Multi-Omics Integrative Analysis to Reveal the Impacts of Shewanella algae on the Development and Lifespan of Marine Nematode Litoditis marina
Abstract
1. Introduction
2. Results
2.1. S. algae Delayed Development and Shortened Lifespan of L. marina
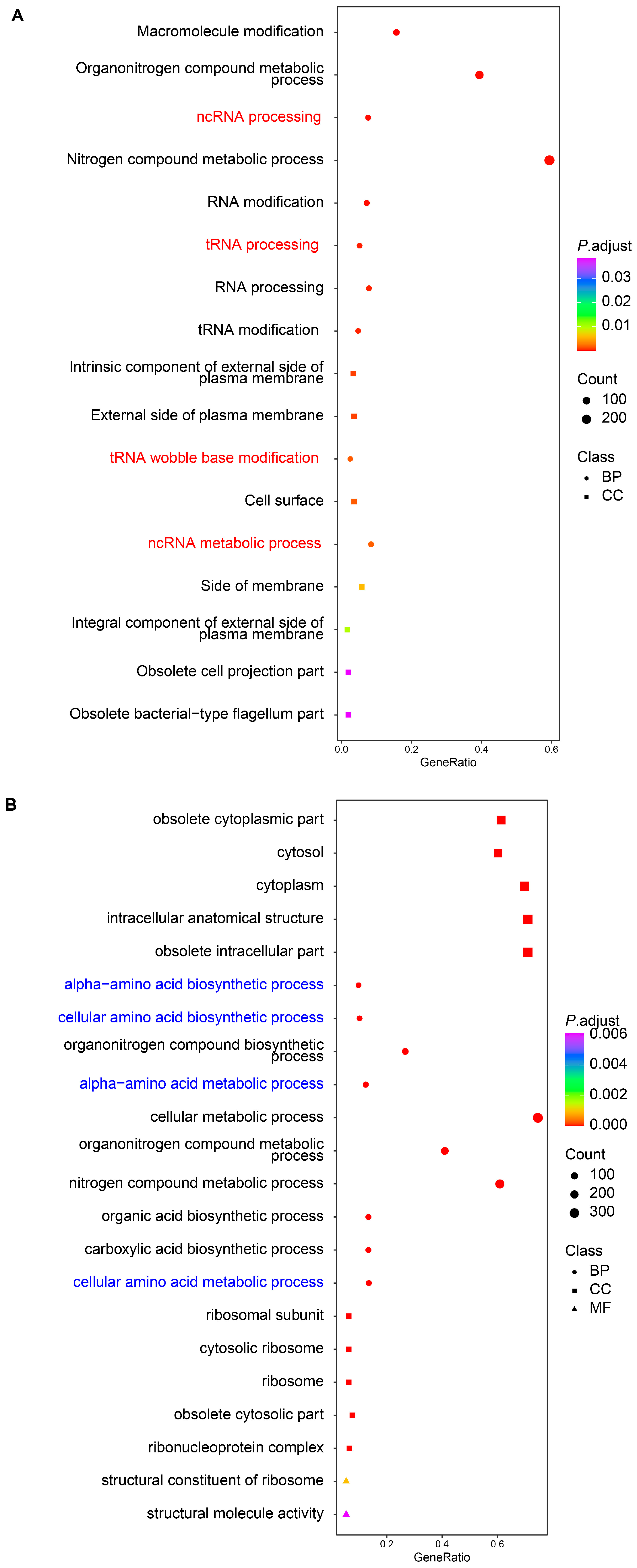
2.2. Transcriptomic Analysis of L. marina Feeding with S. algae versus E. coli OP50
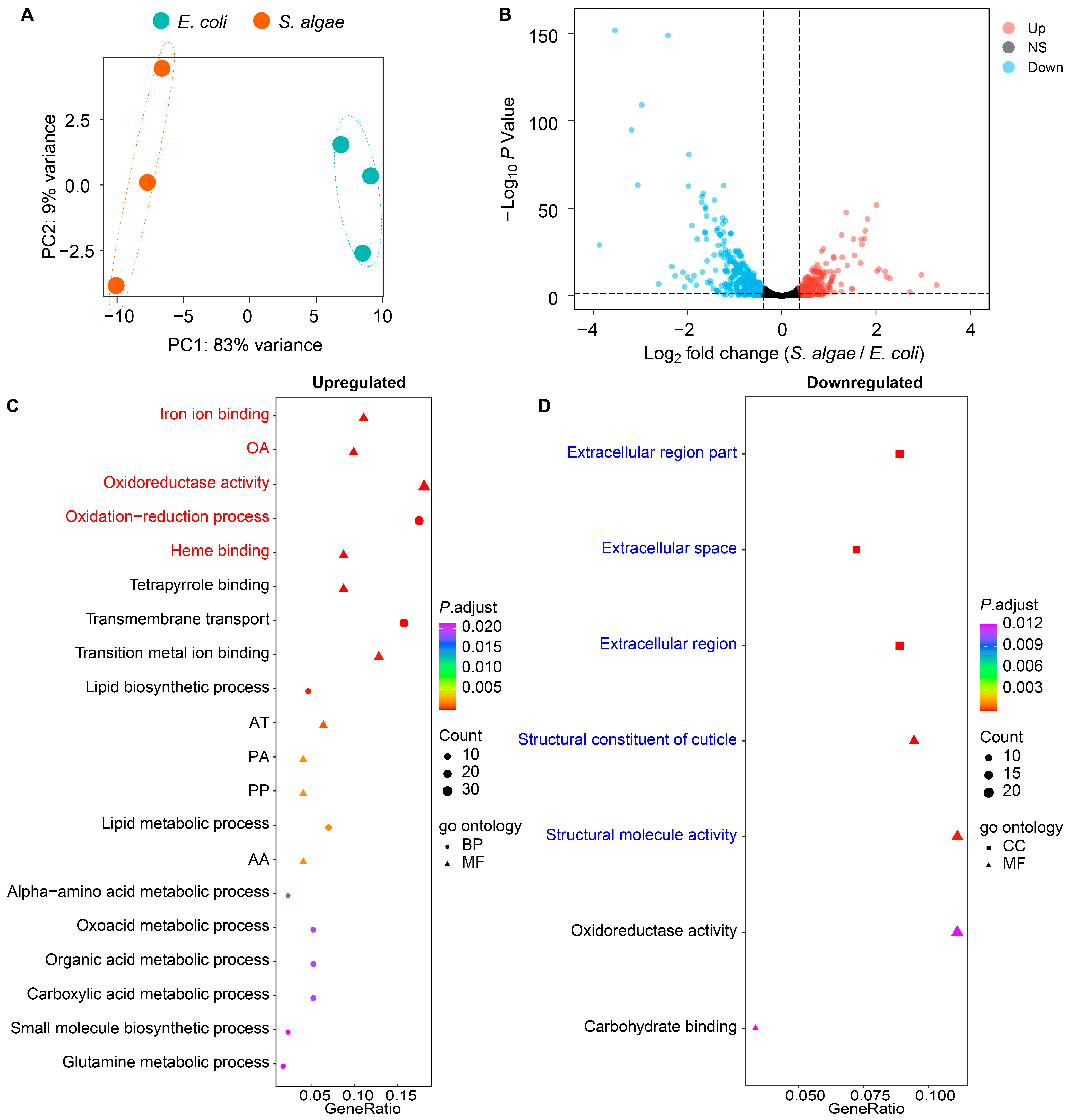
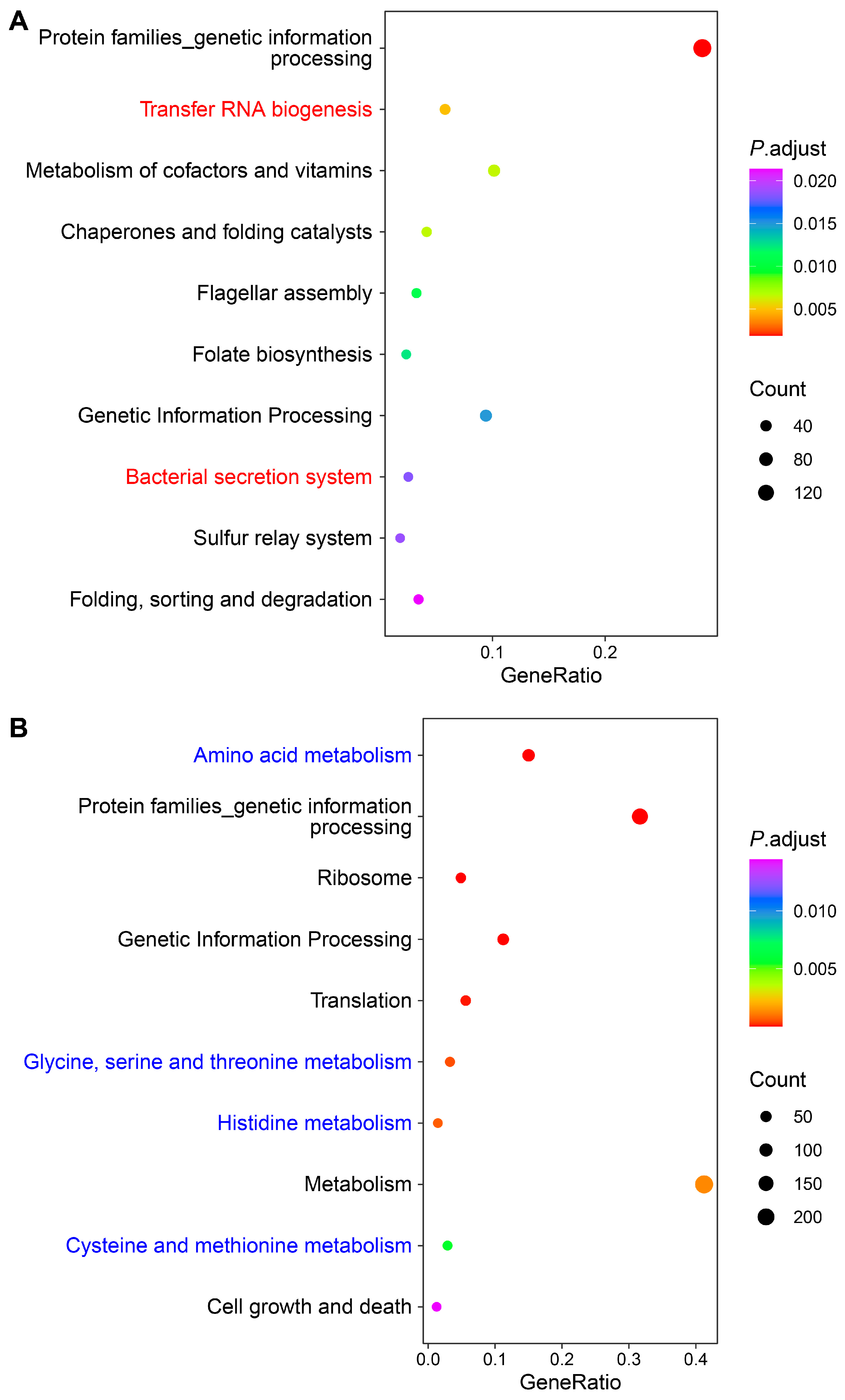
2.3. Transcriptomic Analysis of S. algae versus E. coli OP50
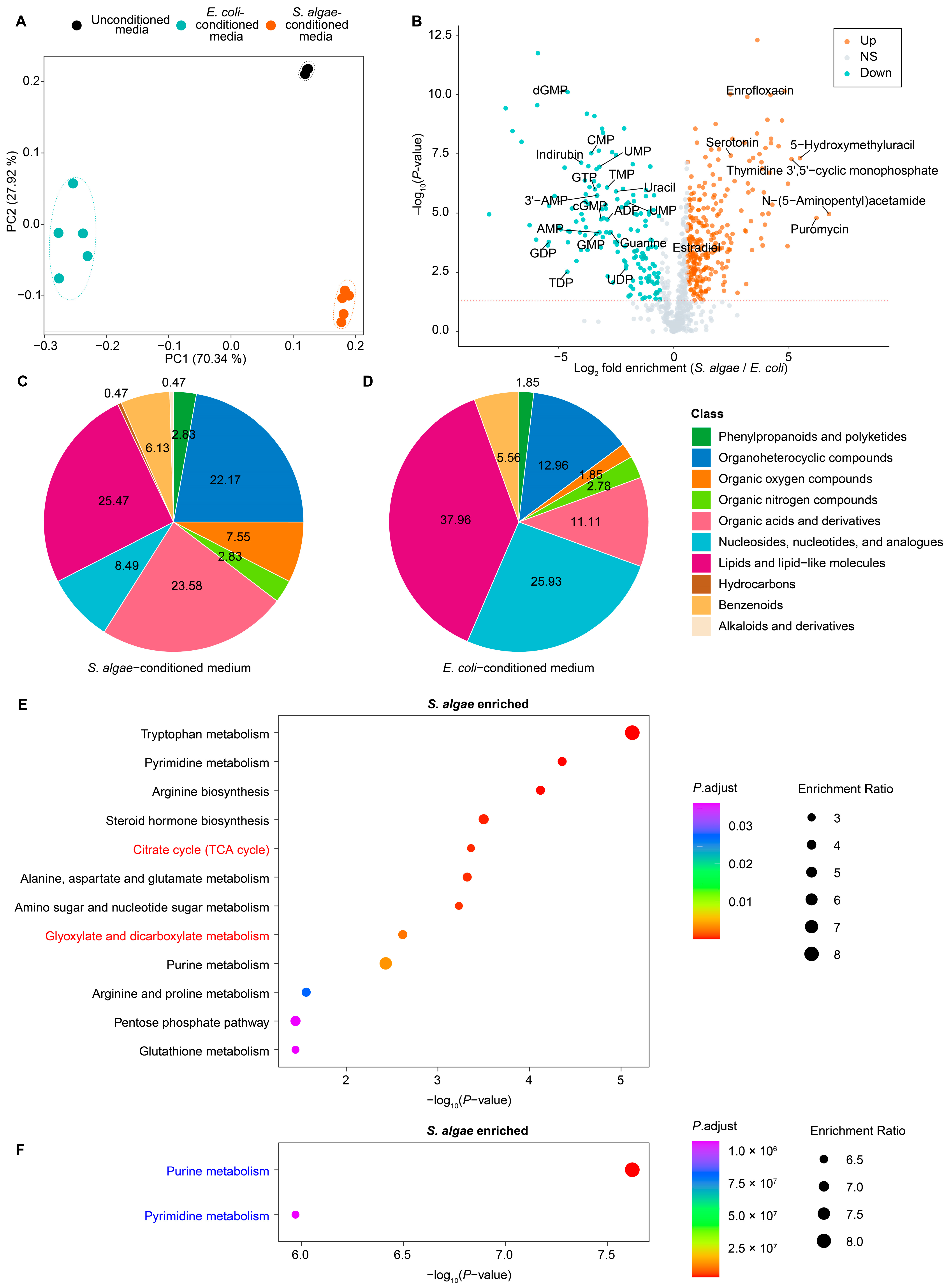
2.4. Metabolic Profiling of S. algae- and E. coli OP50-Conditioned Media
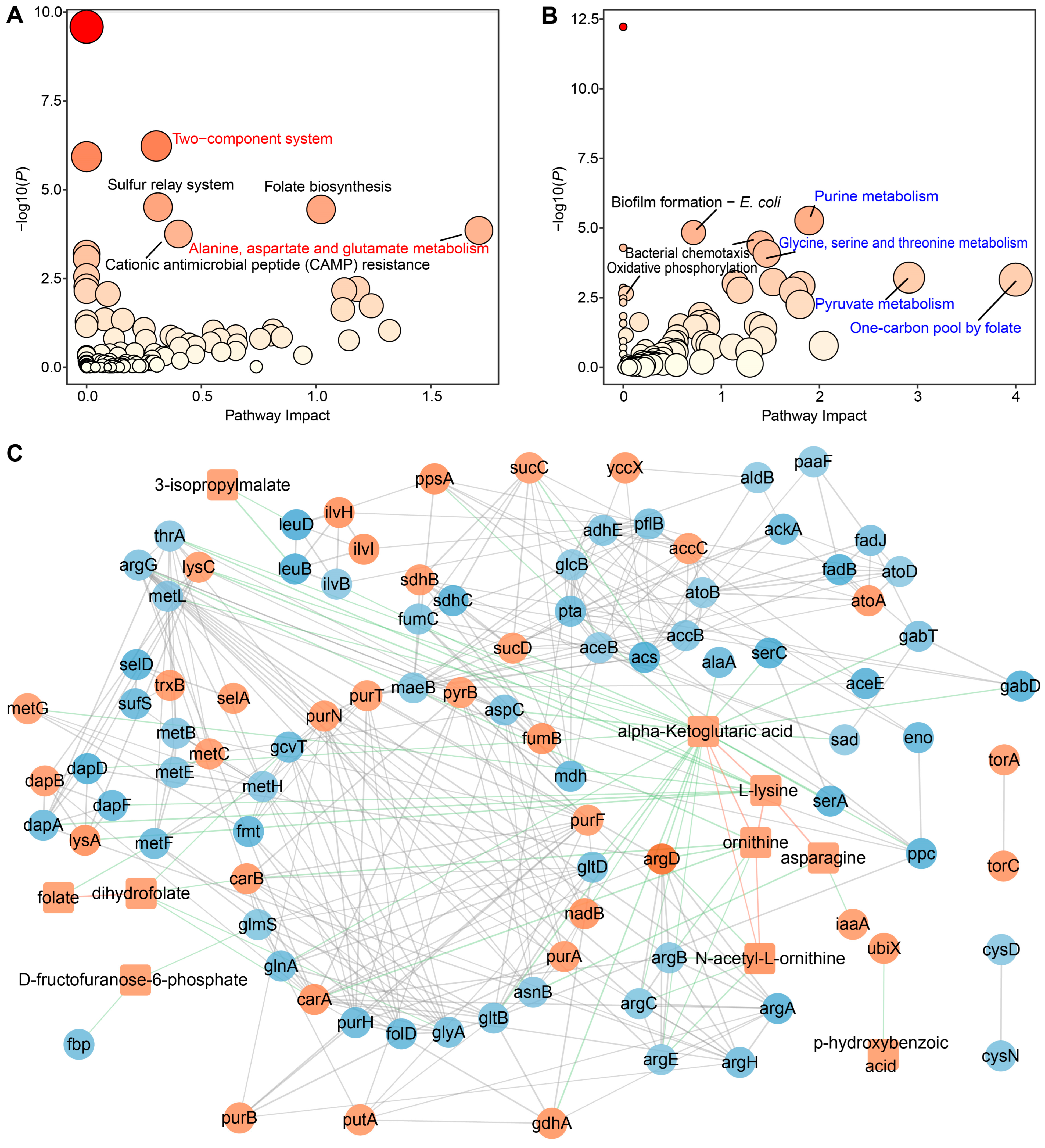
2.5. Joint Pathway Analysis of DAMs and DEGs in S. algae versus E. coli OP50
3. Discussion
3.1. Higher Expression of Genes in Iron Ion Binding and Oxidoreductase Activity Pathways Might Promote Aging of L. marina When Grown on S. algae
3.2. Decreased Expression of Extracellular Structural Components-Related Genes Might Delay the Development of L. marina fed with S. algae
3.3. Downregulated Bacterial Amino Acid Metabolism Genes in S. algae Might Delay Development and Shorten the Lifespan of L. marina
3.4. Higher Expression of Virulence-Related Genes in S. algae Might Contribute to the Delayed Development and Shortened Lifespan of L. marina
3.5. Impact of Toxic Metabolites and Nucleotide on L. marina Development and Lifespan
3.6. “Two-Component System” and “One-Carbon Pool by Folate” Pathways Might Modulate L. marina Physiology
4. Materials and Methods
4.1. Worm and Culture
4.2. Bacteria
4.3. Development and Lifespan Assays
4.4. Sample Collection
4.5. RNA Library Preparation, RNA Sequencing and Transcriptional Analysis of L. marina
4.6. RNA Library Preparation, RNA Sequencing, and Transcriptional Analysis of Bacteria
4.7. Metabolomics
4.8. Bacterial Transcriptome and Metabolism Conjoint Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Baruah, A.; Tomioka, M.; Iino, Y.; Kalita, M.C.; Khan, M. Caenorhabditis elegans: A model to understand host–microbe interactions. Cell. Mol. Life Sci. 2020, 77, 1229–1249. [Google Scholar] [CrossRef]
- Zhang, F.; Berg, M.; Dierking, K.; Félix, M.-A.; Shapira, M.; Samuel, B.S.; Schulenburg, H. Caenorhabditis elegans as a model for microbiome research. Front. Microbiol. 2017, 8, 485. [Google Scholar] [CrossRef]
- Samuel, B.S.; Rowedder, H.; Braendle, C.; Felix, M.A.; Ruvkun, G. Caenorhabditis elegans responses to bacteria from its natural habitats. Proc. Natl. Acad. Sci. USA 2016, 113, E3941–E3949. [Google Scholar] [CrossRef]
- Xue, Y.; Xie, Y.; Cao, X.; Zhang, l. The marine natural microbiome mediates physiological outcomes in host nematodes. bioRxiv 2023. [Google Scholar] [CrossRef]
- Poorbagher, H.; Lamare, M.D.; Barker, M.F. Effects of nutrition on somatic growth and reproductive strategy of the sea urchin Pseudechinus huttoni. Mar. Biol. Res. 2010, 6, 292–301. [Google Scholar] [CrossRef]
- Wang, H.; Ding, J.; Ding, S.; Chang, Y. Metabolomic changes and polyunsaturated fatty acid biosynthesis during gonadal growth and development in the sea urchin Strongylocentrotus intermedius. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 32, 100611. [Google Scholar] [CrossRef]
- del Mar Otero-Villanueva, M.; Kelly, M.S.; Burnell, G. How diet influences energy partitioning in the regular echinoid Psammechinus miliaris; constructing an energy budget. J. Exp. Mar. Biol. Ecol. 2004, 304, 159–181. [Google Scholar] [CrossRef]
- Jane, A.; Rasher, D.B.; Waller, J.; Annis, E.; Frederich, M. Rearing condition influences gene expression in postlarval American lobster (Homarus americanus). PLoS ONE 2024, 19, e0307169. [Google Scholar] [CrossRef]
- Chen, P.; Wu, Z.; Cui, Z.; Liu, C.; Lei, K.; Tian, S.; Mai, K.; Zhang, W. Effects of dietary bile acids levels on growth performance, anti-oxidative capacity, immunity and intestinal microbiota of abalone Haliotis discus hannai. Fish Shellfish Immunol. 2023, 142, 109114. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yu, X.; Chen, P.; Pan, M.; Liu, J.; Sahandi, J.; Zhou, W.; Mai, K.; Zhang, W. Dietary Clostridium autoethanogenum protein has dose-dependent influence on the gut microbiota, immunity, inflammation and disease resistance of abalone Haliotis discus hannai. Fish Shellfish Immunol. 2024, 151, 109737. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.L.I.; Mock, T.S.; Searle, K.; Rocker, M.M.; Turchini, G.M.; Francis, D.S. Growth performance and feed utilisation of Australian hybrid abalone (Haliotis rubra × Haliotis laevigata) fed increasing dietary protein levels at three water temperatures. Br. J. Nutr. 2024, 131, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Abdelsamad, A.E.M.; Said, R.E.M.; Assas, M.; Gaafar, A.Y.; Hamouda, A.H.; Mahdy, A. Effects of dietary supplementation with Bacillus velezensis on the growth performance, body composition, antioxidant, immune-related gene expression, and histology of Pacific white shrimp, Litopenaeus vannamei. BMC Vet. Res. 2024, 20, 368. [Google Scholar] [CrossRef]
- Ye, Y.; Li, S.; Du, X.; Zhang, L.; Bao, N.; Li, Y.; Zhao, Y. Effects of dietary 5-aminolevulinic acid on growth performance and nonspecific immunity of Litopenaeus vannamei, as determined by transcriptomic analysis. Fish Shellfish Immunol. 2024, 151, 109746. [Google Scholar] [CrossRef]
- Liu, R.; Ding, Y.; Jing, F.; Chen, Z.; Su, C.; Pan, L. Effects of dietary glycerol monolaurate on growth and digestive performance, lipid metabolism, immune defense and gut microbiota of shrimp (Penaeus vannamei). Fish Shellfish Immunol. 2024, 151, 109666. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Li, J.; Liu, P.; Li, J.; Zhao, F. Antioxidant, transcriptome and the metabolome response to dietary astaxanthin in Exopalaemon carinicauda. Front. Physiol. 2022, 13, 859305. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, P.; Xue, B.; Cao, X.; Ren, X.; Wang, L.; Sun, Y.; Yang, H.; Zhang, L. Establishment of a marine nematode model for animal functional genomics, environmental adaptation and developmental evolution. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, F.; Gao, S.; Liang, Y.; Long, H.; Lv, Z.; Su, Y.; Ye, N.; Zhang, L.; Zhao, C.; et al. Biodiversity-based development and evolution: The emerging research systems in model and non-model organisms. Sci. China Life Sci. 2021, 64, 1236–1280. [Google Scholar] [CrossRef]
- Vogel, B.F.; Jørgensen, K.; Christensen, H.; Olsen, J.E.; Gram, L. Differentiation of Shewanella putrefaciens and Shewanella alga on the basis of whole-cell protein profiles, ribotyping, phenotypic characterization, and 16S rRNA gene sequence analysis. Appl. Environ. Microbiol. 1997, 63, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Rossello-Mora, R.A.; Caccavo Jr, F.; Osterlehner, K.; Springer, N.; Spring, S.; Schüler, D.; Ludwig, W.; Amann, R.; Vanncanneyt, M.; Schleifer, K.H. Isolation and taxonomic characterization of a halotolerant, facultatively iron-reducing bacterium. Syst. Appl. Microbiol. 1995, 17, 569–573. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.J.; Higdon, S.M.; Thorell, K.; Tellgren-Roth, C.; Sjöling, Å.; Galperin, M.Y.; Krell, T.; Römling, U. Comparative genomics of cyclic di-GMP metabolism and chemosensory pathways in Shewanella algae strains: Novel bacterial sensory domains and functional insights into lifestyle regulation. mSystems 2022, 7, e01518–e01521. [Google Scholar] [CrossRef] [PubMed]
- Jatuyosporn, T.; Laohawutthichai, P.; Romo, J.P.O.; Gallardo-Becerra, L.; Lopez, F.S.; Tassanakajon, A.; Ochoa-Leyva, A.; Krusong, K. White spot syndrome virus impact on the expression of immune genes and gut microbiome of black tiger shrimp Penaeus monodon. Sci. Rep. 2023, 13, 996. [Google Scholar] [CrossRef] [PubMed]
- Nozue, H.; Hayashi, T.; Hashimoto, Y.; Ezaki, T.; Hamasaki, K.; Ohwada, K.; Terawaki, Y. Isolation and characterization of Shewanella alga from human clinical specimens and emendation of the description of S. alga Simidu et al., 1990, 335. Int. J. Syst. Evol. Microbiol. 1992, 42, 628–634. [Google Scholar] [CrossRef]
- Dominguez, H.; Vogel, B.F.; Gram, L.; Hoffmann, S.; Schaebel, S. Shewanella alga bacteremia in two patients with lower leg ulcers. Clin. Infect. Dis. 1996, 22, 1036–1039. [Google Scholar] [CrossRef][Green Version]
- Holt, H.M.; Søgaard, P.; Gahrn-Hansen, B. Ear infections with Shewanella alga: A bacteriologic, clinical and epidemiologic study of 67 cases. Clin. Microbiol. Infect. 1997, 3, 329–334. [Google Scholar] [CrossRef]
- Botelho-Nevers, E.; Gouriet, F.; Rovery, C.; Paris, P.; Roux, V.; Raoult, D.; Brouqui, P. First case of osteomyelitis due to Shewanella algae. J. Clin. Microbiol. 2005, 43, 5388–5390. [Google Scholar] [CrossRef]
- Serrano-Coll, H.; Arrieta, A.; Miranda, J.; López, A.; Mattar, A.S.; Arrieta, G.; Mattar, S. Case Report: A Case of Sepsis due to Shewanella algae Infection in the Colombian Caribbean. Am. J. Trop. Med. Hyg. 2023, 109, 35–37. [Google Scholar] [CrossRef]
- Fernandes, S.; Sérvio, R.; Silva, A.R.; Tavares, R.; Rodrigues, P. Shewanella algae, an emerging human pathogen: A series of four cases from a Portuguese hospital. Cureus 2023, 15, e33686. [Google Scholar] [CrossRef]
- Symanzik, C.; Esser, J.; Pfennigwerth, N.; Reuter, C.; Bronnert, J.; Grade, M. Shewanella algae bacteraemia in a patient with a chronic ulcer after contact with seawater on vacation in turkey: A case report from a German maximum-care hospital. New Microbes New Infect. 2022, 48, 101016. [Google Scholar] [CrossRef]
- Ainoda, Y.; Tanaka, E.; Wajima, T.; Nakaminami, H.; Hirota, Y.; Matsushita, T.; Hirai, Y. A case of Shewanella algae-induced bacteremia in Japan: Case report and literature review. J. Infect. Chemother. 2022, 28, 1430–1432. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, gkae253. [Google Scholar] [CrossRef] [PubMed]
- Beier, D.; Gross, R. Regulation of bacterial virulence by two-component systems. Curr. Opin. Microbiol. 2006, 9, 143–152. [Google Scholar] [CrossRef]
- Feng, M.; Gao, B.; Garcia, L.R.; Sun, Q. Microbiota-derived metabolites in regulating the development and physiology of Caenorhabditis elegans. Front. Microbiol. 2023, 14, 1035582. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Ruiz, D.; Case, H.; Jinkerson, R.E.; Sun, Q. Engineering bacterial warriors: Harnessing microbes to modulate animal physiology. Curr. Opin. Biotechnol. 2024, 87, 103113. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Han, M. Microbial siderophore enterobactin promotes mitochondrial iron uptake and development of the host via interaction with ATP synthase. Cell 2018, 175, 571–582.e11. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Olmedo, M.; Holdorf, A.D.; Shang, Y.; Artal-Sanz, M.; Yilmaz, L.S.; Walhout, A.J. A delicate balance between bacterial iron and reactive oxygen species supports optimal C. elegans development. Cell Host Microbe 2019, 26, 400–411.e3. [Google Scholar] [CrossRef]
- Tian, D.; Han, M. Bacterial peptidoglycan muropeptides benefit mitochondrial homeostasis and animal physiology by acting as ATP synthase agonists. Dev. Cell 2022, 57, 361–372.e5. [Google Scholar] [CrossRef]
- Stuhr, N.L.; Curran, S.P. Bacterial diets differentially alter lifespan and healthspan trajectories in C. elegans. Commun. Biol. 2020, 3, 653. [Google Scholar] [CrossRef]
- Han, B.; Sivaramakrishnan, P.; Lin, C.J.; Neve, I.A.A.; He, J.; Tay, L.W.R.; Sowa, J.N.; Sizovs, A.; Du, G.; Wang, J.; et al. Microbial genetic composition tunes host longevity. Cell 2017, 169, 1249–1262.e13. [Google Scholar] [CrossRef]
- Shin, M.G.; Lee, J.W.; Han, J.S.; Lee, B.; Jeong, J.H.; Park, S.H.; Kim, J.H.; Jang, S.; Park, M.; Kim, S.Y.; et al. Bacteria-derived metabolite, methylglyoxal, modulates the longevity of C. elegans through TORC2/SGK-1/DAF-16 signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 17142–17150. [Google Scholar] [CrossRef]
- Sailer, S.; Keller, M.A.; Werner, E.R.; Watschinger, K. The emerging physiological role of AGMO 10 years after its gene identification. Life 2021, 11, 88. [Google Scholar] [CrossRef]
- Michels, H.; Koopman, M.; Seinstra, R.I.; van Faassen, M. Tryptophan Metabolism in Aging and Disease: Transcriptome Profiling in C. elegans Reveals That TDO and Other Amino Acid Dioxygenases Regulate Mitochondrial Capacity and Healthspan; University of Groningen: Groningen, The Netherlands, 2017. [Google Scholar]
- Dang, H.; Castro-Portuguez, R.; Espejo, L.; Backer, G.; Freitas, S.; Spence, E.; Meyers, J.; Shuck, K.; Gardea, E.A.; Chang, L.M.; et al. On the benefits of the tryptophan metabolite 3-hydroxyanthranilic acid in Caenorhabditis elegans and mouse aging. Nat. Commun. 2023, 14, 8338. [Google Scholar] [CrossRef] [PubMed]
- Sutphin, G.L.; Backer, G.; Sheehan, S.; Bean, S.; Corban, C.; Liu, T.; Peters, M.J.; van Meurs, J.B.; Murabito, J.M.; Johnson, A.D. Caenorhabditis elegans orthologs of human genes differentially expressed with age are enriched for determinants of longevity. Aging Cell 2017, 16, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.P.; Abate, J.P.; Dilks, K.; Landis, J.; Ashraf, J.; Murphy, C.T.; Blackwell, T.K. Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell 2009, 8, 524–541. [Google Scholar] [CrossRef]
- Engelmann, I.; Griffon, A.; Tichit, L.; Montanana-Sanchis, F.; Wang, G.; Reinke, V.; Waterston, R.H.; Hillier, L.W.; Ewbank, J.J. A comprehensive analysis of gene expression changes provoked by bacterial and fungal infection in C. elegans. PLoS ONE 2011, 6, e19055. [Google Scholar] [CrossRef]
- Schmeisser, S.; Priebe, S.; Groth, M.; Monajembashi, S.; Hemmerich, P.; Guthke, R.; Platzer, M.; Ristow, M. Neuronal ROS signaling rather than AMPK/sirtuin-mediated energy sensing links dietary restriction to lifespan extension. Mol. Metab. 2013, 2, 92–102. [Google Scholar] [CrossRef]
- Treitz, C.; Cassidy, L.; Höckendorf, A.; Leippe, M.; Tholey, A. Quantitative proteome analysis of Caenorhabditis elegans upon exposure to nematicidal Bacillus thuringiensis. J. Proteom. 2015, 113, 337–350. [Google Scholar] [CrossRef]
- Sundaram, M.V.; Pujol, N. The Caenorhabditis elegans cuticle and precuticle: A model for studying dynamic apical extracellular matrices in vivo. Genetics 2024, 227, iyae072. [Google Scholar] [CrossRef]
- Ewald, C.Y.; Landis, J.N.; Abate, J.P.; Murphy, C.T.; Blackwell, T.K. Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity. Nature 2015, 519, 97–101. [Google Scholar] [CrossRef]
- Zečić, A.; Dhondt, I.; Braeckman, B.P. The nutritional requirements of Caenorhabditis elegans. Genes Nutr. 2019, 14, 15. [Google Scholar] [CrossRef]
- Edwards, C.; Canfield, J.; Copes, N.; Brito, A.; Rehan, M.; Lipps, D.; Brunquell, J.; Westerheide, S.D.; Bradshaw, P.C. Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans. BMC Genet. 2015, 16, 8. [Google Scholar] [CrossRef]
- Fuchs, S.; Bundy, J.G.; Davies, S.K.; Viney, J.M.; Swire, J.S.; Leroi, A.M. A metabolic signature of long life in Caenorhabditis elegans. BMC Biol. 2010, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Zarse, K.; Schmeisser, S.; Groth, M.; Priebe, S.; Beuster, G.; Kuhlow, D.; Guthke, R.; Platzer, M.; Kahn, C.R.; Ristow, M. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 2012, 15, 451–465. [Google Scholar] [CrossRef]
- Van Der Goot, A.T.; Zhu, W.; Vázquez-Manrique, R.P.; Seinstra, R.I.; Dettmer, K.; Michels, H.; Farina, F.; Krijnen, J.; Melki, R.; Buijsman, R.C. Delaying aging and the aging-associated decline in protein homeostasis by inhibition of tryptophan degradation. Proc. Natl. Acad. Sci. USA 2012, 109, 14912–14917. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wang, H.D.; Wu, J.; Ren, J.; Meng, L.; Wu, Q.; Dong, H.; Wu, J.; Kao, T.Y.; et al. Escherichia coli noncoding RNAs can affect gene expression and physiology of Caenorhabditis elegans. Nat. Commun. 2012, 3, 1073. [Google Scholar] [CrossRef]
- Shippy, D.C.; Fadl, A.A. RNA modification enzymes encoded by the gid operon: Implications in biology and virulence of bacteria. Microb. Pathog. 2015, 89, 100–107. [Google Scholar] [CrossRef]
- Shippy, D.C.; Fadl, A.A. tRNA modification enzymes GidA and MnmE: Potential role in virulence of bacterial pathogens. Int. J. Mol. Sci. 2014, 15, 18267–18280. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, P.; Masiuk, H.; Pruss, A.; Łopusiewicz, Ł.; Sienkiewicz, M.; Wojciechowska-Koszko, I.; Roszkowska, P.; Bania, J.; Guenther, S.; Dołęgowska, B. Clonal diversity, antimicrobial susceptibility and presence of genes encoding virulence factors in Staphylococcus aureus strains isolated from cut wound infections. Curr. Microbiol. 2022, 79, 144. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Páez, A.; Villarroya, M.; Armengod, M.-E. The Escherichia coli RlmN methyltransferase is a dual-specificity enzyme that modifies both rRNA and tRNA and controls translational accuracy. RNA 2012, 18, 1783–1795. [Google Scholar] [CrossRef]
- Goodier, R.I.; Ahmer, B.M. SirA orthologs affect both motility and virulence. J. Bacteriol. 2001, 183, 2249–2258. [Google Scholar] [CrossRef]
- Stauder, M.; Huq, A.; Pezzati, E.; Grim, C.J.; Ramoino, P.; Pane, L.; Colwell, R.R.; Pruzzo, C.; Vezzulli, L. Role of GbpA protein, an important virulence-related colonization factor, for Vibrio cholerae’s survival in the aquatic environment. Environ. Microbiol. Rep. 2012, 4, 439–445. [Google Scholar] [CrossRef]
- Cadona, J.S.; Bustamante, A.V.; Parma, A.E.; Lucchesi, P.M.A.; Sanso, A.M. Distribution of additional virulence factors related to adhesion and toxicity in Shiga toxin-producing Escherichia coli isolated from raw products in Argentina. Lett. Appl. Microbiol. 2013, 56, 449–455. [Google Scholar] [CrossRef]
- Dorrell, N.; Guigue-Talet, P.; Spencer, S.; Foulonge, V.; David, O.; Wren, B.W. Investigation into the role of the response regulator NtrC in the metabolism and virulence of Brucella suis. Microb. Pathog. 1999, 27, 1–11. [Google Scholar] [CrossRef]
- Giordano-Santini, R.; Dupuy, D. Selectable genetic markers for nematode transgenesis. Cell. Mol. Life Sci. 2011, 68, 1917–1927. [Google Scholar] [CrossRef]
- Gomes, M.P.; Brito, J.C.M.; Rocha, D.C.; Navarro-Silva, M.A.; Juneau, P. Individual and combined effects of amoxicillin, enrofloxacin, and oxytetracycline on Lemna minor physiology. Ecotoxicol. Environ. Saf. 2020, 203, 111025. [Google Scholar] [CrossRef] [PubMed]
- Sehonova, P.; Tokanova, N.; Hodkovicova, N.; Kroupova, H.K.; Tumova, J.; Blahova, J.; Marsalek, P.; Plhalova, L.; Doubkova, V.; Dobsikova, R. Oxidative stress induced by fluoroquinolone enrofloxacin in zebrafish (Danio rerio) can be ameliorated after a prolonged exposure. Environ. Toxicol. Pharmacol. 2019, 67, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Song, G.; Liu, X.; Xu, M.; Li, Y. Nucleotides as optimal candidates for essential nutrients in living organisms: A review. J. Funct. Foods 2021, 82, 104498. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Rudolph, F.B.; Van Buren, C.T. The role of dietary sources of nucleotides in immune function: A review. J. Nutr. 1994, 124, 1442S–1446S. [Google Scholar] [CrossRef]
- Wan, Q.-L.; Meng, X.; Fu, X.; Chen, B.; Yang, J.; Yang, H.; Zhou, Q. Intermediate metabolites of the pyrimidine metabolism pathway extend the lifespan of C. elegans through regulating reproductive signals. Aging 2019, 11, 3993. [Google Scholar] [CrossRef]
- Lorenz, M.C.; Fink, G.R. Life and death in a macrophage: Role of the glyoxylate cycle in virulence. Eukaryot. Cell 2002, 1, 657–662. [Google Scholar] [CrossRef]
- Mercado-Lubo, R.; Leatham, M.P.; Conway, T.; Cohen, P.S. Salmonella enterica serovar Typhimurium mutants unable to convert malate to pyruvate and oxaloacetate are avirulent and immunogenic in BALB/c mice. Infect. Immun. 2009, 77, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Alegado, R.A.; Chin, C.Y.; Monack, D.M.; Tan, M.W. The two-component sensor kinase KdpD is required for Salmonella typhimurium colonization of Caenorhabditis elegans and survival in macrophages. Cell. Microbiol. 2011, 13, 1618–1637. [Google Scholar] [CrossRef] [PubMed]
- Cabreiro, F.; Au, C.; Leung, K.-Y.; Vergara-Irigaray, N.; Cochemé, H.M.; Noori, T.; Weinkove, D.; Schuster, E.; Greene, N.D.; Gems, D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 2013, 153, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Annibal, A.; Tharyan, R.G.; Schonewolff, M.F.; Tam, H.; Latza, C.; Auler, M.M.K.; Grönke, S.; Partridge, L.; Antebi, A. Regulation of the one carbon folate cycle as a shared metabolic signature of longevity. Nat. Commun. 2021, 12, 3486. [Google Scholar] [CrossRef]
- Zhang, P.; Xue, B.; Yang, H.; Zhang, L. Transcriptome Responses to Different Salinity Conditions in Litoditis marina, Revealed by Long-Read Sequencing. Genes 2024, 15, 317. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, L. Transcriptomic and proteomic analysis of marine nematode Litoditis marina acclimated to different salinities. Genes 2022, 13, 651. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Mirdita, M.; Steinegger, M.; Söding, J. MMseqs2 desktop and local web server app for fast, interactive sequence searches. Bioinformatics 2019, 35, 2856–2858. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Kuhn, M.; von Mering, C.; Campillos, M.; Jensen, L.J.; Bork, P. STITCH: Interaction networks of chemicals and proteins. Nucleic Acids Res. 2007, 36, D684–D688. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Santos, A.; Von Mering, C.; Jensen, L.J.; Bork, P.; Kuhn, M. STITCH 5: Augmenting protein–chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2016, 44, D380–D384. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Xue, B.; Zhang, L. Multi-Omics Integrative Analysis to Reveal the Impacts of Shewanella algae on the Development and Lifespan of Marine Nematode Litoditis marina. Int. J. Mol. Sci. 2024, 25, 9111. https://doi.org/10.3390/ijms25169111
Xue Y, Xue B, Zhang L. Multi-Omics Integrative Analysis to Reveal the Impacts of Shewanella algae on the Development and Lifespan of Marine Nematode Litoditis marina. International Journal of Molecular Sciences. 2024; 25(16):9111. https://doi.org/10.3390/ijms25169111
Chicago/Turabian StyleXue, Yiming, Beining Xue, and Liusuo Zhang. 2024. "Multi-Omics Integrative Analysis to Reveal the Impacts of Shewanella algae on the Development and Lifespan of Marine Nematode Litoditis marina" International Journal of Molecular Sciences 25, no. 16: 9111. https://doi.org/10.3390/ijms25169111
APA StyleXue, Y., Xue, B., & Zhang, L. (2024). Multi-Omics Integrative Analysis to Reveal the Impacts of Shewanella algae on the Development and Lifespan of Marine Nematode Litoditis marina. International Journal of Molecular Sciences, 25(16), 9111. https://doi.org/10.3390/ijms25169111





