [Google Scholar] [PubMed]
- Chen, J.; Tu, X.; Huang, M.; Xie, Y.; Lin, Y.; Hu, J. Prognostic Value of Platelet Combined with Serum Procalcitonin in Patients with Sepsis. Medicine 2023, 102, e34953. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.F.; Botoni, F.A.; Oliveira, C.R.A.; Silva, C.B.; Pereira, H.A.; Serufo, J.C.; Nobre, V. Procalcitonin Versus C-Reactive Protein for Guiding Antibiotic Therapy in Sepsis: A Randomized Trial. Crit. Care Med. 2013, 41, 2336–2343. [Google Scholar] [CrossRef] [PubMed]
- Henning, D.J.; Hall, M.K.; Watsjold, B.K.; Bhatraju, P.K.; Kosamo, S.; Shapiro, N.I.; Liles, W.C.; Wurfel, M.M. Interleukin-6 Improves Infection Identification When Added to Physician Judgment during Evaluation of Potentially Septic Patients. Am. J. Emerg. Med. 2020, 38, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Park, D.W.; Moon, S.; Cho, H.-J.; Park, J.H.; Seok, H.; Choi, W.S. Diagnostic and Prognostic Value of Interleukin-6, Pentraxin 3, and Procalcitonin Levels among Sepsis and Septic Shock Patients: A Prospective Controlled Study According to the Sepsis-3 Definitions. BMC Infect. Dis. 2019, 19, 968. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yan, W.; Liu, S.; Li, H. Study on the Correlation and Clinical Significance of T Lymphocyte Subsets, IL-6 and PCT in the Severity of Patients with Sepsis. Pak. J. Med. Sci. 2022, 39, 227. [Google Scholar] [CrossRef]
- Kang, R.; Chen, R.; Zhang, Q.; Hou, W.; Wu, S.; Cao, L.; Huang, J.; Yu, Y.; Fan, X.; Yan, Z.; et al. HMGB1 in Health and Disease. Mol. Aspects Med. 2014, 40, 1–116. [Google Scholar] [CrossRef]
- Van Zoelen, M.A.D.; Achouiti, A.; van der Poll, T. The Role of Receptor for Advanced Glycation Endproducts (RAGE) in Infection. Crit. Care 2011, 15, 208. [Google Scholar] [CrossRef]
- Harris, H.E.; Andersson, U.; Pisetsky, D.S. HMGB1: A Multifunctional Alarmin Driving Autoimmune and Inflammatory Disease. Nat. Rev. Rheumatol. 2012, 8, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a Late Mediator of Endotoxin Lethality in Mice. Science 1999, 285, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Karakike, E.; Adami, M.-E.; Lada, M.; Gkavogianni, T.; Koutelidakis, I.M.; Bauer, M.; Giamarellos-Bourboulis, E.J.; Tsangaris, I. Late Peaks of HMGB1 and Sepsis Outcome: Evidence For Synergy With Chronic Inflammatory Disorders. Shock 2019, 52, 334–339. [Google Scholar] [CrossRef]
- Matsuura, R.; Komaru, Y.; Miyamoto, Y.; Yoshida, T.; Yoshimoto, K.; Hamasaki, Y.; Nangaku, M.; Doi, K. Different Biomarker Kinetics in Critically Ill Patients with High Lactate Levels. Diagnostics 2020, 10, 454. [Google Scholar] [CrossRef]
- Brück, E.; Svensson-Raskh, A.; Larsson, J.W.; Caravaca, A.S.; Gallina, A.L.; Eberhardson, M.; Sackey, P.V.; Olofsson, P.S. Plasma HMGB1 Levels and Physical Performance in ICU Survivors. Acta Anaesthesiol. Scand. 2021, 65, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Zhang, Y.; Hao, J.; Zhao, J.; Qi, Y.; Liu, C. Correlation Analysis of Systemic Immune Inflammatory Index, Serum IL-35 and HMGB-1 with the Severity and Prognosis of Sepsis. Pak. J. Med. Sci. 2023, 39, 497. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhang, Y.; Li, C.; Liu, C.; Yao, Y.; Su, M.; Shou, S. The Utility of Presepsin in Diagnosis and Risk Stratification for the Emergency Patients with Sepsis. Am. J. Emerg. Med. 2018, 36, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Enguix-Armada, A.; Escobar-Conesa, R.; García-De La Torre, A.; De La Torre-Prados, M.V. Usefulness of Several Biomarkers in the Management of Septic Patients: C-Reactive Protein, Procalcitonin, Presepsin and Mid-Regional pro-Adrenomedullin. Clin. Chem. Lab. Med. 2016, 54, 163–168. [Google Scholar] [CrossRef]
- Leli, C.; Ferranti, M.; Marrano, U.; Al Dhahab, Z.S.; Bozza, S.; Cenci, E.; Mencacci, A. Diagnostic Accuracy of Presepsin (sCD14-ST) and Procalcitonin for Prediction of Bacteraemia and Bacterial DNAaemia in Patients with Suspected Sepsis. J. Med. Microbiol. 2016, 65, 713–719. [Google Scholar] [CrossRef]
- Prazak, J.; Irincheeva, I.; Llewelyn, M.J.; Stolz, D.; García De Guadiana Romualdo, L.; Graf, R.; Reding, T.; Klein, H.J.; Eggimann, P.; Que, Y.-A. Accuracy of Pancreatic Stone Protein for the Diagnosis of Infection in Hospitalized Adults: A Systematic Review and Individual Patient Level Meta-Analysis. Crit. Care 2021, 25, 182. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.J.; Niggemann, P.; Buehler, P.K.; Lehner, F.; Schweizer, R.; Rittirsch, D.; Fuchs, N.; Waldner, M.; Steiger, P.; Giovanoli, P.; et al. Pancreatic Stone Protein Predicts Sepsis in Severely Burned Patients Irrespective of Trauma Severity: A Monocentric Observational Study. Ann. Surg. 2021, 274, e1179–e1186. [Google Scholar] [CrossRef]
- Pugin, J.; Daix, T.; Pagani, J.-L.; Morri, D.; Giacomucci, A.; Dequin, P.-F.; Guitton, C.; Que, Y.-A.; Zani, G.; Brealey, D.; et al. Serial Measurement of Pancreatic Stone Protein for the Early Detection of Sepsis in Intensive Care Unit Patients: A Prospective Multicentric Study. Crit. Care 2021, 25, 151. [Google Scholar] [CrossRef] [PubMed]
- Filippidis, P.; Hovius, L.; Tissot, F.; Orasch, C.; Flückiger, U.; Siegemund, M.; Pagani, J.-L.; Eggimann, P.; Marchetti, O.; Lamoth, F. Serial Monitoring of Pancreatic Stone Protein for the Detection of Sepsis in Intensive Care Unit Patients with Complicated Abdominal Surgery: A Prospective, Longitudinal Cohort Study. J. Crit. Care 2024, 82, 154772. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wang, G.; Wang, Y.; Tan, Z.; Sun, X.; Zhou, J.; Duan, M.; Zhi, D.; Tang, Z.; Hang, C.; et al. Potential Value of Presepsin Guidance in Shortening Antibiotic Therapy in Septic Patients: A Multicenter, Prospective Cohort Trial. Shock 2022, 57, 63–71. [Google Scholar] [CrossRef]
- Azim, A. Presepsin: A Promising Biomarker for Sepsis. Indian J. Crit. Care Med. 2021, 25, 117–118. [Google Scholar] [CrossRef]
- Hosokawa, K.; Obara, H.; Fukuda, K.; Mastubara, K.; Kitagawa, Y. Specificity of Presepsin as a Biomarker of Bacterial Infection in Mouse Sepsis Models. J. Surg. Res. 2023, 283, 572–580. [Google Scholar] [CrossRef]
- Assal, H.H.; Abdelrahman, S.M.; Abdelbasset, M.A.; Abdelaziz, M.; Sabry, I.M.; Shaban, M.M. Presepsin as a Novel Biomarker in Predicting In-hospital Mortality in Patients With COVID-19 Pneumonia. Int. J. Infect. Dis. 2022, 118, 155–163. [Google Scholar] [CrossRef]
- Patnaik, R.; Azim, A.; Agarwal, V. Neutrophil CD64 a Diagnostic and Prognostic Marker of Sepsis in Adult Critically Ill Patients: A Brief Review. Indian J. Crit. Care Med. 2020, 24, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Ma, T.; Di, X.; Tian, C.; Zhao, M.; Wang, K. Diagnostic Value of Neutrophil CD64, Procalcitonin, and Interleukin-6 in Sepsis: A Meta-Analysis. BMC Infect. Dis. 2021, 21, 384. [Google Scholar] [CrossRef]
- Yeh, C.-F.; Wu, C.-C.; Liu, S.-H.; Chen, K.-F. Comparison of the Accuracy of Neutrophil CD64, Procalcitonin, and C-Reactive Protein for Sepsis Identification: A Systematic Review and Meta-Analysis. Ann. Intensive Care 2019, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.-X.; Zheng, Z.; Wang, M.; Guo, H.-X.; Chen, Y.-J.; Wu, Y.; Li, X.; Li, Q.; Cui, J.-Y.; Ren, X.-X.; et al. Diagnostic Performance of Neutrophil CD64 Index, Procalcitonin, and C-Reactive Protein for Early Sepsis in Hematological Patients. World J. Clin. Cases 2022, 10, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Chen, J.; Wei, Y.; Liu, Y.; Yuan, K.; Chen, J.; He, M.; Liu, N. Multi-Marker Approach Using C-Reactive Protein, Procalcitonin, Neutrophil CD64 Index for the Prognosis of Sepsis in Intensive Care Unit: A Retrospective Cohort Study. BMC Infect. Dis. 2022, 22, 662. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Gu, J.; Zhang, J. Soluble Triggering Receptor Expressed on Myeloid Cell-1 (sTREM-1): A Potential Biomarker for the Diagnosis of Infectious Diseases. Front. Med. 2017, 11, 169–177. [Google Scholar] [CrossRef]
- Qin, Q.; Liang, L.; Xia, Y. Diagnostic and Prognostic Predictive Values of Circulating sTREM-1 in Sepsis: A Meta-Analysis. Infect. Genet. Evol. 2021, 96, 105074. [Google Scholar] [CrossRef] [PubMed]
- Sanger, H.L.; Klotz, G.; Riesner, D.; Gross, H.J.; Kleinschmidt, A.K. Viroids Are Single-Stranded Covalently Closed Circular RNA Molecules Existing as Highly Base-Paired Rod-like Structures. Proc. Natl. Acad. Sci. USA 1976, 73, 3852–3856. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Harries, L.W. Circular RNAs (circRNAs) in Health and Disease. Genes 2017, 8, 353. [Google Scholar] [CrossRef]
- Zhang, T.-N.; Li, D.; Xia, J.; Wu, Q.-J.; Wen, R.; Yang, N.; Liu, C.-F. Non-Coding RNA: A Potential Biomarker and Therapeutic Target for Sepsis. Oncotarget 2017, 8, 91765–91778. [Google Scholar] [CrossRef]
- Qi, L.; Yan, Y.; Chen, B.; Cao, J.; Liang, G.; Xu, P.; Wang, Y.; Ren, Y.; Mao, G.; Huang, Z.; et al. Research Progress of circRNA as a Biomarker of Sepsis: A Narrative Review. Ann. Transl. Med. 2021, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, J.; Di, X.; Cong, S.; Zhao, M.; Wang, K. Exosomal hsa_circRNA_104484 and hsa_circRNA_104670 May Serve as Potential Novel Biomarkers and Therapeutic Targets for Sepsis. Sci. Rep. 2021, 11, 14141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Liu, D.; Ye, J.; Li, B. Circ_0006944 Aggravates LPS-Induced HK2 Cell Injury via Modulating miR-205-5p/UBL4A Pathway. Autoimmunity 2023, 56, 2276066. [Google Scholar] [CrossRef]
- Feng, K.; Huang, W.; Shang, J.; Ping, F.; Tan, Q.; Wang, W.; Li, Y.; Cao, Y. Knockdown of lncRNA-ASLNC12002 Alleviates Epithelial–Mesenchymal Transition of Type II Alveolar Epithelial Cells in Sepsis-Induced Acute Respiratory Distress Syndrome. Hum. Cell 2022, 36, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Liu, H.; Xue, Y.; Xiang, T.; Sun, Z. LncRNA HOTTIP Promotes Inflammatory Response in Acute Gouty Arthritis via miR-101-3p/BRD4 Axis. Int. J. Rheum. Dis. 2023, 26, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhu, W.; Yu, J.; Shi, Y.; Zhao, Y. LncRNA HOTTIP as a Diagnostic Biomarker for Acute Respiratory Distress Syndrome in Patients with Sepsis and to Predict the Short-Term Clinical Outcome: A Case-Control Study. BMC Anesth. 2024, 24, 30. [Google Scholar] [CrossRef]
- Llobat, L.; Gourbault, O. Role of MicroRNAs in Human Osteosarcoma: Future Perspectives. Biomedicines 2021, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Han, L. Downregulation of miR-1184 Serves as a Diagnostic Biomarker in Neonatal Sepsis and Regulates LPS-induced Inflammatory Response by Inhibiting IL-16 in Monocytes. Exp. Ther. Med. 2021, 21, 350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, D.; Gao, J.; Xiang, X.; Hu, X.; Li, S.; Wu, W.; Cai, J.; Tang, C.; Zhang, D.; et al. Discovery and Validation of miR-452 as an Effective Biomarker for Acute Kidney Injury in Sepsis. Theranostics 2020, 10, 11963–11975. [Google Scholar] [CrossRef]
- Zhang, H.; Che, L.; Wang, Y.; Zhou, H.; Gong, H.; Man, X.; Zhao, Q. Deregulated microRNA-22-3p in Patients with Sepsis-Induced Acute Kidney Injury Serves as a New Biomarker to Predict Disease Occurrence and 28-Day Survival Outcomes. Int. Urol. Nephrol. 2021, 53, 2107–2116. [Google Scholar] [CrossRef]
- Liu, H.-C.; Han, D.-S.; Hsu, C.-C.; Wang, J.-S. Circulating MicroRNA-486 and MicroRNA-146a Serve as Potential Biomarkers of Sarcopenia in the Older Adults. BMC Geriatr. 2021, 21, 86. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Guo, S. miR-486-5p Serves as a Diagnostic Biomarker for Sepsis and Its Predictive Value for Clinical Outcomes. J. Inflamm. Res. 2021, 14, 3687–3695. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y.; Lin, S.; Li, Y.; Hua, Y.; Zhou, K. Diagnostic Significance of microRNAs in Sepsis. PLoS ONE 2023, 18, e0279726. [Google Scholar] [CrossRef] [PubMed]
- Erreni, M.; Manfredi, A.A.; Garlanda, C.; Mantovani, A.; Rovere-Querini, P. The Long Pentraxin PTX 3: A Prototypical Sensor of Tissue Injury and a Regulator of Homeostasis. Immunol. Rev. 2017, 280, 112–125. [Google Scholar] [CrossRef]
- Caironi, P.; Masson, S.; Mauri, T.; Bottazzi, B.; Leone, R.; Magnoli, M.; Barlera, S.; Mamprin, F.; Fedele, A.; Mantovani, A.; et al. Pentraxin 3 in Patients with Severe Sepsis or Shock: The ALBIOS Trial. Eur. J. Clin. Investig. 2017, 47, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Moon, S.; Park, D.W.; Cho, H.-J.; Kim, J.Y.; Park, J.; Cha, J.H. Biomarker Combination and SOFA Score for the Prediction of Mortality in Sepsis and Septic Shock: A Prospective Observational Study According to the Sepsis-3 Definitions. Medicine 2020, 99, e20495. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Gong, M.; Chau, A.; Wong, W.T.; Bazoukis, G.; Wong, S.H.; Lampropoulos, K.; Xia, Y.; Li, G.; Wong, M.C.S.; et al. Pentraxin-3 as a Marker of Sepsis Severity and Predictor of Mortality Outcomes: A Systematic Review and Meta-Analysis. J. Infect. 2018, 76, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stasi, A.; Franzin, R.; Divella, C.; Sallustio, F.; Curci, C.; Picerno, A.; Pontrelli, P.; Staffieri, F.; Lacitignola, L.; Crovace, A.; et al. PMMA-Based Continuous Hemofiltration Modulated Complement Activation and Renal Dysfunction in LPS-Induced Acute Kidney Injury. Front. Immunol. 2021, 12, 605212. [Google Scholar] [CrossRef]
- Lundberg, O.H.M.; Lengquist, M.; Spångfors, M.; Annborn, M.; Bergmann, D.; Schulte, J.; Levin, H.; Melander, O.; Frigyesi, A.; Friberg, H. Circulating Bioactive Adrenomedullin as a Marker of Sepsis, Septic Shock and Critical Illness. Crit. Care 2020, 24, 636. [Google Scholar] [CrossRef]
- Andrés, C.; Andaluz-Ojeda, D.; Cicuendez, R.; Nogales, L.; Martín, S.; Martin-Fernandez, M.; Almansa, R.; Calvo, D.; Esteban-Velasco, M.C.; Vaquero-Roncero, L.M.; et al. MR- proADM to Detect Specific Types of Organ Failure in Infection. Eur. J. Clin. Investig. 2020, 50, e13246. [Google Scholar] [CrossRef]
- Schuetz, P.; Hausfater, P.; Amin, D.; Amin, A.; Haubitz, S.; Faessler, L.; Kutz, A.; Conca, A.; Reutlinger, B.; Canavaggio, P.; et al. Biomarkers from Distinct Biological Pathways Improve Early Risk Stratification in Medical Emergency Patients: The Multinational, Prospective, Observational TRIAGE Study. Crit. Care 2015, 19, 377. [Google Scholar] [CrossRef]
- Cander, B.; Visneci, E.F.; Karaoglan, O.; Cakmak, F.; Tuncar, A.; Taslidere, B. Diagnostic and Prognostic Value of MR-pro ADM, Procalcitonin, and Copeptin in Sepsis. Open Med. 2023, 18, 20230865. [Google Scholar] [CrossRef]
- The SepNet Critical Care Trials Group; Elke, G.; Bloos, F.; Wilson, D.C.; Brunkhorst, F.M.; Briegel, J.; Reinhart, K.; Loeffler, M.; Kluge, S.; Nierhaus, A.; et al. The Use of Mid-Regional Proadrenomedullin to Identify Disease Severity and Treatment Response to Sepsis—A Secondary Analysis of a Large Randomised Controlled Trial. Crit. Care 2018, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- Honore, P.M.; Redant, S.; De Bels, D. Reliability of Biomarkers of Sepsis during Extracorporeal Therapies: The Clinician Needs to Know What Is Eliminated and What Is Not. Crit. Care 2020, 24, 553. [Google Scholar] [CrossRef]
- Joffre, J.; Hellman, J.; Ince, C.; Ait-Oufella, H. Endothelial Responses in Sepsis. Am. J. Respir. Crit. Care Med. 2020, 202, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Palud, A.; Parmentier-Decrucq, E.; Pastre, J.; De Freitas Caires, N.; Lassalle, P.; Mathieu, D. Evaluation of Endothelial Biomarkers as Predictors of Organ Failures in Septic Shock Patients. Cytokine 2015, 73, 213–218. [Google Scholar] [CrossRef]
- Constantin, L.; Ungurianu, A.; Streinu-Cercel, A.; Săndulescu, O.; Aramă, V.; Margină, D.; Țârcomnicu, I. Investigation of Serum Endocan Levels in SARS-CoV-2 Patients. Int. J. Mol. Sci. 2024, 25, 3042. [Google Scholar] [CrossRef]
- Gatseva, P.; Blazhev, A.; Yordanov, Z.; Atanasova, V. Early Diagnostic Markers of Late-Onset Neonatal Sepsis. Pediatr. Rep. 2023, 15, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Nakashio, M.; Maruyama, J.; Irie, Y.; Kawano, Y.; Ishikura, H. Validating Plasminogen Activator Inhibitor-1 as a Poor Prognostic Factor in Sepsis. Acute Med. Surg. 2020, 7, e581. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Kitamura, T.; Nakamura, Y.; Irie, Y.; Matsumoto, N.; Kawano, Y.; Ishikura, H. Usefulness of Plasminogen Activator Inhibitor-1 as a Predictive Marker of Mortality in Sepsis. J. Intensive Care 2017, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, Y.; Chen, L.; Mu, H.; Meaney, C.; Fan, Y.; Pillay, J.; Wang, H.; Zhang, J.; Pan, S.; et al. PAI-1 Genetic Polymorphisms Influence Septic Patients’ Outcomes by Regulating Neutrophil Activity. Chin. Med. J. 2023, 136, 1959–1966. [Google Scholar] [CrossRef]
- Bruno, M.E.C.; Mukherjee, S.; Sturgill, J.L.; Cornea, V.; Yeh, P.; Hawk, G.S.; Saito, H.; Starr, M.E. PAI-1 as a Critical Factor in the Resolution of Sepsis and Acute Kidney Injury in Old Age. Front. Cell Dev. Biol. 2024, 11, 1330433. [Google Scholar] [CrossRef]
- Jarahzadeh, M.H.; Jafari, M.; Seifi-Shalamzari, N.; Ferdosian, F.; Bahrami, R.; Raee-Ezzabadi, A.; Nafei, Z.; Shajari, A.; Mirjalili, S.R.; Neamatzadeh, H. Association of PAI-1 4G/5G and ACE I/D Polymorphisms with Susceptibility to Pediatric Sepsis: Evidence from a Meta-Analysis. Fetal Pediatr. Pathol. 2022, 41, 242–258. [Google Scholar] [CrossRef]
- Bloomfield, S.M.; McKinney, J.; Smith, L.; Brisman, J. Reliability of S100B in Predicting Severity of Central Nervous System Injury. Neurocrit Care 2007, 6, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Erikson, K.; Ala-Kokko, T.I.; Koskenkari, J.; Liisanantti, J.H.; Kamakura, R.; Herzig, K.H.; Syrjälä, H. Elevated Serum S-100β in Patients with Septic Shock Is Associated with Delirium. Acta Anaesthesiol. Scand. 2019, 63, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Feng, Q.; Ai, M.-L.; Deng, S.; Liu, Z.-Y.; Huang, L.; Ai, Y.-H.; Zhang, L. The Dynamic Change of Serum S100B Levels from Day 1 to Day 3 Is More Associated with Sepsis-Associated Encephalopathy. Sci. Rep. 2020, 10, 7718. [Google Scholar] [CrossRef]
- Hu, J.; Xie, S.; Li, W.; Zhang, L. Diagnostic and Prognostic Value of Serum S100B in Sepsis-Associated Encephalopathy: A Systematic Review and Meta-Analysis. Front. Immunol. 2023, 14, 1102126. [Google Scholar] [CrossRef] [PubMed]
- Schütze, S.; Drevets, D.A.; Tauber, S.C.; Nau, R. Septic Encephalopathy in the Elderly—Biomarkers of Potential Clinical Utility. Front. Cell Neurosci. 2023, 17, 1238149. [Google Scholar] [CrossRef] [PubMed]
- Custodero, C.; Wu, Q.; Ghita, G.L.; Anton, S.D.; Brakenridge, S.C.; Brumback, B.A.; Efron, P.A.; Gardner, A.K.; Leeuwenburgh, C.; Moldawer, L.L.; et al. Prognostic Value of NT-proBNP Levels in the Acute Phase of Sepsis on Lower Long-Term Physical Function and Muscle Strength in Sepsis Survivors. Crit. Care 2019, 23, 230. [Google Scholar] [CrossRef] [PubMed]
- Varpula, M.; Pulkki, K.; Karlsson, S.; Ruokonen, E.; Pettilä, V. Predictive Value of N-Terminal pro–Brain Natriuretic Peptide in Severe Sepsis and Septic Shock. Crit. Care Med. 2007, 35, 1277–1283. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, R.; Yang, P.; Wang, D. Construction of a Predictive Model and Prognosis of Left Ventricular Systolic Dysfunction in Patients with Sepsis Based on the Diagnosis Using Left Ventricular Global Longitudinal Strain. J. Intensive Care 2022, 10, 29. [Google Scholar] [CrossRef]
- Martín-Rodríguez, F.; Melero-Guijarro, L.; Ortega, G.J.; Sanz-García, A.; De La Torre De Dios, T.; Manzanares, J.Á.; Martín-Conty, J.L.; Castro Villamor, M.A.; Delgado Benito, J.F.; López-Izquierdo, R. Combination of Prehospital NT-proBNP with qSOFA and NEWS to Predict Sepsis and Sepsis-Related Mortality. Dis. Markers 2022, 2022, 5351137. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, J.; Wei, Q.; Wang, H.; Zhao, C.; Hu, C.; Han, Y.; Hui, Z.; Yang, L.; Dai, Q.; et al. Potential of Circulating lncRNA CASC2 as a Biomarker in Reflecting the Inflammatory Cytokines, Multi-Organ Dysfunction, Disease Severity, and Mortality in Sepsis Patients. J. Clin. Lab. Anal. 2022, 36, e24569. [Google Scholar] [CrossRef]
- Liu, J.; Shi, K.; Chen, M.; Xu, L.; Hong, J.; Hu, B.; Yang, X.; Sun, R. Elevated miR-155 Expression Induces Immunosuppression via CD39+ Regulatory T-Cells in Sepsis Patient. Int. J. Infect. Dis. 2015, 40, 135–141. [Google Scholar] [CrossRef]
- Zhao, D.; Li, S.; Cui, J.; Wang, L.; Ma, X.; Li, Y. Plasma miR-125a and miR-125b in Sepsis: Correlation with Disease Risk, Inflammation, Severity, and Prognosis. J. Clin. Lab. Anal. 2020, 34, e23036. [Google Scholar] [CrossRef] [PubMed]
- Na, L.; Ding, H.; Xing, E.; Gao, J.; Liu, B.; Wang, H.; Yu, J.; Yu, C. Lnc-MEG3 Acts as a Potential Biomarker for Predicting Increased Disease Risk, Systemic Inflammation, Disease Severity, and Poor Prognosis of Sepsis via Interacting with miR-21. J. Clin. Lab. Anal. 2020, 34, e23123. [Google Scholar] [CrossRef]
- Yende, S.; Kellum, J.A.; Talisa, V.B.; Peck Palmer, O.M.; Chang, C.-C.H.; Filbin, M.R.; Shapiro, N.I.; Hou, P.C.; Venkat, A.; LoVecchio, F.; et al. Long-Term Host Immune Response Trajectories Among Hospitalized Patients With Sepsis. JAMA Netw. Open 2019, 2, e198686. [Google Scholar] [CrossRef]
- Huang, H.; Xu, R.; Lin, F.; Bao, C.; Wang, S.; Ji, C.; Li, K.; Jin, L.; Mu, J.; Wang, Y.; et al. High Circulating CD39+ Regulatory T Cells Predict Poor Survival for Sepsis Patients. Int. J. Infect. Dis. 2015, 30, 57–63. [Google Scholar] [CrossRef]
- Monneret, G.; Lepape, A.; Voirin, N.; Bohé, J.; Venet, F.; Debard, A.-L.; Thizy, H.; Bienvenu, J.; Gueyffier, F.; Vanhems, P. Persisting Low Monocyte Human Leukocyte Antigen-DR Expression Predicts Mortality in Septic Shock. Intensive Care Med. 2006, 32, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, A.; Movassaghpour, A.; Talebi, M.; Shadvar, K.; Soleimanpour, H. Value of Flow Cytometry (HLA-DR, CD14, CD25, CD13, CD64) in Prediction of Prognosis in Critically Ill Septic Patients Admitted to ICU: A Pilot Study. J. Clin. Anesth. 2020, 61, 109646. [Google Scholar] [CrossRef]
- Abe, T.; Kubo, K.; Izumoto, S.; Shimazu, S.; Goan, A.; Tanaka, T.; Koroki, T.; Saito, K.; Kawana, R.; Ochiai, H. Complement Activation in Human Sepsis Is Related to Sepsis-Induced Disseminated Intravascular Coagulation. Shock 2020, 54, 198–204. [Google Scholar] [CrossRef]
- Zhao, G.-J.; Li, D.; Zhao, Q.; Lian, J.; Hu, T.-T.; Hong, G.; Yao, Y.-M.; Lu, Z.-Q. Prognostic Value of Plasma Tight-Junction Proteins for Sepsis in Emergency Department: An Observational Study. Shock 2016, 45, 326–332. [Google Scholar] [CrossRef]
- Skibsted, S.; Jones, A.E.; Puskarich, M.A.; Arnold, R.; Sherwin, R.; Trzeciak, S.; Schuetz, P.; Aird, W.C.; Shapiro, N.I. Biomarkers of Endothelial Cell Activation in Early Sepsis. Shock 2013, 39, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, J.; Tang, H.; Tu, Q.; Li, Y.; Yuan, X.; Zhang, X.; Cao, J.; Molloy, D.P.; Yin, Y.; et al. Identifying Prokineticin2 as a Novel Immunomodulatory Factor in Diagnosis and Treatment of Sepsis. Crit. Care Med. 2022, 50, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Vetrano, S.; Zhang, L.; Poplis, V.A.; Castellino, F.J. The Protein C Pathway in Tissue Inflammation and Injury: Pathogenic Role and Therapeutic Implications. Blood 2010, 115, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Dahlbäck, B.; Villoutreix, B.O. The Anticoagulant Protein C Pathway. FEBS Lett. 2005, 579, 3310–3316. [Google Scholar] [CrossRef]
- Catenacci, V.; Sheikh, F.; Patel, K.; Fox-Robichaud, A.E. The Prognostic Utility of Protein C as a Biomarker for Adult Sepsis: A Systematic Review and Meta-Analysis. Crit. Care 2022, 26, 21. [Google Scholar] [CrossRef]
- Sinha, P.; Kerchberger, V.E.; Willmore, A.; Chambers, J.; Zhuo, H.; Abbott, J.; Jones, C.; Wickersham, N.; Wu, N.; Neyton, L.; et al. Identifying Molecular Phenotypes in Sepsis: An Analysis of Two Prospective Observational Cohorts and Secondary Analysis of Two Randomised Controlled Trials. Lancet Respir. Med. 2023, 11, 965–974. [Google Scholar] [CrossRef]
- Lacquaniti, A.; Ceresa, F.; Campo, S.; Barbera, G.; Caruso, D.; Palazzo, E.; Patanè, F.; Monardo, P. Acute Kidney Injury and Sepsis after Cardiac Surgery: The Roles of Tissue Inhibitor Metalloproteinase-2, Insulin-like Growth Factor Binding Protein-7, and Mid-Regional Pro-Adrenomedullin. J. Clin. Med. 2023, 12, 5193. [Google Scholar] [CrossRef] [PubMed]
- Van der Slikke, E.C.; Star, B.S.; van Meurs, M.; Henning, R.H.; Moser, J.; Bouma, H.R. Sepsis Is Associated with Mitochondrial DNA Damage and a Reduced Mitochondrial Mass in the Kidney of Patients with Sepsis-AKI. Crit. Care 2021, 25, 36. [Google Scholar] [CrossRef]
- Kerchberger, V.E.; Ware, L.B. The Role of Circulating Cell-Free Hemoglobin in Sepsis-Associated Acute Kidney Injury. Semin. Nephrol. 2020, 40, 148–159. [Google Scholar] [CrossRef] [PubMed]
- He, F.-F.; Wang, Y.-M.; Chen, Y.-Y.; Huang, W.; Li, Z.-Q.; Zhang, C. Sepsis-Induced AKI: From Pathogenesis to Therapeutic Approaches. Front. Pharmacol. 2022, 13, 981578. [Google Scholar] [CrossRef]
- Nusshag, C.; Wei, C.; Hahm, E.; Hayek, S.S.; Li, J.; Samelko, B.; Rupp, C.; Szudarek, R.; Speer, C.; Kälble, F.; et al. suPAR Links a Dysregulated Immune Response to Tissue Inflammation and Sepsis-Induced Acute Kidney Injury. JCI Insight 2023, 8, e165740. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, S.; Zhuo, X.; Lin, M. Predictive Value of suPAR in AKI: A Systematic Review and Meta-Analysis. Clin. Exp. Nephrol. 2023, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Molema, G.; Zijlstra, J.G.; van Meurs, M.; Kamps, J.A.A.M. Renal Microvascular Endothelial Cell Responses in Sepsis-Induced Acute Kidney Injury. Nat. Rev. Nephrol. 2022, 18, 95–112. [Google Scholar] [CrossRef]
- Janz, D.R.; Bastarache, J.A.; Peterson, J.F.; Sills, G.; Wickersham, N.; May, A.K.; Roberts, L.J.; Ware, L.B. Association between Cell-Free Hemoglobin, Acetaminophen, and Mortality in Patients with Sepsis: An Observational Study. Crit. Care Med. 2013, 41, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Adamzik, M.; Hamburger, T.; Petrat, F.; Peters, J.; de Groot, H.; Hartmann, M. Free Hemoglobin Concentration in Severe Sepsis: Methods of Measurement and Prediction of Outcome. Crit. Care 2012, 16, R125. [Google Scholar] [CrossRef] [PubMed]
- Gunay, M.; Mertoglu, C. Endocan, a New Marker for Inflammation and Endothelial Dysfunction, Increases in Acute Kidney Injury. North. Clin. Istanbul 2018, 6, 124–128. [Google Scholar] [CrossRef] [PubMed]
| No. | Title (Year of Publication) | Key Points | Refs. |
|---|---|---|---|
| 1 | Biomarkers for the prediction and judgement of sepsis and sepsis complications: a step towards precision medicine? (2022) |
| [11] |
| 2 | Biomarkers of sepsis: time for a reappraisal (2020) |
| [15] |
| 3 | Biomarkers predicting tissue pharmacokinetics of antimicrobials in sepsis: a review (2022) |
| [16] |
| 4 | How to use biomarkers of infection or sepsis at the bedside: guide to clinicians (2023) |
| [17] |
| 5 | Current evidence and limitation of biomarkers for detecting sepsis and systemic infection (2020) |
| [18] |
| 6 | An update on sepsis biomarkers (2020) |
| [19] |
| 7 | Biomarkers for sepsis: more than just fever and leukocytosis—a narrative review (2022) |
| [20] |
| Biomarker | Source | Response Time | Diagnostic Accuracy | Clinical Significance | Testing Methods | Strengths | Limitations | Refs. |
|---|---|---|---|---|---|---|---|---|
| Commonly used diagnostic biomarkers | ||||||||
| CRP | Liver | Rises within 4–6 h after infection |
|
|
|
|
| [15,22,25,26,27,28,29,30,31,32,33,34] |
| PCT | Thyroid C cells | Rises within 2–4 h after infection |
|
|
|
|
| [17,27,35,36,37,38,39,40,41,42,43,44,45] |
| IL-6 | Immune and non-immune cells | Peaks within 2 h after infection |
|
|
|
|
| [18,46,47,48] |
| HMGB1 | Immune cells (macrophages, monocytes, and neutrophils) | Increases within 4–8 h after infection |
|
|
|
|
| [49,50,51,52,53,54,55,56] |
| PSP | Pancreatic acinar cells | Response time is not well-defined, but it rises rapidly after infection |
|
|
|
|
| [57,58,59,60,61,62,63] |
| Presepsin | Macrophages and monocyte cells | Rises within 2 h after infection |
|
|
|
|
| [64,65,66,67] |
| CD64 | Immune cells (especially neutrophils, monocytes/macrophages) | Upregulated within 6–8 h after infection |
|
|
|
|
| [68,69,70,71,72] |
| sTREM-1 | Myeloid cells | Elevates within 2–4 h after infection |
|
|
|
|
| [73,74] |
| Novel diagnostic biomarkers | ||||||||
| circRNAs | Various tissues and cells, especially cancer cells and neural cells | Response time varies depending on the particular circRNA |
|
|
|
|
| [75,76,77,78,79] |
| HOTTIP | Embryonic stem cells and various cancer cells | Response time is not well-defined |
|
|
|
|
| [81,82,83] |
| microRNA-486-5p | Various tissues, particularly in skeletal muscles, lung tissues, and various cancer cells | Response time is not well-defined, but changes within several hours after infection |
|
|
|
|
| [84,85,86,87,88,89,90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, R.-R.; Yue, G.-L.; Dong, M.-L.; Wang, J.-Q.; Cheng, C. Sepsis Biomarkers: Advancements and Clinical Applications—A Narrative Review. Int. J. Mol. Sci. 2024, 25, 9010. https://doi.org/10.3390/ijms25169010
He R-R, Yue G-L, Dong M-L, Wang J-Q, Cheng C. Sepsis Biomarkers: Advancements and Clinical Applications—A Narrative Review. International Journal of Molecular Sciences. 2024; 25(16):9010. https://doi.org/10.3390/ijms25169010
Chicago/Turabian StyleHe, Rong-Rong, Guo-Li Yue, Mei-Ling Dong, Jia-Qi Wang, and Chen Cheng. 2024. "Sepsis Biomarkers: Advancements and Clinical Applications—A Narrative Review" International Journal of Molecular Sciences 25, no. 16: 9010. https://doi.org/10.3390/ijms25169010
APA StyleHe, R.-R., Yue, G.-L., Dong, M.-L., Wang, J.-Q., & Cheng, C. (2024). Sepsis Biomarkers: Advancements and Clinical Applications—A Narrative Review. International Journal of Molecular Sciences, 25(16), 9010. https://doi.org/10.3390/ijms25169010








