Hsp70 Negatively Regulates Autophagy via Governing AMPK Activation, and Dual Hsp70-Autophagy Inhibition Induces Synergetic Cell Death in NSCLC Cells
Abstract
:1. Introduction
2. Results
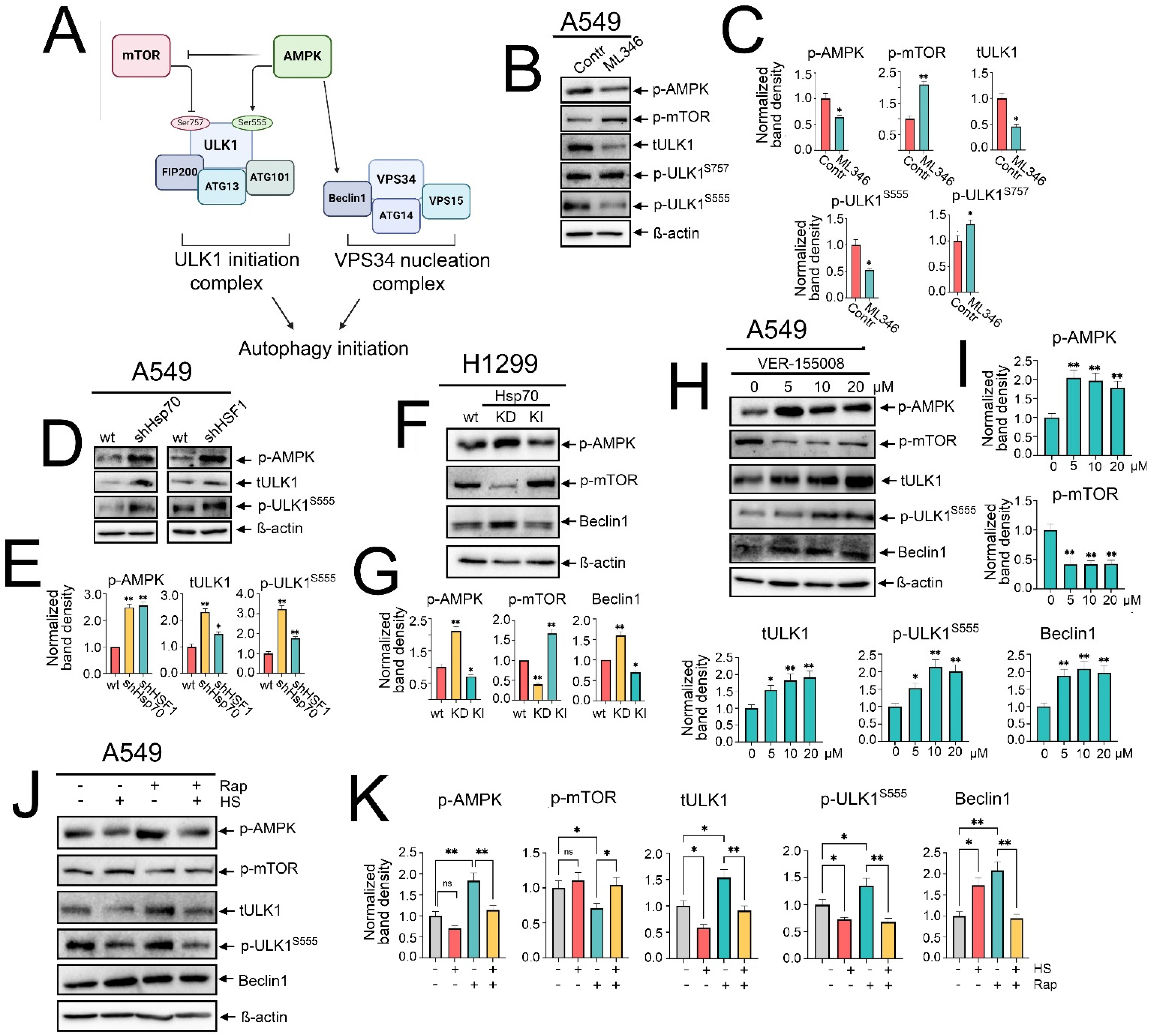
2.1. Autophagy Is Differentially Regulated upon Heat Shock Exposure or Hsp70 Chemical Hyperactivation in NSCLC Cells
2.2. HSF1 Knockdown and Genetic or Chemical Inhibition of Hsp70 Activate Autophagy, While Genetic Hsp70 Overexpression Suppresses Autophagy in NSCLC Cells
2.3. Rapamycin-Induced Autophagy Downregulates Hsp70 Levels, While Inhibition of Autophagy by SAR405 Does Not Influence the Levels of Hsp70 in NSCLC Cells
2.4. Hsp70 Orchestrates the Activation of Autophagy by Upregulating mTOR, Suppressing AMPK and ULK1/Beclin1 Activation
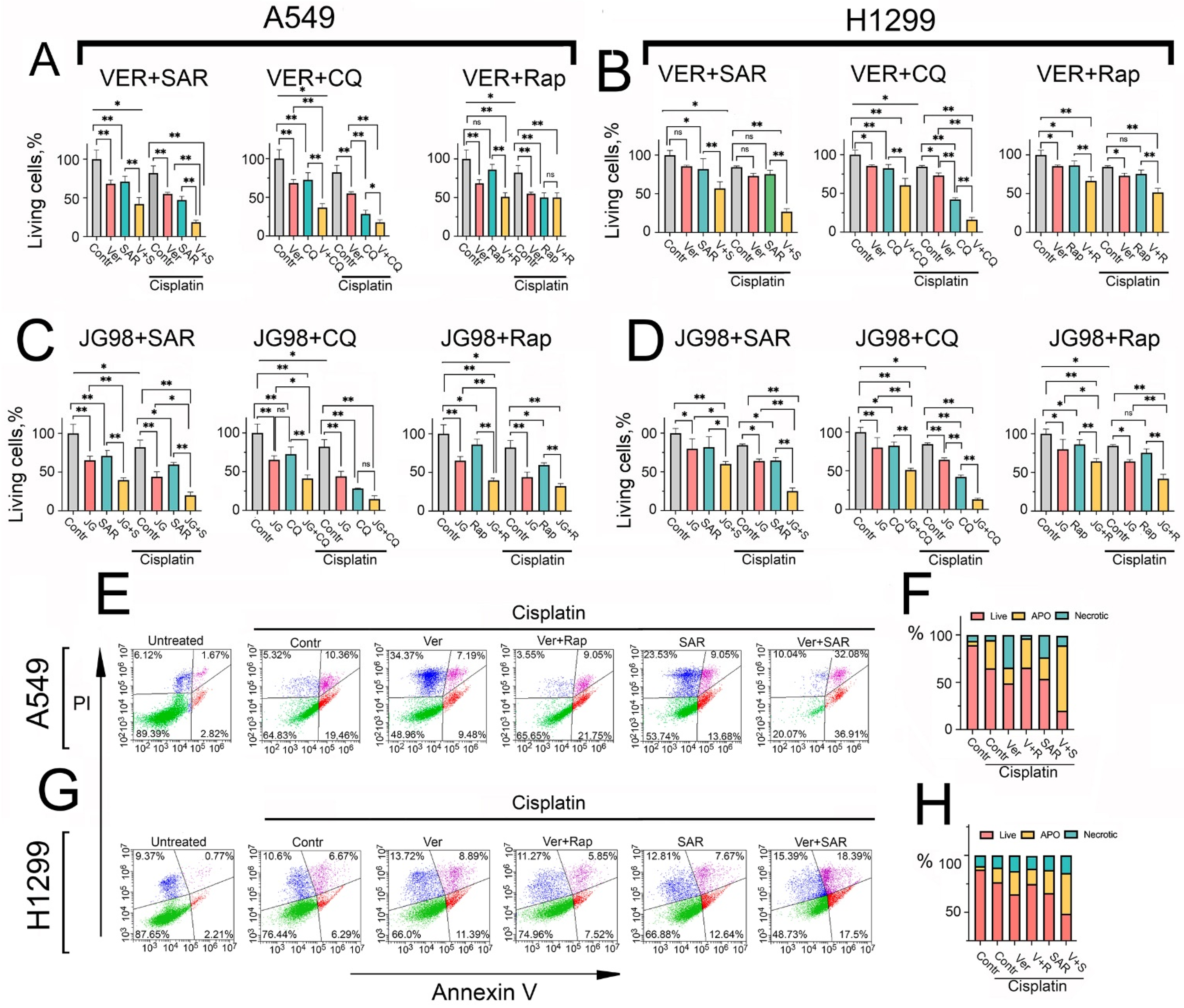
2.5. The Combined Inhibition of Hsp70 and Autophagy Synergistically Enhances Cisplatin-Induced Apoptotic Cell Death in NSCLC Cells
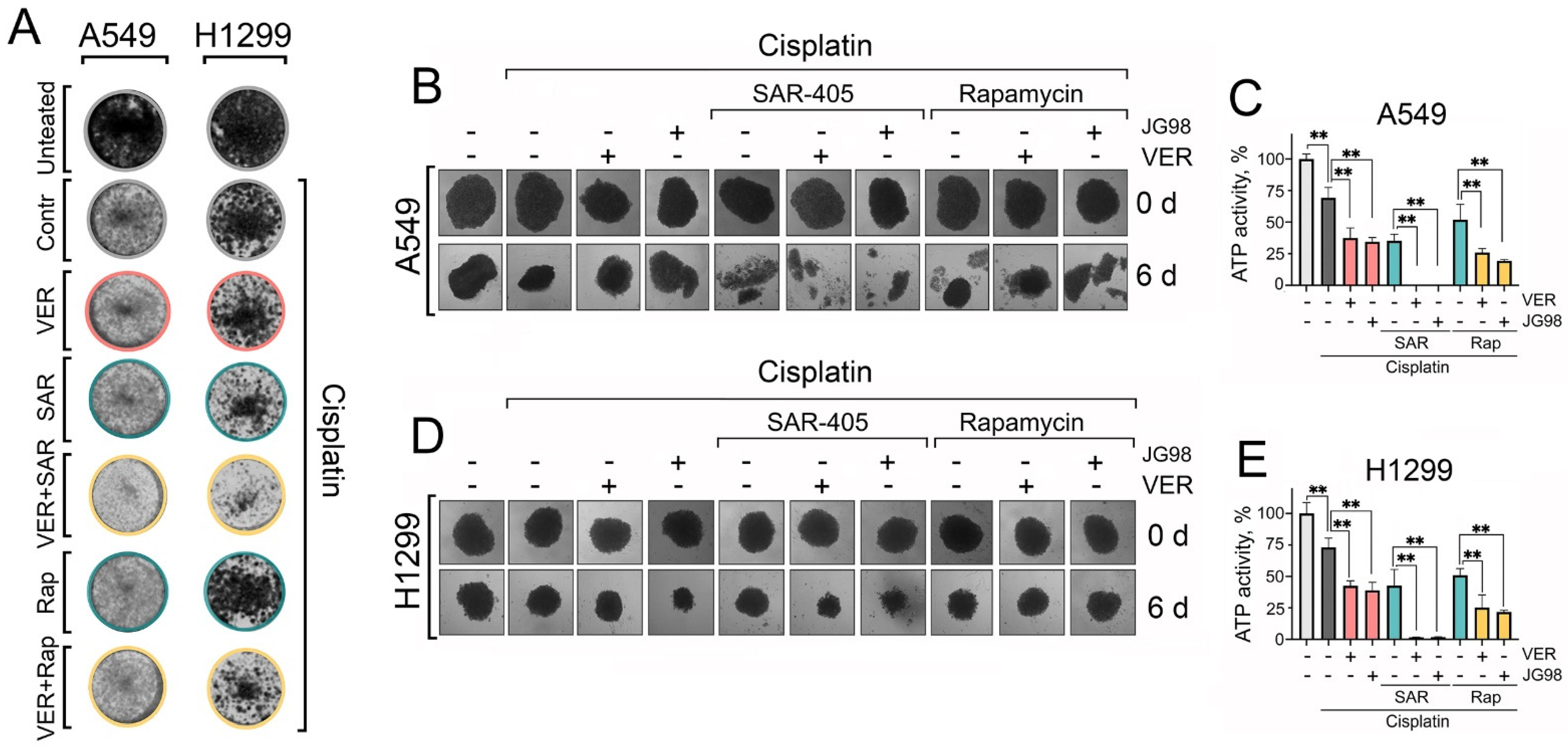
2.6. Dual Targeting of Hsp70 and Autophagy Provokes Substantial Cell Death in Three-Dimensional (3D) Spheroids of NSCLC Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Plasmids
4.3. Chemicals and Drug Treatments
4.4. Heat Shock Treatment
4.5. Western Blot Analysis
4.6. Hsp70 Substrate-Binding Assay
4.7. Cell Viability Assay (MTT Assay)
4.8. Analysis of Drugs Combination Index
4.9. Real-Time PCR
4.10. Colony Formation Assay
4.11. Annexin V/PI Staining Apoptotic Assay
4.12. CellTiter-Glo 3D-Spheroid Viability Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | Adenosine monophosphate-activated protein kinase |
| ATG5 | Autophagy-related protein 5 |
| ATG7 | Autophagy-related protein 7 |
| CMLA | Carboxymethylated lactalbumin |
| CQ | Chloroquine |
| HS | Heat-shock |
| HSF1 | Heat-shock factor 1 |
| Hsp70 | Heat-shock protein 70 kDa |
| HSR | Heat-shock response |
| LC3 I/II | Microtubule-associated protein 1A/1B-light chain 3 I/II |
| mTOR | Mechanistic target of rapamycin |
| NSCLC | Non-small cell lung cancer |
| p62 | Sequestosome-1/ubiquitin-binding protein 62 kDa |
| PSR | Proteotoxic-stress response |
| PIK3C3/VPS34 | Phosphatidylinositol 3-kinase catalytic subunit type 3/vacuolar protein-sorting 34 |
| ULK1 | Unc-51-like autophagy-activating kinase 1 |
| UPR | Unfolded protein response |
| UPS | Ubiquitin–proteasome system |
References
- Liang, R.; Tan, H.; Jin, H.; Wang, J.; Tang, Z.; Lu, X. The tumour-promoting role of protein homeostasis: Implications for cancer immunotherapy. Cancer Lett. 2023, 573, 216354. [Google Scholar] [CrossRef] [PubMed]
- Margulis, B.; Tsimokha, A.; Zubova, S.; Guzhova, I. Molecular Chaperones and Proteolytic Machineries Regulate Protein Homeostasis In Aging Cells. Cells 2020, 9, 1308. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Shen, T.; Fang, S.-J.; Sun, X.-M.; Li, G.-Y.; Li, Y.-F. Protein homeostasis in aging and cancer. Front. Cell Dev. Biol. 2023, 11, 1143532. [Google Scholar] [CrossRef] [PubMed]
- Alhasan, B.A.; Morozov, A.V.; Guzhova, I.V.; Margulis, B.A. The ubiquitin-proteasome system in the regulation of tumor dormancy and recurrence. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2024, 1879, 189119. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Kim, H.J.; Lim, J.H.; Lee, S.H. Heat shock proteins: Agents of cancer development and therapeutic targets in anti-cancer therapy. Cells 2019, 9, 60. [Google Scholar] [CrossRef]
- Wang, X.; Lee, J.; Xie, C. Autophagy Regulation on Cancer Stem Cell Maintenance, Metastasis, and Therapy Resistance. Cancers 2022, 14, 381. [Google Scholar] [CrossRef]
- Dai, C.; Sampson, S.B. HSF1: Guardian of Proteostasis in Cancer. Trends Cell Biol. 2016, 26, 17–28. [Google Scholar] [CrossRef]
- Cyran, A.M.; Zhitkovich, A. Heat Shock Proteins and HSF1 in Cancer. Front. Oncol. 2022, 12, 860320. [Google Scholar] [CrossRef]
- Ambrose, A.J.; Chapman, E. Function, therapeutic potential, and inhibition of Hsp70 chaperones. J. Med. Chem. 2021, 64, 7060–7082. [Google Scholar] [CrossRef]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Pedro, J.M.B.; Cadwell, K.; Cecconi, F.; Choi, A.M.K.; et al. Autophagy in major human diseases. EMBO J. 2021, 40, e108863. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Ro, S.-H.; Cao, J.; Otto, N.M.; Kim, D.-H. mTOR regulation of autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Albakova, Z.; Armeev, G.A.; Kanevskiy, L.M.; Kovalenko, E.I.; Sapozhnikov, A.M. HSP70 Multi-Functionality in Cancer. Cells 2020, 9, 587. [Google Scholar] [CrossRef]
- Alhasan, B.; Mikeladze, M.; Guzhova, I.; Margulis, B. Autophagy, molecular chaperones, and unfolded protein response as promoters of tumor recurrence. Cancer Metastasis Rev. 2023, 42, 217–254. [Google Scholar] [CrossRef]
- Qin, Y.; Ashrafizadeh, M.; Mongiardini, V.; Grimaldi, B.; Crea, F.; Rietdorf, K.; Győrffy, B.; Klionsky, D.J.; Ren, J.; Zhang, W.; et al. Autophagy and cancer drug resistance in dialogue: Pre-clinical and clinical evidence. Cancer Lett. 2023, 570, 216307. [Google Scholar] [CrossRef]
- Bu, F.; Zhang, J.; Shuai, W.; Liu, J.; Sun, Q.; Ouyang, L. Repurposing drugs in autophagy for the treatment of cancer: From bench to bedside. Drug Discov. Today 2022, 27, 1815–1831. [Google Scholar] [CrossRef]
- Lang, B.J.; Prince, T.L.; Okusha, Y.; Bunch, H.; Calderwood, S.K. Heat shock proteins in cell signaling and cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2022, 1869, 119187. [Google Scholar] [CrossRef]
- Sojka, D.R.; Hasterok, S.; Vydra, N.; Toma-jonik, A.; Wieczorek, A.; Gogler-pigłowska, A.; Scieglinska, D. Inhibition of the heat shock protein a (Hspa) family potentiates the anticancer effects of manumycin A. Cells 2021, 10, 1418. [Google Scholar] [CrossRef]
- Lazarev, V.F.; Sverchinsky, D.V.; Mikhaylova, E.R.; Semenyuk, P.I.; Komarova, E.Y.; Niskanen, S.A.; Nikotina, A.D.; Burakov, A.V.; Kartsev, V.G.; Guzhova, I.V.; et al. Sensitizing tumor cells to conventional drugs: HSP70 chaperone inhibitors, their selection and application in cancer models. Cell Death Dis. 2018, 9, 41. [Google Scholar] [CrossRef]
- Sverchinsky, D.V.; Nikotina, A.D.; Komarova, E.Y.; Mikhaylova, E.R.; Aksenov, N.D.; Lazarev, V.F.; Mitkevich, V.A.; Suezov, R.; Druzhilovskiy, D.S.; Poroikov, V.V.; et al. Etoposide-Induced Apoptosis in Cancer Cells Can Be Reinforced by an Uncoupled Link between Hsp70 and Caspase-3. Int. J. Mol. Sci. 2018, 19, 2519. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, Y.; Bai, J.; Yang, Y.; Wang, F.; Feng, Y.; Zhang, R.; Li, F.; Zhang, P.; Lv, N.; et al. Blockade of HSP70 by VER-155008 synergistically enhances bortezomib-induced cytotoxicity in multiple myeloma. Cell Stress Chaperones 2020, 25, 357–367. [Google Scholar] [CrossRef]
- Hyun, S.Y.; Le, H.T.; Min, H.-Y.; Pei, H.; Lim, Y.; Song, I.; Nguyen, Y.T.K.; Hong, S.; Han, B.W.; Lee, H.-Y. Evodiamine inhibits both stem cell and non-stem-cell populations in human cancer cells by targeting heat shock protein 70. Theranostics 2021, 11, 2932–2952. [Google Scholar] [CrossRef] [PubMed]
- Dower, C.M.; Bhat, N.; Gebru, M.T.; Chen, L.; Wills, C.A.; Miller, B.A.; Wang, H.-G. Targeted Inhibition of ULK1 Promotes Apoptosis and Suppresses Tumor Growth and Metastasis in Neuroblastoma. Mol. Cancer Ther. 2018, 17, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, B. SAR405, a PIK3C3/Vps34 inhibitor that prevents autophagy and synergizes with MTOR inhibition in tumor cells. Autophagy 2015, 11, 725–726. [Google Scholar] [CrossRef]
- Anand, K.; Niravath, P.; Patel, T.; Ensor, J.; Rodriguez, A.; Boone, T.; Wong, S.T.; Chang, J.C. A Phase II Study of the Efficacy and Safety of Chloroquine in Combination With Taxanes in the Treatment of Patients With Advanced or Metastatic Anthracycline-refractory Breast Cancer. Clin. Breast Cancer 2021, 21, 199–204. [Google Scholar] [CrossRef]
- Marastoni, S.; Madariaga, A.; Pesic, A.; Nair, S.N.; Li, Z.J.; Shalev, Z.; Ketela, T.; Colombo, I.; Mandilaras, V.; Cabanero, M.; et al. Repurposing Itraconazole and Hydroxychloroquine to Target Lysosomal Homeostasis in Epithelial Ovarian Cancer. Cancer Res. Commun. 2022, 2, 293–306. [Google Scholar] [CrossRef]
- Nikotina, A.D.; Vladimirova, S.A.; Kokoreva, N.E.; Komarova, E.Y.; Aksenov, N.D.; Efremov, S.; Leonova, E.; Pavlov, R.; Kartsev, V.G.; Zhang, Z.; et al. Combined Cytotoxic Effect of Inhibitors of Proteostasis on Human Colon Cancer Cells. Pharmaceuticals 2022, 15, 923. [Google Scholar] [CrossRef] [PubMed]
- Calamini, B.; Silva, M.C.; Madoux, F.; Hutt, D.M.; Khanna, S.; Chalfant, M.A.; Allais, C.; Ouizem, S.; Saldanha, S.A.; Ferguson, J.; et al. ML346: A novel modulator of proteostasis for protein conformational diseases. In Probe Reports from the NIH Molecular Libraries Program [Internet]; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2013. [Google Scholar]
- Komarova, E.Y.; Afanasyeva, E.A.; Bulatova, M.M.; Cheetham, M.E.; Margulis, B.A.; Guzhova, I.V. Downstream caspases are novel targets for the antiapoptotic activity of the molecular chaperone hsp70. Cell Stress Chaperones 2004, 9, 265. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, S.; Shunmei, E.; Zou, J. Induction of macroautophagy by heat. Mol. Biol. Rep. 2009, 36, 2323–2327. [Google Scholar] [CrossRef]
- Liao, Q.; Ozawa, F.; Friess, H.; Zimmermann, A.; Takayama, S.; Reed, J.C.; Kleeff, J.; Büchler, M.W. The anti-apoptotic protein BAG-3 is overexpressed in pancreatic cancer and induced by heat stress in pancreatic cancer cell lines. FEBS Lett. 2001, 503, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kodiha, M.; Rassi, J.G.; Brown, C.M.; Stochaj, U. Localization of AMP kinase is regulated by stress, cell density, and signaling through the MEK→ERK1/2 pathway. Am. J. Physiol.-Cell Physiol. 2007, 293, C1427–C1436. [Google Scholar] [CrossRef]
- Wen, W.; Liu, W.; Shao, Y.; Chen, L. VER-155008, a small molecule inhibitor of HSP70 with potent anti-cancer activity on lung cancer cell lines. Exp. Biol. Med. 2014, 239, 638–645. [Google Scholar] [CrossRef]
- Li, X.; Colvin, T.; Rauch, J.N.; Acosta-Alvear, D.; Kampmann, M.; Dunyak, B.; Hann, B.; Aftab, B.T.; Murnane, M.; Cho, M.; et al. Validation of the Hsp70–Bag3 Protein–Protein Interaction as a Potential Therapeutic Target in Cancer. Mol. Cancer Ther. 2015, 14, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Jiang, J. Profiling of apoptosis- and autophagy-associated molecules in human lung cancer A549 cells in response to cisplatin treatment using stable isotope labeling with amino acids in cell culture. Int. J. Oncol. 2019, 54, 1071–1085. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.; Bentivoglio, V.; Varani, M.; Signore, A. Three-Dimensional In Vitro Tumor Spheroid Models for Evaluation of Anticancer Therapy: Recent Updates. Cancers 2023, 15, 4846. [Google Scholar] [CrossRef]
- Shevtsov, M.; Multhoff, G.; Mikhaylova, E.; Shibata, A.; Guzhova, I.; Margulis, B. Combination of anti-cancer drugs with molecular chaperone inhibitors. Int. J. Mol. Sci. 2019, 20, 5284. [Google Scholar] [CrossRef]
- Su, K.-H.; Cao, J.; Tang, Z.; Dai, S.; He, Y.; Sampson, S.B.; Benjamin, I.J.; Dai, C. HSF1 critically attunes proteotoxic stress sensing by mTORC1 to combat stress and promote growth. Nat. Cell Biol. 2016, 18, 527–539. [Google Scholar] [CrossRef]
- Su, K.-H.; Dai, C. mTORC1 senses stresses: Coupling stress to proteostasis. BioEssays 2017, 39, 1600268. [Google Scholar] [CrossRef]
- Chou, S.-D.; Prince, T.; Gong, J.; Calderwood, S.K. mTOR Is Essential for the Proteotoxic Stress Response, HSF1 Activation and Heat Shock Protein Synthesis. PLoS ONE 2012, 7, e39679. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Tang, Z.; Cao, J.; Zhou, W.; Li, H.; Sampson, S.; Dai, C. Suppression of the HSF1-mediated proteotoxic stress response by the metabolic stress sensor AMPK. EMBO J. 2015, 34, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Su, K.-H.; Dai, S.; Tang, Z.; Xu, M.; Dai, C. Heat shock factor 1 is a direct antagonist of AMP-activated protein kinase. Mol. Cell 2019, 76, 546–561. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rubín-de-Celis, S. The Role of Beclin 1-Dependent Autophagy in Cancer. Biology 2020, 9, 4. [Google Scholar] [CrossRef]
- Zhou, B.; Lu, Q.; Liu, J.; Fan, L.; Wang, Y.; Wei, W.; Wang, H.; Sun, G. Melatonin Increases the Sensitivity of Hepatocellular Carcinoma to Sorafenib through the PERK-ATF4-Beclin1 Pathway. Int. J. Biol. Sci. 2019, 15, 1905–1920. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Li, X.; Cai, Q.; Zhang, C.; Yu, Q.; Jiang, Y.; Lee, J.-H.; Hawke, D.; Wang, Y.; Xia, Y.; et al. Phosphoglycerate Kinase 1 Phosphorylates Beclin1 to Induce Autophagy. Mol. Cell 2017, 65, 917–931.e6. [Google Scholar] [CrossRef]
- Su, H.; Yang, F.; Wang, Q.; Shen, Q.; Huang, J.; Peng, C.; Zhang, Y.; Wan, W.; Wong, C.C.L.; Sun, Q.; et al. VPS34 Acetylation Controls Its Lipid Kinase Activity and the Initiation of Canonical and Non-canonical Autophagy. Mol. Cell 2017, 67, 907–921.e7. [Google Scholar] [CrossRef]
- Das, S.; Shukla, N.; Singh, S.S.; Kushwaha, S.; Shrivastava, R. Mechanism of interaction between autophagy and apoptosis in cancer. Apoptosis 2021, 26, 512–533. [Google Scholar] [CrossRef]
- Sakai, K.; Inoue, M.; Mikami, S.; Nishimura, H.; Kuwabara, Y.; Kojima, A.; Toda, M.; Ogawa-Kobayashi, Y.; Kikuchi, S.; Hirata, Y.; et al. Functional inhibition of heat shock protein 70 by VER-155008 suppresses pleural mesothelioma cell proliferation via an autophagy mechanism. Thorac. Cancer 2021, 12, 491–503. [Google Scholar] [CrossRef]
- Sannino, S.; Guerriero, C.J.; Sabnis, A.J.; Stolz, D.B.; Wallace, C.T.; Wipf, P.; Watkins, S.C.; Bivona, T.G.; Brodsky, J.L. Compensatory increases of select proteostasis networks after Hsp70 inhibition in cancer cells. J. Cell Sci. 2018, 131, jcs217760. [Google Scholar] [CrossRef]
- Sannino, S.; Yates, M.E.; Schurdak, M.E.; Oesterreich, S.; Lee, A.V.; Wipf, P.; Brodsky, J.L. Unique integrated stress response sensors regulate cancer cell susceptibility when Hsp70 activity is compromised. eLife 2021, 10, e64977. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Xia, T.; Gu, M.; Zhang, Z.; Zhang, Q.; Shen, J.; Fan, Y.; Yao, H.; Pan, S.; Lu, Y.; et al. AMPK-mTOR-Mediated Activation of Autophagy Promotes Formation of Dormant Polyploid Giant Cancer Cells. Cancer Res. 2022, 82, 846–858. [Google Scholar] [CrossRef]
- Feng, J.; Xi, Z.; Jiang, X.; Li, Y.; Nik Nabil, W.N.; Liu, M.; Song, Z.; Chen, X.; Zhou, H.; Dong, Q.; et al. Saikosaponin A enhances Docetaxel efficacy by selectively inducing death of dormant prostate cancer cells through excessive autophagy. Cancer Lett. 2023, 554, 216011. [Google Scholar] [CrossRef]
- Nikotina, A.D.; Vladimirova, S.A.; Kokoreva, N.E.; Nevdakha, V.A.; Lazarev, V.F.; Kuznetcova, L.S.; Komarova, E.Y.; Suezov, R.V.; Efremov, S.; Leonova, E.; et al. Novel mechanism of drug resistance triggered by tumor-associated macrophages through Heat Shock Factor-1 activation. Cancer Immunol. Immunother. 2024, 73, 25. [Google Scholar] [CrossRef] [PubMed]
- Dokladny, K.; Zuhl, M.N.; Mandell, M.; Bhattacharya, D.; Schneider, S.; Deretic, V.; Moseley, P.L. Regulatory Coordination between Two Major Intracellular Homeostatic Systems: Heat Shock Response and Autophagy. J. Biol. Chem. 2013, 288, 14959–14972. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Marjaneh, R.; Paseban, M.; Moradi Marjaneh, M. Hsp70 inhibitors: Implications for the treatment of colorectal cancer. IUBMB Life 2019, 71, 1834–1845. [Google Scholar] [CrossRef]
- Goloudina, A.R.; Demidov, O.N.; Garrido, C. Inhibition of HSP70: A challenging anti-cancer strategy. Cancer Lett. 2012, 325, 117–124. [Google Scholar] [CrossRef]
- Ferretti, G.D.S.; Quaas, C.E.; Bertolini, I.; Zuccotti, A.; Saatci, O.; Kashatus, J.A.; Sharmin, S.; Lu, D.Y.; Poli, A.N.R.; Quesnelle, A.F.; et al. HSP70-mediated mitochondrial dynamics and autophagy represent a novel vulnerability in pancreatic cancer. Cell Death Differ. 2024, 31, 881–896. [Google Scholar] [CrossRef]



| Gene of Interest | Forward | Reverse |
|---|---|---|
| Hsp70 | 5′-AGAAGGACATCAGCCAGAACAA-3′ | 5′-AGAAGTCGATGCCCTCAAACA-3′ |
| ULK1 | 5′-CTGCTGGGGAAGGAAATCAAAAT-3′ | 5′-AACCAGGTAGACAGAATTAGCCAT-3′ |
| ATG7 | 5′-AAGCCATGATGTCGTCTTCCTAT-3′ | 5′-GCATTGATGACCAGCTTTCTCTT-3′ |
| p62 | 5′-TCAGGAGGAGATGATGACTGGA-3′ | 5′-TTGGCCCTTCGGATTCTGG-3′ |
| Actin | 5′-CCATCATGAAGTGTGACGTGC-3′ | 5′-GTCCGCCTAGAAGCATTTGCG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhasan, B.; Gladova, Y.A.; Sverchinsky, D.V.; Aksenov, N.D.; Margulis, B.A.; Guzhova, I.V. Hsp70 Negatively Regulates Autophagy via Governing AMPK Activation, and Dual Hsp70-Autophagy Inhibition Induces Synergetic Cell Death in NSCLC Cells. Int. J. Mol. Sci. 2024, 25, 9090. https://doi.org/10.3390/ijms25169090
Alhasan B, Gladova YA, Sverchinsky DV, Aksenov ND, Margulis BA, Guzhova IV. Hsp70 Negatively Regulates Autophagy via Governing AMPK Activation, and Dual Hsp70-Autophagy Inhibition Induces Synergetic Cell Death in NSCLC Cells. International Journal of Molecular Sciences. 2024; 25(16):9090. https://doi.org/10.3390/ijms25169090
Chicago/Turabian StyleAlhasan, Bashar, Yana A. Gladova, Dmitry V. Sverchinsky, Nikolai D. Aksenov, Boris A. Margulis, and Irina V. Guzhova. 2024. "Hsp70 Negatively Regulates Autophagy via Governing AMPK Activation, and Dual Hsp70-Autophagy Inhibition Induces Synergetic Cell Death in NSCLC Cells" International Journal of Molecular Sciences 25, no. 16: 9090. https://doi.org/10.3390/ijms25169090






