Advances in Molecular Mechanisms of Kidney Disease: Integrating Renal Tumorigenesis of Hereditary Cancer Syndrome
Abstract
1. Introduction
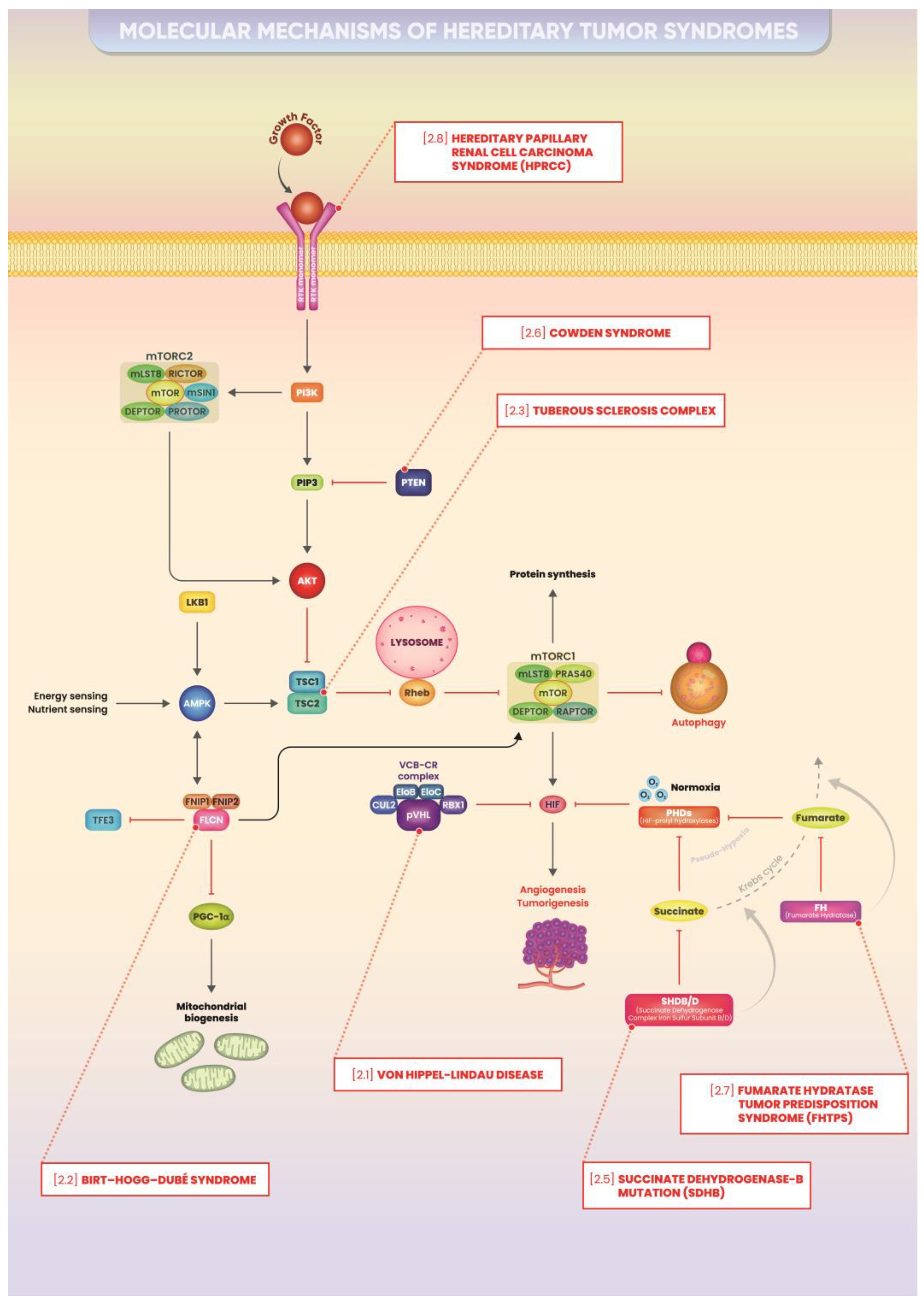
2. Hereditary Cancer Syndromes: Main Molecular Mechanisms
2.1. Von Hippel–Lindau Disease
2.1.1. Molecular Mechanism
2.1.2. Focus on Kidney Cancer
2.2. Birt–Hogg–Dubé Syndrome
2.2.1. Molecular Mechanism
2.2.2. Focus on Kidney Cancer
2.3. Tuberous Sclerosis Complex
2.3.1. Molecular Mechanism
2.3.2. Focus on Kidney Cancer
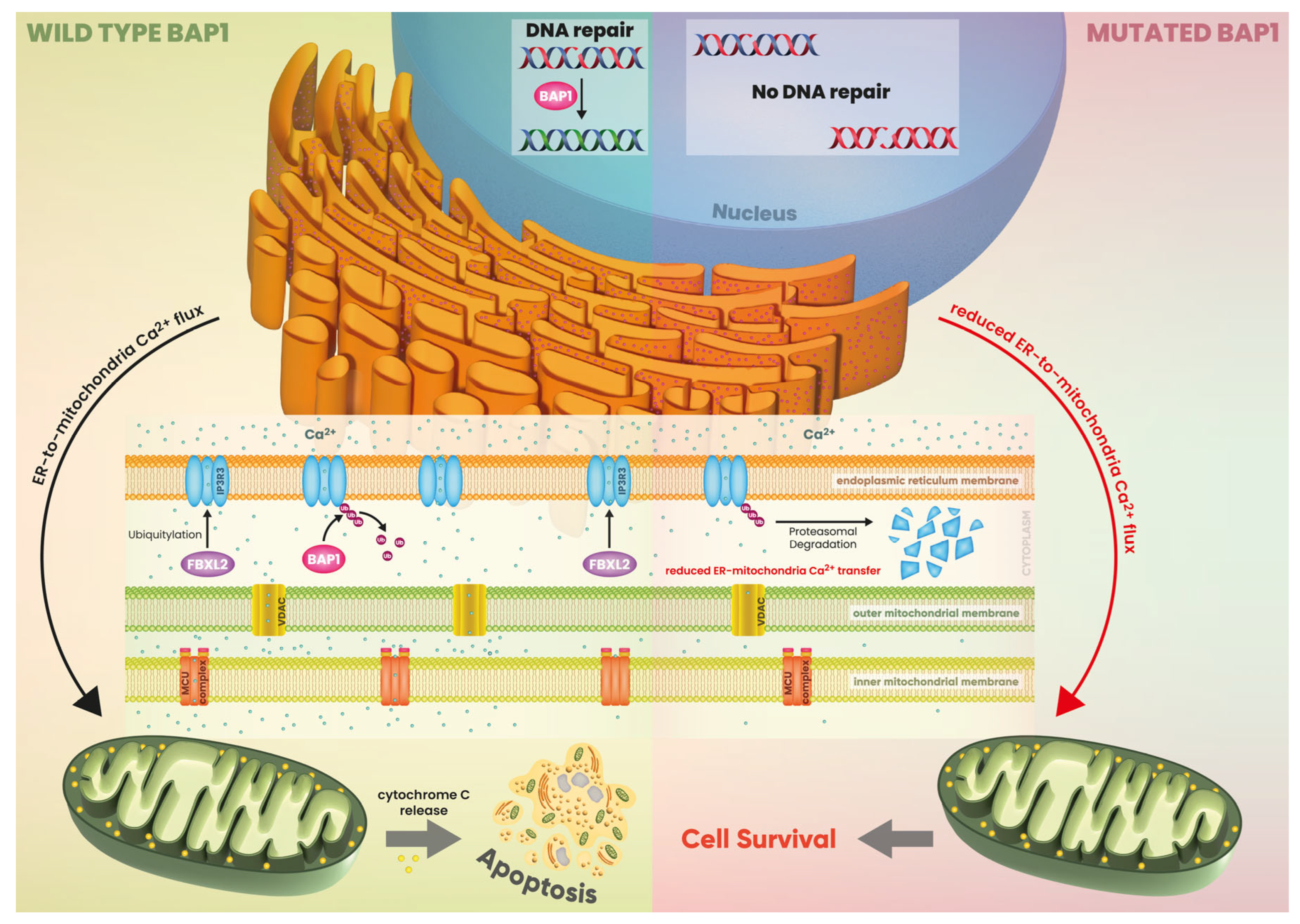
2.4. BRCA-1 Associated Protein-1 (BAP-1)-Associated Renal Cell Carcinoma
2.4.1. Molecular Mechanism
2.4.2. Focus on Kidney Cancer
2.5. Hereditary Paraganglioma/Pheochromocytoma (HPP) Syndrome/Succinate Dehydrogenase-B Mutation (SDHB)
2.5.1. Molecular Mechanism
2.5.2. Focus on Kidney Cancer
2.6. Cowden Syndrome
2.6.1. Molecular Mechanism
2.6.2. Focus on Kidney Cancer
2.7. Fumarate Hydratase Tumor Predisposition Syndrome (FHTPS)
2.7.1. Molecular Mechanism
2.7.2. Focus on Kidney Cancer
2.8. Hereditary Papillary Renal Cell Carcinoma Syndrome (HPRCC)
2.8.1. Molecular Mechanism
2.8.2. Focus on Kidney Cancer
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal Cell Carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef]
- Capitanio, U.; Montorsi, F. Identifying Patients for Adjuvant Therapy after Nephrectomy. Lancet 2022, 400, 1080–1081. [Google Scholar] [CrossRef] [PubMed]
- Alchoueiry, M.; Cornejo, K.; Henske, E.P. Kidney Cancer: Links between Hereditary Syndromes and Sporadic Tumorigenesis. Semin. Diagn. Pathol. 2024, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A.G. Hereditary Cancer: Two Hits Revisited. J. Cancer Res. Clin. Oncol. 1996, 122, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Mighton, C.; Lerner-Ellis, J.P. Principles of Molecular Testing for Hereditary Cancer. Genes. Chromosomes Cancer 2022, 61, 356–381. [Google Scholar] [CrossRef] [PubMed]
- Imyanitov, E.N.; Kuligina, E.S.; Sokolenko, A.P.; Suspitsin, E.N.; Yanus, G.A.; Iyevleva, A.G.; Ivantsov, A.O.; Aleksakhina, S.N. Hereditary Cancer Syndromes. World J. Clin. Oncol. 2023, 14, 40–68. [Google Scholar] [CrossRef]
- Knudson, A.G. Mutation and Cancer: Statistical Study of Retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef]
- Latif, F.; Tory, K.; Gnarra, J.; Yao, M.; Duh, F.M.; Orcutt, M.L.; Stackhouse, T.; Kuzmin, I.; Modi, W.; Geil, L. Identification of the von Hippel-Lindau Disease Tumor Suppressor Gene. Science 1993, 260, 1317–1320. [Google Scholar] [CrossRef]
- Giornale Europeo Di Genetica Umana. Prevalenza, Incidenza Alla Nascita e Penetranza Della Malattia Di von Hippel-Lindau (vHL) in Danimarca. Available online: https://www.nature.com/articles/ejhg2016173 (accessed on 24 March 2024).
- Maher, E.R.; Iselius, L.; Yates, J.R.; Littler, M.; Benjamin, C.; Harris, R.; Sampson, J.; Williams, A.; Ferguson-Smith, M.A.; Morton, N. Von Hippel-Lindau Disease: A Genetic Study. J. Med. Genet. 1991, 28, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Maddock, I.R.; Moran, A.; Maher, E.R.; Teare, M.D.; Norman, A.; Payne, S.J.; Whitehouse, R.; Dodd, C.; Lavin, M.; Hartley, N.; et al. A Genetic Register for von Hippel-Lindau Disease. J. Med. Genet. 1996, 33, 120–127. [Google Scholar] [CrossRef]
- Chittiboina, P.; Lonser, R.R. Von Hippel–Lindau Disease. Handb. Clin. Neurol. 2015, 132, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Maher, E.R.; Neumann, H.P.; Richard, S. Von Hippel-Lindau Disease: A Clinical and Scientific Review. Eur. J. Hum. Genet. 2011, 19, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A.G. Genetics of Human Cancer. Annu. Rev. Genet. 1986, 20, 231–251. [Google Scholar] [CrossRef] [PubMed]
- Stolle, C.; Glenn, G.; Zbar, B.; Humphrey, J.S.; Choyke, P.; Walther, M.; Pack, S.; Hurley, K.; Andrey, C.; Klausner, R.; et al. Improved Detection of Germline Mutations in the von Hippel-Lindau Disease Tumor Suppressor Gene. Hum. Mutat. 1998, 12, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Prowse, A.H.; Webster, A.R.; Richards, F.M.; Richard, S.; Olschwang, S.; Resche, F.; Affara, N.A.; Maher, E.R. Somatic Inactivation of the VHL Gene in Von Hippel-Lindau Disease Tumors. Am. J. Hum. Genet. 1997, 60, 765–771. [Google Scholar] [PubMed]
- Wait, S.D.; Vortmeyer, A.O.; Lonser, R.R.; Chang, D.T.; Finn, M.A.; Bhowmick, D.A.; Pack, S.D.; Oldfield, E.H.; Zhuang, Z. Somatic Mutations in VHL Germline Deletion Kindred Correlate with Mild Phenotype. Ann. Neurol. 2004, 55, 236–240. [Google Scholar] [CrossRef]
- Friedrich, C.A. Von Hippel-Lindau Syndrome. A Pleomorphic Condition. Cancer 1999, 86, 2478–2482. [Google Scholar]
- Iliopoulos, O.; Ohh, M.; Kaelin, W.G. pVHL19 Is a Biologically Active Product of the von Hippel-Lindau Gene Arising from Internal Translation Initiation. Proc. Natl. Acad. Sci. USA 1998, 95, 11661–11666. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Neumann, M.; Stearman, R.; Stauber, R.; Pause, A.; Pavlakis, G.N.; Klausner, R.D. Transcription-Dependent Nuclear-Cytoplasmic Trafficking Is Required for the Function of the von Hippel-Lindau Tumor Suppressor Protein. Mol. Cell Biol. 1999, 19, 1486–1497. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chen, D.Y.; Humphrey, J.S.; Gnarra, J.R.; Linehan, W.M.; Klausner, R.D. Nuclear/Cytoplasmic Localization of the von Hippel-Lindau Tumor Suppressor Gene Product Is Determined by Cell Density. Proc. Natl. Acad. Sci. USA 1996, 93, 1770–1775. [Google Scholar]
- Li, M.; Kim, W.Y. Two Sides to Every Story: The HIF-Dependent and HIF-Independent Functions of pVHL. J. Cell Mol. Med. 2011, 15, 187–195. [Google Scholar] [CrossRef]
- Wang, V.; Davis, D.A.; Haque, M.; Huang, L.E.; Yarchoan, R. Differential Gene Up-Regulation by Hypoxia-Inducible Factor-1alpha and Hypoxia-Inducible Factor-2alpha in HEK293T Cells. Cancer Res. 2005, 65, 3299–3306. [Google Scholar] [CrossRef]
- Mandriota, S.J.; Turner, K.J.; Davies, D.R.; Murray, P.G.; Morgan, N.V.; Sowter, H.M.; Wykoff, C.C.; Maher, E.R.; Harris, A.L.; Ratcliffe, P.J.; et al. HIF Activation Identifies Early Lesions in VHL Kidneys: Evidence for Site-Specific Tumor Suppressor Function in the Nephron. Cancer Cell 2002, 1, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G. Molecular Basis of the VHL Hereditary Cancer Syndrome. Nat. Rev. Cancer 2002, 2, 673–682. [Google Scholar] [CrossRef]
- Crossey, P.A.; Eng, C.; Ginalska-Malinowska, M.; Lennard, T.W.; Wheeler, D.C.; Ponder, B.A.; Maher, E.R. Molecular Genetic Diagnosis of von Hippel-Lindau Disease in Familial Phaeochromocytoma. J. Med. Genet. 1995, 32, 885–886. [Google Scholar] [CrossRef][Green Version]
- PubMed. Coinvolgimento Renale Nella Malattia Di von Hippel-Lindau. Available online: https://pubmed.ncbi.nlm.nih.gov/8872970/ (accessed on 7 May 2024).
- Ball, M.W.; An, J.Y.; Gomella, P.T.; Gautam, R.; Ricketts, C.J.; Vocke, C.D.; Schmidt, L.S.; Merino, M.J.; Srinivasan, R.; Malayeri, A.A.; et al. Growth Rates of Genetically Defined Renal Tumors: Implications for Active Surveillance and Intervention. J. Clin. Oncol. 2020, 38, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.M.; Rhodes, L.; Blanco, I.; Chung, W.K.; Eng, C.; Maher, E.R.; Richard, S.; Giles, R.H. Von Hippel-Lindau Disease: Genetics and Role of Genetic Counseling in a Multiple Neoplasia Syndrome. J. Clin. Oncol. 2016, 34, 2172–2181. [Google Scholar] [CrossRef]
- Steinbach, F.; Novick, A.C.; Zincke, H.; Miller, D.P.; Williams, R.D.; Lund, G.; Skinner, D.G.; Esrig, D.; Richie, J.P.; deKernion, J.B. Treatment of Renal Cell Carcinoma in von Hippel-Lindau Disease: A Multicenter Study. J. Urol. 1995, 153, 1812–1816. [Google Scholar] [PubMed]
- Duffey, B.G.; Choyke, P.L.; Glenn, G.; Grubb, R.L.; Venzon, D.; Linehan, W.M.; Walther, M.M. The Relationship between Renal Tumor Size and Metastases in Patients with von Hippel-Lindau Disease. J. Urol. 2004, 172, 63–65. [Google Scholar] [CrossRef]
- Iacovelli, R.; Arduini, D.; Ciccarese, C.; Pierconti, F.; Strusi, A.; Piro, G.; Carbone, C.; Foschi, N.; Daniele, G.; Tortora, G. Targeting Hypoxia-Inducible Factor Pathways in Sporadic and Von Hippel-Lindau Syndrome-Related Kidney Cancers. Crit. Rev. Oncol. Hematol. 2022, 176, 103750. [Google Scholar] [CrossRef]
- Cho, H.; Du, X.; Rizzi, J.P.; Liberzon, E.; Chakraborty, A.A.; Gao, W.; Carvo, I.; Signoretti, S.; Bruick, R.K.; Josey, J.A.; et al. On-Target Efficacy of a HIF-2α Antagonist in Preclinical Kidney Cancer Models. Nature 2016, 539, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.-E.; Daccord, C.; Taffé, P.; Lazor, R. Prevalence of Birt-Hogg-Dubé Syndrome Determined through Epidemiological Data on Spontaneous Pneumothorax and Bayes Theorem. Front. Med. (Lausanne) 2021, 8, 631168. [Google Scholar] [CrossRef]
- Yang, Y.; Padilla-Nash, H.M.; Vira, M.A.; Abu-Asab, M.S.; Val, D.; Worrell, R.; Tsokos, M.; Merino, M.J.; Pavlovich, C.P.; Ried, T.; et al. The UOK 257 Cell Line—A Novel Model for Studies of the Human Birt-Hogg-Dubé Gene Pathway. Cancer Genet. Cytogenet. 2008, 180, 100–109. [Google Scholar] [CrossRef]
- Possik, E.; Jalali, Z.; Nouët, Y.; Yan, M.; Gingras, M.-C.; Schmeisser, K.; Panaite, L.; Dupuy, F.; Kharitidi, D.; Chotard, L.; et al. Folliculin Regulates Ampk-Dependent Autophagy and Metabolic Stress Survival. PLoS Genet. 2014, 10, e1004273. [Google Scholar] [CrossRef]
- Hasumi, Y.; Baba, M.; Ajima, R.; Hasumi, H.; Valera, V.A.; Klein, M.E.; Haines, D.C.; Merino, M.J.; Hong, S.-B.; Yamaguchi, T.P.; et al. Homozygous Loss of BHD Causes Early Embryonic Lethality and Kidney Tumor Development with Activation of mTORC1 and mTORC2. Proc. Natl. Acad. Sci. USA 2009, 106, 18722–18727. [Google Scholar] [CrossRef]
- Tsun, Z.-Y.; Bar-Peled, L.; Chantranupong, L.; Zoncu, R.; Wang, T.; Kim, C.; Spooner, E.; Sabatini, D.M. The Folliculin Tumor Suppressor Is a GAP for the RagC/D GTPases That Signal Amino Acid Levels to mTORC1. Mol. Cell 2013, 52, 495–505. [Google Scholar] [CrossRef] [PubMed]
- PubMed. Ruolo Protettivo dell’autofagia Contro la Citolisina di Vibrio Cholerae, una Tossina Formante Pori Di V. Cholerae. Available online: https://pubmed.ncbi.nlm.nih.gov/17267617/ (accessed on 3 August 2024).
- Shintani, T.; Klionsky, D.J. Cargo Proteins Facilitate the Formation of Transport Vesicles in the Cytoplasm to Vacuole Targeting Pathway. J. Biol. Chem. 2004, 279, 29889–29894. [Google Scholar] [CrossRef]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Vasquez, D.A.; Joshi, A.; Gwinn, D.M.; Taylor, R.; Asara, J.M.; et al. Phosphorylation of ULK1 (hATG1) by AMP-Activated Protein Kinase Connects Energy Sensing to Mitophagy. Science 2011, 331, 456–461. [Google Scholar]
- Djeddi, A.; Michelet, X.; Culetto, E.; Alberti, A.; Barois, N.; Legouis, R. Induction of Autophagy in ESCRT Mutants Is an Adaptive Response for Cell Survival in C. Elegans. J. Cell Sci. 2012, 125, 685–694. [Google Scholar] [CrossRef]
- Samokhvalov, V.; Scott, B.A.; Crowder, C.M. Autophagy Protects from Hypoxic Injury in C. Elegans. Autophagy 2008, 4, 1034–1041. [Google Scholar] [PubMed]
- Betschinger, J.; Nichols, J.; Dietmann, S.; Corrin, P.D.; Paddison, P.J.; Smith, A. Exit from Pluripotency Is Gated by Intracellular Redistribution of the bHLH Transcription Factor Tfe3. Cell 2013, 153, 335–347. [Google Scholar] [CrossRef]
- Mathieu, J.; Detraux, D.; Kuppers, D.; Wang, Y.; Cavanaugh, C.; Sidhu, S.; Levy, S.; Robitaille, A.M.; Ferreccio, A.; Bottorff, T.; et al. Folliculin Regulates mTORC1/2 and WNT Pathways in Early Human Pluripotency. Nat. Commun. 2019, 10, 632. [Google Scholar] [CrossRef]
- Di Malta, C.; Siciliano, D.; Calcagni, A.; Monfregola, J.; Punzi, S.; Pastore, N.; Eastes, A.N.; Davis, O.; De Cegli, R.; Zampelli, A.; et al. Transcriptional Activation of RagD GTPase Controls mTORC1 and Promotes Cancer Growth. Science 2017, 356, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, E.A.; Seifan, S.; Claessens, T.; Behrends, C.; Kamps, M.A.; Rozycka, E.; Kemp, A.J.; Nookala, R.K.; Blenis, J.; Coull, B.J.; et al. FLCN, a Novel Autophagy Component, Interacts with GABARAP and Is Regulated by ULK1 Phosphorylation. Autophagy 2014, 10, 1749–1760. [Google Scholar] [CrossRef]
- PMC. I Tumori Renali di Birt-Hogg-Dubé Sono Geneticamente Distinti dalle Altre Neoplasie Renali e Sono Associati ad una Sovraregolazione dell’Espressione Genica Mitocondriale. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3012009/ (accessed on 21 May 2024).
- Luijten, M.N.H.; Basten, S.G.; Claessens, T.; Vernooij, M.; Scott, C.L.; Janssen, R.; Easton, J.A.; Kamps, M.A.F.; Vreeburg, M.; Broers, J.L.V.; et al. Birt-Hogg-Dube Syndrome Is a Novel Ciliopathy. Hum. Mol. Genet. 2013, 22, 4383–4397. [Google Scholar] [CrossRef]
- Glykofridis, I.E.; Knol, J.C.; Balk, J.A.; Westland, D.; Pham, T.V.; Piersma, S.R.; Lougheed, S.M.; Derakhshan, S.; Veen, P.; Rooimans, M.A.; et al. Loss of FLCN-FNIP1/2 Induces a Non-Canonical Interferon Response in Human Renal Tubular Epithelial Cells. eLife 2021, 10, e61630. [Google Scholar] [CrossRef] [PubMed]
- El-Houjeiri, L.; Possik, E.; Vijayaraghavan, T.; Paquette, M.; Martina, J.A.; Kazan, J.M.; Ma, E.H.; Jones, R.; Blanchette, P.; Puertollano, R.; et al. The Transcription Factors TFEB and TFE3 Link the FLCN-AMPK Signaling Axis to Innate Immune Response and Pathogen Resistance. Cell Rep. 2019, 26, 3613–3628.e6. [Google Scholar] [CrossRef] [PubMed]
- Endoh, M.; Baba, M.; Endoh, T.; Hirayama, A.; Nakamura-Ishizu, A.; Umemoto, T.; Hashimoto, M.; Nagashima, K.; Soga, T.; Lang, M.; et al. A FLCN-TFE3 Feedback Loop Prevents Excessive Glycogenesis and Phagocyte Activation by Regulating Lysosome Activity. Cell Rep. 2020, 30, 1823–1834.e5. [Google Scholar] [CrossRef]
- Napolitano, G.; Di Malta, C.; Esposito, A.; de Araujo, M.E.G.; Pece, S.; Bertalot, G.; Matarese, M.; Benedetti, V.; Zampelli, A.; Stasyk, T.; et al. A Substrate-Specific mTORC1 Pathway Underlies Birt–Hogg–Dubé Syndrome. Nature 2020, 585, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Taya, M.; Hammes, S.R. Glycoprotein Non-Metastatic Melanoma Protein B (GPNMB) and Cancer: A Novel Potential Therapeutic Target. Steroids 2018, 133, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.A.N.; Biondini, M.; Curiel, R.; Siegel, P.M. Targeting GPNMB with Glembatumumab Vedotin: Current Developments and Future Opportunities for the Treatment of Cancer. Pharmacol. Ther. 2017, 179, 127–141. [Google Scholar] [CrossRef]
- Näf, E.; Laubscher, D.; Hopfer, H.; Streit, M.; Matyas, G. Birt–Hogg–Dubé Syndrome: Novel FLCN Frameshift Deletion in Daughter and Father with Renal Cell Carcinomas. Fam. Cancer 2016, 15, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Pavlovich, C.P.; Grubb, R.L.; Hurley, K.; Glenn, G.M.; Toro, J.; Schmidt, L.S.; Torres-Cabala, C.; Merino, M.J.; Zbar, B.; Choyke, P.; et al. Evaluation and Management of Renal Tumors in the Birt-Hogg-Dubé Syndrome. J. Urol. 2005, 173, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Data from: Renal Imaging in 199 Dutch Patients with Birt-Hogg-Dubé Syndrome: Screening Compliance and Outcome. Available online: https://researchinformation.amsterdamumc.org/en/datasets/data-from-renal-imaging-in-199-dutch-patients-with-birt-hogg-dub%C3%A9 (accessed on 22 May 2024).
- Vocke, C.D.; Yang, Y.; Pavlovich, C.P.; Schmidt, L.S.; Nickerson, M.L.; Torres-Cabala, C.A.; Merino, M.J.; Walther, M.M.; Zbar, B.; Linehan, W.M. High Frequency of Somatic Frameshift BHD Gene Mutations in Birt-Hogg-Dubé-Associated Renal Tumors. J. Natl. Cancer Inst. 2005, 97, 931–935. [Google Scholar] [CrossRef]
- Castellucci, R.; Marchioni, M.; Valenti, S.; Sortino, G.; Borgonovo, G.; Pesenti, N.; Vismara, A.C.A.; Circo, M.C.; Sessa, B.; Micheli, E.; et al. Multiple Chromophobe and Clear Cell Renal Cancer in a Patient Affected by Birt-Hogg-Dubè Syndrome: A Case Report. Urologia 2017, 84, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Linehan, W.M. Evaluation and Screening for Hereditary Renal Cell Cancers. Can. Urol. Assoc. J. 2013, 7, 324–325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ball, M.W.; Srinivasan, R. Kidney Cancer in 2017: Challenging and Refining Treatment Paradigms. Nat. Rev. Urol. 2018, 15, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Carlo, M.I.; Hakimi, A.A.; Stewart, G.D.; Bratslavsky, G.; Brugarolas, J.; Chen, Y.-B.; Linehan, W.M.; Maher, E.R.; Merino, M.J.; Offit, K.; et al. Familial Kidney Cancer: Implications of New Syndromes and Molecular Insights. Eur. Urol. 2019, 76, 754–764. [Google Scholar] [CrossRef]
- Rosenkrantz, A.B.; Hindman, N.; Fitzgerald, E.F.; Niver, B.E.; Melamed, J.; Babb, J.S. MRI Features of Renal Oncocytoma and Chromophobe Renal Cell Carcinoma. AJR Am. J. Roentgenol. 2010, 195, W421–W427. [Google Scholar] [CrossRef] [PubMed]
- Bratslavsky, G.; Mendhiratta, N.; Daneshvar, M.; Brugarolas, J.; Ball, M.W.; Metwalli, A.; Nathanson, K.L.; Pierorazio, P.M.; Boris, R.S.; Singer, E.A.; et al. Genetic Risk Assessment for Hereditary Renal Cell Carcinoma: Clinical Consensus Statement. Cancer 2021, 127, 3957–3966. [Google Scholar] [CrossRef]
- Gomella, P.T.; Linehan, W.M.; Ball, M.W. Precision Surgery and Kidney Cancer: Knowledge of Genetic Alterations Influences Surgical Management. Genes 2021, 12, 261. [Google Scholar] [CrossRef]
- Stamatakis, L.; Metwalli, A.R.; Middelton, L.A.; Marston Linehan, W. Diagnosis and Management of BHD-Associated Kidney Cancer. Fam. Cancer 2013, 12, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Yao, M.; Sano, F.; Sakata, R.; Tatenuma, T.; Makiyama, K.; Nakaigawa, N.; Kubota, Y. A case of metastatic renal cell carcinoma associated with Birt-Hogg-Dubé syndrome treated with molecular-targeting agents. Hinyokika Kiyo 2013, 59, 503–506. [Google Scholar]
- Hinton, R.B.; Prakash, A.; Romp, R.L.; Krueger, D.A.; Knilans, T.K.; International Tuberous Sclerosis Consensus Group. Cardiovascular Manifestations of Tuberous Sclerosis Complex and Summary of the Revised Diagnostic Criteria and Surveillance and Management Recommendations from the International Tuberous Sclerosis Consensus Group. J. Am. Heart Assoc. 2014, 3, e001493. [Google Scholar] [CrossRef] [PubMed]
- Northrup, H.; Krueger, D.A.; International Tuberous Sclerosis Complex Consensus Group. Tuberous Sclerosis Complex Diagnostic Criteria Update: Recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr. Neurol. 2013, 49, 243–254. [Google Scholar] [CrossRef]
- DiMario, F.J.; Sahin, M.; Ebrahimi-Fakhari, D. Tuberous Sclerosis Complex. Pediatr. Clin. N. Am. 2015, 62, 633–648. [Google Scholar] [CrossRef]
- Sadowski, K.; Kotulska, K.; Schwartz, R.A.; Jóźwiak, S. Systemic Effects of Treatment with mTOR Inhibitors in Tuberous Sclerosis Complex: A Comprehensive Review. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 586–594. [Google Scholar] [CrossRef]
- MacKeigan, J.P.; Krueger, D.A. Differentiating the mTOR Inhibitors Everolimus and Sirolimus in the Treatment of Tuberous Sclerosis Complex. Neuro Oncol. 2015, 17, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.H.; Ng, S.M.; Parker, A. Annual Review of Children with Tuberous Sclerosis. Arch. Dis. Child.-Educ. Pract. 2015, 100, 114–121. [Google Scholar] [CrossRef]
- Hoogeveen-Westerveld, M.; Ekong, R.; Povey, S.; Mayer, K.; Lannoy, N.; Elmslie, F.; Bebin, M.; Dies, K.; Thompson, C.; Sparagana, S.P.; et al. Functional Assessment of TSC2 Variants Identified in Individuals with Tuberous Sclerosis Complex. Hum. Mutat. 2013, 34, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Zhu, T.; Guan, K.-L. TSC2 Mediates Cellular Energy Response to Control Cell Growth and Survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- PMC. Renal Manifestations of Tuberous Sclerosis Complex. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7478169/ (accessed on 22 May 2024).
- Bissler, J.J.; Kingswood, J.C. Renal Angiomyolipomata. Kidney Int. 2004, 66, 924–934. [Google Scholar] [CrossRef]
- Brook-Carter, P.T.; Peral, B.; Ward, C.J.; Thompson, P.; Hughes, J.; Maheshwar, M.M.; Nellist, M.; Gamble, V.; Harris, P.C.; Sampson, J.R. Deletion of the TSC2 and PKD1 Genes Associated with Severe Infantile Polycystic Kidney Disease—A Contiguous Gene Syndrome. Nat. Genet. 1994, 8, 328–332. [Google Scholar] [CrossRef]
- Armour, E.A.; Carson, R.P.; Ess, K.C. Cystogenesis and Elongated Primary Cilia in Tsc1-Deficient Distal Convoluted Tubules. Am. J. Physiol. Ren. Physiol. 2012, 303, F584–F592. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, H.; Jia, C.; Song, Q.; Chen, J.; Zhang, Y.; Lai, P.; Fan, X.; Zhou, X.; Liu, M.; et al. Activation of mTORC1 in Collecting Ducts Causes Hyperkalemia. J. Am. Soc. Nephrol. 2014, 25, 534–545. [Google Scholar] [CrossRef]
- Wilson, C.; Bonnet, C.; Guy, C.; Idziaszczyk, S.; Colley, J.; Humphreys, V.; Maynard, J.; Sampson, J.R.; Cheadle, J.P. Tsc1 Haploinsufficiency without Mammalian Target of Rapamycin Activation Is Sufficient for Renal Cyst Formation in Tsc1+/− Mice. Cancer Res. 2006, 66, 7934–7938. [Google Scholar] [CrossRef]
- Bonsib, S.M.; Boils, C.; Gokden, N.; Grignon, D.; Gu, X.; Higgins, J.P.T.; Leroy, X.; McKenney, J.K.; Nasr, S.H.; Phillips, C.; et al. Tuberous Sclerosis Complex: Hamartin and Tuberin Expression in Renal Cysts and Its Discordant Expression in Renal Neoplasms. Pathol. Res. Pract. 2016, 212, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Zomer, A.; Maynard, C.; Verweij, F.J.; Kamermans, A.; Schäfer, R.; Beerling, E.; Schiffelers, R.M.; de Wit, E.; Berenguer, J.; Ellenbroek, S.I.J.; et al. In Vivo Imaging Reveals Extracellular Vesicle-Mediated Phenocopying of Metastatic Behavior. Cell 2015, 161, 1046–1057. [Google Scholar] [CrossRef]
- Patel, B.; Patel, J.; Cho, J.-H.; Manne, S.; Bonala, S.; Henske, E.; Roegiers, F.; Markiewski, M.; Karbowniczek, M. Exosomes Mediate the Acquisition of the Disease Phenotypes by Cells with Normal Genome in Tuberous Sclerosis Complex. Oncogene 2016, 35, 3027–3036. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, E. Rag GTPase in Amino Acid Signaling. Amino Acids 2016, 48, 915–928. [Google Scholar] [CrossRef]
- Zoncu, R.; Bar-Peled, L.; Efeyan, A.; Wang, S.; Sancak, Y.; Sabatini, D.M. mTORC1 Senses Lysosomal Amino Acids through an Inside-out Mechanism That Requires the Vacuolar H+-ATPase. Science 2011, 334, 678–683. [Google Scholar] [CrossRef]
- Kim, H.; Xu, H.; Yao, Q.; Li, W.; Huang, Q.; Outeda, P.; Cebotaru, V.; Chiaravalli, M.; Boletta, A.; Piontek, K.; et al. Ciliary Membrane Proteins Traffic through the Golgi via a Rabep1/GGA1/Arl3-Dependent Mechanism. Nat. Commun. 2014, 5, 5482. [Google Scholar] [CrossRef]
- Pema, M.; Drusian, L.; Chiaravalli, M.; Castelli, M.; Yao, Q.; Ricciardi, S.; Somlo, S.; Qian, F.; Biffo, S.; Boletta, A. mTORC1-Mediated Inhibition of Polycystin-1 Expression Drives Renal Cyst Formation in Tuberous Sclerosis Complex. Nat. Commun. 2016, 7, 10786. [Google Scholar] [CrossRef]
- Anglickis, M.; Anglickienė, G.; Andreikaitė, G.; Skrebūnas, A. Microwave Thermal Ablation versus Open Partial Nephrectomy for the Treatment of Small Renal Tumors in Patients Over 70 Years Old. Medicina 2019, 55, 664. [Google Scholar] [CrossRef]
- Gupta, S.; Jimenez, R.E.; Herrera-Hernandez, L.; Lohse, C.M.; Thompson, R.H.; Boorjian, S.A.; Leibovich, B.C.; Cheville, J.C. Renal Neoplasia in Tuberous Sclerosis: A Study of 41 Patients. Mayo Clin. Proc. 2021, 96, 1470–1489. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Turkbey, B.; Choyke, P. Hereditary Renal Tumor Syndromes: Update on Diagnosis and Management. Semin. Ultrasound CT MRI 2017, 38, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Alva, A.; Baine, M.; Beckermann, K.; Carlo, M.I.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; et al. Kidney Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 71–90. [Google Scholar] [CrossRef]
- Hatano, T.; Matsu-Ura, T.; Mori, K.-I.; Inaba, H.; Endo, K.; Tamari, M.; Egawa, S. Effect of Everolimus Treatment for Regrown Renal Angiomyolipoma Associated with Tuberous Sclerosis Complex after Transcatheter Arterial Embolization. Int. J. Clin. Oncol. 2018, 23, 1134–1139. [Google Scholar] [CrossRef]
- Pirson, Y. Tuberous Sclerosis Complex-Associated Kidney Angiomyolipoma: From Contemplation to Action. Nephrol. Dial. Transpl. 2013, 28, 1680–1685. [Google Scholar] [CrossRef]
- Bissler, J.; Cappell, K.; Charles, H.; Song, X.; Liu, Z.; Prestifilippo, J.; Hulbert, J. Rates of Interventional Procedures in Patients with Tuberous Sclerosis Complex-Related Renal Angiomyolipoma. Curr. Med. Res. Opin. 2015, 31, 1501–1507. [Google Scholar] [CrossRef]
- Testa, J.R.; Cheung, M.; Pei, J.; Below, J.E.; Tan, Y.; Sementino, E.; Cox, N.J.; Dogan, A.U.; Pass, H.I.; Trusa, S.; et al. Germline BAP1 Mutations Predispose to Malignant Mesothelioma. Nat. Genet. 2011, 43, 1022–1025. [Google Scholar] [CrossRef]
- PubMed. BAP1 Regola Il Flusso di Ca2+ Mediato da IP3R3 Verso i Mitocondri Sopprimendo la Trasformazione Cellulare. Available online: https://pubmed.ncbi.nlm.nih.gov/28614305/ (accessed on 23 May 2024).
- Peña-Llopis, S.; Vega-Rubín-de-Celis, S.; Liao, A.; Leng, N.; Pavía-Jiménez, A.; Wang, S.; Yamasaki, T.; Zhrebker, L.; Sivanand, S.; Spence, P.; et al. BAP1 Loss Defines a New Class of Renal Cell Carcinoma. Nat. Genet. 2012, 44, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.; Pilarski, R.; Cebulla, C.M.; Abdel-Rahman, M.H. Comprehensive Review of BAP1 Tumor Predisposition Syndrome with Report of Two New Cases. Clin. Genet. 2016, 89, 285–294. [Google Scholar] [CrossRef]
- Slane, B.G.; Aykin-Burns, N.; Smith, B.J.; Kalen, A.L.; Goswami, P.C.; Domann, F.E.; Spitz, D.R. Mutation of Succinate Dehydrogenase Subunit C Results in Increased O2.−, Oxidative Stress, and Genomic Instability. Cancer Res. 2006, 66, 7615–7620. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, C.J.; Shuch, B.; Vocke, C.D.; Metwalli, A.R.; Bratslavsky, G.; Middelton, L.; Yang, Y.; Wei, M.-H.; Pautler, S.E.; Peterson, J.; et al. Succinate Dehydrogenase Kidney Cancer: An Aggressive Example of the Warburg Effect in Cancer. J. Urol. 2012, 188, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.J.; Hes, O.; Papathomas, T.; Šedivcová, M.; Tan, P.H.; Agaimy, A.; Andresen, P.A.; Kedziora, A.; Clarkson, A.; Toon, C.W.; et al. Succinate Dehydrogenase (SDH)-Deficient Renal Carcinoma: A Morphologically Distinct Entity: A Clinicopathologic Series of 36 Tumors from 27 Patients. Am. J. Surg. Pathol. 2014, 38, 1588–1602. [Google Scholar] [CrossRef]
- Benn, D.E.; Robinson, B.G.; Clifton-Bligh, R.J. 15 YEARS OF PARAGANGLIOMA: Clinical Manifestations of Paraganglioma Syndromes Types 1–5. Endocr. Relat. Cancer 2015, 22, T91–T103. [Google Scholar] [CrossRef]
- Benn, D.E.; Gimenez-Roqueplo, A.-P.; Reilly, J.R.; Bertherat, J.; Burgess, J.; Byth, K.; Croxson, M.; Dahia, P.L.M.; Elston, M.; Gimm, O.; et al. Clinical Presentation and Penetrance of Pheochromocytoma/Paraganglioma Syndromes. J. Clin. Endocrinol. Metab. 2006, 91, 827–836. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-Activated Protein Kinase: A Cellular Energy Sensor with a Key Role in Metabolic Disorders and in Cancer. Biochem. Soc. Trans. 2011, 39, 1–13. [Google Scholar] [CrossRef]
- Alao, J.P.; Stavropoulou, A.V.; Lam, E.W.-F.; Coombes, R.C. Role of Glycogen Synthase Kinase 3 Beta (GSK3β) in Mediating the Cytotoxic Effects of the Histone Deacetylase Inhibitor Trichostatin A (TSA) in MCF-7 Breast Cancer Cells. Mol. Cancer 2006, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A Nutrient and Energy Sensor That Maintains Energy Homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Ricketts, C.J.; Forman, J.R.; Rattenberry, E.; Bradshaw, N.; Lalloo, F.; Izatt, L.; Cole, T.R.; Armstrong, R.; Kumar, V.K.A.; Morrison, P.J.; et al. Tumor Risks and Genotype-Phenotype-Proteotype Analysis in 358 Patients with Germline Mutations in SDHB and SDHD. Hum. Mutat. 2010, 31, 41–51. [Google Scholar] [CrossRef]
- Tomitsuka, E.; Hirawake, H.; Goto, Y.; Taniwaki, M.; Harada, S.; Kita, K. Direct Evidence for Two Distinct Forms of the Flavoprotein Subunit of Human Mitochondrial Complex II (Succinate-Ubiquinone Reductase). J. Biochem. 2003, 134, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Bayley, J.-P.; Devilee, P.; Taschner, P.E.M. The SDH Mutation Database: An Online Resource for Succinate Dehydrogenase Sequence Variants Involved in Pheochromocytoma, Paraganglioma and Mitochondrial Complex II Deficiency. BMC Med. Genet. 2005, 6, 39. [Google Scholar] [CrossRef]
- Sun, F.; Huo, X.; Zhai, Y.; Wang, A.; Xu, J.; Su, D.; Bartlam, M.; Rao, Z. Crystal Structure of Mitochondrial Respiratory Membrane Protein Complex II. Cell 2005, 121, 1043–1057. [Google Scholar] [CrossRef]
- Timmers, H.J.L.M.; Kozupa, A.; Eisenhofer, G.; Raygada, M.; Adams, K.T.; Solis, D.; Lenders, J.W.M.; Pacak, K. Clinical Presentations, Biochemical Phenotypes, and Genotype-Phenotype Correlations in Patients with Succinate Dehydrogenase Subunit B-Associated Pheochromocytomas and Paragangliomas. J. Clin. Endocrinol. Metab. 2007, 92, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, N.; Yorita, K.; Nagasaki, M.; Harada, Y.; Ohe, C.; Jeruc, J.; Raspollini, M.R.; Michal, M.; Hes, O.; Amin, M.B. Review of Succinate Dehydrogenase-Deficient Renal Cell Carcinoma with Focus on Clinical and Pathobiological Aspects. Pol. J. Pathol. 2016, 67, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.R.; Eble, J.N.; Amin, M.B.; Gupta, N.S.; Smith, S.C.; Sholl, L.M.; Montironi, R.; Hirsch, M.S.; Hornick, J.L. Succinate Dehydrogenase-Deficient Renal Cell Carcinoma: Detailed Characterization of 11 Tumors Defining a Unique Subtype of Renal Cell Carcinoma. Mod. Pathol. 2015, 28, 80–94. [Google Scholar] [CrossRef]
- Tufton, N.; Shapiro, L.; Sahdev, A.; Kumar, A.V.; Martin, L.; Drake, W.M.; Akker, S.A.; Storr, H.L. An Analysis of Surveillance Screening for SDHB-Related Disease in Childhood and Adolescence. Endocr. Connect. 2019, 8, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Pilarski, R. Cowden Syndrome: A Critical Review of the Clinical Literature. J. Genet. Couns. 2009, 18, 13–27. [Google Scholar] [CrossRef]
- Lloyd, K.M.; Dennis, M. Cowden’s Disease. A Possible New Symptom Complex with Multiple System Involvement. Ann. Intern. Med. 1963, 58, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, D.D.; Taher, A.; Wong, V.K.; Elsaiey, A.; Consul, N.; Mahmoud, H.S.; Mujtaba, B.; Stanietzky, N.; Elsayes, K.M. PTEN Hamartoma Tumor Syndrome/Cowden Syndrome: Genomics, Oncogenesis, and Imaging Review for Associated Lesions and Malignancy. Cancers 2021, 13, 3120. [Google Scholar] [CrossRef] [PubMed]
- Maehama, T.; Dixon, J.E. The Tumor Suppressor, PTEN/MMAC1, Dephosphorylates the Lipid Second Messenger, Phosphatidylinositol 3,4,5-Trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378. [Google Scholar] [CrossRef] [PubMed]
- Gammon, A.; Jasperson, K.; Champine, M. Genetic Basis of Cowden Syndrome and Its Implications for Clinical Practice and Risk Management. Appl. Clin. Genet. 2016, 9, 83. [Google Scholar] [CrossRef]
- Squarize, C.H.; Castilho, R.M.; Gutkind, J.S. Chemoprevention and Treatment of Experimental Cowden’s Disease by mTOR Inhibition with Rapamycin. Cancer Res. 2008, 68, 7066–7072. [Google Scholar] [CrossRef]
- Mester, J.L.; Eng, C. Estimate of de Novo Mutation Frequency in Probands with PTEN Hamartoma Tumor Syndrome. Genet. Med. 2012, 14, 819–822. [Google Scholar] [CrossRef]
- Tan, M.-H.; Mester, J.L.; Ngeow, J.; Rybicki, L.A.; Orloff, M.S.; Eng, C. Lifetime Cancer Risks in Individuals with Germline PTEN Mutations. Clin. Cancer Res. 2012, 18, 400–407. [Google Scholar] [CrossRef]
- Carlo, M.I. Hereditary Renal Cell Carcinoma Syndromes. Hematol./Oncol. Clin. N. Am. 2023, 37, 841–848. [Google Scholar] [CrossRef]
- Kamihara, J.; Schultz, K.A.; Rana, H.Q. FH Tumor Predisposition Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Alam, N.A.; Bevan, S.; Churchman, M.; Barclay, E.; Barker, K.; Jaeger, E.E.; Nelson, H.M.; Healy, E.; Pembroke, A.C.; Friedmann, P.S.; et al. Localization of a Gene (MCUL1) for Multiple Cutaneous Leiomyomata and Uterine Fibroids to Chromosome 1q42.3-Q43. Am. J. Hum. Genet. 2001, 68, 1264–1269. [Google Scholar] [CrossRef]
- Sulkowski, P.L.; Sundaram, R.K.; Oeck, S.; Corso, C.D.; Liu, Y.; Noorbakhsh, S.; Niger, M.; Boeke, M.; Ueno, D.; Kalathil, A.N.; et al. Krebs-Cycle-Deficient Hereditary Cancer Syndromes Are Defined by Defects in Homologous-Recombination DNA Repair. Nat. Genet. 2018, 50, 1086–1092. [Google Scholar] [CrossRef]
- Ricketts, C.J.; Killian, J.K.; Vocke, C.D.; Wang, Y.; Merino, M.J.; Meltzer, P.S.; Linehan, W.M. Kidney Tumors Associated with Germline Mutations of FH and SDHB Show a CpG Island Methylator Phenotype (CIMP). PLoS ONE 2022, 17, e0278108. [Google Scholar] [CrossRef]
- Toro, J.R.; Glenn, G.; Hou, L.; Duray, P.; Clark, B.; Merino, M.; Zbar, B.; Linehan, M.; Turner, M.L. Facial Papules, Spontaneous Pneumothorax, and Renal Tumors. J. Am. Acad. Dermatol. 2003, 48, 111–114. [Google Scholar] [CrossRef]
- Zhang, L.; Walsh, M.F.; Jairam, S.; Mandelker, D.; Zhong, Y.; Kemel, Y.; Chen, Y.-B.; Musheyev, D.; Zehir, A.; Jayakumaran, G.; et al. Fumarate Hydratase FH c.1431_1433dupAAA (p.Lys477dup) Variant Is Not Associated with Cancer Including Renal Cell Carcinoma. Hum. Mutat. 2020, 41, 103–109. [Google Scholar] [CrossRef]
- Alam, N.A.; Olpin, S.; Leigh, I.M. Fumarate Hydratase Mutations and Predisposition to Cutaneous Leiomyomas, Uterine Leiomyomas and Renal Cancer. Br. J. Dermatol. 2005, 153, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Menko, F.H.; Maher, E.R.; Schmidt, L.S.; Middelton, L.A.; Aittomäki, K.; Tomlinson, I.; Richard, S.; Linehan, W.M. Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC): Renal Cancer Risk, Surveillance and Treatment. Fam. Cancer 2014, 13, 637–644. [Google Scholar] [CrossRef]
- Lehtonen, H.J.; Kiuru, M.; Ylisaukko-Oja, S.K.; Salovaara, R.; Herva, R.; Koivisto, P.A.; Vierimaa, O.; Aittomäki, K.; Pukkala, E.; Launonen, V.; et al. Increased Risk of Cancer in Patients with Fumarate Hydratase Germline Mutation. J. Med. Genet. 2006, 43, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Merino, M.J.; Torres-Cabala, C.; Pinto, P.; Linehan, W.M. The Morphologic Spectrum of Kidney Tumors in Hereditary Leiomyomatosis and Renal Cell Carcinoma (HLRCC) Syndrome. Am. J. Surg. Pathol. 2007, 31, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, A.; Gopal, N.; Firouzabadi, F.D.; Anari, P.Y.; Wakim, P.; Ball, M.W.; Jones, E.C.; Turkbey, B.; Huda, F.; Marston Linehan, W.; et al. Role of Ultra-High b-Value DWI in the Imaging of Hereditary Leiomyomatosis and Renal Cell Carcinoma (HLRCC). Abdom. Radiol. 2023, 48, 340–349. [Google Scholar] [CrossRef]
- Toro, J.R.; Nickerson, M.L.; Wei, M.H.; Warren, M.B.; Glenn, G.M.; Turner, M.L.; Stewart, L.; Duray, P.; Tourre, O.; Sharma, N.; et al. Mutations in the Fumarate Hydratase Gene Cause Hereditary Leiomyomatosis and Renal Cell Cancer in Families in North America. Am. J. Hum. Genet. 2003, 73, 95–106. [Google Scholar] [CrossRef]
- Zbar, B.; Tory, K.; Merino, M.; Schmidt, L.; Glenn, G.; Choyke, P.; Walther, M.M.; Lerman, M.; Linehan, W.M. Hereditary Papillary Renal Cell Carcinoma. J. Urol. 1994, 151, 561–566. [Google Scholar] [CrossRef]
- Zbar, B.; Glenn, G.; Lubensky, I.; Choyke, P.; Walther, M.M.; Magnusson, G.; Bergerheim, U.S.; Pettersson, S.; Amin, M.; Hurley, K. Hereditary Papillary Renal Cell Carcinoma: Clinical Studies in 10 Families. J. Urol. 1995, 153, 907–912. [Google Scholar] [PubMed]
- Yang, Y.; Ricketts, C.J.; Vocke, C.D.; Killian, J.K.; Padilla-Nash, H.M.; Lang, M.; Wei, D.; Lee, Y.H.; Wangsa, D.; Sourbier, C.; et al. Characterization of Genetically Defined Sporadic and Hereditary Type 1 Papillary Renal Cell Carcinoma Cell Lines. Genes. Chromosomes Cancer 2021, 60, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.; Duh, F.M.; Chen, F.; Kishida, T.; Glenn, G.; Choyke, P.; Scherer, S.W.; Zhuang, Z.; Lubensky, I.; Dean, M.; et al. Germline and Somatic Mutations in the Tyrosine Kinase Domain of the MET Proto-Oncogene in Papillary Renal Carcinomas. Nat. Genet. 1997, 16, 68–73. [Google Scholar] [CrossRef] [PubMed]
- PubMed. Met, Metastasi, Motilità e Altro. Available online: https://pubmed.ncbi.nlm.nih.gov/14685170/ (accessed on 18 June 2024).
- Birchmeier, W.; Brinkmann, V.; Niemann, C.; Meiners, S.; DiCesare, S.; Naundorf, H.; Sachs, M. Role of HGF/SF and c-Met in Morphogenesis and Metastasis of Epithelial Cells. Ciba Found. Symp. 1997, 212, 230–240; discussion 240–246. [Google Scholar] [CrossRef]
- Webster, B.R.; Gopal, N.; Ball, M.W. Tumorigenesis Mechanisms Found in Hereditary Renal Cell Carcinoma: A Review. Genes 2022, 13, 2122. [Google Scholar] [CrossRef] [PubMed]
- Novel Germline MET Pathogenic Variants in French Patients with Papillary Renal Cell Carcinomas Type I—Sebai—2022—Human Mutation—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/humu.24313 (accessed on 18 June 2024).
- Maher, E.R. Hereditary Renal Cell Carcinoma Syndromes: Diagnosis, Surveillance and Management. World J. Urol. 2018, 36, 1891–1898. [Google Scholar] [CrossRef]
- Macher-Goeppinger, S.; Keith, M.; Endris, V.; Penzel, R.; Tagscherer, K.E.; Pahernik, S.; Hohenfellner, M.; Gardner, H.; Grüllich, C.; Schirmacher, P.; et al. MET Expression and Copy Number Status in Clear-Cell Renal Cell Carcinoma: Prognostic Value and Potential Predictive Marker. Oncotarget 2017, 8, 1046–1057. [Google Scholar] [CrossRef]
- Twardowski, P.W.; Tangen, C.M.; Wu, X.; Plets, M.R.; Plimack, E.R.; Agarwal, N.; Vogelzang, N.J.; Wang, J.; Tao, S.; Thompson, I.M.; et al. Parallel (Randomized) Phase II Evaluation of Tivantinib (ARQ197) and Tivantinib in Combination with Erlotinib in Papillary Renal Cell Carcinoma: SWOG S1107. Kidney Cancer 2017, 1, 123–132. [Google Scholar] [CrossRef] [PubMed]

| Disease | Gene | Extrarenal Tumors | Incidence | Imaging Presentation | Histological Presentation |
|---|---|---|---|---|---|
| Von Hippel–Lindau disease | VHL | Hemangioblastomas, pancreatic neuroendocrine tumors, pheochromocytomas, paragangliomas | 0.3–3.2% | Multiple tumors in both kidneys | Clear cell |
| Birt–Hogg–Dubé syndrome | FLCN | Cystic lung disease, pneumothorax, skin lesions | 0.3–1.6% | Bilateral and multifocal renal tumors | 50–67% are hybrid oncocytic–chromophobe, 23–34% are chromophobe, 7–9% are clear cell, 3–5% are oncocytomas, and 2% are papillary |
| Tuberous sclerosis | TSC1/TSC2 | SEGA (subependymal giant cell astrocytomas, cerebral cortical tubers), heart (rhabdomyomas), lung (lymphangioleiomyomatosis or LAM), and skin (cutaneous and subungual angiofibromas, shagreen patch), retinal hamartomas | 0.2–2.6% | Bilateral and multifocal cysts/angiomyolipomas/RCC | Hybrid oncocytic–chromophobe, chromophobe, clear cell, oncocytomas, and papillary |
| Succinate dehydrogenase-B Mutation (SDHB)/HPP syndrome | SDHB, SDHC, SDHD, SDHA | PPGLs, thyroid carcinomas, GIST, pituitary adenomas | 0.7–0.9% | 70% unilateral kidney tumors, 30% multifocal or bilateral | Chromophobe, clear cell, papillary |
| Hereditary papillary renal cell carcinoma | MET | None | 0.4% | Multifocal papillary renal cancer | Papillary Renal cell Carcinoma (RCC) |
| Fumarate hydratase deficiency | FH | Uterine leiomyomas | 0.2–5.2% | Single growths in one kidney | Papillary, oncocytoma, clear cell RCC |
| BAP1 tumor predisposition syndrome | BAP1 | Uveal melanomas, malignant mesothelioma | 0.3–1.6% | Multifocality | High Fuhrman grade, all RCC subtypes |
| Cowden syndrome | PTEN | Breast carcinomas, thyroid carcinomas, endometrial carcinomas | 0.3% | Solitary renal lesions | Papillary and clear cell RCC |
| Disease | Tumor Surveillance | Therapy | Surgical Approach |
|---|---|---|---|
| Von Hippel–Lindau disease | Biannual TC mdc/MRI from 15 years of age | Anti-HIF2-alpha therapy (belzutifan) | Lesions > 3 cm in diameter |
| Birt–Hogg–Dubé syndrome | Biannual TC mdc/MRI from 20 years of age | / | Lesions > 3 cm in diameter |
| Tuberous sclerosis | TC mdc/MRI every 1–3 years from childhood; annual renal function assessment | mTOR inhibitors (everolimus) | “Watch and wait” for angiomyolipomas and insufficient evidence for RCC |
| Succinate dehydrogenase-B Mutation (SDHB)/HPP syndrome | TC mdc/MRI every 2–3 years from childhood | / | Immediate surgical removal |
| Hereditary papillary renal cell carcinoma | Annual TC mdc/MRI from 30 years of age | MET inhibitors | Lesions > 3 cm in diameter |
| Fumarate hydratase deficiency | Annual TC mdc/MRI from 8–10 years of age | Bevacizumab + erlotinib | Immediate surgical removal |
| BAP1-tumor predisposition syndrome | Annual TC mdc/MRI from 30 years of age | / | Insufficient evidence, but immediate surgical removal is suggested |
| Cowden syndrome | Biannual ultrasound from 40 years of age | / | Insufficient evidence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicchetti, R.; Basconi, M.; Litterio, G.; Mascitti, M.; Tamborino, F.; Orsini, A.; Digiacomo, A.; Ferro, M.; Schips, L.; Marchioni, M. Advances in Molecular Mechanisms of Kidney Disease: Integrating Renal Tumorigenesis of Hereditary Cancer Syndrome. Int. J. Mol. Sci. 2024, 25, 9060. https://doi.org/10.3390/ijms25169060
Cicchetti R, Basconi M, Litterio G, Mascitti M, Tamborino F, Orsini A, Digiacomo A, Ferro M, Schips L, Marchioni M. Advances in Molecular Mechanisms of Kidney Disease: Integrating Renal Tumorigenesis of Hereditary Cancer Syndrome. International Journal of Molecular Sciences. 2024; 25(16):9060. https://doi.org/10.3390/ijms25169060
Chicago/Turabian StyleCicchetti, Rossella, Martina Basconi, Giulio Litterio, Marco Mascitti, Flavia Tamborino, Angelo Orsini, Alessio Digiacomo, Matteo Ferro, Luigi Schips, and Michele Marchioni. 2024. "Advances in Molecular Mechanisms of Kidney Disease: Integrating Renal Tumorigenesis of Hereditary Cancer Syndrome" International Journal of Molecular Sciences 25, no. 16: 9060. https://doi.org/10.3390/ijms25169060
APA StyleCicchetti, R., Basconi, M., Litterio, G., Mascitti, M., Tamborino, F., Orsini, A., Digiacomo, A., Ferro, M., Schips, L., & Marchioni, M. (2024). Advances in Molecular Mechanisms of Kidney Disease: Integrating Renal Tumorigenesis of Hereditary Cancer Syndrome. International Journal of Molecular Sciences, 25(16), 9060. https://doi.org/10.3390/ijms25169060







