Identification and Expression Analysis of Sulfate Transporter Genes Family and Function Analysis of GmSULTR3;1a from Soybean
Abstract
:1. Introduction
2. Results
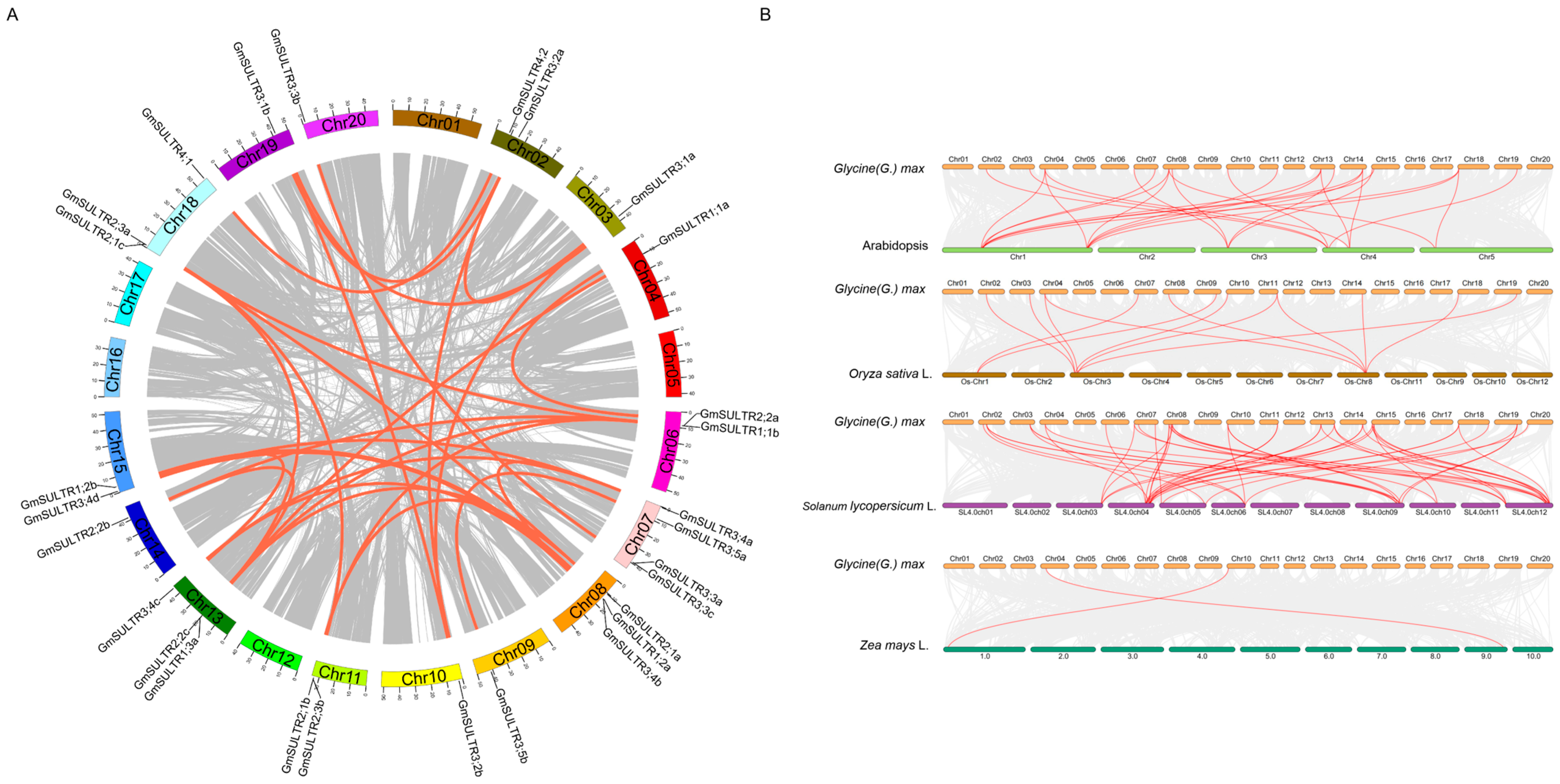
2.1. Identification of the GmSULTR Gene Family and Syntenic Analysis of SULTR Genes in Soybean
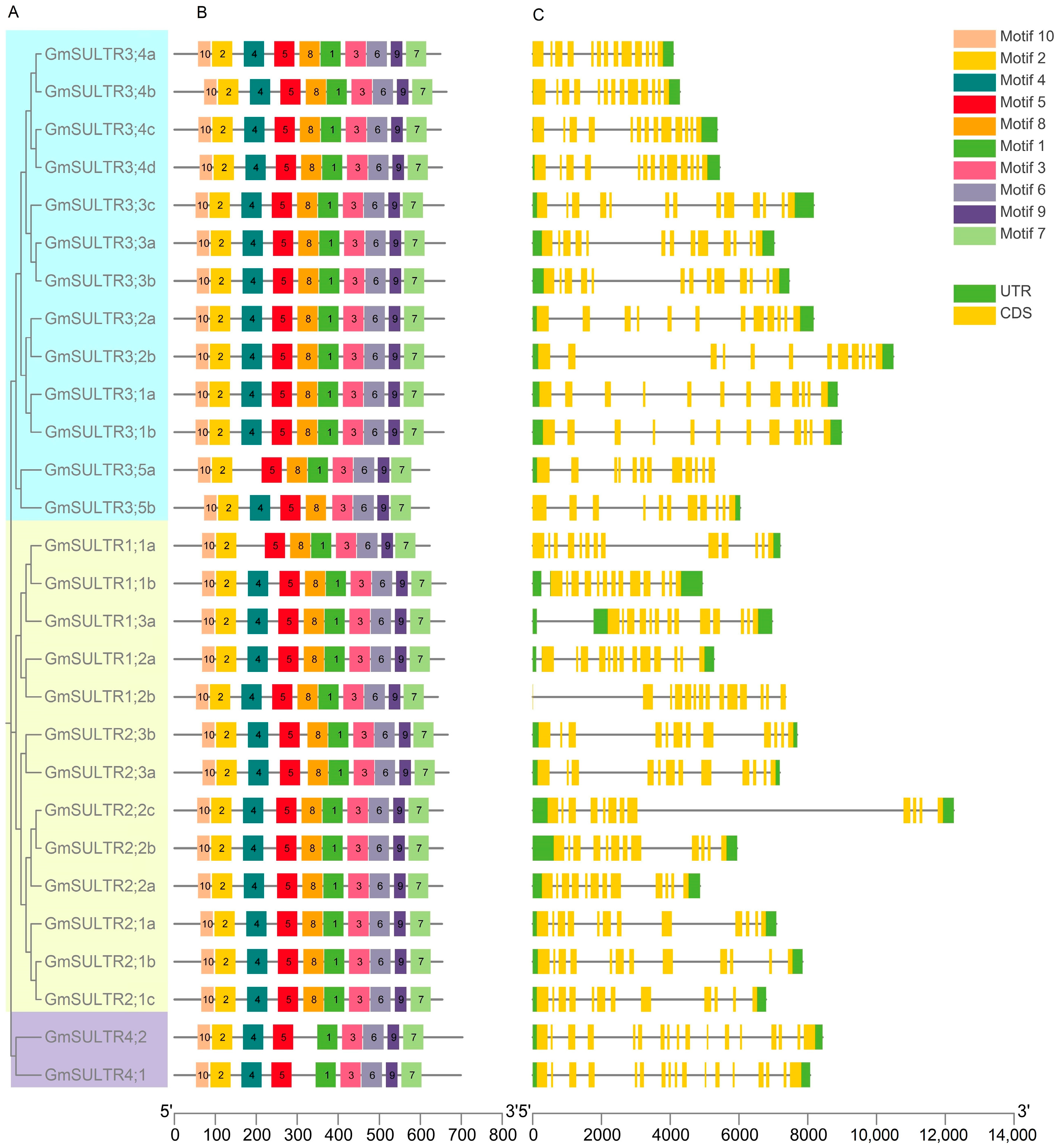
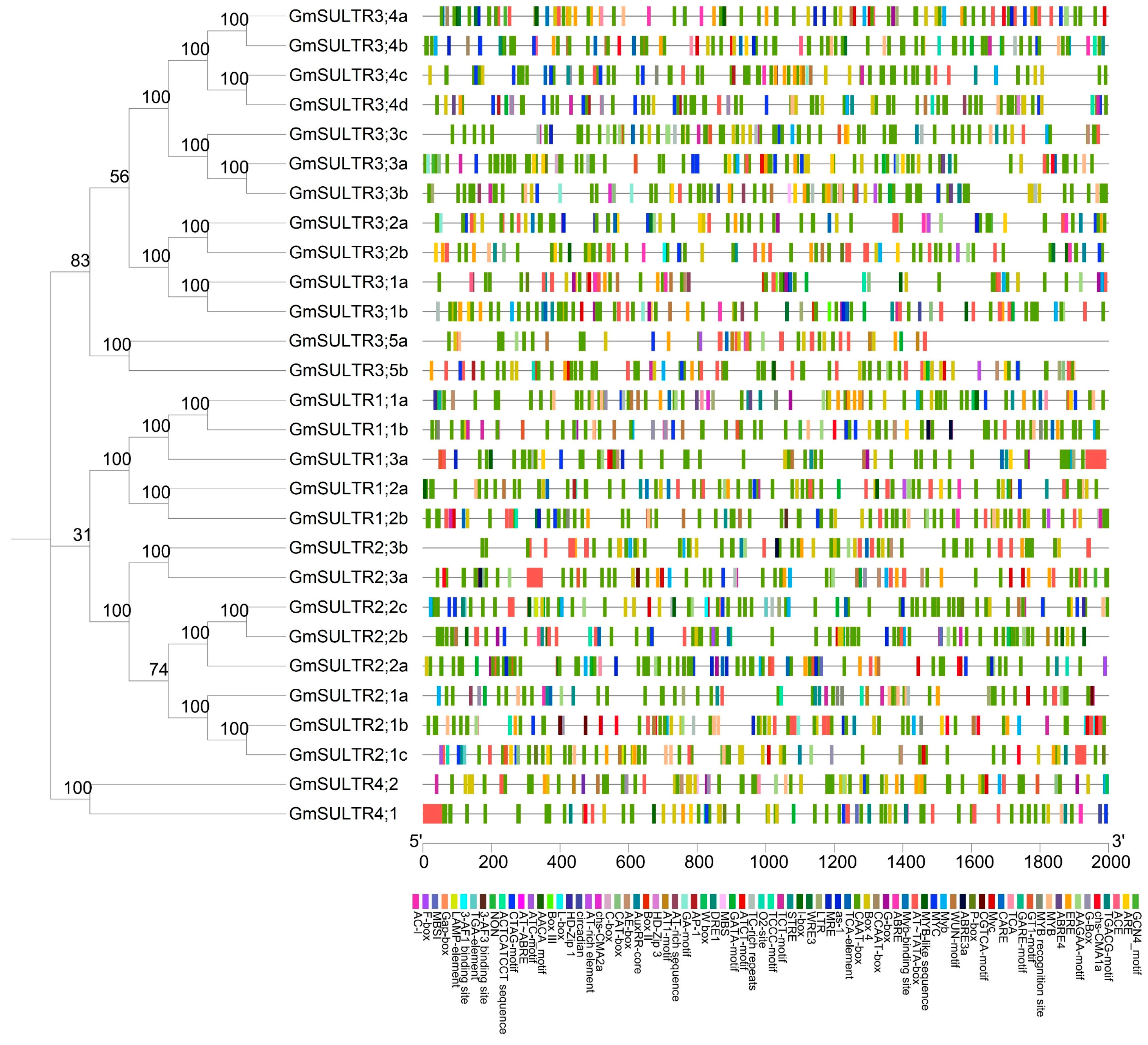
2.2. Analysis of Protein Conserved Motisf, Gene Structure, and Cis-Elements of SULTRs in Soybean
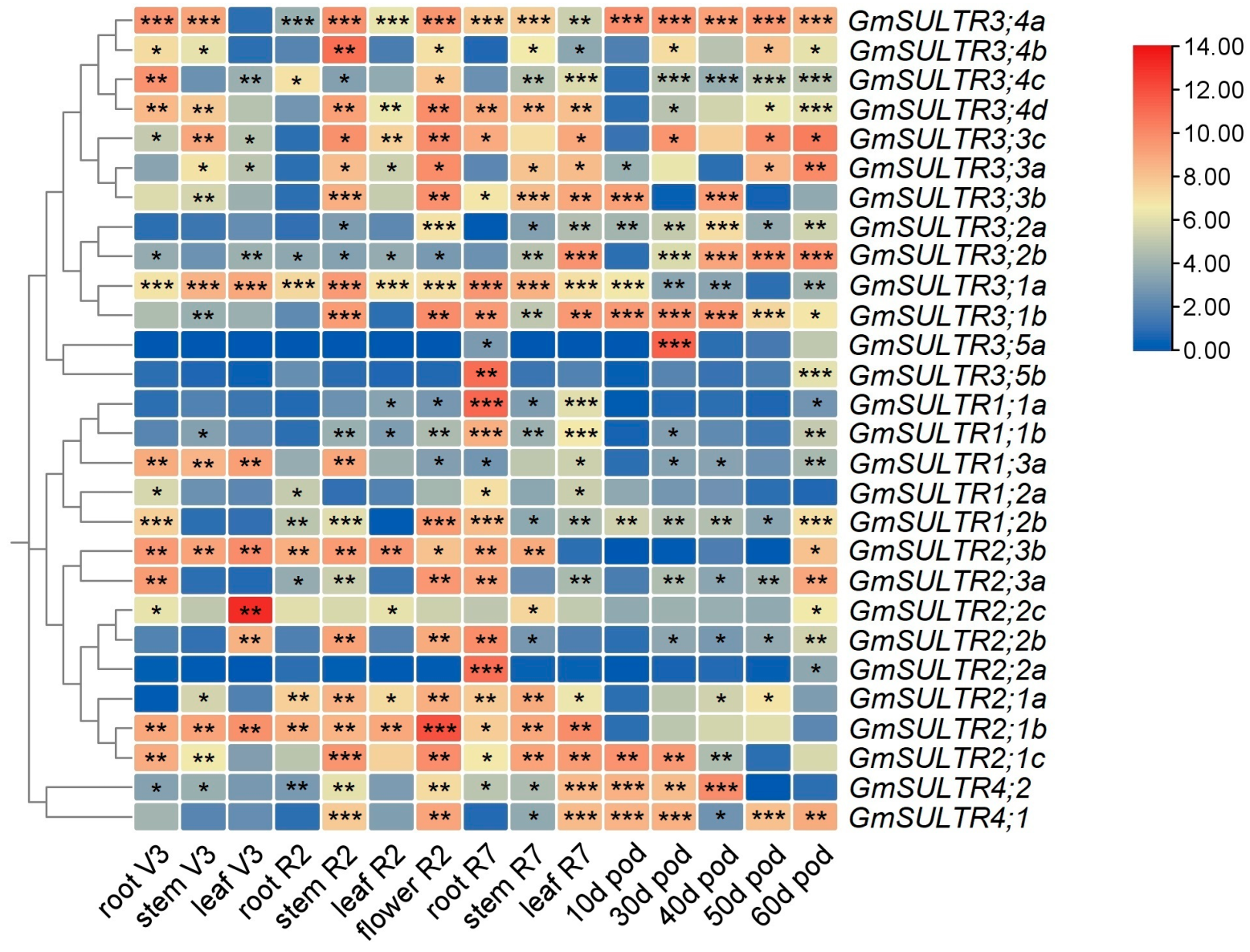
2.3. Expression Profiling of GmSULTRs in Various Tissues
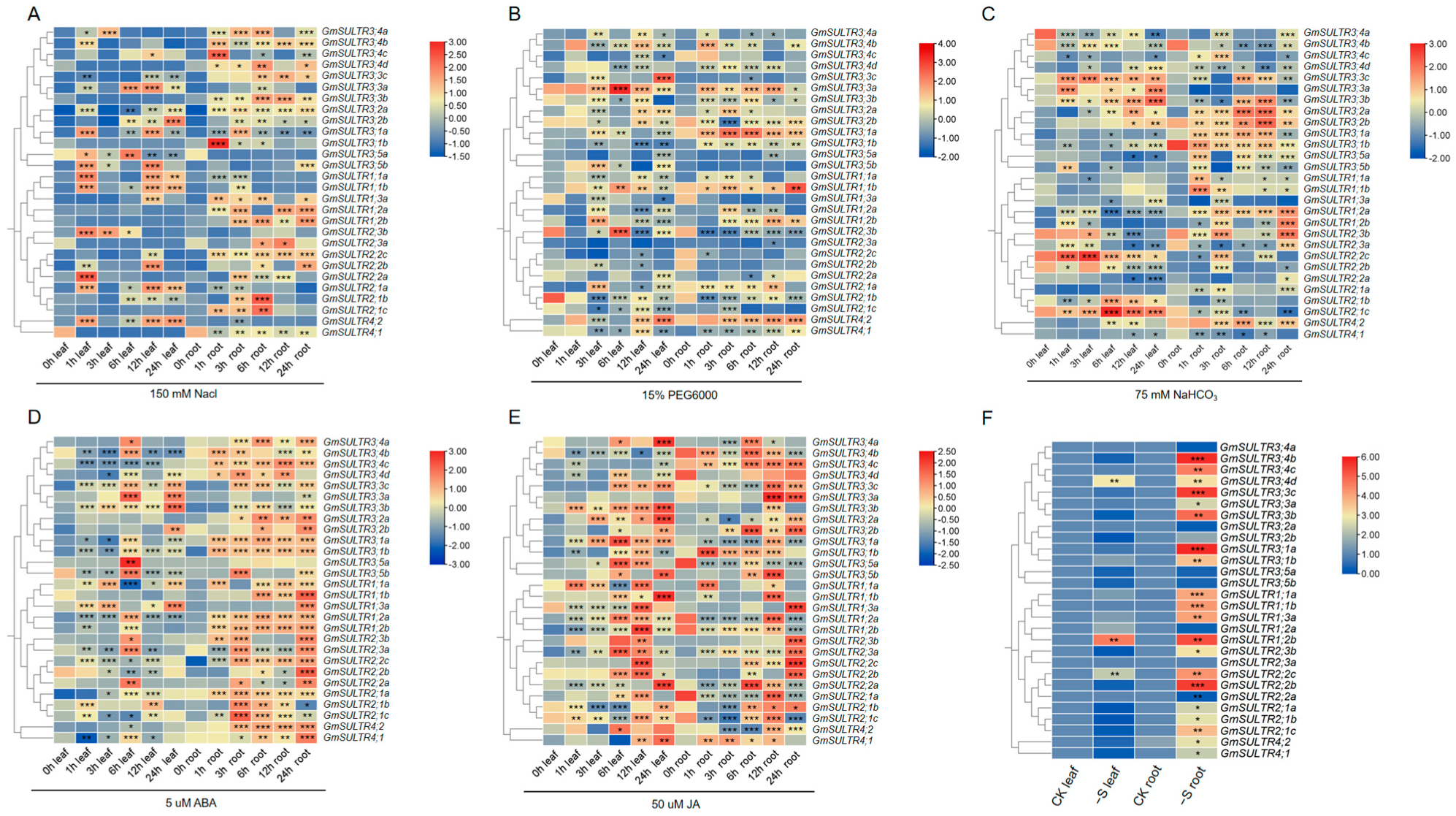
2.4. GmSULTRs Expression in Response to Abiotic Stress and Plant Hormones Treatments
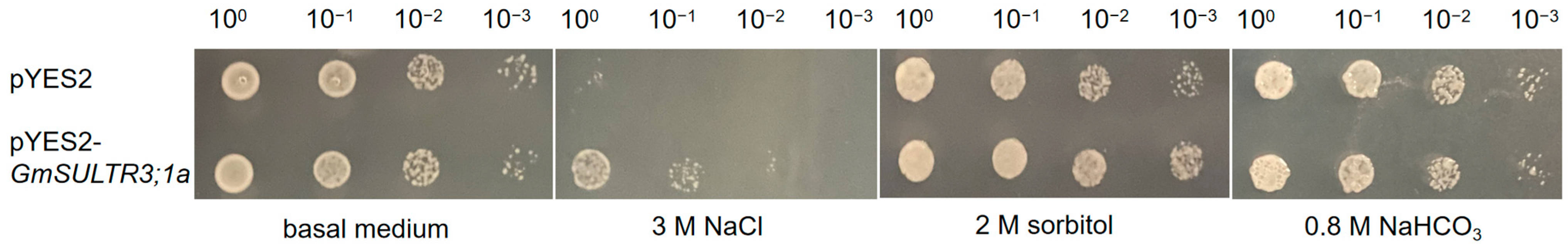
2.5. GmSULTR3;1a Improved Salt Tolerance
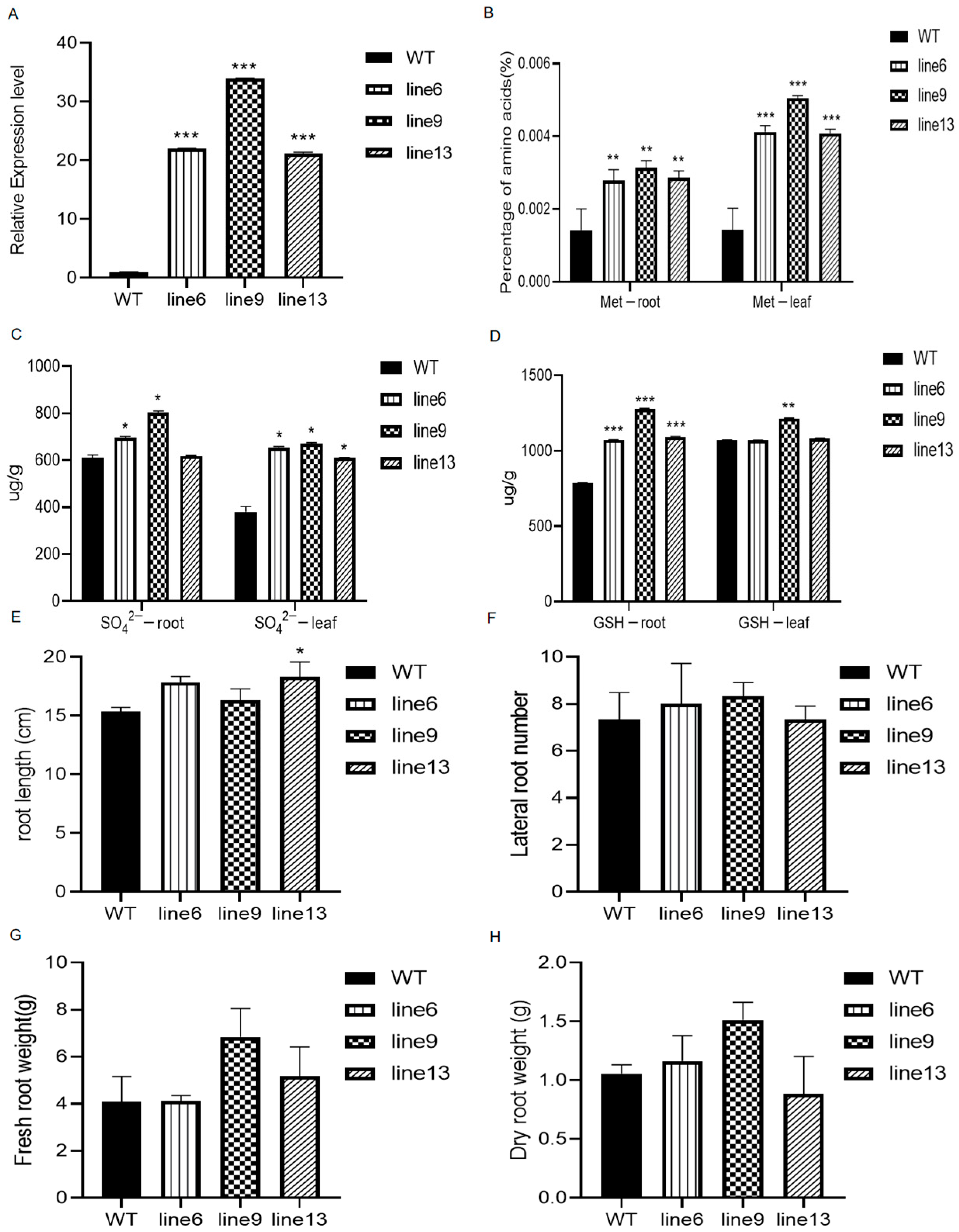
2.6. GmSULTR3;1a Alters Sulfur-Containing Compounds and Root Phenotypes of Transgenic Soybean Hairy Roots
3. Discussion
4. Materials and Methods
4.1. Gene Structure, Conserved Motifs, and Promoter Analysis of GmSULTRs
4.2. Syntenic Analysis between Glycine and Other Species
4.3. Plant Materials and Treatments
4.4. Total RNA Extraction and qRT-PCR Analysis
4.5. Stress Tolerance Analysis
4.6. Preparation of Transgenic Soybean Hairy Roots
4.7. Determination of Quality Index of Transgenic Soybean Hairy Roots
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xun, M.; Song, J.; Shi, J.; Li, J.; Shi, Y.; Yan, J.; Zhang, W.; Yang, H. Genome-Wide Identification of Sultr Genes in Malus domestica and Low Sulfur-Induced MhSultr3;1a to Increase Cysteine-Improving Growth. Front. Plant Sci. 2021, 12, 748242. [Google Scholar] [CrossRef]
- Hernández, L.E.; Sobrino-Plata, J.; Montero-Palmero, M.B.; Carrasco-Gil, S.; Flores-Cáceres, M.L.; Ortega-Villasante, C.; Escobar, C. Contribution of glutathione to the control of cellular redox homeostasis under toxic metal and metalloid stress. J. Exp. Bot. 2015, 66, 2901–2911. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-H.; Yi, H.-L.; Xiu-Ping, L.; Qi, H.-X. Sulfur dioxide enhance drought tolerance of wheat seedlings through H2S signaling. Ecotoxicol. Environ. Saf. 2021, 207, 111248. [Google Scholar] [CrossRef]
- Yi, H.; Ravilious, G.E.; Galant, A.; Krishnan, H.B.; Jez, J.M. From sulfur to homoglutathione: Thiol metabolism in soybean. Amino Acids 2010, 39, 963–978. [Google Scholar] [CrossRef]
- Ernst, L.; Goodger, J.Q.D.; Alvarez, S.; Marsh, E.L.; Berla, B.; Lockhart, E.; Jung, J.; Li, P.; Bohnert, H.J.; Schachtman, D.P. Sulphate as a xylem-borne chemical signal precedes the expression of ABA biosynthetic genes in maize roots. J. Exp. Bot. 2010, 61, 3395–3405. [Google Scholar] [CrossRef]
- Malcheska, F.; Ahmad, A.; Batool, S.; Müller, H.M.; Ludwig-Müller, J.; Kreuzwieser, J.; Randewig, D.; Hänsch, R.; Mendel, R.R.; Hell, R.; et al. Drought-Enhanced Xylem Sap Sulfate Closes Stomata by Affecting ALMT12 and Guard Cell ABA Synthesis. Plant Physiol. 2017, 174, 798–814. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Q.; Gao, L.; Yu, X.-M.; Fang, P.; Oliver, D.J.; Xiang, C.-B. Isolation and characterization of low-sulphur-tolerant mutants of Arabidopsis. J. Exp. Bot. 2010, 61, 3407–3422. [Google Scholar] [CrossRef]
- Vatansever, R.; Koc, I.; Ozyigit, I.I.; Sen, U.; Uras, M.E.; Anjum, N.A.; Pereira, E.; Filiz, E. Genome-wide identification and expression analysis of sulfate transporter (SULTR) genes in potato (Solanum tuberosum L.). Planta 2016, 244, 1167–1183. [Google Scholar] [CrossRef] [PubMed]
- Akbudak, M.A.; Filiz, E.; Kontbay, K. Genome-wide identification and cadmium induced expression profiling of sulfate transporter (SULTR) genes in sorghum (Sorghum bicolor L.). BioMetals 2017, 31, 91–105. [Google Scholar] [CrossRef]
- Buchner, P.; Parmar, S.; Kriegel, A.; Carpentier, M.; Hawkesford, M.J. The Sulfate Transporter Family in Wheat: Tissue-Specific Gene Expression in Relation to Nutrition. Mol. Plant 2010, 3, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Honsel, A.; Kojima, M.; Haas, R.; Frank, W.; Sakakibara, H.; Herschbach, C.; Rennenberg, H. Sulphur limitation and early sulphur deficiency responses in poplar: Significance of gene expression, metabolites, and plant hormones. J. Exp. Bot. 2012, 63, 1873–1893. [Google Scholar] [CrossRef]
- Heidari, P.; Hasanzadeh, S.; Faraji, S.; Ercisli, S.; Mora-Poblete, F. Genome-Wide Characterization of the Sulfate Transporter Gene Family in Oilseed Crops: Camelina sativa and Brassica napus. Plants 2023, 12, 628. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, X.; Zhang, J.; Wang, L.; Wang, Y.; Li, N.; Guo, L.; Ren, H.; Zeng, J. Genome-wide identification of SULTR genes in tea plant and analysis of their expression in response to sulfur and selenium. Protoplasma 2021, 259, 127–140. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, X.; Yang, R.; Sun, Z.; Yang, S.; Zhang, H.; Xing, B.; Li, Y.; Liu, Q.; Lu, Q.; et al. Genome-wide identification and expression-pattern analysis of sulfate transporter (SULTR) gene family in cotton under multiple abiotic stresses and fiber development. Funct. Integr. Genom. 2024, 24, 108. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, M.; Xia, Z. The SULTR gene family in maize (Zea mays L.): Gene cloning and expression analyses under sulfate starvation and abiotic stress. J. Plant Physiol. 2018, 220, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhou, X.; Zuo, L.; Wang, H.; Yu, D. Identification and functional characterization of the sulfate transporter gene GmSULTR1;2b in soybean. BMC Genom. 2016, 17, 373. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Inoue, E.; Saito, K.; Yamaya, T.; Takahashi, H. Phloem-Localizing Sulfate Transporter, Sultr1;3, Mediates Re-Distribution of Sulfur from Source to Sink Organs in Arabidopsis. Plant Physiol. 2003, 131, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Watanabe-Takahashi, A.; Smith, F.W.; Blake-Kalff, M.; Hawkesford, M.J.; Saito, K. The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J. 2000, 23, 171–182. [Google Scholar] [PubMed]
- Cao, M.J.; Wang, Z.; Wirtz, M.; Hell, R.; Oliver, D.J.; Xiang, C.B. SULTR3;1 is a chloroplast-localized sulfate transporter in Arabidopsis thaliana. Plant J. 2012, 73, 607–616. [Google Scholar] [CrossRef]
- Kataoka, T.; Hayashi, N.; Yamaya, T.; Takahashi, H. Root-to-Shoot Transport of Sulfate in Arabidopsis. Evidence for the Role of SULTR3;5 as a Component of Low-Affinity Sulfate Transport System in the Root Vasculature. Plant Physiol. 2004, 136, 4198–4204. [Google Scholar] [CrossRef] [PubMed]
- Zuber, H.; Davidian, J.-C.; Aubert, G.; Aimé, D.; Belghazi, M.; Lugan, R.; Heintz, D.; Wirtz, M.; Hell, R.; Thompson, R.; et al. The Seed Composition of Arabidopsis Mutants for the Group 3 Sulfate Transporters Indicates a Role in Sulfate Translocation within Developing Seeds. Plant Physiol. 2010, 154, 913–926. [Google Scholar] [CrossRef]
- Kataoka, T.; Watanabe-Takahashi, A.; Hayashi, N.; Ohnishi, M.; Mimura, T.; Buchner, P.; Hawkesford, M.J.; Yamaya, T.; Takahashi, H. Vacuolar Sulfate Transporters Are Essential Determinants Controlling Internal Distribution of Sulfate in Arabidopsis. Plant Cell 2004, 16, 2693–2704. [Google Scholar] [CrossRef] [PubMed]
- Puresmaeli, F.; Heidari, P.; Lawson, S. Insights into the Sulfate Transporter Gene Family and Its Expression Patterns in Durum Wheat Seedlings under Salinity. Genes. 2023, 14, 333. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, X.-Q.; Broughton, S.; Westcott, S.; Wu, D.; Lance, R.; Li, C. A nonsense mutation in a putative sulphate transporter gene results in low phytic acid in barley. Funct. Integr. Genom. 2011, 11, 103–110. [Google Scholar] [CrossRef]
- Zhao, H.; Frank, T.; Tan, Y.; Zhou, C.; Jabnoune, M.; Arpat, A.B.; Cui, H.; Huang, J.; He, Z.; Poirier, Y.; et al. Disruption of OsSULTR3;3 reduces phytate and phosphorus concentrations and alters the metabolite profile in rice grains. New Phytol. 2016, 211, 926–939. [Google Scholar] [CrossRef]
- Yamaji, N.; Takemoto, Y.; Miyaji, T.; Mitani-Ueno, N.; Yoshida, K.T.; Ma, J.F. Reducing phosphorus accumulation in rice grains with an impaired transporter in the node. Nature 2016, 541, 92–95. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, L.; Wu, Y.; He, L. Genome-wide identification of ETHYLENE INSENSITIVE 2 in Triticeae species reveals that TaEIN2-4D.1 regulates cadmium tolerance in Triticum aestivum. Plant Physiol. Biochem. 2023, 203, 108009. [Google Scholar] [CrossRef]
- Narayan, O.P.; Verma, N.; Jogawat, A.; Dua, M.; Johri, A.K. Sulfur transfer from the endophytic fungus Serendipita indica improves maize growth and requires the sulfate transporter SiSulT. Plant Cell 2021, 33, 1268–1285. [Google Scholar] [CrossRef]
- Armbruster, L.; Uslu, V.V.; Wirtz, M.; Hell, R. The Recovery from Sulfur Starvation Is Independent from the mRNA Degradation Initiation Enzyme PARN in Arabidopsis. Plants 2019, 8, 380. [Google Scholar] [CrossRef]
- Cao, M.J.; Wang, Z.; Zhao, Q.; Mao, J.L.; Speiser, A.; Wirtz, M.; Hell, R.; Zhu, J.K.; Xiang, C.B. Sulfate availability affects ABA levels and germination response to ABA and salt stress in Arabidopsis thaliana. Plant J. 2014, 77, 604–615. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Takahashi, H.; Smith, F.W.; Yamaya, T.; Saito, K. Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsis roots. Plant J. 2002, 29, 465–473. [Google Scholar] [CrossRef]
- Batool, S.; Uslu, V.V.; Rajab, H.; Ahmad, N.; Waadt, R.; Geiger, D.; Malagoli, M.; Xiang, C.-B.; Hedrich, R.; Rennenberg, H.; et al. Sulfate is Incorporated into Cysteine to Trigger ABA Production and Stomatal Closure. Plant Cell 2018, 30, 2973–2987. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, P.-X.; Miao, Z.-Q.; Qi, G.-F.; Wang, Z.; Yuan, Y.; Ahmad, N.; Cao, M.-J.; Hell, R.; Wirtz, M.; et al. SULTR3s Function in Chloroplast Sulfate Uptake and Affect ABA Biosynthesis and the Stress Response. Plant Physiol. 2019, 180, 593–604. [Google Scholar] [CrossRef]
- Howarth, J.R.; Fourcroy, P.; Davidian, J.-C.; Smith, F.W.; Hawkesford, M.J. Cloning of two contrasting high-affinity sulfate transporters from tomato induced by low sulfate and infection by the vascular pathogen Verticillium dahliae. Planta 2003, 218, 58–64. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Gautam, R.; Ahmed, I.; Shukla, P.; Meena, R.K.; Kirti, P.B. Genome-wide characterization of ALDH Superfamily in Brassica rapa and enhancement of stress tolerance in heterologous hosts by BrALDH7B2 expression. Sci. Rep. 2019, 9, 7012. [Google Scholar] [CrossRef]
- Kereszt, A.; Li, D.; Indrasumunar, A.; Nguyen, C.D.T.; Nontachaiyapoom, S.; Kinkema, M.; Gresshoff, P.M. Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat. Protoc. 2007, 2, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Lancilli, C.; Giacomini, B.; Lucchini, G.; Davidian, J.C.; Cocucci, M.; Sacchi, G.A.; Nocito, F.F. Cadmium exposure and sulfate limitation reveal differences in the transcriptional control of three sulfate transporter (Sultr1;2) genes in Brassica juncea. BMC Plant Biol. 2014, 14, 132. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Wang, Y.-T.; Pan, X.-B.; Xi, Z.-M. Amelioration of cold-induced oxidative stress by exogenous 24-epibrassinolide treatment in grapevine seedlings: Toward regulating the ascorbate–glutathione cycle. Sci. Hortic. 2019, 244, 379–387. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Dong, Y.; Liu, Y.; Huang, Y.; Jiang, W.; Zheng, X.; Zhang, H.; Gong, N.; Bai, X. Identification and Expression Analysis of Sulfate Transporter Genes Family and Function Analysis of GmSULTR3;1a from Soybean. Int. J. Mol. Sci. 2024, 25, 9080. https://doi.org/10.3390/ijms25169080
Zhou J, Dong Y, Liu Y, Huang Y, Jiang W, Zheng X, Zhang H, Gong N, Bai X. Identification and Expression Analysis of Sulfate Transporter Genes Family and Function Analysis of GmSULTR3;1a from Soybean. International Journal of Molecular Sciences. 2024; 25(16):9080. https://doi.org/10.3390/ijms25169080
Chicago/Turabian StyleZhou, Jingwen, Yue Dong, Yue Liu, Yifan Huang, Wenjing Jiang, Xiangmin Zheng, Huimin Zhang, Na Gong, and Xi Bai. 2024. "Identification and Expression Analysis of Sulfate Transporter Genes Family and Function Analysis of GmSULTR3;1a from Soybean" International Journal of Molecular Sciences 25, no. 16: 9080. https://doi.org/10.3390/ijms25169080




