Maternal Salivary miR-423-5p Is Linked to Neonatal Outcomes and Periodontal Status in Cardiovascular-High-Risk Pregnancies
Abstract
1. Introduction
2. Results
2.1. Demographic Data
2.2. Pregestational Body Mass Index (pre-BMI), Oral Status, and Neonatal Outcomes
2.3. Selection Strategy for Relevant miRNAs
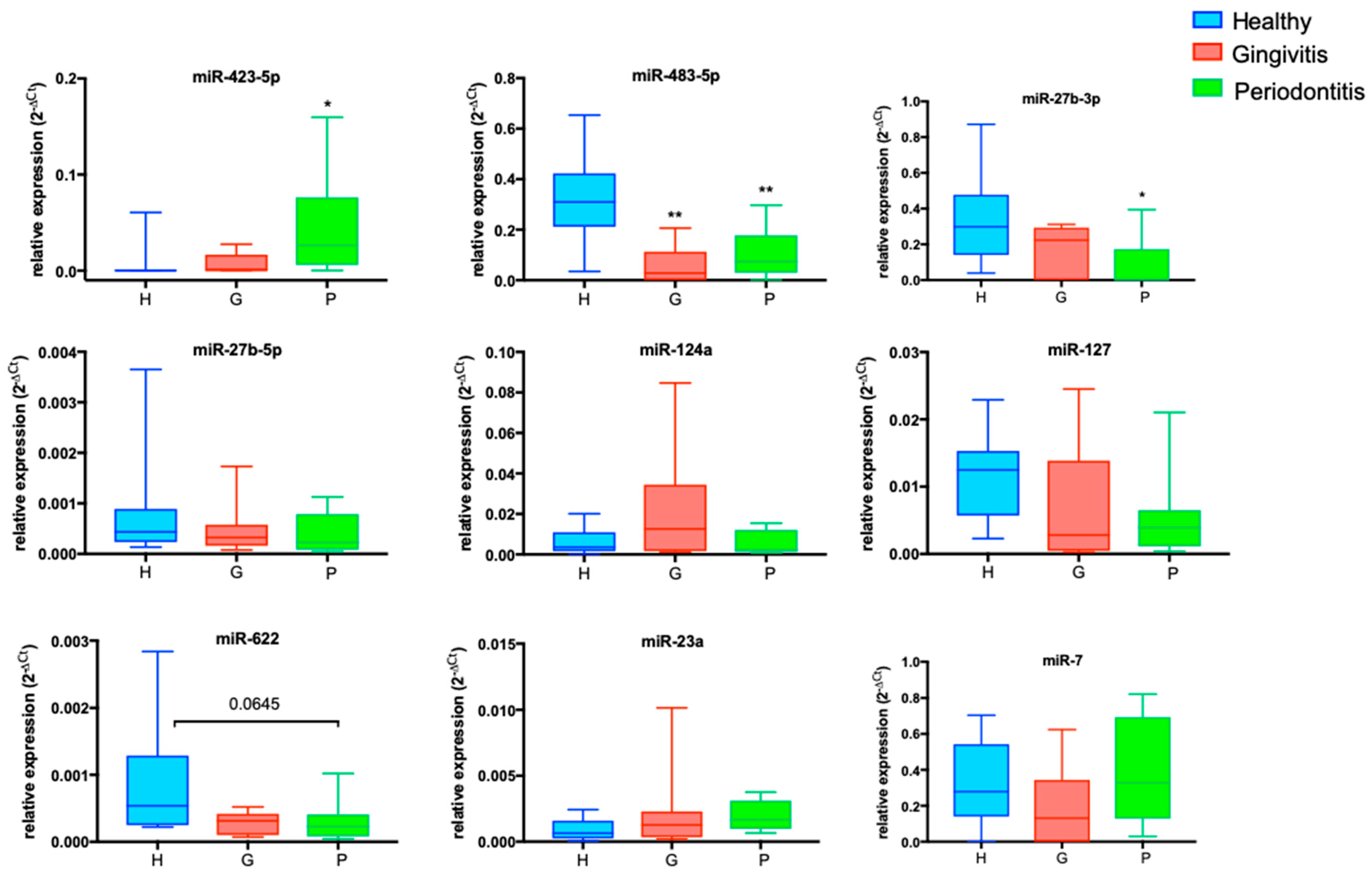
2.4. Differentially Expressed miRNAs in the P-Group: miR-423-5p and miR-27b-3p
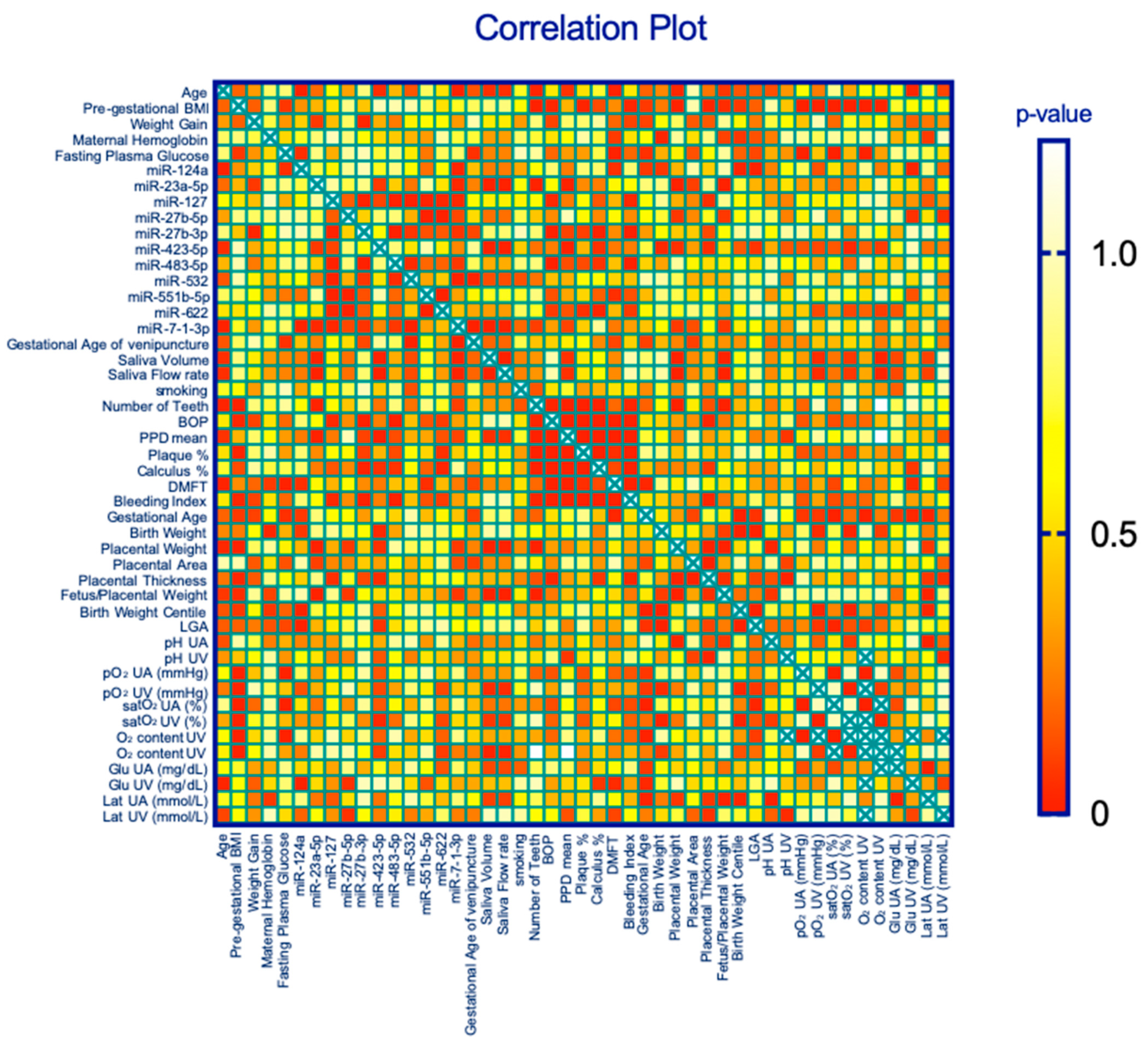
2.5. Correlations between miRNAs and Maternal Periodontal Health Parameters
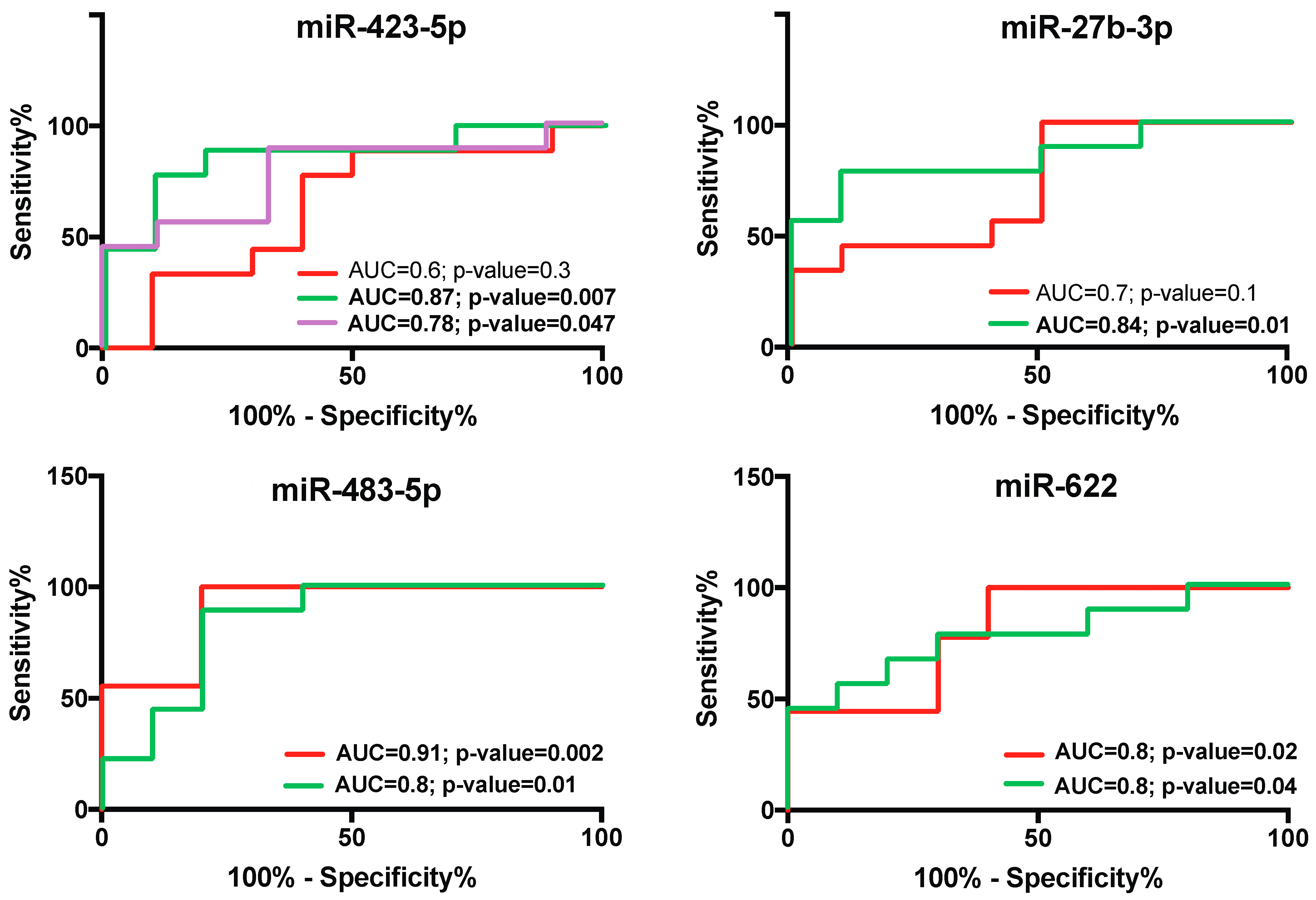
2.6. Diagnostic Specificity and Sensitivity of Salivary miR-423-5p, miR-483-5p, and miR-27b-3p for Periodontal Diseases during Pregnancy
3. Materials and Methods
3.1. Study Participants
3.2. Biological Sample Collection
3.3. Isolation of Extracellular Vesicles (EVs) and miRNA Detection
3.4. Data Analysis and Statistics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kristiansson, P.; Wang, J.X. Reproductive hormones and blood pressure during pregnancy. Hum. Reprod. 2001, 16, 13–17. [Google Scholar] [CrossRef]
- Savona-Ventura, C.; Mahmood, T.; Mukhopadhyay, S.; Louwen, F. Omega-3 fatty acid supply in pregnancy for risk reduction of preterm and early preterm birth: A position statement by the European Board and College of Obstetrics and Gynaecology (EBCOG). Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 295, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, W.; Li, J.; Cui, L.; Chen, Z.J. Periodontal Disease and Adverse Neonatal Outcomes: A Systematic Review and Meta-Analysis. Front. Pediatr. 2022, 10, 799740. [Google Scholar] [CrossRef]
- Dasanayake, A.P.; Gennaro, S.; Hendricks-Munoz, K.D.; Chhun, N. Maternal periodontal disease, pregnancy, and neonatal outcomes. MCN Am. J. Matern. Child. Nurs. 2008, 33, 45–49. [Google Scholar] [CrossRef]
- Xie, Y.; Xiong, X.; Elkind-Hirsch, K.E.; Pridjian, G.; Maney, P.; Delarosa, R.L.; Buekens, P. Change of periodontal disease status during and after pregnancy. J. Periodontol. 2013, 84, 725–731. [Google Scholar] [CrossRef]
- Raju, K.; Berens, L. Periodontology and pregnancy: An overview of biomedical and epidemiological evidence. Periodontol. 2000 2021, 87, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Pileri, P.; Villa, A.; Calabrese, S.; Ottolenghi, L.; Abati, S. Pathogenic mechanisms linking periodontal diseases with adverse pregnancy outcomes. Reprod. Sci. 2012, 19, 633–641. [Google Scholar] [CrossRef]
- Tsuchida, S.; Nakayama, T. Recent Clinical Treatment and Basic Research on the Alveolar Bone. Biomedicines 2023, 11, 843. [Google Scholar] [CrossRef] [PubMed]
- Larvin, H.; Kang, J.; Aggarwal, V.R.; Pavitt, S.; Wu, J. Risk of incident cardiovascular disease in people with periodontal disease: A systematic review and meta-analysis. Clin. Exp. Dent. Res. 2021, 7, 109–122. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Lo Giudice, A. Analysis of galectin-3 levels as a source of coronary heart disease risk during periodontitis. J. Periodontal Res. 2021, 56, 597–605. [Google Scholar] [CrossRef]
- Leng, Y.; Hu, Q.; Ling, Q.; Yao, X.; Liu, M.; Chen, J.; Yan, Z.; Dai, Q. Periodontal disease is associated with the risk of cardiovascular disease independent of sex: A meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1114927. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, I.Z.; Debrey, S.; Oladubu, M.; Ugarte, R. Markers of systemic bacterial exposure in periodontal disease and cardiovascular disease risk: A systematic review and meta-analysis. J. Periodontol. 2007, 78, 2289–2302. [Google Scholar] [CrossRef] [PubMed]
- Foratori-Junior, G.A.; da Silva, B.M.; da Silva Pinto, A.C.; Honorio, H.M.; Groppo, F.C.; de Carvalho Sales-Peres, S.H. Systemic and periodontal conditions of overweight/obese patients during pregnancy and after delivery: A prospective cohort. Clin. Oral Investig. 2020, 24, 157–165. [Google Scholar] [CrossRef]
- Ye, C.; Kapila, Y. Oral microbiome shifts during pregnancy and adverse pregnancy outcomes: Hormonal and Immunologic changes at play. Periodontol. 2000 2021, 87, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef]
- Stewart, R.; West, M. Increasing Evidence for an Association Between Periodontitis and Cardiovascular Disease. Circulation 2016, 133, 549–551. [Google Scholar] [CrossRef]
- Zambon, M.; Mando, C.; Lissoni, A.; Anelli, G.M.; Novielli, C.; Cardellicchio, M.; Leone, R.; Monari, M.N.; Massari, M.; Cetin, I.; et al. Inflammatory and Oxidative Responses in Pregnancies With Obesity and Periodontal Disease. Reprod. Sci. 2018, 25, 1474–1484. [Google Scholar] [CrossRef]
- Kononen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- Gurav, A.N. The implication of periodontitis in vascular endothelial dysfunction. Eur. J. Clin. Investig. 2014, 44, 1000–1009. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- La Sala, L.; Tagliabue, E.; Mrakic-Sposta, S.; Uccellatore, A.C.; Senesi, P.; Terruzzi, I.; Trabucchi, E.; Rossi-Bernardi, L.; Luzi, L. Lower miR-21/ROS/HNE levels associate with lower glycemia after habit-intervention: DIAPASON study 1-year later. Cardiovasc. Diabetol. 2022, 21, 35. [Google Scholar] [CrossRef]
- Garavelli, S.; Bruzzaniti, S.; Tagliabue, E.; Prattichizzo, F.; Di Silvestre, D.; Perna, F.; La Sala, L.; Ceriello, A.; Mozzillo, E.; Fattorusso, V.; et al. Blood Co-Circulating Extracellular microRNAs and Immune Cell Subsets Associate with Type 1 Diabetes Severity. Int. J. Mol. Sci. 2020, 21, 477. [Google Scholar] [CrossRef]
- Mando, C.; Abati, S.; Anelli, G.M.; Favero, C.; Serati, A.; Dioni, L.; Zambon, M.; Albetti, B.; Bollati, V.; Cetin, I. Epigenetic Profiling in the Saliva of Obese Pregnant Women. Nutrients 2022, 14, 2122. [Google Scholar] [CrossRef]
- Lusardi, T.A.; Phillips, J.I.; Wiedrick, J.T.; Harrington, C.A.; Lind, B.; Lapidus, J.A.; Quinn, J.F.; Saugstad, J.A. MicroRNAs in Human Cerebrospinal Fluid as Biomarkers for Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 1223–1233. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.Y.; Lee, Y.M.; Lee, Y.K. MicroRNAs as biomarkers for dental diseases. Singap. Dent. J. 2015, 36, 18–22. [Google Scholar] [CrossRef]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol. 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef]
- Gorr, S.U. Antimicrobial peptides in periodontal innate defense. Front. Oral Biol. 2012, 15, 84–98. [Google Scholar] [CrossRef]
- Kapur, V.K.; Wilsdon, A.G.; Au, D.; Avdalovic, M.; Enright, P.; Fan, V.S.; Hansel, N.N.; Heckbert, S.R.; Jiang, R.; Krishnan, J.A.; et al. Obesity is associated with a lower resting oxygen saturation in the ambulatory elderly: Results from the cardiovascular health study. Respir. Care 2013, 58, 831–837. [Google Scholar] [CrossRef]
- Bianchi, C.; Taricco, E.; Cardellicchio, M.; Mando, C.; Massari, M.; Savasi, V.; Cetin, I. The role of obesity and gestational diabetes on placental size and fetal oxygenation. Placenta 2021, 103, 59–63. [Google Scholar] [CrossRef]
- Santana, D.D.; Kac, G.; Dos Santos, P.P.T.; da Silva, T.C.; Benaim, C.; Cocate, P.G.; Trindade de Castro, M.B.; Heitmann, B.L.; Adegboye, A.R.A. Association between Pre-Pregnancy BMI and Inflammatory Profile Trajectories during Pregnancy and Postpartum in Brazilian Women with Periodontitis: The IMPROVE Trial. Int. J. Environ. Res. Public Health 2022, 19, 2705. [Google Scholar] [CrossRef]
- Gao, S.; Tian, J.; Li, Y.; Liu, T.; Li, R.; Yang, L.; Xing, Z. Periodontitis and Number of Teeth in the Risk of Coronary Heart Disease: An Updated Meta-Analysis. Med. Sci. Monit. 2021, 27, e930112. [Google Scholar] [CrossRef]
- Serati, A.; Novielli, C.; Anelli, G.M.; Mandalari, M.; Parisi, F.; Cetin, I.; Paleari, R.; Mando, C. Characterization of Maternal Circulating MicroRNAs in Obese Pregnancies and Gestational Diabetes Mellitus. Antioxidants 2023, 12, 515. [Google Scholar] [CrossRef]
- Drewlo, S.; Levytska, K.; Sobel, M.; Baczyk, D.; Lye, S.J.; Kingdom, J.C. Heparin promotes soluble VEGF receptor expression in human placental villi to impair endothelial VEGF signaling. J. Thromb. Haemost. 2011, 9, 2486–2497. [Google Scholar] [CrossRef]
- Diceglie, C.; Anelli, G.M.; Martelli, C.; Serati, A.; Lo Dico, A.; Lisso, F.; Parisi, F.; Novielli, C.; Paleari, R.; Cetin, I.; et al. Placental Antioxidant Defenses and Autophagy-Related Genes in Maternal Obesity and Gestational Diabetes Mellitus. Nutrients 2021, 13, 1303. [Google Scholar] [CrossRef]
- Audette, M.C.; Levytska, K.; Lye, S.J.; Melamed, N.; Kingdom, J.C. Parental ethnicity and placental maternal vascular malperfusion pathology in healthy nulliparous women. Placenta 2018, 66, 40–46. [Google Scholar] [CrossRef]
- Baptiste-Roberts, K.; Salafia, C.M.; Nicholson, W.K.; Duggan, A.; Wang, N.Y.; Brancati, F.L. Maternal risk factors for abnormal placental growth: The national collaborative perinatal project. BMC Pregnancy Childbirth 2008, 8, 44. [Google Scholar] [CrossRef]
- Page, R.C.; Eke, P.I. Case Definitions for Use in Population-Based Surveillance of Periodontitis. J. Periodontol. 2007, 78 (Suppl. 7S), 1387–1399. [Google Scholar] [CrossRef]
- Irwandi, R.A.; Vacharaksa, A. The role of microRNA in periodontal tissue: A review of the literature. Arch. Oral Biol. 2016, 72, 66–74. [Google Scholar] [CrossRef]
- Dabelea, D.; Crume, T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes 2011, 60, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M. The inheritance of cardiovascular disease risk. Acta Paediatr. 2019, 108, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, O.; Roland, M.C.; Zucknick, M.; Reine, T.M.; Kolset, S.O.; Henriksen, T.; Lekva, T.; Michelsen, T. Maternal body mass index and placental weight: A role for fetal insulin, maternal insulin and leptin. J. Endocrinol. Investig. 2022, 45, 2105–2121. [Google Scholar] [CrossRef] [PubMed]
- Perri, R.; Nares, S.; Zhang, S.; Barros, S.P.; Offenbacher, S. MicroRNA modulation in obesity and periodontitis. J. Dent. Res. 2012, 91, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lapehn, S.; Paquette, A.G. The Placental Epigenome as a Molecular Link Between Prenatal Exposures and Fetal Health Outcomes Through the DOHaD Hypothesis. Curr. Environ. Health Rep. 2022, 9, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Celik, D.; Kantarci, A. Vascular Changes and Hypoxia in Periodontal Disease as a Link to Systemic Complications. Pathogens 2021, 10, 1280. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhao, Y.; Sun, H.; Xu, J.; Li, W.; Gao, L. MiR-423-5p activated by E2F1 promotes neovascularization in diabetic retinopathy by targeting HIPK2. Diabetol. Metab. Syndr. 2021, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.V.; Gusar, V.A.; Kan, N.E.; Prozorovskaya, K.N.; Karapetyan, A.O.; Bayev, O.R.; Chagovets, V.V.; Kliver, S.F.; Iakovishina, D.Y.; Frankevich, V.E.; et al. Identification of potential early biomarkers of preeclampsia. Placenta 2018, 61, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Costantini, E.; Sinjari, B.; Di Giovanni, P.; Aielli, L.; Caputi, S.; Muraro, R.; Murmura, G.; Reale, M. TNFalpha, IL-6, miR-103a-3p, miR-423-5p, miR-23a-3p, miR-15a-5p and miR-223-3p in the crevicular fluid of periodontopathic patients correlate with each other and at different stages of the disease. Sci. Rep. 2023, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Al Gashaamy, Z.J.; Alomar, T.; Al-Sinjary, L.; Wazzan, M.; Saeed, M.H.; Al-Rawi, N.H. MicroRNA expression in apical periodontitis and pulpal inflammation: A systematic review. PeerJ 2023, 11, e14949. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zhu, S.; Li, X.; Weng, J.; Chen, S. miR-27b-3p Suppressed Osteogenic Differentiation of Maxillary Sinus Membrane Stem Cells by Targeting Sp7. Implant Dent. 2017, 26, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, Q.; Yang, Q.; Yu, Y.; Meng, M.; Zou, J. Epigenetic regulation of osteogenic differentiation of periodontal ligament stem cells in periodontitis. Oral Dis. 2023, 29, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Garavelli, S.; Bruzzaniti, S.; Tagliabue, E.; Di Silvestre, D.; Prattichizzo, F.; Mozzillo, E.; Fattorusso, V.; La Sala, L.; Ceriello, A.; Puca, A.A.; et al. Plasma circulating miR-23~27~24 clusters correlate with the immunometabolic derangement and predict C-peptide loss in children with type 1 diabetes. Diabetologia 2020, 63, 2699–2712. [Google Scholar] [CrossRef]
- Zhou, Q.; Gallagher, R.; Ufret-Vincenty, R.; Li, X.; Olson, E.N.; Wang, S. Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23~27~24 clusters. Proc Natl Acad Sci USA. 2011, 108, 8287–8292. [Google Scholar] [CrossRef] [PubMed]
| Healthy (H) (n = 10) | With Gingivitis (G) (n = 9) | With Periodontitis (P) (n = 9) | Overall p Value | H-G p Value | H-P p Value | G-P p Value | |
|---|---|---|---|---|---|---|---|
| Maternal data | |||||||
| Age B, years | 29.6 ± 4.93 | 31.9 ± 3.65 | 34.8 ± 3.87 | 0.04 | 0.5 | 0.033 | 0.3 |
| Pregestational BMI BC, kg/m2 | 26.5 ± 7.59 | 28.3 ± 7.85 | 31.4 ± 11.52 | 0.51 | 0.9 | 0.5 | 0.7 |
| NW, n (%) OB, n (%) | 6 (60.0) 4 (40.0) | 4 (44.4) 5 (55.6) | 4 (44.4) 5 (55.6) | ||||
| Pregestational hypertension C, n (%) | 2 (20.0) | 0 (0.0) | 2 (22.2) | ||||
| Gestational diabetes (GDM) C, n (%) | 1 (10.0) | 5 (55.6) | 3 (33.3) | ||||
| Fasting glycemia B, mg/dl | 87.7 ± 12.1 | 84.9 ± 9.90 | 89.5 ± 15.7 | 0.74 | 0.9 | 0.9 | 0.7 |
| 1-hour postload glycemia B, mg/dl | 111.8 ± 37.5 | 136.0 ± 39.1 | 132.3 ± 52.6 | 0.43 | 0.5 | 0.6 | 0.9 |
| 2-hour postload glycemia B, mg/dl | 92.0 ± 21.3 | 138.7 ± 46.3 | 108.0 ± 43.6 | 0.04 | 0.03 | 0.6 | 0.2 |
| Gestational age at sampling A, weeks | 33.1 ± 2.33 | 33.3 ± 2.62 | 33.3 ± 1.95 | 0.97 | 0.99 | 0.99 | 1.0 |
| Number of teeth B | 27.9 ± 0.33 | 27.0 ± 1.50 | 24.3 ± 3.54 | 0.004 | 0.6 | 0.004 | 0.04 |
| Gingival BOP B, % sites | 6.35 ± 14.5 | 23.2 ± 19.3 | 59.6 ± 25.7 | <0.0001 | 0.18 | <0.0001 | 0.002 |
| Gingival PPD B, mm | 2.01 ± 0.12 | 2.25 ± 0.25 | 3.30 ± 0.61 | <0.0001 | 0.37 | <0.0001 | <0.0001 |
| Plaque index A, % | 11.1 ± 18.2 | 39.7 ± 18.0 | 68.0 ± 33.3 | 0.0001 | 0.04 | <0.0001 | 0.04 |
| Calculus A, % | 9.92 ± 16.5 | 29.3 ± 14.1 | 69.2 ± 25.2 | <0.0001 | 0.09 | <0.0001 | 0.0005 |
| Dental health index (DMFT) A | 5.56 ± 3.68 | 5.33 ± 3.00 | 9.78 ± 4.15 | 0.02 | 0.99 | 0.04 | 0.04 |
| Maternal, Placental, and Neonatal Data at Delivery | |||||||
| Gestational age A, weeks | 39.4 ± 1.03 | 39.5 ± 0.91 | 39.9 ± 1.27 | 0.6 | 0.9 | 0.6 | 0.7 |
| Maternal GWG B, kg | 10.8 ± 7.00 | 11.8 ± 3.93 | 11.8 ± 5.02 | 0.9 | 0.9 | 0.9 | 1 |
| Neonatal weight A, g | 3369.5 ± 380.2 | 3357.8 ± 315.0 | 3480.6 ± 353.4 | 0.7 | 0.99 | 0.8 | 0.7 |
| Neonatal weight centile AC | 50.7 ± 29.7 | 55.0 ± 27.0 | 56.7 ± 31.5 | 0.9 | 0.95 | 0.9 | 0.99 |
| AGA, n (%) LGA, n (%) | 8 (80.0) 2 (20.0) | 8 (88.9) 1 (11.1) | 6 (66.7) 3 (33.3) | ||||
| Neonatal sex C M, n (%) F, n (%) | 6 (60.0) 4 (40.0) | 3 (33.3) 6 (66.7) | 3 (33.3) 6 (66.7) | ||||
| Placental weight A, g | 450.8 ± 81.4 | 470.0 ± 77.8 | 526.7 ± 104.8 | 0.2 | 0.9 | 0.2 | 0.4 |
| F/P weight ratio B | 7.71 ± 1.62 | 7.32 ± 1.40 | 6.81 ± 1.40 | 0.4 | 0.8 | 0.4 | 0.7 |
| Placental area B, cm2 | 278.9 ± 55.3 | 276.4 ± 57.4 | 248.2 ± 39.1 | 0.4 | 1.0 | 0.4 | 0.5 |
| Placental thickness A, cm | 1.67 ± 0.45 | 1.74 ± 0.32 | 2.19 ± 0.57 | 0.04 | 0.9 | 0.05 | 0.1 |
| (A) | ||||||||
| Neonatal Outcomes | mir-23a-5p | mir-423-5p | mir-127 | mir-124a | ||||
| p value | p value | p value | p value | |||||
| Neonatal Weight (kg) | - | 0.034 | - | - | ||||
| Placental Weight (kg) | 0.003 | 0.055 | - | - | ||||
| Placenta Thickness (cm) | - | 0.043 | 0.050 | - | ||||
| Fetus–Placental Weight (Ratio) | 0.013 | - | - | - | ||||
| Weight Newborn Centile | - | - | - | 0.016 | ||||
| LGA Newborn | - | 0.063 | - | 0.007 | ||||
| (B) | ||||||||
| Neonatal Outcomes | Maternal Oral Outcomes | |||||||
| N teeth | BOP | DMFT | ||||||
| Gestational Age (GA) | - | - | 0.03 | |||||
| r (rho) | - | - | −0.39 | |||||
| Placental Weight (PW, kg) | 0.00 | - | - | |||||
| r (rho) | −0.59 | - | - | |||||
| Placental Thickness (PT, cm) | - | 0.03 | - | |||||
| r (rho) | - | 0.44 | - | |||||
| Weight Fetus/Placenta | 0.00 | - | - | |||||
| r (rho) | 0.59 | - | - | |||||
| (C) | ||||||||
| Maternal Oral Outcomes | miRs | |||||||
| 23a-5p | 423-5p | 7-1-3p | 127 | 27b-3p | 483-5p | 551b-5p | 622 | |
| p value | p value | p value | p value | p value | p value | p value | p value | |
| Saliva Volume | 0.005 | 0.001 | 0.009 | - | - | - | - | - |
| Flow Rate (mL/min) | 0.005 | 0.001 | 0.009 | - | - | - | - | - |
| Number of Teeth (nT) | 0.016 | - | - | - | - | - | - | - |
| BOP | - | - | - | 0.033 | 0.000 | 0.004 | - | - |
| PPD | 0.008 | 0.001 | - | - | - | - | - | - |
| % Plaque | - | - | - | - | - | - | 0.053 | |
| % Calculus | - | 0.056 | - | - | 0.006 | - | - | 0.019 |
| DMFT | - | - | - | - | - | - | 0.051 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Sala, L.; Carlini, V.; Mandò, C.; Anelli, G.M.; Pontiroli, A.E.; Trabucchi, E.; Cetin, I.; Abati, S. Maternal Salivary miR-423-5p Is Linked to Neonatal Outcomes and Periodontal Status in Cardiovascular-High-Risk Pregnancies. Int. J. Mol. Sci. 2024, 25, 9087. https://doi.org/10.3390/ijms25169087
La Sala L, Carlini V, Mandò C, Anelli GM, Pontiroli AE, Trabucchi E, Cetin I, Abati S. Maternal Salivary miR-423-5p Is Linked to Neonatal Outcomes and Periodontal Status in Cardiovascular-High-Risk Pregnancies. International Journal of Molecular Sciences. 2024; 25(16):9087. https://doi.org/10.3390/ijms25169087
Chicago/Turabian StyleLa Sala, Lucia, Valentina Carlini, Chiara Mandò, Gaia Maria Anelli, Antonio E. Pontiroli, Emilio Trabucchi, Irene Cetin, and Silvio Abati. 2024. "Maternal Salivary miR-423-5p Is Linked to Neonatal Outcomes and Periodontal Status in Cardiovascular-High-Risk Pregnancies" International Journal of Molecular Sciences 25, no. 16: 9087. https://doi.org/10.3390/ijms25169087
APA StyleLa Sala, L., Carlini, V., Mandò, C., Anelli, G. M., Pontiroli, A. E., Trabucchi, E., Cetin, I., & Abati, S. (2024). Maternal Salivary miR-423-5p Is Linked to Neonatal Outcomes and Periodontal Status in Cardiovascular-High-Risk Pregnancies. International Journal of Molecular Sciences, 25(16), 9087. https://doi.org/10.3390/ijms25169087







