The Complex World of Kynurenic Acid: Reflections on Biological Issues and Therapeutic Strategy
Abstract
1. Introduction
2. Biological Issues
2.1. Generation and Movement of Kynurenic Acid
2.1.1. Cells and Transporters
2.1.2. IDO1 Expression
2.1.3. IDO1 Efflux
2.1.4. IDO1-Independent Production
2.1.5. Kynurenic Acid Concentrations
2.1.6. Factors Affecting Concentration–Effect Relationships
2.1.7. Kynurenic Acid in the CNS
3. Sites of Action
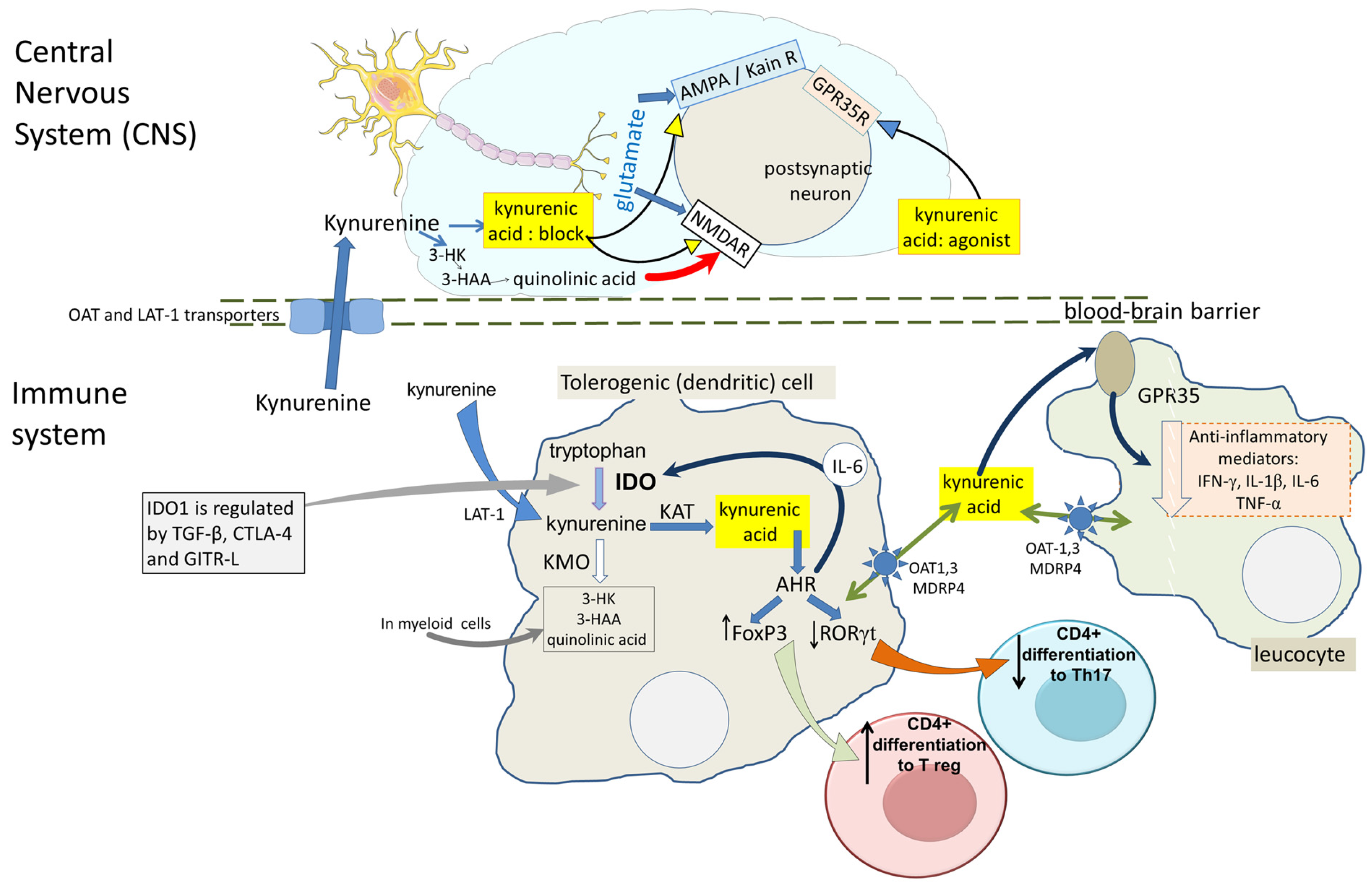
3.1. Glutamate Receptors
3.2. G-Protein Coupled Receptor-35 (GPR35)
3.3. Aryl Hydrocarbon Receptors (AHRs)
3.4. Hydroxy-Carboxylic Acid Receptors (HCAR)
3.5. Nicotinic Receptors
4. Emerging Targets and Mechanisms
4.1. Is the KP a Cause or an Effect?
4.2. CNS Disorders
4.2.1. Neurodegeneration
4.2.2. Neurodevelopmental Disorders: Schizophrenia
4.2.3. Depression
4.2.4. The Need for Drug Selectivity
4.3. Peripheral Tissues
4.3.1. Cytoprotection: Cytoskeletal Modulation and Wound Healing
4.3.2. Anti-Inflammatory Properties
4.4. Dietary and Metabolic Factors
4.4.1. Vitamins
4.4.2. Diabetes and Pancreatic Function
4.5. Kynurenic Acid and Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AHR | Aryl Hydrocarbon Receptor |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| BD | Bipolar depressive disorder |
| CD26 | Cluster of Differentiation marker 26, and a dipeptidyl-peptidase-4 enzyme |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| CTLA-4 | Cytotoxic Lymphocyte Antigen-4 (CTLA-4) |
| cyp 1A1 | Cytochrome P450, family 1 metabolic enzyme |
| GiT | Gastrointestinal tract |
| GITR-L | Glucose-Induced TNF Receptor-Related Ligand |
| GPR35 | G-Protein Coupled Receptor-3 |
| HCAR | Hydroxy-carboxylic acid receptors |
| 5-HIAA | 5-hydroxyindole-acetic acid |
| 3-HK | 3-hydroxykynurenine |
| 5-HT | 5-hydroxytryptamine, serotonin |
| HtrA-1 | High temperature requiring protein A-1 |
| IDO | Indoleamine-2,3-dioxygenase |
| IFN-γ | Interferon-γ |
| IL- | Interleukin- |
| IL4i1 | Interleukin-4-induced protein-1 |
| I3PA | Indole-3-propionic acid |
| I3PyA | Indole-3-pyruvic acid |
| JNK | Jun-N-terminal kinases |
| KAT | Kynurenine aminotransferase |
| KMO | Kynurenine-3-mono-oxygenase |
| KP | Kynurenine pathway |
| K/Q ratio | Kynurenic acid/quinolinic acid ratio |
| K/T ratio | Kynurenine/tryptophan ratio |
| LAT-1 | Large Neutral Amino Acid Transporter-1 |
| LPS | Bacterial lipopolysaccharides |
| MDD | Major depressive disorder |
| MRP4 | Multidrug resistance associated protein 4 |
| NAD | Nicotinamide adenine dinucleotide (NAD+) |
| NMDA | N-methyl-D-aspartate |
| OAT | Organic Anion Transporter |
| PAH | Para-aminohippuric acid |
| PBMCs | Peripheral blood mononuclear cells |
| PFC | Prefrontal cortex |
| PSA | Prostate Specific Antigen |
| Ro61-8048 | 3,4-dimethoxy-N-[4-(3-nitrophenyl)thiazol-2-yl]benzenesulphonamide |
| TDO | Tryptophan-2,3-dioxygenase |
| TGF-β1 | Transforming Growth Factor-β1 |
| TLR | Toll-Like Receptors |
| TNF | Tumour Necrosis Factor-α |
References
- Yoshida, R.; Imanishi, J.; Oku, T.; Kishida, T.; Hayaishi, O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc. Natl. Acad. Sci. USA 1981, 78, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W. The Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 1993, 45, 309–379. [Google Scholar] [PubMed]
- Stone, T.W.; Clanchy, F.I.L.; Huang, Y.-S.; Chiang, N.Y.; Darlington, L.G.; Williams, R.O. An integrated cytokine and kynurenine network as the basis of neuroimmune communication. Front. Neurosci. 2022, 16, 1002004. [Google Scholar] [CrossRef]
- Stone, T.W.; Darlington, L.G. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Brit. J. Pharmacol. 2013, 169, 1211–1227. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Williams, R.O. Tryptophan metabolism as a ‘reflex’ feature of neuroimmune communication: Sensor and effector functions for the indoleamine-2, 3-dioxygenase kynurenine pathway. J. Neurochem. 2024, in press. [Google Scholar] [CrossRef]
- Von Liebig, J. Uber Kynurensäure. Ann. Der Chem. 1853, 86, 125–126. [Google Scholar] [CrossRef]
- Badawy, A.A.B. Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Badawy, A.A.B. Hypothesis kynurenic and quinolinic acids: The main players of the kynurenine pathway and opponents in inflammatory disease. Med. Hypotheses 2018, 118, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.S.; Sundaram, G.; Heng, B.; Krishnamurthy, S.; Brew, B.J.; Guillemin, G.J. Recent advances in clinical trials targeting the kynurenine pathway. Pharmacol. Therap. 2022, 236, 108055. [Google Scholar] [CrossRef]
- Swartz, K.J.; During, M.J.; Freese, A.; Beal, M.F. Cerebral synthesis and release of kynurenic acid-an endogenous antagonist of excitatory amino-acid receptors. J. Neurosci. 1990, 10, 2965–2973. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Q.; Fuxe, K.; Schwarcz, R. Neuronal A1 receptors mediate increase in extracellular kynurenic acid after local intrastriatal adenosine infusion. J. Neurochem. 2004, 90, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Ceresoli-Borroni, G.; Rassoulpour, A.; Wu, H.Q.; Guidetti, P.; Schwarcz, R. Chronic neuroleptic treatment reduces endogenous kynurenic acid levels in rat brain. J. Neural Transm. 2006, 113, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Kiss, C.; Ceresoli-Borroni, G.; Guidetti, P.; Zielke, C.L.; Zielke, H.R.; Schwarcz, R. Kynurenate production by cultured human astrocytes. J. Neural Transm. 2003, 110, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stazka, J.; Luchowski, P.; Wielosz, M.; Kleinrok, Z.; Urbanska, E.M. Endothelium-dependent production and liberation of kynurenic acid by rat aortic rings exposed to L-kynurenine. Europ. J. Pharmacol. 2002, 448, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Stazka, J.; Luchowski, P.; Urbanska, E.M. Homocysteine, a risk factor for atherosclerosis, biphasically changes the endothelial production of kynurenic acid. Europ. J. Pharmacol. 2005, 517, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Turski, W.A.; Gramsbergen, J.B.; Traitler, H.; Schwarcz, R. Rat brain slices produce and liberate kynurenic acid upon exposure to L-kynurenine. J. Neurochem. 1989, 52, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Herédi, J.; Cseh, E.K.; Berkó, A.M.; Veres, G.; Zádori, D.; Toldi, J.; Kis, Z.; Vécsei, L.; Ono, E.; Gellért, L. Investigating KYNA production and kynurenergic manipulation on acute mouse brain slice preparatis. Brain Res. Bull. 2019, 146, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Notarangelo, F.M.; Beggiato, S.; Schwarcz, R. Assessment of prenatal kynurenine metabolism using tissue slices: Focus on the neosynthesis of kynurenic acid in mice. Develop. Neurosci. 2019, 41, 102–111. [Google Scholar] [CrossRef]
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood-brain barrier transport of kynurenines: Implications for brain synthesis and metabolism. J. Neurochem. 1991, 56, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Speciale, C.; Hares, K.; Schwarcz, R.; Brookes, N. High-affinity uptake of l-kynurenine by a na+-independent transporter of neutral amino-acids in astrocytes. J. Neurosci. 1989, 9, 2066–2072. [Google Scholar] [CrossRef] [PubMed]
- Uwai, Y.; Honjo, H.; Iwamoto, K. Interaction and transport of kynurenic acid via human organic anion transporters hOAT1 and hOAT3. Pharmacol. Res. 2012, 65, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Uwai, Y.; Hara, H.; Iwamoto, K. Transport of Kynurenic Acid by Rat Organic Anion Transporters rOAT1 and rOAT3: Species Difference between Human and Rat in OAT1. Int. J. Tryptophan Res. 2013, 6, S11206. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.R.; Ran, F.L.; Xin, M.Y.; Gou, X.Y.; Wang, X.Y.; Wu, X.A. Albumin-bound kynurenic acid is an appropriate endogenous biomarker for assessment of the renal tubular OATs-MRP4 channel. J. Pharm. Anal. 2023, 13, 1205–1220. [Google Scholar] [CrossRef]
- Tang, J.; Shen, H.; Zhao, X.F.; Holenarsipur, V.K.; Mariappan, T.T.; Zhang, Y.P.; Panfen, E.; Zheng, J.; Humphreys, W.G.; Lai, Y.R. Endogenous Plasma Kynurenic Acid in Human: A Newly Discovered Biomarker for Drug-Drug Interactions Involving Organic Anion Transporter 1 and 3 Inhibition. Drug Metab. Dispos. 2021, 49, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Molnár, K.; Lőrinczi, B.; Fazakas, C.; Szatmári, I.; Fülöp, F.; Kmetykó, N.; Berkecz, R.; Ilisz, I.; Krizbai, I.A.; Wilhelm, I.; et al. SZR-104, a Novel Kynurenic Acid Analogue with High Permeability through the Blood–Brain Barrier. Pharmaceutics 2021, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, L.V.; Neyens, D.; Ramsay, G.; Taylor, P.M.; Cantrell, D.A. Single cell analysis of kynurenine and System L amino acid transport in T cells. Nat. Commun. 2018, 9, 1981. [Google Scholar] [CrossRef]
- Ogbechi, J.; Wright, H.L.; Balint, S.; Topping, L.M.; Kristina, Z.; Huang, Y.S.; Pantazi, E.; Swart, M.; Windell, D.; Marin, E.; et al. LAT1 enables T cell activation under inflammatory conditions: A new therapeutic target for rheumatoid arthritis. J. Autoimmun. 2023, 138, 103031. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, Y.D.; Babu, E.; Ganapathy, V. Interferon-γ induces a tryptophan-selective amino acid transporter in human colonic epithelial cells and mouse dendritic cells. Biochim. Biophys. Acta Biomembr. 2015, 1848, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Silk, J.D.; Lakhal, S.; Laynes, R.; Vallius, L.; Karydis, I.; Marcea, C.; Boyd, C.A.R.; Cerundolo, V. IDO Induces Expression of a Novel Tryptophan Transporter in Mouse and Human Tumor Cells. J. Immunol. 2011, 187, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Gonzalez, G.; Jacobs, K.R.; Don, E.; Cole, N.J.; Adams, S.; Lim, C.K.; Lovejoy, D.B.; Guillemin, G.J. Kynurenine 3-monooxygenase activity in human primary neurons and effect on cellular bioenergetics identifies new neurotoxic mechanisms. Neurotox. Res. 2019, 35, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Sathyasaikumar, K.V.; de la Cruz, V.P.; Pineda, B.; Cervantes, G.I.V.; Ortega, D.R.; Donley, D.W.; Severson, P.L.; West, B.L.; Giorgini, F.; Fox, J.H.; et al. Cellular localization of kynurenine 3-monooxygenase in the brain: Challenging the dogma. Antioxidants 2022, 11, 315. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, P.; Hoffman, G.E.; Melendez-Ferro, M.; Albuquerque, E.X.; Schwarcz, R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia 2007, 55, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Zmarowski, A.; Wu, H.Q.; Brooks, J.M.; Potter, M.C.; Pellicciari, R.; Schwarcz, R.; Bruno, J.P. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Europ. J. Neurosci. 2009, 29, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Perkins, M.N. Quinolinic acid: An endogenous neuronal excitant at glutamate receptors in the CNS. Europ. J. Pharmacol. 1981, 72, 411–412. [Google Scholar] [CrossRef]
- Perkins, M.N.; Stone, T.W. The pharmacology and regional variations of quinolinic acid evoked excitations in the rat CNS. J. Pharmacol. Exp. Therap. 1983, 226, 551–557. [Google Scholar]
- Guillemin, G.J. Quinolinic acid, the inescapable toxin. FEBS J. 2012, 279, 1356–1365. [Google Scholar] [CrossRef]
- Stone, T.W.; Forrest, C.M.; Darlington, L.G. kynurenine pathway inhibition as a therapeutic strategy for neuroprotection. FEBS J. 2012, 279, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.L.; Xu, X.D.; Bai, Y.L.; Wang, X.P.; Wu, Y.B.; Zhong, J.; Wu, Q.Y.; Luo, Y.J.; Shang, T.B.; Shen, R.P.; et al. Therapeutic potential of targeting kynurenine pathway in neurodegenerative diseases. Europ. J. Med. Chem. 2023, 251, 115258. [Google Scholar] [CrossRef] [PubMed]
- Walczak, K.; Dabrowski, W.; Langner, E.; Zgrajka, W.; Pilat, J.; Kocki, T.; Rzeski, W.; Turski, W.A. Kynurenic acid synthesis and kynurenine aminotransferases expression in colon derived normal and cancer cells. Scand. J. Gastroenterol. 2011, 46, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, J. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host and Microbe 2016, 23, 716–724. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Panahi, H.K.S.; Guillemins, G.J. Microorganisms, Tryptophan Metabolism, and Kynurenine Pathway: A Complex Interconnected Loop Influencing Human Health Status. Int. J. Tryptophan Res. 2019, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Turski, M.P.; Turska, M.; Paluszkiewicz, P.; Parada-Turska, J.; Oxenkrug, G.F. Kynurenic Acid in the Digestive System-New Facts, New Challenges. Int. J. Tryptophan Res. 2013, 6, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Turska, M.; Paluszkiewicz, P.; Turski, W.A.; Parada-Turska, J. A review of the health benefits of food enriched with kynurenic acid. Nutrients 2022, 14, 4182. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand, B.; Scheers, N.; Rasmussen, M.K.; Young, J.F.; Ross, A.B.; Landberg, R. Brain foods—The role of diet in brain performance and health. Nutr. Rev. 2021, 79, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ju, Z.J.; Zuo, T. Time for food: The impact of diet on gut microbiota and human health. Nutrition 2018, 51, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F. Intestinal microbiota, diet and health. Brit. J. Nutr. 2014, 111, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lawson, M.A.; Dantzer, R.; Kelley, K.W. LPS-induced indoleamine 2,3-dioxygenase is regulated in an interferon-gamma-independent manner by a JNK signaling pathway in primary murine microglia. BrainBehav. Immun. 2010, 24, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Fujigaki, H.; Saito, K.; Fujigaki, S.; Takemura, M.; Sudo, K.; Ishiguro, H.; Seishima, M. The signal transducer and activator of transcription-1-alpha and interferon regulatory factor-1 are not essential for the induction of indoleamine-2,3-dioxygenase by lipopolysaccharide: Involvement of p38 mitogen-activated protein kinase and nuclear factor kappaB pathways, and synergistic effect of several proinflammatory cytokines. J. Biochem. 2006, 139, 655–662. [Google Scholar] [CrossRef]
- Campbell, B.M.; Charych, E.; Lee, A.W.; Möller, T. Kynurenines in CNS disease: Regulation by inflammatory cytokines. Front. Neurosci. 2014, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Clanchy, F.I.L.; Huang, Y.S.; Ogbechi, J.; Darlington, L.G.; Williams, R.O.; Stone, T.W. Induction of IDO1 and Kynurenine by Serine Proteases Subtilisin, Prostate Specific Antigen, CD26 and HtrA: A New Form of Immunosuppression? Front Immunol. 2022, 13, 832989. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W. Dependence and Guidance Receptors—DCC and neogenin—In partial EMT and the actions of serine proteases. Front. Oncol. 2020, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Forrest, C.M.; McNair, K.; Vincenten, M.C.J.; Darlington, L.G.; Stone, T.W. Selective depletion of tumour suppressors Deleted in Colorectal Cancer (DCC) and neogenin by environmental and endogenous serine proteases: Linking diet and cancer. BMC Cancer 2016, 16, 772. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Williams, R.O. Interactions of IDO and the kynurenine pathway with Cell Transduction Systems and Metabolism at the Inflammation–Cancer Interface. Cancers 2023, 15, 2895. [Google Scholar] [CrossRef] [PubMed]
- McNair, K.; Forrest, C.M.; Vincenten, M.C.J.; Darlington, L.G.; Stone, T.W. Serine protease modulation of Dependence Receptors and EMT protein expression. Cancer Biol. Ther. 2019, 20, 349–367. [Google Scholar] [CrossRef] [PubMed]
- Walczak, K.; Langner, E.; Szalast, K.; Makuch-Kocka, A.; Pozarowski, P.; Plech, T. A Tryptophan Metabolite, 8-Hydroxyquinaldic Acid, Exerts antiproliferative and anti-migratory effects on colorectal cancer cells. Molecules 2020, 25, 1655. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.J.; DuHadaway, J.B.; Donover, P.S.; Sutanto-Ward, E.; Prendergast, G.C. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 2005, 11, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, G.C.; Metz, R.; Muller, A.J. Towards a Genetic Definition of Cancer-Associated Inflammation of the IDO Pathway. Am. J. Pathol. 2010, 176, 2082–2087. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, G.C.; Malachowski, W.J.; Mondal, A.; Scherle, P.; Muller, A.J. Indoleamine 2,3-Dioxygenase and Its Therapeutic Inhibition in Cancer. Int. Rev. Cell Mol. Biol. 2018, 336, 175–203. [Google Scholar] [CrossRef] [PubMed]
- Nowicka-Stazka, P.; Langner, E.; Turski, W.; Rzeski, W.; Parada-Turska, J. Quinaldic acid in synovial fluid of patients with rheumatoid arthritis and osteoarthritis and its effect on synoviocytes in vitro. Pharmacol. Rep. 2018, 70, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Langner, E.; Jeleniewicz, W.; Turski, W.A.; Plech, T. Quinaldic acid induces changes in the expression of p53 tumor suppressor both on protein and gene level in colon cancer LS180 cells. Pharmacol. Rep. 2019, 71, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Noto, Y.; Okamoto, H. Inhibition By Kynurenine Metabolites Of Proinsulin Synthesis In Isolated Pancreatic-Islets. Acta Diabetol. 1978, 15, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.W.; Hajjar, J.; Hwu, P.; Naing, A. Targeting the indoleamine 2,3-dioxygenase pathway in cancer. J. Immunother. Cancer 2015, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Iizasa, H.; Kartika, A.V.; Fekadu, S.; Okada, S.; Onomura, D.; Wadi, A.F.A.A.; Khatun, M.M.; Moe, T.M.; Nishikawa, J.; Yoshiyama, H. Development of Epstein-Barr virus-associated gastric cancer: Infection, inflammation, and oncogenesis. World J. Gastroenterol. 2022, 28, 6249–6257. [Google Scholar] [CrossRef] [PubMed]
- Larrain, M.T.I.; Rabassa, M.E.; Lacunza, E.; Barbera, A.; Creton, A.; Segal-Eiras, A.; Croce, M.V. IDO is highly expressed in breast cancer and breast cancer-derived circulating microvesicles and associated to aggressive types of tumors by in silico analysis. Tumor Biol. 2014, 35, 6511–6519. [Google Scholar] [CrossRef]
- Haghighitalab, A.; Matin, M.M.; Amin, A.; Minaee, S.; Bidkhori, H.R.; Doeppner, T.R.; Bahrami, A.R. Investigating the effects of IDO1, PTGS2, and TGF-beta 1 overexpression on immunomodulatory properties of hTERT-MSCs and their extracellular vesicles. Sci. Rep. 2021, 11, 7825. [Google Scholar] [CrossRef]
- Joo, H.; Oh, M.K.; Kang, J.Y.; Park, H.S.; Chae, D.H.; Kim, J.; Lee, J.H.; Yoo, H.M.; Choi, U.; Kim, D.K.; et al. Extracellular vesicles from thapsigargin-treated mesenchymal stem cells ameliorated experimental colitis via enhanced immunomodulatory properties. Biomedicines 2021, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- de Castro, L.L.; Lopes-Pacheco, M.; Weiss, D.J.; Cruz, F.F.; Rocco, P.R.M. Current understanding of the immunosuppressive properties of mesenchymal stromal cells. J. Mol. Med. 2019, 97, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Deregibus, M.C.; Camussi, G. The secretome of mesenchymal stromal cells: Role of extracellular vesicles in immunomodulation. Immunol. Lett. 2015, 168, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.Z.; Tang, C.H.; Zhang, Z.; Zhou, W.; Zhao, R.; Wang, L.N.; Xu, J.C.; Wu, Y.Y.; Wu, J.; Zhang, X.; et al. Simultaneous Detection of Exosomal Membrane Protein and RNA by Highly Sensitive Aptamer Assisted Multiplex-PCR. ACS Appl. BioMater. 2020, 3, 2560–2567. [Google Scholar] [CrossRef]
- Serejo, T.R.T.; Silva-Carvalho, A.E.; Braga, L.D.D.F.; Neves, F.D.R.; Pereira, R.W.; de Carvalho, J.L.; Saldanha-Araujo, F. Assessment of the immunosuppressive potential of INF-gamma licensed adipose mesenchymal stem cells, their secretome and extracellular vesicles. Cells 2019, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Romani, R.; Pirisinu, I.; Calvitti, M.; Pallotta, M.T.; Gargaro, M.; Bistoni, G.; Vacca, C.; Di Michele, A.; Orabona, C.; Rosati, J.; et al. Stem cells from human amniotic fluid exert immunoregulatory function via secreted indoleamine 2,3-dioxygenase1. J. Cell Mol. Med. 2015, 19, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- De Pedro, M.A.; Gómez-Serrano, M.; Marinaro, F.; López, E.; Pulido, M.; Preusser, C.; von Strandmann, E.P.; Sánchez-Margallo, F.M.; Alvarez, V.; Casado, J.G. IFN-γ and TNF-α as a priming strategy to enhance the immunomodulatory capacity of secretomes from menstrual blood-derived stromal cells. Int. J. Mol. Sci. 2021, 22, 12177. [Google Scholar] [CrossRef] [PubMed]
- De Pedro, M.A.; Pulido, M.; Alvarez, V.; Marinaro, F.; Marchena, A.M.; Sánchez-Margallo, F.M.; Casado, J.G.; López, E. Menstrual blood-derived stromal cells: Insights into their secretome in acute hypoxia conditions. Mol. Med. 2023, 29, 48. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.B.; Guillemin, G. The Plasma [Kynurenine]/[Tryptophan] Ratio and Indoleamine-2,3-Dioxygenase: Time for Appraisal. Int. J. Tryptophan Res. 2019, 12, 1178646919868978. [Google Scholar] [CrossRef] [PubMed]
- Marttila, S.; Jylhava, J.; Eklund, C.; Hervonen, A.; Jylha, M.; Hurme, M. Aging-associated increase in indoleamine 2,3-dioxygenase (IDO) activity appears to be unrelated to the transcription of the IDO1 or IDO2 genes in peripheral blood mononuclear cells. Immun. Ageing 2011, 8, 9. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Antoniadi, G.; Liakopoulos, V.; Stefanidis, I.; Galaktidou, G. Plasma indoleamine 2,3-dioxygenase concentration is increased in hemodialysis patients and may contribute to the pathogenesis of coronary heart disease. Ren. Fail. 2012, 34, 68–72. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Yiannaki, E.; Antoniadi, G.; Liakopoulos, V.; Pissas, G.; Galaktidou, G.; Stefanidis, I. Plasma indoleamine 2,3-dioxygenase and arginase type i may contribute to decreased blood t-cell count in hemodialysis patients. Ren. Fail. 2012, 34, 1118–1122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zoga, M.; Oulis, P.; Chatzipanagiotou, S.; Masdrakis, V.G.; Pliatsika, P.; BoufIdou, F.; Foteli, S.; Soldatos, C.R.; Nikolaou, C.; Papageorgiou, C. Indoleamine 2,3-dioxygenase and Immune Changes Under Antidepressive Treatment in Major Depression in Females. In Vivo 2014, 28, 633–638. [Google Scholar] [PubMed]
- Zhong, W.C.; Gao, L.; Zhou, Z.T.; Lin, H.Y.; Chen, C.; Huang, P.; Huang, W.L.; Zhou, C.Y.; Huang, S.H.; Nie, L.H.; et al. Indoleamine 2,3-dioxygenase 1 deficiency attenuates CCl4-induced fibrosis through Th17 cells down-regulation and tryptophan 2,3-dioxygenase compensation. Oncotarget 2017, 8, 40486–40500. [Google Scholar] [CrossRef] [PubMed]
- Unal, D.; Ustundag-Budak, Y.; Sambel, M.; Turkoglu, A.R.; Huysal, K.; Coban, S.; Demirbas, M.; Guzelsoy, M. Serum levels of indoleamine 2,3 dioxygenase in erectile-dysfunction patients. J. Clin. Anal. Med. 2017, 8, 449–452. [Google Scholar] [CrossRef]
- Grzegorzewska, A.E.; Winnicka, H.; Warchol, W.; Mostowska, A.; Jagodzinski, P.P. Correlations of indoleamine 2,3-dioxygenase, interferon-lambda 3, and anti-HBs antibodies in hemodialysis patients. Vaccine 2018, 36, 4454–4461. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.N.; Gao, N.; Chang, Q.; Meng, X.C.; Wang, W.H. The role of IDO, IL-10, and TGF-beta in the HCV-associated chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. J. Med. Virol. 2019, 91, 265–271. [Google Scholar] [CrossRef]
- Lushnikova, A.; Bohr, J.; Wickbom, A.; Münch, A.; Sjöberg, K.; Hultgren, O.; Wirén, A.; Hörnquist, E.H. Patients with microscopic colitis have altered levels of inhibitory and stimulatory biomarkers in colon biopsies and sera compared to non-inflamed controls. Front. Med. 2021, 8, 727412. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.S.; Liu, K.Y.; Liu, D.H.; Xu, L.P.; Chen, H.; Huang, X.J. Potential immunosuppressive function of plasma indoleamine 2,3-dioxygenase in patients with aGVHD after allo-HSCT. Clin. Transplant. 2011, 25, E304–E311. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Lin, F.M.; Wu, L.F.; Tan, L.N.; Lu, L.L.; Xie, X.L.; Zhang, Y.; Bao, Y.N.; Ma, Y.C.; Huang, X.Q.; et al. The prevalence and the effect of interferon in the comorbidity of rheumatoid arthritis and depression. Behav. Brain Res. 2023, 439, 114237. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.N.; Wang, W.; Chen, M.; Chen, K.F.; Xia, X.H.; Zhou, S.N.; Yang, H.H. GBP1 Facilitates Indoleamine 2,3-Dioxygenase Extracellular Secretion to Promote the Malignant Progression of Lung Cancer. Front. Immunol. 2021, 11, 622467. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Huang, X.; Zeng, C.; Sun, D.R.; Liu, F.; Zhang, J.Y.; Liao, Q.; Luo, S.H.; Xu, W.Y.; Xiao, Y.Q.; et al. The role of indoleamine 2,3-dioxygenase 1 in early-onset post-stroke depression. Front. Immunol. 2023, 14, 1125634. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Geng, X.F. Research progress on the kynurenine pathway in the prevention and treatment of Parkinson’s disease. J. Enzyme Inhib. Med. Chem. 2023, 38, 2225800. [Google Scholar] [CrossRef] [PubMed]
- Arinola, G.O.; Abdullahi, I.; Rahamon, S.K.; Fasasi, Z.B.; Adedeji, O.O.; Kehinde, A.; Bakare, A.A. Activities of plasma indoleamine-2, 3-dioxygenase (IDO) enzyme in Nigerian patients with lung diseases: Basis for tryptophan supplementation or IDO inhibitor use. Egypt. J. Bronchol. 2023, 17, 2. [Google Scholar] [CrossRef]
- Odabasi, M.S.; Yazici, S.; Ozkaya, G.; Baskan, E.B.; Oral, A.Y. Serum indoleamine-2,3-dioxygenase level and diagnostic value in patients with rosacea. Dermatol. Sin. 2023, 41, 25–30. [Google Scholar] [CrossRef]
- Sadik, A.; Patterson, L.F.S.; Öztürk, S.; Mohapatra, S.R.; Panitz, V.; Secker, P.F.; Pfänder, P.; Loth, S.; Salem, H.; Prentzell, M.T.; et al. IL4i1 Is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell 2020, 182, 1252–1270. [Google Scholar] [CrossRef] [PubMed]
- Politi, V.; Lavaggi, M.V.; Distazio, G.; Margonelli, A. Indole-3-pyruvic acid as a direct precursor of kynurenic acid. Adv. Exp. Med. Biol. 1991, 294, 515–522. [Google Scholar] [PubMed]
- Moroni, F.; Russi, P.; Carla, V.; Deluca, G.; Politi, V. The regulation of brain kynurenic acid content-focus on indole-3-pyruvic acid. Adv. Exp. Med. Biol. 1991, 294, 299–308. [Google Scholar] [PubMed]
- Grobben, Y.; den Ouden, J.E.; Aguado, C.; van Altena, A.M.; Kraneveld, A.D.; Zaman, G.J.R. Amino Acid-Metabolizing Enzymes in Advanced High-Grade Serous Ovarian Cancer Patients: Value of Ascites as Biomarker Source and Role for IL4i1 and IDO1. Cancers 2023, 15, 893. [Google Scholar] [CrossRef] [PubMed]
- Seegers, N.; van Doornmalen, A.M.; Uitdehaag, J.C.M.; de Man, J.; Buijsman, R.C.; Zaman, G.J.R. High-Throughput Fluorescence-Based Screening Assays for Tryptophan-Catabolizing Enzymes. J. Biomol. Screen. 2014, 19, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Castellano, F.; Prevost-Blondel, A.; Cohen, J.L.; Molinier-Frenkel, V. What role for AHR activation in IL4i1-mediated immunosuppression ? Oncoimmunology 2021, 10, 1924500. [Google Scholar] [CrossRef] [PubMed]
- Castellano, F.; Molinier-Frenkel, V. An overview of l-amino acid oxidase functions from bacteria to mammals: Focus on the immunoregulatory phenylalanine oxidase IL4i1. Molecules 2017, 22, 2151. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Magid, A.F. Inhibitors of Interleukin 4 Induced Protein 1 (IL4i1) as Potential Treatment for Cancer. ACS Med. Chem. Lett. 2023, 14, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Presset, M.; Djordevic, D.; Dupont, A.; Gall, E.L.; Miliner-Frenkel, V.; Castellano, F. Identification of inhibitors of the immunosuppressive enzyme IL4i1. Bio. Org. Chem. 2020, 94, 103463. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Chávez, L.A.; Huitrón, R.L.; Esquivel, D.G.; Pineda, B.; Ríos, C.; Silva-Adaya, D.; Sánchez-Chapul, L.; Roldán-Roldán, G.; de la Cruz, V.P. Relevance of alternative routes of kynurenic acid production in the brain. Oxid. Med. Cell Longev. 2018, 2018, 5272741. [Google Scholar] [CrossRef] [PubMed]
- Ayala, T.B.; Huitrón, R.L.; Aparício, L.C.; Ortega, D.R.; Esquivel, D.G.; Chaverri, J.P.; de la Cruz, G.P.; Ríos, C.; Schwarcz, R.; de la Cruz, V.P. Alternative kynurenic acid synthesis routes studied in the rat cerebellum. Front. Cell Neurosci. 2015, 9, 178. [Google Scholar] [CrossRef]
- Guidetti, P.; Schwarcz, R. 3-hydroxykynurenine potentiates quinolinate but not NMDA toxicity in the rat striatum. Europ. J. Neurosci. 1999, 11, 3857–3863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Collier, M.E.W.; Heyes, D.J.; Giorgini, F.; Scrutton, N.S. Advantages of brain penetrating inhibitors of kynurenine-3-monooxygenase for treatment of neurodegenerative diseases. Arch. Biochem. Biophys. 2021, 697, 108702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Sakuma, M.; Deora, G.S.; Levy, C.W.; Klausing, A.; Breda, C.; Read, K.D.; Edlin, C.D.; Ross, B.P.; Muelas, M.W.; et al. A brain-permeable inhibitor of the neurodegenerative disease target kynurenine 3-monooxygenase prevents accumulation of neurotoxic metabolites. Commun. Biol. 2019, 2, 271. [Google Scholar] [CrossRef] [PubMed]
- Forrest, C.M.; Khalil, O.S.; Pisar, M.; McNair, K.; Kornisiuk, E.; Snitcofsky, M.; Gonzalez, N.; Jerusalinsky, D.; Darlington, L.G.; Stone, T.W. Changes in synaptic transmission and protein expression in the brains of adult offspring after prenatal inhibition of the kynurenine pathway. Neuroscience 2013, 254, 241–259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Forrest, C.M.; Khalil, O.S.; Pisar, M.; Darlington, L.G.; Stone, T.W. Prenatal inhibition of the tryptophan-kynurenine pathway alters synaptic plasticity and protein expression in the rat hippocampus. Brain Res. 2013, 1504, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Giorgini, F.; Huang, S.Y.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Thomas, M.A.R.; Tararina, M.; Wu, H.Q.; Schwarcz, R.; Muchowski, P.J. Targeted Deletion of Kynurenine 3-Monooxygenase in Mice: A new tool for studying kynurenine pathway metabolism in periphery and brain. J. Biol. Chem. 2013, 288, 36554–36566. [Google Scholar] [CrossRef]
- Erhardt, S.; Pocivavsek, A.; Repici, M.; Liu, X.C.; Imbeault, S.; Maddison, D.C.; Thomas, M.A.R.; Smalley, J.L.; Larsson, M.K.; Muchowski, P.J.; et al. Adaptive and Behavioral Changes in Kynurenine 3-Monooxygenase Knockout Mice: Relevance to Psychotic Disorders. Biol. Psychiatry 2017, 82, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Röver, S.; Cesura, A.M.; Huguenin, P.; Kettler, R.; Szente, A. Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)-benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. J. Med. Chem. 1997, 40, 4378–4385. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.L.; Ancellin, N.; Beaufils, B.; Bergeal, M.; Binnie, M.; Bouillot, A.; Clapham, D.; Denis, A.; Haslan, C.P.; Holmes, D.S.; et al. Development of a series of kynurenine 3-monooxygenase inhibitors leading to a clinical candidate for the treatment of acute pancreatitis. J. Med. Chem. 2017, 60, 3383–3404. [Google Scholar] [CrossRef] [PubMed]
- Modoux, M.; Rolhion, N.; Mani, S.; Sokol, H. Tryptophan metabolism as a pharmacological target. Trends Pharmacol. Sci. 2021, 42, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.D.; Guner, O.F.; Iradukunda, E.C.; Phillips, R.S.; Bowen, J.P. The kynurenine pathway and kynurenine-3-monooxygenase inhibitors. Molecules 2022, 27, 273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Y.-S.; Ogbechi, J.; Clanchy, F.; Williams, R.O.; Stone, T.W. Kynurenine metabolites in peripheral disorders and neuroinflammation. Front. Immunol. 2020, 11, 388. [Google Scholar] [CrossRef]
- Connor, T.J.; Starr, N.; O’Sullivan, J.B.; Harkin, A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: A role for IFN-γ? Neurosci. Lett. 2008, 441, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.-B.; Dougherty, D.M. Assessment of the human kynurenine pathway: Comparisons and clinical implications of ethnic and gender differences in plasma tryptophan, kynurenine metabolites, and enzyme expressions at baseline and after acute tryptophan loading and depletion. Int. J. Tryptophan Res. 2016, 9, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef]
- Moyer, B.J.; Rojas, I.Y.; Kerley-Hamilton, J.S.; Hazlett, H.F.; Nemani, K.V.; Trask, H.W.; West, R.J.; Lupien, L.; Collins, A.J.; Ringelberg, C.S.; et al. Inhibition of the aryl hydrocarbon receptor prevents Western diet-induced obesity. Model for AHR activation by kynurenine via oxidized-LDL, TLR2/4, TGFβ, and IDO. Toxicol. Appl. Pharmacol. 2016, 300, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Terramani, T.; Lynch, G.; Baudry, M. A Glycine Site Associated With N-Methyl-D-aspartic acid receptors-characterization and identification of a new class of antagonists. J. Neurochem. 1989, 52, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Kuc, D.; Zgrajka, W.; Parada-Turska, J.; Urbanik-Sypniewska, T.; Turski, W.A. Micromolar concentration of kynurenic acid in rat small intestine. Amino Acids 2008, 35, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Paluszkiewicz, P.; Zgrajka, W.; Saran, T.; Schabowski, J.; Piedra, J.L.V.; Fedkiv, O.; Rengman, S.; Pierzynowski, S.G.; Turski, W.A. High concentration of kynurenic acid in bile and pancreatic juice. Amino Acids 2009, 37, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Schefold, J.C.; Zeden, J.P.; Fotopoulou, C.; von Haehling, S.; Pschowski, R.; Hasper, D.; Volk, H.D.; Schuett, C.; Reinke, P. Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: A possible link between chronic inflammation and uraemic symptoms. Nephrol. Dial. Transpl. 2009, 24, 1901–1908. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.N.; Stone, T.W. Actions of kynurenic acid and quinolinic acid in the rat hippocampus in vivo. Exp. Neurol. 1985, 88, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Carla, V.; Lombardi, G.; Beni, M.; Russi, P.; Moneti, G.; Moroni, F. Identification and measurement of kynurenic acid in the rat-brain and other organs. Analytic. Biochem. 1988, 169, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Moroni, F.; Russi, P.; Carla, V.; Lombardi, G. Kynurenic acid is present in the rat-brain and its content increases during development and aging processes. Neurosci. Lett. 1988, 94, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Baran, H.; Cairns, N.; Lubec, B.; Lubec, G. Increased kynurenic acid levels and decreased brain kynurenine aminotransferase I in patients with Down syndrome. Life Sci. 1996, 58, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Jauch, D.; Urbanska, E.M.; Guidetti, P.; Bird, E.D.; Vonsattel, J.P.G.; Whetsell, W.O.; Schwarcz, R. Dysfunction of brain kynurenic acid metabolism in Huntingtons-disease-focus on kynurenine aminotransferases. J. Neurol. Sci. 1995, 130, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Cai, T.; Tagle, D.A.; Li, J.Y. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell Mol. Life Sci. 2010, 67, 353–368. [Google Scholar] [CrossRef]
- Rossi, F.; Miggiano, R.; Ferraris, D.M.; Rizzi, M. The synthesis of kynurenic acid in mammals: An updated kynurenine aminotransferase structural KATalogue. Front. Mol. Biosci. 2019, 6, 7. [Google Scholar] [CrossRef]
- Okuno, E.; Schmidt, W.; Parks, D.A.; Nakamura, M.; Schwarcz, R. Measurement of rat-brain kynurenine aminotransferase at physiological kynurenine concentrations. J. Neurochem. 1991, 57, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, P.; Okuno, E.; Schwarcz, R. Characterization of rat brain kynurenine aminotransferases I and II. J. Neurosci. Res. 1997, 50, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Rzeski, W.; Kocki, T.; Dybel, A.; Wejksza, K.; Zdzisinska, B.; Kandefer-Szerszén, M.; Turski, W.A.; Okuno, E.; Albrecht, J. Demonstration of kynurenine aminotransferases I and II and characterization of kynurenic acid synthesis in cultured cerebral cortical neurons. J. Neurosci. Res. 2005, 80, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Carpenedo, R.; Meli, E.; Peruginelli, F.; Pellegrini-Giampietro, D.E.; Moroni, F. Kynurenine 3-mono-oxygenase inhibitors attenuate post-ischemic neuronal death in organotypic hippocampal slice cultures. J. Neurochem. 2002, 82, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Carpenedo, R.; Pittaluga, A.; Cozzi, A.; Attucci, S.; Galli, A.; Raiteri, M.; Moroni, F. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Europ. J. Neurosci. 2001, 13, 2141–2147. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, E.M.; Luchowski, P.; Luchowska, E.; Pniewski, J.; Wozniak, R.; Chodakowska-Zebrowska, M.; Lazarewicz, J. Serum kynurenic acid positively correlates with cardiovascular disease risk factor, homocysteine: A study in stroke patients. Pharmacol. Rep. 2006, 58, 507–511. [Google Scholar] [PubMed]
- Hartai, Z.; Juhász, A.; Rimanóczy, A.; Janáky, T.; Donkó, T.; Dux, L.; Penke, B.; Tóth, G.K.; Janka, Z.; Kálmán, J. Decreased serum and red blood cell kynurenic acid levels i Alzheimer’s disease. Neurochem. Int. 2007, 50, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Kepplinger, B.; Baran, H.; Kainz, A.; Ferraz-Leite, H.; Newcombe, J.; Kalina, P. Age-related increase of kynurenic acid in human cerebrospinal fluid-IgG and β2-microglobulin changes. Neurosignals 2005, 14, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Gramsbergen, J.B.P.; Schmidt, W.; Turski, W.A.; Schwarcz, R. Age-Related-Changes In Kynurenic Acid Production In Rat-Brain. Brain Res. 1992, 588, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Guillemin, G.J.; Mansour, H.; Chan-Ling, T.; Grant, R. Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. FEBS J. 2011, 278, 4425–4434. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, S.; Blennow, K.; Nordin, C.; Skogh, E.; Lindström, L.H.; Engberg, G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci. Lett. 2001, 313, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Linderholm, K.R.; Skogh, E.; Olsson, S.K.; Dahl, M.L.; Holtze, M.; Engberg, G.; Samuelsson, M.; Erhardt, S. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr. Bull. 2012, 38, 426–432. [Google Scholar] [CrossRef]
- Sathyasaikumar, K.V.; Stachowski, E.K.; Amori, L.; Guidetti, P.; Muchowski, P.J.; Schwarcz, R. Dysfunctional kynurenine pathway metabolism in the R6/2 mouse model of Huntington’s disease. J. Neurochem. 2010, 113, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Kegel, M.E.; Johansson, V.; Wetterberg, L.; Bhat, M.; Schwieler, L.; Cannon, T.D.; Schuppe-Koistinen, I.; Engberg, G.; Landén, M.; Hultman, C.M.; et al. Kynurenic acid and psychotic symptoms and personality traits in twins with psychiatric morbidity. Psychiatry Res. 2017, 247, 105–112. [Google Scholar] [CrossRef] [PubMed]
- González-Sánchez, M.; Jiménez, J.; Narváez, A.; Antequera, D.; Llamas-Velasco, S.; Herrero-San Martín, A.; Arjona, J.A.M.; de Munain, A.L.; Bisa, A.L.; Marco, M.P.; et al. Kynurenic Acid Levels are Increased in the CSF of Alzheimer’s Disease Patients. Biomolecules 2020, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Knapskog, A.B.; Aksnes, M.; Edwin, T.H.; Ueland, P.M.; Ulvik, A.; Fang, E.F.; Eldholm, R.S.; Halaas, N.B.; Saltvedt, I.; Giil, L.M.; et al. Higher concentrations of kynurenic acid in CSF are associated with the slower clinical progression of Alzheimer’s disease. Alzheimer’s Dement. 2023, 19, 5573–5582. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.N.; Stone, T.W. An iontophoretic investigation of the action of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982, 247, 184–187. [Google Scholar] [CrossRef]
- Laube, B.; Hirai, H.; Sturgess, M.; Betz, H.; Kuhse, J. Molecular determinants of agonist discrimination by NMDA receptor subunits: Analysis of the glutamate binding site on the NR2B subunit. Neuron 1997, 18, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Anson, L.C.; Chen, P.E.; Wyllie, D.J.; Colquhoun, D.; Schoepfer, R. Identification of amino acid residues of the NR2A subunit that control glutamate potency in recombinant NR1/NR2A NMDA receptors. J. Neurosci. 1998, 18, 581–589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chou, T.H.; Tajima, N.; Romero-Hernandez, A.; Furukawa, H. Structural Basis of Functional Transitions in Mammalian NMDA Receptors. Cell 2020, 182, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Kloc, R.; Luchowska, E.; Wieosz, M.; Owe-Larsson, B.; Urbanska, E.M. Memantine increases brain production of kynurenic acid via protein kinase A-dependent mechanism. Neurosci. Lett. 2008, 435, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, A.E.; Milligan, G. The emerging pharmacology and function of GPR35 in the nervous system. Neuropharmacology 2017, 113, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Quon, T.; Lin, L.C.; Ganguly, A.; Tobin, A.B.; Milligan, G. Therapeutic Opportunities and Challenges in Targeting the Orphan G Protein-Coupled Receptor GPR35. ACS Pharmacol. Transl. Sci. 2020, 3, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Im, D.S. Recent advances in GPR35 pharmacology; 5-HIAA serotonin metabolite becomes a ligand. Arch. Pharmacal Res. 2023, 46, 550–563. [Google Scholar] [CrossRef] [PubMed]
- De Giovanni, M.; Tam, H.; Valet, C.; Xu, Y.; Looney, M.R.; Cyster, J.G. GPR35 promotes neutrophil recruitment in response to serotonin metabolite 5-HIAA. Cell 2022, 185, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Nilsson-Todd, L.K.; Nordin, C.; Jönsson, E.G.; Skogh, E.; Erhardt, S. Cerebrospinal fluid kynurenic acid in male patients with schizophrenia: Correlation with monoamine metabolites. Acta Neuropsychiatr. 2007, 19, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, L.; Alvarez-Curto, E.; Campbell, K.; de Munnik, S.; Canals, M.; Schlyer, S.; Milligan, G. Agonist activation of the G protein-coupled receptor GPR35 involves transmembrane domain III and is transduced via Gα13 and β-arrestin-2. Brit. J. Pharmacol. 2011, 162, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Barth, M.C.; Ahluwalia, N.; Anderson, T.J.T.; Hardy, G.J.; Sinha, S.; Alvarez-Cardona, J.A.; Pruitt, I.E.; Rhee-Colvin, R.A.; Gerszten, R.E. Kynurenic Acid Triggers Firm Arrest of Leukocytes to Vascular Endothelium under Flow Conditions. J. Biol. Chem. 2009, 284, 19189–19195. [Google Scholar] [CrossRef] [PubMed]
- Resta, F.; Masi, A.; Sili, M.; Laurino, A.; Moroni, F.; Mannaioni, G. Kynurenic acid and zaprinast induce analgesia by modulating HCN channels through GPR35 activation. Neuropharmacology 2016, 108, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Cosi, C.; Mannaioni, G.; Cozzi, A.; Carlà, V.; Sili, M.; Cavone, L.; Maratea, D.; Moroni, F. G-protein coupled receptor-35 (GPR35) activation and inflammatory pain: Studies on the antinociceptive effects of kynurenic acid and zaprinast. Neuropharmacology 2011, 60, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Berlinguer-Palmini, R.; Masi, A.; Narducci, R.; Cavone, L.; Maratea, D.; Cozzi, A.; Sili, M.; Moroni, F.; Mannaioni, G. GPR35 Activation Reduces Ca2+ Transients and Contributes to the Kynurenic Acid-Dependent Reduction of Synaptic Activity at CA3-CA1 Synapses. PLoS ONE 2013, 8, e82180. [Google Scholar] [CrossRef]
- Alkondon, M.; Pereira, E.F.R.; Todd, S.W.; Randall, W.R.; Lane, M.V.; Albuquerque, E.X. Functional G-protein-coupled receptor-35 is expressed by neurons in the CA1 field of the hippocampus. Biochem. Pharmacol. 2015, 93, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.Y.; Xie, R.Q.; He, H.B.; Xie, Q.Q.; Zhao, X.Q.; Kang, G.J.; Cheng, C.; Yin, W.W.; Cong, J.J.; Li, J.; et al. Kynurenic acid ameliorates NLRP3 inflammasome activation by blocking calcium mobilization via GPR35. Front. Immunol. 2022, 13, 1019365. [Google Scholar] [CrossRef] [PubMed]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Guenther, J. Kynurenic Acid: The Janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front. Immunol. 2018, 8, 1957. [Google Scholar] [CrossRef] [PubMed]
- Tiszlavicz, Z.; Németh, B.; Fülöp, F.; Vécsei, L.; Tápai, K.; Ocsovszky, I.; Mándi, Y. Different inhibitory effects of kynurenic acid and a novel kynurenic acid analogue on tumour necrosis factor-α (TNF-α) production by mononuclear cells, HMGB1 production by monocytes and HNP1-3 secretion by neutrophils. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 383, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Elizei, S.; Poormasjedi-Meibod, M.-S.; Wang, X.; Kheirandish, M.; Ghahary, A. Kynurenic acid downregulates IL-17/1L-23 axis in vitro. Mol. Cell. Biochem. 2017, 431, 55–65. [Google Scholar] [CrossRef]
- Garrison, A.M.; Parrott, J.M.; Tuñon, A.; Delgado, J.; Redus, L.; O’Connor, J.C. kynurenine pathway metabolic balance influences microglia activity: Targeting kynurenine monooxygenase to dampen neuroinflammation. Psychoneuroendocrinology 2018, 94, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fallarini, S.; Magliulo, L.; Paoletti, T.; de Lalla, C.; Lombardi, G. Expression of functional GPR35 in human iNKT cells. Biochem. Biophys. Res. Commun. 2010, 398, 420–425. [Google Scholar] [CrossRef] [PubMed]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef]
- Kaszaki, J.; Érces, D.; Varga, G.; Szabó, A.; Vécsei, L.; Boros, M. Kynurenines and intestinal neurotransmission: The role of NMDA receptors. J. Neural Transm. 2012, 119, 211–223. [Google Scholar] [CrossRef]
- Lanis, J.M.; Alexeev, E.E.; Curtis, V.F.; Kitzenberg, D.A.; Kao, D.J.; Battista, K.D.; Gerich, M.E.; Glover, L.E.; Kominsky, D.J.; Colgan, S.P. Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol. 2017, 10, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.J.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An Interaction between Kynurenine and the Aryl Hydrocarbon Receptor Can Generate Regulatory T cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef] [PubMed]
- Wirthgen, E.; Hoeflich, A. Endotoxin-induced tryptophan degradation along the kynurenine pathway: The role of indolamine 2, 3-dioxygenase and aryl hydro-carbon receptor-mediated immunosuppressive effects in endotoxin tolerance and cancer and its implications for immunoparalysis. J. Amino Acids 2015, 2015, 973548. [Google Scholar] [CrossRef]
- Szelest, M.; Walczak, K.; Plech, T. A New Insight into the Potential Role of Tryptophan-Derived AHR Ligands in Skin Physiological and Pathological Processes. Int. J. Mol. Sci. 2021, 22, 1104. [Google Scholar] [CrossRef]
- Dawood, S.; Badawy, A.A.-B. Molecular docking of FICZ (6-formylindolo [3,2-b]-carbazole) to kynurenine pathway enzymes: Biological basis of a potential drug. J. Pharmacol. Pharm. 2024, 1, 1004. [Google Scholar]
- Solvay, M.; Holfelder, P.; Klaessens, S.; Pilotte, L.; Stroobant, V.; Lamy, J.; Naulaerts, S.; Spillier, Q.; Frédérick, R.; De Plaen, E.; et al. Tryptophan depletion sensitizes the AHR pathway by increasing AHR expression and GCN2/LAT1-mediated kynurenine uptake, and potentiates induction of regulatory T lymphocytes. J. Immunother. Cancer 2023, 11, e006728. [Google Scholar] [CrossRef] [PubMed]
- Litzenburger, U.M.; Opitz, C.A.; Sahm, F.; Rauschenbach, K.J.; Trump, S.; Winter, M.; Ott, M.; Ochs, K.; Lutz, C.; Liu, X.; et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget 2014, 5, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Bankoti, J.; Rase, B.; Simones, T.; Shepherd, D.M. Functional and phenotypic effects of AHR activation in inflammatory dendritic cells. Toxicol. Appl. Pharmacol. 2010, 246, 18–28. [Google Scholar] [CrossRef]
- Li, Q.; Harden, J.L.; Anderson, C.D.; Egilmez, N.K. Tolerogenic phenotype of IFN-gamma-induced IDO+ dendritic cells is maintained via an autocrine IDO-kynurenine/AHR-IDO loop. J. Immunol. 2016, 197, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Novikov, O.; Wang, Z.; Stanford, E.A.; Parks, A.J.; Ramirez-Cardenas, A.; Landesman, E.; Laklouk, I.; Sarita-Reyes, C.; Gusenleitner, D.; Li, A.; et al. An Aryl Hydrocarbon Receptor-Mediated Amplification Loop That Enforces Cell Migration in ER-/PR-/Her2- Human Breast Cancer Cells. Mol. Pharmacol. 2016, 90, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Stanford, E.A.; Ramirez-Cardenas, A.; Wang, Z.Y.; Novikov, O.; Alamoud, K.; Koutrakis, P.; Mizgerd, J.P.; Genco, C.A.; Kukuruzinska, M.; Monti, S.; et al. Role for the aryl hydrocarbon receptor and diverse ligands in oral squamous cell carcinoma migration and tumorigenesis. Mol. Cancer Res. 2016, 14, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Stanford, E.A.; Wang, Z.Y.; Novikov, O.; Mulas, F.; Landesman-Bollag, E.; Monti, S.; Smith, B.W.; Seldin, D.C.; Murphy, G.J.; Sherr, D.H. The role of the aryl hydrocarbon receptor in the development of cells with the molecular and functional characteristics of cancer stem-like cells. BMC Biol. 2016, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Stroisch, T.J.; Vorac, J.; Herrmann, N.; Leib, N.; Schnautz, S.; Kirins, H.; Förster, I.; Weighardt, H.; Bieber, T. AHR mediates an anti-inflammatory feedback mechanism in human Langerhans cells involving FcεRI and IDO. Allergy 2017, 72, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Nakahama, T.; Le, D.H.; VanSon, L.; Chu, H.H.; Kishimoto, T. Aryl hydrocarbon receptor and kynurenine:recent advances in autoimmune disease research. Front. Immunol. 2014, 5, 551. [Google Scholar] [CrossRef]
- Bessede, A.; Gargaro, M.; Pallotta, M.T.; Matino, D.; Servillo, G.; Brunacci, C.; Bicciato, S.; Mazza, E.M.C.; Macchiarulo, A.; Vacca, C.; et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 2014, 511, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Jaronen, M.; Quintana, F.J. Immunological relevance of the coevolution of IDO1 and AHR. Front. Immunol. 2014, 5, 521. [Google Scholar] [CrossRef] [PubMed]
- Belladonna, M.L.; Orabona, C.; Grohmann, U.; Puccetti, P. TGF-β and kynurenines as the key to infectious tolerance. Trend Mol. Med. 2009, 15, 41–49. [Google Scholar] [CrossRef]
- Kaiser, H.; Parker, E.; Hamrick, M.W. Kynurenine signaling through the aryl hydrocarbon receptor: Implications for aging and healthspan. Exp. Gerontol. 2020, 130, 110797. [Google Scholar] [CrossRef]
- Tomblin, J.K.; Arthur, S.; Primerano, D.A.; Chaudhry, A.R.; Fan, J.; Denvir, J.; Salisbury, T.B. Aryl hydrocarbon receptor (AHR) regulation of L-Type Amino Acid Transporter 1 (LAT-1) expression in MCF-7 and MDA-MB-231 breast cancer cells. Biochem. Pharmacol. 2016, 106, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Sekine, A.; Okamoto, M.; Kanatani, Y.; Sano, M.; Shibata, K.; Fukuwatari, T. Amino acids inhibit kynurenic acid formation via suppression of kynurenine uptake or kynurenic acid synthesis in rat brain in vitro. SpringerPlus 2015, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Sekine, A.; Kuroki, Y.; Urata, T.; Mori, N.; Fukuwatari, T. Inhibition of Large Neutral Amino Acid Transporters Suppresses Kynurenic Acid Production Via Inhibition of Kynurenine Uptake in Rodent Brain. Neurochem. Res. 2016, 41, 2256–2266. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan Catabolites from Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity via IL-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Walczak, K.; Langner, E.; Makuch-Kocka, A.; Szelest, M.; Szalast, K.; Marciniak, S.; Plech, T. Effect of Tryptophan-Derived AHR Ligands, Kynurenine, Kynurenic Acid and FICZ, on Proliferation, Cell Cycle Regulation and Cell Death of Melanoma Cells-In Vitro Studies. Int. J. Mol. Sci. 2020, 21, 7946. [Google Scholar] [CrossRef] [PubMed]
- Offermanns, S. Hydroxy-Carboxylic Acid Receptor Actions in Metabolism. Trends Endocrinol. Metab. 2017, 28, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Kapolka, N.J.; Taghon, G.J.; Rowe, J.B.; Morgan, W.M.; Enten, J.F.; Lambert, N.A.; Isom, D.G. DCyFIR: A high-throughput CRISPR platform for multiplexed G protein-coupled receptor profiling and ligand discovery. Proc. Nat. Acad. Sci. USA 2020, 117, 13117–13126. [Google Scholar] [CrossRef] [PubMed]
- Wnorowski, A.; Wnorowska, S.; Kurzepa, J.; Parada-Turska, J. Alterations in Kynurenine and NAD+ Salvage Pathways during the Successful Treatment of Inflammatory Bowel Disease Suggest HCAR3 and NNMT as Potential Drug Targets. Int. J. Mol. Sci. 2021, 22, 13497. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W. Relationships and interactions between ionotropic glutamate receptors and nicotinic receptors in the CNS. Neuroscience 2021, 468, 321–365. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W. Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. J. Neurochem. 2020, 152, 627–649. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W. Kynurenic acid blocks nicotinic synaptic transmission to hippocampal interneurons in young rats. Europ. J. Neurosci. 2007, 25, 2656–2665. [Google Scholar] [CrossRef] [PubMed]
- World Intellectual Property Organization. 2024. Available online: https://patentscope.wipo.int/search/en/result.jsf?_vid=P21-LXBZOE-54265 (accessed on 1 July 2024).
- Nestor, M.S.; Berman, B.; Fischer, D.L.; Han, H.W.; Gade, A.; Arnold, D.; Lawson, A. A Randomized, Double-Blind, Active- and Placebo-Controlled Trial Evaluating a Novel Topical Treatment for Keloid Scars. J. Drugs Dermatol 2021, 20, 964–968. [Google Scholar] [CrossRef] [PubMed]
- BirchBioMed. 2024. Available online: http://www.birchbiomed.com/our-science/ (accessed on 1 July 2024).
- Herhaus, B.; Joisten, N.; Wessels, I.; Zimmer, P.; Petrowski, K. How acute physical and psychological stress differentially influence the kynurenine pathway: A randomized cross-over trial. Psychoneuroendocrinology 2021, 134, 105433. [Google Scholar] [CrossRef] [PubMed]
- Joisten, N.; Ruas, J.L.; Braidy, N.; Guillemin, G.J.; Zimmer, P. The kynurenine pathway in chronic diseases: A compensatory mechanism or a driving force? Trends Mol. Med. 2021, 27, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Arefayene, M.; Philips, S.; Cao, D.H.; Mamidipalli, S.; Desta, Z.; Flockhart, D.A.; Wilkes, D.S.; Skaar, T.C. Identification of genetic variants in the human indoleamine 2,3-dioxygenase (IDO1) gene, which have altered enzyme activity. Pharmacogenetics Genom. 2009, 19, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Boros, F.A.; Bohár, Z.; Vécsei, L. Genetic alterations affecting the genes encoding the enzymes of the kynurenine pathway and their association with human diseases. Mutation Res. Rev. 2018, 776, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Boros, F.A.; Vécsei, L. Immunomodulatory Effects of Genetic Alterations Affecting the Kynurenine Pathway. Front. Immunol. 2019, 10, 2570. [Google Scholar] [CrossRef] [PubMed]
- Pellicciari, R.; Venturoni, F.; Bellocchi, D.; Carotti, A.; Marinozzi, M.; Macchiarulo, A.; Amori, L.; Schwarcz, R. Sequence variants in kynurenine aminotransferase II (KAT II) orthologs determine different potencies of the inhibitor S-ESBA. Chem. Med. Chem. 2008, 3, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Wonodi, I.; McMahon, R.P.; Krishna, N.; Mitchell, B.D.; Liu, J.; Glassman, M.; Hong, L.E.; Gold, J.M. Influence of kynurenine 3-monooxygenase (KMO) gene polymorphism on cognitive function in schizophrenia. Schizophr. Res. 2014, 160, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Holtze, M.; Saetre, P.; Engberg, G.; Schwieler, L.; Werge, T.; Andreassen, O.A.; Hall, H.; Terenius, L.; Agartz, I.; Jönsson, E.G.; et al. Kynurenine 3-monooxygenase polymorphisms: Relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls. J. Psychiatry Neurosci. 2012, 37, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Guillemin, G.J. Kynurenine pathway Metabolites in Humans: Disease and Healthy States. Int. J. Tryptophan Res. 2009, 2, IJTR-S2097. [Google Scholar] [CrossRef] [PubMed]
- Vecsei, L.; Szalardy, L.; Fulop, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Disc. 2013, 12, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Ostapiuk, A.; Urbanska, E.M. Kynurenic acid in neurodegenerative disorders-unique neuroprotection or double-edged sword? CNS Neurosci. Therap. 2022, 28, 19–35. [Google Scholar] [CrossRef]
- Lim, C.K.; Fernández-Gomez, F.J.; Braidy, N.; Estrada, C.; Costa, C.; Costa, S.; Bessede, A.; Fernandez-Villalba, E.; Zinger, A.; Herrero, M.T.; et al. Involvement of the kynurenine pathway in the pathogenesis of Parkinson’s disease. Progr. Neurobiol. 2017, 155, SI:76–95. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Guidetti, P.; Sathyasaikumar, K.V.; Muchowski, P.J. Of mice, rats and men: Revisiting the quinolinic acid hypothesis of Huntington’s disease. Progr. Neurobiol. 2010, 90, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Bates, G.P.; Dorsey, R.; Gusella, J.F.; Hayden, M.R.; Kay, C.; Leavitt, B.R.; Nance, M.; Ross, C.A.; Scahill, R.I.; Wetzel, R.; et al. Huntington disease. Nat. Rev. Dis. Primers 2015, 1, 15005. [Google Scholar] [CrossRef] [PubMed]
- Kincses, Z.T.; Toldi, J.; Vécsei, L. Kynurenines, neurodegeneration and Alzheimer’s disease. J. Cell. Mol. Med. 2010, 14, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Zadori, D.; Veres, G.; Szalardy, L.; Kliv, P.; Vecsei, L. Alzheimer’s Disease: Recent Concepts on the Relation of Mitochondrial Disturbances, Excitotoxicity, Neuroinflammation, and Kynurenines. J. Alzheimer’s Dis. 2018, 62, 523–547. [Google Scholar] [CrossRef]
- Savonije, K.; Meek, A.; Weaver, D.E. Indoleamine 2,3-dioxygenase as a therapeutic target for Alzheimer’s disease and geriatric depression. Brain Sci. 2023, 13, 852. [Google Scholar] [CrossRef]
- Chen, C.M.; Huang, C.Y.; Lai, C.H.; Chen, Y.C.; Hwang, Y.T.; Lin, C.Y. Neuroprotection effects of kynurenic acid-loaded micelles for the Parkinson’s disease models. J. Liposome Res. 2024, 1–12. [Google Scholar] [CrossRef]
- Majláth, Z.; Tajti, J.; Vécsei, L. Kynurenines and other novel therapeutic strategies in the treatment of dementia. Therap. Adv. Neurol. Dis. 2013, 6, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Tóth, F.; Cseh, E.K.; Vécsei, L. Natural Molecules and Neuroprotection: Kynurenic Acid, Pantethine and α-Lipoic Acid. Int. J. Mol. Sci. 2021, 22, 403. [Google Scholar] [CrossRef]
- Szalardy, L.; Zadori, D.; Toldi, J.; Fulop, F.; Klivenyi, P.; Vecsei, L. Manipulating Kynurenic Acid Levels in the Brain–On the Edge Between Neuroprotection and Cognitive Dysfunction. Curr. Topics Med. Chem. 2012, 12, 1797–1806. [Google Scholar] [CrossRef]
- Szalárdy, L.; Klivényi, P.; Zádori, D.; Fülöp, F.; Toldi, J.; Vécsei, L. Mitochondrial Disturbances, Tryptophan Metabolites and Neurodegeneration: Medicinal Chemistry Aspects. Curr. Med. Chem. 2012, 19, 1899–1920. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.G.; Tasca, C.I.; Santos, C.E.S.; Alves, L.B.; Porciúncula, L.O.; Emanuelli, T.; Souza, D.O. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem. Int. 2002, 40, 621–627. [Google Scholar] [CrossRef]
- Ting, K.K.; Brew, B.; Guillemin, G. The involvement of astrocytes and kynurenine pathway in Alzheimer’s disease. Neurotox. Res. 2007, 12, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, P.; Bates, G.P.; Graham, R.K.; Hayden, M.R.; Leavitt, B.R.; MacDonald, M.E.; Slow, E.J.; Wheeler, V.C.; Woodman, B.; Schwarcz, R. Elevated brain 3-hydroxykynurenine and quinolinate levels in Huntington disease mice. Neurobiol. Dis. 2006, 23, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Swaih, A.M.; Breda, C.; Sathyasaikumar, K.V.; Allcock, N.; Collier, M.E.W.; Mason, R.P.; Feasby, A.; Herrera, F.; Outeiro, T.F.; Schwarcz, R.; et al. Kynurenine 3-monooxygenase interacts with huntingtin at the outer mitochondrial membrane. Biomedicines 2022, 10, 2294. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Tan, V.; Lovejoy, D.; Braidy, N.; Rowe, D.B.; Brew, B.J.; Guillemin, G.J. Involvement of quinolinic acid in the neuropathogenesis of amyotrophic lateral sclerosis. Neuropharmacology 2017, 112, 346–364. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Franco, N.F.; Ng, M.L.; Pai, S.; Lim, C.K.; Guillemin, G.J.; Brew, B.J. Current evidence for a role of the kynurenine pathway of tryptophan metabolism in multiple sclerosis. Front. Immunol. 2016, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Tan, V.X.; Guillemin, G.J. kynurenine pathway metabolites as biomarkers for amyotrophic lateral sclerosis. Front. Neurosci. 2019, 13, 1013. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, G.; Bessede, A.; Gilot, D.; Pires, A.S.; Sherman, L.S.; Brew, B.J.; Guillemin, G.J. Prophylactic and Therapeutic Effect of Kynurenine for Experimental Autoimmune Encephalomyelitis (EAE) Disease. Int. J. Tryptophan Res. 2022, 15, 11786469221118657. [Google Scholar] [CrossRef] [PubMed]
- Turska-Kozlowska, M.; Pedraz-Petrozzi, B.; Paluszkiewicz, P.; Parada-Turska, J. Different kynurenine pathway Dysregulation in Systemic Sclerosis in Men and Women. Int. J. Mol. Sci. 2024, 25, 3842. [Google Scholar] [CrossRef] [PubMed]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef]
- Massarali, A.; Adhya, D.; Srivastava, D.P.; Baron-Cohen, S.; Kotter, M.R. Virus-Induced Maternal Immune Activation as an Environmental Factor in the Etiology of Autism and Schizophrenia. Front. Neurosci. 2022, 16, 834058. [Google Scholar] [CrossRef] [PubMed]
- Lipner, E.; Murphy, S.K.; Ellman, L.M. Prenatal Maternal Stress and the Cascade of Risk to Schizophrenia Spectrum Disorders in Offspring. Curr. Psychiatry Rept. 2019, 21, 99. [Google Scholar] [CrossRef]
- Guma, E.; Plitrnan, E.; Chakravarty, M.M. The role of maternal immune activation in altering the neurodevelopmental trajectories of offspring: A translational review of neuroimaging studies with implications for autism spectrum disorder and schizophrenia. Neurosci. Biobehav. Revs. 2019, 104, 141–157. [Google Scholar] [CrossRef]
- Choudhury, Z.; Lennox, B. Maternal Immune Activation and Schizophrenia-Evidence for an Immune Priming Disorder. Front. Psychiatry 2021, 12, 585742. [Google Scholar] [CrossRef]
- Mawson, E.R.; Morris, B.J. A consideration of the increased risk of schizophrenia due to prenatal maternal stress, and the possible role of microglia. Progr. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 125, 110773. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, C.A.; Southcott, S.; Sacco, C.; Wagner, A.D.; Ghose, S. Glutamate Dysfunction in Hippocampus: Relevance of Dentate Gyrus and CA3 Signaling. Schizophr. Bull. 2012, 38, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, A.; Zukin, R.S. Schizophrenia: Evidence implicating hippocampal gluN2B protein and rest epigenetics in psychosis pathophysiology. Neuroscience 2015, 309, 233–242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Millan, M.J. NMDA receptors as a target for improved antipsychotic agents: Novel insights and clinical perspectives. Psychopharmacology 2005, 179, 30–53. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J.; Agid, Y.; Brüne, M.; Bullmore, E.T.; Carter, C.S.; Clayton, N.S.; Connor, R.; Davis, S.; Deakin, B.; DeRubeis, R.J.; et al. Cognitive dysfunction in psychiatric disorders: Characteristics, causes and the quest for improved therapy. Nature Rev. Drug Disc. 2012, 11, 141–168. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J.; Andrieux, A.; Bartzokis, G.; Cadenhead, K.; Dazzan, P.; Fusar-Poli, P.; Gallinat, J.; Giedd, J.; Grayson, D.R.; Heinrichs, M.; et al. Altering the course of schizophrenia: Progress and perspectives. Nature Rev. Drug Disc. 2016, 15, 485–515. [Google Scholar] [CrossRef] [PubMed]
- Kindler, J.; Lim, C.K.; Weickert, C.S.; Boerrigter, D.; Galletly, C.; Liu, D.; Jacobs, K.R.; Balzan, R.; Bruggemann, J.; O’Donnell, M.; et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol. Psychiatry 2020, 25, 2860–2872. [Google Scholar] [CrossRef] [PubMed]
- Khalil, O.S.; Pisar, M.; Forrest, C.M.; Vincenten, M.C.J.; Darlington, L.G.; Stone, T.W. Prenatal inhibition of the kynurenine pathway leads to structural changes in the hippocampus of adult rat offspring. Europ. J. Neurosci. 2014, 39, 1558–1571. [Google Scholar] [CrossRef] [PubMed]
- Pisar, M.; Forrest, C.M.; Khalil, O.S.; McNair, K.; Vincenten, M.C.J.; Qasem, S.; Darlington, L.G.; Stone, T.W. Modified neocortical and cerebellar protein expression and morphology in adult rats following prenatal inhibition of the kynurenine pathway. Brain Res. 2014, 1576, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Notarangelo, F.M.; Schwarcz, R. A single prenatal lipopolysaccharide injection has acute, but not long-lasting, effects on cerebral kynurenine pathway metabolism in mice. Europ. J. Neurosci. 2021, 54, 5968–5981. [Google Scholar] [CrossRef] [PubMed]
- Pocivavsek, A.; Wu, H.Q.; Elmer, G.I.; Bruno, J.P.; Schwarcz, R. Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. Europ. J. Neurosci. 2012, 35, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Pocivavsek, A.; Elmer, G.I.; Schwarcz, R. Inhibition of kynurenine aminotransferase II attenuates hippocampus-dependent memory deficit in adult rats treated prenatally with kynurenine. Hippocampus 2019, 29, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, S.; Schwieler, L.; Nilsson, L.; Linderholm, K.; Engberg, G. The kynurenic acid hypothesis of schizophrenia. Physiol. Behav. 2007, 92, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Kegel, M.E.; Bhat, M.; Skogh, E.; Samuelsson, M.; Lundberg, K.; Dahl, M.L.; Sellgren, C.; Schwieler, L.; Engberg, G.; Schuppe-Koistinen, I.; et al. Imbalanced kynurenine pathway in Schizophrenia. Int. J. Tryptophan Res. 2014, 7, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Klausing, A.D.; Fukuwatari, T.; Bucci, D.J.; Schwarcz, R. Stress-induced impairment in fear discrimination is causally related to increased kynurenic acid formation in the prefrontal cortex. Psychopharmacology 2020, 237, 1931–1941. [Google Scholar] [CrossRef]
- Chirico, M.; Custer, J.; Shoyombo, I.; Cooper, C.; Meldrum, S.; Dantzer, R.; Trivedi, M.H.; Rathouz, P.; Toups, M.S. Kynurenine pathway, metabolites selectively associate with impaired associative memory function in depression. Brain Behav. Immun. Health 2020, 8, 100126. [Google Scholar] [CrossRef] [PubMed]
- Sathyasaikumar, K.V.; Stachowski, E.K.; Wonodi, I.; Roberts, R.C.; Rassoulpour, A.; McMahon, R.P.; Schwarcz, R. Impaired kynurenine pathway Metabolism in The Prefrontal Cortex of Individuals With Schizophrenia. Schizophr. Bull. 2011, 37, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Sapienza, J.; Spangaro, M.; Guillemin, G.J.; Comai, S.; Bosia, M. Importance of the dysregulation of the kynurenine pathway on cognition in schizophrenia: A systematic review of clinical studies. Europ. Arch. Psychiatry Clin. Neurosci. 2023, 273, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Schwieler, L.; Larsson, M.K.; Skogh, E.; Kegel, M.E.; Orhan, F.; Abdelmoaty, S.; Finn, A.; Bhat, M.; Samuelsson, M.; Lundberg, K.; et al. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia-significance for activation of the kynurenine pathway. J. Psychiatry Neurosci. 2015, 40, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Sellgren, C.M.; Kegel, M.E.; Bergen, S.E.; Ekman, C.J.; Olsson, S.; Larsson, M.; Vawter, M.P.; Backlund, L.; Sullivan, P.F.; Sklar, P.; et al. A genome-wide association study of kynurenic acid in cerebrospinal fluid: Implications for psychosis and cognitive impairment in bipolar disorder. Mol. Psychiatry 2016, 21, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, S.; Olsson, S.K.; Engberg, G. Pharmacological Manipulation of Kynurenic Acid Potential in the Treatment of Psychiatric Disorders. CNS Drugs 2009, 23, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Beggiato, S.; Ieraci, A.; Tomasini, M.C.; Schwarcz, R.; Ferraro, L. Prenatal THC exposure raises kynurenic acid levels in the prefrontal cortex of adult rats. Progr. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 100, 109883. [Google Scholar] [CrossRef] [PubMed]
- Beggiato, S.; Ieraci, A.; Zuccarini, M.; Di Iorio, P.; Schwarcz, R.; Ferraro, L. Alterations in rat prefrontal cortex kynurenic acid levels are involved in the enduring cognitive dysfunctions induced by tetrahydrocannabinol exposure during the adolescence. Front. Psychiatry 2022, 13, 996406. [Google Scholar] [CrossRef] [PubMed]
- Jorratt, P.; Hoschl, C.; Ovsepian, S.V. Endogenous antagonists of NMDA receptor in schizophrenia. Alzheimers Dement. 2021, 17, 888–905. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J.; Drevets, W.C.; Wurfel, B.E.; Ford, B.N.; Bellgowan, P.S.F.; Victor, T.A.; Bodurka, J.; Teague, T.K.; Dantzer, R. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain Behav. Immun. 2015, 46, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J.; Drevets, W.C.; Smith, C.M.; Victor, T.A.; Wurfel, B.E.; Bellgowan, P.S.F.; Bodurka, J.; Teague, T.K.; Dantzer, R. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 2015, 40, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Gibney, S.M.; McGuinness, B.; Prendergast, C.; Harkin, A.; Connor, T.J. Poly I:C-induced activation of the immune response is accompanied by depression and anxiety-like behaviours, kynurenine pathway activation and reduced BDNF expression. Brain Behav. Immun 2013, 28, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2023, 28, 3243–3256. [Google Scholar] [CrossRef] [PubMed]
- Mackay, G.M.; Forrest, C.M.; Christofides, J.; Bridel, M.A.; Mitchell, S.; Cowlard, R.; Stone, T.W.; Darlington, L.G. Kynurenine metabolites and inflammation markers in depressed patients treated with fluoxetine or counselling. Clin Exp. Pharmacol. Physiol. 2009, 36, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Lawson, M.A.; Kelley, K.W. Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology 2011, 36, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.; Cordeiro, T.M.E.; Suchting, R.; de Dios, C.; Leal, V.A.C.; Soares, J.C.; Dantzer, R.; Teixeira, A.L.; Selvaraj, S. Effect of immune activation on the kynurenine pathway and depression symptoms—A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 118, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Mondanelli, G.; Coletti, A.; Greco, F.A.; Pallotta, M.T.; Orabona, C.; Iacono, A.; Belladonna, M.L.; Albini, E.; Panfili, E.; Fallarino, F.; et al. Positive allosteric modulation of indoleamine 2,3-dioxygenase 1 restrains neuroinflammation. Proc. Nat. Acad. Sci. USA 2020, 117, 3848–3857. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; Walker, A.K. Is there a role for glutamate-mediated excitotoxicity in inflammation-induced depression? J. Neural Transm. 2014, 121, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Comai, S.; Melloni, E.; Lorenzi, C.; Bollettini, I.; Vai, B.; Zanardi, R.; Colombo, C.; Valtorta, F.; Benedetti, F.; Poletti, S. Selective association of cytokine levels and kynurenine/tryptophan ratio with alterations in white matter microstructure in bipolar but not in unipolar depression. Europ. Neuropsychopharmacol. 2022, 55, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, T.; Murakami, Y.; Mouri, A.; Imamura, Y.; Nabeshima, T.; Yamamoto, Y.; Saito, K. Kynurenine 3-monooxygenase is implicated in antidepressants-responsive depressive-like behaviors and monoaminergic dysfunctions. Behav. Brain Res. 2017, 317, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Parrott, J.M.; Redus, L.; Santana-Coelho, D.; Morales, J.; Gao, X.; O’Connor, J.C. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl. Psychiatry 2016, 6, e918. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Misiak, B.O.D.; Callovini, T.; Cavaleri, D.; Cioni, R.M.; Crocamo, C.; Savitz, J.B.; Carrà, G. The kynurenine pathway in bipolar disorder: A meta-analysis on the peripheral blood levels of tryptophan and related metabolites. Mol. Psychiatry 2021, 26, 3419–3429. [Google Scholar] [CrossRef] [PubMed]
- Ogyu, K.; Kubo, K.; Noda, Y.; Iwata, Y.; Tsugawa, S.; Omura, Y.; Wada, M.; Tarumi, R.; Plitman, E.; Moriguchi, S.; et al. Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 90, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P.; et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: A meta-analysis of 101 studies. Mol. Psychiatry 2021, 26, 4158–4178. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.X.; Teague, T.K.; Yeh, F.C.; Burrows, K.; Figueroa-Hall, L.K.; Aupperle, R.L.; Khalsa, S.S.; Paulus, M.P.; Savitz, J. C-Reactive protein and the kynurenic acid to quinolinic acid ratio are independently associated with white matter integrity in major depressive disorder. Brain Behav. Immun 2022, 105, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Pothula, S.; Liu, R.J.; Wu, M.; Sliby, A.N.; Picciotto, M.R.; Banerjee, P.; Duman, R.S. Positive modulation of NMDA receptors by AGN-241751 exerts rapid antidepressant-like effects via excitatory neurons. Neuropsychopharmacology 2021, 46, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.; Zarina, S.; Bano, S. Docking studies of antidepressants against single crystal structure of tryptophan 2, 3-dioxygenase using Molegro Virtual Docker software. Pak. J. Pharm. Sci. 2014, 27, 1529–1539. [Google Scholar] [PubMed]
- Dawood, S.; Bano, S.; Badawy, A.A. Inflammation and serotonin deficiency in major depressive disorder: Molecular docking of antidepressant and anti-inflammatory drugs to tryptophan and indoleamine 2,3-dioxygenases. Biosci Rep. 2022, 42, BSR20220426. [Google Scholar] [CrossRef]
- Badawy, A.A.-B.; Dawood, S.; Bano, S. Kynurenine pathway of tryptophan metabolism in pathophysiology and therapy of major depressive disorder. World J. Psychiatry 2023, 13, 141–148. [Google Scholar] [CrossRef]
- Beal, M.F.; Swartz, K.J.; Hyman, B.T.; Storey, E.; Finn, S.F.; Koroshetz, W. Aminooxyacetic Acid Results In Excitotoxin Lesions By A Novel Indirect Mechanism. J. Neurochem. 1991, 57, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, E.; Ikonomidou, C.; Sieklucka, M.; Turski, W.A. Aminooxyacetic acid produces excitotoxic lesions in the rat striatum. Synapse 1991, 9, 129–135. [Google Scholar] [CrossRef] [PubMed]
- McMaster, O.G.; Du, F.; French, E.D.; Schwarcz, R. Focal injection of aminooxyacetic acid produces seizures and lesions in rat hippocampus: Evidence for mediation by NMDA receptors. Exp. Neurol. 1991, 113, 378–385. [Google Scholar] [CrossRef]
- Pierozan, P.; Biasibetti-Brendler, H.; Schmitz, F.; Ferreira, F.; Pessoa-Pureur, R.; Wyse, A.T.S. Kynurenic acid prevents cytoskeletal disorganization induced by quinolinic acid in mixed cultures of rat striatum. Mol. Neurobiol. 2018, 55, 5111–5124. [Google Scholar] [CrossRef]
- Santamaría, A.; Galván-Arzate, S.; Lisy, V.; Ali, S.F.; Duhart, H.M.; Osorio-Rico, L.; Ríos, C.; St’astny, F. Quinolinic acid induces oxidative stress in rat brain synaptosomes. Neuroreport 2001, 12, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Martinez, J.-M.; Forrest, C.M.; Darlington, L.G.; Smith, R.A.; Stone, T.W. Quinolinic acid induces neuritogenesis in SH-SY5Y neuroblastoma cells independently of NMDA receptor activation. Europ. J. Neurosci. 2016, 45, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Elizei, S.S.; Poormasjedi-Meibod, M.S.; Li, Y.Y.; Jalili, R.B.; Ghahary, A. Effects of kynurenine on CD3+ and macrophages in wound healing. Wound Repair Regen. 2015, 23, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Poormasjedi-Meibod, M.S.; Elizei, S.S.; Leung, V.; Jalili, R.B.; Ko, F.; Ghahary, A. Kynurenine Modulates MMP-1 and Type-I collagen expression via aryl hydrocarbon receptor activation in dermal fibroblasts. J. Cell. Physiol. 2016, 231, 2749–2760. [Google Scholar] [CrossRef] [PubMed]
- Summers, B.S.; Broome, S.T.; Pang, T.W.R.; Mundell, H.D.; Belic, N.K.; Tom, N.C.; Ng, M.L.; Yap, M.; Sen, M.K.; Sedaghat, S.; et al. A review of the evidence for tryptophan and the kynurenine pathway as a regulator of stem cell niches in health and disease. Int. J. Trp. Res. 2024, 17, 11786469241248287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pang, A.; Yang, Y. Hydrogels containing KYNA promote angiogenesis and inhibit inflammation to improve the survival rate of multi-territory perforator flaps. Biomed. Pharmacother. 2024, 174, 116454. [Google Scholar] [CrossRef]
- O’Reilly, K.; O’Farrell, K.; Midttun, O.; Rakovets, Y.; Bercholz, J.D.; Harkin, A. Kynurenic Acid Protects Against Reactive Glial-associated Reductions in the Complexity of Primary Cortical Neurons. J. Neuroimmune Pharmacol. 2021, 16, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Misztal, T.; Roszkowicz-Ostrowska, K.; Kowalczyk, P.; Młotkowska, P.; Marciniak, E. Kynurenic Acid Modulates the Expression of Genes and Activity of Cellular Antioxidant Enzymes in the Hypothalamus and Hippocampus in Sheep. Preprints 2024, in press. [Google Scholar] [CrossRef]
- Bagasrawala, I.; Zecevic, N.; Radonjic, N.V. N-Methyl D-Aspartate Receptor Antagonist Kynurenic Acid Affects Human Cortical Development. Front. Neurosci. 2016, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Clark, S.M.; Vaughn, C.N.; Nicholson, J.D.; Murphy, K.J.; Mou, T.C.M.; Schwarcz, R.; Hoffman, G.E.; Tonelli, L.H. Quantitative analysis of kynurenine aminotransferase ii in the adult rat brain reveals high expression in proliferative zones and corpus callosum. Neuroscience 2018, 369, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wyant, G.A.; Yu, W.; Doulamis, I.P.; Nomoto, R.S.; Saeed, M.Y.; Duignan, T.; McCully, J.D.; Kaelin, G.G. Mitochondrial Remodeling and Ischemic Protection by G-protein coupled receptor 35 Agonists. Science 2022, 377, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Malaczewska, J.; Siwicki, A.K.; Wójcik, R.M.; Turski, W.A.; Kaczorek, E. The effect of kynurenic acid on the synthesis of selected cytokines by murine splenocytes-in vitro and ex vivo studies. Central Europ. J. Immunol. 2016, 41, 39–46. [Google Scholar] [CrossRef] [PubMed]
- La Russa, D.; Di Santo, C.; Lizasoain, I.; Moraga, A.; Bagetta, G.; Amantea, D. Tumor Necrosis Factor (TNF)-α-Stimulated Gene 6 (TSG-6): A Promising Immunommodulatory Target in Acute Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 1162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, G.; Cao, K.; Liu, K.L.; Xue, Y.Q.; Roberts, A.I.; Li, F.Y.; Han, Y.Y.; Rabson, A.B.; Wang, Y.; Shi, Y.F. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018, 25, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.C.; Fang, J.K.; Liu, Z.H.; Hou, P.B.; Cao, L.J.; Zhang, Y.Y.; Liu, R.; Li, Y.A.; Shang, Q.W.; Chen, Y.J.; et al. Inflammatory cytokines-stimulated human muscle stem cells ameliorate ulcerative colitis via the IDO-TSG6 axis. Stem Cell Res. Ther. 2021, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Mándi, Y.; Endrész, V.; Mosolygó, T.; Burián, K.; Lantos, I.; Fülöp, F.; Szatmári, I.; Lorinczi, B.; Balog, A.; Vécsei, L. The opposite effects of kynurenic acid and different kynurenic acid analogs on tumor necrosis factor-α (TNF-α) production and tumor necrosis factor-stimulated gene-6 (TSG-6) expression. Front. Immunol. 2019, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Musso, G.; Contran, N.; Basso, D. Inflammation, Autoinflammation and Autoimmunity in Inflammatory Bowel Diseases. Curr. Issues Mol. Biol. 2023, 45, 5534–5557. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Taghizadieh, M.; Mehdizadehfar, E.; Hasani, A.; Fard, J.K.; Feizi, H.; Hamishehkar, H.; Ansarin, M.; Yekani, M.; Memar, M.Y. Gut microbiota in neurological diseases: Melatonin plays an important regulatory role. Biomed. Pharmacother. 2024, 174, 116487. [Google Scholar] [CrossRef] [PubMed]
- Dudzinska, E.; Szymona, K.; Kloc, R.; Gil-Kulik, P.; Kocki, T.; Swistowska, M.; Bogucki, J.; Kocki, J.; Urbanska, E.M. Increased expression of kynurenine aminotransferases mRNA in lymphocytes of patients with inflammatory bowel disease. Therap. Adv. Gastroenterol. 2019, 12, 1756284819881304. [Google Scholar] [CrossRef] [PubMed]
- Zhen, D.L.; Liu, J.J.; Zhang, X.D.; Song, Z.H. Kynurenic Acid Acts as a signaling molecule regulating energy expenditure and is closely associated with metabolic diseases. Front. Endocrinol. 2022, 13, 847611. [Google Scholar] [CrossRef] [PubMed]
- Zhen, D.L.; Ding, L.A.; Wang, B.; Wang, X.L.; Hou, Y.L.; Ding, W.Y.; Portha, B.; Liu, J.J. Oral administration of kynurenic acid delays the onset of type 2 diabetes in Goto-Kakizaki rats. Heliyon 2023, 9, e17733. [Google Scholar] [CrossRef] [PubMed]
- Jayawickrama, G.S.; Nematollahi, A.; Sun, G.C.; Gorrell, M.D.; Church, W.B. Inhibition of human kynurenine aminotransferase isozymes by estrogen and its derivatives. Sci. Repts. 2017, 7, 17559. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Q.; Ungerstedt, U.; Schwarcz, R. L-alpha-aminoadipic acid as a regulator of kynurenic acid production inthe hippocampus-a microdialysis study in freely moving rats. Europ. J. Pharmacol. 1995, 281, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hodgkins, P.S.; Wu, H.Q.; Zielke, H.R.; Schwarcz, R. 2-Oxoacids regulate kynurenic acid production in the rat brain: Studies in vitro and in vivo. J. Neurochem. 1999, 72, 643–651. [Google Scholar] [CrossRef]
- Heischmann, S.; Gano, L.B.; Quinn, K.; Liang, L.P.; Klepacki, J.; Christians, U.; Reisdorph, N.; Patel, M. Regulation of kynurenine metabolism by a ketogenic diet. J. Lipid Res. 2018, 59, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Zarnowska, I.; Wróbel-Dudzinska, D.; Tulidowicz-Bielak, M.; Kocki, T.; Mitosek-Szewczyk, K.; Gasior, M.; Turski, W.A. Changes in tryptophan and kynurenine pathway metabolites in the blood of children treated with ketogenic diet for refractory epilepsy. Seizure-Europ. J. Epilepsy 2019, 69, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Zarnowski, T.; Choragiewicz, T.J.; Schuettauf, F.; Zrenner, E.; Rejdak, R.; Gasior, M.; Zarnowska, I.; Thaler, S. Ketogenic Diet Attenuates NMDA-induced damage to rat’s retinal ganglion cells in an age-dependent manner. Ophthalmic Res. 2015, 53, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Zarnowski, T.; Tulidowicz-Bielak, M.; Zarnowska, I.; Mitosek-Szewczyk, K.; Wnorowski, A.; Józwiak, K.; Gasior, M.; Turski, W.A. Kynurenic Acid and Neuroprotective Activity of the Ketogenic Diet in the Eye. Curr. Med. Chem. 2017, 24, 3547–3558. [Google Scholar] [CrossRef] [PubMed]
- Rejdak, R.; Junemann, A.; Grieb, P.; Thaler, S.; Schuettauf, F.; Choragiewicz, T.; Zarnowski, T.; Turski, W.A.; Zrenner, E. Kynurenic acid and kynurenine aminotransferases in retinal aging and neurodegeneration. Pharmacol. Rep. 2011, 63, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Chmiel-Perzynska, I.; Kloc, R.; Perzynski, A.; Rudzki, S.; Urbanska, E.M. Novel aspect of ketone action: β-hydroxybutyrate increases brain synthesis of kynurenic acid in vitro. Neurotox. Res. 2011, 20, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Chmiel-Perzynska, I.; Perzynski, A.; Urbanska, E.M. Experimental diabetes mellitus type 1 increases hippocampal content of kynurenic acid in rats. Pharmacol. Rep. 2014, 66, 1134–1139. [Google Scholar] [CrossRef]
- Rosser, E.C.; Piper, C.J.M.; Matei, D.E.; Blair, P.A.; Rendeiro, A.F.; Orford, M.; Alber, D.G.; Krausgruber, T.; Catalan, D.; Klein, N.; et al. Microbiota-Derived Metabolites Suppress Arthritis by Amplifying Aryl-Hydrocarbon Receptor Activation in Regulatory B Cells. Cell. Metab. 2020, 31, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Luchowska, E.; Kloc, R.; Olajossy, B.; Wnuk, S.; Wielosz, M.; Owe-Larsson, B.; Urbanska, E.M. β-adrenergic enhancement of brain kynurenic acid production mediated via cAMP-related protein kinase A signaling. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Fujigaki, H.; Kato, K.; Yamazaki, K.; Fujigaki, S.; Kunisawa, K.; Yamamoto, Y.; Mouri, A.; Oda, A.; Nabeshima, T.; et al. Selective and competitive inhibition of kynurenine aminotransferase-2 by glycyrrhizic acid and its analogues. Sci. Rept. 2019, 9, 10243. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Jamie, J.; Guillemin, G.J. Kynurenine-3-monooxygenase: A review of structure, mechanism, and inhibitors. Drug Disc. Today 2016, 21, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.F.; Shao, M.M.; Wu, T. Kynurenine-3-monooxygenase: A new direction for the treatment in different diseases. Food Sci. Nutr. 2020, 8, 711–719. [Google Scholar] [CrossRef]
- Ulvik, A.; Theofylaktopoulou, D.; Midttun, O.; Nygård, O.; Eussen, S.J.P.M.; Ueland, P.M. Substrate product ratios of enzymes in the kynurenine pathway measured in plasma as indicators of functional vitamin B-6 status. Amer. J. Clin. Nutr. 2013, 98, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Theofylaktopoulou, D.; Midttun, O.; Ulvik, A.; Ueland, P.M.; Tell, G.S.; Vollset, S.E.; Nygård, O.; Eussen, S.J.P.M. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: The Hordaland Health Study. Clin. Exp. Immunol. 2013, 173, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Theofylaktopoulou, D.; Ulvik, A.; Midttun, O.; Ueland, P.M.; Vollset, S.E.; Nygård, O.; Hustad, S.; Tell, G.S.; Eussen, S.J.P.M. Vitamins B2 and B6 as determinants of kynurenines and related markers of interferon-γ-mediated immune activation in the community-based Hordaland Health Study. Brit. J. Nutr. 2014, 112, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.Q.; Yang, N.D.; Ding, Y.Q.; Han, L.Q.; Zhou, Z.Q.; Guo, X.L.; Fang, Z.J.; Bai, H.; Peng, B.; Zhang, C.W.; et al. Mitochondrial-Targeting Vitamin B3 ameliorates the phenotypes of parkinson’s disease in vitro and in vivo by restoring mitochondrial function. Adv. Ther. 2022, 5, 2200094. [Google Scholar] [CrossRef]
- Connick, J.H.; Stone, T.W. The Role of Kynurenines in Diabetes-Mellitus. Med. Hypotheses 1985, 18, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Rebnord, E.W.; Strand, E.; Midttun, O.; Svingen, G.F.T.; Christensen, M.H.E.; Ueland, P.M.; Mellgren, G.; Njolstad, P.R.; Tell, G.S.; Nygard, O.K.; et al. The kynurenine: Tryptophan ratio as a predictor of incident type 2 diabetes mellitus in individuals with coronary artery disease. Diabetologia 2017, 60, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, J.; Staniszewska, M.; Sadok, I.; Sypniewska, G.; Stefanska, A. The kynurenine pathway in Obese Middle-Aged Women with Normoglycemia and Type 2 Diabetes. Metabolites 2022, 12, 492. [Google Scholar] [CrossRef] [PubMed]
- Koziel, K.; Urbanska, E.M. kynurenine pathway in Diabetes Mellitus-Novel Pharmacological Target? Cells 2023, 12, 460. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.N.; Ma, Z.J.; Jin, L.Z.; Nizhamuding, X.; Zeng, J.; Zhang, T.J.; Zhang, J.T.; Wang, J.; Zhao, H.J.; Zhou, W.Y.; et al. Altered neopterin and IDO in kynurenine metabolism based on LC-MS/MS metabolomics study: Novel therapeutic checKPoints for type 2 diabetes mellitus. Clin. Chim. Acta 2024, 557, 117859. [Google Scholar] [CrossRef] [PubMed]
- Bednarz, K.; Koziel, K.; Urbanska, E.M. Novel Activity of Oral Hypoglycemic Agents Linked with Decreased Formation of Tryptophan Metabolite, Kynurenic Acid. Life Basel 2024, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Mole, D.J.; Webster, S.P.; Uings, I.; Zheng, X.Z.; Binnie, M.; Wilson, K.; Hutchinson, J.P.; Mirguet, O.; Walker, A.; Beaufils, B.; et al. Kynurenine-3-monooxygenase inhibition prevents multiple organ failure in rodent models of acute pancreatitis. Nat. Med. 2016, 22, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.J.; Zheng, X.Z.; O’Kelly, J.; Neyton, L.P.A.; Bochkina, N.A.; Uings, I.; Liddle, J.; Baillie, J.K.; Just, G.; Binnie, M.; et al. Kynurenine monooxygenase regulates inflammation during critical illness and recovery in experimental acute pancreatitis. Cell Rep. 2023, 42, 112763. [Google Scholar] [CrossRef] [PubMed]
- Bakker, O.J.; Issa, Y.; van Santvoort, H.C.; Besselink, M.G.; Schepers, N.J.; Bruno, M.J.; Boermaster, M.A.; Gooszen, H.G. Treatment optionis for acute pancreatities. Nature Rev. Gastroenterol. Hepatol. 2014, 11, 462–469. [Google Scholar] [CrossRef]
- Hutchinson, J.P.; Rowland, P.; Taylor, M.R.D.; Christodoulou, E.M.; Haslam, C.; Hobbs, C.I.; Holmes, D.S.; Homes, P.; Liddle, J.; Mole, D.J.; et al. Structural and mechanistic basis of differentiated inhibitors of the acute pancreatitis target kynurenine-3-monooxygenase. Nature Commun. 2017, 8, 15827. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.J.; Manfredi, M.G.; Zakharia, Y.; Prendergast, G.C. Inhibiting IDO pathways to treat cancer: Lessons from the ECHO-301 trial and beyond. Seminars Immunopathol. 2019, 41, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Williams, R.O. Modulation of T cells by tryptophan metabolites in the kynurenine pathway. Trends Pharmacol. Sci. 2023, 44, 442–456. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kato, S.; Nesline, M.K.; Conroy, J.M.; DePietro, P.; Pabla, S.; Kurzrock, R. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 2022, 110, 102461. [Google Scholar] [CrossRef] [PubMed]
- Gotina, L.; Seo, S.H.; Kim, C.W.; Lim, S.M.; Pae, A.N. Pharmacophore-Based Virtual Screening of Novel Competitive Inhibitors of the Neurodegenerative Disease Target Kynurenine-3-Monooxygenase. Molecules 2021, 26, 3314. [Google Scholar] [CrossRef] [PubMed]
- Rebai, R.; Carmena-Bargueño, M.; Toumi, M.E.; Derardja, I.; Jasmin, L.; Pérez-Sánchez, H.; Boudah, A. Identification of potent inhibitors of kynurenine-3-monooxygenase from natural products: In silico and in vitro approaches. Heliyon 2024, 10, e30287. [Google Scholar] [CrossRef] [PubMed]
- Walczak, K.; Wnorowski, A.; Turski, W.A.; Plech, T. Kynurenic acid and cancer: Facts and controversies. Cell Mol. Life Sci. 2020, 77, 1531–1550. [Google Scholar] [CrossRef] [PubMed]
- Perez-Castro, L.; Garcia, R.; Venkateswaran, N.; Barnes, S.; Conacci-Sorrell, M. Tryptophan and its metabolites in normal physiology and cancer etiology. FEBS J. 2021, 290, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.-B. Tryptophan metabolism and disposition in cancer biology and immunotherapy. Biosci. Rep. 2022, 42, BSR20221682. [Google Scholar] [CrossRef] [PubMed]
- Hezaveh, K.; Shinde, R.S.; Klotgen, A.; Halaby, M.J.; Lamorte, S.; Ciudad, M.T.; Quevedo, R.; Neufeld, L.; Liu, Z.Q.; Jin, R.; et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumour immunity. Immunity 2022, 55, 324–340. [Google Scholar] [CrossRef] [PubMed]
- Papp, A.; Hartwell, R.; Evans, M.; Ghahary, A. The safety and tolerability of topically delivered kynurenic acid in humans. A phase 1 randomized double-blind clinical trial. J Pharm. Sci. 2018, 107, 1572–1576. [Google Scholar] [CrossRef] [PubMed]
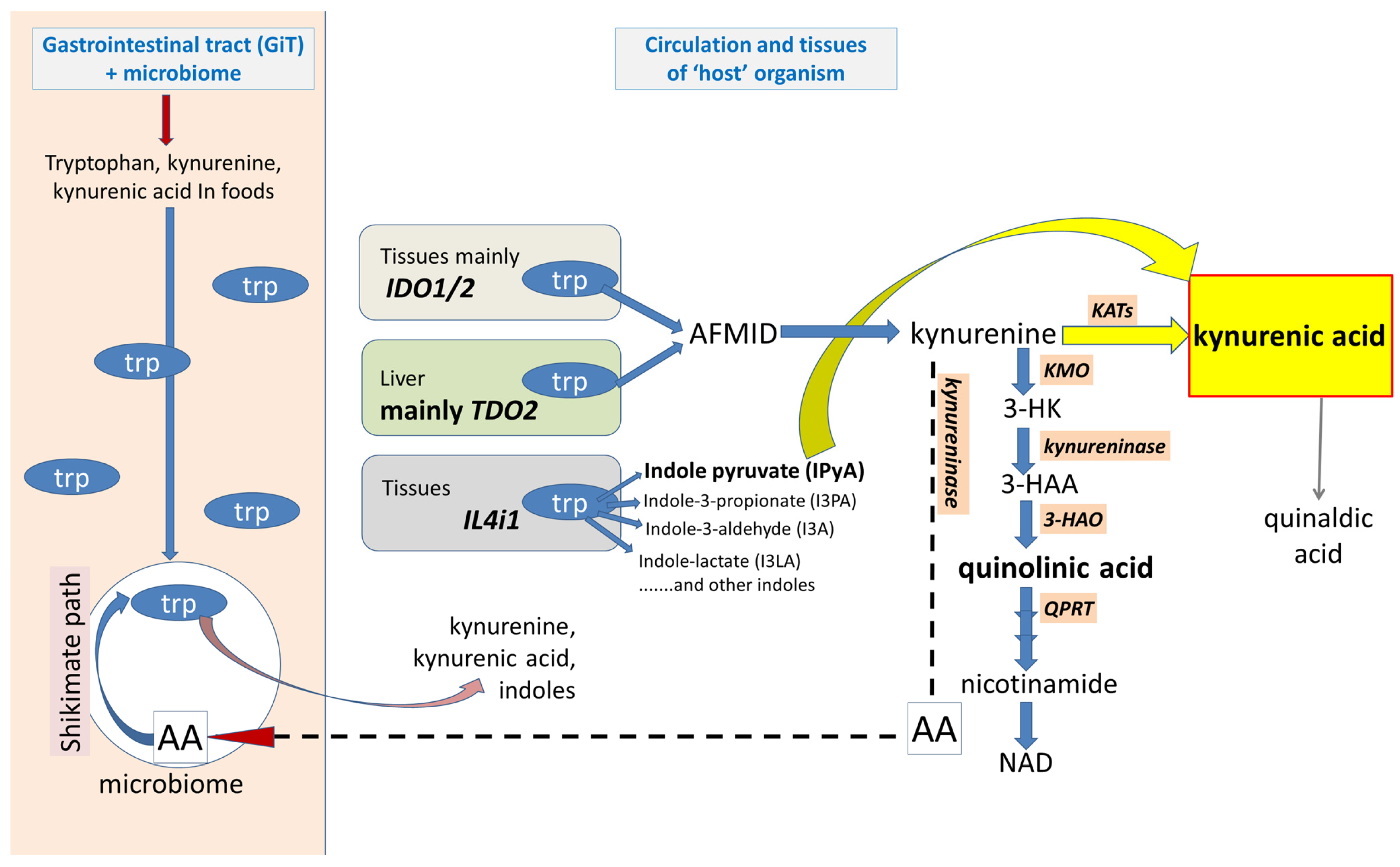
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stone, T.W.; Darlington, L.G.; Badawy, A.A.-B.; Williams, R.O. The Complex World of Kynurenic Acid: Reflections on Biological Issues and Therapeutic Strategy. Int. J. Mol. Sci. 2024, 25, 9040. https://doi.org/10.3390/ijms25169040
Stone TW, Darlington LG, Badawy AA-B, Williams RO. The Complex World of Kynurenic Acid: Reflections on Biological Issues and Therapeutic Strategy. International Journal of Molecular Sciences. 2024; 25(16):9040. https://doi.org/10.3390/ijms25169040
Chicago/Turabian StyleStone, Trevor W., L. Gail Darlington, Abdulla A.-B. Badawy, and Richard O. Williams. 2024. "The Complex World of Kynurenic Acid: Reflections on Biological Issues and Therapeutic Strategy" International Journal of Molecular Sciences 25, no. 16: 9040. https://doi.org/10.3390/ijms25169040
APA StyleStone, T. W., Darlington, L. G., Badawy, A. A.-B., & Williams, R. O. (2024). The Complex World of Kynurenic Acid: Reflections on Biological Issues and Therapeutic Strategy. International Journal of Molecular Sciences, 25(16), 9040. https://doi.org/10.3390/ijms25169040







