Integrated Multi-Omics Analysis Reveals Mountain-Cultivated Ginseng Ameliorates Cold-Stimulated Steroid-Resistant Asthma by Regulating Interactions among Microbiota, Genes, and Metabolites
Abstract
1. Introduction
2. Results
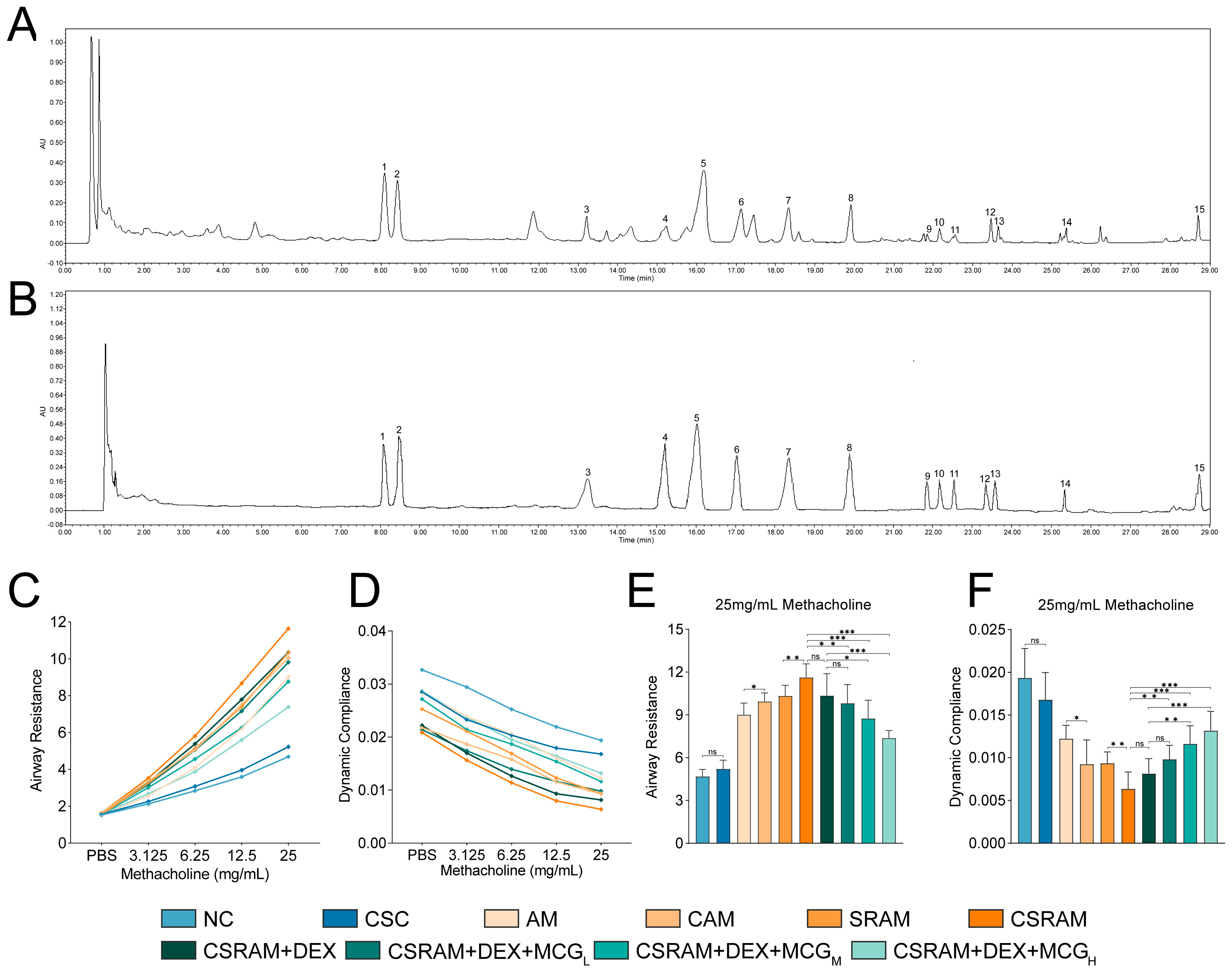
2.1. Chemical Content Analysis of MCG
2.2. Combination of DEX and MCG Attenuated Lung Function Impairment
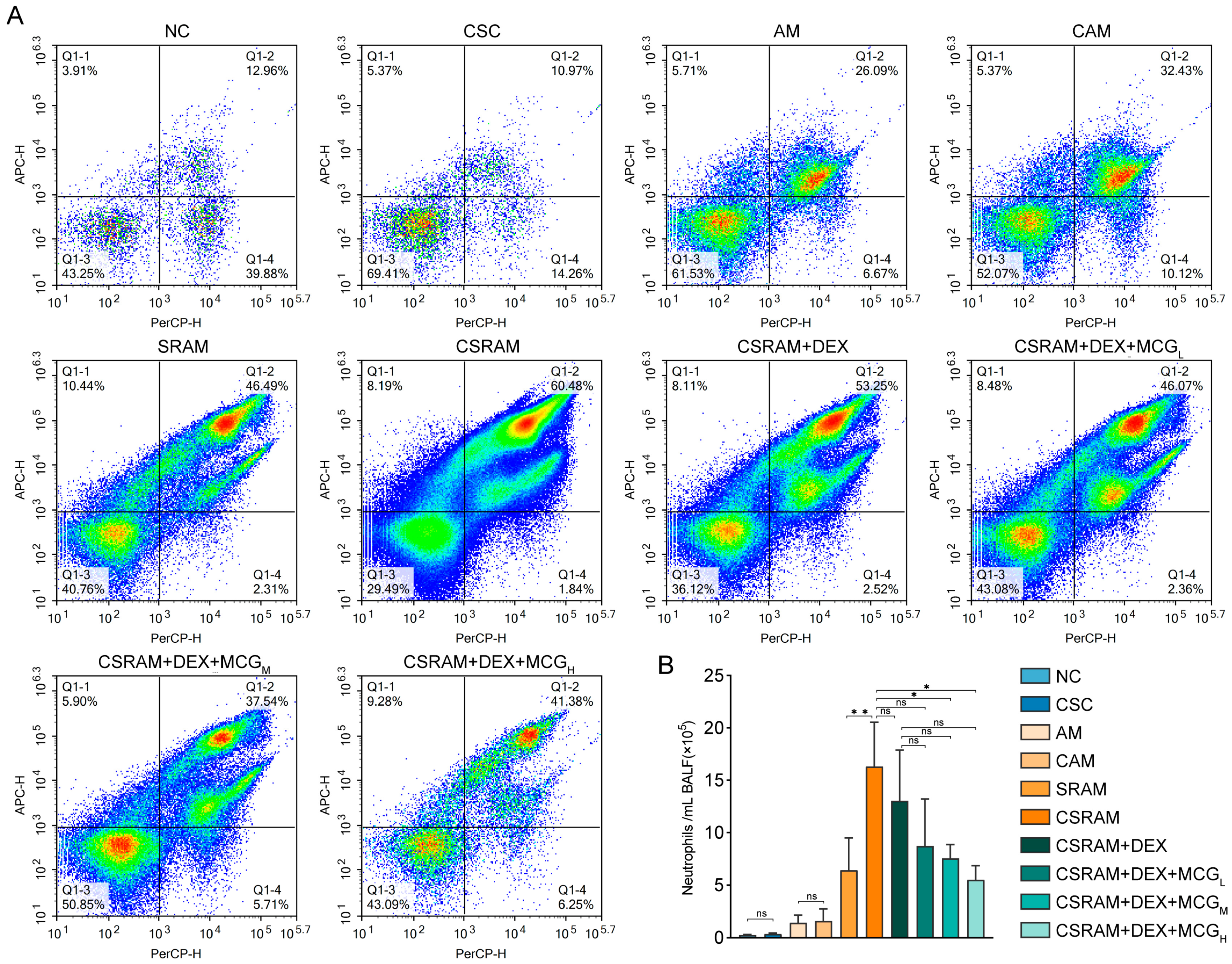
2.3. Combination of DEX and MCG Reduced the Proportion and Count of Neutrophils
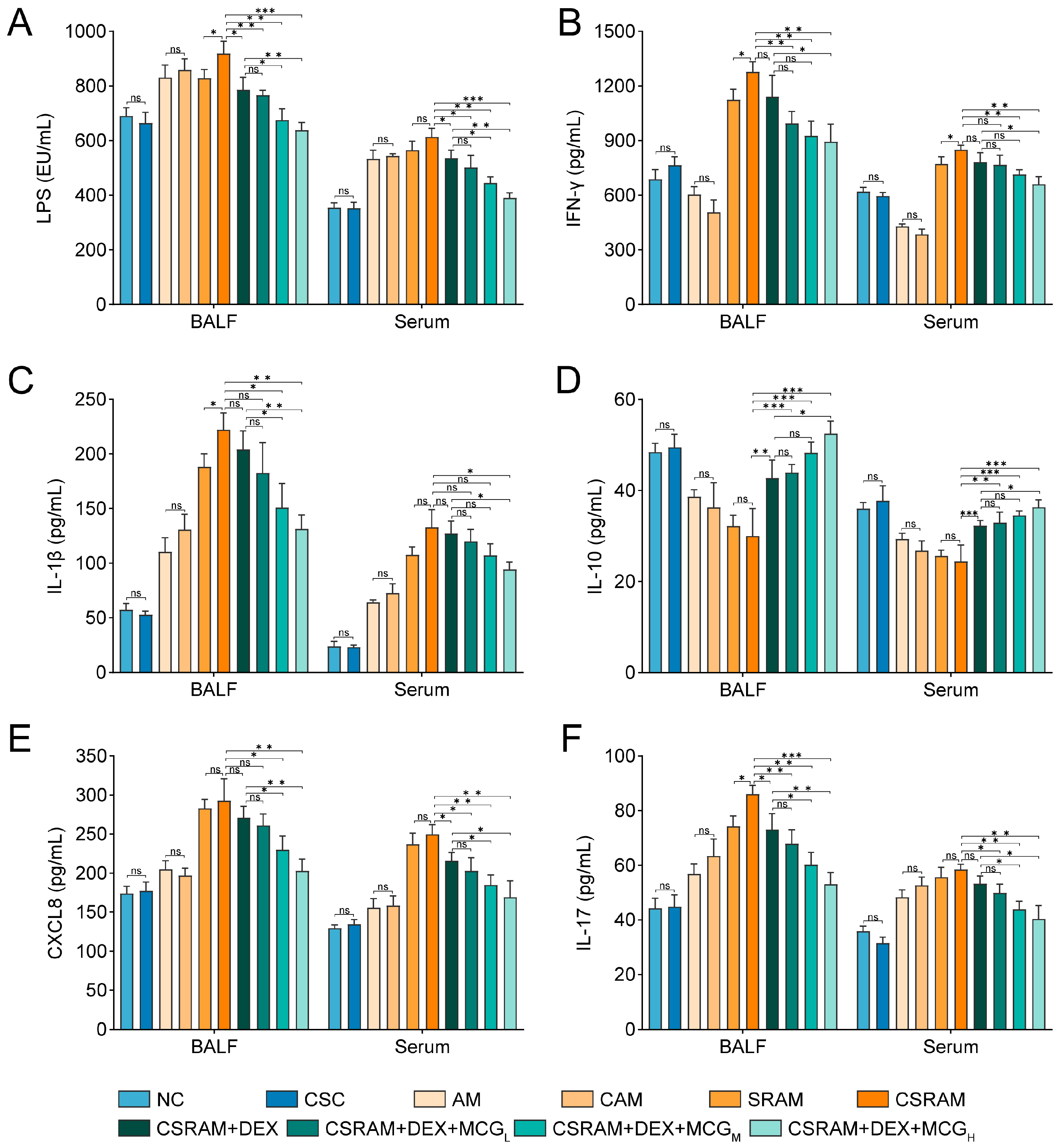
2.4. Combination of DEX and MCG Modulated the Levels of Cytokines
2.5. Combination Treatment of DEX and MCG Alleviated Airway Remodeling and Mucus Hypersecretion
2.6. Combination of DEX and MCG Inhibited the Expression of MUC5AC and Enhanced the Expression of TJs
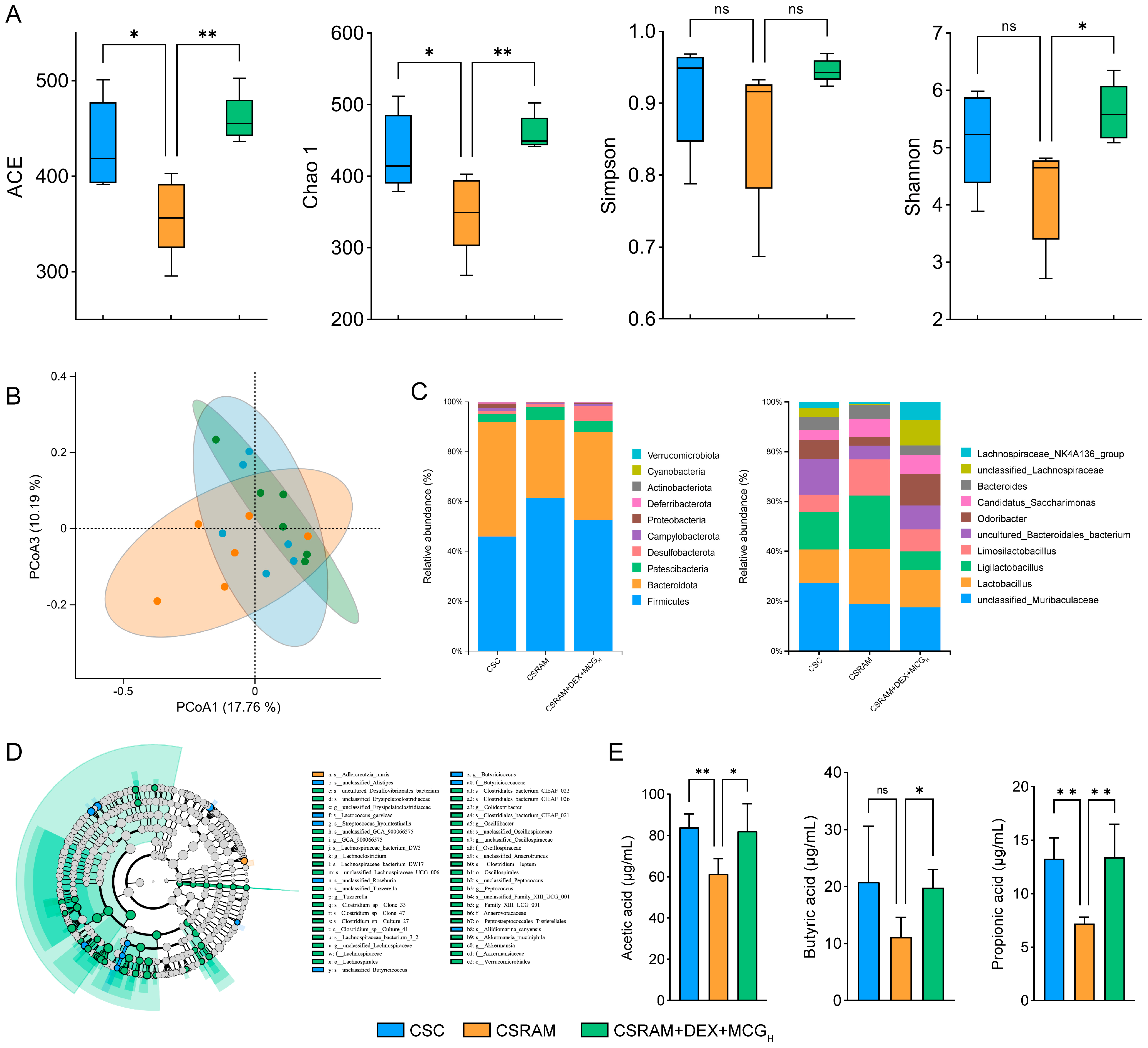
2.7. Combination of DEX and MCG Regulated the Gut Microbiota and Increased the Synthesis of SCFAs
2.8. Combination of DEX and MCG Regulated Gene Transcription Levels
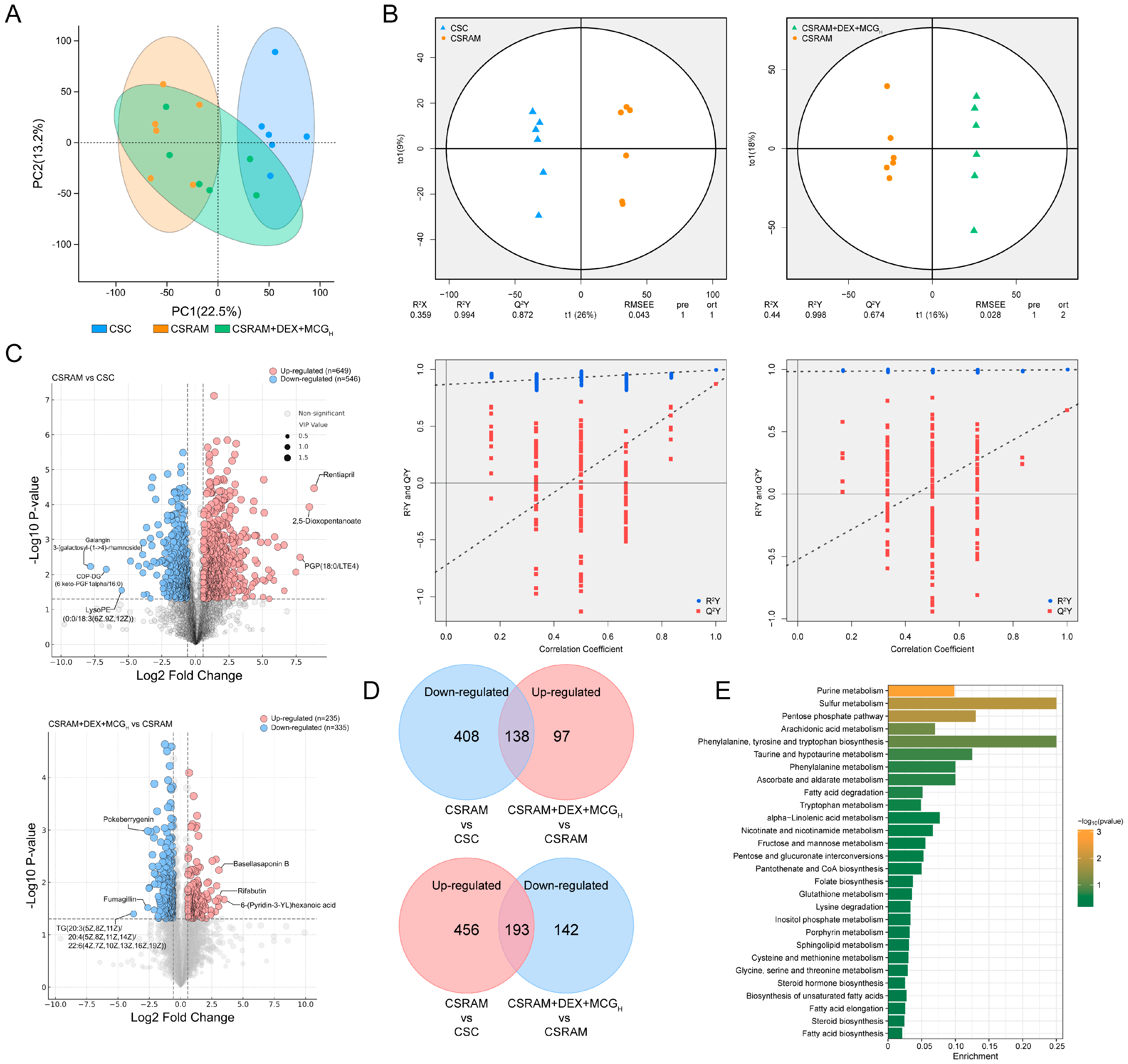
2.9. Combination of DEX and MCG Modulated Metabolite Levels
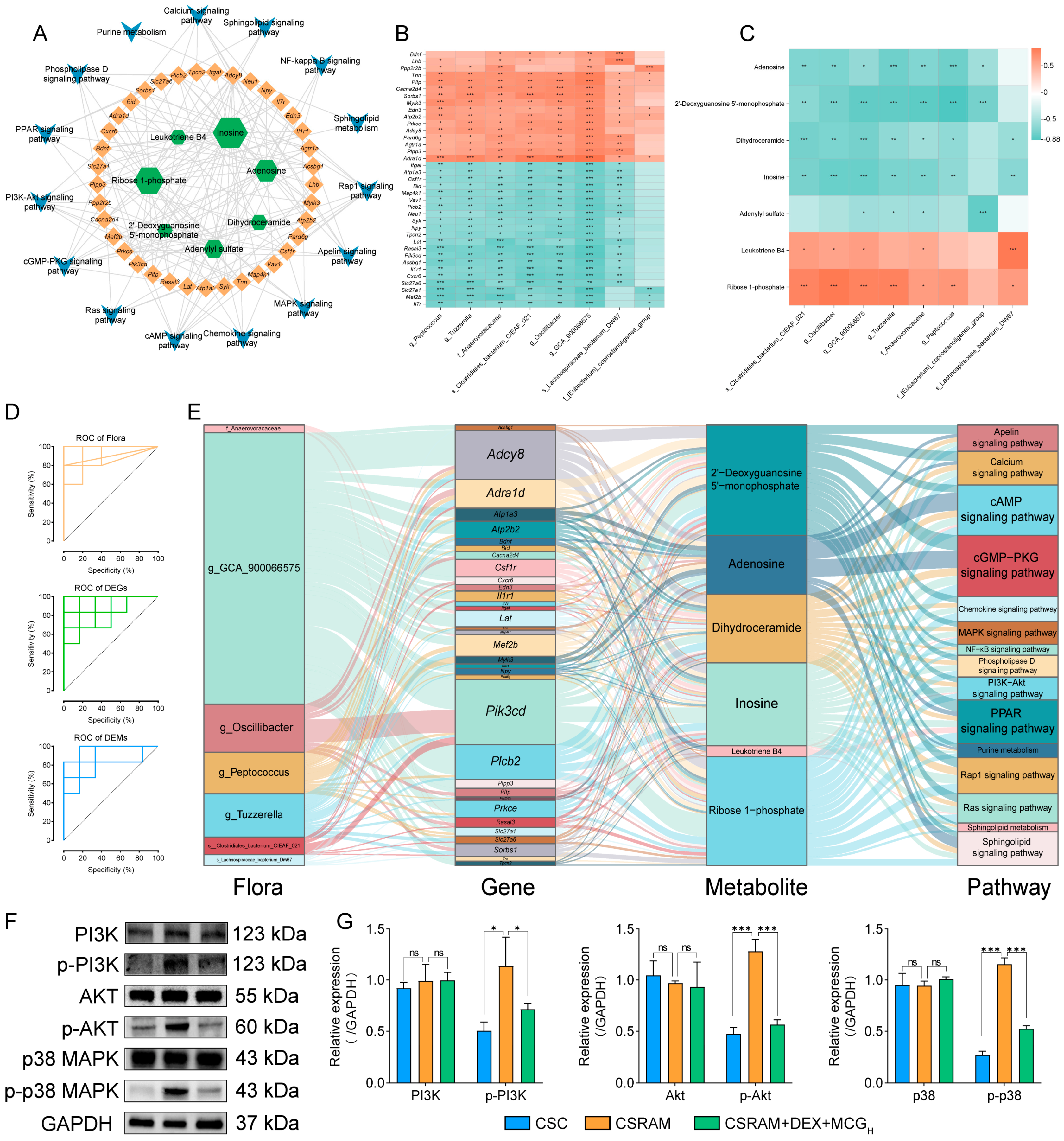
2.10. Multi-Omics Integrated Analysis
2.11. Combination of DEX and MCG Inhibits Activation of the PI3K-Akt/MAPK Pathway
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation and Analysis of MCG
4.3. Animals and Grouping
4.4. Pulmonary Function Examination
4.5. Neutrophil Counting in the Bronchoalveolar Lavage Fluid (BALF)
4.6. Detection of LPS and Cytokines in Serum and BALF
4.7. Histopathologic Examination of Lung Inflammation and Mucus Hypersecretion
4.8. Detection of MUC5AC and Tight Junction Protein (TJ) Expression in Lung Tissues
4.9. Analysis of Microbiomics and Microbial Metabolites
4.10. Analysis of Transcriptomics
4.11. Analysis of Non-Target Metabolomics
4.12. Integrated Multi-Omics Analysis
4.13. Verification of Key Pathways and Targets
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bateman, E.D.; Hurd, S.S.; Barnes, P.J.; Bousquet, J.; Drazen, J.M.; FitzGerald, M.; Gibson, P.; Ohta, K.; O’Byrne, P.; Pedersen, S.E.; et al. Global strategy for asthma management and prevention: GINA executive summary. Eur. Respir. J. 2008, 31, 143–178. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.C.; Kerr, S.; Dunican, E.M.; Woodruff, P.G.; Fajt, M.L.; Levy, B.D.; Israel, E.; Phillips, B.R.; Mauger, D.T.; Comhair, S.A.; et al. Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids. J. Allergy Clin. Immunol. 2019, 143, 104–113.e14. [Google Scholar] [CrossRef]
- Schoettler, N.; Strek, M.E. Recent Advances in Severe Asthma. Chest 2020, 157, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Hansbro, P.M.; Kim, R.Y.; Starkey, M.R.; Donovan, C.; Dua, K.; Mayall, J.R.; Liu, G.; Hansbro, N.G.; Simpson, J.L.; Wood, L.G.; et al. Mechanisms and treatments for severe, steroid-resistant allergic airway disease and asthma. Immunol. Rev. 2017, 278, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, Z.; Zuo, X.; Pan, H.; Gu, Y.; Yuan, Y.; Wang, G.; Wang, S.; Zheng, R.; Liu, Z.; et al. The role of NTHi colonization and infection in the pathogenesis of neutrophilic asthma. Respir. Res. 2020, 21, 170. [Google Scholar] [CrossRef]
- Wang, G.; Pang, Z.; Hsu, A.C.-Y.; Guan, X.; Ran, N.; Yuan, Y.; Wang, Z.; Guo, Y.; Zheng, R.; Wang, F. Combined treatment with SB203580 and dexamethasone suppresses non-typeable Haemophilus influenzae-induced Th17 inflammation response in murine allergic asthma. Eur. J. Pharmacol. 2019, 862, 172623. [Google Scholar] [CrossRef]
- Li, M.; Li, Q.; Yang, G.; Kolosov, V.P.; Perelman, J.M.; Zhou, X.D. Cold temperature induces mucin hypersecretion from normal human bronchial epithelial cells in vitro through a transient receptor potential melastatin 8 (TRPM8)–mediated mechanism. J. Allergy Clin. Immunol. 2011, 128, 626–634.e5. [Google Scholar] [CrossRef]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef]
- Hyrkäs-Palmu, H.; Ikäheimo, T.M.; Laatikainen, T.; Jousilahti, P.; Jaakkola, M.S.; Jaakkola, J.J.K. Cold weather increases respiratory symptoms and functional disability especially among patients with asthma and allergic rhinitis. Sci. Rep. 2018, 8, 10131. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, D.; Fu, J.; Zhang, Y.; Zhao, Y.; Liu, Y.; Chang, Z.G.; Liu, Y.; Liu, X.; Xu, K.; et al. Associations between ambient temperature and adult asthma hospitalizations in Beijing, China: A time-stratified case-crossover study. Respir. Res. 2022, 23, 38. [Google Scholar] [CrossRef]
- Barratt, B.; Quint, J.K. Asthma hospitalisations and air pollution. Thorax 2016, 71, 1076–1077. [Google Scholar] [CrossRef]
- Deng, L.; Ma, P.; Wu, Y.; Ma, Y.; Yang, X.; Li, Y.; Deng, Q. High and low temperatures aggravate airway inflammation of asthma: Evidence in a mouse model. Environ. Pollut. 2020, 256, 113433. [Google Scholar] [CrossRef]
- Pirogov, A.B.; Prikhodko, A.G.; Pirogova, N.A.; Perelman, J.M. Clinical and pathogenetic aspects of neutrophilic bronchial inflammation in asthma patients with cold-induced airway hyperresponsiveness (literature review). Bull. Sib. Med. 2023, 22, 143–152. [Google Scholar] [CrossRef]
- Gibson, P.G.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; Peters, M.J.; Marks, G.B.; Baraket, M.; et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): A randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, Å.; Bowen, K.; et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N. Engl. J. Med. 2021, 384, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Zhang, D.; Chen, W.; Wang, S.; He, Z.; Pang, S.; Qu, S.; Wang, Y. Identification of anti-inflammatory components in Panax ginseng of Sijunzi Decoction based on spectrum-effect relationship. Chin. Herb. Med. 2023, 15, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Bingze, C.; Yuzhen, P. Observation on the clinical efficacy of Yupingfeng oral solution combined with ginseng and schizandra soup in treating lung and spleen qi deficiency during the remission period of bronchial asthma. Pract. Chin. West. Med. Clin. 2018, 18, 150–152. [Google Scholar]
- Lim, C.-Y.; Moon, J.-M.; Kim, B.-Y.; Lim, S.-H.; Lee, G.-S.; Yu, H.-S.; Cho, S.-I. Comparative study of Korean White Ginseng and Korean Red Ginseng on efficacies of OVA-induced asthma model in mice. J. Ginseng Res. 2015, 39, 38–45. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, J.; Li, D.; Zhou, D.; Zhang, Y.; Wang, J.; Hu, B.; Ju, A.; Ye, Z. Nontargeted metabolomics approach for the differentiation of cultivation ages of mountain cultivated ginseng leaves using UHPLC/QTOF-MS. J. Pharm. Biomed. Anal. 2017, 141, 108–122. [Google Scholar] [CrossRef]
- Mizuno, M.; Yamada, J.; Terai, H.; Kozukue, N.; Lee, Y.S.; Tsuchida, H. Differences in immunomodulating effects between wild and cultured Panax ginseng. Biochem. Biophys. Res. Commun. 1994, 200, 1672–1678. [Google Scholar] [CrossRef]
- Lee, H.Y.; Jung, J.G.; Kim, S.C.; Cho, D.Y.; Kim, M.J.; Lee, A.R.; Son, K.-H.; Lee, J.H.; Lee, D.-H.; Cho, K.M. Comprehensive comparison of nutritional constituents and antioxidant activity of cultivated ginseng, mountain-cultivated ginseng, and whole plant parts of mountain-cultivated ginseng. J. Appl. Biol. Chem. 2021, 64, 453–463. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, C.; Cheng, S.; Wang, X.; Meng, X.; Li, L.; Du, J.; Liu, Q.; Guo, Y.; Meng, Y.; et al. Ginsenoside Rh1 Improves the Effect of Dexamethasone on Autoantibodies Production and Lymphoproliferation in MRL/lpr Mice. Evid. Based Complement. Altern. Med. 2015, 2015, 727650. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chu, S.; Li, J.; Li, J.; Zhang, Z.; Xia, C.; Heng, Y.; Zhang, M.; Hu, J.; Wei, G.; et al. Anti-inflammatory function of ginsenoside Rg1 on alcoholic hepatitis through glucocorticoid receptor related nuclear factor-kappa B pathway. J. Ethnopharmacol. 2015, 173, 231–240. [Google Scholar] [CrossRef]
- Li, F.; Feng, Y.; Liu, H.; Kong, D.; Hsueh, C.-Y.; Shi, X.; Wu, Q.; Li, W.; Wang, J.; Zhang, Y.; et al. Gut Microbiome and Metabolome Changes in Mice With Acute aVestibular Deficit. Front. Cell. Infect. Microbiol. 2022, 12, 821780. [Google Scholar]
- Zhao, Y.; Huang, Z.; Wang, S.; Hu, J.; Xiao, J.; Li, X.; Liu, T.; Zeng, W.; Guo, L.; Du, Q.; et al. Morbidity burden of respiratory diseases attributable to ambient temperature: A case study in a subtropical city in China. Environ. Health 2019, 18, 89. [Google Scholar] [CrossRef]
- Kaur, R.; Chupp, G. Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J. Allergy Clin. Immunol. 2019, 144, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Tan, W.S.D.; Wong, W.S.F. Andrographolide Restores Steroid Sensitivity To Block Lipopolysaccharide/IFN-γ–Induced IL-27 and Airway Hyperresponsiveness in Mice. J. Immunol. 2016, 196, 4706–4712. [Google Scholar] [CrossRef]
- Pang, Z.; Ran, N.; Yuan, Y.; Wang, C.; Wang, G.; Lin, H.; Hsu, A.C.-Y.; Liu, J.; Wang, F. Phenotype-Specific Therapeutic Effect of Rhodiola wallichiana var. cholaensis Combined with Dexamethasone on Experimental Murine Asthma and Its Comprehensive Pharmacological Mechanism. Int. J. Mol. Sci. 2019, 20, 4216. [Google Scholar] [CrossRef]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil Function: From Mechanisms to Disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef]
- Han, X.A.; Jie, H.Y.; Wang, J.H.; Zhang, X.M.; Wang, J.; Yu, C.X.; Zhang, J.L.; He, J.; Chen, J.Q.; Lai, K.F.; et al. Necrostatin-1 Ameliorates Neutrophilic Inflammation in Asthma by Suppressing MLKL Phosphorylation to Inhibiting NETs Release. Front. Immunol. 2020, 11, 666. [Google Scholar] [CrossRef]
- Davis, M.S.; Freed, A.N. Repeated Hyperventilation Causes Peripheral Airways Inflammation, Hyperreactivity, and Impaired Bronchodilation in Dogs. Am. J. Respir. Crit. Care Med. 2001, 164, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.S.; Williams, C.C.; Meinkoth, J.H.; Malayer, J.R.; Royer, C.M.; Williamson, K.K.; McKenzie, E.C. Influx of neutrophils and persistence of cytokine expression in airways of horses after performing exercise while breathing cold air. J. Am. Vet. Med. Assoc. 2007, 68, 185–189. [Google Scholar]
- Eimonte, M.; Paulauskas, H.; Daniuseviciute, L.; Eimantas, N.; Vitkauskiene, A.; Dauksaite, G.; Solianik, R.; Brazaitis, M. Residual effects of short-term whole-body cold-water immersion on the cytokine profile, white blood cell count, and blood markers of stress. Int. J. Hyperth. 2021, 38, 696–707. [Google Scholar] [CrossRef]
- Al-Kouba, J.; Wilkinson, A.N.; Starkey, M.R.; Rudraraju, R.; Werder, R.B.; Liu, X.; Law, S.-C.; Horvat, J.C.; Brooks, J.F.; Hill, G.R.; et al. Allergen-encoding bone marrow transfer inactivates allergic T cell responses, alleviating airway inflammation. JCI Insight 2017, 2, e85742. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.; Nong, G.; Ward, J.; Seumois, G.; Prince, L.R.; Wilson, S.J.; Cornelius, V.; Dent, G.; Djukanovic, R. Prosurvival activity for airway neutrophils in severe asthma. Thorax 2010, 65, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.K.; Al Heialy, S.; Hamid, Q. Role of IL-17 in asthma pathogenesis and its implications for the clinic. Expert Rev. Respir. Med. 2019, 13, 1057–1068. [Google Scholar] [CrossRef]
- Onishi, R.M.; Gaffen, S.L. Interleukin-17 and its target genes: Mechanisms of interleukin-17 function in disease. Immunology 2010, 129, 311–321. [Google Scholar] [CrossRef]
- de Morales, J.M.G.R.; Puig, L.; Daudén, E.; Cañete, J.D.; Pablos, J.L.; Martín, A.O.; Juanatey, C.G.; Adán, A.; Montalbán, X.; Borruel, N.; et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun. Rev. 2020, 19, 102429. [Google Scholar] [CrossRef]
- Karasawa, T.; Komada, T.; Yamada, N.; Aizawa, E.; Mizushina, Y.; Watanabe, S.; Baatarjav, C.; Matsumura, T.; Takahashi, M. Cryo-sensitive aggregation triggers NLRP3 inflammasome assembly in cryopyrin-associated periodic syndrome. eLife 2022, 11, e75166. [Google Scholar] [CrossRef]
- Du, C.; Kang, J.; Yu, W.; Chen, M.; Li, B.; Liu, H.; Wang, H. Repeated exposure to temperature variation exacerbates airway inflammation through TRPA1 in a mouse model of asthma. Respirology 2018, 24, 238–245. [Google Scholar] [CrossRef]
- Bonser, L.; Erle, D. Airway Mucus and Asthma: The Role of MUC5AC and MUC5B. J. Clin. Med. 2017, 6, 112. [Google Scholar] [CrossRef]
- Koeppen, M.; McNamee, E.N.; Brodsky, K.S.; Aherne, C.M.; Faigle, M.; Downey, G.P.; Colgan, S.P.; Evans, C.M.; Schwartz, D.A.; Eltzschig, H.K. Detrimental role of the airway mucin Muc5ac during ventilator-induced lung injury. Mucosal Immunol. 2013, 6, 762–775. [Google Scholar] [CrossRef]
- Suarez, M.F.; Echenique, J.; López, J.M.; Medina, E.; Irós, M.; Serra, H.M.; Fini, M.E. Transcriptome Analysis of Pterygium and Pinguecula Reveals Evidence of Genomic Instability Associated with Chronic Inflammation. Int. J. Mol. Sci. 2021, 22, 12090. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, T.; Zhang, X. Low temperature affects airway mucin secretion and promotes acute exacerbation of chronic obstructive pulmonary disease. Chin. Crit. Care Med. 2020, 32, 1273–1276. [Google Scholar]
- Campbell, H.K.; Maiers, J.L.; DeMali, K.A. Interplay between tight junctions & adherens junctions. Exp. Cell Res. 2017, 358, 39–44. [Google Scholar] [PubMed]
- Xiao, C.; Puddicombe, S.M.; Field, S.; Haywood, J.; Broughton-Head, V.; Puxeddu, I.; Haitchi, H.M.; Vernon-Wilson, E.; Sammut, D.; Bedke, N.; et al. Defective epithelial barrier function in asthma. J. Allergy Clin. Immunol. 2011, 128, 549–556.e12. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Lee, S.-H.; Jeong, S.; Hong, S.-J. Protease-Activated Receptors 2-Antagonist Suppresses Asthma by Inhibiting Reactive Oxygen Species-Thymic Stromal Lymphopoietin Inflammation and Epithelial Tight Junction Degradation. Allergy Asthma Immunol. Res. 2019, 11, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Tanga, A.; Saidi, A.; Jourdan, M.-L.; Dallet-Choisy, S.; Zani, M.-L.; Moreau, T. Protection of lung epithelial cells from protease-mediated injury by trappin-2 A62L, an engineered inhibitor of neutrophil serine proteases. Biochem. Pharmacol. 2012, 83, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Sekiyama, A.; Gon, Y.; Terakado, M.; Takeshita, I.; Kozu, Y.; Maruoka, S.; Matsumoto, K.; Hashimoto, S. Glucocorticoids enhance airway epithelial barrier integrity. Int. Immunopharmacol. 2012, 12, 350–357. [Google Scholar] [CrossRef]
- Li, H.; Shang, Z.; Liu, X.; Qiao, Y.; Wang, K.; Qiao, J. Clostridium butyricum Alleviates Enterotoxigenic Escherichia coli K88-Induced Oxidative Damage through Regulating the p62-Keap1-Nrf2 Signaling Pathway and Remodeling the Cecal Microbial Community. Front. Immunol. 2021, 12, 771826. [Google Scholar] [CrossRef]
- Richards, L.B.; Li, M.; Folkerts, G.; Henricks, P.A.J.; Garssen, J.; van Esch, B.C.A.M. Butyrate and Propionate Restore the Cytokine and House Dust Mite Compromised Barrier Function of Human Bronchial Airway Epithelial Cells. Int. J. Mol. Sci. 2020, 22, 65. [Google Scholar] [CrossRef]
- Alhamwe, B.A.; Gao, Z.; Alhamdan, F.; Harb, H.; Pichene, M.; Garnier, A.; Andari, J.E.; Kaufmann, A.; Graumann, P.L.; Kesper, D.; et al. Intranasal administration of Acinetobacter lwoffii in a murine model of asthma induces IL-6-mediated protection associated with cecal microbiota changes. Allergy 2023, 78, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Chen, Y.; Li, H.; Yang, Y.; Zhang, H.; Ke, K.; Shi, X.-N.; Liu, X.; Li, L.; Ma, J.; et al. The antipsychotic agent flupentixol is a new PI3K inhibitor and potential anticancer drug for lung cancer. Int. J. Biol. Sci. 2019, 15, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.E.; Porto, B.N.; Puga, R.; Stein, R.T.; Pitrez, P.M. Identifying a biomarker network for corticosteroid resistance in asthma from bronchoalveolar lavage samples. Mol. Biol. Rep. 2016, 43, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Xu, H.; Dai, W.; Zhu, C.; Wu, L.; Yan, S.; Ge, X.; Zhou, W.; Chen, C.; Dai, Y. The role of HDAC2 in cigarette smoke–induced airway inflammation in a murine model of asthma and the effect of intervention with roxithromycin. J. Asthma 2017, 55, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.Y.; Horvat, J.C.; Pinkerton, J.W.; Starkey, M.R.; Essilfie, A.T.; Mayall, J.R.; Nair, P.M.; Hansbro, N.G.; Jones, B.; Haw, T.J.; et al. MicroRNA-21 drives severe, steroid-insensitive experimental asthma by amplifying phosphoinositide 3-kinase–mediated suppression of histone deacetylase 2. J. Allergy Clin. Immunol. 2017, 139, 519–532. [Google Scholar] [CrossRef]
- Alam, R.; Gorska, M.M. Mitogen-activated protein kinase signalling and ERK1/2 bistability in asthma. Clin. Exp. Allergy 2010, 41, 149–159. [Google Scholar] [CrossRef]
- Dong, B.; Wang, C.; Zhang, J.; Zhang, J.; Gu, Y.; Guo, X.; Zuo, X.; Pan, H.; Hsu, A.C.-Y.; Wang, G.; et al. Exosomes from human umbilical cord mesenchymal stem cells attenuate the inflammation of severe steroid-resistant asthma by reshaping macrophage polarization. Stem Cell Res. Ther. 2021, 12, 204. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Y.; Liang, K.Y.; Xu, S.; Zhou, X.J.; Tan, K.; Lin, J.; Bai, X.C.; Yang, C.L. Rheb1 deletion in myeloid cells aggravates OVA-induced allergic inflammation in mice. Sci. Rep. 2017, 7, 42655. [Google Scholar] [CrossRef]
- Barnes, P.J. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2018, 18, 454–466. [Google Scholar] [CrossRef]
- Welsh, K.G.; Rousseau, K.; Fisher, G.; Bonser, L.R.; Bradding, P.; Brightling, C.E.; Thornton, D.J.; Gaillard, E.A. MUC5AC and a Glycosylated Variant of MUC5B Alter Mucin Composition in Children With Acute Asthma. Chest 2017, 152, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Miao, X.; Zhu, L.; Liu, J.; Lin, Y.; Xiang, G.; Wu, X.; Wang, X.; Ni, Z.; Li, S. Autocrine TGF-alpha is associated with Benzo(a)pyrene-induced mucus production and MUC5AC expression during allergic asthma. Ecotoxicol. Environ. Saf. 2022, 241, 113833. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.-H.; Tang, J.-H.; Chen, G.U.O.; Lai, Y.-M.; Chen, Q.-G.; Li, Z.A.O.; Yang, W.E.I.; Luo, X.-M.; Wang, X.-B. Resveratrol inhibits mucus overproduction and MUC5AC expression in a murine model of asthma. Mol. Med. Rep. 2016, 13, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.T.T.; Hagner, S.; Ruchti, F.; Radzikowska, U.; Tan, G.; Altunbulakli, C.; Eljaszewicz, A.; Moniuszko, M.; Akdis, M.; Akdis, C.A.; et al. Tight junction, mucin, and inflammasome-related molecules are differentially expressed in eosinophilic, mixed, and neutrophilic experimental asthma in mice. Allergy 2018, 74, 294–307. [Google Scholar] [CrossRef]
- Zhao, Q.; Song, S.-Y.; Zhang, Y.-Q.; Ren, X.; Zhang, P.; Li, X.; Fu, X.-M.; Wang, C.-Y. The underlying mechanisms of anti-hepatitis B effects of formula Le-Cao-Shi and its single herbs by network pharmacology and gut microbiota analysis. Biomed. Pharmacother. 2022, 148, 112692. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Li, W.; Zheng, B.; Wang, J.; Chen, S.; Wang, Y.; Ge, F.; Qin, B.; Zheng, X.; et al. Proline metabolism reprogramming of trained macrophages induced by early respiratory infection combined with allergen sensitization contributes to development of allergic asthma in childhood of mice. Front. Immunol. 2022, 13, 977235. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Zhu, M.-J. A sensitive GC/MS detection method for analyzing microbial metabolites short chain fatty acids in fecal and serum samples. Talanta 2019, 196, 249–254. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Chen, C.-C.; Lin, Y.-T.; Wu, W.-K.; Chang, L.C.; Lai, C.-H.; Wu, M.-S.; Kuo, C.-H. Evaluation and optimization of sample handling methods for quantification of short-chain fatty acids in human fecal samples by GC-MS. J. Proteome Res. 2019, 18, 1948–1957. [Google Scholar] [CrossRef]
- Zhu, W.; Qi, Y.; Wang, X.; Shi, X.; Chang, L.; Liu, J.; Zhu, L.; Jiang, J. Multi-Omics Approaches Revealed the Associations of Host Metabolism and Gut Microbiome With Phylogeny and Environmental Adaptation in Mountain Dragons. Front. Microbiol. 2022, 13, 913700. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Shen, X.; Liu, J.; Zhao, D.; Sun, Y.; Wang, L.; Liu, Y.; Gong, X.; Liu, Y.; et al. Serum metabolomics for early diagnosis of esophageal squamous cell carcinoma by UHPLC-QTOF/MS. Metabolomics 2016, 12, 116. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, D.; Wang, C.; Liu, H.; Wu, J.; Tan, L.; Liu, S.; Lv, H.; Wang, C.; Wang, F.; Liu, J. Integrated Multi-Omics Analysis Reveals Mountain-Cultivated Ginseng Ameliorates Cold-Stimulated Steroid-Resistant Asthma by Regulating Interactions among Microbiota, Genes, and Metabolites. Int. J. Mol. Sci. 2024, 25, 9110. https://doi.org/10.3390/ijms25169110
Tang D, Wang C, Liu H, Wu J, Tan L, Liu S, Lv H, Wang C, Wang F, Liu J. Integrated Multi-Omics Analysis Reveals Mountain-Cultivated Ginseng Ameliorates Cold-Stimulated Steroid-Resistant Asthma by Regulating Interactions among Microbiota, Genes, and Metabolites. International Journal of Molecular Sciences. 2024; 25(16):9110. https://doi.org/10.3390/ijms25169110
Chicago/Turabian StyleTang, Daohao, Chao Wang, Hanlin Liu, Junzhe Wu, Luying Tan, Sihan Liu, Haoming Lv, Cuizhu Wang, Fang Wang, and Jinping Liu. 2024. "Integrated Multi-Omics Analysis Reveals Mountain-Cultivated Ginseng Ameliorates Cold-Stimulated Steroid-Resistant Asthma by Regulating Interactions among Microbiota, Genes, and Metabolites" International Journal of Molecular Sciences 25, no. 16: 9110. https://doi.org/10.3390/ijms25169110
APA StyleTang, D., Wang, C., Liu, H., Wu, J., Tan, L., Liu, S., Lv, H., Wang, C., Wang, F., & Liu, J. (2024). Integrated Multi-Omics Analysis Reveals Mountain-Cultivated Ginseng Ameliorates Cold-Stimulated Steroid-Resistant Asthma by Regulating Interactions among Microbiota, Genes, and Metabolites. International Journal of Molecular Sciences, 25(16), 9110. https://doi.org/10.3390/ijms25169110






