NMR Dynamic View of the Stabilization of the WW4 Domain by Neutral NaCl and Kosmotropic Na2SO4 and NaH2PO4
Abstract
:1. Introduction
2. Results
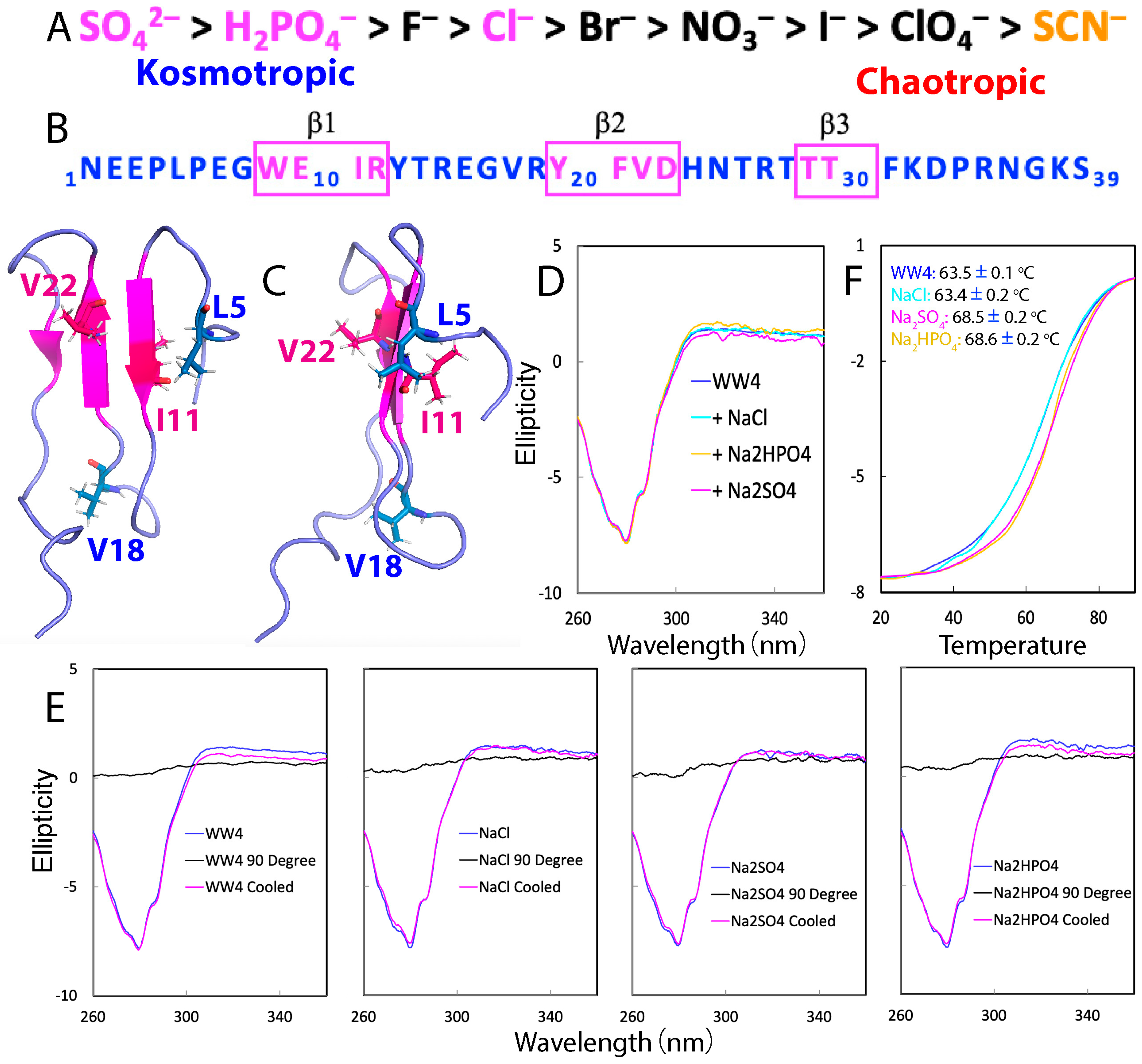
2.1. CD Characterization of the Effects of NaCl, Na2SO4, and Na2HPO4 on WW4
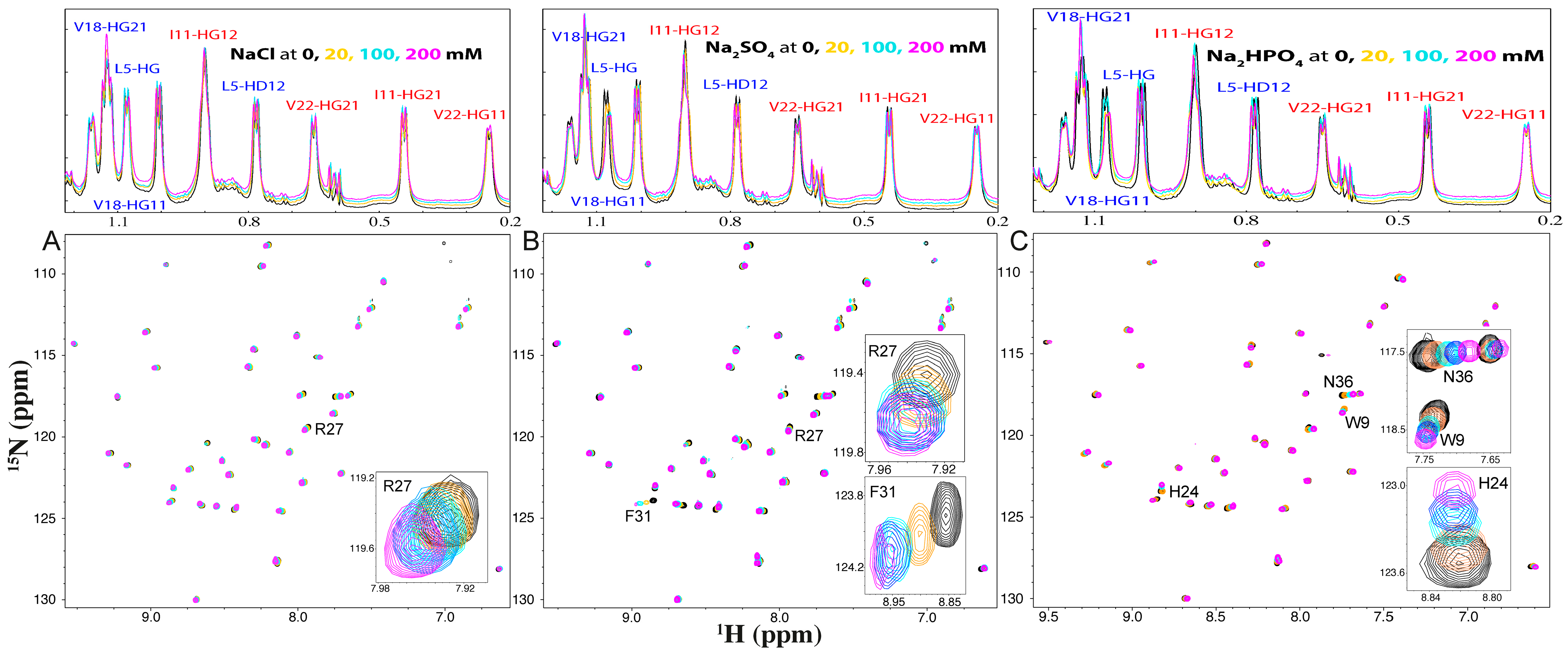
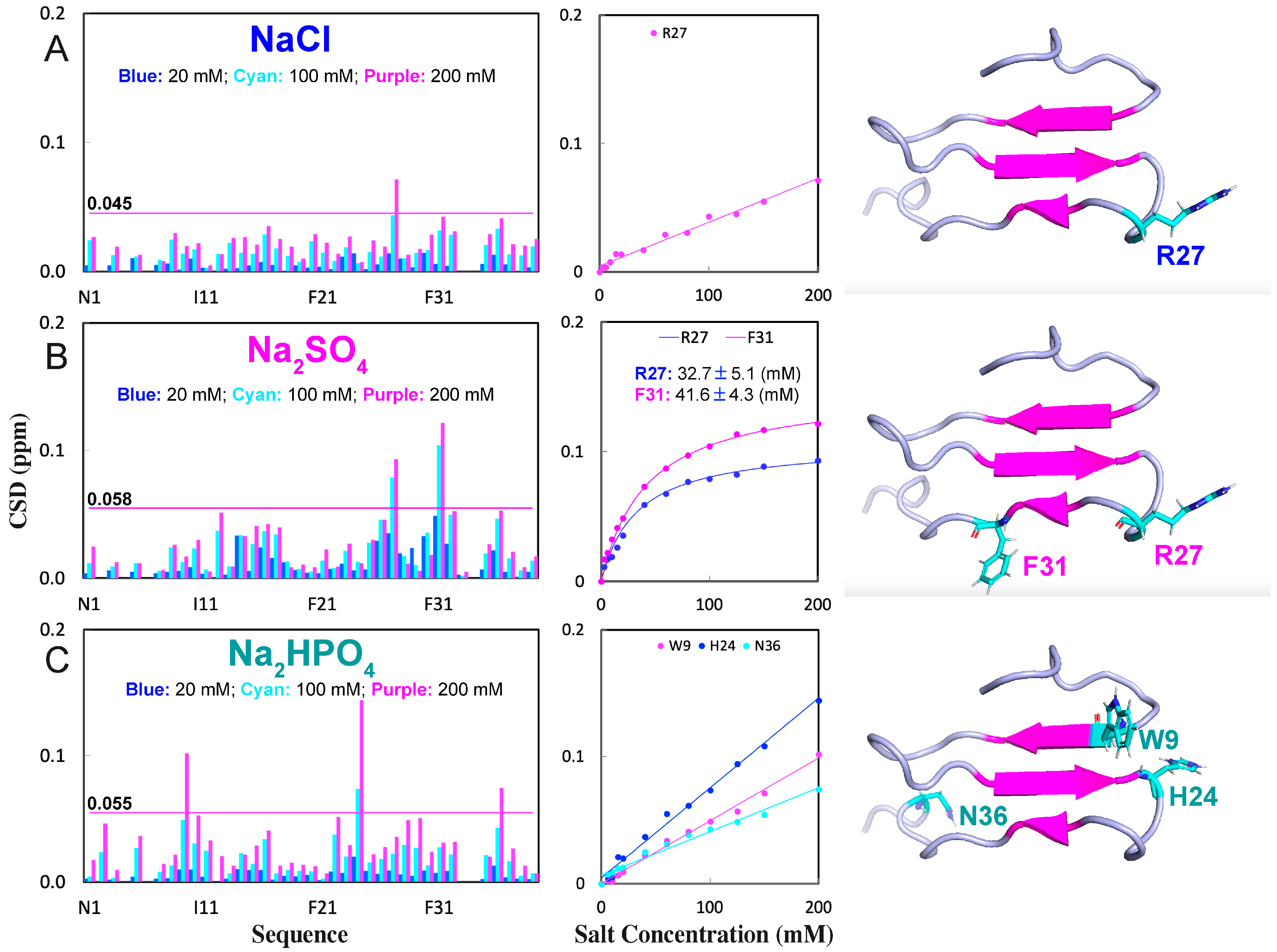
2.2. NMR Characterization of the Binding of NaCl, Na2SO4, and Na2HPO4 with WW4
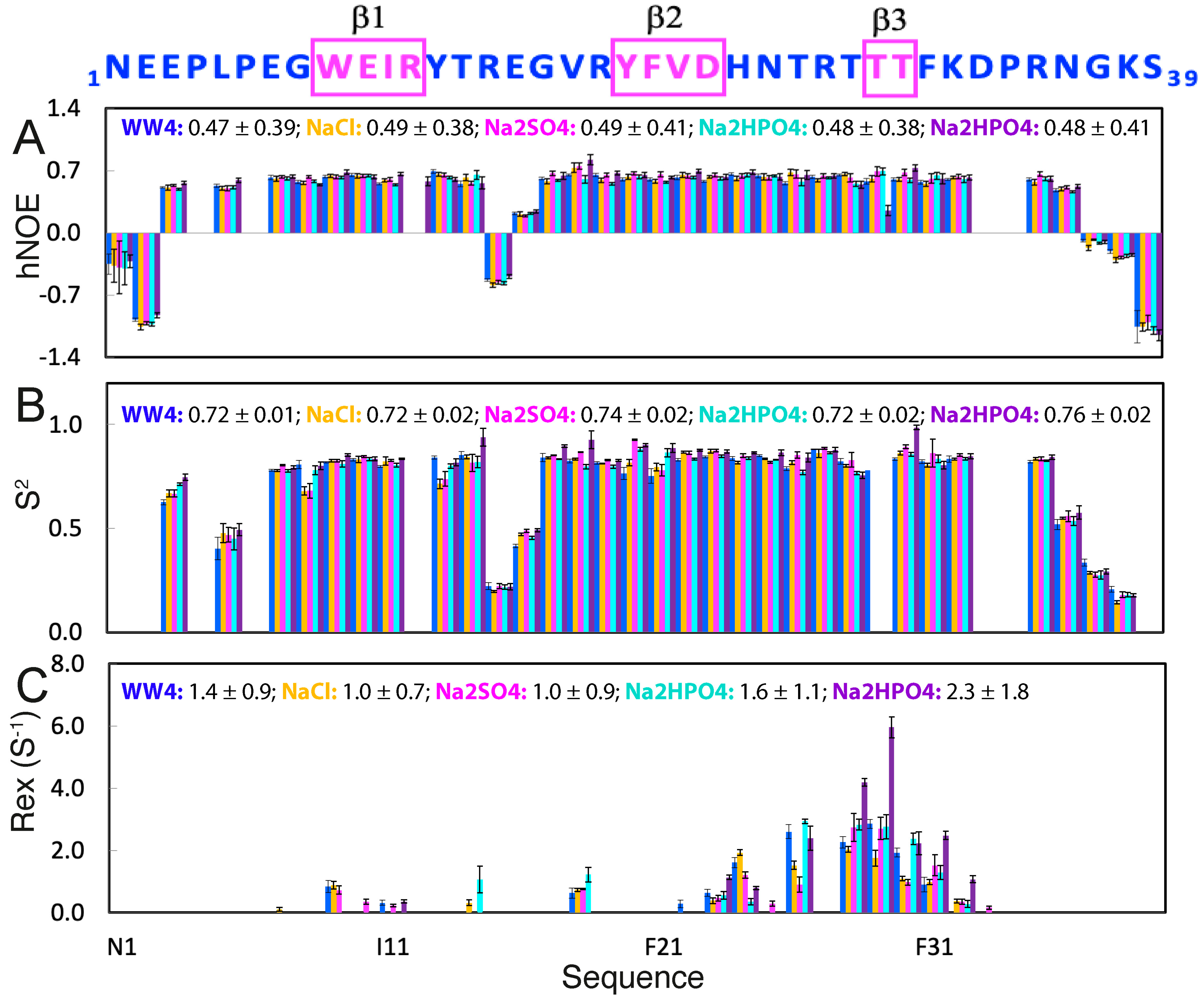
2.3. Effects of NaCl, Na2SO4, and Na2HPO4 on ps-ns Backbone Dynamics of WW4
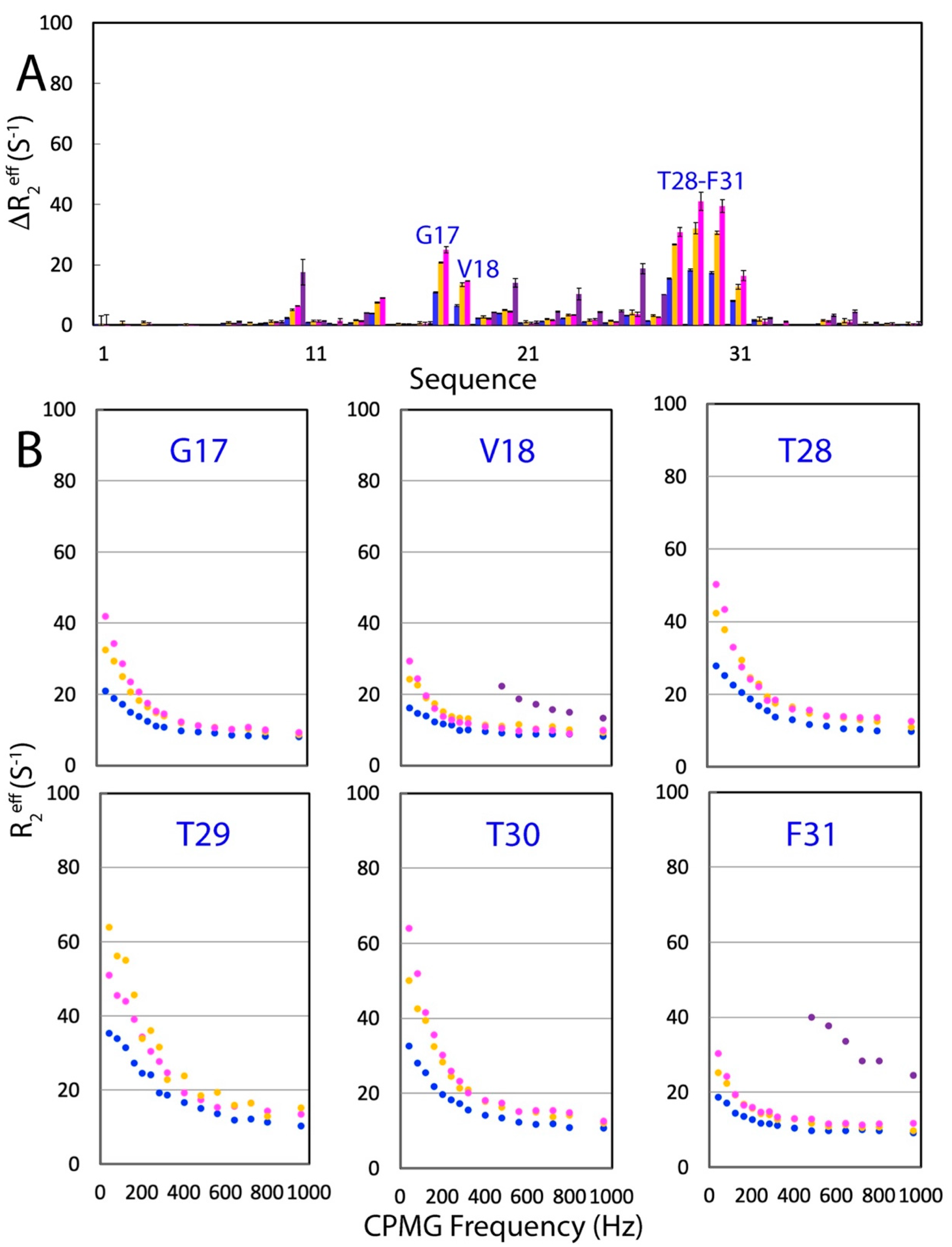
2.4. Effects of NaCl, Na2SO4, and Na2HPO4 on µs-ns Backbone Dynamics of WW4
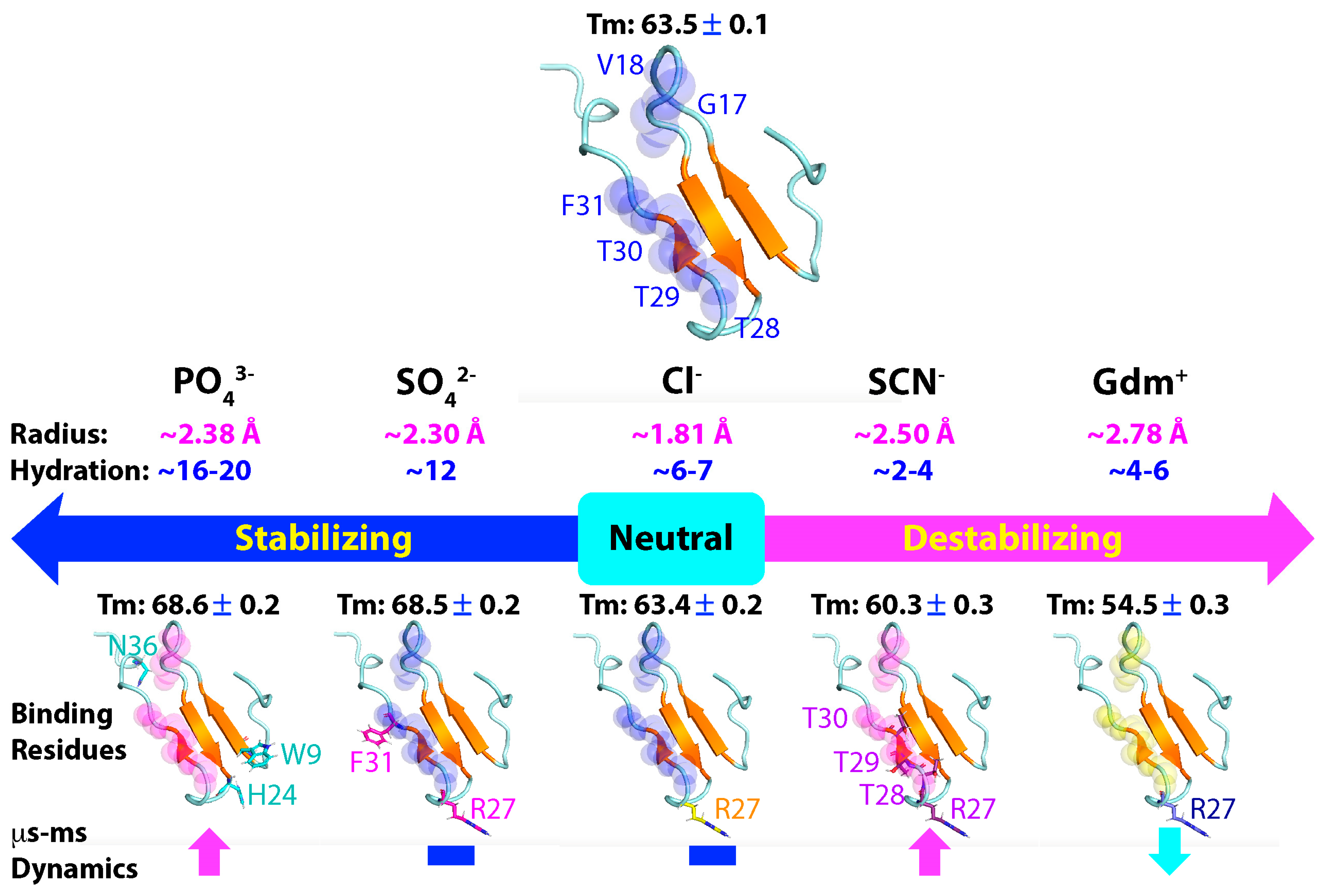
3. Discussion
4. Materials and Methods
4.1. Expression and Purification of WW4
4.2. Circular Dichroism (CD) Experiments
4.3. NMR Titration of NaCl, Na2SO4, and Na2HPO4 to WW4
4.4. Calculation of CSD and Data Fitting to Obtain Kd
4.5. NMR Characterization of 15N Backbone Dynamics on the ps-ns Time Scale
4.6. NMR Characterization of 15N Backbone Dynamics on the µs-ms Time Scale
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anfinsen, C.B. Principles that govern the folding of protein chains. Science 1973, 181, 223–230. [Google Scholar] [CrossRef]
- Baldwin, R.L. Early days of studying the mechanism of protein folding. In Protein Folding Handbook; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 1–23. [Google Scholar]
- Kim, D.E.; Gu, H.; Baker, D. The sequences of small proteins are not extensively optimized for rapid folding by natural selection. Proc. Natl. Acad. Sci. USA 1998, 95, 4982–4986. [Google Scholar] [CrossRef]
- Baldwin, R.L. How Hofmeister ion interactions affect protein stability. Biophys. J. 1996, 4, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Palmer AG 3rd. NMR probes of molecular dynamics: Overview and comparison with other techniques. Annu. Rev. Biophys. Biomol. Struct. 2001, 30, 129–155. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, A.; Kay, L.E. An NMR View of Protein Dynamics in Health and Disease. Annu. Rev. Biophys. 2019, 48, 297–319. [Google Scholar] [CrossRef]
- Gonzalez, N.A.; Li, B.A.; McCully, M.E. The stability and dynamics of computationally designed proteins. Protein Eng. Des. Sel. 2022, 35, gzac001. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2023, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Hofmeister, F. Zur Lehre von der Wirkung der Salze. Arch. Exp. Pathol. Pharmakol. 1888, 24, 247–260. [Google Scholar] [CrossRef]
- ASalis, B.W. Ninham Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 2014, 43, 7358–7377. [Google Scholar]
- Okur, H.I.; Hladílková, J.; Rembert, K.B.; Cho, Y.; Heyda, J.; Dzubiella, J.; Cremer, P.S.; Jungwirth, P. Beyond the Hofmeister Series: Ion-Specific Effects on Proteins and Their Biological Functions. J. Phys. Chem. B 2017, 121, 1997–2014. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.K.; Rosgen, J.; Englander, S.W. Urea, but not guanidinium, destabilizes proteins by forming hydrogen bonds to the peptide group. Proc. Natl. Acad. Sci. USA 2009, 106, 2595–2600. [Google Scholar] [CrossRef] [PubMed]
- Street, T.O.; Bolen, D.W.; Rose, G.D. A molecular mechanism for osmolyte-induced protein stability. Proc. Natl. Acad. Sci. USA 2006, 103, 13997–14002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cremer, P.S. Interactions between macromolecules and ions: The Hofmeister series. Curr. Opin. Chem. Biol. 2006, 10, 658–663. [Google Scholar] [CrossRef]
- Mráček, A.; Varhaníková, J.; Lehocký, M.; Gřundělová, L.; Pokopcová, A.; Velebný, V. The influence of Hofmeister series ions on hyaluronan swelling and viscosity. Molecules 2008, 13, 1025–1034. [Google Scholar] [CrossRef]
- Gregory, K.P.; Wanless, E.J.; Webber, G.B.; Craig, V.S.J.; Page, A.J. The electrostatic origins of specific ion effects: Quantifying the Hofmeister series for anions. Chem. Sci. 2021, 12, 15007–15015. [Google Scholar] [CrossRef]
- Lim, L.-Z.; Song, J. NMR Dynamic View of the Destabilization of WW4 Domain by Chaotropic GdmCl and NaSCN. Int. J. Mol. Sci. 2024, 25, 7344. [Google Scholar] [CrossRef]
- Qin, H.; Pu, H.X.; Li, M.; Ahmed, S.; Song, J. Identification and structural mechanism for a novel interaction between a ubiquitin ligase WWP1 and Nogo-A, a key inhibitor for central nervous system regeneration. Biochemistry 2008, 47, 13647–13658. [Google Scholar] [CrossRef]
- Zuiderweg, E.R. Mapping protein–protein interactions in solution by NMR spectroscopy. Biochemistry 2002, 41, 1–7. [Google Scholar] [CrossRef]
- Williamson, M.P. Using chemical shift perturbation to characterise ligand binding. Prog. Nucl. Magn. Reson. Spectrosc. 2013, 73, 1–16. [Google Scholar] [CrossRef]
- Miao, L.; Qin, H.; Koehl, P.; Song, J. Selective and specific ion binding on proteins at physiologically-relevant concentrations. FEBS Lett. 2011, 585, 3126–3132. [Google Scholar] [CrossRef] [PubMed]
- Kukic, P.; O’Meara, F.; Hewage, C.; Nielsen, J.E. Coupled effect of salt and pH on proteins probed with NMR spectroscopy. Chem. Phys. Lett. 2013, 579, 114–121. [Google Scholar] [CrossRef]
- Kang, J.; Lim, L.; Song, J. ATP binds and inhibits the neurodegeneration-associated fibrillization of the FUS RRM domain. Commun. Biol. 2019, 2, 223. [Google Scholar] [CrossRef]
- Lu, Y.; Lim, L.; Song, J. RRM domain of ALS/FTD-causing FUS characteristic of irreversible unfolding spontaneously self-assembles into amyloid fibrils. Sci. Rep. 2017, 7, 1043. [Google Scholar] [CrossRef]
- Kang, J.; Lim, L.; Song, J. ATP induces folding of ALS-causing C71G-hPFN1 and nascent hSOD1. Commun. Chem. 2023, 6, 186. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Gilquin, B.; Jamin, N.; Drakopoulou, E.; Guenneugues, M.; Dauplais, M.; Vita, C.; Ménez, A. NMR solution structure of a two-disulfide derivative of charybdotoxin: Structural evidence for conservation of scorpion toxin alpha/beta motif and its hydrophobic side chain packing. Biochemistry 1997, 36, 3760–3766. [Google Scholar] [CrossRef]
- Song, J.; Jamin, N.; Gilquin, B.; Vita, C.; Ménez, A. A gradual disruption of tight side-chain packing: 2D 1H-NMR characterization of acid-induced unfolding of CHABII. Nat. Struct. Biol. 1999, 6, 129–134. [Google Scholar] [CrossRef]
- Wei, Z.; Song, J. Molecular mechanism underlying the thermal stability and pH-induced unfolding of CHABII. J. Mol. Biol. 2005, 348, 205–218. [Google Scholar] [CrossRef]
- Zarrine-Afsar, A.; Mittermaier, A.; Kay, L.E.; Davidson, A.R. R Protein stabilization by specific binding of guanidinium to a functional arginine-binding surface on an SH3 domain. Protein Sci. 2006, 15, 162–170. [Google Scholar] [CrossRef]
- Farrow, N.A.; Muhandiram, R.; Singer, A.U.; Pascal, S.M.; Kay, C.M.; Gish, G.; Shoelson, S.E.; Pawson, T.; Forman-Kay, J.D.; Kay, L.E. Backbone dynamics of a free and phosphopeptide-complexed Src homology 2 domain studied by 15N NMR relaxation. Biochemistry 1994, 33, 5984–6003. [Google Scholar] [CrossRef]
- Dyson, H.J.; Wright, P.E. Unfolded proteins and protein folding studied by NMR. Chem. Rev. 2004, 104, 3607–3622. [Google Scholar] [CrossRef] [PubMed]
- Fushman, D.; Cahill, S.; Cowburn, D. The main-chain dynamics of the dynamin pleckstrin homology (PH) domain in solution: Analysis of 15N relaxation with monomer/dimer equilibration. J. Mol. Biol. 1997, 266, 173–194. [Google Scholar] [CrossRef]
- Lipari, G.; Szabo, A. Model-free approach to the interpretation of Nuclear Magnetic Resonance relaxation in macromolecules. 1. Theory and range of validity. J. Am. Chem. Soc. 1982, 104, 4546–4559. [Google Scholar] [CrossRef]
- Clore, G.M.; Driscoll, P.C.; Wingfield, P.T.; Gronenborn, A.M. Analysis of the backbone dynamics of interleukin-1 b using two-dimensional inverse detected heteronuclear 15N–1H NMR spectroscopy. Biochemistry 1990, 29, 7387–7401. [Google Scholar] [CrossRef]
- Qin, H.; Lim, L.; Song, J. Dynamic principle for designing antagonistic/agonistic molecules for EphA4 receptor, the only known ALS modifier. ACS Chem. Biol. 2015, 10, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Lim, L.Z.; Wei, Y.; Song, J. TDP-43 N terminus encodes a novel ubiquitin-like fold and its unfolded form in equilibrium that can be shifted by binding to ssDNA. Proc. Natl. Acad. Sci. USA 2014, 111, 18619–18624. [Google Scholar] [CrossRef] [PubMed]
- Walker, O.; Varadan, R.; Fushman, D. Efficient and accurate determination of the overall rotational diffusion tensor of a molecule from 15N relaxation data using computer program ROTDIF. J. Magn. Reson. 2004, 168, 336–345. [Google Scholar] [CrossRef]
- Hall, J.B.; Fushman, D. Characterization of the overall and local dynamics of a protein with intermediate rotational anisotropy: Differentiating between conformational exchange and anisotropic diffusion in the B3 domain of protein G. J. Biomol. NMR 2003, 27, 261–275. [Google Scholar] [CrossRef]
- Mulder, F.A.; Hon, B.; Mittermaier, A.; Dahlquist, F.W.; Kay, L.E. Slow internal dynamics in proteins: Application of NMR relaxation dispersion spectroscopy to methyl groups in a cavity mutant of T4 lysozyme. J. Am. Chem. Soc. 2002, 124, 1443–1451. [Google Scholar] [CrossRef]
- Palmer, A.G. NMR characterization of the dynamics of biomacromolecules. Chem. Rev. 2004, 104, 3623–3640. [Google Scholar] [CrossRef]
- Kleckner, I.R.; Foster, M.P. An introduction to NMR-based approaches for measuring protein dynamics. Biochim. Biophys. Acta 2011, 1814, 942–968. [Google Scholar] [CrossRef] [PubMed]
- Millet, O.; Loria, J.P.; Kroenke, C.D.; Pons, M.; Palmer, A.G. III The static magnetic field dependence of chemical exchange line broadening defines the NMR chemical shift time scale. J. Am. Chem. Soc. 2000, 122, 2867–2877. [Google Scholar] [CrossRef]
- Kleckner, I.R.; Foster, M.P. GUARDD: User-friendly MATLAB software for rigorous analysis of CPMG RD NMR data. J. Biomol. NMR 2012, 52, 11–22. [Google Scholar] [CrossRef]
- Pfuhl, M.; Chen, H.A.; Kristensen, S.M.; Driscoll, P.C. NMR exchange broadening arising from specific low affinity protein self-association: Analysis of nitrogen-15 nuclear relaxation for rat CD2 domain 1. J. Biomol. NMR 1999, 14, 307–320. [Google Scholar] [CrossRef]
- Akerud, T.; Thulin, E.; Van Etten, R.L.; Akke, M. Intramolecular dynamics of low molecular weight protein tyrosine phosphatase in monomer-dimer equilibrium studied by NMR: A model for changes in dynamics upon target binding. J. Mol. Biol. 2002, 322, 137–152. [Google Scholar] [CrossRef]
- Ragucci, S.; Ruggiero, A.; Russo, R.; Landi, N.; Valletta, M.; Chambery, A.; Russo, L.; Di Maro, A. Correlation of structure; function; protein dynamics in myoglobins from Eurasian woodcock; chicken; ostrich. J. Biomol. Struct. Dyn. 2021, 39, 851–866. [Google Scholar] [CrossRef]
- Nam, K.; Wolf-Watz, M. Protein dynamics: The future is bright and complicated! Struct. Dyn. 2023, 10, 014301. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef]
- Song, J. Why do proteins aggregate? “Intrinsically insoluble proteins” and “dark mediators” revealed by studies on “insoluble proteins” solubilized in pure water. F1000Research 2013, 2, 94. [Google Scholar] [CrossRef]
- Taylor, J.P.; Brown RHJr Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef]
- Silva, J.L.; Foguel, D.; Ferreira, V.F.; Vieira, T.C.R.G.; Marques, M.A.; Ferretti, G.D.S.; Outeiro, T.F.; Cordeiro, Y.; de Oliveira, G.A. Targeting Biomolecular Condensation and Protein Aggregation against Cancer. Chem. Rev. 2023, 123, 9094–9138. [Google Scholar] [CrossRef] [PubMed]
- Diteepeng, T.; Del Monte, F.; Luciani, M. The long and winding road to target protein misfolding in cardiovascular diseases. Eur. J. Clin. Invest. 2021, 51, e13504. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, J.; Ran, X.; Fang, M.; Shi, J.; Qin, H.; Goh, J.M.; Song, J. Resurrecting abandoned proteins with pure water: CD and NMR studies of protein fragments solubilized in salt-free water. Biophys. J. 2006, 91, 4201–4209. [Google Scholar] [CrossRef] [PubMed]
- Pinna, S.; Kunz, C.; Halpern, A.; Harrison, S.A.; Jordan, S.F.; Ward, J.; Werner, F.; Lane, N. A prebiotic basis for ATP as the universal energy currency. PLoS Biol. 2022, 20, e3001437. [Google Scholar] [CrossRef]
- Leningher, A. Principles of Biochemistry; W. H. Freeman and Company: New York, NY, USA, 2005. [Google Scholar]
- Patel, A.; Malinovska, L.; Saha, S.; Wang, J.; Alberti, S.; Krishnan, Y.; Hyman, A.A. ATP as a biological hydrotrope. Science 2017, 356, 753–756. [Google Scholar] [CrossRef]
- Rice, A.M.; Rosen, M.K. ATP controls the crowd. Science 2017, 356, 701–702. [Google Scholar] [CrossRef]
- Song, J. ATP energy-independently controls protein homeostasis with unique structure and diverse mechanisms. Protein Sci. 2021, 30, 1277–1293. [Google Scholar] [CrossRef] [PubMed]
- Song, J. Adenosine Triphosphate: The Primordial Molecule That Controls Protein Homeostasis and Shapes the Genome–Proteome Interface. Biomolecules 2024, 14, 500. [Google Scholar] [CrossRef]
- Collins, K.D.; Washabaugh, M.W. The Hofmeister effect and the behaviour of water at interfaces. Q Rev. Biophys. 1985, 18, 323–422. [Google Scholar] [CrossRef]
- Marcus, Y. Effect of Ions on the Structure of Water: Structure Making and Breaking. Chem. Rev. 2009, 109, 1346–1370. [Google Scholar] [CrossRef]
- England, J.L.; Haran, G. Role of solvation effects in protein denaturation: From thermodynamics to single molecules and back. Annu. Rev. Phys. Chem. 2011, 62, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Mason PE, Neilson GW, Dempsey CE, Barnes AC, Cruickshank JM The hydration structure of guanidinium and thiocyanate ions: Implications for protein stability in aqueous solution. Proc. Natl. Acad. Sci. USA 2003, 100, 4557–4561. [CrossRef]
- Scott, J.N.; Nucci, N.V.; Vanderkooi, J.M. Changes in water structure induced by the guanidinium cation and implications for protein denaturation. J. Phys. Chem. A 2008, 112, 10939–10948. [Google Scholar] [CrossRef]
- Gregory, K.P.; Elliott, G.R.; Robertson, H.; Kumar, A.; Wanless, E.J.; Webber, G.B.; Craig, V.S.J.; Andersson, G.G.; Page, A.J. Understanding specific ion effects and the Hofmeister series. Phys. Chem. Chem. Phys. 2022, 24, 12682–12718. [Google Scholar] [CrossRef]
- O’Brien, E.P.; Dima, R.I.; Brooks, B.; Thirumalai, D. Interactions between hydrophobic and ionic solutes in aqueous guanidinium chloride and urea solutions: Lessons for protein denaturation mechanism. J. Am. Chem. Soc. 2007, 129, 7346–7353. [Google Scholar] [CrossRef] [PubMed]
- Kubíčková, A.; Křížek, T.; Coufal, P.; Wernersson, E.; Heyda, J.; Jungwirth, P. Guanidinium cations pair with positively charged arginine side chains in water. J. Phys. Chem. Lett. 2011, 2, 1387–1389. [Google Scholar] [CrossRef]
- Bennion, B.J.; Daggett, V. The molecular basis for the chemical denaturation of proteins by urea. Proc. Natl. Acad. Sci. USA 2003, 100, 5142–5147. [Google Scholar] [CrossRef]
- TiradoRives, J.; Orozco, M.; Jorgensen, W.L. Molecular dynamics simulations of the unfolding of barnase in water and 8 m aqueous urea. Biochemistry 1997, 36, 7313–7329. [Google Scholar] [CrossRef]
- Caflisch, A.; Karplus, M. Structural details of urea binding to barnase: A molecular dynamics analysis. Structure 1999, 7, 477–488. [Google Scholar] [CrossRef]
- Jencks, W.P. On the attribution and additivity of binding energies. Proc. Natl. Acad. Sci. USA 1981, 78, 4046. [Google Scholar] [CrossRef]
- Ryde, U. A fundamental view of enthalpy–entropy compensation. Med. Chem. Commun. 2014, 5, 1324–1336. [Google Scholar] [CrossRef]
- Baldwin, R.L. Dynamic hydration shell restores Kauzmann’s 1959 explanation of how the hydrophobic factor drives protein folding. Proc. Natl. Acad. Sci. USA 2014, 111, 12056–13052. [Google Scholar] [CrossRef]
- Levy, Y.; Onuchic, J.N. Water mediation in protein folding and molecular recognition. Annu. Rev. Biophys. Biomol. Struct. 2006, 35, 389–415. [Google Scholar] [CrossRef] [PubMed]
- Ball, P. Water as an active constituent in cell biology. Chem. Rev. 2008, 108, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Song, J. Insight into “insoluble proteins” with pure water. FEBS Lett. 2009, 583, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Bellissent-Funel, M.C.; Hassanali, A.; Havenith, M.; Henchman, R.; Pohl, P.; Sterpone, F.; Van Der Spoel, D.; Xu, Y.; Garcia, A.E. Water determines the structure and dynamics of proteins. Chem. Rev. 2016, 116, 7673–7697. [Google Scholar] [CrossRef]
- Laage, D.; Elsaesser, T.; Hynes, J.T. Water dynamics in the hydration shells of biomolecules. Chem. Rev. 2017, 117, 10694–10725. [Google Scholar] [CrossRef]
- Mazza, M.G.; Stokely, K.; Pagnotta, S.E.; Bruni, F.; Stanley, H.E.; Franzese, G. More than one dynamic crossover in protein hydration water. Proc. Natl. Acad. Sci. USA 2011, 108, 19873–19878. [Google Scholar] [CrossRef]
- Roche, J.; Caro, J.A.; Norberto, D.R.; Barthe, P.; Roumestand, C.; Schlessman, J.L.; Garcia, A.E.; García-Moreno, E.B.; Royer, C.A. Cavities determine the pressure unfolding of proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 6945–6950. [Google Scholar] [CrossRef]
- Spyracopoulos, L. Thermodynamic interpretation of protein dynamics from NMR relaxation measurements. Protein Pept. Lett. 2005, 12, 235–240. [Google Scholar] [CrossRef]
- Doan-Nguyen, V.; Loria, J.P. The effects of cosolutes on protein dynamics: The reversal of denaturant-induced protein fluctuations by trimethylamine N-oxide. Protein Sci. 2007, 16, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Kamerzell, T.J.; Middaugh, C.R. The complex inter-relationships between protein flexibility and stability. J. Pharm. Sci. 2008, 97, 3494–3517. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, D.; Libera, V.; Tombari, E.; Peters, J.; Natali, F.; Petrillo, C.; Comez, L.; Capaccioli, S.; Paciaroni, A. Dynamic Personality of Proteins and Effect of the Molecular Environment. J. Phys. Chem. Lett. 2024, 15, 5543–5548. [Google Scholar] [CrossRef]
- Gray, M.J.; Wholey, W.-Y.; Wagner, N.O.; Cremers, C.M.; Mueller-Schickert, A.; Hock, N.T.; Krieger, A.G.; Smith, E.M.; Bender, R.A.; Bardwell, J.C.; et al. Polyphosphate is a primordial chaperone. Mol. Cell 2014, 53, 689–699. [Google Scholar] [CrossRef]
- Rao, N.N.; Gómez-García, M.R.; Kornberg, A. Inorganic polyphosphate: Essential for growth and survival. Annu. Rev. Biochem. 2009, 78, 605–647. [Google Scholar] [CrossRef]
- Delaglio, F.; Grzesiek, S.; Vuister, G.W.; Zhu, G.; Pfeifer, J.; Bax, A. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 1995, 6, 277. [Google Scholar] [CrossRef]
- Johnson, B.A.; Blevins, R.A. NMRView: A computer program for the visualization and analysis of NMR data. J. Biomol. NMR 1994, 4, 603. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Roldan, J.L.; Blackledge, M.; Jensen, M.R. Characterizing Protein-Protein Interactions Using Solution NMR Spectroscopy. Methods Mol. Biol. 2018, 1764, 73–85. [Google Scholar]
- Giri, M.; Gupta, P.; Maulik, A.; Gracias, M.; Singh, M. Structure and DNA binding analysis of AT-rich interaction domain present in human BAF-B specific subunit BAF250b. Protein Sci. 2022, 31, e4294. [Google Scholar] [CrossRef]
- Bottini, A.; Wu, B.; Barile, E.; De, S.K.; Leone, M.; Pellecchia, M. High-Throughput Screening (HTS) by NMR Guided Identification of Novel Agents Targeting the Protein Docking Domain of YopH. ChemMedChem 2016, 11, 919–927. [Google Scholar] [CrossRef]
- Fan, D.; Zheng, Y.; Yang, D.; Wang, J. NMR solution structure and dynamics of an exchangeable apolipoprotein, Locusta migratoria apolipophorin III. J. Biol. Chem. 2003, 278, 21212. [Google Scholar] [CrossRef]
- Ran, X.; Qin, H.; Liu, J.; Fan, J.S.; Shi, J.; Song, J. NMR structure and dynamics of human ephrin-B2 ectodomain: The functionally critical C-D and G-H loops are highly dynamic in solution. Proteins 2008, 72, 1019. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, L.-Z.; Song, J. NMR Dynamic View of the Stabilization of the WW4 Domain by Neutral NaCl and Kosmotropic Na2SO4 and NaH2PO4. Int. J. Mol. Sci. 2024, 25, 9091. https://doi.org/10.3390/ijms25169091
Lim L-Z, Song J. NMR Dynamic View of the Stabilization of the WW4 Domain by Neutral NaCl and Kosmotropic Na2SO4 and NaH2PO4. International Journal of Molecular Sciences. 2024; 25(16):9091. https://doi.org/10.3390/ijms25169091
Chicago/Turabian StyleLim, Liang-Zhong, and Jianxing Song. 2024. "NMR Dynamic View of the Stabilization of the WW4 Domain by Neutral NaCl and Kosmotropic Na2SO4 and NaH2PO4" International Journal of Molecular Sciences 25, no. 16: 9091. https://doi.org/10.3390/ijms25169091




