The Pathophysiological Mechanism and Clinical Treatment of Polycystic Ovary Syndrome: A Molecular and Cellular Review of the Literature
Abstract
1. Introduction
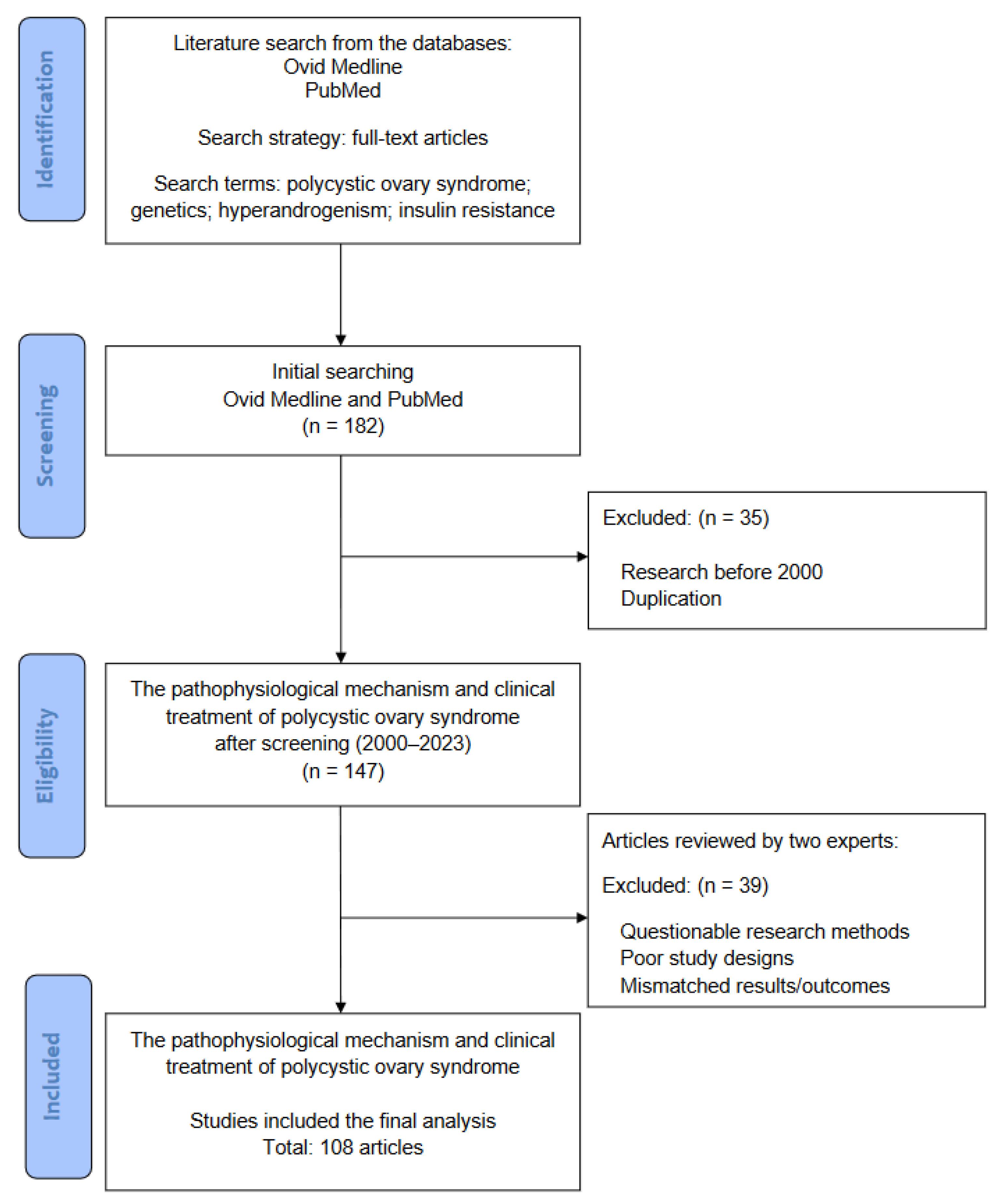
2. Research Method and Literature Review
3. Diagnosis of PCOS
3.1. The NIH Criteria
3.2. The Rotterdam Criteria
3.3. The AE-PCOS Criteria
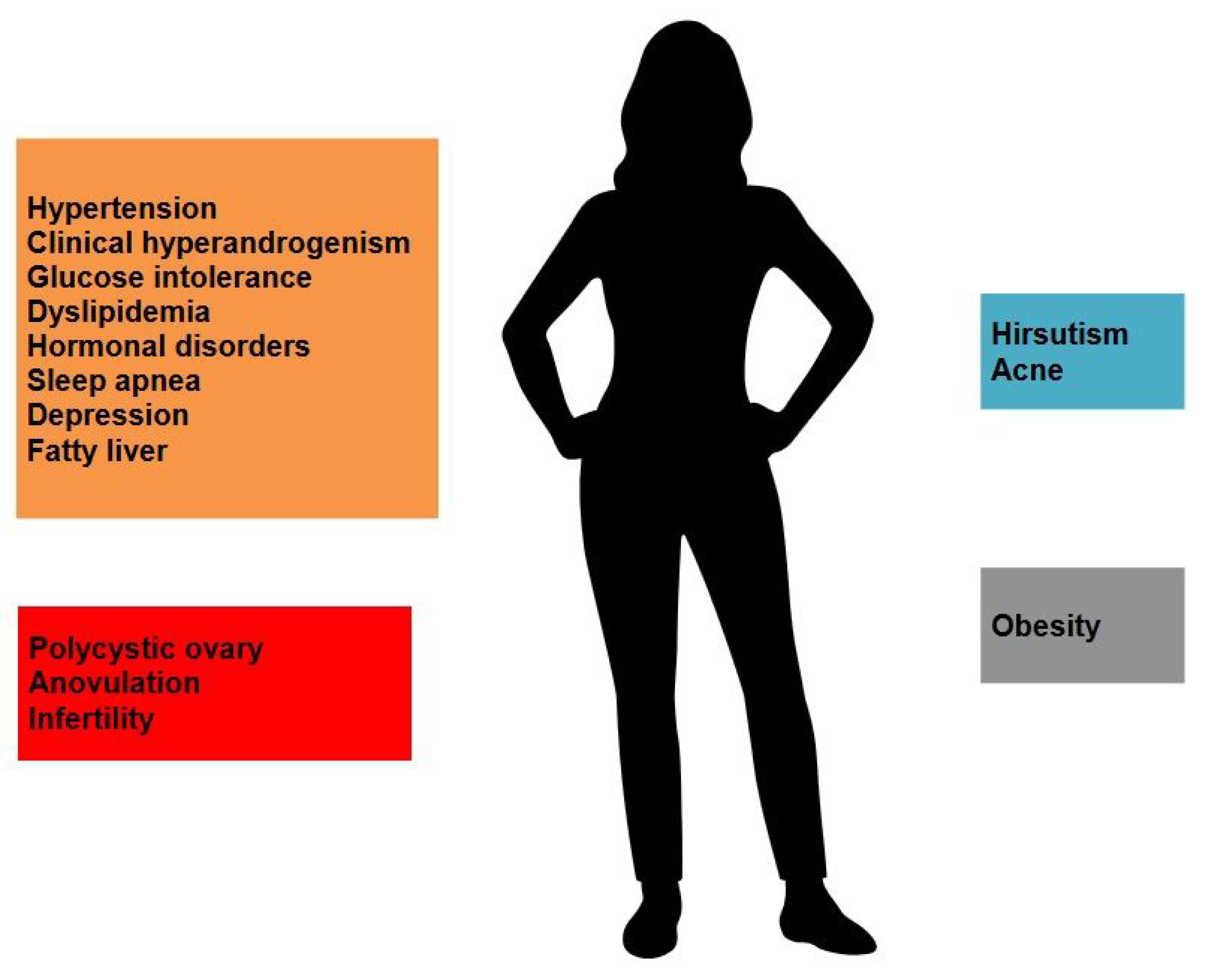
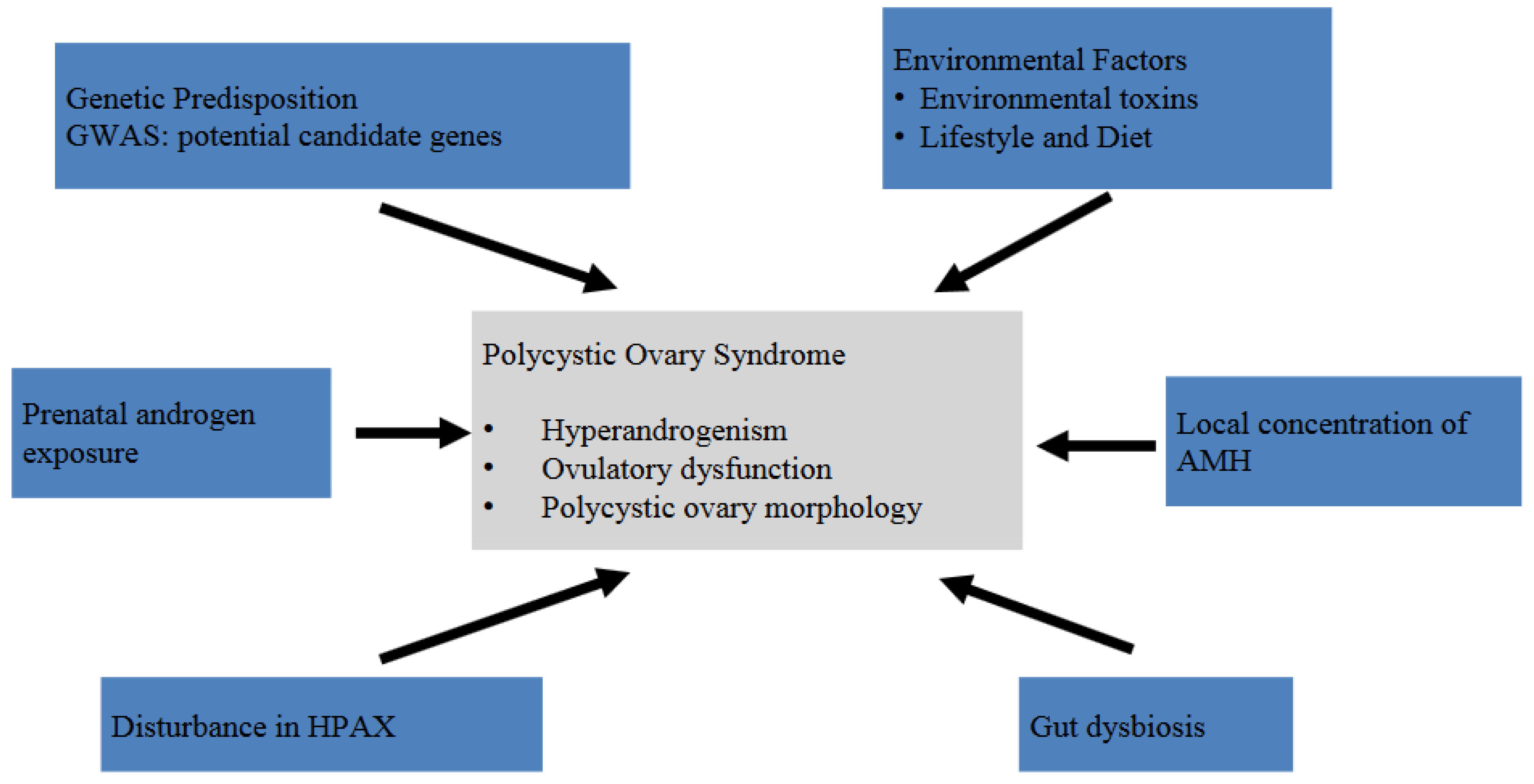
4. Pathogenesis and Pathophysiology
4.1. Genetic Predisposition
4.1.1. The Genetics of PCOS
4.1.2. The Candidate Genes for PCOS
4.2. Hyperandrogenism
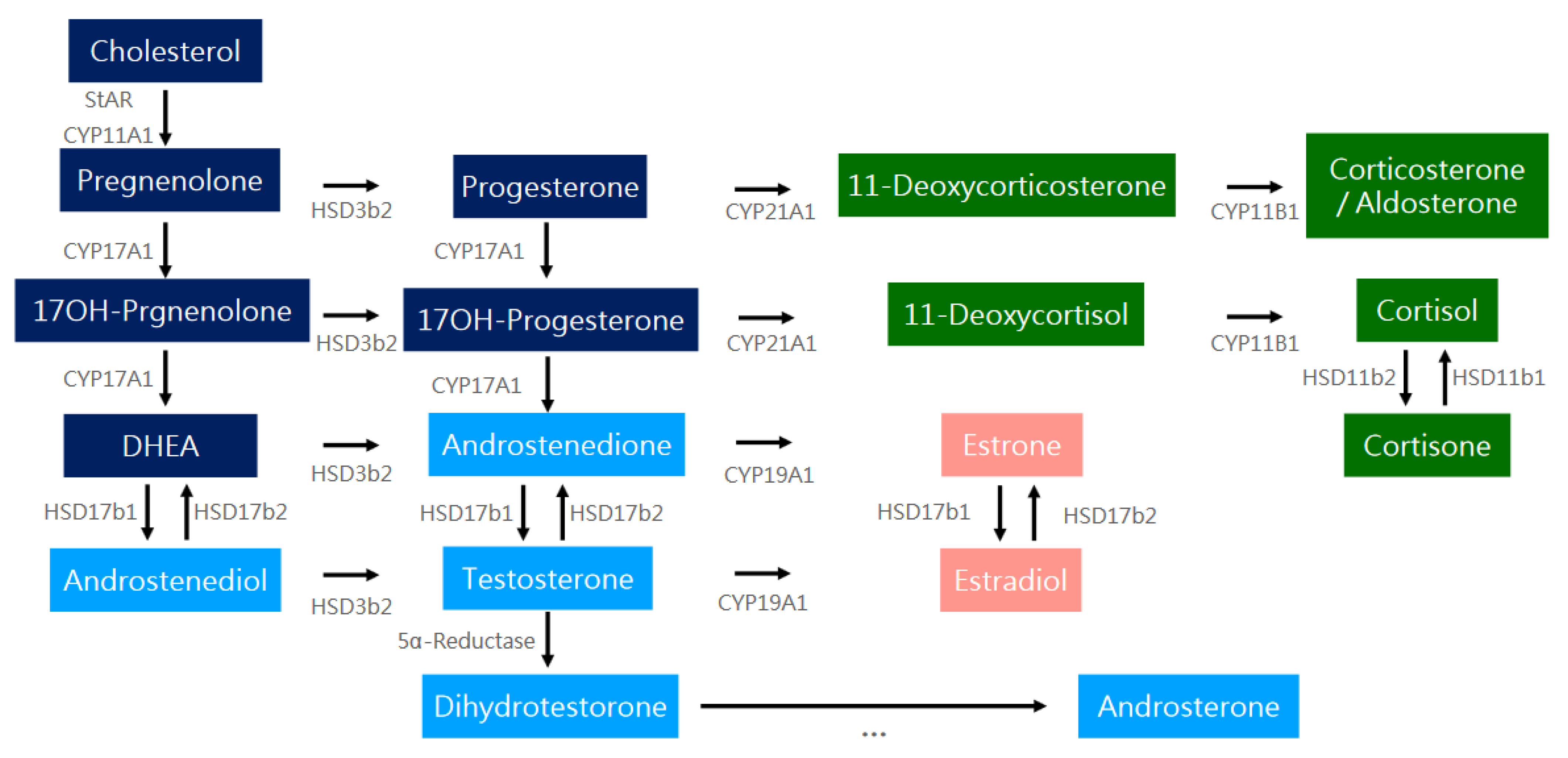
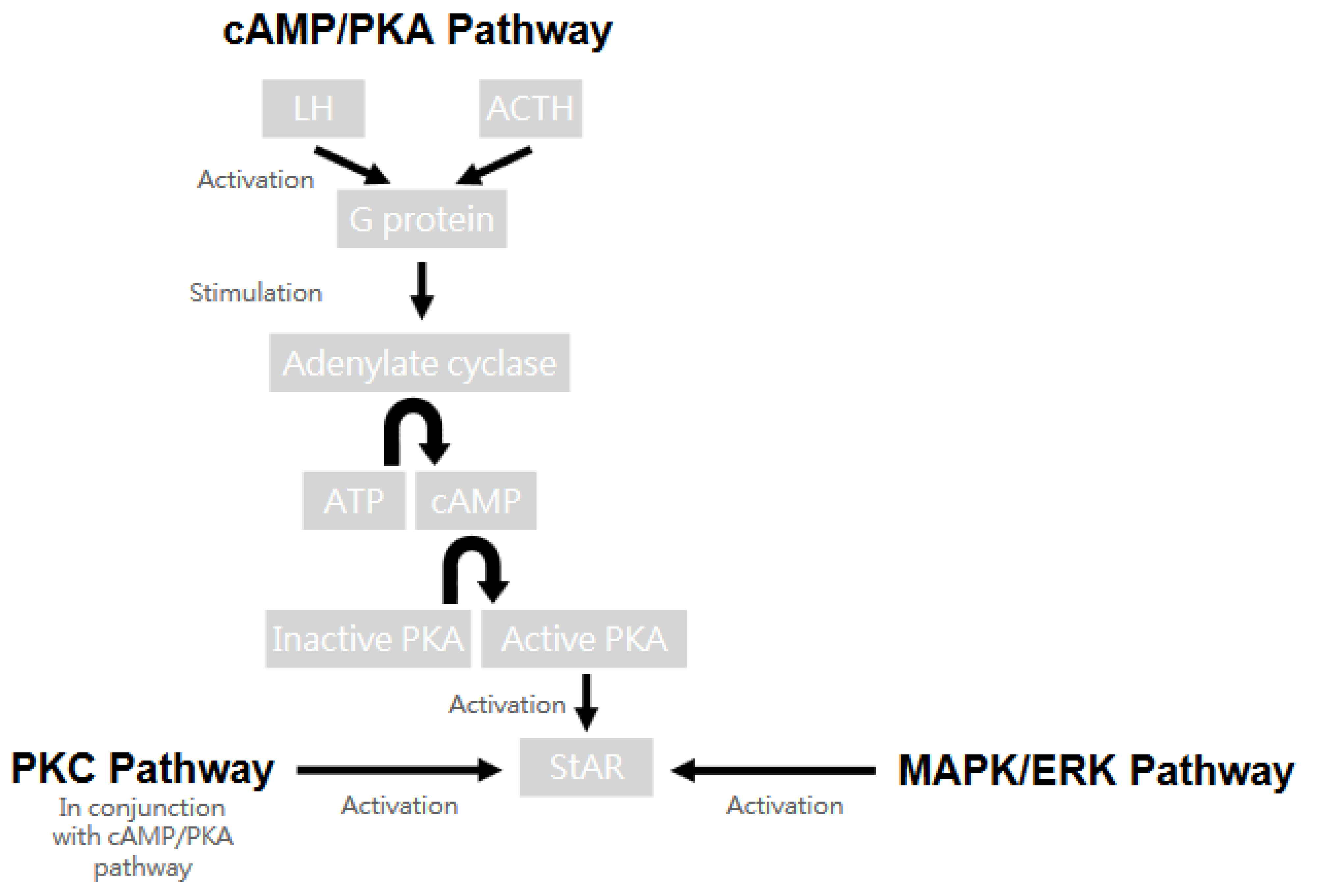
4.2.1. Steroidogenesis
4.2.2. Genes Involved in Hyperandrogenism
CYP11A
CYP17
CYP19
CYP21
Brief Summary of CYP Genes
4.3. Hyperinsulinemia and Insulin Resistance
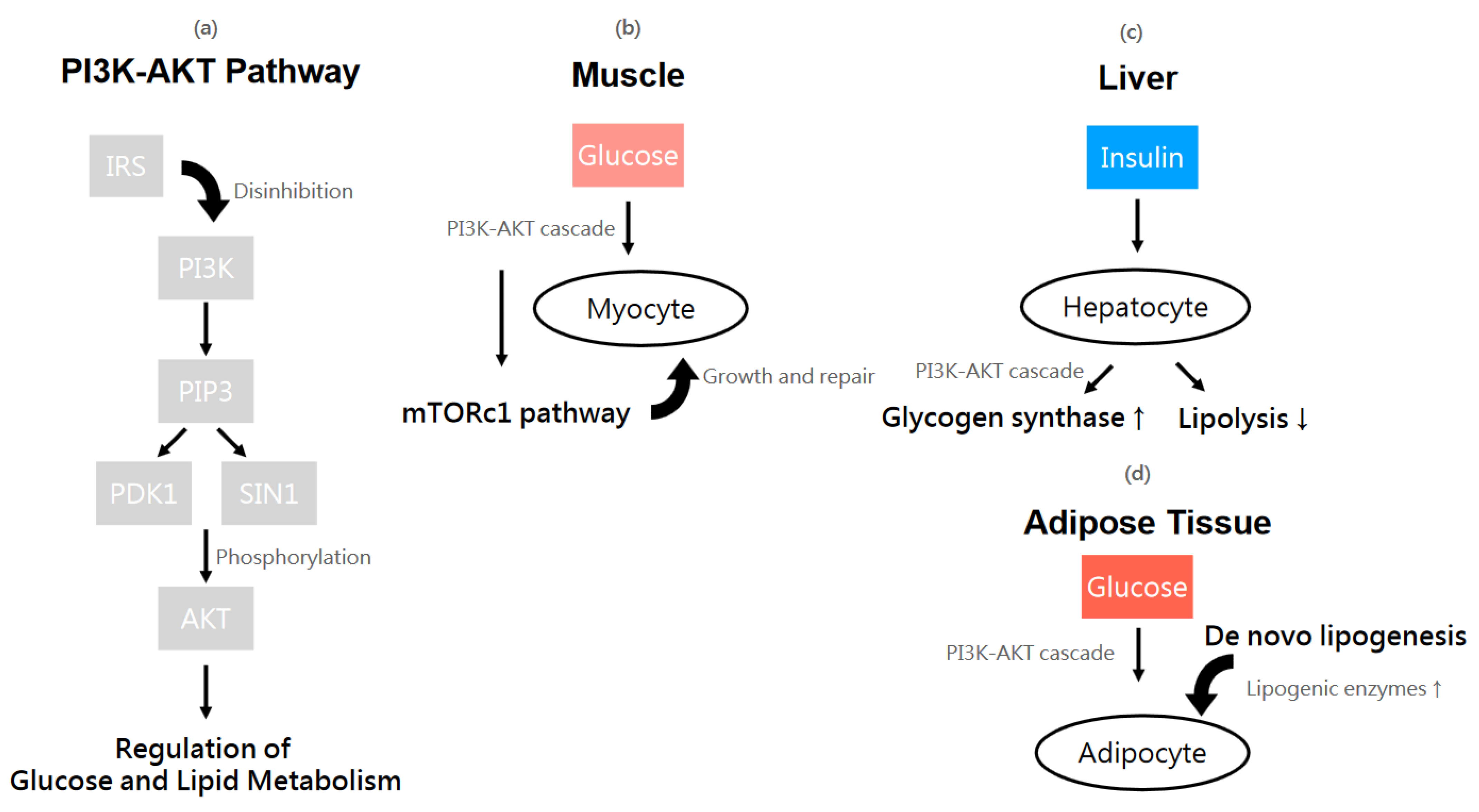
4.3.1. Insulin Signaling Pathways
4.3.2. Insulin Function and Glucose Utilization in Different Sites of Human Body
4.3.3. Genes Involved in Hyperinsulinemia and Insulin Resistance (IR)
4.4. Hyperandrogenism (HA), Insulin Resistance (IR), and PCOS
5. Management of PCOS
5.1. Lifestyle and Diet Modification
5.2. Medication of PCOS
5.2.1. Improvement in Hyperandrogenic Features
5.2.2. Management of Metabolic Disorders
Metformin
Thiazolidinediones (TZDs)
Other Drugs for Metabolic Control
5.2.3. Fertility Concerns: Ovulation and Contraception
Ovulation
Contraception
5.3. Surgical Intervention
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- McCartney, C.R.; Marshall, J.C. Clinical Practice. Polycystic Ovary Syndrome. N. Engl. J. Med. 2016, 375, 54–64. [Google Scholar] [CrossRef]
- Ndefo, U.A.; Eaton, A.; Green, M.R. Polycystic Ovary Syndrome: A Review of Treatment Options with a Focus on Pharmacological Approaches. Pharm. Ther. 2013, 38, 336–355. [Google Scholar]
- Deswal, R.; Narwal, V.; Dang, A.; Pundir, C.S. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Hum. Reprod. Sci. 2020, 13, 261–271. [Google Scholar]
- Goh, J.E.; Farrukh, M.J.; Keshavarzi, F.; Yap, C.S.; Saleem, Z.; Salman, A.; John, A. Assessment of Prevalence, Knowledge of Polycystic Ovary Syndrome and Health-related Practices among Women in Klang Valley: A Cross-sectional Survey. Front. Endocrinol. 2022, 13, 985588. [Google Scholar] [CrossRef]
- Wolf, W.M.; Wattick, R.A.; Kinkade, O.N.; Olfert, M.D. Geographical Prevalence of Polycystic Ovary Syndrome as Determined by Region and Race/Ethnicity. Int. J. Environ. Res. Public Health 2018, 15, 2589. [Google Scholar] [CrossRef] [PubMed]
- Engmann, L.; Jin, S.; Sun, F.; Legro, R.S.; Polotsky, A.J.; Hansen, K.R.; Coutifaris, C.; Diamond, M.P.; Eisenberg, E.; Zhang, H.; et al. Racial and Ethnic Differences in the Polycystic Ovary Syndrome Metabolic Phenotype. Am. J. Obstet. Gynecol. 2017, 216, e1–e493. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiao, J. Ethnic Differences in the Phenotypic Expression of Polycystic Ovary Syndrome. Steroids 2013, 78, 755–760. [Google Scholar] [CrossRef]
- Singh, S.; Pal, N.; Shubham, S.; Sarma, D.K.; Verma, V.; Marotta, F.; Kumar, M. Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. J. Clin. Med. 2023, 12, 1454. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, J.; Yang, S.; Li, Z. Risk Factors of Polycystic Ovarian Syndrome Among Li People. Asian Pac. J. Trop. Med. 2015, 8, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Peña, A.S.; Codner, E.; Witchel, S. Criteria for Diagnosis of Polycystic Ovary Syndrome during Adolescence: Literature Review. Diagnostics 2022, 12, 1931. [Google Scholar] [CrossRef]
- Christ, J.P.; Cedars, M.I. Current Guidelines for Diagnosing PCOS. Diagnostics 2023, 13, 1113. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Polycystic Ovary Syndrome: Definition, Aetiology, Diagnosis and Treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef]
- Khan, M.J.; Ullah, A.; Basit, S. Genetic Basis of Polycystic Ovary Syndrome (PCOS): Current Perspectives. Appl. Clin. Genet. 2019, 12, 249–260. [Google Scholar] [CrossRef]
- Rutkowska, A.Z.; Diamanti-Kandarakis, E. Polycystic Ovary Syndrome and Environmental Toxins. Fertil. Steril. 2016, 106, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, W.; Shi, Y.; Zhang, J.; Cui, L.; Chen, Z.J. Lifestyle and Environmental Contributions to Ovulatory Dysfunction in Women of Polycystic Ovary Syndrome. BMC Endocr. Disord. 2020, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Nabi, M.; Rasool, S.U.A.; Rashid, F.; Amin, S. Hyperandrogenism in Polycystic Ovarian Syndrome and Role of CYP Gene Variants: A Review. Egypt. J. Med. Hum. Genet. 2019, 20, 25. [Google Scholar] [CrossRef]
- Weiss, M.; Steiner, D.F.; Philipson, L.H. Insulin Biosynthesis, Secretion, Structure, and Structure-Activity Relationships. In Comprehensive Free Online Endocrinology Book; MDText.com Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279029/ (accessed on 21 April 2024).
- Riestenberg, C.; Jagasia, A.; Markovic, D.; Buyalos, R.P.; Azziz, R. Health Care-Related Economic Burden of Polycystic Ovary Syndrome in the United States: Pregnancy-Related and Long-Term Health Consequences. J. Clin. Endocrinol. Metab. 2022, 107, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Islam, H.; Masud, J.; Islam, Y.N.; Haque, F.K.M. An update on polycystic ovary syndrome: A review of the current state of knowledge in diagnosis, genetic etiology, and emerging treatment options. Women’s Health (Lond) 2022, 18, 17455057221117966. [Google Scholar] [CrossRef]
- Chang, S.; Dunaif, A. Diagnosis of Polycystic Ovary Syndrome: Which Criteria to Use and When? Endocrinol. Metab. Clin. N. Am. 2021, 50, 11–23. [Google Scholar] [CrossRef]
- Smet, M.E.; McLennan, A. Rotterdam criteria, the end. Australas. J. Ultrasound Med. 2018, 21, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, G.; Gainder, S.; Suri, V.; Sachdeva, N.; Chopra, S. Comparison of the Different PCOS Phenotypes Based on Clinical Metabolic, and Hormonal Profile, and their Response to Clomiphene. Indian. J. Endocrinol. Metab. 2019, 23, 326–331. [Google Scholar] [PubMed]
- Clark, N.M.; Podolski, A.J.; Brooks, E.D.; Chizen, D.R.; Pierson, R.A.; Lehotay, D.C.; Lujan, M.E. Prevalence of Polycystic Ovary Syndrome Phenotypes Using Updated Criteria for Polycystic Ovarian Morphology: An Assessment of Over 100 Consecutive Women Self-reporting Features of Polycystic Ovary Syndrome. Reprod. Sci. 2014, 21, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Bani Mohammad, M.; Majdi Seghinsara, A. Polycystic Ovary Syndrome (PCOS), Diagnostic Criteria, and AMH. Asian Pac. J. Cancer Prev. 2017, 18, 17–21. [Google Scholar]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: The complete task force report. Fertil. Steril. 2009, 91, 456–488. [Google Scholar] [CrossRef]
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Farhadi-Azar, M.; Aminorroaya, A.; Heidari-Beni, M.; Khamseh, F.; Nouri, M.; Kazerouni, F.; Amini, M. The Prevalence of Polycystic Ovary Syndrome, Its Phenotypes and Cardio-Metabolic Features in a Community Sample of Iranian Population: Tehran Lipid and Glucose Study. Front. Endocrinol. (Lausanne) 2022, 13, 825528. [Google Scholar] [CrossRef]
- Wawrzkiewicz-Jałowiecka, A.; Kowalczyk, K.; Trybek, P.; Duleba, A.J.; Kurzawa, R.; Bednarek, W.; Skrzypulec-Plinta, V.; Meczekalski, B.; Głowacka, I. In Search of New Therapeutics-Molecular Aspects of the PCOS Pathophysiology: Genetics, Hormones, Metabolism and Beyond. Int. J. Mol. Sci. 2020, 21, 7054. [Google Scholar] [CrossRef]
- Ajmal, N.; Khan, S.Z.; Shaikh, R. Polycystic ovary syndrome (PCOS) and genetic predisposition: A review article. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 3, 100060. [Google Scholar] [CrossRef] [PubMed]
- Hiam, D.; Moreno-Asso, A.; Teede, H.J.; Laven, J.S.; Stepto, N.K.; Moran, L.J. The Genetics of Polycystic Ovary Syndrome: An Overview of Candidate Gene Systematic Reviews and Genome-Wide Association Studies. J. Clin. Med. 2019, 8, 1606. [Google Scholar] [CrossRef]
- Crespo, R.P.; Bachega, T.A.S.S.; Mendonça, B.B.; Gomes, L.G. An update of genetic basis of PCOS pathogenesis. Arch. Endocrinol. Metab. 2018, 62, 352–361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nautiyal, H.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Afzal, M.; Alzarea, S.I.; Güven, E.; Al-Abbasi, F.A.; Kazmi, I. Polycystic Ovarian Syndrome: A Complex. Disease with a Genetics Approach. Biomedicines 2022, 10, 540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Urbanek, M.; Legro, R.S.; Driscoll, D.A.; Azziz, R.; Ehrmann, D.A.; Norman, R.J.; Strauss, J.F., III; Spielman, R.S.; Dunaif, A. Thirty-seven candidate genes for polycystic ovary syndrome: Strongest evidence for linkage is with follistatin. Proc. Natl. Acad. Sci. USA 1999, 96, 8573–8578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franks, S.; Gharani, N.; McCarthy, M. Candidate genes in polycystic ovary syndrome. Hum. Reprod. Update 2001, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Schade, D.S.; Shey, L.; Eaton, R.P. Cholesterol Review: A Metabolically Important Molecule. Endocr. Pract. 2020, 26, 1514–1523. [Google Scholar] [CrossRef]
- Shi, Q.; Yin, S.; Wang, J.; Liang, S.; Duan, Z. Intracellular Cholesterol Synthesis and Transport. Front. Cell Dev. Biol. 2022, 10, 819281. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Pramanik, J.; Mahata, B. Revisiting steroidogenesis and its role in immune regulation with the advanced tools and technologies. Genes. Immun. 2021, 22, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M.; Zhao, A.H.; Tu, L.N.; Morohaku, K.; Selvaraj, V. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: More complicated than we thought. Mol. Endocrinol. 2005, 19, 2647–2659. [Google Scholar] [CrossRef]
- Liang, J.J.; Rasmusson, A.M. Overview of the Molecular Steps in Steroidogenesis of the GABAergic Neurosteroids Allopregnanolone and Pregnanolone. Chronic Stress (Thousand Oaks) 2018, 2, 2470547018818555. [Google Scholar] [CrossRef]
- Gharani, N.; Waterworth, D.M.; Batty, S.; White, D.; Gilling-Smith, C.; Conway, G.S.; McCarthy, M.I. Association of the steroid synthesis gene CYP11a with polycystic ovary syndrome and hyperandrogenism. Hum. Mol. Genet. 1997, 6, 397–402. [Google Scholar] [CrossRef]
- Ashraf, S.; Rashid, F.; Nabi, M.; Shah, M.; Rasool, S.U.A.; Fazili, K.M.; Amin, S. CYP17 gene polymorphic sequence variation is associated with hyperandrogenism in Kashmiri women with polycystic ovarian syndrome. Gynecol. Endocrinol. 2021, 37, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.M.; Munir, S.; Akhtar, T.; Hanif, M.; Mazhar, F. Association of CYP17 gene polymorphism (rs743572) with polycystic ovary syndrome. Meta Gene 2022, 31, 100996. [Google Scholar] [CrossRef]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef]
- Ashraf, S.; Rasool, S.U.A.; Nabi, M.; Ganie, M.A.; Masoodi, S.R.; Amin, S. Impact of rs2414096 Polymorphism of CYP19 Gene on Susceptibility of Polycystic Ovary Syndrome and Hyperandrogenism in Kashmiri Women. Sci. Rep. 2021, 11, 12942. [Google Scholar] [CrossRef] [PubMed]
- Petry, C.J.; Ong, K.K.; Michelmore, K.F.; Balen, A.H.; Dunger, D.B. Association of Aromatase (CYP 19) Gene Variation with Features of Hyperandrogenism in Two Populations of Young Women. Hum. Reprod. 2005, 20, 1837–1843. [Google Scholar] [CrossRef]
- Xita, N.; Georgiou, I.; Lazaros, L.; Psofaki, V.; Kolios, G.; Tsatsoulis, A. CYP19 Gene: A Genetic Modifier of Polycystic Ovary Syndrome Phenotype. Fertil. Steril. 2010, 94, 250–254. [Google Scholar] [CrossRef]
- Speiser, P.W.; Azziz, R.; Baskin, L.S.; Ghizzoni, L.; Hensle, T.W.; Merke, D.P.; Meyer-Bahlburg, H.F.; Montori, V.M.; Oberfield, S.E.; Ritzen, E.M. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 4043–4088. [Google Scholar] [CrossRef] [PubMed]
- Witchel, S.F.; Aston, C.E. The Role of Heterozygosity for CYP21 in the Polycystic Ovary Syndrome. J. Pediatr. Endocrinol. Metab. 2000, 13 (Suppl. S5), 1315–1317. [Google Scholar] [PubMed]
- Witchel, S.F.; Oberfield, S.E.; Rosenfield, R.L. Prevalence of CYP21 Mutations and IRS1 Variant among Women with Polycystic Ovary Syndrome and Adrenal Androgen Excess. Fertil. Steril. 2005, 83, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.C. Understanding Insulin and Its Receptor from Their Three-Dimensional Structures. Mol. Metab. 2021, 52, 101255. [Google Scholar] [CrossRef]
- Landreh, M.; Kenney, J.W.; Ortiz-Perez, A.; Bouley, R.; Vigna, S.R.; Ng, S.S.; Ward, R.E.; Aponte, G.W. The Structure, Molecular Interactions and Bioactivities of Proinsulin C-Peptide Correlate with a Tripartite Molecule. Biomol. Concepts 2014, 5, 109–118. [Google Scholar] [CrossRef]
- Kitabchi, A.E. Proinsulin and C-Peptide: A Review. Metabolism 1977, 26, 547–587. [Google Scholar] [CrossRef]
- Leighton, E.; Sainsbury, C.A.; Jones, G.C. A Practical Review of C-Peptide Testing in Diabetes. Diabetes Ther. 2017, 8, 475–487. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- De Meyts, P. The Insulin Receptor and Its Signal Transduction Network. In Comprehensive Free Online Endocrinology Book; MDText.com Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK378978/ (accessed on 20 May 2024).
- White, M.F.; Kahn, C.R. Insulin Action at a Molecular Level—100 Years of Progress. Mol. Metab. 2021, 52, 101304. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [PubMed]
- Sylow, L.; Kleinert, M.; Richter, E.A.; Jensen, T.E. The Many Actions of Insulin in Skeletal Muscle, the Paramount Tissue Determining Glycemia. Cell Metab. 2021, 33, 758–780. [Google Scholar] [CrossRef]
- Edgerton, D.S.; Lautz, M.; Scott, M.; Everett, C.A.; Stettler, K.M.; Neal, D.W.; Chu, C.A.; Williams, P.E.; Cherrington, A.D. Insulin’s Direct Effects on the Liver Dominate the Control of Hepatic Glucose Production. J. Clin. Investig. 2006, 116, 521–527. [Google Scholar] [CrossRef]
- Guerra, S.; Gastaldelli, A. The Role of the Liver in the Modulation of Glucose and Insulin in Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes. Curr. Opin. Pharmacol. 2020, 55, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Titchenell, P.M.; Lazar, M.A.; Birnbaum, M.J. Unraveling the Regulation of Hepatic Metabolism by Insulin. Trends Endocrinol. Metab. 2017, 28, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; McGraw, T.E.; Kahn, B.B. Insulin Action in Adipocytes, Adipose Remodeling, and Systemic Effects. Cell Metab. 2021, 33, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.; Mitrou, P.; Lambadiari, V.; Maratou, E.; Raptis, S.A. Insulin Effects in Muscle and Adipose Tissue. Diabetes Res. Clin. Pract. 2011, 93 (Suppl. S1), S52–S59. [Google Scholar] [CrossRef]
- Brown, A.E.; Walker, M. Genetics of Insulin Resistance and the Metabolic Syndrome. Curr. Cardiol. Rep. 2016, 18, 75. [Google Scholar] [CrossRef]
- Parikh, H.M.; Chan, Y.Y.; Lin, S.J.; Liao, C.W.; Wu, C.S.; Chen, C.J.; Chen, Y.T. Relationship Between Insulin Sensitivity and Gene Expression in Human Skeletal Muscle. BMC Endocr. Disord. 2021, 21, 32. [Google Scholar] [CrossRef]
- Chen, Z.; McIntosh, C.; Aronow, B.; Davenport, M.L.; Fortunato, R.S.; Menon, R.K.; Radovick, S.; Porter, K.L. Functional Screening of Candidate Causal Genes for Insulin Resistance in Human Preadipocytes and Adipocytes. Circ. Res. 2020, 126, 330–346. [Google Scholar] [CrossRef] [PubMed]
- Semple, R.K.; Savage, D.B.; Chatterjee, V.K.; O’Rahilly, S. Genetic Syndromes of Severe Insulin Resistance. Endocr. Rev. 2011, 32, 498–514. [Google Scholar] [CrossRef] [PubMed]
- Harada, M. Pathophysiology of Polycystic Ovary Syndrome Revisited: Current Understanding and Perspectives Regarding Future Research. Reprod. Med. Biol. 2022, 21, e12487. [Google Scholar] [CrossRef]
- Balen, A. The Pathophysiology of Polycystic Ovary Syndrome: Trying to Understand PCOS and Its Endocrinology. Best. Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 685–706. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Jozkowiak, M.; Imakawa, K.; Takahashi, T. Endocrine Disrupting Chemicals in Polycystic Ovary Syndrome: The Relevant Role of the Theca and Granulosa Cells in the Pathogenesis of the Ovarian Dysfunction. Cells 2022, 12, 174. [Google Scholar] [CrossRef]
- Lentscher, J.A.; Decherney, A.H. Clinical Presentation and Diagnosis of Polycystic Ovarian Syndrome. Clin. Obstet. Gynecol. 2021, 64, 3–11. [Google Scholar] [CrossRef]
- Sidra, S.; Tariq, M.H.; Farrukh, M.J.; Mohsin, M. Evaluation of Clinical Manifestations, Health Risks, and Quality of Life Among Women with Polycystic Ovary Syndrome. PLoS ONE 2019, 14, e0223329. [Google Scholar] [CrossRef]
- Ranathunga, I.; Rameez, M.F.; Ranasinghe, P.; Katulanda, P. Evaluation of Socio-Demographic and Clinical Characteristics of PCOS Patients Attending a Tertiary Care Institute in Colombo. BMC Endocr. Disord. 2022, 22, 289. [Google Scholar] [CrossRef]
- McManus, S.S.; Levitsky, L.L.; Misra, M. Polycystic Ovary Syndrome: Clinical Presentation in Normal-Weight Compared with Overweight Adolescents. Endocr. Pract. 2013, 19, 471–478. [Google Scholar] [CrossRef][Green Version]
- Lim, S.S.; Hutchison, S.K.; Van Ryswyk, E.; Norman, R.J.; Teede, H.J. Lifestyle Changes in Women with Polycystic Ovary Syndrome. Cochrane Database Syst. Rev. 2019, 3, Cd007506. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Luo, W.; Huang, Y.; Li, M.; Jiang, L.; Liu, H. Dietary Interventions: A Promising Treatment for Polycystic Ovary Syndrome. Ann. Nutr. Metab. 2021, 77, 313–323. [Google Scholar] [CrossRef]
- Szczuko, M.; Skowronek, M.; Ziętek, M.; Kikut, J.; Czerwińska, E.; Maciejewska, D.; Komorniak, N. Nutrition Strategy and Life Style in Polycystic Ovary Syndrome-Narrative Review. Nutrients 2021, 13, 2452. [Google Scholar] [CrossRef]
- Cowan, S.; Ogutu, S.; Small, C. Lifestyle Management in Polycystic Ovary Syndrome—Beyond Diet and Physical Activity. BMC Endocr. Disord. 2023, 23, 14. [Google Scholar] [CrossRef]
- Rashid, R.; Attia, G.R.; Shafik, H.E. Polycystic Ovarian Syndrome-Current Pharmacotherapy and Clinical Implications. Taiwan. J. Obstet. Gynecol. 2022, 61, 40–50. [Google Scholar] [CrossRef]
- Radosh, L. Drug Treatments for Polycystic Ovary Syndrome. Am. Fam. Physician 2009, 79, 671–676. [Google Scholar]
- Ehrmann, D.A. Polycystic Ovary Syndrome. N. Engl. J. Med. 2005, 352, 1223–1236. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, Y. Metformin and Its Benefits for Various Diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The Mechanisms of Action of Metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Attia, G.M.; Almouteri, M.M.; Alnakhli, F.T. Role of Metformin in Polycystic Ovary Syndrome (PCOS)-Related Infertility. Cureus 2023, 15, e44493. [Google Scholar] [CrossRef]
- Vasudevan, A.R.; Balasubramanyam, A. Thiazolidinediones: A Review of Their Mechanisms of Insulin Sensitization, Therapeutic Potential, Clinical Efficacy, and Tolerability. Diabetes Technol. Ther. 2004, 6, 850–863. [Google Scholar] [CrossRef]
- Chiarelli, F.; Di Marzio, D. Peroxisome Proliferator-Activated Receptor-Gamma Agonists and Diabetes: Current Evidence and Future Perspectives. Vasc. Health Risk Manag. 2008, 4, 297–304. [Google Scholar] [PubMed]
- Froment, P.; Touraine, P. Thiazolidinediones and Fertility in Polycystic Ovary Syndrome (PCOS). PPAR Res. 2006, 2006, 73986. [Google Scholar] [CrossRef]
- Zhao, H.; Lin, S.; Li, Y.; Li, X.; Wang, W.; Zhang, W. Comparative Efficacy of Oral Insulin Sensitizers Metformin, Thiazolidinediones, Inositol, and Berberine in Improving Endocrine and Metabolic Profiles in Women with PCOS: A Network Meta-Analysis. Reprod. Health 2021, 18, 171. [Google Scholar] [CrossRef]
- Duleba, A.J. The Role of Heterozygosity for CYP21 PCOS. Steroids 2012, 77, 306–311. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Siddiqui, M.F.; Alnaimy, A.R.; Islam, N. A Review of Therapeutic Options for Managing the Metabolic Aspects of Polycystic Ovary Syndrome. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820938305. [Google Scholar] [CrossRef]
- Cunha, A.; Póvoa, A.M. Infertility Management in Women with Polycystic Ovary Syndrome: A Review. Porto Biomed. J. 2021, 6, e116. [Google Scholar] [CrossRef] [PubMed]
- Dickey, R.P.; Holtkamp, D.E. Development, Pharmacology and Clinical Experience with Clomiphene Citrate. Hum. Reprod. Update 1996, 2, 483–506. [Google Scholar] [CrossRef] [PubMed]
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Taylor, A.E.; Lambertini, L.; Sookhai, L.; Palomba, S.; et al. The Management of Anovulatory Infertility in Women with Polycystic Ovary Syndrome: An Analysis of the Evidence to Support the Development of Global WHO Guidance. Hum. Reprod. Update 2016, 22, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.P.; Dowsett, M.; Bhatnagar, A.S.; Santner, S.J.; Tormey, D.C.; Stoll, B.A.; Miller, W.R. The Pharmacology of Letrozole. J. Steroid Biochem. Mol. Biol. 2003, 87, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.G.; Kalyani, A.; Das, S.; Gupta, S.; Chatterjee, S. Letrozole: Pharmacology, Toxicity and Potential Therapeutic Effects. Life Sci. 2022, 310, 121074. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Zhang, Q.; Xue, Q. Letrozole Compared with Clomiphene Citrate for Polycystic Ovarian Syndrome: A Systematic Review and Meta-Analysis. Obstet. Gynecol. 2023, 141, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Tsiami, A.P.; Karkanaki, A.; Dimitriadis, G.K.; Christodoulaki, C.; Calis, K.A.; Piperi, C. Higher Ovulation Rate with Letrozole as Compared with Clomiphene Citrate in Infertile Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hormones (Athens) 2021, 20, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Elnashar, A.M. The Role of Metformin in Ovulation Induction: Current Status. Middle East. Fertil. Soc. J. 2011, 16, 175–181. [Google Scholar] [CrossRef]
- Johnson, N.P. Metformin Use in Women with Polycystic Ovary Syndrome. Ann. Transl. Med. 2014, 2, 56. [Google Scholar]
- Teal, S.; Edelman, A. Contraception Selection, Effectiveness, and Adverse Effects: A Review. JAMA 2021, 326, 2507–2518. [Google Scholar] [CrossRef]
- Forslund, M.; Jensen, M.L.; Grinsted, J.; Glintborg, D.; Ravn, P.; Andersen, M.S. Different Kinds of Oral Contraceptive Pills in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Eur. J. Endocrinol. 2023, 189, S1–S16. [Google Scholar] [CrossRef]
- de Melo, A.S.; Dias, S.V.; Cavalli, R.D.C.; Cardoso, V.C.; Bettiol, H.; Barbieri, M.A.; Simões, M.J. Hormonal Contraception in Women with Polycystic Ovary Syndrome: Choices, Challenges, and Noncontraceptive Benefits. Open Access J. Contracept. 2017, 8, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Gomel, V.; Yarali, H. Surgical Treatment of Polycystic Ovary Syndrome Associated with Infertility. Reprod. Biomed. Online 2004, 9, 35–42. [Google Scholar] [CrossRef]
- Della Corte, L.; Barra, F.; Farthing, A.; Bifulco, G.; Di Martino, G.; Ferrero, S.; Giampaolino, P. Is There Still a Place for Surgery in Patients with PCOS? A Review. Life 2023, 13, 1270. [Google Scholar] [CrossRef]
- Rath, S.K.; Sharma, R.K.; Duggal, B.S. Surgical Approach for Polycystic Ovarian Syndrome in Management of Infertility. Med. J. Armed Forces India 2006, 62, 119–122. [Google Scholar] [CrossRef][Green Version]
- Samarasinghe, S.N.S.; Woods, C.; Miras, A.D. Bariatric Surgery in Women with Polycystic Ovary Syndrome. Metabolism 2024, 151, 155745. [Google Scholar] [CrossRef]
- Bhandari, M.; Naik, N.; Lalwani, S.; Londhe, R.; Parmar, C.; Zaveri, H.; Kalyani, A.; Shah, M.; Adalja, M.; Ramachandran, S. Effects of Bariatric Surgery on People with Obesity and Polycystic Ovary Syndrome: A Large Single Center Study from India. Obes. Surg. 2022, 32, 3305–3312. [Google Scholar] [CrossRef]
- McGill, D.J.; Hutchison, C.; McKenzie, E.; McSherry, E.; Mackay, I.R. Laser hair removal in women with polycystic ovary syndrome. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.N. Electrolysis for the treatment of hypertrichosis and hirsutism. Skin. Ther. Lett. 1999, 4, 3–4. [Google Scholar] [PubMed]
- Xue, Z.; Feng, Y.; Lin, X.; Qiao, J. Research Progress on the Mechanism Between Polycystic Ovary Syndrome and Abnormal Endometrium. Front. Physiol. 2021, 12, 788772. [Google Scholar] [CrossRef] [PubMed]


| Feature | The NIH Criteria | The Rotterdam Criteria | The AE-PCOS Criteria |
|---|---|---|---|
| Hyperandrogenism (HA) | Biochemical or clinical evidence | Biochemical or clinical evidence | Biochemical or clinical evidence |
| Ovulatory dysfunction (OD) | Chronic oligo-anovulation | Chronic oligo-anovulation | Chronic oligo-anovulation |
| Polycystic ovarian morphology (PCO) | At least 12 follicles at the size of 2–9 mm in diameter or an ovarian volume > 10 cm3 in one or both ovaries | Polycystic ovarian appearance on imaging | |
| Features required for diagnosis | Both HA + OD | 2 of 3 | HA + at least one other criteria |
| Phenotypes | HA + OD + PCO | HA + OD HA + PCO OD + PCO HA + OD + PCO | HA + OD HA + PCO HA + OD + PCO |
| Measures | Details |
|---|---|
| Lifestyle and diet modification (first-line treatment) |
|
| Medication |
|
| Surgical intervention |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.-J.; Chen, J.-H.; Chen, K.-H. The Pathophysiological Mechanism and Clinical Treatment of Polycystic Ovary Syndrome: A Molecular and Cellular Review of the Literature. Int. J. Mol. Sci. 2024, 25, 9037. https://doi.org/10.3390/ijms25169037
Chang K-J, Chen J-H, Chen K-H. The Pathophysiological Mechanism and Clinical Treatment of Polycystic Ovary Syndrome: A Molecular and Cellular Review of the Literature. International Journal of Molecular Sciences. 2024; 25(16):9037. https://doi.org/10.3390/ijms25169037
Chicago/Turabian StyleChang, Kai-Jung, Jie-Hong Chen, and Kuo-Hu Chen. 2024. "The Pathophysiological Mechanism and Clinical Treatment of Polycystic Ovary Syndrome: A Molecular and Cellular Review of the Literature" International Journal of Molecular Sciences 25, no. 16: 9037. https://doi.org/10.3390/ijms25169037
APA StyleChang, K.-J., Chen, J.-H., & Chen, K.-H. (2024). The Pathophysiological Mechanism and Clinical Treatment of Polycystic Ovary Syndrome: A Molecular and Cellular Review of the Literature. International Journal of Molecular Sciences, 25(16), 9037. https://doi.org/10.3390/ijms25169037







