S100B Serum Levels in Chronic Heart Failure Patients: A Multifaceted Biomarker Linking Cardiac and Cognitive Dysfunction
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
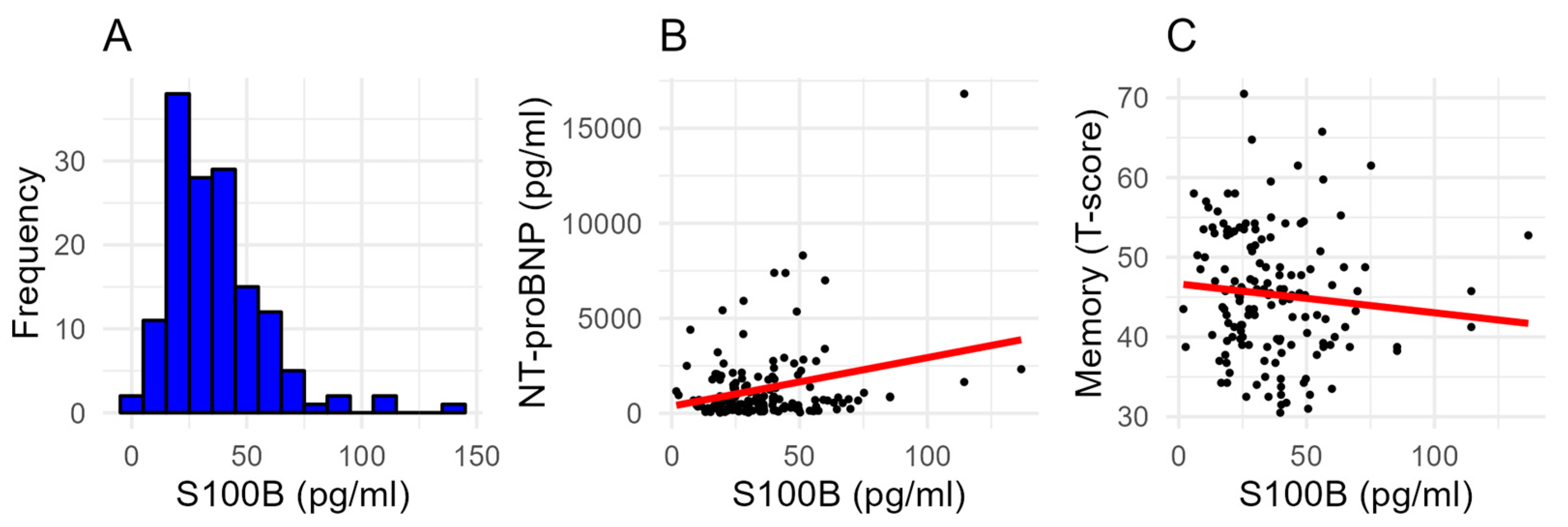
2.2. S100B Serum Levels
2.3. Association of Clinical Variables with S100B Serum Levels
2.4. Predictors of Mild Cognitive Impairment
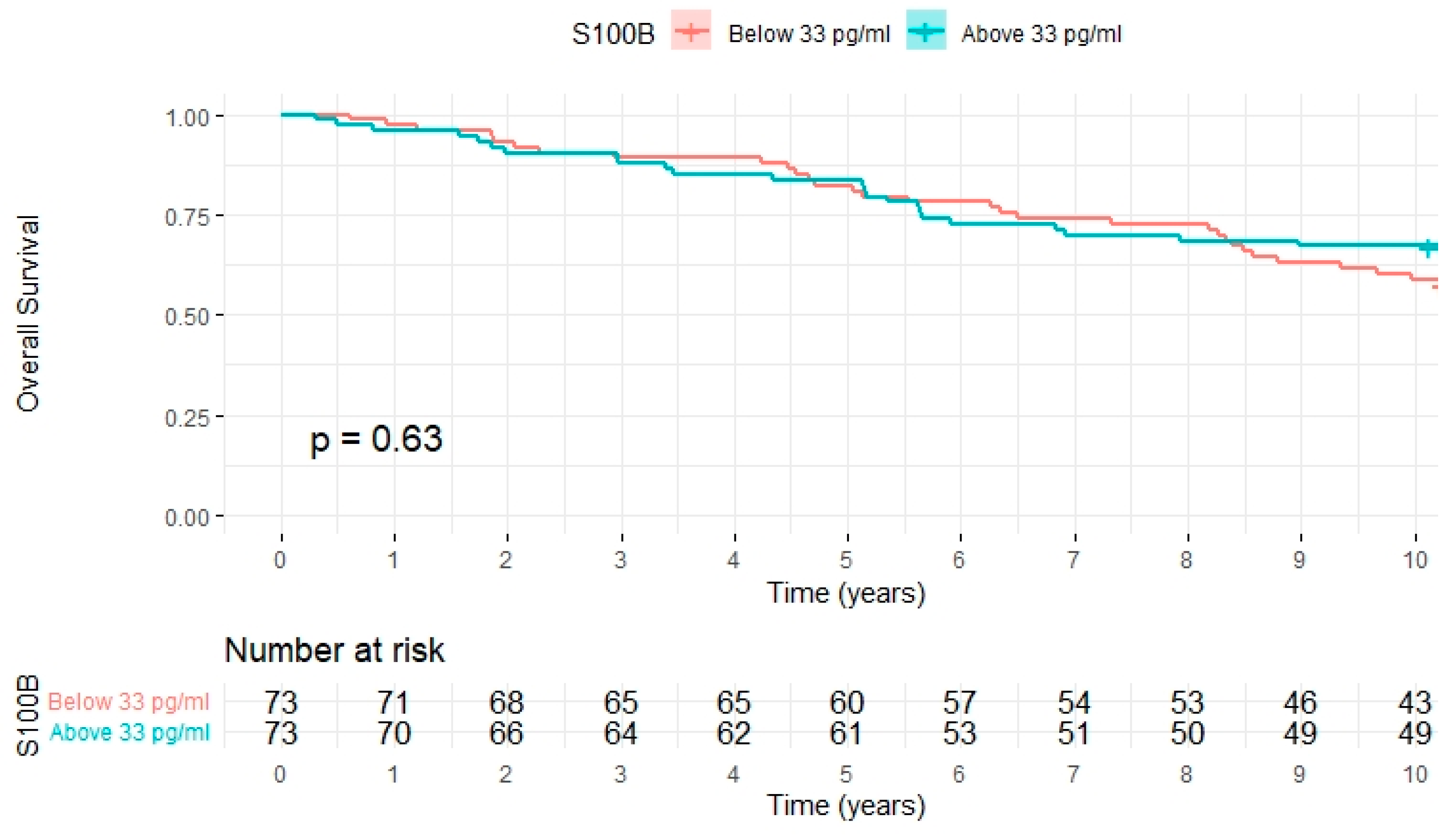
2.5. All-Cause Mortality
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. S100B Measurements
4.3. Neuropsychological Testing
4.4. Statistical Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marenholz, I.; Heizmann, C.W.; Fritz, G. S100 proteins in mouse and man: From evolution to function and pathology (including an update of the nomenclature). Biochem. Biophys. Res. Commun. 2004, 322, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Zurek, J.; Fedora, M. The usefulness of S100B, NSE, GFAP, NF-H, secretagogin and Hsp70 as a predictive biomarker of outcome in children with traumatic brain injury. Acta Neurochir. 2012, 154, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Gayger-Dias, V.; Vizuete, A.F.; Rodrigues, L.; Wartchow, K.M.; Bobermin, L.; Leite, M.C.; Quincozes-Santos, A.; Kleindienst, A.; Goncalves, C.A. How S100B crosses brain barriers and why it is considered a peripheral marker of brain injury. Exp. Biol. Med. 2023, 248, 2109–2119. [Google Scholar] [CrossRef]
- Imbalzano, E.; Mandraffino, G.; Casciaro, M.; Quartuccio, S.; Saitta, A.; Gangemi, S. Pathophysiological mechanism and therapeutic role of S100 proteins in cardiac failure: A systematic review. Heart Fail. Rev. 2016, 21, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Tsoporis, J.N.; Marks, A.; Kahn, H.J.; Butany, J.W.; Liu, P.P.; O’Hanlon, D.; Parker, T.G. S100beta inhibits alpha1-adrenergic induction of the hypertrophic phenotype in cardiac myocytes. J. Biol. Chem. 1997, 272, 31915–31921. [Google Scholar] [CrossRef]
- Li, J.P.; Lu, L.; Wang, L.J.; Zhang, F.R.; Shen, W.F. Increased serum levels of S100B are related to the severity of cardiac dysfunction, renal insufficiency and major cardiac events in patients with chronic heart failure. Clin. Biochem. 2011, 44, 984–988. [Google Scholar] [CrossRef]
- Mazzini, G.S.; Schaf, D.V.; Vinade, E.R.; Horowitz, E.; Bruch, R.S.; Brunm, L.M.; Goncalves, C.A.; Bacal, F.; Souza, D.O.; Portela, L.V.; et al. Increased S100B serum levels in dilated cardiomyopathy patients. J. Card. Fail. 2007, 13, 850–854. [Google Scholar] [CrossRef]
- Mazzini, G.S.; Schaf, D.V.; Oliveira, A.R.; Goncalves, C.A.; Bello-Klein, A.; Bordignon, S.; Bruch, R.S.; Campos, G.F.; Vassallo, D.V.; Souza, D.O.; et al. The ischemic rat heart releases S100B. Life Sci. 2005, 77, 882–889. [Google Scholar] [CrossRef]
- Raquel, H.A.; Perego, S.M.; Masson, G.S.; Jensen, L.; Colquhoun, A.; Michelini, L.C. Blood-brain barrier lesion-a novel determinant of autonomic imbalance in heart failure and the effects of exercise training. Clin. Sci. 2023, 137, 1049–1066. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Yao, M.; Chen, L.; Chen, W.; Liu, X. Association of S100B 3’UTR polymorphism with risk of chronic heart failure in a Chinese Han population. Medicine 2020, 99, e21018. [Google Scholar] [CrossRef]
- Traub, J.; Frey, A.; Stork, S. Chronic Neuroinflammation and Cognitive Decline in Patients with Cardiac Disease: Evidence, Relevance, and Therapeutic Implications. Life 2023, 13, 329. [Google Scholar] [CrossRef]
- Dardiotis, E.; Giamouzis, G.; Mastrogiannis, D.; Vogiatzi, C.; Skoularigis, J.; Triposkiadis, F.; Hadjigeorgiou, G.M. Cognitive impairment in heart failure. Cardiol. Res. Pract. 2012, 2012, 595821. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Weng, X.; Cao, L.; Liang, D.; Zeng, F.; Chen, S.; Zhang, Y.; Huang, H.; Gao, M. Correlation Analysis of Serum 3-NT, NPASDP-4, and S100beta Protein Levels with Cognitive Function in Patients Diagnosed with Cerebral Infarction. Altern. Ther. Health Med. 2024, 30, 54–59. [Google Scholar] [PubMed]
- Hu, J.; Xie, S.; Li, W.; Zhang, L. Diagnostic and prognostic value of serum S100B in sepsis-associated encephalopathy: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1102126. [Google Scholar] [CrossRef]
- Glimmerveen, A.; Verhulst, M.; Verbunt, J.; Van Heugten, C.; Hofmeijer, J. Predicting Long-Term Cognitive Impairments in Survivors after Cardiac Arrest: A Systematic Review. J. Rehabil. Med. 2023, 55, jrm00368. [Google Scholar] [CrossRef]
- Tulner, D.M.; Smith, O.R.; de Jonge, P.; van Melle, J.P.; Slomp, J.; Storm, H.; Quere, M.; den Boer, J.A.; Honig, A.; Korf, J. Circulating cerebral S100B protein is associated with depressive symptoms following myocardial infarction. Neuropsychobiology 2009, 59, 87–95. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, S.G.; Kim, H.J.; Song, Y.R. Serum S100B protein is associated with depressive symptoms in patients with end-stage renal disease. Clin. Biochem. 2012, 45, 1573–1577. [Google Scholar] [CrossRef]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef]
- Pham, N.; Fazio, V.; Cucullo, L.; Teng, Q.; Biberthaler, P.; Bazarian, J.J.; Janigro, D. Extracranial sources of S100B do not affect serum levels. PLoS ONE 2010, 5, e12691. [Google Scholar] [CrossRef]
- Gebhardt, C.; Lichtenberger, R.; Utikal, J. Biomarker value and pitfalls of serum S100B in the follow-up of high-risk melanoma patients. J. Dtsch. Dermatol. Ges. 2016, 14, 158–164. [Google Scholar] [CrossRef]
- Gross, S.; Homan van der Heide, J.J.; van Son, W.J.; Gans, R.O.; Foell, D.; Navis, G.; Bakker, S.J. Body mass index and creatinine clearance are associated with steady-state serum concentrations of the cell damage marker S100B in renal transplant recipients. Med. Sci. Monit. 2010, 16, CR318–CR324. [Google Scholar] [PubMed]
- Steiner, J.; Schiltz, K.; Walter, M.; Wunderlich, M.T.; Keilhoff, G.; Brisch, R.; Bielau, H.; Bernstein, H.G.; Bogerts, B.; Schroeter, M.L.; et al. S100B serum levels are closely correlated with body mass index: An important caveat in neuropsychiatric research. Psychoneuroendocrinology 2010, 35, 321–324. [Google Scholar] [CrossRef]
- Fiedorczuk, P.; Olszewska, E.; Polecka, A.; Walasek, M.; Mroczko, B.; Kulczynska-Przybik, A. Investigating the Role of Serum and Plasma IL-6, IL-8, IL-10, TNF-alpha, CRP, and S100B Concentrations in Obstructive Sleep Apnea Diagnosis. Int. J. Mol. Sci. 2023, 24, 13875. [Google Scholar] [CrossRef] [PubMed]
- Polyakova, M.; Sander, C.; Arelin, K.; Lampe, L.; Luck, T.; Luppa, M.; Kratzsch, J.; Hoffmann, K.T.; Riedel-Heller, S.; Villringer, A.; et al. First evidence for glial pathology in late life minor depression: S100B is increased in males with minor depression. Front. Cell. Neurosci. 2015, 9, 406. [Google Scholar] [CrossRef]
- Calluy, E.; Beaudart, C.; Alokail, M.S.; Al-Daghri, N.M.; Bruyere, O.; Reginster, J.Y.; Cavalier, E.; Ladang, A. Confounding factors of the expression of mTBI biomarkers, S100B, GFAP and UCH-L1 in an aging population. Clin. Chem. Lab. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Routsi, C.; Stamataki, E.; Nanas, S.; Psachoulia, C.; Stathopoulos, A.; Koroneos, A.; Zervou, M.; Jullien, G.; Roussos, C. Increased levels of serum S100B protein in critically ill patients without brain injury. Shock 2006, 26, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, K.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.J.; Ponikowski, P.; Poole-Wilson, P.A.; Stromberg, A.; van Veldhuisen, D.J.; Atar, D.; Hoes, A.W.; et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur. J. Heart Fail. 2008, 10, 933–989. [Google Scholar] [CrossRef]
- McMurray, J.J.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Bohm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2012, 33, 1787–1847. [Google Scholar] [CrossRef]
- Geiger, J.; Both, S.; Kircher, S.; Neumann, M.; Rosenwald, A.; Jahns, R. Hospital-integrated Biobanking as a Service—The Interdisciplinary Bank of Biomaterials and Data Wuerzburg (ibdw). Open J. Bioresour. 2018, 5, 6. [Google Scholar] [CrossRef]
| Full Sample (n = 146) | S100B < 33 pg/mL (n = 73) | S100B ≥ 33 pg/mL (n = 73) | p Value (Two-Sided) | |
|---|---|---|---|---|
| Age (years) | 64 (56–72) | 63 (54–71) | 65 (58–74) | 0.097 |
| Female sex | 22 (15) | 12 (16) | 10 (14) | 0.644 |
| Body mass index (kg/m2) | 28 (26–32) | 28 (26–32) | 29 (25–32) | 0.813 |
| Systolic blood pressure (mmHg) | 135 (124–151) | 137 (124–150) | 135 (123–153) | 0.423 |
| Diastolic blood pressure (mmHg) | 80 (75–89) | 81 (75–90) | 80 (75–88) | 0.455 |
| Resting heart rate (1/min) | 63 (58–70) | 64 (58–71) | 62 (57–70) | 0.151 |
| Time since diagnosis of heart failure (years) | 4 (1–10) | 3 (1–7) | 5 (2–12) | 0.014 |
| Ischemic heart failure | 96 (66) | 48 (66) | 48 (66) | >0.999 |
| New York Heart Association functional class I | 39 (27) | 19 (26) | 20 (27) | 0.852 |
| New York Heart Association functional class II | 88 (60) | 47 (64) | 41 (56) | 0.310 |
| New York Heart Association functional class III | 19 (13) | 7 (10) | 12 (16) | 0.219 |
| 6 min walking distance (m) | 400 (340–460) | 380 (340–460) | 420 (350–460) | 0.224 |
| NT-proBNP (pg/mL) | 672 (237–1736) | 632 (219–1475) | 703 (265–1926) | 0.063 |
| Left ventricular ejection fraction (%) | 44 (38–48) | 43 (38–48) | 44 (38–49) | 0.241 |
| Heart failure with preserved ejection fraction | 24 (16) | 9 (12) | 15 (21) | 0.180 |
| Heart failure with midrange ejection fraction | 72 (49) | 41 (56) | 31 (43) | 0.098 |
| Heart failure with reduced ejection fraction | 50 (34) | 23 (32) | 27 (37) | 0.485 |
| Left atrial volume index (mL/m2) | 38 (29–52) | 38 (29–48) | 39 (31–55) | 0.041 |
| Left ventricular end-diastolic volume (mL) | 167 (110–162) | 127 (112–166) | 124 (110–153) | 0.244 |
| E/e’ ratio | 10 (8–13) | 10 (8–12) | 10 (7–13) | 0.872 |
| Deceleration time (ms) | 188 (146–231) | 195 (159–241) | 172 (144–221) | 0.069 |
| Arterial hypertension | 116 (80) | 54 (74) | 62 (85) | 0.101 |
| Diabetes mellitus | 42 (29) | 24 (33) | 18 (25) | 0.273 |
| Hyperlipidemia | 105 (72) | 53 (73) | 52 (71) | 0.854 |
| Smoking (ever) | 88 (60) | 44 (60) | 44 (60) | >0.999 |
| Coronary artery disease | 100 (69) | 51 (70) | 49 (67) | 0.722 |
| History of myocardial infarction | 80 (55) | 42 (58) | 38 (55) | 0.506 |
| Atrial fibrillation | 33 (23) | 14 (19) | 19 (26) | 0.322 |
| Angiotensin-converting enzyme inhibitor | 86 (59) | 48 (66) | 38 (52) | 0.093 |
| Angiotensin-1 receptor antagonist | 48 (33) | 19 (26) | 29 (40) | 0.078 |
| Sacubitril/valsartan | 0 (0) | 0 (0) | 0 (0) | >0.999 |
| Beta-blocker | 131 (90) | 65 (89) | 66 (90) | 0.785 |
| Mineralocorticoid receptor antagonist | 54 (37) | 26 (36) | 28 (38) | 0.732 |
| Sodium glucose linked transporter 2 inhibitor | 0 (0) | 0 (0) | 0 (0) | >0.999 |
| Statins | 101 (69) | 53 (73) | 48 (66) | 0.370 |
| Ezetimibe | 9 (6) | 4 (6) | 5 (7) | >0.999 |
| Loop diuretic | 80 (55) | 36 (49) | 44 (60) | 0.183 |
| Thiazide | 23 (16) | 9 (12) | 14 (19) | 0.256 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 68 (55–80) | 74 (59–84) | 61 (48–76) | 0.009 |
| Alanine transferase (U/L) | 24 (19–34) | 23 (19–34) | 25 (18–34) | 0.749 |
| Aspartate transferase (U/L) | 26 (21–31) | 26 (21–31) | 25 (22–31) | 0.702 |
| Albumin (g/dL) | 4.6 (4.4–4.7) | 4.6 (4.4–4.8) | 4.6 (4.4–4.7) | 0.466 |
| Creatine kinase (U/L) | 102 (75–153) | 95 (70–139) | 113 (77–168) | 0.163 |
| C-reactive protein (mg/dL) | 0.2 (0.1–0.5) | 0.2 (0.1–0.6) | 0.2 (0.1–0.4) | 0.139 |
| Hemoglobin (g/dL) | 14.5 (13.6–15.3) | 14.3 (13.6–15.4) | 14.5 (13.7–15.2) | 0.804 |
| Chronic kidney disease G1 | 15 (10) | 7 (10) | 8 (11) | 0.785 |
| Chronic kidney disease G2 | 79 (54) | 47 (64) | 32 (44) | 0.013 |
| Chronic kidney disease G3 | 47 (32) | 19 (26) | 28 (38) | 0.111 |
| Chronic kidney disease G4 | 5 (3) | 0 (0) | 5 (7) | 0.058 |
| Chronic kidney disease G5 | 0 (0) | 0 (0) | 0 (0) | >0.999 |
| Mild cognitive impairment | 86 (59) | 36 (50) | 50 (69) | 0.023 |
| Intensity of attention (T-score) | 42 (37–47) | 42 (38–46) | 40 (35–47) | 0.152 |
| Visual/verbal memory (T-score) | 45 (39–51) | 46 (41–53) | 43 (39–48) | 0.006 |
| Executive functions (T-score) | 45 (42–50) | 45 (41–50) | 45 (42–50) | 0.469 |
| S100B Serum Level (pg/mL) | Univariable Regression | Multivariable Regression | |||
|---|---|---|---|---|---|
| R2 | T-Value | p Value | T-Value | p Value | |
| Age (years) | 0.01 | 1.43 | 0.155 | 2.02 | 0.046 |
| Sex (female) | 0.00 | -0.46 | 0.646 | 1.18 | 0.243 |
| Body mass index (kg/m2) | 0.01 | 1.33 | 0.186 | 1.17 | 0.244 |
| 6 min walking distance (m) | 0.01 | 1.03 | 0.303 | 1.40 | 0.164 |
| New York Heart Association functional class | 0.00 | −0.01 | 0.988 | −0.73 | 0.466 |
| NT-proBNP (pg/mL) | 0.07 | 3.34 | 0.001 | 2.27 | 0.026 |
| Left ventricular ejection fraction (%) | 0.01 | 0.96 | 0.339 | 0.70 | 0.486 |
| Left atrial volume index (mL/m2) | 0.01 | 0.96 | 0.340 | −1.04 | 0.299 |
| Left ventricular end-diastolic volume (mL) | 0.01 | −1.04 | 0.299 | −0.83 | 0.410 |
| E/e’ ratio | 0.00 | 0.07 | 0.947 | −0.35 | 0.730 |
| Deceleration time (ms) | 0.01 | −1.01 | 0.312 | −1.08 | 0.281 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 0.07 | −3.29 | 0.001 | −1.90 | 0.061 |
| Alanine transferase (U/L) | 0.00 | 0.20 | 0.842 | 1.81 | 0.074 |
| Aspartate transferase (U/L) | 0.00 | 0.53 | 0.596 | −1.30 | 0.198 |
| Albumin (g/dL) | 0.01 | −1.06 | 0.291 | −1.09 | 0.280 |
| Creatine kinase (U/L) | 0.02 | 1.69 | 0.094 | 0.94 | 0.350 |
| C-reactive protein (mg/dL) | 0.01 | −0.86 | 0.389 | −0.76 | 0.450 |
| Hemoglobin (g/dL) | 0.00 | −0.16 | 0.870 | 0.97 | 0.332 |
| Arterial hypertension | 0.02 | −1.62 | 0.108 | −1.25 | 0.214 |
| Diabetes mellitus | 0.01 | 1.07 | 0.288 | 0.64 | 0.524 |
| Hyperlipidemia | 0.00 | 0.06 | 0.954 | −0.38 | 0.705 |
| Smoking (ever) | 0.00 | −0.23 | 0.817 | −1.89 | 0.062 |
| Coronary artery disease | 0.01 | 1.08 | 0.283 | 0.13 | 0.900 |
| History of myocardial infarction | 0.01 | 1.05 | 0.294 | −0.51 | 0.612 |
| Atrial fibrillation | 0.03 | 2.04 | 0.043 | 0.84 | 0.400 |
| Angiotensin-converting enzyme inhibitor | 0.03 | 2.23 | 0.027 | 0.60 | 0.552 |
| Angiotensin-1 receptor antagonist | 0.02 | −1.50 | 0.135 | −0.53 | 0.597 |
| Beta-blocker | 0.00 | −0.85 | 0.397 | −1.31 | 0.192 |
| Mineralocorticoid receptor antagonist | 0.00 | −0.57 | 0.567 | −0.78 | 0.440 |
| Statins | 0.01 | 1.35 | 0.178 | 1.89 | 0.062 |
| Ezetimibe | 0.00 | 0.56 | 0.577 | −0.23 | 0.819 |
| Loop diuretic | 0.02 | −1.89 | 0.061 | −0.47 | 0.640 |
| Thiazide | 0.01 | −1.09 | 0.278 | −0.95 | 0.346 |
| Mild Cognitive Impairment | Univariable Regression | Multivariable Regression | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| S100B (pg/mL) | 1.01 (0.99–1.03) | 0.213 | 1.03 (1.00–1.06) | 0.083 |
| Age (years) | 1.03 (1.00–1.06) | 0.073 | 0.98 (0.92–1.04) | 0.474 |
| Sex (female) | 2.02 (0.74–5.51) | 0.170 | 0.38 (0.06–2.59) | 0.326 |
| Body mass index (kg/m2) | 0.97 (0.91–1.04) | 0.414 | 0.94 (0.83–1.06) | 0.280 |
| 6 min walking distance (m) | 1.00 (0.99–1.00) | 0.014 | 1.00 (0.99–1.00) | 0.389 |
| New York Heart Association functional class | 1.56 (0.90–2.71) | 0.111 | 3.05 (1.18–7.88) | 0.021 |
| NT-proBNP (pg/mL) | 1.00 (1.00–1.00) | 0.486 | 1.00 (1.00–1.00) | 0.463 |
| Left ventricular ejection fraction (%) | 1.02 (0.98–1.06) | 0.333 | 1.11 (1.02–1.21) | 0.015 |
| Left atrial volume index (mL/m2) | 1.01 (0.99–1.03) | 0.431 | 1.00 (0.96–1.04) | 0.873 |
| Left ventricular end-diastolic volume (mL) | 1.00 (0.99–1.01) | 0.531 | 1.01 (0.99–1.02) | 0.392 |
| E/e’ ratio | 0.97 (0.89–1.04) | 0.386 | 0.94 (0.84–1.06) | 0.343 |
| Deceleration time (ms) | 1.00 (0.99–1.00) | 0.185 | 0.99 (0.98–1.00) | 0.229 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 0.99 (0.97–1.00) | 0.122 | 1.00 (0.97–1.03) | 0.991 |
| Alanine transferase (U/L) | 0.99 (0.96–1.01) | 0.272 | 0.98 (0.93–1.03) | 0.434 |
| Aspartate transferase (U/L) | 1.01 (0.97–1.04) | 0.769 | 1.05 (0.98–1.13) | 0.161 |
| Albumin (g/dL) | 0.48 (0.13–1.76) | 0.269 | 2.26 (0.22–23.30) | 0.493 |
| Creatine kinase (U/L) | 1.00 (0.99–1.00) | 0.637 | 1.00 (0.99–1.00) | 0.299 |
| C-reactive protein (mg/dL) | 1.09 (0.72–1.67) | 0.674 | 1.35 (0.70–2.61) | 0.373 |
| Hemoglobin (g/dL) | 0.90 (0.71–1.14) | 0.381 | 0.73 (0.45–1.20) | 0.212 |
| Arterial hypertension | 0.68 (0.30–1.54) | 0.354 | 1.42 (0.37–5.47) | 0.609 |
| Diabetes mellitus | 0.86 (0.41–1.79) | 0.685 | 0.51 (0.14–1.77) | 0.286 |
| Hyperlipidemia | 1.62 (0.75–3.48) | 0.217 | 1.40 (0.32–6.15) | 0.657 |
| Smoking (ever) | 2.25 (1.11–4.55) | 0.024 | 5.59 (1.62–19.22) | 0.006 |
| Coronary artery disease | 0.85 (0.42–1.72) | 0.641 | 0.16 (0.02–1.30) | 0.086 |
| History of myocardial infarction | 1.10 (0.57–2.15) | 0.772 | 3.07 (0.57–16.49) | 0.191 |
| Atrial fibrillation | 1.07 (0.49–2.37) | 0.863 | 1.19 (0.30–4.70) | 0.799 |
| Angiotensin-converting enzyme inhibitor | 1.18 (0.60–2.32) | 0.628 | 5.13 (0.76–34.54) | 0.093 |
| Angiotensin-1 receptor antagonist | 1.21 (0.60–2.43) | 0.598 | 6.97 (0.85–57.26) | 0.071 |
| Beta-blocker | 0.76 (0.26–2.23) | 0.620 | 0.41 (0.09–1.82) | 0.242 |
| Mineralocorticoid receptor antagonist | 0.89 (0.45–1.77) | 0.734 | 0.90 (0.31–2.61) | 0.847 |
| Statins | 1.30 (0.63–2.69) | 0.484 | 1.08 (0.15–7.93) | 0.938 |
| Ezetimibe | 2.56 (0.59–11.16) | 0.210 | 6.28 (0.74–53.54) | 0.093 |
| Loop diuretic | 0.66 (0.34–1.29) | 0.228 | 0.77 (0.24–2.45) | 0.655 |
| Thiazide | 0.93 (0.37–2.30) | 0.868 | 0.69 (0.16–2.90) | 0.614 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traub, J.; Schuhmann, M.K.; Sell, R.; Frantz, S.; Störk, S.; Stoll, G.; Frey, A. S100B Serum Levels in Chronic Heart Failure Patients: A Multifaceted Biomarker Linking Cardiac and Cognitive Dysfunction. Int. J. Mol. Sci. 2024, 25, 9094. https://doi.org/10.3390/ijms25169094
Traub J, Schuhmann MK, Sell R, Frantz S, Störk S, Stoll G, Frey A. S100B Serum Levels in Chronic Heart Failure Patients: A Multifaceted Biomarker Linking Cardiac and Cognitive Dysfunction. International Journal of Molecular Sciences. 2024; 25(16):9094. https://doi.org/10.3390/ijms25169094
Chicago/Turabian StyleTraub, Jan, Michael K. Schuhmann, Roxanne Sell, Stefan Frantz, Stefan Störk, Guido Stoll, and Anna Frey. 2024. "S100B Serum Levels in Chronic Heart Failure Patients: A Multifaceted Biomarker Linking Cardiac and Cognitive Dysfunction" International Journal of Molecular Sciences 25, no. 16: 9094. https://doi.org/10.3390/ijms25169094
APA StyleTraub, J., Schuhmann, M. K., Sell, R., Frantz, S., Störk, S., Stoll, G., & Frey, A. (2024). S100B Serum Levels in Chronic Heart Failure Patients: A Multifaceted Biomarker Linking Cardiac and Cognitive Dysfunction. International Journal of Molecular Sciences, 25(16), 9094. https://doi.org/10.3390/ijms25169094






