Regeneration and Plasticity Induced by Epidural Stimulation in a Rodent Model of Spinal Cord Injury
Abstract
:1. Introduction
2. Results
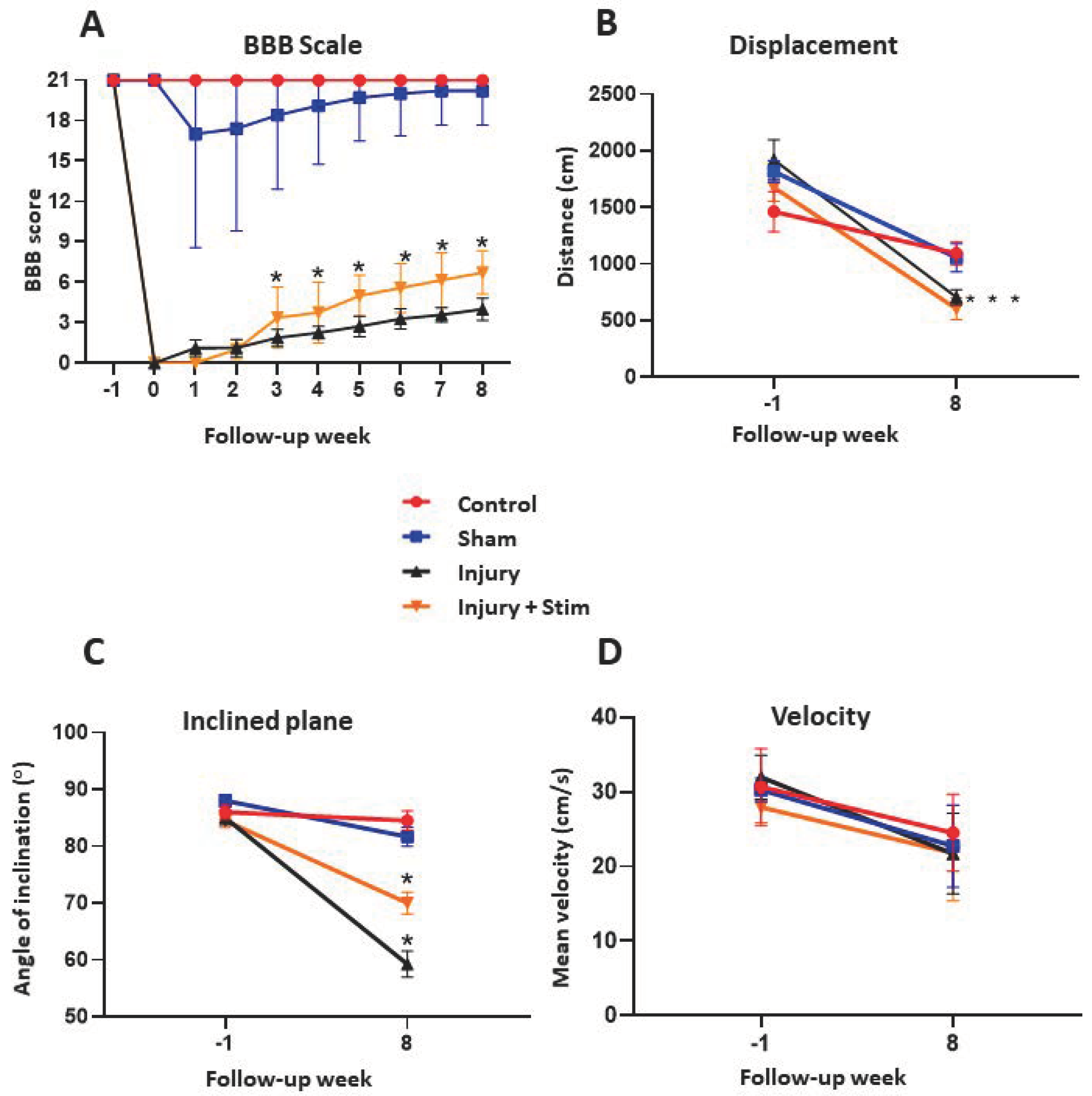
2.1. BBB
2.2. Inclined Plane
2.3. Open Field
2.4. Histological Analysis
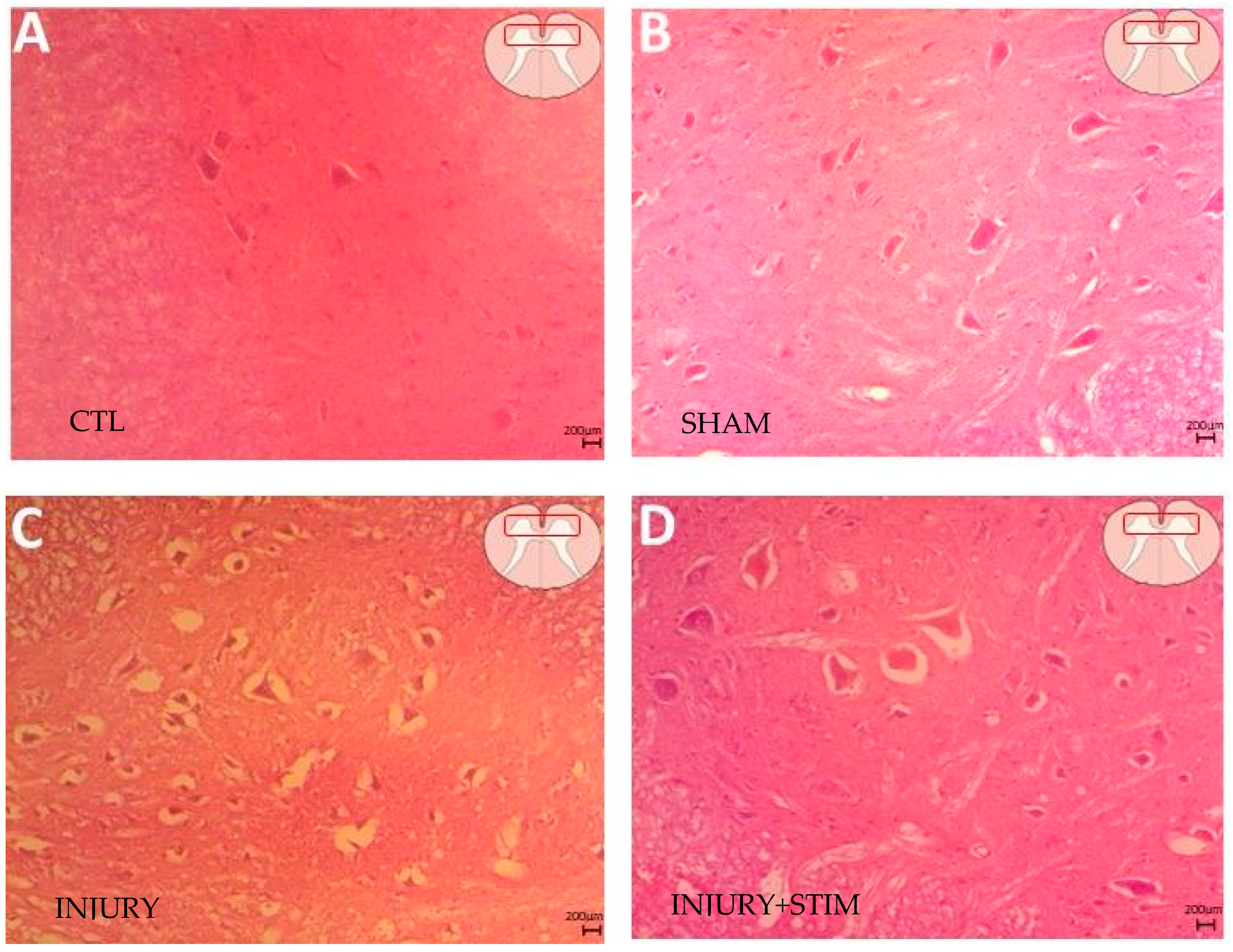
2.4.1. Hematoxylin and Eosin
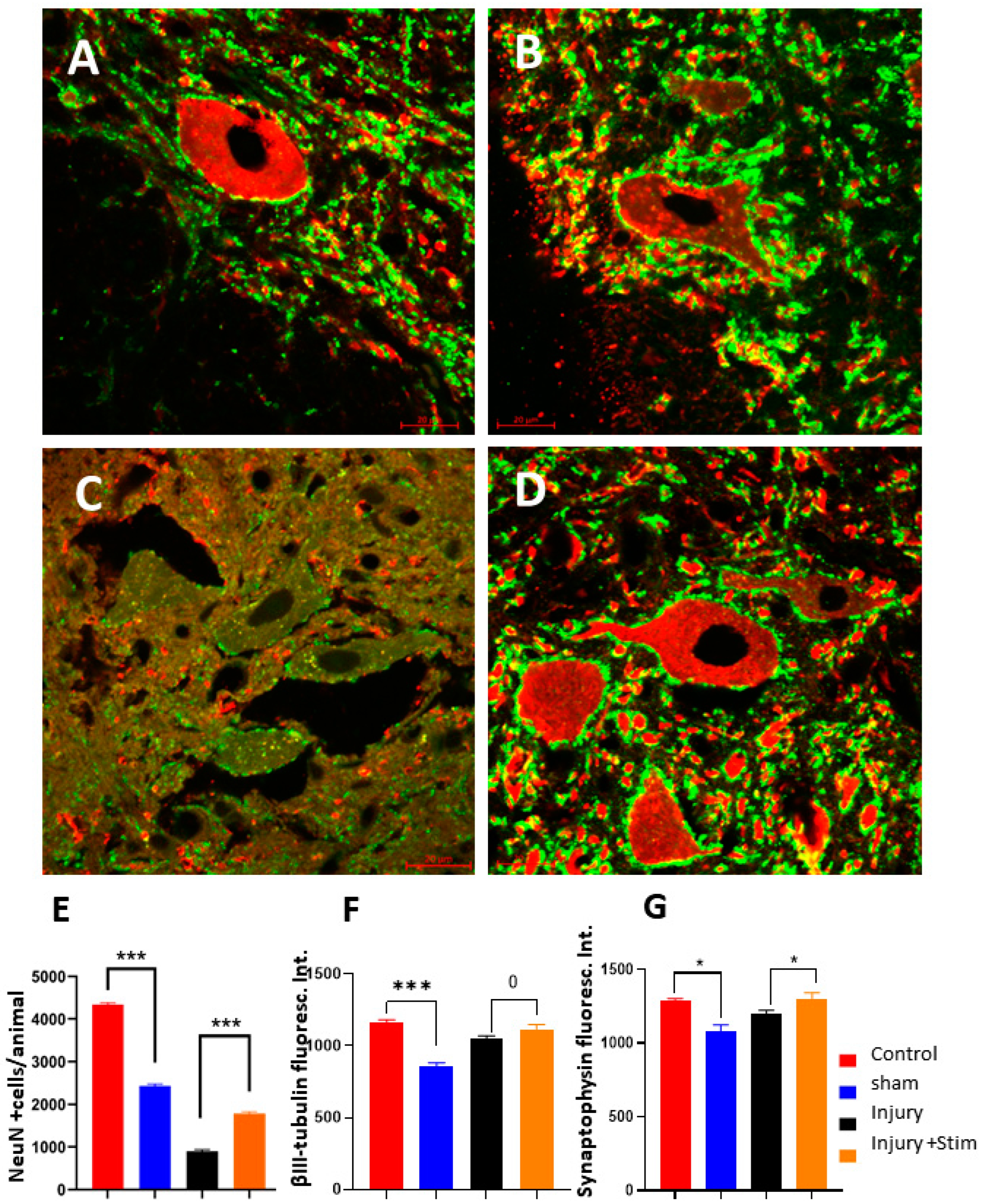
2.4.2. Stereology for NeuN-Immunopositive Cells
2.4.3. Synaptohysin and β-III-Tubulin Fluorescence
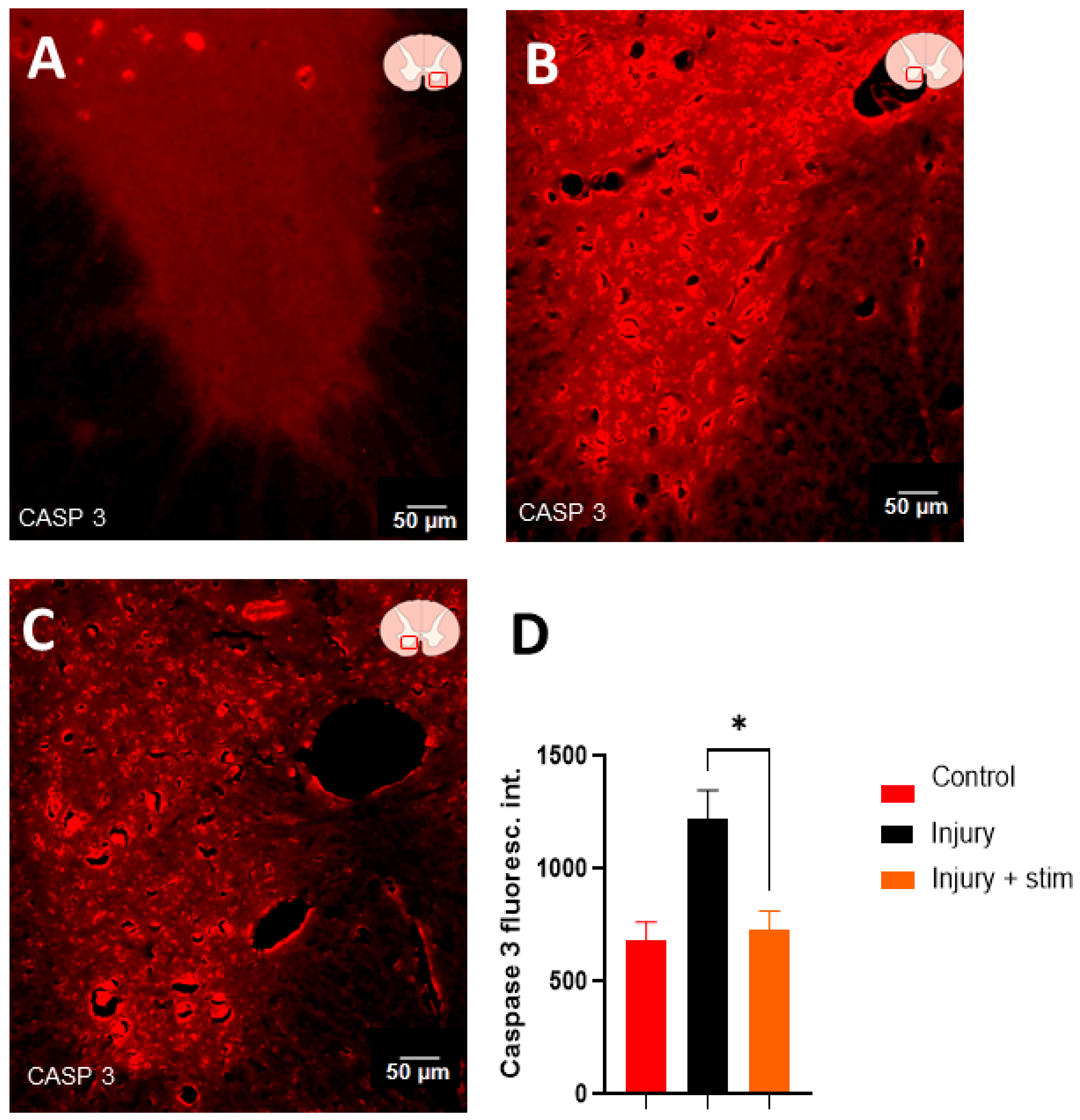
2.4.4. Caspase 3 Levels
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics
4.2. Experimental Design
4.3. Impact Lesion Model
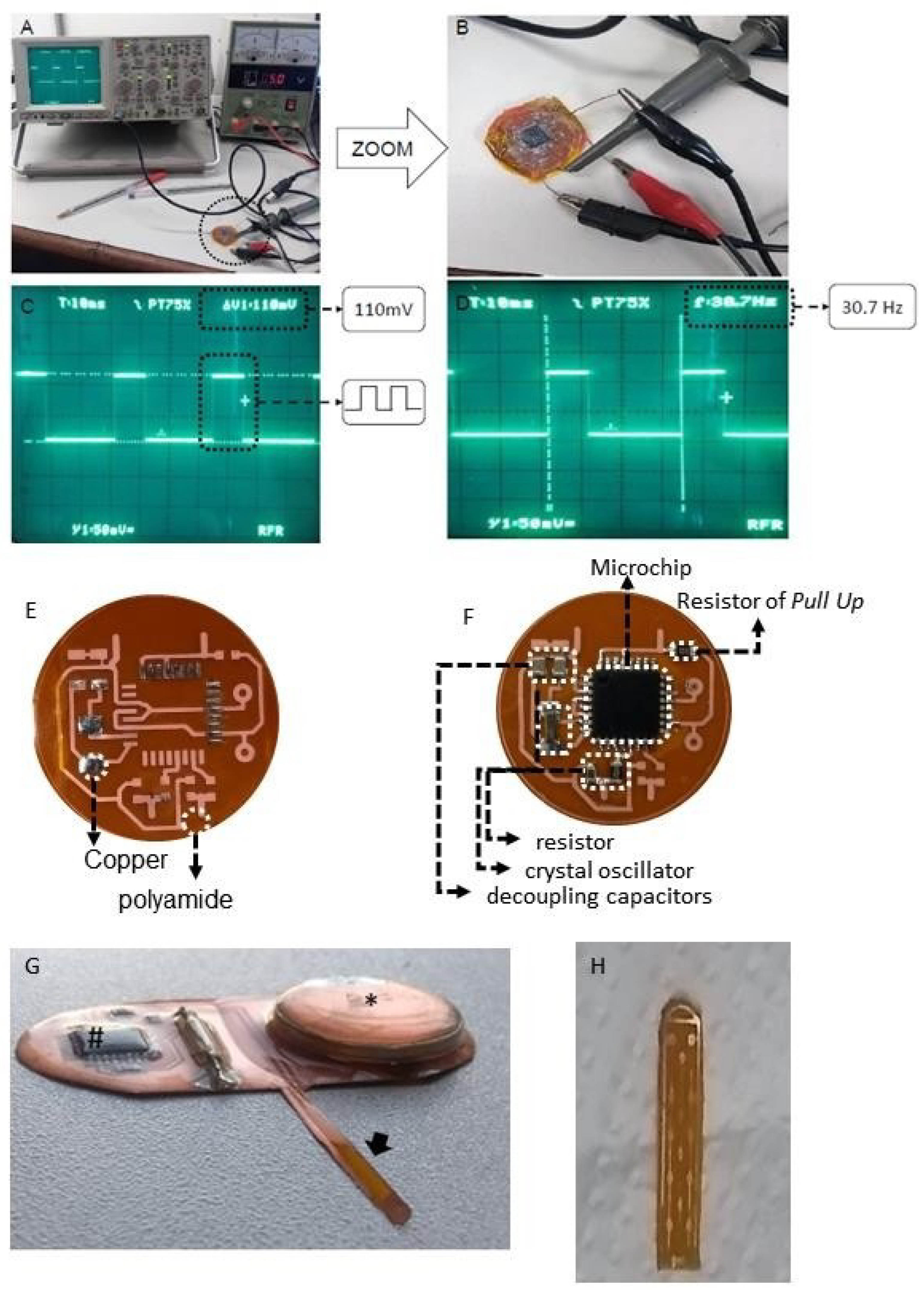
4.4. Spinal Cord Stimulation
4.5. Behavioral Analysis
4.5.1. Motricity/Basso, Beattie, Bresnahan (BBB) Locomotor Rating Scale
4.5.2. Inclined Plane
4.5.3. Open Field
4.6. Immunohistochemistry and Cell Quantification
4.6.1. Estimates of NeuN-Immunopositive Cells
4.6.2. Fluorescence Quantifications
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- GBD Collaborator Network. Global, regional, and national burden of disorders affecting the nervous system, 1990-2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef] [PubMed]
- GBD Collaborator Network. Global, regional, and national burden of spinal cord injury, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2023, 22, 1026–1047. [Google Scholar] [CrossRef] [PubMed]
- Hachem, L.D.; Ahuja, C.S.; Fehlings, M.G. Assessment and management of acute spinal cord injury: From point of injury to rehabilitation. J. Spinal Cord. Med. 2017, 40, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Mariano, E.D.; Batista, C.M.; Barbosa, B.J.; Marie, S.K.; Teixeira, M.J.; Morgalla, M.; Tatagiba, M.; Li, J.; Lepski, G. Current perspectives in stem cell therapy for spinal cord repair in humans: A review of work from the past 10 years. Arq. Neuropsiquiatr. 2014, 72, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lepski, G. Cell transplantation for spinal cord injury: A systematic review. Biomed. Res. Int. 2013, 2013, 786475. [Google Scholar] [CrossRef]
- Moraud, E.M.; Capogrosso, M.; Formento, E.; Wenger, N.; DiGiovanna, J.; Courtine, G.; Micera, S. Mechanisms Underlying the Neuromodulation of Spinal Circuits for Correcting Gait and Balance Deficits after Spinal Cord Injury. Neuron 2016, 89, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Moghaddamjou, A.; Harrop, J.S.; Stanford, R.; Ball, J.; Aarabi, B.; Freeman, B.J.C.; Arnold, P.M.; Guest, J.D.; Kurpad, S.N.; et al. Safety and Efficacy of Riluzole in Acute Spinal Cord Injury Study (RISCIS): A Multi-Center, Randomized, Placebo-Controlled, Double-Blinded Trial. J. Neurotrauma 2023, 40, 1878–1888. [Google Scholar] [CrossRef] [PubMed]
- Casha, S.; Zygun, D.; McGowan, M.D.; Bains, I.; Yong, V.W.; Hurlbert, R.J. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 2012, 135 Pt 4, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yamazaki, M.; Okawa, A.; Sakuma, T.; Kato, K.; Hashimoto, M.; Hayashi, K.; Furuya, T.; Fujiyoshi, T.; Kawabe, J.; et al. Neuroprotective therapy using granulocyte colony-stimulating factor for acute spinal cord injury: A phase I/IIa clinical trial. Eur. Spine J. 2012, 21, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Lepski, G.; Batista, C.M.; Mariano, E.D.; Dale, C.S.; Cristante, A.F.; Britto, L.R.; Otoch, J.P.; Teixeira, M.J.; Morgalla, M. Pain inhibition through transplantation of fetal neuronal progenitors into the injured spinal cord in rats. Neural Regen. Res. 2019, 14, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Lepski, G. Transplantation of GABAergic precursors into the spinal cord to alleviate neuropathic pain. Ann. Palliat. Med. 2020, 9, 2437–2441. [Google Scholar] [CrossRef] [PubMed]
- Fessler, R.G.; Ehsanian, R.; Liu, C.Y.; Steinberg, G.K.; Jones, L.; Lebkowski, J.S.; Wirth, E.D., III; McKenna, S.L. A phase 1/2a dose-escalation study of oligodendrocyte progenitor cells in individuals with subacute cervical spinal cord injury. J. Neurosurg. Spine 2022, 37, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Pahwa, B.; Agrawal, D.; Singh, P.K.; Gujjar, H.; Mishra, S.; Jagdevan, A.; Misra, M. Efficacy and outcome of bone marrow derived stem cells transplanted via intramedullary route in acute complete spinal cord injury—A randomized placebo controlled trial. J. Clin. Neurosci. 2022, 100, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Albu, S.; Kumru, H.; Coll, R.; Vives, J.; Vallés, M.; Benito-Penalva, J.; Rodríguez, L.; Codinach, M.; Hernández, J.; Navarro, X.; et al. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: A randomized controlled study. Cytotherapy 2021, 23, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Bydon, M.; Qu, W.; Moinuddin, F.M.; Hunt, C.L.; Garlanger, K.L.; Reeves, R.K.; Windebank, A.J.; Zhao, K.D.; Jarrah, R.; Trammell, B.C.; et al. Intrathecal delivery of adipose-derived mesenchymal stem cells in traumatic spinal cord injury: Phase I trial. Nat. Commun. 2024, 15, 2201. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Sasaki, M.; Kataoka-Sasaki, Y.; Nakazaki, M.; Nagahama, H.; Oka, S.; Oshigiri, T.; Takebayashi, T.; Yamashita, T.; Kocsis, J.D.; et al. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury. Neuroscience 2016, 335, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Courtine, G.; Gerasimenko, Y.; van den Brand, R.; Yew, A.; Musienko, P.; Zhong, H.; Song, B.; Ao, Y.; Ichiyama, R.M.; Lavrov, I.; et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 2009, 12, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C.; Anderson, D.K.; Faden, A.I.; Gruner, J.A.; Holford, T.; Hsu, C.; Noble, L.; Nockels, R.; et al. MASCIS evaluation of open field locomotor scores: Effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J. Neurotrauma 1996, 13, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Rivlin, A.S.; Tator, C.H. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J. Neurosurg. 1977, 47, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Lepski, G.; Jannes, C.E.; Wessolleck, J.; Kobayashi, E.; Nikkhah, G. Equivalent neurogenic potential of wild-type and GFP-labeled fetal-derived neural progenitor cells before and after transplantation into the rodent hippocampus. Transplantation 2011, 91, 390–397. [Google Scholar] [PubMed]
- Lepski, G.; Jannes, C.E.; Strauss, B.; Marie, S.K.; Nikkhah, G. Survival and neuronal differentiation of mesenchymal stem cells transplanted into the rodent brain are dependent upon microenvironment. Tissue Eng. Part A 2010, 16, 2769–2782. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Danner, S.M.; Freundl, B.; Binder, H.; Mayr, W.; Rattay, F.; Minassian, K. Periodic modulation of repetitively elicited monosynaptic reflexes of the human lumbosacral spinal cord. J. Neurophysiol. 2015, 114, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Sayenko, D.G.; Angeli, C.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P. Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J. Neurophysiol. 2014, 111, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Prochazka, A.; Gorassini, M. Ensemble firing of muscle afferents recorded during normal locomotion in cats. J. Physiol. 1998, 507 Pt 1, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Prochazka, A.; Gorassini, M. Models of ensemble firing of muscle spindle afferents recorded during normal locomotion in cats. J. Physiol. 1998, 507 Pt 1, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Capogrosso, M.; Wenger, N.; Raspopovic, S.; Musienko, P.; Beauparlant, J.; Bassi Luciani, L.; Courtine, G.; Micera, S. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J. Neurosci. 2013, 33, 19326–19340. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, Y.P.; Lavrov, I.A.; Courtine, G.; Ichiyama, R.M.; Dy, C.J.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J. Neurosci. Methods 2006, 157, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Wenger, N.; Moraud, E.M.; Raspopovic, S.; Bonizzato, M.; DiGiovanna, J.; Musienko, P.; Morari, M.; Micera, S.; Courtine, G. Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Sci. Transl. Med. 2014, 6, 255ra133. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Hinckley, C.A.; Hilde, K.L.; Driscoll, S.P.; Poon, T.H.; Montgomery, J.M.; Pfaff, S.L. Identification of a cellular node for motor control pathways. Nat. Neurosci. 2014, 17, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Clarac, F.; Cattaert, D.; Le Ray, D. Central control components of a “simple” stretch reflex. Trends Neurosci. 2000, 23, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J. Physiol. 1910, 40, 28–121. [Google Scholar] [CrossRef] [PubMed]
- Pierrot-Deseilligny, E. Peripheral and descending control of neurones mediating non-monosynaptic Ia excitation to motoneurones: A presumed propriospinal system in man. Prog. Brain Res. 1989, 80, 305–314. [Google Scholar] [PubMed]
- Borton, D.; Micera, S.; Millán Jdel, R.; Courtine, G. Personalized neuroprosthetics. Sci. Transl. Med. 2013, 5, 210rv2. [Google Scholar] [CrossRef] [PubMed]
- Rattay, F.; Minassian, K.; Dimitrijevic, M.R. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. quantitative analysis by computer modeling. Spinal Cord. 2000, 38, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Edgerton, V.R.; Gerasimenko, Y.P.; Harkema, S.J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 2014, 137 Pt 5, 1394–1409. [Google Scholar] [CrossRef]
- Danner, S.M.; Hofstoetter, U.S.; Freundl, B.; Binder, H.; Mayr, W.; Rattay, F.; Minassian, K. Human spinal locomotor control is based on flexibly organized burst generators. Brain 2015, 138 Pt 3, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, V.R.; Courtine, G.; Gerasimenko, Y.P.; Lavrov, I.; Ichiyama, R.M.; Fong, A.J.; Cai, L.L.; Otoshi, C.K.; Tillakaratne, N.J.; Burdick, J.W.; et al. Training locomotor networks. Brain Res. Rev. 2008, 57, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Talpalar, A.E.; Endo, T.; Löw, P.; Borgius, L.; Hägglund, M.; Dougherty, K.J.; Ryge, J.; Hnasko, T.S.; Kiehn, O. Identification of minimal neuronal networks involved in flexor-extensor alternation in the mammalian spinal cord. Neuron 2011, 71, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, J.; Fedirchuk, B.; Gosgnach, S.; McCrea, D.A. Group I disynaptic excitation of cat hindlimb flexor and bifunctional motoneurones during fictive locomotion. J. Physiol. 2000, 525 Pt 2, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Rybak, I.A.; Stecina, K.; Shevtsova, N.A.; McCrea, D.A. Modelling spinal circuitry involved in locomotor pattern generation: Insights from the effects of afferent stimulation. J. Physiol. 2006, 577 Pt 2, 641–658. [Google Scholar] [CrossRef] [PubMed]
- Bang, W.S.; Han, I.; Mun, S.A.; Hwang, J.M.; Noh, S.H.; Son, W.; Cho, D.-C.; Kim, B.-J.; Kim, C.H.; Choi, H.; et al. Electrical stimulation promotes functional recovery after spinal cord injury by activating endogenous spinal cord-derived neural stem/progenitor cell: An in vitro and in vivo study. Spine J. 2024, 24, 534–553. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Telezhkin, V.; Jiang, W.; Gu, Y.; Wang, Y.; Hong, W.; Tian, W.; Yarova, P.; Zhang, G.; Lee, S.M.-Y.; et al. Electric field stimulation boosts neuronal differentiation of neural stem cells for spinal cord injury treatment via PI3K/Akt/GSK-3β/β-catenin activation. Cell Biosci. 2023, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Gary, D.; Rosenzweig, E.; Grill, W.; McDonald, J. Functional electrical stimulation helps replenish progenitor cells in the injured spinal cord of adult rats. Exp. Neurol. 2010, 222, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Barrera, R.; Rivas-González, M.; García-Sánchez, J.; Mojica-Torres, D.; Ibarra, A. Neurogenesis after spinal cord injury: State of the art. Cells 2021, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
- Havelikova, K.; Smejkalova, B.; Jendelova, P. Neurogenesis as a tool for spinal cord injury. Int. J. Mol. Sci. 2022, 23, 3728. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Barrera, R.; Flores-Romero, A.; García, E.; Fernández-Presas, A.M.; Incontri-Abraham, D.; Navarro-Torres, L.; García-Sánchez, J.; Whaley, J.J.J.; Madrazo, I.; Ibarra, A. Immunization with neural-derived peptides increases neurogenesis in rats with chronic spinal cord injury. CNS Neurosci. Ther. 2020, 26, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Dorrian, R.M.; Berryman, C.F.; Lauto, A.; Leonard, A.V. Electrical stimulation for the treatment of spinal cord injuries: A review of the cellular and molecular mechanisms that drive functional improvements. Front. Cell. Neurosci. 2023, 17, 1095259. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.S.; Ding, Y.; Xu, H.Y.; Zeng, X.; Lai, B.Q.; Li, G.; Ma, Y.H. Electro-acupuncture and its combination with adult stem cell transplantation for spinal cord injury treatment: A summary of current laboratory findings and a review of literature. CNS Neurosci. Ther. 2022, 28, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Yip, P.K.; Malaspina, A. Spinal cord trauma and the molecular point of no return. Mol. Neurodegener. 2012, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Lepski, G.; Jannes, C.E.; Nikkhah, G.; Bischofberger, J. cAMP promotes the differentiation of neural progenitor cells in vitro via modulation of voltage-gated calcium channels. Front. Cell Neurosci. 2013, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Hieber, C.; Jonas, P.; Bischofberger, J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 2004, 429, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Furlanetti, L.L.; Cordeiro, J.G.; Cordeiro, K.K.; Garcia, J.A.; Winkler, C.; Lepski, G.A.; Coenen, V.A.; Nikkhah, G.; Döbrössy, M.D. Continuous High-Frequency Stimulation of the Subthalamic Nucleus Improves Cell Survival and Functional Recovery Following Dopaminergic Cell Transplantation in Rodents. Neurorehabil. Neural Repair. 2015, 29, 1001–1012. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelin, L.G.; Carreño, M.N.P.; Otoch, J.P.; de Resende, J.C.F.; Arévalo, A.; Motta-Teixeira, L.C.; Seelaender, M.C.L.; Lepski, G. Regeneration and Plasticity Induced by Epidural Stimulation in a Rodent Model of Spinal Cord Injury. Int. J. Mol. Sci. 2024, 25, 9043. https://doi.org/10.3390/ijms25169043
Angelin LG, Carreño MNP, Otoch JP, de Resende JCF, Arévalo A, Motta-Teixeira LC, Seelaender MCL, Lepski G. Regeneration and Plasticity Induced by Epidural Stimulation in a Rodent Model of Spinal Cord Injury. International Journal of Molecular Sciences. 2024; 25(16):9043. https://doi.org/10.3390/ijms25169043
Chicago/Turabian StyleAngelin, Leonidas Gomes, Marcelo Nelson Páez Carreño, Jose Pinhata Otoch, Joyce Cristina Ferreira de Resende, Analía Arévalo, Lívia Clemente Motta-Teixeira, Marilia Cerqueira Leite Seelaender, and Guilherme Lepski. 2024. "Regeneration and Plasticity Induced by Epidural Stimulation in a Rodent Model of Spinal Cord Injury" International Journal of Molecular Sciences 25, no. 16: 9043. https://doi.org/10.3390/ijms25169043




